Abstract
Background
A potential role for coagulation factors in pulmonary arterial hypertension has been recently described, but the mechanism of action is currently not known. Here, we investigated the interactions between thrombin and the nitric oxide-cGMP pathway in pulmonary endothelial cells and experimental pulmonary hypertension.
Principal Findings
Chronic treatment with the selective thrombin inhibitor melagatran (0.9 mg/kg daily via implanted minipumps) reduced right ventricular hypertrophy in the rat monocrotaline model of experimental pulmonary hypertension. In vitro, thrombin was found to have biphasic effects on key regulators of the nitric oxide-cGMP pathway in endothelial cells (HUVECs). Acute thrombin stimulation led to increased expression of the cGMP-elevating factors endothelial nitric oxide synthase (eNOS) and soluble guanylate cyclase (sGC) subunits, leading to increased cGMP levels. By contrast, prolonged exposition of pulmonary endothelial cells to thrombin revealed a characteristic pattern of differential expression of the key regulators of the nitric oxide-cGMP pathway, in which specifically the factors contributing to cGMP elevation (eNOS and sGC) were reduced and the cGMP-hydrolyzing PDE5 was elevated (qPCR and Western blot). In line with the differential expression of key regulators of the nitric oxide-cGMP pathway, a reduction of cGMP by prolonged thrombin stimulation was found. The effects of prolonged thrombin exposure were confirmed in endothelial cells of pulmonary origin (HPAECs and HPMECs). Similar effects could be induced by activation of protease-activated receptor-1 (PAR-1).
Conclusion
These findings suggest a link between thrombin generation and cGMP depletion in lung endothelial cells through negative regulation of the nitric oxide-cGMP pathway, possibly mediated via PAR-1, which could be of relevance in pulmonary arterial hypertension.
Introduction
Pulmonary arterial hypertension is a life-threatening progressive disease, which is characterized by functional and structural alterations of the pulmonary arterial tree, increased pulmonary vascular resistance and subsequent right heart failure [1]. Anticoagulation with warfarin is recommended for the treatment of patients with pulmonary arterial hypertension in the American College of Chest Physicians Clinical Practice guidelines [2], [3] despite the lack of conclusive experimental or clinical evidence to support its use [4], [5]. Recently, we have shown that inhibition of the coagulation factor Xa (FXa) with the oral, direct and specific FXa inhibitor rivaroxaban reduced right ventricular (RV) hypertrophy and dysfunction in monocrotaline (MCT)-induced pulmonary hypertension [6], suggesting that activation of coagulation factors is involved in disease progression. However, the mechanism of how activated clotting factors might contribute to pulmonary arterial hypertension is not known.
Coagulation factor Xa is the critical juncture of the intrinsic and extrinsic pathways of the coagulation cascade, the first step of the final, common pathway leading to thrombin generation and fibrin formation. Besides their role in clot formation, FXa and thrombin have profound and diverse direct effects on vascular cells [7]–[12], mediated at least in part through the protease-activated receptors (PARs). In particular, both FXa and thrombin have been proposed to interact with the nitric oxide-cGMP pathway, although the reported effects are partly conflicting [13]–[15].
Nitric oxide (NO) is produced by endothelial NO-synthase (eNOS) and stimulates the soluble guanylate cyclase (sGC) to synthesize cyclic guanosine monophosphate (cGMP) which, in turn, is hydrolyzed by specific phosphodiesterases (PDEs) to the inactive 5′-GMP [16]. The nitric oxide-cGMP pathway has multiple effects on the vasculature [17], and its dysfunction leading to decreased cGMP levels has been implicated in various cardiovascular diseases including pulmonary hypertension [18], [19]. Importantly, pharmacological modulation of the nitric oxide-cGMP pathway aiming at increasing cGMP, e.g. by inhaled NO or PDE5 inhibitors, has become a clinically established and highly effective treatment paradigm [20]–[27].
In light of the recent description of the role of coagulation factor Xa in experimental pulmonary hypertension, and given the increasingly established pharmacological relevance of the nitric oxide-cGMP pathway in this disease, the goal of the present study was to investigate the interplay between both pathways in pulmonary endothelial cells and in experimental pulmonary hypertension. The findings of the present study suggest a link between the activation of coagulation factors and the nitric oxide-cGMP pathway in pulmonary endothelial cells that could be mimicked by a direct peptide activator of PAR-1, and might be of relevance in pulmonary arterial hypertension.
Results
Thrombin inhibition reduces RV hypertrophy in experimental pulmonary hypertension
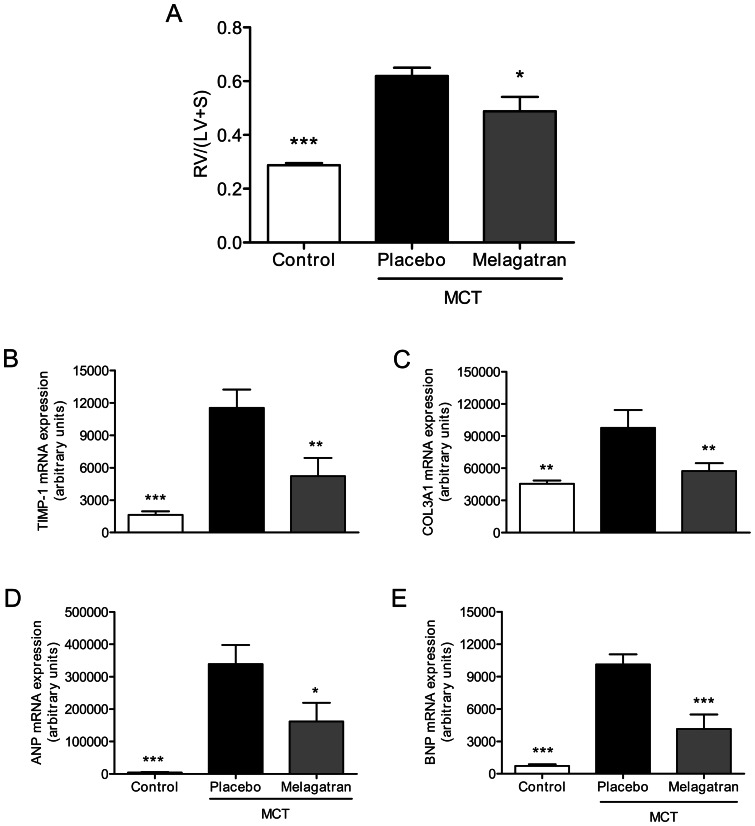
Inhibition of coagulation factor Xa was recently shown to inhibit the development of RV hypertension and hypertrophy in the rat model of MCT-induced pulmonary hypertension [6]. Since the downstream target of FXa is prothrombin that is cleaved to active thrombin, we tested the effects of direct thrombin inhibition in the same experimental model. Rats injected with MCT were treated with the specific and direct thrombin inhibitor melagatran, administered by an osmotic minipump to provide constant plasma levels over 28 days. Here, thrombin was inhibited 54±9% at day 28 post MCT application (data not shown). One rat in each group died in the last days prior to scheduled assessment of RV hypertrophy. The MCT-injected rats treated with placebo developed significant RV hypertrophy 28 days after MCT administration: from 0.29±0.01 (controls without MCT) to 0.62±0.03 (MCT group) (p<0.001). Chronic treatment with melagatran reduced the RV hypertrophy to 0.49±0.05 (p<0.05 vs. MCT group) (Fig. 1A). In line with this, the expression of marker genes of remodeling TIMP-1 and Col3A1, and the pressure sensitive marker genes ANP and BNP were elevated in the RV of rats in the MCT-group in comparison to healthy controls, but significantly reduced under treatment with melagatran (Fig. 1B–E). These findings indicate that contribution of FXa to RV pressure increase and hypertrophy in experimental pulmonary hypertension is mediated through its downstream effector thrombin.
Figure 1. Thrombin inhibition reduces right ventricular hypertrophy in experimental pulmonary hypertension.
(A) MCT-induced RV hypertrophy is significantly reduced by treatment with the selective thrombin inhibitor melagatran. Increased expression of remodeling marker genes TIMP-1 (B) and Col3A1 (C) and the pressure sensitive marker genes ANP (D) and BNP (E) in the right ventricle of rats with MCT-induced PH is reduced by melagatran. White bars: controls without MCT; black bars: MCT with placebo treatment; grey bars: MCT and melagatran treatment. Data shown as mean ± SEM (n = 8–10/group). *p<0.05, **p<0.01, **p<0.001 vs placebo.
Cyclic GMP levels in lung homogenates were elevated from 7.7±1.0 pmol/g lung (controls without MCT) to 23.4±5.3 pmol/g lung (MCT group), confirming the previously published findings in the MCT model [28]–[30].
Thrombin modulates the expression of key regulators of the nitric oxide-cGMP pathway in endothelial cells in a biphasic manner
First, we tested whether FXa itself directly modulates the expression levels of eNOS, sGC subunits and PDE5 in HUVECs. Although prolonged incubation with FXa reduced eNOS and increased PDE5 mRNA expression; the effects were significant only at the highest concentration of 30 nM (Fig. 2A–D). No effect on sGC alpha 1 or beta 1 levels was found. Hence, coagulation factor Xa has only marginal effects on the nitric oxide-cGMP pathway in endothelial cells.
Figure 2. Marginal effects of FXa on key regulators of the nitric oxide-cGMP pathway in endothelial cells.
Incubation of HUVECs with FXa for 6 hours reduces eNOS (A) and increases PDE5 (D) mRNA expression significantly only at the highest concentration of 30 nM. No effect on sGC alpha 1 (B) and sGC beta 1 (C) is detectable. Data shown as mean ± SEM (n = 6/group). *p<0.05, ***p<0.001 vs cells not exposed to thrombin.
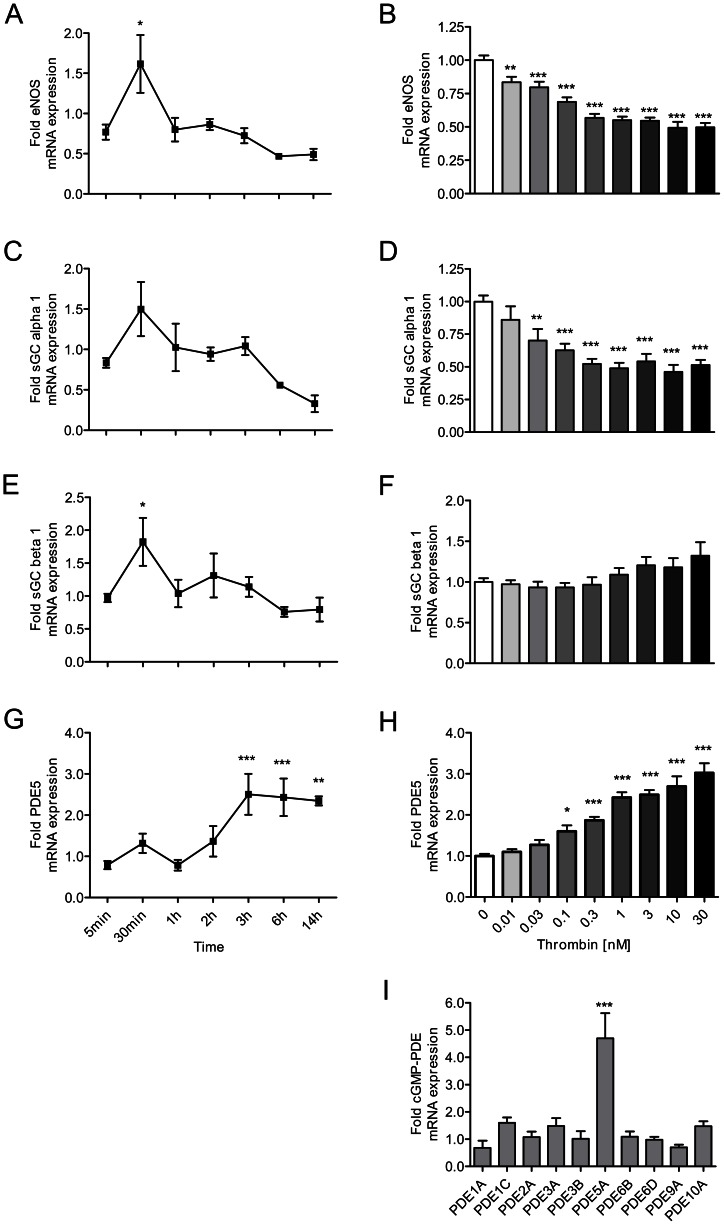
Next, we stimulated endothelial cells with thrombin. Short-time stimulation of HUVECs with thrombin was found to increase the expression of eNOS and the beta 1 subunit of sGC; increased expression of the sGC subunit alpha 1 was also found, but this change did not reach the level of statistical significance. However, these effects were short-lived, and the essentially opposite pattern was found after 6 hours of incubation with thrombin: a downregulation of eNOS, confirming published findings [15] and of the alpha 1 subunit of sGC, combined with an overexpression of PDE5 (Fig. 3A–H). Interestingly, thrombin exposition selectively elevated the expression of PDE5, but not that of any other of the cGMP-hydrolyzing PDEs (Fig. 3I). Changes in expression levels were concentration-dependent, starting at thrombin concentrations as low as 10 pM (eNOS reduction), 30 pM (sGC alpha1 reduction) and 100 pM (PDE5 upregulation), respectively. Hence, exposure to thrombin revealed a striking pattern of differential expression of key regulators of the nitric oxide-cGMP pathway. Short-term stimulation led to an upregulation of factors that contribute to cGMP elevation. By contrast, prolonged incubation reduced the expression of cGMP-elevating factors, and concomitantly increased that of the cGMP-degrading PDE5.
Figure 3. Thrombin modulates the expression of key regulators of the NO-cGMP pathway in a biphasic manner.
Left panel: Short time stimulation of HUVECs with thrombin (50 nM) increases (A) eNOS and (E) sGC beta 1 expression. Right panel: By contrast, chronic stimulation decreases (B) eNOS and (D) sGC alpha 1 expression, whereas (H) PDE5 expression is increased. At late time points, all effects are concentration-dependent (B, D, H). (I) PDE5 is the only cGMP-hydrolyzing PDE to be upregulated by thrombin (30 nM). Data shown as mean ± SEM (A, C, E, G: n = 3–5/group; B, D, F, H: n = 11/group; I: n = 3/group). *p<0.05, **p<0.01, ***p<0.001 vs cells not exposed to thrombin.
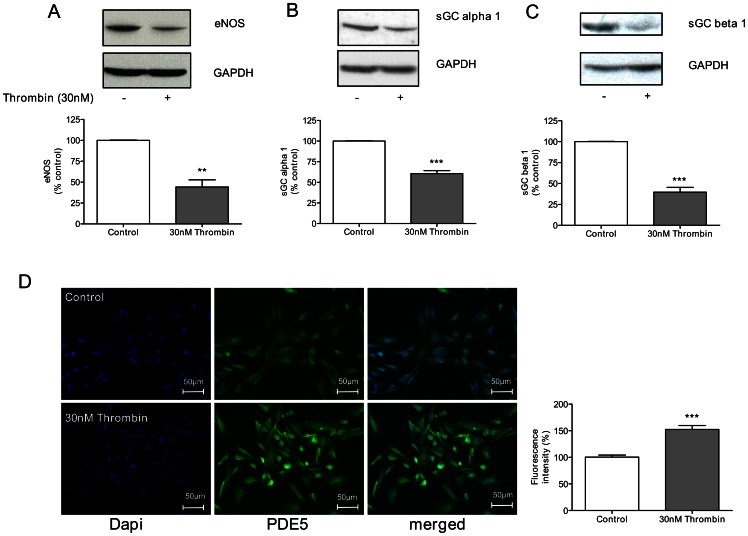
The protein levels of the regulators of the nitric oxide-cGMP pathway were investigated after prolonged thrombin exposure, in order to confirm the mRNA expression data. A significant decrease on eNOS (Fig. 4A) and the sGC subunits alpha 1 (Fig. 4B) and beta 1 (Fig. 4C) expression to approximately 40.1±7.8% (p<0.01), 60.6±3.1% (p<0.001) and 39.6±5.1% (p<0.001) of control level, respectively, was found by immunoblotting. Interestingly, thrombin also induced a significant decrease on sGC beta 1 (Fig. 4C) protein expression, which was not detectable at mRNA level (Fig. 3E–F). Furthermore, increased PDE5 protein expression was seen by immunofluorescence staining of cells exposed to thrombin (p<0.001) (Fig. 4D). Hence, the pattern of differential mRNA expression levels of factors of the nitric oxide-cGMP pathway was confirmed at the protein level.
Figure 4. Thrombin modulates protein expression of key regulators of the nitric oxide-cGMP pathway in endothelial cells.
Exposure of HUVECs to thrombin (30 nM) over 14 hours leads to a reduced expression of (A) eNOS, (B) sGC alpha 1 and (C) sGC beta 1, whereas (D) PDE5 protein expression is increased. Data shown as mean ± SEM (A, B, C: n = 5/group; D: n = 3/group). **p<0.01, ***p<0.001 vs cells not exposed to thrombin.
Thrombin has biphasic effects on cGMP levels in endothelial cells
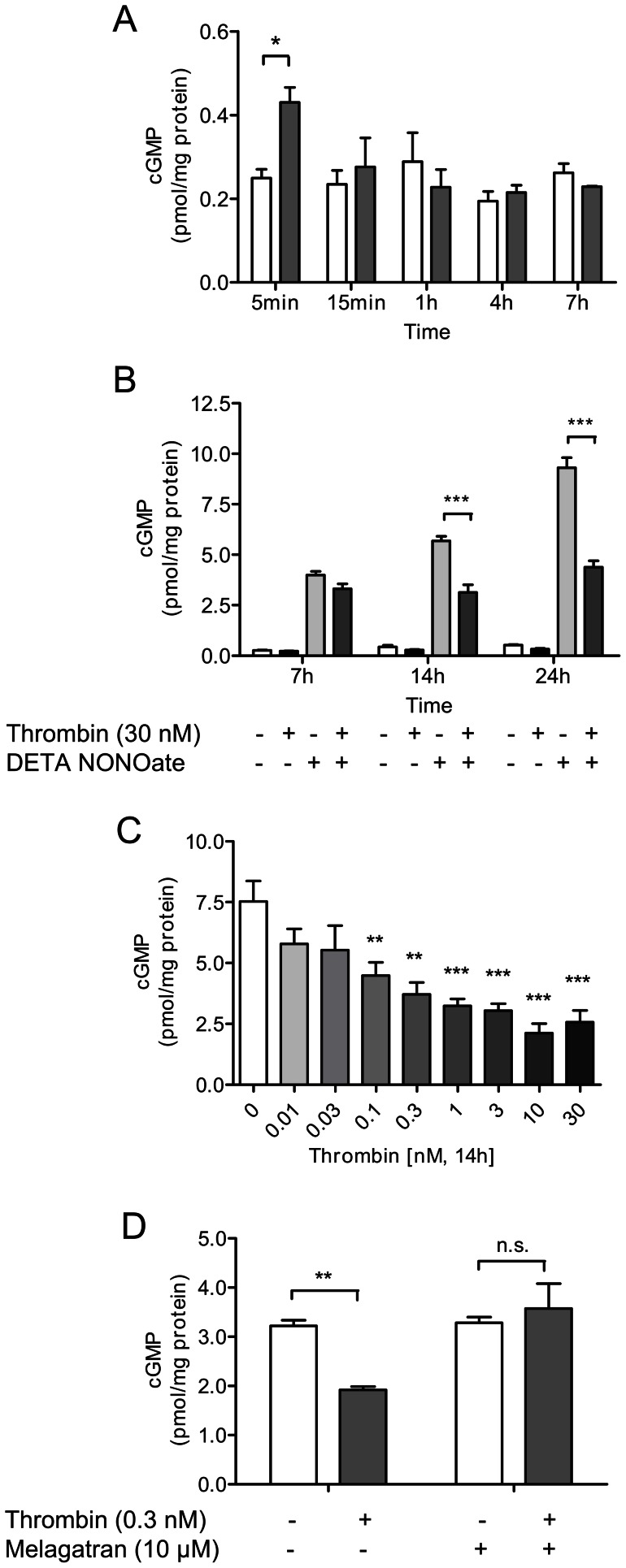
Next, we investigated the functional implications of thrombin exposure. The aim was to investigate, whether the differential expression of factors of the nitric oxide-cGMP pathway seen after acute vs. prolonged thrombin exposure translated into increased and reduced cGMP levels in the expected manner. Indeed, an increase of cGMP was seen in HUVECs after 5 min of thrombin exposition (Fig. 5A), in line with the increased expression of eNOS and sGC beta 1 induced by acute thrombin exposure. The effect, however, was transient. Similarly, in cells with increased cGMP levels obtained by stimulation with IBMX, a further cGMP elevation was found by exposure to thrombin, but the thrombin effect was transient as well (Figure S1). The effects of prolonged thrombin exposure were investigated by measuring the cGMP content with thrombin exposure at 7, 14 and 24 hours in cells stimulated with DETA NONOate (Fig. 5B). NO-donation for 7, 14 and 24 hours caused an increase of cGMP at all time points, and the effect was significantly blunted by simultaneous exposure to thrombin over 14 to 24 hours (Fig. 5B). Thrombin-induced reduction of cGMP was concentration-dependent, starting at a concentration as low as 100 pM (Fig. 5C) after 14 hours of exposition. The specificity of thrombin-induced reduction of cGMP levels was verified by co-incubation with the specific thrombin inhibitor melagatran. As shown in figure 5D, the effect of thrombin was completely abolished by melagatran.
Figure 5. Thrombin has biphasic effects on cGMP levels in endothelial cells.
(A) Thrombin (30 nM) acutely increases cGMP content in HUVECs; the effects, however, are transient (white bars: controls; grey bars: with thrombin). (B) cGMP elevation by incubation with the NO donor DETA NONOate (100 µM) is partly blunted by thrombin over 14 to 24 hours. (C) Reduction of cGMP levels in DETA NONOate-stimulated cells is concentration-dependent. (D) Thrombin-induced reduction of cGMP levels over 14 hours in DETA NONOate-stimulated cells is completely abolished by direct thrombin inhibitor melagatran (10 µM). Data shown as mean ± SEM (A, B, C: n = 3; D: n = 4/group). *p<0.05, **p<0.01, ***p<0.001 vs cells not exposed to thrombin.
In summary, the differential regulation of key factors of the nitric oxide-cGMP pathway seen at the mRNA and protein levels was found to translate into a concordant reduction in cellular cGMP.
Contribution of eNOS, sGC and PDE5 to thrombin-induced cGMP depletion in endothelial cells
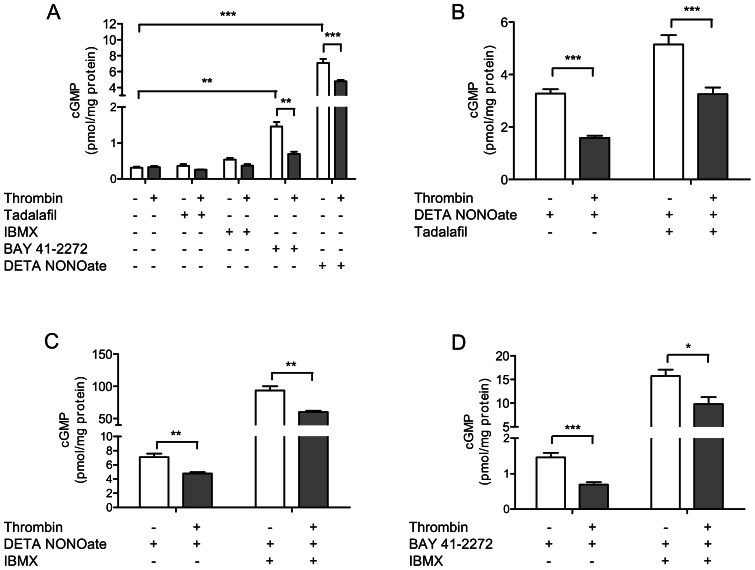
We sought to differentiate, whether the observed reduction of cGMP levels by prolonged thrombin stimulation were due to reduced production or increased degradation of cGMP, or both. First, we exposed HUVECs to the specific PDE5 inhibitor tadalafil and the unspecific PDE inhibitor IBMX: no significant increase in cGMP was found by either compound (Fig. 6A). By contrast, cGMP was increased by exposure to tadalafil or by IBMX when endothelial cells were co-stimulated with the NO-donor DETA NONOate (Fig. 6B, 6C). In this setting, co-incubation with thrombin significantly reduced the cGMP-elevating effect of the PDE5 inhibitor tadalafil as well as that of the unspecific PDE inhibitor IBMX. Since thrombin was found to maintain its cGMP-reducing effect even when PDE5 was inhibited, upregulation of PDE5 could be excluded as cause of thrombin-induced cGMP depletion. Next, we tested the role of factors that increase cGMP production. Incubation of endothelial cells with the direct sGC stimulator BAY 41–2272 or with the NO-donor DETA NONOate significantly increased cGMP levels (Fig. 6A). This effect was significantly blunted by thrombin. The effects were further investigated by co-stimulation with IBMX (Fig. 6C, 6D), which led to further increase of cGMP content, but, again, this effect was significantly reduced by thrombin. Since DETA NONOate and BAY 41–2272 directly stimulate the sGC, eNOS downregulation can be excluded as the driver of thrombin-induced cGMP reduction. By contrast, thrombin effects could be explained by the reduced expression of the sGC subunits. Importantly, however, cGMP levels could still be increased by drugs targeting the nitric oxide-cGMP pathway even in the presence of thrombin.
Figure 6. cGMP-reducing effects of thrombin in the presence of pharmacological modulators of the nitric oxide-cGMP pathway.
(A) Direct sGC-stimulator BAY 41–2272 (1 µM) und NO donor DETA NONOate (100 µM) elevate cGMP, but the prolonged thrombin challenge (0.3 nM) over 14 hours still reduces cGMP (white bars: controls; grey bars: thrombin). (B) When co-stimulated with DETA NONOate, both (B) Tadalafil and (C) IBMX elevate cGMP; again, however, cGMP-decreasing effects of thrombin were still present under stimulation with these agents. (D) The combination of IBMX and BAY 41–2272 increases cGMP more strongly than either compound alone, but thrombin-induced cGMP reduction is still present. Data shown as mean ± SEM (A: n = 5/group; B: n = 12/group; C: n = 3–5/group; D: n = 5/group). *p<0.05, **p<0.01, ***p<0.001.
Prolonged exposure of endothelial cells of pulmonary origin to thrombin differentially regulates key factors of the nitric oxide-cGMP pathway and causes cGMP depletion
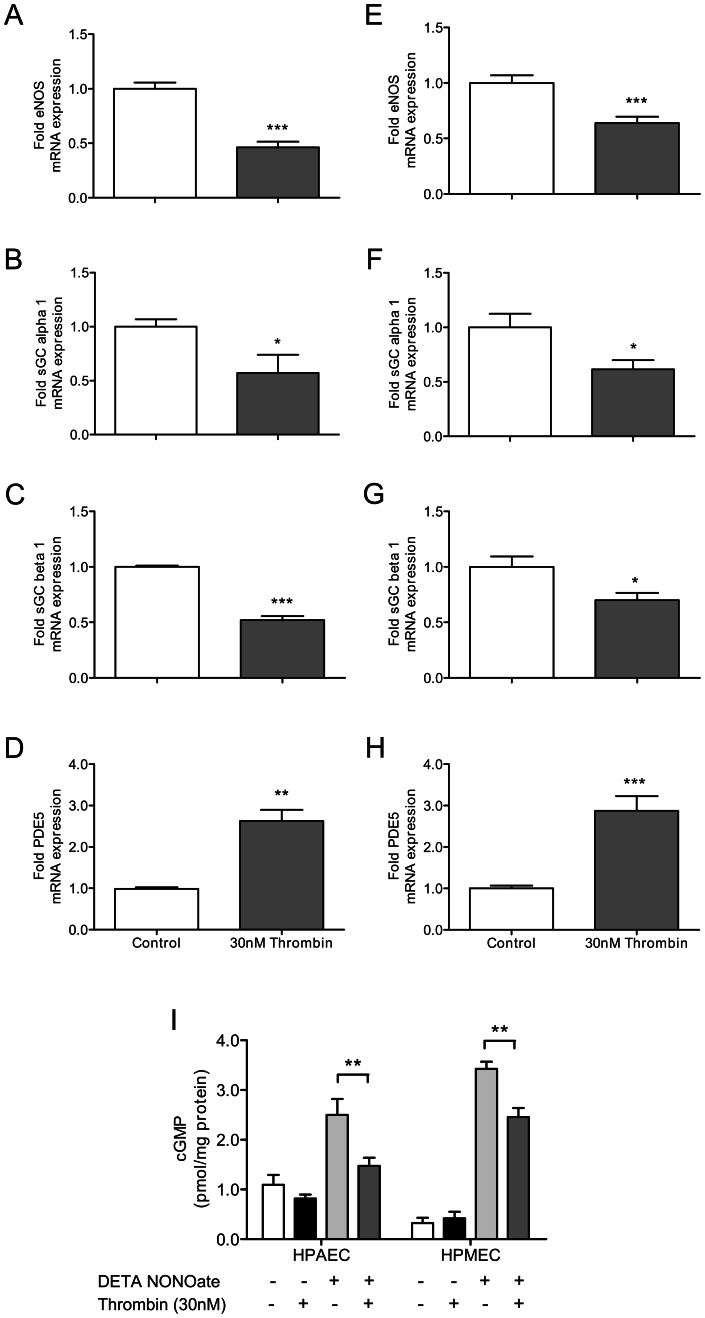
Next, we tested, whether the effects seen in HUVECs could also be observed in endothelial cells of pulmonary origin: primary human pulmonary artery endothelial cells (HPAECs) and human pulmonary microvascular endothelial cells (HPMECs). Similar to the findings in HUVECs, prolonged thrombin exposure decreased eNOS (Fig. 7A, 7E) and sGC alpha 1 (Fig. 7B, 7F) expression, and increased PDE5 expression (Fig. 7D, 7H) both in HPAECs (Fig. 7A–D) and HPMECs (Fig. 7E–H). Moreover, thrombin-induced decrease of sGC beta 1 also reached the level of statistical significance, in contrast to the findings in HUVECs (Fig. 7C, 7G). Finally, prolonged thrombin exposition was found to blunt NO-induced cGMP elevation both in HPAECs and HPMECs significantly (p<0.01).
Figure 7. Chronic thrombin stimulation leads to nitric oxide-cGMP pathway dysfunction in pulmonary endothelial cells.
Thrombin (30 nM) stimulation over 6 hours reduces the expression of (A, E) eNOS, (B, F) sGC alpha 1 and (C, G) sGC beta 1 in pulmonary artery (A–D) and pulmonary microvascular (E–H) endothelial cells, whereas (D, H) PDE5 expression is increased (White bars: control cells; grey bars: with thrombin). (I) Prolonged thrombin exposition over 14 hours leads to cGMP depletion in pulmonary endothelial cells in presence of DETA NONOate (100 µM). Data shown as mean ± SEM (A, B, C, D: n = 6/group; E, F, G, H: n = 12/group; I: n = 5–6). *p<0.05, **p<0.01 vs cells not exposed to thrombin.
Direct activation of PAR-1 induces changes in the nitric oxide-cGMP pathway similarly to thrombin
Thrombin is known to activate the protease-activated receptors 1, 3 and 4 [31]. To explore the mechanism by which thrombin regulates the key factors of the nitric oxide-cGMP pathway, we stimulated HUVECs with different concentration of TRAP-10, which directly and specifically activates PAR-1, and a PAR-4 activating peptide, respectively, for 6h.
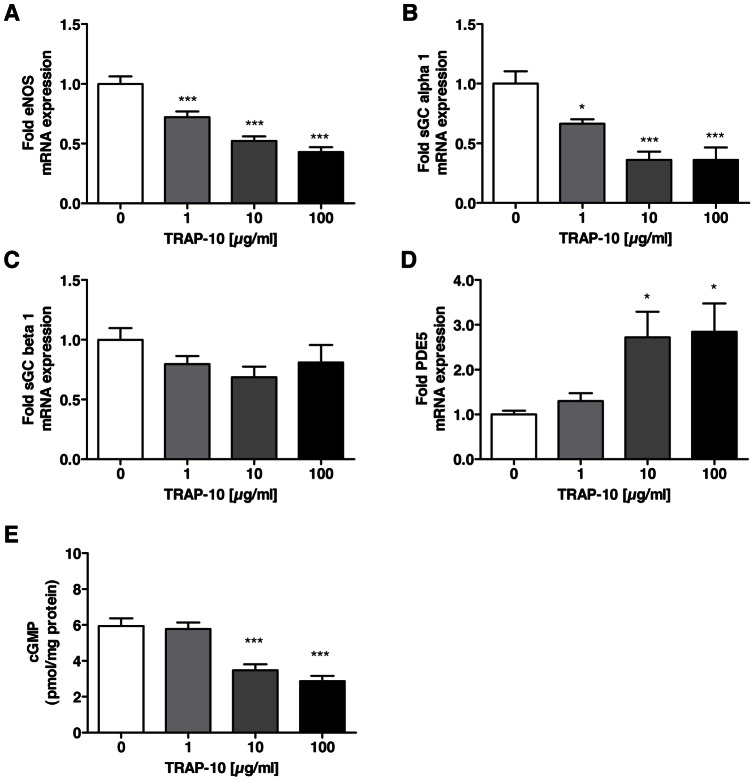
Similar to the findings with thrombin, prolonged TRAP-10 exposure decreased eNOS (Fig. 8A) and sGC alpha 1 (Fig. 8B) expression, and increased PDE5 expression (Fig. 8D) in a concentration-dependent manner. The expression of sGC beta 1 subunit was not affected (Fig. 8C). In contrast, no effect on the nitric-oxide-cGMP pathway was detectable when HUVECs were stimulated with a PAR-4 activating peptide (data not shown). Since PAR-3 is a co-factor for the activation of the other PARs, the activation of this receptor was not analyzed.
Figure 8. Thrombin-induced nitric oxide-cGMP pathway dysfunction is mediated via activation of protease-activated receptor-1.
Exposure of HUVECs to different concentration of the PAR-1 activating peptide TRAP-1 (1 to 100 µg/ml) over 6 hours leads to reduced expression of (A) eNOS, (B) sGC alpha 1 and (C) sGC beta 1, whereas (D) PDE5 protein expression is increased. (E) Prolonged TRAP-10 exposition over 14 hours leads to cGMP depletion in HUVECs in presence of DETA NONOate (100 µM). Data shown as mean ± SEM (A, B, C, D: n = 6/group; E: n = 9/group). *p<0.05, **p<0.01, ***p<0.001 vs cells not exposed to TRAP-10.
Previously, it was shown that PAR-2 can be activated by cleaved PAR-1 and that this transactivation is recruiting PAR-2 into the thrombin response in HUVECs [32]. To analyze whether the effect of the PAR-1 activating peptide TRAP-10 on the nitric-oxide-cGMP pathway is exclusively mediated through PAR-1 activation or additionally through transactivation of PAR-2, HUVECs were also stimulated with a PAR-2 activating peptide for 6 h. The PAR-2 specific peptide did not affect the expression of eNOS, sGC alpha 1 and sGC beta 1, but increased the expression of PDE5 (data not shown).
In addition, we analyzed the effect of TRAP-10 and the PAR-2 specific peptide on cGMP level in HUVECs. In line with the results obtained after exposure to thrombin, prolonged exposure to TRAP-10 was found to blunt NO-induced cGMP elevation significantly in a concentration-dependent manner (Fig. 8E). In contrast, the exposure to the PAR-2 specific peptide did not affect the NO-induced cGMP elevation in a significant manner (data not shown).
Thus, exposure to TRAP-10 revealed a similar pattern of differential expression of key regulators of the nitric oxide-cGMP pathway, as exposure to thrombin; these data suggest that the effects of thrombin on endothelial cells in the context of pulmonary hypertension are, at least in part, mediated via PAR-1.
Discussion
Based on the recent observation that the coagulation factor Xa is involved in RV hypertension and hypertrophy in experimental pulmonary hypertension [6], we investigated the role of its downstream effector thrombin. Even moderate chronic thrombin inhibition in vivo was found to also reduce RV hypertrophy in the MCT model of pulmonary hypertension, indicating that the previously shown effect of FXa is mediated through thrombin. In vitro, thrombin was found to have biphasic effects on the key regulators of the nitric oxide-cGMP pathway in endothelial cells. Whereas short-term exposure to thrombin resulted in an increase of cGMP, chronic exposition was found to induce the opposite effect. A pattern of differential expression of the key regulators of the nitric oxide-cGMP pathway was revealed, in which the factors contributing to cGMP elevation (eNOS and sGC) were increased by acute thrombin stimulation, whereas prolonged thrombin exposure reduced the expression of cGMP-elevating factors and increased that of the cGMP-hydrolyzing PDE5 in a remarkably specific manner. Finally, activation of PAR-1 was found to reproduce the effects seen with thrombin, indicating that thrombin-induced negative regulation of the nitric oxide-cGMP pathway in endothelial cells is, at least in part, mediated via PAR-1.
Nitric oxide-cGMP pathway in pulmonary hypertension
The nitric oxide-cGMP pathway is known to have vasoprotective, anti-inflammatory and antioxidant effects on endothelial cells [33]–[35], and has been proposed to prevent endothelial dysfunction and intimal lesions in pulmonary arterioles of patients with pulmonary arterial hypertension [36], [37]. The clinical importance of this pathway in pulmonary arterial hypertension is well established, and drugs targeting its elements to increase cGMP levels in the pulmonary vasculature are highly therapeutically effective [22]–[27], [38], [39]. Despite these clinical successes, there is substantial controversy regarding the actual levels of expression and function of the nitric oxide-cGMP pathway in the pulmonary vasculature in animal models of pulmonary hypertension or in patients with this disease [24], [40]–[45]. In our study we observed that right ventricular hypertrophy was accompanied by the elevation of cGMP levels that confirms previous published data [28]–[30].
Thrombin and the nitric oxide-cGMP pathway
Both FXa and thrombin have been proposed to interact with the nitric oxide-cGMP pathway in endothelial cells, although the reported effects are partly conflicting [13]–[15]. Here we sought to specifically investigate the interactions between thrombin and this pathway in the context of pulmonary arterial hypertension. Using primary endothelial cells, we found that short-term exposure to thrombin induced the increased expression of the cGMP-elevating factors eNOS and the beta 1 subunit of sGC; this effect was associated with increased cGMP as expected. However, we found these effects to be short-lived and to be reversed as early as one hour after start of thrombin exposure. By contrast, prolonged thrombin stimulation induced a striking pattern of differential expression of regulators of the nitric oxide-cGMP pathway, in which specifically the expression of factors contributing to cGMP elevation were reduced (eNOS and sGC subunits), and the expression of the cGMP-hydrolyzing PDE5 was elevated. These findings combined suggest opposite functions of acute and chronic thrombin exposition on nitric oxide-cGMP pathway in endothelial cells.
The differential expression of eNOS, sGC subunits and PDE5 seen after prolonged thrombin stimulation would be expected to work together to reduce cGMP expression which, indeed, was found to be the case in subsequent experiments. Further experiments using pharmacological tools suggested sGC-downregulation to be the main driver of cGMP lowering by thrombin, although a combined role for eNOS, sGC and PDE5 could not be ruled out. It is remarkable, however, that all three factors differentially regulated by thrombin are also specific targets for pharmacotherapy of pulmonary arterial hypertension, where the drugs act in the opposite direction of the effects seen in vitro for thrombin: eNOS activation (target for L-arginine), sGC stimulation (target for riociguat), and PDE5 inhibition (target for sildenafil and tadalafil) [22]–[27], [38], [39]. The relevance of the present findings for the clinical setting is unclear at present; however, it is important to note that all pharmacological compounds (NO-donors, sGC-stimulators and PDE5 inhibitors) retained their cGMP-increasing effects even in the presence of prolonged thrombin exposure.
By contrast to thrombin, chronic FXa exposition modulated only the expression of eNOS and PDE5 expression in HUVECs, and these changes were significant only at the highest concentration of 30 nM. The relevance of these changes is questionable by comparison with thrombin, which showed significant changes of eNOS, sGC subunits and PDE5 expression at concentrations as low as 10 to 100 pM. Furthermore, it is estimated that the activation of one molecule FXa results in the generation of 1000 molecules of thrombin [46] and, accordingly, the plasma concentration of FX (170 nM) is substantially lower than that of prothrombin (1.4 µM) [47]. These findings indicate that the marginal direct effect of FXa on nitric oxide-cGMP pathway in endothelial cells seen in vitro is most likely not physiologically relevant in vivo.
A role for thrombin in pulmonary arterial hypertension?
Several authors have proposed a role for coagulation factors in the pathogenesis and progression of pulmonary arterial hypertension [4], [5], [48], [49]. Until recently, however, this concept was based solely on descriptive data from animal experiments and retrospective observations in patients with pulmonary arterial hypertension, whereas conclusive prospective interventional data were lacking.
Recently, the results from two experimental studies have reinforced the concept. A mouse overexpressing tissue factor pathway inhibitor (TFPI) was found to have reduced pulmonary hypertension when exposed to hypoxia [50]. We have recently shown that pharmacological inhibition of FXa by the direct, selective inhibitor rivaroxaban is beneficial in the MCT model of experimental pulmonary hypertension [6]. The present study is the first to show that specific inhibition of thrombin, the final enzymatic step of the coagulation cascade, is sufficient to reduce pulmonary hypertension in the MCT model.
The mode of action of thrombin in disease initiation or progression is, however, not clear. Available evidence suggests two possible mechanisms. Clot formation by thrombin-induced fibrin generation and platelet accumulation would be expected to obstruct pulmonary arteries up to total occlusion, thereby leading to resistance increase and pulmonary arterial hypertension [4], [5], [51]. In support of this hypothesis, clots are frequently found in the pulmonary vasculature of patients who have died of pulmonary arterial hypertension [52], [53]. Alternatively, a direct effect of coagulation factors on the endothelium of pulmonary arteries has been postulated. Indeed, interactions have been described between the plasma coagulation system and the endothelium, the cells that are in direct contact with plasma [7]–[9]. Specifically, FXa and thrombin have been reported to exert direct effects on endothelial cells including activation, angiogenesis, vascular leakage and inflammation [7]–[9], [21]. In line with this, thrombin has been shown to have a physiological role in modulating vascular tone by activating endothelial cells independent of its procoagulant properties [15], [54], [55].
The findings in the present study reinforce these concepts by demonstrating a dysfunction of the nitric oxide-cGMP pathway after prolonged thrombin exposition, and could indicate that direct interactions with the pulmonary endothelium might lead to a pathophysiology reminescent of “endothelial dysfunction”, ultimately leading to initiation or aggravation of pulmonary hypertension [56].
The cell-mediated effects of thrombin might be mediated through protease-activated receptors (PARs) [57]–[60]. Published evidence indicates that PAR-1 plays a role in arterial wall thickening after vascular injury [61], [62]. In addition, previous data indicate that thrombin induced a sustained contraction in normal pulmonary arteries by activating PAR-1 [63]. Recently, data were published that indicate a role for PAR-2 expressed on vascular smooth muscle cells in vascular remodeling in pulmonary hypertension [64].
We found that exposure to the PAR-1 activating peptide TRAP-10 revealed the same pattern of differential expression of key regulators of the nitric oxide-cGMP pathway as exposure to thrombin. In contrast, the exposure to a specific PAR-2 activating peptide did not influence the nitric oxide-cGMP pathway in our studies using endothelial cells. These findings indicate that the effects of thrombin on the nitroc oxide-cGNP pathway in endothelial cells might be mediated via PAR-1.
In conclusion, the findings presented here suggest a previously unrecognized link between the coagulation system and the nitric oxide-cGMP pathway, possibly mediated via PAR-1, that might be of relevance in pulmonary arterial hypertension. If confirmed by further studies, these findings could form an additional rationale for the clinical use and accepted efficacy of drugs targeting the nitric oxide-cGMP pathway, and for the additional use of anticoagulants in this indication.
Materials and Methods
In Vivo Experiments
All animal experiments were performed in accordance with the European Community guidelines for the use of experimental animals and with the German Animal Protection Act (Deutsches Tierschutzgesetz). The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996), and was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz of Nordrhein-Westfalen/Germany (LANUV).
Monocrotaline Application and Pharmacological Treatments
Adult male Sprague-Dawley rats weighing 200 to 250 g were purchased from Charles River Laboratories (Sulzfeld, Germany). Rats were randomized to one of the treatment groups, before receiving a single subcutaneous (s.c.) injection of saline or 60 mg/kg monocrotaline (MCT) (Sigma-Aldrich Chemie GmbH, Munich, Germany) as described previously [65], [66]. The rats were randomly assigned to one of three groups (n = 10/group): (1) control animals, (2) MCT + placebo, (3) MCT + melagatran (0.9 mg/kg/d via alzet® osmotic pumps 2ML4 (Durect Corporation, Cupertino, CA, USA)) (AstraZeneca, Mölndal, Sweden). The pumps were implanted under the skin dorsally in the area of the neck under isoflorane anesthesia. All pumps were incubated in physiological saline for 24 h at 37°C before implantation. Activated pumps were then implanted one day before MCT-injection.
Assessment of RV Hypertrophy and Tissue Preparation
On day 28 after MCT injection, the rats were anesthetized with intraperitoneal inactin (180 mg/kg) (Sigma-Aldrich Chemie GmbH, Munich, Germany). The animals were exsanguinated via abdominal aorta and the heart was excised. The RV wall was separated from the left ventricular wall and the ventricular septum. The ratio of the right ventricle to left ventricle plus septum weight (RV/(LV+S)) was calculated as a body weight-independent index of right ventricular hypertrophy as described previously [6], [66]. The right ventricles were snap-frozen on dry ice for RNA extraction and quantitative real-time polymerase chain reaction.
Ecarin Fluorogenic Assay
The ecarin fluorogenic assay is a modification from the ecarin chromogenic assay (ECA) [67]. Plasma samples (20 µl), water (20 µl) and fluorogenic thrombin substrate (50 µM, 20 µl; Boc-Val-Pro-Arg-AMC; Bachem, Bubendorf, Switzerland) and Ecarin (0.4 U/ml) (Sigma-Aldrich Chemie GmbH, Munich, Germany) in Ca2+-Buffer (2.5 mM, 20 µl) were mixed and fluorescence was measured immediately for 20 minutes with 390 nm excitation and 465 nm emission wavelength. The thrombin inhibition was related to control plasma (0%).
In Vitro Experiments
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Basel, Switzerland). Human pulmonary artery endothelial cells (HPAECs) and human pulmonary microvascular endothelial cells (HPMECs) were purchased from Promocell (Heidelberg, Germany) and cultured in the medium provided from the manufacturers. For the present studies, cells between passage 2 and 5 were used. Before treatment, the cells were serum-starved for 1 hour using the adequate medium without any supplements. In all subsequent steps, cells were maintained in this serum free medium.
RNA Extraction, cDNA Synthesis and Quantitative Real-Time Polymerase Chain Reaction
Cells were grown in 12 multiwell plates until confluence, serum-starved for 1 hour and then treated with different concentration of either Factor Xa, thrombin (0.01 to 30 nM; Kordia, Leiden, The Netherlands), TRAP-10 (JPT Peptide Technologies, Berlin, Germany), a PAR-2 activating peptide (1 to 100 µg/ml; Bachem, Merseyside, UK) or a PAR-4 activating peptide (1 to 100 µg/ml; Bachem, Merseyside, UK) for 6 hours or with 50 nM thrombin over different time periods. Total RNA was isolated using the RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturers protocol. From snap-frozen RV samples total RNA was extracted by using TRIzol (Invitrogen, Darmstadt, Germany) [68]. Isolated RNA was reversed transcribed using Im-Prom-II Reverse Transcription System (Promega, Madison, WI, USA) and amplified using TaqMan real-time PCR as described previously [6]. Primer sequences are shown in Table S1 and detailed methodology is provided in the Materials and Methods S1.
Western Blot Analysis
After treatment HUVECs were harvested in ice-cold lysis buffer (Cell signaling, Danvers, MA, USA) in the presence of protease inhibitors (Complete; Roche, Basel, Switzerland). Cell lysates were subjected to Western blot analysis as described in the Materials and Methods S1 section using commercially available antibodies. eNOS (BD Transduction Laboratories, Basel, Switzerland), sGC alpha 1 (abcam, Cambridge, USA), sGC beta 1 (Cayman Chemical, Ann Arbor, Ml, USA) and GAPDH (Cell Signaling, Danvers, MA, USA). Detection was performed by the ECL method (Amersham Biosciences, Freiburg, Germany) and the protein levels were determined by densitometric analysis of the specific protein bands (GS-800 Calibrated Densitometer, Quantity One Analysis Software; Biorad, Munich, Germany).
Immuncytochemistry
HUVECs were plated onto gelatin (0.25%) coated glass chamber slides (Nunc, Naperville, IL, USA) and treated as above with 30 nM thrombin (Kordia, Leiden, The Netherlands). After treatment, cells were washed with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton-X 100, and stained for immunocytochemistry with a polyclonal rabbit antibody against human PDE5 (1∶1.000) (abcam, Cambridge, USA) used in conjunction with a fluorescent (Alexa 488) secondary antibody (Invitrogen, Darmstadt, Germany). Nuclei were stained with Dapi (1∶10.000; Sigma-Aldrich Chemie GmbH, Munich, Germany). The intracellular localization and expression of PDE5 at baseline and in response to thrombin was visualized by fluorescence microscopy (Axio Imager Z1; Zeiss, Göttingen, Germany) with excitation at 495 nm and emission at 519 nm for Alexa Flour 488 and excitation at 364 nm and emission at 454 nm for Dapi bound to dsDNA. In each case, fluorescence intensity was measured in levels of grey after removal of background fluorescence from four different sections by two blinded investigators using Axiovision 4 Software (Zeiss, Göttingen, Germany).
Measurement of cGMP
Cells were grown in 12 multiwell plates until confluence, serum-starved for 1 hour and then treated as described below.
Three different protocols were performed for treating cells before measuring of cGMP: (1) For analyzing the acute effect of thrombin, cells were exposed to 30 nM Thrombin (Kordia, Leiden, The Netherlands) over different time points in presence and absence of the unspecific PDE inhibitor IBMX (750 µM) (Sigma-Aldrich Chemie GmbH, Munich, Germany). (2) For analyzing the chronic effect of thrombin, cells were simultaneously exposed to thrombin and 100 µM of the long-acting NO-donor DETA NONOate (Cayman Chemicals, Ann Arbor, Ml, USA) (stock solution 10 mM in 0.01 mM NaOH) over different time points and different concentrations of thrombin over 14 hours, respectively. In a separate experiment, the cells were additionally incubated with the direct thrombin inhibitor melagatran, started 30 min prior thrombin and DETA NONOate exposition over 14 hours. (3) cells were exposed to thrombin over 14 hours, medium was aspirated and cells were then incubated with either the specific PDE5 inhibitor Tadalafil (10 µM) (Cialis®, Lilli Pharma, Indianapolis, IN, USA), the unspecific PDE inhibitor IBMX (750 µM) (Sigma-Aldrich Chemie GmbH, Munich, Germany), the direct sGC stimulator BAY 41–2272 (1 µM) (Bayer Schering Pharma AG, Wuppertal, Germany) or DETA NONOate for 15 minutes. Since no cGMP elevation was found with Tadalafil incubation over 15 min in combination with DETA NONOate, protocol (2) was applied for this group (2).
Following treatments, plates were placed on ice, medium was aspirated and 70% icecold EtOH were added to the wells for cGMP extraction. Plates were stored at −20°C over night and the extracts were collected. Cyclic GMP was lyophilized before the cGMP enzyme immunoassay kit (Amersham Biosciences, Freiburg, Germany) according to the manufactures instruction was performed. Cell remnants were include with 0.1 M NaOH and used for protein determination via BCA protein assay (Pierce, Rockford, USA). Values are presented at pmol/mg protein.
To measure pulmonary cGMP levels, lungs were snap-frozen on dry ice and cGMP was extracted according to the manufactures instructions of the cGMP enzyme immunoassay kit (Amersham Biosciences, Freiburg, Germany). Values are presented at pmol/g lung.
Statistical Analysis
All data are shown as means ± SEM. Statistical comparisons between groups were performed using one-way ANOVA, followed by Student-Newman-Keuls post-hoc analysis, or an unpaired Students t test (two-tailed) using GraphPad Prism 5.03 Software (GraphPad Software Inc., San Diego, CA, USA). A p value of less than 0.05 was considered significant.
Supporting Information
Thrombin-induced cGMP elevation in IBMX-stimulated endothelial cells is transient. Thrombin (30 nM) acutely increases cGMP content in HUVECs; the effects, however, are transient (white bars: controls; grey bars: with thrombin). Data shown as mean ± SEM (n = 3). White bars: control cells; grey bars: with thrombin. *p<0.05, ***p<0.001 vs cells not exposed to thrombin.
(TIF)
(DOC)
Primer and probe sequences for real-time PCR.
(PPT)
Acknowledgments
We are grateful to Janina Nowatzky for assistance in immunostaining and to Johannes-Peter Stasch for expert advice.
Funding Statement
Katrin Nickel received funding from Bayer Pharma AG. Volker Laux and Georges v. Degenfeld are employees of Bayer Pharma AG. Bayer Pharma provided funding for the in vivo studies and took part in the design, data aquisition and analysis of the in vivo experiments. Bayer Pharma had no role in the interpretation of the data and decision to publish. The authors declare that funding by Bayer Pharma does not influence their adherence to the PLOS ONE policies on sharing data and materials.
References
- 1. Dorfmüller P, Humbert M, Capron F, Müller KM (2003) Pathology and aspects of pathogenesis in Pulmonary Arterial Hypertension. Sarcoidosis Vasc Diffuse Lung Dis 20: 9–19. [PubMed] [Google Scholar]
- 2. Badesch DB, Abman SH, Ahearn GS, Barst RJ, McCrory DC, et al. (2004) Medical Therapy for Pulmonary Arterial Hypertension: ACCP Evidence-Based Clinical Practice Guidelines. Chest 126: 35S–62S. [DOI] [PubMed] [Google Scholar]
- 3. Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV (2007) Medical Therapy for Pulmonary Arterial Hypertension – Updated ACCP Evidence-Based Clinical Practice Guidelines. Chest 131: 1917–1928. [DOI] [PubMed] [Google Scholar]
- 4. Johnson SR, Mehta S, Granton JT (2006) Anticoagulation in pulmonary arterial hypertension: a qualitative systemic review. Eur Respir J 28: 999–1004. [DOI] [PubMed] [Google Scholar]
- 5. Grünig E, Ehlken N, Nagel C (2008) Antikoagulation bei der pulmonal arteriellen Hypertonie. Hämostaseologie 28: 225–230. [PubMed] [Google Scholar]
- 6. Delbeck M, Nickel KF, Perzborn E, Ellinghaus P, Strassburger J, et al. (2011) A role for coagulation factor Xa in experimental pulmonary hypertension. Cardiovasc Res 92: 159–168. [DOI] [PubMed] [Google Scholar]
- 7. Krupiczojc MA, Scotton CJ, Chambers RC (2008) Coagulation signaling following tissue injury: Focus on the role of factor Xa. Int J Biochem Cell Biol 40: 1228–1237. [DOI] [PubMed] [Google Scholar]
- 8. Borensztajn K, Peppelenbosch MP, Spek CA (2008) Factor Xa: at the crossroads between coagulation and signaling in physiology and disease. Trends Mol Med 14: 429–440. [DOI] [PubMed] [Google Scholar]
- 9. Borissoff JI, Spronk HMH, Heeneman S, ten Cate H (2009) Is thrombin a keyplayer in the ‘coagulation-atherogenesis’ maze? Cardiovas Res 82: 392–403. [DOI] [PubMed] [Google Scholar]
- 10. Licari LG, Kovacic JP (2009) Thrombin physiology and pathophysiology. J Vet Emerg Crit Care 19: 11–22. [DOI] [PubMed] [Google Scholar]
- 11. Borensztajn K, Stiekema J, Nijmeijer S, Reitsma PH, Peppelenbosch MP, et al. (2008) Factor Xa stimulates proinflammatory and profibrotic responses in fibroblasts via protease-activated receptor-2 activation. Am J Pathol 172: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scotton CJ, Krupiczojc MA, Königshoff M, Mercer PF, Lee YC, et al. (2009) Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest 1199: 2550–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papapetropoulos A, Piccardoni P, Cirino G, Bucci M, Sorrentino R, et al. (1998) Hypotension and inflammatory cytokine gene expression triggered by factor Xa-nitride oxide signaling. Proc Natl Acad Sci 95: 4738–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motley ED, Eguchi K, Patterson MM, Palmer PD, Suzuki H, et al. (2007) Mechanism of endothelial nitric oxide synthase phosphorylation and activation by thrombin. Hypertension 49: 577–583. [DOI] [PubMed] [Google Scholar]
- 15. Eto M, Barandier C, Rathgeb L, Kozai T, Joch H, et al. (2001) Thrombin Suppresses Endothelial Nitric Oxide Synthase and Upregulates Endothelin-Converting Enzyme-1 Expression by Distinct Pathways: Role of Rho/ROCK and Mitogen-Activated Protein Kinase. Circ Res 89: 583–590. [DOI] [PubMed] [Google Scholar]
- 16. Friebe A, Koesling D (2003) Regulation of Nitric Oxide-Sensitive Guanylyl Cyclase. Circ Res 93: 96–105. [DOI] [PubMed] [Google Scholar]
- 17. Kemp-Harper B, Schmidt HH (2009) cGMP in the vasculature. Handb Exp Pharmacol (191): 447–467. [DOI] [PubMed] [Google Scholar]
- 18. Stasch JP, Hobbs AJ (2009) NO-independent, haem-dependent soluble guanylate cyclase stimlators. Handb Exp Pharmacol (191): 277–308. [DOI] [PubMed] [Google Scholar]
- 19. Wilkins MR, Wharton J, Grimminger F, Ghofrani HA (2008) Phosphodiesterase inhibitors for the treatment of pulmonary hypertension. Eur Respir J 32: 198–209. [DOI] [PubMed] [Google Scholar]
- 20. Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J (1991) Inhaled nitric oxide as a cause of selective pulmonary vasodilation in pulmonary hypertension. Lancet 338: 1173–1174. [DOI] [PubMed] [Google Scholar]
- 21. Steiner MK, Preston IR, Klinger JR, Hill NS (2005) Pulmonary hypertension: inhaled nitric oxide, sildenafil and natriuretic peptides. Curr Opin Pharmacol 5: 245–250. [DOI] [PubMed] [Google Scholar]
- 22. Ramani GV, Park MH (2010) Update on the clinical utility of sildenafil in the treatment of pulmonary arterial hypertension. Drug Des Devel Ther 4: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levin YD, White RJ (2011) Novel therapeutic approaches in pulmonary arterial hypertension: focus on tadalafil. Drugs Today (Barc) 47: 145–156. [DOI] [PubMed] [Google Scholar]
- 24. Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Müller D, et al. (2008) Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J 32: 881–891. [DOI] [PubMed] [Google Scholar]
- 25. Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, et al. (2009) First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Resir J 33: 785–792. [DOI] [PubMed] [Google Scholar]
- 26. Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, et al. (2009) Discovery of riociguat (BAY 63–2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. Chem Med Chem 4: 853–865. [DOI] [PubMed] [Google Scholar]
- 27. Stasch JP, Pacher P, Evgenov OV (2011) Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sawamura F, Kato M, Fujita K, Nakazawa T, Beardsworth A (2009) Tadalafil, a long-acting inhibitor of PDE5, improves pulmonary hemodynamics and survival rate of monocrotaline-induced pulmonary artery hypertension in rats. J Pharmacol Sci 111: 235–243. [DOI] [PubMed] [Google Scholar]
- 29. Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, et al. (2004) Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med 169: 39–45. [DOI] [PubMed] [Google Scholar]
- 30. Kodama K, Adachi H (1999) Improvement of mortality by long-term E4010 treatment in monocrotaline-induced pulmonary hypertensive rats. J Pharmacol Exp Ther 290: 748–52. [PubMed] [Google Scholar]
- 31. Borissoff JI, Spronk HM, Heeneman S, ten Cate H (2009) Is thrombin a key player in the ‘coagulation-atherogenesis’ maze? Cardiovasc Res 82: 392–403. [DOI] [PubMed] [Google Scholar]
- 32. O'Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, et al. (2000) Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J Biol Chem 275: 13502–13509. [DOI] [PubMed] [Google Scholar]
- 33. Boerrigter G, Burnett JC Jr (2007) Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41–2272 in cardiovascular disease. Cardiovasc Drug Rev 25: 30–45. [DOI] [PubMed] [Google Scholar]
- 34. Ahluwalia A, Foster P, Scotland RS, McLean PG, Mathur A, et al. (2004) Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependnet down-regulation of P-selectin expression and leucocyte recruitment. PNAS 101: 1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephens RS, Rentsendorj O, Servinsky LE, Moldobaeva A, Damico R, et al. (2010) cGMP increases antioxidant function and attenuates oxidant cell death in mouse lung microvascular endothelial cells by a protein kinase G-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 299: L323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jurasz P, Courtman D, Babaie S, Stewart DJ (2010) Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther 126: 1–8. [DOI] [PubMed] [Google Scholar]
- 37. Budhiraja R, Tuder RM, Hassoun PM (2004) Endothelial dysfunction in pulmonary hypertension. Circulation 109: 159–165. [DOI] [PubMed] [Google Scholar]
- 38. Mehta S, Stewart DJ, Langleben D, Levy RD, et al. (1995) Short-term pulmonary vasodilation with L-arginine in pulmonary hypertension. Circulation 92: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 39. Nagaya N, Uematsu M, Oya H, Sato N, Sakamaki F, et al. (2001) Short-term oral administration of L-arginine improves hemodynamics and exercise capacity inpatients with precapillary pulmonary hypertension. Am J Respir Crit Care Med 163: 887–891. [DOI] [PubMed] [Google Scholar]
- 40. Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, et al. (1998) High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Path 185: 313–318. [DOI] [PubMed] [Google Scholar]
- 41. Mathew R, Yuan N, Rosenfeld L, Gewitz MH, Kumar A (2002) Effects of monocrotaline on endothelial nitric oxide synthase expression and sulfhydryl levels in rat lungs. Heart Dis 4: 152–158. [DOI] [PubMed] [Google Scholar]
- 42. Huang J, Wolk JH, Gewitz MH, Mathew R (2010) Progressive endothelial cell damage in an inflammatory model of pulmonary hypertension. Exp Lung Res 36: 57–66. [DOI] [PubMed] [Google Scholar]
- 43. Giordano D, De Stefano ME, Citro G, Modica A, Giorgi M (2001) Expression of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in mouse tissues and cell lines using an antibody against the enzyme amino-terminal domain. Biochim Biophys Acta 1539: 16–27. [DOI] [PubMed] [Google Scholar]
- 44. Corbin JD, Beasley A, Blount MA, Francis SH (2005) High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun 334: 930–938. [DOI] [PubMed] [Google Scholar]
- 45. Wharton J, Strange JW, Møller GMO, Growcott EJ, Ren X, et al. (2005) Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med 172: 105–113. [DOI] [PubMed] [Google Scholar]
- 46. Mann KG, Brummel K, Butenas S (2003) What is all that thrombin for? J Thromb Haemost 1: 1504–1514. [DOI] [PubMed] [Google Scholar]
- 47. Butenas S, Mann KG (2002) Blood coagulation. Biochemistry (Mosc) 67: 3–12. [DOI] [PubMed] [Google Scholar]
- 48. Schultze AE, Roth RA (1998) Chronic pulmonary hypertension – the monocrotaline model and involvement of the hemostatic system. J Toxicol Environ Health B Crit Rev Part B 1: 271–346. [DOI] [PubMed] [Google Scholar]
- 49. Berger G, Azzam ZS, Hoffmann R, Yigla M (2009) Coagulation and anticoagulation in pulmonary arterial hypertension. IMAJ 11: 376–379. [PubMed] [Google Scholar]
- 50. White TA, Witt TA, Pan S, Mueske CS, Kleppe LS, et al. (2010) Tissue factor pathway inhibitor overexpression inhibits hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol 43: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hassell KL (1998) Altered hemostasis in pulmonary hypertension. Blood Coagul Fibrinolysis 9: 107–117. [DOI] [PubMed] [Google Scholar]
- 52. Bjornsson J, Edwards WD (1985) Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc 60: 16–25. [DOI] [PubMed] [Google Scholar]
- 53. Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, et al. (1989) Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National heart, lung, and blood institute, primary pulmonary hypertension registry. Circulation 80: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 54. De Mey JG, Claeys M, Vanhoutte PM (1982) Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther 222: 166–173. [PubMed] [Google Scholar]
- 55. Lüscher TF, Diederich D, Siebenmann R, Lehmann K, Stulz P, et al. (1988) Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med 319: 462–467. [DOI] [PubMed] [Google Scholar]
- 56. Budhiraja R, Tuder RM, Hassoun PM (2004) Endothelial dysfunction in pulmonary hypertension. Circulation 109: 159–165. [DOI] [PubMed] [Google Scholar]
- 57. Vu TK, Wheaton VI, Hung DT, Charo I, Coughlin SR (1991) Domains specifying thrombin-receptor interaction. Nature 353: 674–677. [DOI] [PubMed] [Google Scholar]
- 58. Chen J, Ishii M, Wang L, Ishii K, Coughlin SR (1994) Thrombin receptor activation. Confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intermolecular liganding mode. J Biol Chem 269: 16041–16045. [PubMed] [Google Scholar]
- 59. Coughlin SR (1999) How the protease thrombin talks to cells. Proc Natl Acad Sci U S A 96: 11023–11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R (2001) Proteinase-activated receptors. Pharmacol Rev 53: 245–282. [PubMed] [Google Scholar]
- 61. Coughlin SR (2000) Thrombin signalling and protease-activated receptors. Nature 407: 258–264. [DOI] [PubMed] [Google Scholar]
- 62. Cheung WM, D'Andrea MR, Andrade-Gordon P, Damiano BP (1999) Altered vascular injury responses in mice deficient in protease-activated receptor-1. Arterioscler Thromb Vasc Biol 19: 3014–3024. [DOI] [PubMed] [Google Scholar]
- 63. Maki J, Hirano M, Hoka S, Kanaide H, Hirano K (2010) Thrombin activation of proteinase-activated receptor 1 potentiates the myofilament Ca2+ sensitivity and induces vasoconstriction in porcine pulmonary arteries. Br J Pharmacol 159: 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kwapiszewska G, Markart P, Dahal BK, Kojonazarov B, Marsh LM, et al. (2012) PAR-2 inhibition reverses experimental pulmonary hypertension. Circ Res 110: 1179–1791. [DOI] [PubMed] [Google Scholar]
- 65. Schermuly RT, Dony E, Gofhrani HA, Pullamsetti S, Savai R, et al. (2005) Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Klein M, Schermuly RT, Ellinghaus P, Milting H, Riedl B, et al. (2008) Combined tyrosine and serine/threonine kinase inhibition by sorafenib prevents progression of experimental pulmonary hypertension and myocardial remodeling. Circulation 118: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 67. Lange U, Nowak G, Bucha E (2003) Ecarin chromogenic assay – a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol Haemost Thromb 33: 184–191. [DOI] [PubMed] [Google Scholar]
- 68. Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thrombin-induced cGMP elevation in IBMX-stimulated endothelial cells is transient. Thrombin (30 nM) acutely increases cGMP content in HUVECs; the effects, however, are transient (white bars: controls; grey bars: with thrombin). Data shown as mean ± SEM (n = 3). White bars: control cells; grey bars: with thrombin. *p<0.05, ***p<0.001 vs cells not exposed to thrombin.
(TIF)
(DOC)
Primer and probe sequences for real-time PCR.
(PPT)