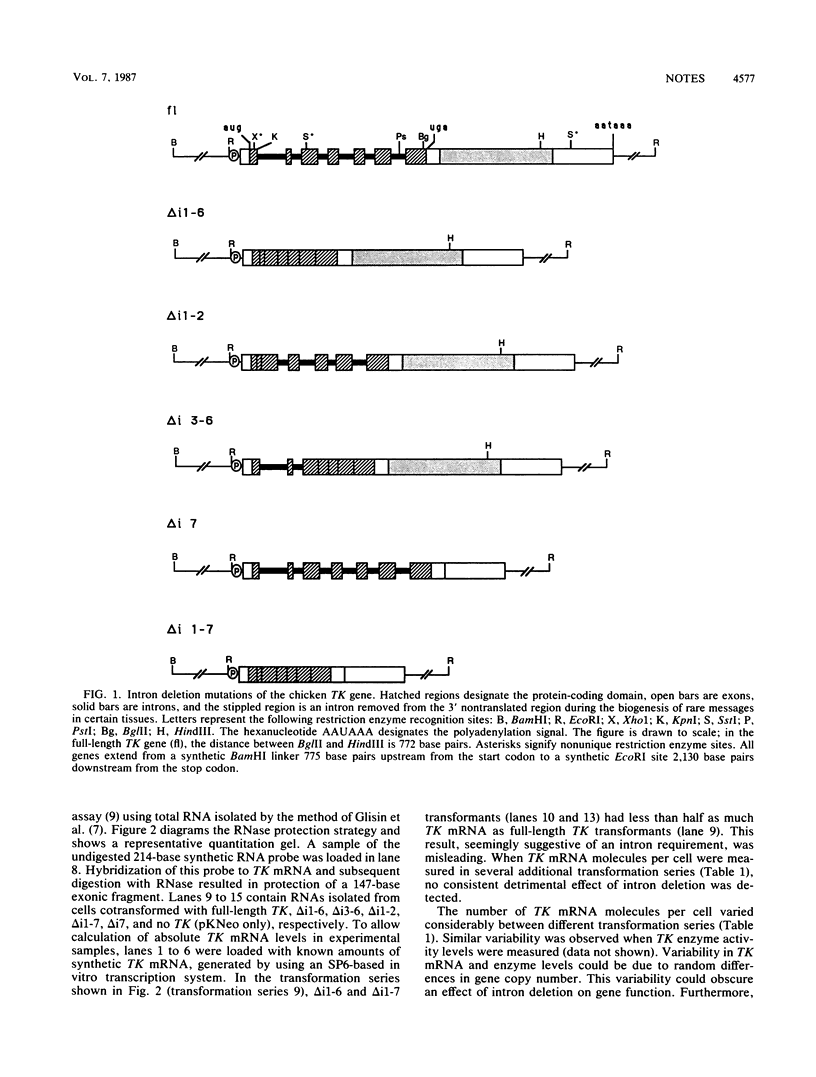
Abstract
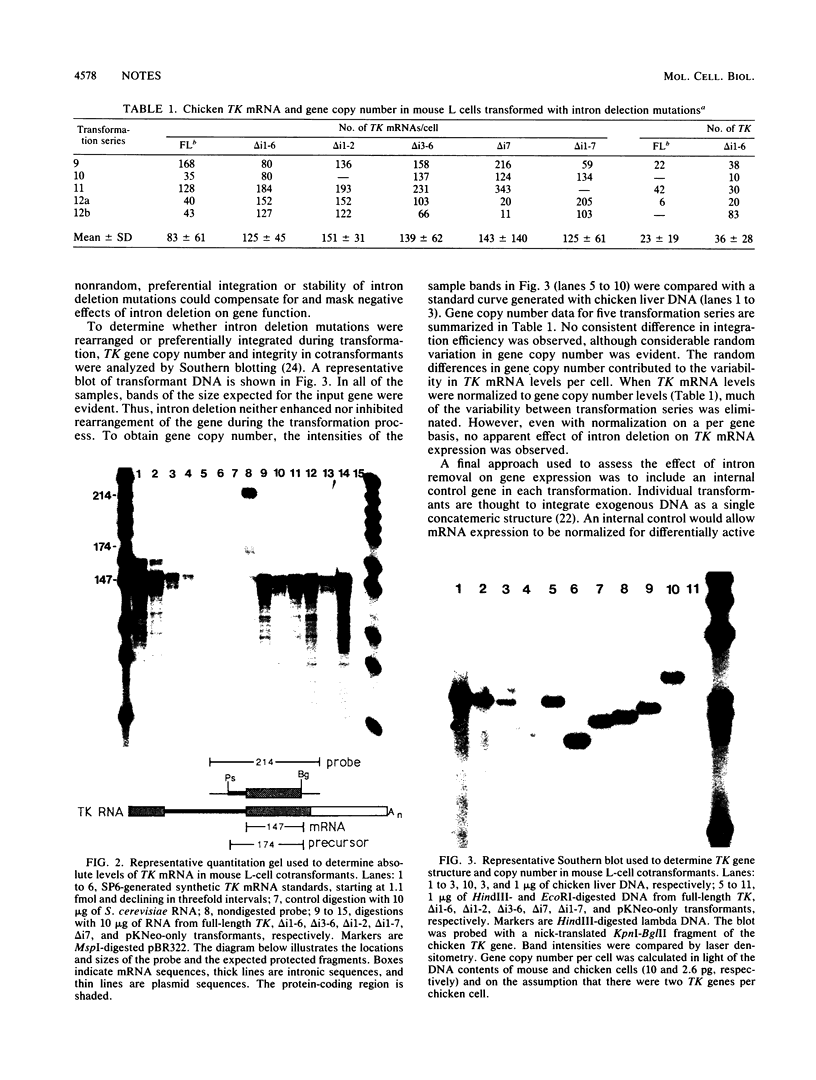
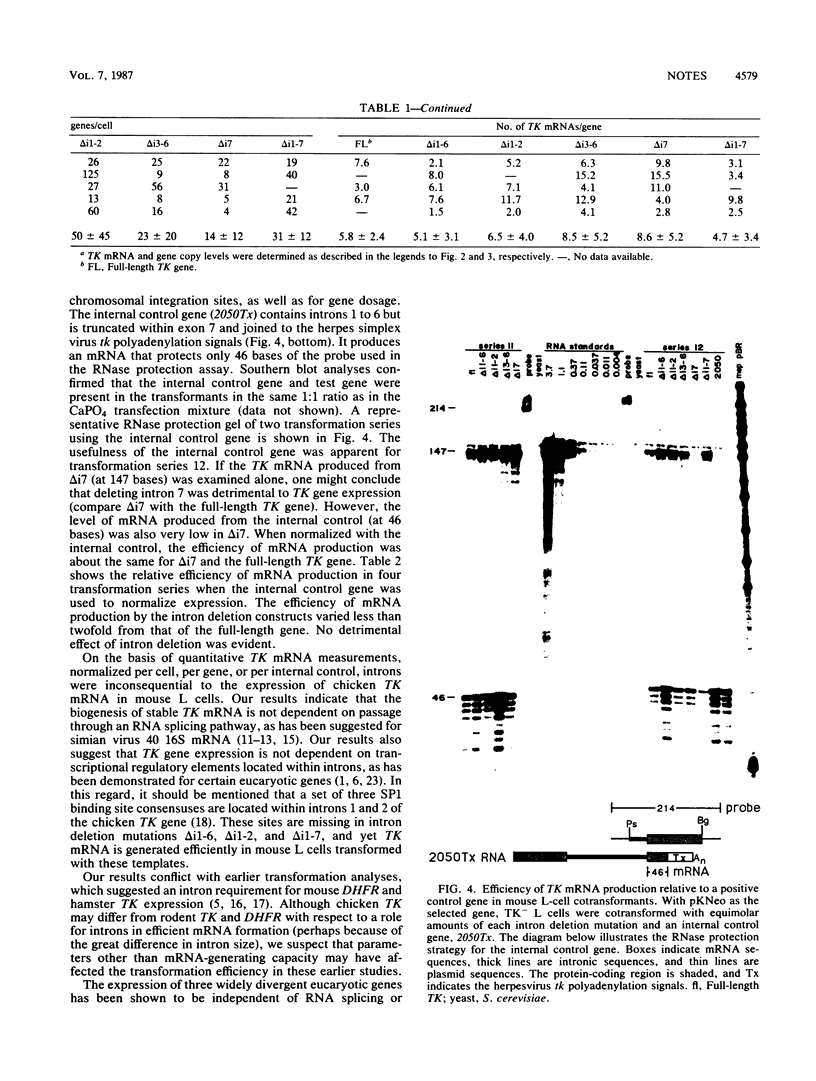
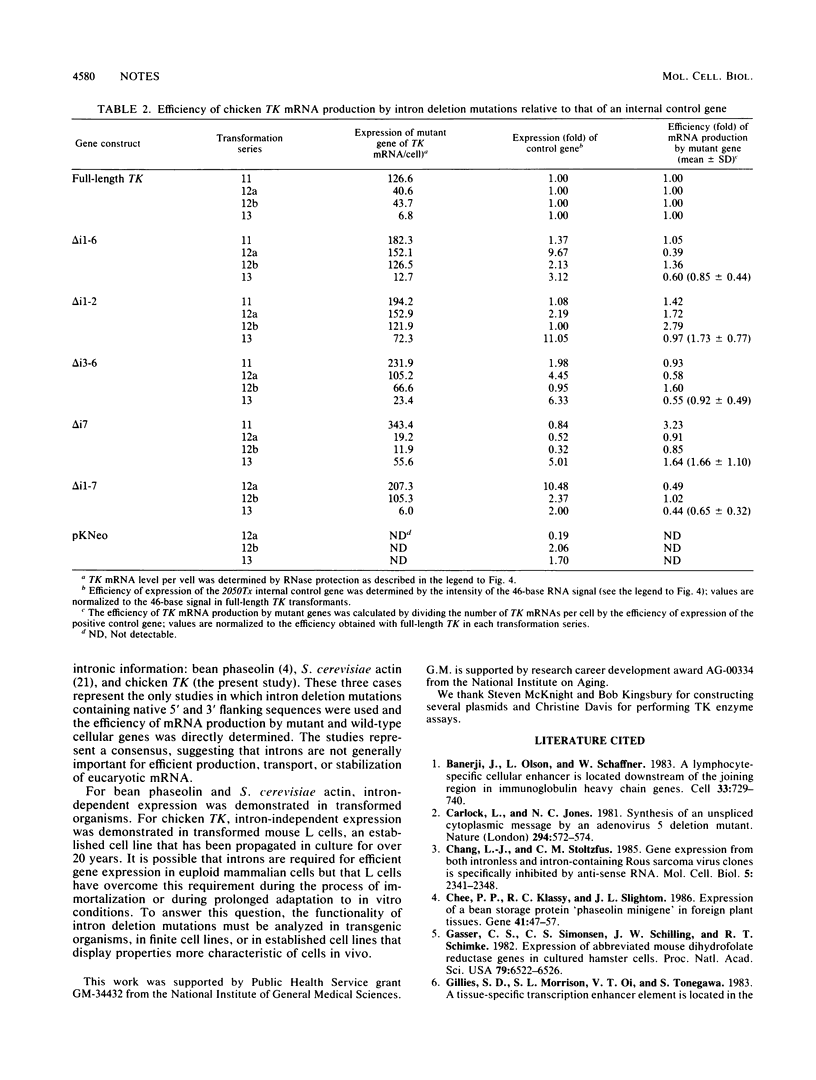
TK mRNA levels were determined in mouse L cells transformed with intron deletion mutations of the chicken TK gene. Whether normalized per cell, per integrated gene, or per internal control signal, intron deletion did not diminish the efficiency of TK mRNA formation in transformed L cells. The results demonstrated that introns are not required for efficient biogenesis of cellular mRNA in transformed mouse L cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Carlock L., Jones N. C. Synthesis of an unspliced cytoplasmic message by an adenovirus 5 deletion mutant. Nature. 1981 Dec 10;294(5841):572–574. doi: 10.1038/294572a0. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Stoltzfus C. M. Gene expression from both intronless and intron-containing Rous sarcoma virus clones is specifically inhibited by anti-sense RNA. Mol Cell Biol. 1985 Sep;5(9):2341–2348. doi: 10.1128/mcb.5.9.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee P. P., Klassy R. C., Slightom J. L. Expression of a bean storage protein 'phaseolin minigene' in foreign plant tissues. Gene. 1986;41(1):47–57. doi: 10.1016/0378-1119(86)90266-0. [DOI] [PubMed] [Google Scholar]
- Gasser C. S., Simonsen C. C., Schilling J. W., Schimke R. T. Expression of abbreviated mouse dihydrofolate reductase genes in cultured hamster cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6522–6526. doi: 10.1073/pnas.79.21.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gross M. K., Kainz M. S., Merrill G. F. The chicken thymidine kinase gene is transcriptionally repressed during terminal differentiation: the associated decline in TK mRNA cannot account fully for the disappearance of TK enzyme activity. Dev Biol. 1987 Aug;122(2):439–451. doi: 10.1016/0012-1606(87)90308-3. [DOI] [PubMed] [Google Scholar]
- Gruss P., Khoury G. Rescue of a splicing defective mutant by insertion of an heterologous intron. Nature. 1980 Aug 7;286(5773):634–637. doi: 10.1038/286634a0. [DOI] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Smith K. D., Boyer S. H., Leder P. SV40 recombinants carrying rabbit beta-globin gene coding sequences. Cell. 1979 Jul;17(3):725–735. doi: 10.1016/0092-8674(79)90279-4. [DOI] [PubMed] [Google Scholar]
- Hofbauer R., Müllner E., Seiser C., Wintersberger E. Cell cycle regulated synthesis of stable mouse thymidine kinase mRNA is mediated by a sequence within the cDNA. Nucleic Acids Res. 1987 Jan 26;15(2):741–752. doi: 10.1093/nar/15.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Khoury G. Deletion mutants of simian virus 40 defective in biosynthesis of late viral mRNA. Proc Natl Acad Sci U S A. 1979 Jan;76(1):71–75. doi: 10.1073/pnas.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. A. Structure and expression of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1986 Jun;6(6):1998–2010. doi: 10.1128/mcb.6.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Harland R. M., Groudine M., McKnight S. L. Genetic and physical analysis of the chicken tk gene. Mol Cell Biol. 1984 Sep;4(9):1769–1776. doi: 10.1128/mcb.4.9.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Hauschka S. D., McKnight S. L. tk Enzyme expression in differentiating muscle cells is regulated through an internal segment of the cellular tk gene. Mol Cell Biol. 1984 Sep;4(9):1777–1784. doi: 10.1128/mcb.4.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill G. F., Tufaro F. D. Structural and functional analysis of an alternatively spliced chicken TK messenger RNA. Nucleic Acids Res. 1986 Aug 11;14(15):6281–6297. doi: 10.1093/nar/14.15.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Domdey H., Larson G., Rossi J. J., Abelson J. A test for intron function in the yeast actin gene. Nature. 1985 Mar 14;314(6007):183–184. doi: 10.1038/314183a0. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Svensson C., Pettersson U., Akusjärvi G. Splicing of adenovirus 2 early region 1A mRNAs is non-sequential. J Mol Biol. 1983 Apr 15;165(3):475–495. doi: 10.1016/s0022-2836(83)80214-9. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]