Abstract
The Age-Related Eye Diseases Study 2 (AREDS2) clinical trial is assessing the effects of higher dietary xanthophyll (lutein and zeaxanthin) and long-chain n3 polyunsaturated fatty acid (LCPUFA) docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) intake on progression to advanced age-related macular degeneration (AMD). This study’s purpose was to examine the retinal effects of the AREDS2 formulation on Chemokine (C-C motif) ligand 2 (Ccl2−/−)/CX3C chemokine receptor 1 (Cx3cr1−/−) mice on Crumbs homolog 1 retinal degeneration phenotype 8 (Crb1rd8) background (DKO), which develop focal retinal lesions with certain features similar to AMD. DKO and C57BL/6N rd8 background mice (WT) were bred and randomized into 4 groups. Two groups, WT mice on AREDS2 diet (A-WT) and DKO mice on AREDS2 diet (A-DKO), were supplemented daily with 1.76 μmol of lutein, 35.1 μmol of zeaxanthin, 215 μmol EPA, and 107 μmol of DHA, and 2 control groups, WT mice on control diet (C-WT) and DKO mice on control diet (C-DKO), were fed an isocaloric diet. All mice had monthly fundus photos and were killed after 3 mo for biochemical and histologic analyses. After 3 mo, 81% of A-DKO mice had lesion regression compared with 25% of C-DKO mice (P < 0.05). Toxic retinal 2-[2,6-dimethyl-8-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1E,3E,5E,7E-octatetra-enyl]–1-(2-hydroxyethyl)–4-[4-methyl-6(2,6,6-trimethyl-1-cyclohexen-1-yl) 1E,3E,5E,7E-hexatrienyl]-pyridinium (A2E) concentrations were significantly lower in A-DKO compared with C-DKO mice. The outer nuclear layer thickness in A-DKO mice was significantly greater than that in C-DKO mice. Retinal expression of inducible nitric oxide synthase (iNos) tumor necrosis factor-α (Tnf-α), Cyclooxygenase-2 (Cox-2), interleukin1beta (IL-1β), and vascular endothelial growth factor (Vegf) was significantly lower in A-DKO compared with C-DKO mice. Xanthophylls and LCPUFAs have antiinflammatory, neuroprotective, and antiangiogenic properties. Our data provide potential mechanisms by which the AREDS2 formula has a protective effect on retinal lesions in DKO mice.
Introduction
Age-related macular degeneration (AMD)10 is the leading cause of irreversible central vision loss in the elderly population in the United States and the world (1). Most patients with AMD experience slowly progressive, high-resolution central vision loss after age 60 y, making activities such as driving and reading difficult. The pathogenesis of AMD is not well understood, but it is thought that cumulative oxygen and light exposure over a lifetime might lead to oxidative stress and inflammation, which injure the retinal pigment epithelium (RPE), resulting in loss of retinal outer layers and photoreceptor cells (2). Although vascular endothelial growth factor (VEGF) has been a drug target for patients with neovascular AMD, the majority of patients with geographic atrophic AMD have no therapeutic options.
Free radical species expedite oxidative stress-induced damage of RPE cells, and nutritional supplements that quench these species have been a great source of interest in AMD research. The Age-Related Eye Disease Study was a large-scale, randomized, controlled clinical trial that showed a beneficial effect of supplementation with vitamins C and E, β-carotene, and zinc with copper in reducing the risk of progression to advanced AMD in patients with intermediate or advanced AMD (3). Observational studies (4) demonstrated an inverse relationship between macular xanthophylls (lutein and zeaxanthin) and n3 long-chain PUFA (LCPUFA) supplementation and advanced AMD (5, 6). The Age-Related Eye Disease Study 2 (AREDS2), a large, multi-center, randomized clinical trial, is prospectively evaluating the efficacy of lutein, zeaxanthin, and n3 LCPUFA supplementation in participants who are at risk of developing advanced AMD.
The purpose of our study is to evaluate the effects of a diet supplemented with lutein, zeaxanthin, DHA, and EPA in DKO mice on clinical and histopathological retinal lesions, retinal inflammatory gene expression, VEGF expression, 2-[2,6-dimethyl-8-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1E,3E,5E,7E-octatetra-enyl]–1-(2-hydroxyethyl)–4-[4-methyl-6(2,6,6-trimethyl-1-cyclohexen-1-yl) 1E,3E,5E,7E-hexatrienyl]-pyridinium (A2E) concentrations, and photoreceptor loss. We selected the Chemokine (C-C motif) ligand 2 (Ccl2) −/−/ CX3C chemokine receptor 1 (Cx3cr1)−/− mouse on Crumbs homolog 1 retinal degeneration phenotype 8 (Crb1rd8) background (DKO), which develops focal retinal lesions that have clinical, biochemical, and pathological features of AMD. In addition to the lesions of Crb1rd8 (7, 8), these mice have focal photoreceptor and RPE degeneration and atrophy and a few drusenoid deposits (9, 10).
Materials and Methods
Mice.
DKO mice and C57BL/6N rd8/rd8 (WT) mice were bred in-house. These mice were termed DKO in our previous publications (9, 11), and now DKO refers to DKO on an rd8 background (7). The study was conducted in compliance with the Association for Research in Vision and Ophthalmology statement for the ethical use of animals. Two of 54 mice died during the experiment from a fight with a mouse in the same cage, so data from these mice were excluded from analysis. All animal experiments were performed under protocols approved by the National Eye Institute (NEI)’s Institutional Animal Care and Use Committee.
Experimental protocol.
DKO and WT mice were separated from their mothers at 3 wk of age, randomly assigned to 2 groups, and separately fed the standard diet. Feeding habits were observed and we calculated that the mean consumption of both WT and DKO mice was 4.0 ± 0.2 g/d diet and the mean body weight was 16 ± 0.3 g. The doses of these ingredients in the experimental formula were determined using the human AREDS2 clinical trial dose (12) and converting this to the mouse dose using allometry formulas (A diet). The 2 pelleted, purified animal diets used (provided by Dyets) were based on the AIN-93G formulation (13, 14) with several modifications. The isocaloric control diet is (C diet) identical to the AIN-93G diet with the exception of an increased amount of soybean oil (117 vs. 70 g/kg diet) and the experimental diet is identical to the AIN-93G diet with the following modifications: no soybean oil, 1.76 mmol zeaxanthin/kg diet, 17.6 mmol lutein/kg diet, 54.9 mmol EPA/kg diet, and 25.2 mmol DHA/kg diet (Supplemental Table 1). The effective daily dose is: 1.76 μmol lutein, 35.1 μmol zeaxanthin, 215 μmol EPA, and 107 μmol DHA. The ratio of EPA:DHA is 2:1. The animal diets were also quantitatively tested for the presence of baseline concentrations of lutein, zeaxanthin, and DHA+EPA at 0, 4, and 9 wk at Covance (Supplemental Table 2). DKO and WT mice were fed for 3 mo then killed for further studies. Of the 4 experimental groups, 2 groups (A-WT and A-DKO) were supplemented daily with 1.76mmol of lutein, 35.1mmol of zeaxanthin, 215mmol EPA, and 107mmol of DHA (AREDS2 formula). C-WT and C-DKO were fed an isocaloric diet (control formula). Only one eye per mouse was assayed for each analysis. The experiment was repeated 3 times. In total, each group had a mean of 25 mice.
Fundus photography.
After pupil dilation, sequential funduscopic examinations and photography were performed every month from 1 mo of age until 4 mo of age using methods previously described (14). Retinal lesion change was evaluated by comparing the sequential photos taken over time in the same fundus area. The lesions were quantitatively evaluated using a previously described scale (15) in which a negative integer indicates lesion regression and a positive integer represents lesion progression. Grading of the pictures was conducted by a masked ophthalmologist.
Histopathology: light microscopy and retinal thickness measurements.
Eyes were harvested following killing of the mice at 4 mo of age. The eyes were processed using previously described methods (14). The photoreceptor outer nuclear layer (ONL) thickness was evaluated on photomicrographs of retinal sections with a 20× objective of a photomicroscope (E800; Nikon) and a digital camera (DXM1200; Nikon). Histopathological examination and measurement of ONL thicknesses in 10 different areas were performed on 4 A-DKO eyes, 3 C-DKO eyes, 4 A-WT eyes, and 3 C-WT eyes. For each eye, 10 measurements were made across the inferior and superior retinas every 100 μm from the optic nerve head.
Transmission electron microscopy.
One eye from 2 mice in each diet group was used for transmission electron microscopic study. Eye cups were fixed in 2% paraformaldehyde in 0.1 mmol/L phosphate buffer. The strips of tissue, inclusive of the neural retina, RPE, and choroid, were embedded in Epon-812 resin. Six 1-μm-thick sections stained with toluidine blue were examined under light microscopy. The lesions shown on thick sections guided the selection of ultrathin sections, which were stained with uranyl acetate and lead citrate for examination under a JEOL1010 electron microscope.
A2E extraction and quantification.
A fluorophore found in lipofuscin and RPE phagolysosomes, A2E, is generated during the visual cycle. Quantitative A2E measurement was performed on 10 A-DKO eyes, 10 CT-DKO eyes, 10 A-WT eyes, and 10 C-WT eyes. The mice were kept in the dark for >12 h before being killed. Whole eyes were removed in a dark room under dim red light and homogenized. A2E was extracted with chloroform/methanol as previously described (16). Detection and quantification of A2E was performed as we previously described (14).
ELISA.
Because n3 LCPUFA supplementation can influence the metabolism of arachidonic acids (AAs), we measured PGE2, an inflammatory, biologically active metabolite of AAs. Serum was collected from all experimental groups. PGE2 levels were determined by monoclonal antibody-based ELISA. Assays were performed using the EIA kit (Cayman Chemical) following the manufacturer’s instructions.
Quantification of gene expression by RT-PCR.
Approximately 100 retinal cells (RPE and neuronal cells) were microdissected from a frozen section of an ocular slide. The primers for TNFα, IL-6, and VEGF were synthesized by SuperArray and supplied as the RT2 Real-Time Gene Expression Assay kit. RT-PCR was performed using previously described methods (14). The levels of the target mRNAs were quantified, using masked procedures, relative to the level of the housekeeping gene, β-actin, by the comparative ΔΔCT method. The formula is the fold of = 10^ΔΔCt/standard curve slope, ΔΔCt = [Ct (target gene of the tissue) − Ct (β-actin of the tissue)] – [Ct (target gene of the reference) − Ct (β-actin of the reference)]. In the event that an individual mRNA level was more than 2 SDs above the group when not included, it was considered an outlier and excluded. The results were calculated by using universal total RNA as the reference (SABiosciences). Each sample was analyzed twice.
Retina fatty acid analyses.
Five eyes in each treatment group were used for retinal fatty acid analyses. Total cellular lipids were extracted from the retina samples as previously described (17). Briefly, retinas were manually homogenized in a small volume of ice-cold buffer (50 mmol/L Trizma, 1 mmol/L EDTA, pH 7.4). After acidification with 0.1 nmol/L HCL, total lipids were extracted with chloroform-methanol (2:1). The organic phase was concentrated under a stream of nitrogen gas. FAMEs were prepared by base-catalyzed methylation (0.5 mol/L sodium methoxide in methanol) and analyzed using an Agilent Technologies gas chromatograph equipped with 60- × 0.25-mm i.d.-fused silica capillary column with a 0.15-μm film thickness (DB-23 column). FAMEs were identified by comparing retention times with those from commercial standards. Data are expressed as mol/100 mol of the total FAMEs identified.
Statistical analysis.
Median fundus lesion scores were compared between A-DKO and C-DKO at 1, 2, and 3 mo of treatment using a 2-tailed unpaired Mann-Whitney U test. Rates of progression and regression between C-DKO and A-DKO were analyzed by chi-squared analysis at 1, 2, and 3 mo of treatment. Data are presented with chi-squared statistic (df) (P value). A2E concentrations (C-DKO vs. A-DKO and C-WT vs. A-WT), gene expression level (A-DKO vs. C-DKO), retinal outer layer thickness (C-WT vs. A-WT and C-DKO vs. A-DKO), serum PGE2 concentration (A-DKO vs. C-DKO), and retinal fatty acid concentrations (A-WT vs. C-WT and A-DKO vs. C-DKO) were compared after 3 mo of treatment using a 2-tailed unpaired Mann-Whitney U test. Values in the text are presented as median (minimum value, maximum value) (U = Mann-Whitney U value, P-value). Differences were considered significant at P < 0.05 for chi-squared and Mann-Whitney U analyses. XLSTAT software version 2011.4.02 and Microsoft Excel were used for statistical analysis.
Results
Three independent experiments were performed on DKO and WT mice fed either the control diet (C-DKO and C-WT) or the AREDS2-supplemented diet (A-DKO and A-WT) for 3 mo. The results of all 3 experiments were comparable. Data (fundoscopic photography, histopathology, fatty acid analysis, etc.) were pooled and are presented below. Dietary analysis performed and confirmed that these nutrients were present at the expected levels in the diets and did not change over time (Supplemental Table 2). Analyses of the diets revealed that the amounts of the starting ingredients were 100-140% of the expected values.
Clinical ocular features.
Fundus photographs were scored with comparison to baseline photographs using the aforementioned standardized scale. We measured lesion progression as a positive score and regression as a negative score. C-WT and A-WT mice did not develop retinal lesions and therefore had a score of 0 (Supplemental Fig. 1). As previously described, by 4 wk of age, all DKO mice developed a retinal phenotype of progressive focal photoreceptor loss and RPE mottling (Fig. 1A).
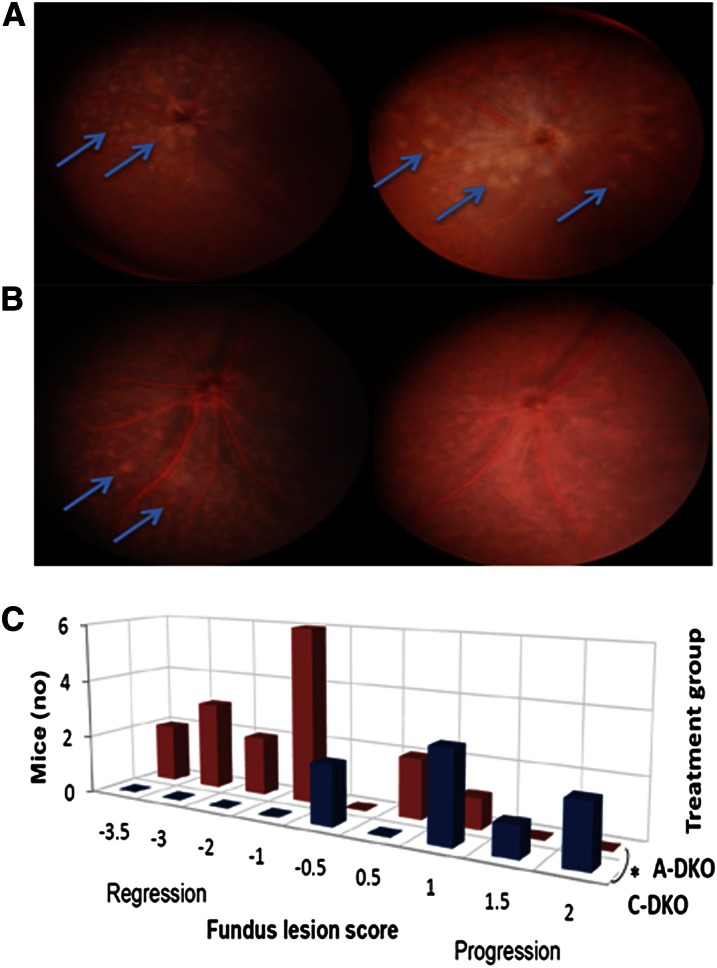
FIGURE 1.
Periodic monitoring of fundus lesions in DKO mice after treatment with a control or AREDS2 diet. C-DKO mice (A) and A-DKO mice (B) are shown at 1 mo of age prior to treatment (left) and after 3 mo of treatment (right). C-DKO mice had an increase in the number and size of lesions (arrows), whereas A-DKO mice had lesion regression (arrows). (C) A 3D contingency table of the fundus lesion scores of A-DKO and C-DKO mice showing a significant difference in the rate of lesion progression after 3 mo of treatment, n = 16 (A-DKO), 12 (C-DKO), P = 0.008. A-DKO, DKO on AREDS2 diet; AREDS2, Age-Related Eye Diseases Study 2; C-DKO, DKO on control diet; Cx3cr1, CX3C chemokine receptor 1; DKO, Ccl2−/−/Cx3cr1−/−.
The fundus scores were 0.75 (−2, 2) in C-DKO mice and −0.25 (−2, 2) in A-DKO mice at 1 mo (U = 152; P = 0.09); 1 (−0.5, 2) in C-DKO mice and −0.5 (−3, 0) in A-DKO mice (U = 118; P = 0.001) at 2 mo; and 1 (−0.5, 2) in C-DKO mice and −1 (−3.5, 1) in A-DKO mice at 3 mo (U = 121; P = 0.001). The contingency table of fundus scores of 54 mice during 3 mo (one eye per mouse from 3 separate experiments) is shown (Fig. 1B). At the end of 3 mo of treatment, more A-DKO than C-DKO mice had lesion regression [χ2 = 20.6 (8); P = 0.008].
Histological and ultrastructural features.
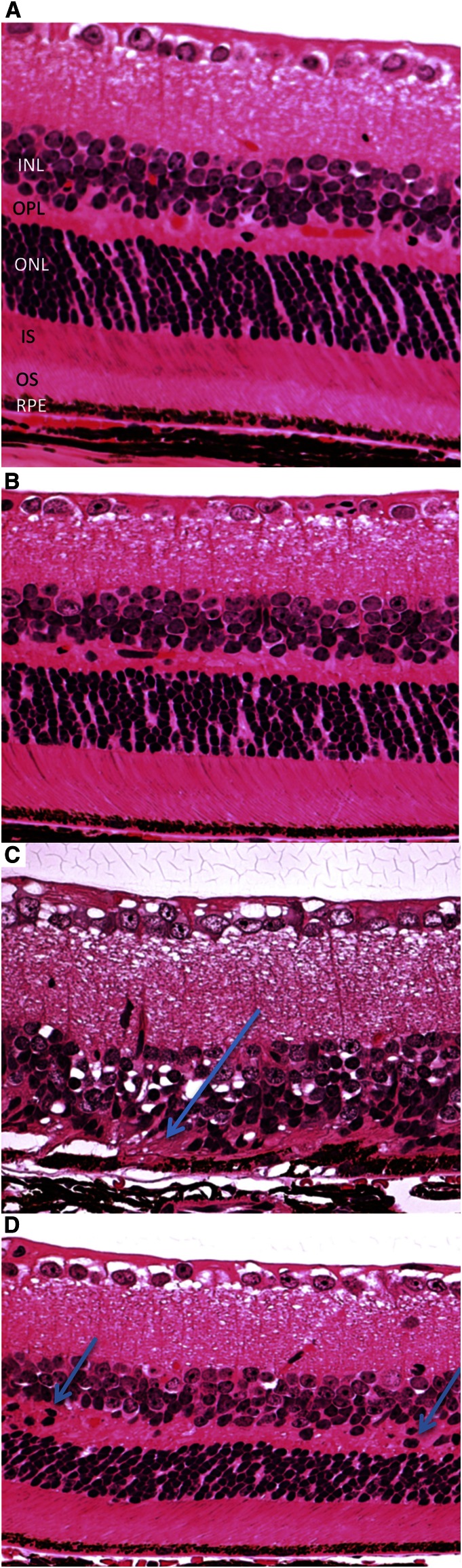
Histopathological examination was conducted on 8 A-DKO, 4 C-DKO, 8 A-WT, and 6 C-WT eyes. C-WT and A-WT eyes had a similar histopathology regardless of diet, with normal morphology of the outer and inner segments (OSs/ISs), inner nuclear layer (INL) and ONL, and RPE cell layer (Fig. 2A,B). C-DKO mice had focal RPE hypertrophy and hypopigmentation, loss of the OS and IS layers, and focal disorganization of the ONLs and INLs with abundant photoreceptor loss (Fig. 2C). In contrast, an A-DKO eye had preserved organization of the OSs/ISs and normal RPE morphology. Although there were some areas of retinal dystrophy (photoreceptor nuclear migration to the outer plexiform layer) characteristic for mice with the rd8 background (18), no pseudorosettes or retinal folds were detected in these mice. Photoreceptor architecture and separation of the INL and ONL was preserved in A-DKO eyes (Fig. 2D).
FIGURE 2.
Ocular photomicrographs of all treatment groups after treatment with a control or AREDS2 diet. Representative photomicrographs taken after 3 mo of treatment. (A,B) In C-WT and A-WT eyes, there is a clear INL, OPL, ONL, IS, OS, and RPE. The RPE appears homogenous in size and character. (C) C-DKO mice have focal areas of photoreceptor loss (arrow), loss of the IS/OS and OPL, and collapse of the ONL and INL. RPE shows vacuole accumulation, atrophy, and hypertrophy. (D) In contrast, A-DKO mice have preservation of retinal architecture and a relatively normal RPE, OS, IS, and OLM with only scattered areas of nuclear photoreceptor migration (arrows) in the OPL (hematoxylin and eosin, original magnification ×200). AREDS2, Age-Related Eye Diseases Study 2; A-DKO, DKO on AREDS2 diet; A-WT, WT on AREDS2 diet; C-DKO, DKO on control diet; C-WT, WT on control diet; INL, inner nuclear layer; IS, inner segment; OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segment; RPE, retinal pigment epithelium; WT, C57BL/6N retinal degeneration phenotype 8 background.
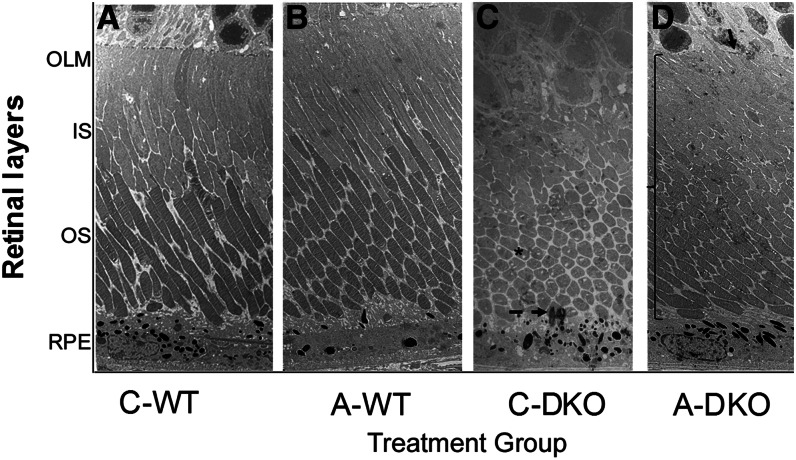
At the ultrastructural level, A-WT and C-WT eyes had normal RPE morphology and pigmentation, clear demarcation of the OS/IS, and an outer limiting membrane (OLM) (Fig. 3A,B). In C-DKO eyes, liposomes and lipofuscin accumulated in the RPE, with pigment extravasation, a shorter OS/IS, and complete loss of the OLM. The photoreceptor IS appeared vacuolated, collapsed, and disorganized (Fig. 3C). In contrast, the RPE cell in the A-DKO eye showed normal melanosomes and intact structure without excess lipofuscin granules. The OS/IS of the photoreceptors were more organized, appear healthy without vacuolization, and had preserved length. Additionally, the OLM was easily identified (Fig. 3D, arrow).
FIGURE 3.
Retinal ultrastructural images of all treatment groups after treatment with a control or AREDS2 diet. In a normal RPE, a distinct OLM, IS, and OS are seen clearly in C-WT (A) and A-WT (B) mice. (C) In C-DKO mice, the IS and OS are shortened and photoreceptors have vacuoles (asterisk). RPE cells have abundant lysosomes, lipofuscin granules, loss of infoldings, and pigment extravasation (dashed arrow). (D) In A-DKO mice, the IS and OS appear normal and intact with fewer photoreceptors vacuoles. The OLM is preserved (arrow) and RPE cells are normal (magnification ×2500). A-DKO, DKO on AREDS2 diet; A-WT, WT on AREDS2 diet; AREDS2, Age-Related Eye Diseases Study 2; C-DKO, DKO on control diet; C-WT, WT on control diet; IS, inner segment; OLM, outer limiting membrane; OS, outer segment; RPE, retinal pigment epithelium.
Retinal outer layer thicknesses.
The results of the histopathological examination and measurement of ONL thicknesses were summarized with mean thickness and SD. The retinal ONL thicknesses (μm) in C-WT eyes [33.4 (30.0, 39.0)] were similar to A-WT eyes [32.7 (20.7, 101)]. The ONL thickness (μm) in a C-DKO eye was 0.75 (0, 11.2) and <18.3 (0, 29.5) in the A-DKO eye (U = 33.5; P < 0.0001). Overall, ONL thickness was significantly greater in A-DKO eyes than in C-DKO eyes.
Quantification of a biochemical component in RPE phagolysosomes.
Quantitative A2E measurement was performed on 10 each of A-DKO, CT-DKO, A-WT, and C-WT eyes. A2E concentrations were significantly higher in C-DKO eyes than in C-WT eyes, as previously published (10, 11, 19). The retinal A2E concentration in C-WT eyes did not differ from that in A-WT eyes. A2E (pmol/eye) in C-DKO eyes [62.3 (53.8, 77.6)] was greater than in A-DKO eyes [38.0 (25.9, 51.7)] (U = 90; P = 0.0003) and did not differ from C-WT concentrations.
Retinal transcript expression profile.
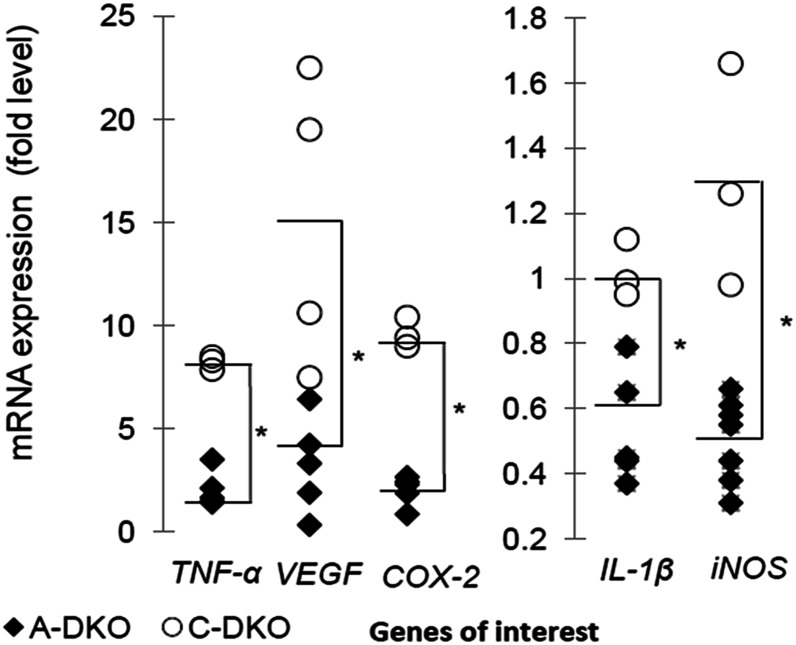
We investigated the retinal mRNA expression of several pathologic proinflammatory genes, including tumor necrosis factor-α (Tnf-α), cyclooxygenase-2 (Cox-2), interleukin-1β (Il-1β), and inducible nitric oxide synthase (iNOS), in our treatment groups (Fig. 4). The expression of Tnf-α (U = 18; P = 0.028), Cox-2 (U = 15; P = 0.036), Il-1β (U = 15; P = 0.036), and iNOS (U = 21; P = 0.017) in A-DKO mice was lower than in C-DKO mice. Vegf levels were also significantly higher in C-DKO mice compared with A-DKO mice (U = 22; P = 0.04). A-DKO mice had lower expression of inflammatory and proangiogenic genes linked to advanced AMD.
FIGURE 4.
Gene transcripts in the retina of A-WT and A-DKO mice after treatment. Retinal gene expression of Tnf-α, Vegf, Cox2-2, Il-1β, and iNos in A-WT and A-DKO mice are plotted on a scatter plot. The median values are noted with a dash, n = 7 (A-DKO), 4 (C-DKO). The asterisk indicates that medians differ, P < 0.05. A-DKO, DKO on AREDS2 diet C-DKO, Ccl2−/−/Cx3cr1−/− Crb1rd8 mice fed isocaloric control; Cox-2, Cyclooxygenase-2; Cx3cr1, CX3C chemokine receptor 1; DKO, Ccl−/−/Cx3cr1−/−; A-WT, WT on AREDS2 diet; iNos, inducible nitric oxide synthase; Vegf, vascular endothelial growth factor.
Retinal accumulation of fatty acids and reduction in AA concentrations.
To ascertain that dietary consumption of fatty acids results in retinal accumulation, analyses of fatty acid concentrations were performed using 5 eyes from each treatment group. AA concentrations (nmol/mg retina) were lower in A-WT eyes [1.7 (1.5, 2.5)] compared with C-WT eyes [3.0 (2.8, 3.2)] (U = 25; P = 0.008) and in A-DKO eyes [1.2 (1.2, 1.6)] compared with C-DKO eyes [2.6 (2.5, 3.1)] (U = 25; P = 0.008). There were higher concentrations of EPA (nmol/mg of retina) in A-WT mice [0.40 (0.38, 0.44)] compared with C-WT mice [0.067 (0, 0.15)] (U = 0; P = 0.011) and in A-DKO mice [0.53 (0.50, 0.57)] compared with C-DKO mice [0.11 (0, 0.14)] (U = 0; P = 0.012). Retinal DHA concentrations (nmol/mg retina) were 9.4 (8.8, 9.9) in C-WT eyes, <11.0 (10.3, 11.4) in A-WT eyes (U = 0; P = 0.008), and 9.1 (8.6, 9.4) in C-DKO mice, significantly <10 (9.9, 11) in A-DKO mice (U = 0; P = 0.008).
Profile of AA derivatives.
The concentration of PGE2, a proinflammatory metabolite of AA, was measured in all treatment groups (56 retinas). The median PGE2 serum concentration (nmol/L) of C-DKO mice [5.49 (0.32, 22.7)] did not significantly differ from that of A-DKO mice [9.78 (0.32, 11.4)].
Discussion
In this study, DKO and WT mice were fed either a diet supplemented with lutein, zeaxanthin, and n3 LCPUFAs or an isocaloric diet. The results demonstrate a benefit of the AREDS2 diet on retinal AMD-like lesions, RPE lipofuscin and A2E accumulation, pathologic gene expression, and preservation of photoreceptors. The DKO mouse model was generated by knocking out a chemokine (Ccl2) and a chemokine receptor (Cx3cr1) on a Crb1rd8 background. Although nonprimate models do not have a macula, this model mimics the A2E accumulation and focal photoreceptor and RPE degeneration found in AMD. The rd8 background may contribute to focal retinal dystrophic lesions in both WT and DKO mice. DKO mice develop RPE pathology (lipofuscin accumulation, hypertrophy, and hypotrophy) and photoreceptor and synaptic degeneration that is more characteristic in AMD (20).
The n3 LCPUFA supplementation fed during gestation ameliorated retinal lesions, reduced retinal A2E levels, and decreased concentrations of serum AA metabolites in DKO mice (14). In the retina, n3 LCPUFAs are antiangiogenic, neuroprotective, and influence retinal cell gene expression, cellular differentiation, and survival (21). DHA acts as a transcription factor for PPARα, which prevents endothelial cell dysfunction, photoreceptor death, and vascular remodeling (22–24). Humans lack the capacity for de novo synthesis of the precursors of EPA and DHA, necessitating dietary supplementation. AMD patients who reported the highest intake of n3 LCPUFAs were less likely to have neovascular AMD at baseline or to progress to advanced AMD at the end of 12 y (25, 26).
In this study, LCPUFAs, lutein, and zeaxanthin were administered in combination to mice after 1 mo of age, and clinical and histopathological improvement was observed after only 4 wk of nutritional supplementation. The addition of omega-3 LCPUFA to oral supplementation of lutein/zeaxanthin for 6 months did not change the serum levels of lutein and zeaxanthin in persons with or without AMD (27). There may be a greater protective effect of the combination of xanthophylls and LCPUFAs on the retina compared with LCPUFAs alone in AREDS2, a long-term clinical trial, but one limitation of this study is that the nutrients were not studied individually. It has been shown in humans that dietary lutein supplementation increases serum and macular retinal concentrations of lutein (28). Because of the extremely low serum and tissue concentrations of lutein and zeaxanthin in rodents, these carotenoids were not measured in the experimental mice in this study. It therefore cannot be determined if the effects of these carotenoids on the retina are direct or indirect. Lutein supplementation was shown to be beneficial in the LAST trial (29) and there is an inverse relationship between dietary consumption and advanced AMD (30–32). Lutein suppresses STAT3 activation by inflammatory cytokines and extracellular signal-regulated kinase (ERK) activation, slowing DNA damage and preserving a-wave ERG amplitude in mouse models (33). Lutein attenuates hypoxia inducible factor 1α expression, inhibits reactive oxidative species production, and decreases VEGF expression (34).
Incomplete degradation of the photoreceptor OS in lysosomes of RPE cells creates lipofuscin granules (35) and produces A2E, which correlates with poor RPE longevity and photoreceptor degeneration in the central retina. Retinal A2E is directly related to complement activation, destabilization of cell membranes, and choroidal neovascularization in vivo (36–38). A2E accumulation is an appropriate biomarker of AMD pathology in mice, because classic AMD Bruch’s membrane changes and drusen are not usually seen (10). It is of great importance that the A-DKO mice had lower A2E levels.
We measured the retinal expression of genes that are important in AMD pathology. NO mediates the proangiogenic responses of VEGF (39), and NO inhibition attenuates VEGF-induced angiogenesis (40). Expression of iNOS produces large amounts of NO for a prolonged period, leading to free radical production and posterior retinal degeneration (41, 42). Submacular choroidal blood flow control is also mediated in part by iNOS (43). Our study shows that supplementation with the AREDS2 diet significantly reduces retinal iNOS expression (Fig. 4).
TNFα, an inflammatory cytokine expressed by a subset of microglia associated with retinal vessels (44), is directly responsible for photoreceptor death (45) and choroidal neovascularization (CNV). TNF inhibitors reduce the size and leakiness of laser-induced CNV lesions in many animal models (46). We reported lower ocular TNF-α transcripts in DKO mice treated with naloxone, an inhibitor of microglia activation (47), and improved retinal lesions in DKO mice treated with a TNF-inducible gene 6 protein (48). These same findings with the AREDS2 treatment suggest that lower ocular TNF-α may be important in healing retinal lesions in DKO mice.
AA is the substrate for the synthesis of many mediators of leukocyte chemotaxis and inflammatory cytokine production, including prostaglandins, thromboxanes, and leukotrienes. EPA supplementation results in incorporation into phospholipid membranes at the expense of prostaglandins (49), decreasing AA concentrations. COX-2 is the major enzyme that converts AA to PGs in central nervous system inflammatory cells. AA and COX-2 were both significantly reduced in the A-DKO mice. Whereas n3 LCPUFA supplementation alone significantly reduced PGE2 concentrations (14), a significant difference was not found in our study.
IL-1β is produced in RPE cells and CNV membranes. It is a diverse proinflammatory cytokine whose upregulation promotes angiogenesis and induces and perpetuates neuroinflammation (50). The inhibition of IL-1β by intravitreal injections of recombinant IL-1 receptor antagonists has also been shown to prevent CNV in a mouse model (51). Retinal IL-1β was significantly reduced in A-DKO mice compared with C-DKO mice.
VEGF has been widely related to advanced AMD, especially CNV (52). We reported that local delivery of AAV-5 mediated sFLT01 gene therapy can stabilize retinal lesions in DKO mice (15). Our group has shown that supplementation with n3 LCPUFAs alone did not alter expression of VEGF (14). In this study, A-DKO mice had significantly less retinal VEGF expression after 3 mo compared with C-DKO mice.
In addition, deranged RPE and photoreceptor metabolism likely improved with the supplementation of DHA and lutein based on the lower A2E levels, fewer retinal lesions, and preservation of the ONL. Retinal lesion regression in A-DKO mice may indicate that oxidative damage was counteracted early enough to halt RPE damage and photoreceptor loss. Overall, the results of our study are promising for this murine model. These findings in a homogenous mouse population may not necessarily translate to findings in humans. We look forward to the AREDS2 controlled clinical trial results to assess the potential benefit of these nutrients in AMD patients.
Supplementary Material
Acknowledgments
The authors thank Dr. Ginger Tansey at the National Eye Institute for helping with the experimental protocol and animal care, Mark Milbank from DSM Nutritional Products for providing the nutrients used in this study, Technical Marketing Analytical Services for performing stability analyses, Dr. Kevin Fritsche at the University of Missouri for performing the fatty acid analyses, and Dr. Ronald Bush at the National Eye Institute for facilitating the retinal thickness measurements. C.-C.C. and E.Y.C. designed research; H.L.R., J.T., D.F.S., J.Z., and X.C. conducted research; H.L.R., C.-C.C., J.T., J.Z., and D.F.S. analyzed data; and H.L.R. wrote the paper with C.-C.C. and both parties have final responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; A-DKO, DKO mice on AREDS2 diet; A2E, 2-[2,6-dimethyl-8-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1E,3E,5E,7E-octatetra-enyl]–1-(2-hydroxyethyl)–4-[4-methyl-6(2,6,6-trimethyl-1-cyclohexen-1-yl) 1E,3E,5E,7E-hexatrienyl]-pyridinium; AMD, age-related macular degeneration; AREDS2, Age-Related Eye Diseases Study 2; A-WT, WT mice on AREDS2 diet Ccl2, Chemokine (C-C motif) ligand 2; C-DKO, DKO mice on control diet; COX-2, Cyclooxygenase-2; Crb1, Crumbs homolog 1; C-WT, WT mice on control diet; Cx3cr1, CX3C chemokine receptor 1; DKO, Ccl2−/−/Cx3cr1−/−; INL, inner nuclear layer; iNOS, inducible nitric oxide synthase; IS, inner segment; long-chain PUFA, LCPUFA; OLM, outer limiting membrane; ONL, outer nuclear layer; OS, outer segment; rd8, retinal degeneration phenotype 8; RPE, retinal pigment epithelium; VEGF, vascular endothelial growth factor; WT, C57BL/6N retinal degeneration phenotype 8 background.
Literature Cited
- 1.WHO. Magnitude and causes of visual impairment. Geneva: WHO; 2009. [Google Scholar]
- 2.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss, AREDS Report No. 8. Arch Ophthalmol. 2001;119:1417–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson JH, Erie JC, Bakri SJ. Nutritional supplementation and age-related macular degeneration. Semin Ophthalmol. 2011;26:131–6. [DOI] [PubMed] [Google Scholar]
- 5.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009;127:656–65. [DOI] [PubMed] [Google Scholar]
- 6.Chiu CJ, Klein R, Milton RC, Gensler G, Taylor A. Does eating particular diets alter the risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br J Ophthalmol. 2009;93:1241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR. The rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012;53:2921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luhmann UF, Lange CA, Robbie S, Munro PM, Cowing JA, Armer HE, Luong V, Carvalho LS, Maclaren RE, Fitzke FW, et al. Differential modulation of retinal degeneration by ccl2 and cx3cr1 chemokine signalling. PLoS ONE. 2012;7:e35551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD). Prog Retin Eye Res. 2010;29:169–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CC, Ross RJ, Shen D, Ding X, Majumdar Z, Bojanowski CM, Zhou M, Salem N, Jr, Bonner R, Tuo J. Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration. Ophthalmic Res. 2008;40:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew EY, Clemons T, Sangiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012;119:2282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 14.Tuo J, Ross RJ, Herzlich AA, Shen D, Ding X, Zhou M, Coon SL, Hussein N, Salem N, Jr, Chan CC. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009;175:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuo J, Pang JJ, Cao X, Shen D, Zhang J, Scaria A, Wadsworth SC, Pechan P, Boye SL, Hauswirth WW, et al. AAV5-mediated sFLT01 gene therapy arrests retinal lesions in Ccl2(−/−)/Cx3cr1(−/−) mice. Neurobiol Aging. 2012;33:433 e1–10. [DOI] [PMC free article] [PubMed]
- 16.Karan G, Lillo C, Yang Z, Cameron DJ, Locke KG, Zhao Y, Thirumalaichary S, Li C, Birch DG, Vollmer-Snarr HR, et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc Natl Acad Sci USA. 2005;102:4164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Aleman TS, Cideciyan AV, Aguirre GK, Huang WC, Mullins CL, Roman AJ, Sumaroka A, Olivares MB, Tsai FF, Schwartz SB, et al. Human CRB1-associated retinal degeneration: comparison with the rd8 Crb1-mutant mouse model. Invest Ophthalmol Vis Sci. 2011;52:6898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross RJ, Zhou M, Shen D, Fariss RN, Ding X, Bojanowski CM, Tuo J, Chan CC. Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration. Exp Eye Res. 2008;86:675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Tuo J, Shen D, Li W, Chan C-C. Early degeneration of photoreceptor synapse in Ccl2/Cx3cr1 deficient mice on Crb1rd8 background. Synapse. 2013;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. [DOI] [PubMed] [Google Scholar]
- 22.Lin Q, Ruuska SE, Shaw NS, Dong D, Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor alpha. Biochemistry. 1999;38:185–90. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Houghton LA, Brenna JT, Noy N. Docosahexaenoic acid modulates the interactions of the interphotoreceptor retinoid-binding protein with 11-cis-retinal. J Biol Chem. 1996;271:20507–15. [DOI] [PubMed] [Google Scholar]
- 24.Organisciak DT, Darrow RM, Jiang YL, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest Ophthalmol Vis Sci. 1996;37:2243–57. [PubMed] [Google Scholar]
- 25.SanGiovanni JP, Agron E, Clemons TE, Chew EY. Omega-3 long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration. Arch Ophthalmol. 2009;127:110–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SanGiovanni JP, Chew EY, Agron E, Clemons TE, Ferris FL III, Gensler G, Lindblad AS, Milton RC, Seddon JM, Klein R, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang LL, Coleman HR, Kim J, de Monasterio F, Wong WT, Schleicher RL, Ferris FL III, Chew EY. Oral supplementation of lutein/zeaxanthin and omega-3 long chain polyunsaturated fatty acids in persons aged 60 years or older, with or without AMD. Invest Ophthalmol Vis Sci. 2008;49:3864–9. [DOI] [PubMed] [Google Scholar]
- 28.Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133:992–8. [DOI] [PubMed] [Google Scholar]
- 29.Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST Study (Lutein Antioxidant Supplementation Trial). Optometry. 2004;75:216–30. [DOI] [PubMed] [Google Scholar]
- 30.Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S. Carotenoids and antioxidants in age-related maculopathy Italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology. 2008;115:324–33 e2. [DOI] [PubMed] [Google Scholar]
- 31.Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB, Mares JA. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Arch Ophthalmol. 2006;124:1151–62. [DOI] [PubMed] [Google Scholar]
- 32.SanGiovanni JP, Chew EY, Clemons TE, Ferris FL III, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol. 2007;125:1225–32. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K. Neuroprotective effects of lutein in the retina. Curr Pharm Des. 2012;18:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SW, Cho CS, Jun HO, Ryu NH, Kim JH, Yu YS, Kim JS. Anti-angiogenic effect of luteolin on retinal neovascularization via blockade of reactive oxygen species production. Invest Ophthalmol Vis Sci. 2012;53:7718–26 [DOI] [PubMed] [Google Scholar]
- 35.Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–6. [DOI] [PubMed] [Google Scholar]
- 36.Sparrow JR. Bisretinoids of RPE lipofuscin: trigger for complement activation in age-related macular degeneration. Adv Exp Med Biol. 2010;703:63–74. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc Natl Acad Sci USA. 2010;107:7275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iriyama A, Inoue Y, Takahashi H, Tamaki Y, Jang WD, Yanagi Y. A2E, a component of lipofuscin, is pro-angiogenic in vivo. J Cell Physiol. 2009;220:469–75. [DOI] [PubMed] [Google Scholar]
- 39.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawson VL, Dawson TM. Physiological and toxicological actions of nitric oxide in the central nervous system. Adv Pharmacol. 1995;34:323–42. [DOI] [PubMed] [Google Scholar]
- 42.Chiou GC. Review: effects of nitric oxide on eye diseases and their treatment. J Ocul Pharmacol Ther. 2001;17:189–98. [DOI] [PubMed]
- 43.Bhutto IA, Baba T, Merges C, McLeod DS, Lutty GA. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD). Exp Eye Res. 2010;90:155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter DA, Dick AD. Lipopolysaccharide/interferon-gamma and not transforming growth factor beta inhibits retinal microglial migration from retinal explant. Br J Ophthalmol. 2003;87:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakazawa T, Kayama M, Ryu M, Kunikata H, Watanabe R, Yasuda M, Kinugawa J, Vavvas D, Miller JW. Tumor necrosis factor-alpha mediates photoreceptor death in a rodent model of retinal detachment. Invest Ophthalmol Vis Sci. 2011;52:1384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lichtlen P, Lam TT, Nork TM, Streit T, Urech DM. Relative contribution of VEGF and TNF-alpha in the cynomolgus laser-induced CNV model: comparing the efficacy of bevacizumab, adalimumab, and ESBA105. Invest Ophthalmol Vis Sci. 2010;51:4738–45. [DOI] [PubMed] [Google Scholar]
- 47.Shen D, Cao X, Zhao L, Tuo J, Wong WT, Chan CC. Naloxone ameliorates retinal lesions in Ccl2/Cx3cr1 double-deficient mice via modulation of microglia. Invest Ophthalmol Vis Sci. 2011;52:2897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuo J, Cao X, Shen D, Wang Y, Zhang J, Oh JY, Prockop DJ, Chan CC. Anti-inflammatory recombinant TSG-6 stabilizes the progression of focal retinal degeneration in a murine model. J Neuroinflammation. 2012;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calder PC. Long-chain n-3 fatty acids and inflammation: potential application in surgical and trauma patients. Brazilian J Med Biol Res. 2003;36:433–46. [DOI] [PubMed]
- 50.Liu Y, Biarnes Costa M, Gerhardinger C. IL-1beta is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1beta autostimulation. PLoS ONE. 2012;7:e36949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavalette S, Raoul W, Houssier M, Camelo S, Levy O, Calippe B, Jonet L, Behar-Cohen F, Chemtob S, Guillonneau X, et al. Interleukin-1beta inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am J Pathol. 2011;178:2416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res. 2008;27:372–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.