Abstract
The prevalence of Salmonella enterica serovar Enteritidis is gradually decreasing in poultry flocks in the EU, which may result in the demand for a vaccine that allows for the differentiation of vaccinated flocks from those infected by wild-type S. Enteritidis. In this study, we therefore constructed a (Salmonella Pathogenicity Island 1) SPI1-lon mutant with or without fliC encoding for S. Enteritidis flagellin. The combination of SPI1-lon mutations resulted in attenuated but immunogenic mutant suitable for oral vaccination of poultry. In addition, the vaccination of chickens with the SPI1-lon-fliC mutant enabled the serological differentiation of vaccinated and infected chickens. The absence of fliC therefore did not affect the immunogenicity of the vaccine strain and allowed for serological differentiation of the vaccinated chickens. The SPI1-lon-fliC mutant is therefore a suitable marker vaccine strain for oral vaccination of poultry.
Introduction
Salmonella enterica serovar Enteritidis (S. Enteritidis) colonises chickens usually without any gross clinical signs, however, inflammation can be recorded in the intestinal tract, caecum in particular [1]–[3]. Susceptibility of chickens to S. Enteritidis decreases with age and 6 week old chickens are usually quite resistant to S. Enteritidis infection [4], [5]. The prevalence of S. Enteritidis in poultry flocks is gradually decreasing in the EU member states [6]. One of the reasons for such a decrease is the use of vaccination in egg producing flocks, usually with live, attenuated Salmonella vaccines. Current commercial vaccines are therefore of great importance in Salmonella control programs. However, with a decreasing prevalence, the demand for a simple differentiation of vaccinated flocks from those infected by wild-type S. Enteritidis will increase and this is something that the current commercial vaccines cannot provide.
Several laboratories therefore initiated research on a Salmonella marker vaccine [7], [8]. In our previous study, we showed that deletion of the fliC gene from S. Enteritidis might be an interesting option of how to construct a marker vaccine [9]. This genetic modification has a considerable advantage when compared with other approaches since there is a commercially available ELISA kit detecting the presence of anti-S. Enteritidis flagellin antibodies in chicken serum. However, flagellin is also one of the pathogen associated molecular patterns recognized by TLR5 [10], [11]. In agreement with this, the deletion of flagella in S. Typhimurium led to its less efficient recognition by the host immune system and a temporary increase in the virulence in the early stages of chicken infection [12]. On the other hand, over-expression of flagella resulted in a lower invasiveness of S. Enteritidis, perhaps due to efficient TLR5 dependent recognition of the vaccine [13]. Due to the dual role of flagella both as a major T and B cell antigen and pathogen associated molecular pattern, there are therefore concerns that if a Salmonella vaccine strain stimulates the production of anti-flagella antibodies, these may then bind to flagella expressed by the invading wild-type S. Enteritidis and interfere with its correct recognition by TLR5, as has been shown in E. coli in cattle [14]. On the other hand, an aflagellated vaccine not inducing anti-flagella antibodies may allow for the efficient recognition of challenge Salmonella by innate TLR5-dependent recognition and specific immunity against all remaining Salmonella antigens, as observed in S. enterica immunized and challenged mice [7], [15].
The virulence of Salmonella enterica can be attenuated by many different approaches. By understanding the function of type III secretion systems encoded by two different pathogenicity islands, SPI1 and SPI2, the mutants disabled in these virulence factors were constructed and used as live, attenuated vaccines. Interestingly, whilst SPI2 mutants of S. enterica are attenuated in all warm-blooded hosts, SPI1 mutants seem to be attenuated only in hosts for which an enteric type of disease is characteristic and these genes are dispensable when the output of the infection is a typhoid disease [16]–[18]. In agreement with the previous statement, the removal of SPI1 genes from S. Enteritidis or S. Typhimurium, i.e. the serovars which cause a mild enteric disease in chickens, results in a decrease in virulence with preserved immunogenicity in these hosts [5], [19], [20]. Moreover, SPI1 mutants are defective in early interactions with macrophages which may enable the macrophage’s proper antigen processing and presentation [21]–[23] though the role of SPI1 in the interactions with other antigen presenting cells in the chicken is less clear. This may finally result in an efficient specific immune response, as we have shown recently [5].
The above-mentioned results suggest that the ΔSPI1-fliC mutant might be an interesting vaccine strain because it should be attenuated in virulence and also should enable serological differentiation of vaccinated and infected chickens. However, given the concerns on increased virulence of flagella defective mutants [12], we were thinking of additional independent attenuation. One of the possibilities was the inactivation of gene encoding Lon protease what results in a mucoid colony phenotype [24]. Lon protease also is a negative regulator of SPI1 genes [25] and is required for the resistance to multiple environmental stresses [26]. We have shown earlier that the removal of lon reduces the virulence of S. Enteritidis even for highly sensitive Balb/C mice [17] and the production of mucoid colonies due to the overproduction of capsular polysaccharides may enable simple differentiation of the vaccine strain from those circulating in the environment. Finally, there are reports on the attenuation of lon mutants for chickens, originally for S. Gallinarum and recently also for S. Enteritidis [26]–[29]. In this study, we have therefore constructed a triple SPI1-lon-fliC mutant of S. Enteritidis and tested its efficacy as a live attenuated marker vaccine for the oral vaccination of poultry.
Results
Vaccine Strain Characterisation
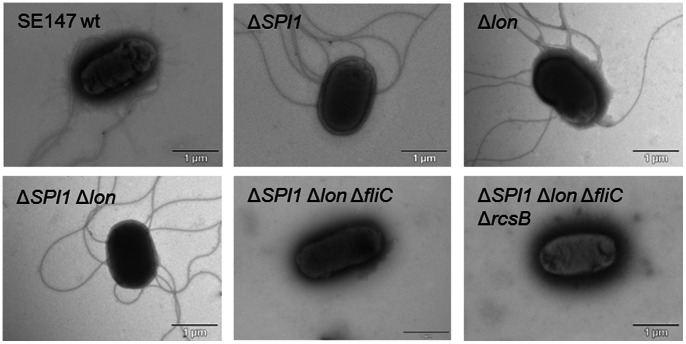
Inactivation of lon resulted in a mucoid colony phenotype which was observed in all the lon mutants except for the SPI1-lon::Cm-fliC-rcsB::Kan mutant (Fig. 1). All the mutants harboring the fliC mutation were free of flagella on their surface (Fig. 2) and non-motile when inoculated in semisolid 0.3% agar (not shown).
Figure 1. Colony morphology of the wild-type S. Enteritidis, SPI1- lon::Cm-fliC mutant and SPI1-lon::Cm-fliC-rcsB::Kan mutant.
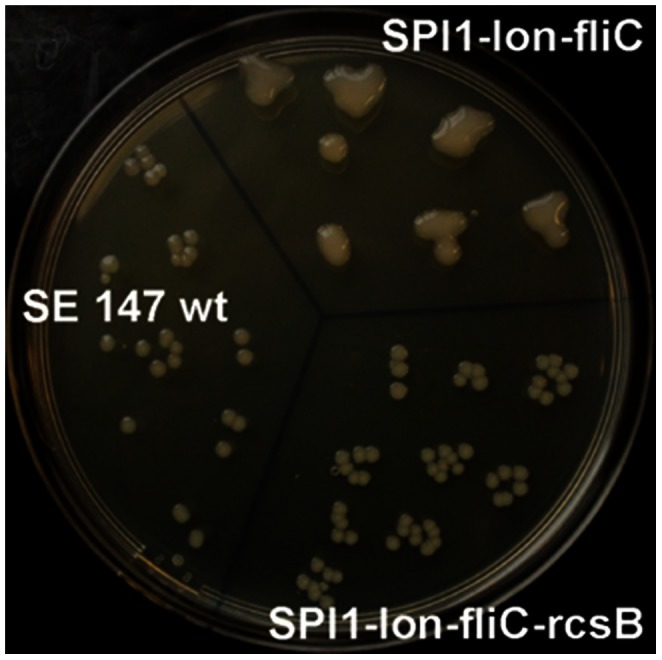
Inactivation of lon resulted in a mucoid colony phenotype which was observed in all the mutants with the lon mutation except for the mutant in which the rcsB mutation has been introduced. The overproduction of capsular polysaccharides in the vaccine strain enables simple differentiation of the vaccine strain from those circulating in the environment.
Figure 2. Electron microscopy of flagella in S. Enteritidis.
Flagella could be visualised in all the strains and mutants with intact fliC after negative staining with ammonium molybdate.
Experiment 1, Vaccination with SPI1 and lon Single Mutants
The protective capacity of the SPI1 and lon mutants for chickens was tested in the first vaccination trial. Three weeks after the first vaccination on the day of hatching, the SPI1 mutant efficiently colonized both the liver and caecum. After revaccination and prior to challenge on day 42 of life, the birds vaccinated with the SPI1 mutant were free of the vaccine strain in the liver but half of the birds remained positive in the spleen and 1 out of 6 tested chickens was positive in the caecum. The lon mutant was isolated from the vaccinated chickens with a lower frequency than the SPI1 mutant at day 42 although this difference did not reach statistical significance (Table 1). Four days post challenge, the SPI1 and lon mutant vaccinated chickens were protected against colonization of the liver and spleen but not the caecum. Fourteen days post infection, a positive effect of vaccination was observed also in the caecum as significantly less chickens tested positive when compared with the non-vaccinated controls (Table 1).
Table 1. Persistence, attenuation and protective capacity of the SPI1 and lon mutants for chickens.
| day 21& | day 42 | day 46 | day 56 | |||||||||
| vaccination | liver | spleen | caecum | liver | spleen | caecum | liver | spleen | caecum | liver | spleen | caecum |
| ΔSPI1 | 5/6# | n.d.∧ | 6/6 | 0/6 | 3/6 | 1/6 | 2/6 | 1/6* | 5/6 | 0/6 | 0/6* | 1/6* |
| Δlon | 1/6 | n.d. | 0/6 | 0/6 | 1/6 | 0/6 | 1/6* | 0/6* | 6/6 | 0/6 | 0/6* | 2/6 |
| non vaccinated | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5/6 | 6/6 | 6/6 | 2/6 | 6/6 | 6/6 |
data for days 21 and 42 of life indicate persistence of the vaccine strains, data for days 46 and 56 of life indicate colonization by the challenge wild-type S. Enteritidis.
number of positive chickens/number of tested.
n.d., not determined due to the small size of some of the spleens of 21-day-old chickens.
significantly different from non-vaccinated controls by χ2 test at P<0.05.
Experiment 2, Oral Vaccination with the SPI1-lon, SPI1-lon-fliC and SPI1-lon-fliC-rcsB Mutants
Although removal of SPI1 results in attenuation of S. Enteritidis for chickens [5], we hypothesized that the removal of fliC may increase its virulence [12]. That is why we combined both attenuating mutations, i.e. SPI1 and lon. However, as the lon mutants overproduce capsular polysaccharides, we suppressed the overproduction of a capsule by the introduction of the rcsB mutation into SPI1-lon-fliC mutant. All the constructed vaccine strains were then tested as attenuated vaccines.
At 4 DPI, chickens vaccinated with the SPI1-lon-fliC vaccine were protected against oral challenge with wild-type S. Enteritidis as only one chicken tested positive in the liver and none of the challenged chickens tested positive in the spleen or caecum. Vaccination with the remaining two mutants, i.e. the SPI1-lon mutant and the quadruple SPI1-lon-fliC-rcsB mutant did not prevent early caecum, liver and spleen colonization in the challenged chickens at 4 DPI (Table 2).
Table 2. Protective capacity of the SPI1-lon, SPI1-lon-fliC and SPI1-lon-fliC-rcsA mutants after oral-oral vaccination and oral or intravenous challenge in chickens.
| day 42 of life Challenge | 4 DPI | 14 DPI | |||||
| Vaccination | Liver | spleen | caecum | liver | spleen | caecum | |
| SPI1-lon::Cm | oral | 1/6# | 2/6 | 5/6& | 1/6 | 0/6 | 3/6 |
| SPI1-lon::Cm -fliC | 1/6 | 0/6 | 0/6 | 0/6 | 1/6 | 2/6 | |
| SPI1-lon::Cm-fliC-rcsB::Kan | 4/6 | 3/6 | 6/6& | 0/6 | 0/6 | 3/6 | |
| non-vaccinated | 2/6 | 2/6 | 6/6& | 4/6 | 4/6 | 6/6 | |
| SPI1-lon::Cm | intravenous | 3.94±0.47* | 5.20±0.24* | 6/6 | 3/6 | 2.40±1.41* | 2/6 |
| SPI1-lon::Cm -fliC | 2.92±1.37* | 4.89±0.72* | 6/6 | 5/6 | 3.29±1.07 | 2/6 | |
| SPI1-lon::Cm-fliC-rcsB::Kan | 3.97±0.20* | 5.34±0.25* | 6/6 | 6/6 | 3.58±0.31* | 4/6 | |
| non-vaccinated | 4.71±0.39 | 6.68±0.42 | 6/6 | 5/5 | 4.16±0.38 | 5/5 | |
number of positive chickens/number of tested.
t-test different from the non-vaccinated control chickens at P<0.05.
χ2 test different from the chickens vaccinated with the SPI1-lon-fliC mutant in caecum at 4 DPI (P<0.05).
Fourteen days post infection, chickens vaccinated with any one of the vaccine strains exhibited protection as lower numbers of positive chickens were observed when compared with the non-vaccinated controls. The protective effect was observed mainly in the liver and spleen and, to a lesser extent, also in the caecum (Table 2).
Intravenous challenge resulted in extensive tissue colonization. At 4 DPI, all three tested vaccines significantly reduced the bacterial load in the liver and spleen but not in the caecum. Between 4 and 14 DPI, one chicken in the non-vaccinated group died. Besides this, an approx. 2 log decrease in counts of challenge S. Enteritidis was observed in all groups (Table 2). In comparison with the non-vaccinated chickens, significantly lower S. Enteritidis counts were observed in the spleens of chickens vaccinated with the SPI1-lon and SPI1-lon-fliC-rcsB mutants at 14 DPI.
Experiment 3, Intravenous Vaccination with the SPI1-lon and SPI1-lon-fliC Mutants
In the last experiment we were interested whether we could further increase chicken immunity by an intravenous application of the vaccine strain after two oral vaccination doses. In addition, this experiment allowed us to demonstrate the absence of anti-flagellin antibodies in the chickens vaccinated with the mutants harboring the fliC mutation. To reduce the number of treated animals, this experiment was performed with only the SPI1-lon and SPI1-lon-fliC mutants as the quadruple SPI1-lon-fliC-rcsB mutant appeared as the least immunogenic in the previous experiment (Table 2).
Although the i.v. vaccinated chickens were protected against challenge both at 4 and 14 DPI when compared with the non-vaccinated chickens, no additional protection after intravenous re-vaccination followed by oral challenge was observed when compared with the chickens vaccinated only orally in experiment 2 (compare tabs. 2 and 3). However, when the intravenously re-vaccinated chickens were challenged via the i.v. route, approx. 10 times better protection was achieved when compared with the chickens vaccinated only orally, though such comparison must be considered with a certain care since the challenged chickens were not of the same age. The increase in protective capacity after i.v. vaccination was significant when SPI1-lon mutant was used for the vaccination but did not reach statistical significance when the SPI1-lon-fliC mutant was used for the vaccination. Similar to the oral vaccination only, an efficient protection from caecum colonization by the challenge strain was achieved after the oral/oral/i.v. mode of vaccination with the SPI1-lon-fliC mutant as early as 4 DPI (Table 3).
Table 3. Protective capacity of the SPI1-lon and SPI1-lon-fliC mutants after oral-oral-intravenous vaccination, followed by oral or intravenous challenge in chickens.
| 4 DPV$ | 14 DPV | day 62of lifechallen. | 4 DPI | 14 DPI | |||||||||
| vaccination | liver | spleen | caecum | Liver | spleen | caecum | liver | spleen | caecum | liver | spleen | caecum | |
| SPI1-lon | 4/6# | 4.42±0.36 | 0/6 | 2/6 | 5/6 | 0/6 | Oral | 2/6 | 5/6 | 1/6* | 0/6 | 2/6 | 0/6 |
| SPI1-lon-fliC | 4/6 | 4.21±0.48 | 0/6 | 1/6 | 6/6 | 0/6 | 0/6* | 3/6 | 2/6 | 0/5 | 1/5 | 0/5 | |
| non-vaccinated | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 6/6 | 6/6 | 6/6 | 2/6 | 3/6 | 0/6 | |
| SPI1-lon | i.v | 1.94±0.94& | 4.27±0.38& | 4/6 | 4/6 | 2.25±1.26& | 0/6 | ||||||
| SPI1-lon-fliC | 2.26±0.89& | 4.49±0.41& | 0/6* | 1/6* | 2.17±1.19& | 0/6 | |||||||
| non-vaccinated | 4.73±0.70 | 6.50±0.56 | 5/6 | 6/6 | 4.06±0.67 | 1/6 | |||||||
DPV, days post intravenous vaccination.
number of positive chickens/number of tested.
χ2 test different from the non-vaccinated control chickens at P<0.05.
t-test different from the non-vaccinated control chickens at P<0.05.
Antibody Production after Infection in Experiment 2 and 3
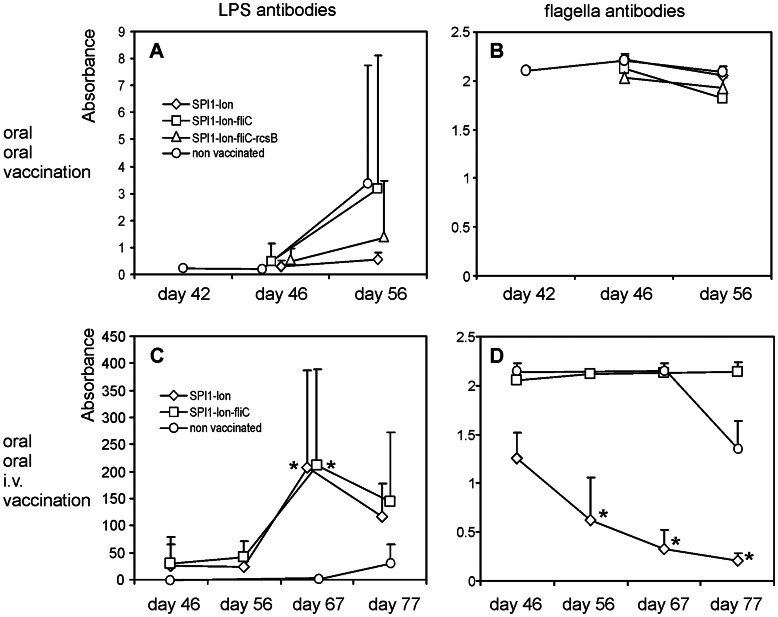
Oral challenge in orally vaccinated chickens resulted in only a moderate antibody production. Anti-LPS antibodies increased weakly at 4 DPI in all groups of vaccinated chickens and the increase in antibody production continued up to 14 DPI. However, this increase was caused by two or three highly responding chickens what resulted in high within-group variation and insignificance statistical insignificance (Fig. 3A). Anti-flagellin antibodies were not produced by any of the orally vaccinated and orally challenged chickens, perhaps due to too short duration of the experiment (Fig. 3B).
Figure 3. Antibody production in immunized and challenged chickens.
Panel A, anti-LPS antibodies after oral vaccination and oral challenge on day 42 of the chicken’s life. Panel B, the same as in panel A except for the data shown for anti-flagellin antibodies. Panel C, anti LPS antibodies after oral vaccination and i.v. revaccination followed by oral challenge on day 63 of the chicken’s life. Panel D, the same as in panel C except for the data shown for anti-flagellin antibodies. As competitive ELISA was used, the increase in anti-flagellin antibody is characterized by a decrease in absorbance. Diamonds, SPI1-lon::Cm vaccinated chickens; squares, SPI1- lon::Cm-fliC vaccinated chickens; triangles, SPI1- lon::Cm-fliC-rcsB::Kan vaccinated chickens; circles, non-vaccinated chickens. * - significantly different from the non-infected controls sacrificed on day 42 by Kruskal-Wallis and post hoc Dunn’s test at P<0.05 (panels A and B) or day 46 (panels C and D).
Chickens vaccinated twice orally and revaccinated intravenously produced high levels of anti-LPS antibodies, independent of the vaccine strain used. The antibodies appeared as early as 4 days after the i.v. revaccination and reached statistical significance at 4 DPI when compared with the non-infected controls sacrificed on day 46 (Fig. 3). Chickens vaccinated with the SPI1-lon-fliC did not produce anti-flagellin antibodies at all, even after oral challenge with the wild type S. Enteritidis. On the other hand, anti-flagellin antibodies appeared in the group of chickens vaccinated with the SPI1-lon mutant 4 days after the i.v. revaccination and gradually increased as the experiment continued. Additionally, in this experiment we recorded the production of anti-flagellin antibodies also in the control group of non-vaccinated and orally challenged chickens at 14 DPI (Fig. 3).
Discussion
The key characteristics for a new generation of live, attenuated Salmonella vaccine, besides the attenuation and immunogenicity, include an absence of any antibiotic resistance markers in the vaccine strain, the possibility of a simple vaccine strain differentiation and the possibility to differentiate vaccinated from naturally infected flocks [7], [9]. Except for the presence antibiotic resistance (chloramphenicol or kanamycine), vaccine strains described in this study provided all the remaining characteristics – and even the antibiotic resistance could be easily removed prior its commercial and widespread use.
Although we did not sacrifice orally vaccinated chickens on day 42 in experiments 2 and 3, and we therefore do not have data on antibody levels in these chickens, it is likely that these were very low because even 4 days after oral challenge with the wild type S. Enteritidis there were very low levels of anti-LPS or anti-flagellin antibodies. However, using intravenous vaccination we proved that the SPI1-lon-fliC mutant never induced production of anti-flagellin antibodies whilst these could be easily detected after intravenous vaccination with the SPI1-lon mutant. The use of the SPI1-lon-fliC mutant will therefore not result in anti-flagellin antibodies, which will enable the differentiation of vaccinated flocks from those naturally infected.
In the first experiment, we confirmed the protective capacity of the SPI1 and lon mutants of S. Enteritidis, as described previously for S. Gallinarum [27]. Based on these results we constructed 3 additional mutants. SPI1-lon and SPI1-lon-fliC mutants were designed to be of a similar attenuation differing only in their ability to stimulate the production of anti-flagellin antibodies. The third mutant SPI1-lon-fliC-rcsB was constructed to suppress the mucoid phenotype of the lon mutation. However, the SPI1-lon-fliC-rcsB mutant was the least protective, either due to an additional attenuation caused by the rcsB mutation [30], [31] or due to the suppression of the mucoid phenotype by capsule overproduction, which may increase the immunogenicity of the lon mutants. Indeed, the lon mutants, though attenuated, exhibit a prolonged persistence in mice [17].
When the immunogenicity SPI1-lon mutants, with or without intact fliC was compared, vaccination with the SPI1-lon-fliC mutant resulted in slightly more efficient protection of chickens than the vaccination with the SPI1-lon mutant. This might be related to the fact that flagellin is a ligand for TLR5. The vaccination with the flagellin-positive SPI1-lon mutant led to the production of anti-flagellin antibodies which may bind to flagellin of the challenge strain and prevent its recognition by TLR5 [14]. Chickens vaccinated by the SPI1-lon mutant therefore responded to S. Enteritidis challenge by a well-developed specific immune response, but unlike the SPI1-lon-fliC vaccinated chickens, perhaps without the activation of the TLR5-dependent innate immune response. A similar negative effect of anti-flagella antibodies to challenge has been reported in mice infected S. enterica or Pseudomonas aeruginosa [7], [15], [32].
Materials and Methods
Ethics Statement
The handling of animals in the study was performed in accordance with current Czech legislation (Animal protection and welfare Act No. 246/1992 Coll. of the Government of the Czech Republic). The specific experiments were approved by the Ethics Committee of the Veterinary Research Institute (permit number 48/2010) followed by the Committee for Animal Welfare of the Ministry of Agriculture of the Czech Republic (permit number MZe 1226).
Bacterial Strains
S. Enteritidis 147 with proven virulence and ability to colonize the chicken gut was used [20]. The construction of the SPI1 mutant with the whole pathogenicity island SPI1 removed from the chromosome has been described earlier [20], [33]. lon::Cm, fliC::Cm and rcsB::Kan mutations were constructed by λ red recombination [34] and transferred to final recipients by P22-mediated transduction [20]. Each of the mutation was verified by PCR and primer pairs used for the construction of lon::Cm, fliC::Cm and rcsB::Kan mutations and PCR verifications are listed in Table 4. After each transduction, the resulting transductant was checked for sensitivity to P22 phage and, if necessary, the chloramphenicol gene cassette was excised from the chromosome by transient transformation with plasmid pCP20 [34]. Genotypes of the resulting mutants therefore were ΔSPI1, Δlon, ΔSPI1 lon::Cm, ΔSPI1 lon::Cm ΔfliC and ΔSPI1 lon::Cm ΔfliC rcsB::Kan. To simplify enumeration, the wild-type S. Enteritidis and all mutants were spontaneously resistant to nalidixic acid which, to our best knowledge, does not affect this strain virulence.
Table 4. List of primers used in this study for the construction of fliC, rcsB and lon mutants.
| Name* | Primer 5′-3′ |
| fliC_44F | GTCGGTGAATCAATCGCCGGATTAACGCAGTAAAGAGAGGACGT |
| fliC_44R | AGTCATTAATACAAACAGCCTGTCGCTGTTGACCCAGAATAACC |
| rcsB_44F | ATGAACAATATGAACGTAATTATTGCCGATGACCACCCGATTGT |
| rcsB_44R | TTATTCTTTGTCTGTCGGACTCAGGGTGACAGAAGAGAGATAGT |
| lon_51F | CAGCTATACTATCTGATTACCTGGCGGACACTAAACTAAGAGAGAGCTCTT |
| lon_50R | CGAAATAGCCTGCCAGCCCTGTTTTTATTAGCGCTATTTGCGCGAGGTCA |
| fliC_FCTR | TGGCGAGATATTTTTTAACC |
| fliC_RCTR | AGTAGTTAAGCGCGTTATCG |
| rcsB_FCTR | GGCTATTATGCGCTATTTGT |
| rcsB_RCTR | ATATTGTTCTGAGCGATGTG |
| lon_FCTR | GCAGGCTTCTGGCGAATAAT |
| lon_RCTR | CGACCGCGCAGCAGTTATAT |
For primers used for the amplification of pKD3 or pKD4, only the gene specific overhangs are shown. „CTR“ primers, either Forward (F) or Reverse (R) were used for the verification of the final contructs.
Experimental Animals
Male, newly-hatched ISA Brown Chickens (Hendrix Genetics, Boxmeer, The Netherlands) were used in this study. The chickens were reared in perforated plastic boxes with free access to water and feed. Each of the experimental or control groups was kept in a separate room.
Experimental Design
In the first vaccination trial (Experiment 1), 60 chickens were divided into 2 experimental groups of 24 chickens each (group 1 and 2), and a control group of 12 non-vaccinated chickens (group 3). Group 1 was orally vaccinated with the SPI1 mutant and group 2 with the lon mutant. The chickens were vaccinated orally on day 1 of life and revaccinated on day 21 with 107 CFU of appropriate vaccine strain per chicken. On days 21 and 42, 6 vaccinated chickens from each group were sacrificed and the remaining chickens were orally challenged with 3×107 CFU of the wild type S. Enteritidis in LB broth. Six birds from each group were euthanized 4 and 14 days post infection (DPI), respectively.
In the second vaccination trial (Experiment 2), 102 chickens were divided into 3 experimental groups of 24 birds each (group 1, 2 and 3), and a control group of 30 non-vaccinated chickens (group 4). Group 1 was orally vaccinated with the SPI1-lon mutant, group 2 with the SPI1-lon-fliC mutant and group 3 with SPI1-lon-fliC-rcsB mutant. The chickens were vaccinated on day 1 of life and revaccinated on day 21 with 107 CFU of appropriate vaccine strain per chicken in LB broth. On day 42, 6 non-vaccinated chickens were sacrificed and the remaining chickens in each group were challenged with wild type S. Enteritidis. Half of the chickens were challenged orally with 3×107 CFU of S. Enteritidis in LB broth and the remaining half were intravenously challenged with 107 CFU of S. Enteritidis in 0.1 ml of PBS.
Six birds from each group were euthanized 4 and 14 DPI, respectively. The intravenous challenge in experiment 2 and experiment 3 (see below) was performed to assess the resistance of the vaccinated birds to an extreme level of systemic infection and to get strong serological response to LPS and flagella.
In the last vaccination trial (Experiment 3), 102 chickens were divided into 2 experimental groups of 36 birds each (group 1 and 2), and a control group of 30 non-vaccinated chickens (group 3). Group 1 was vaccinated with the SPI1-lon mutant and group 2 with the SPI1-lon-fliC mutant. The chickens in group 1 and 2 were orally vaccinated on day 1 of life, orally revaccinated on day 21 and intravenously revaccinated on day 42 with 107 CFU of appropriate vaccine strain per chicken. The chickens in group 3 served as non-vaccinated controls. On day 63, the chickens were either orally or intravenously challenged as described above and 6 birds from each group were euthanized 4 and 14 DPI, respectively.
Sample Collection and Processing
At the end of each experiment, blood from each bird was collected for serological tests and samples of the liver, spleen and cecal content were processed for enumeration of S. Enteritidis. These samples were homogenized in peptone water, tenfold serially diluted and plated on XLD agar plates (HiMedia) supplemented with 20 µg/ml nalidixic acid. Detection limit of direct plating was 500 CFU/g of sample. Samples negative after direct plating were subjected to enrichment in modified semi-solid Rappaport-Vassiliadis medium (Oxoid) for qualitative S. Enteritidis determination. Counts of S. Enteritidis positive after direct plating were logarithmically transformed. Samples positive only after enrichment were assigned a value of 1 and negative samples were assigned a value of 0.
ELISA Detection of Anti-LPS and Flagella Antibodies
A commercial FLOCKSCREEN™ Salmonella Enteritidis Antibody ELISA kit (x-OvO Limited) was used for the detection of anti-LPS serum antibodies. For anti-flagella antibodies, a FlockCheck kit was used as recommended by the manufacturer (IDEXX Laboratories, USA). Both ELISA tests were performed as recommended by the manufacturers except that the sera were diluted from 1∶10 up to 1∶8000 using sample dilution buffer to reach the absorbance which could be measured by the spectrophotometer, i.e. ranging from 0.2 to 1.8. The real absorbances were then calculated knowing the read absorbance and particular dilution, and such data are used throughout this study.
Transmission Electron Microscopy
A formvar-coated copper grid was placed on a single drop of overnight culture for 5 min. The grid was washed twice in a drop of water, stained with 1% ammonium molybdate and observed with a Philips EM 208 transmission electron microscope under an acceleration of 80 kV.
Statistical Analysis
The χ2 square test and Student’s t-test were used for bacteria counts analysis as indicated in the text. Antibody response was analysed by Kruskal-Wallis test followed by post hoc Dunn’s test. SPSS v.14 software was used for statistical calculations.
Acknowledgments
Authors wish to thank Peter Eggenhuizen for his English language corrections.
Funding Statement
This work has been supported by the projects MZE0002716202 and QJ1310019 of the Czech Ministry of Agriculture and AdmireVet project CZ.1.05/2.1.00/01.0006– ED0006/01/01 from the Czech Ministry of Education. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Berndt A, Wilhelm A, Jugert C, Pieper J, Sachse K, et al. (2007) Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect Immun 75: 5993–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matulova M, Rajova J, Vlasatikova L, Volf J, Stepanova H, et al. (2012) Characterization of chicken spleen transcriptome after infection with Salmonella enterica serovar Enteritidis. PLoS One 7: e48101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matulova M, Varmuzova K, Sisak F, Havlickova H, Babak V, et al. (2013) Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis. Vet Res. [DOI] [PMC free article] [PubMed]
- 4. Beal RK, Wigley P, Powers C, Hulme SD, Barrow PA, et al. (2004) Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet Immunol Immunopathol 100: 151–64. [DOI] [PubMed] [Google Scholar]
- 5. Matulova M, Havlickova H, Sisak F, Rychlik I (2012) Vaccination of chickens with Salmonella Pathogenicity Island (SPI) 1 and SPI2 defective mutants of Salmonella enterica serovar Enteritidis. Vaccine 30: 2090–7. [DOI] [PubMed] [Google Scholar]
- 6.Lahuerta A, Westrell T, Takkinen J, Boelaert F, Rizzi V, et al.. (2011) Zoonoses in the European Union: origin, distribution and dynamics - the EFSA-ECDC summary report 2009. Euro Surveill 16: pii: 19832. [PubMed]
- 7. Adriaensen C, De Greve H, Tian JQ, De Craeye S, Gubbels E, et al. (2007) A live Salmonella enterica serovar Enteritidis vaccine allows serological differentiation between vaccinated and infected animals. Infect Immun 75: 2461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Selke M, Meens J, Springer S, Frank R, Gerlach GF (2007) Immunization of pigs to prevent disease in humans: construction and protective efficacy of a Salmonella enterica serovar Typhimurium live negative-marker vaccine. Infect Immun 75: 2476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Methner U, Barrow PA, Berndt A, Rychlik I (2011) Salmonella Enteritidis with double deletion in phoPfliC–a potential live Salmonella vaccine candidate with novel characteristics for use in chickens. Vaccine 29: 3248–53. [DOI] [PubMed] [Google Scholar]
- 10. Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167: 1882–5. [DOI] [PubMed] [Google Scholar]
- 11. Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, et al. (2001) The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–103. [DOI] [PubMed] [Google Scholar]
- 12. Iqbal M, Philbin VJ, Withanage GS, Wigley P, Beal RK, et al. (2005) Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar Typhimurium. Infect Immun 73: 2344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kremer CJ, O’Meara KM, Layton SL, Hargis BM, Cole K (2011) Evaluation of recombinant Salmonella expressing the flagellar protein fliC for persistence and enhanced antibody response in commercial turkeys. Poult Sci 90: 752–8. [DOI] [PubMed] [Google Scholar]
- 14. McNeilly TN, Naylor SW, Mahajan A, Mitchell MC, McAteer S, et al. (2008) Escherichia coli O157: H7 colonization in cattle following systemic and mucosal immunization with purified H7 flagellin. Infect Immun 76: 2594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kodama C, Matsui H (2004) Salmonella flagellin is not a dominant protective antigen in oral immunization with attenuated live vaccine strains. Infect Immun 72: 2449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones MA, Wigley P, Page KL, Hulme SD, Barrow PA (2001) Salmonella enterica serovar Gallinarum requires the Salmonella pathogenicity island 2 type III secretion system but not the Salmonella pathogenicity island 1 type III secretion system for virulence in chickens. Infect Immun 69: 5471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karasova D, Sebkova A, Vrbas V, Havlickova H, Sisak F, et al. (2009) Comparative analysis of Salmonella enterica serovar Enteritidis mutants with a vaccine potential. Vaccine 27: 5265–70. [DOI] [PubMed] [Google Scholar]
- 18. Murray RA, Lee CA (2000) Invasion genes are not required for Salmonella enterica serovar typhimurium to breach the intestinal epithelium: evidence that salmonella pathogenicity island 1 has alternative functions during infection. Infect Immun 68: 5050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dieye Y, Ameiss K, Mellata M, Curtiss R III (2009) The Salmonella Pathogenicity Island (SPI) 1 contributes more than SPI2 to the colonization of the chicken by Salmonella enterica serovar Typhimurium. BMC Microbiol 9: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rychlik I, Karasova D, Sebkova A, Volf J, Sisak F, et al. (2009) Virulence potential of five major pathogenicity islands (SPI-1 to SPI-5) of Salmonella enterica serovar Enteritidis for chickens. BMC Microbiol 9: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monack DM, Raupach B, Hromockyj AE, Falkow S (1996) Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci U S A 93: 9833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pavlova B, Volf J, Ondrackova P, Matiasovic J, Stepanova H, et al. (2011) SPI-1-encoded type III secretion system of Salmonella enterica is required for the suppression of porcine alveolar macrophage cytokine expression. Vet Res 42: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pullinger GD, Paulin SM, Charleston B, Watson PR, Bowen AJ, et al. (2007) Systemic translocation of Salmonella enterica serovar Dublin in cattle occurs predominantly via efferent lymphatics in a cell-free niche and requires type III secretion system 1 (T3SS-1) but not T3SS-2. Infect Immun 75: 5191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gottesman S, Trisler P, Torres-Cabassa A (1985) Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol 162: 1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takaya A, Tomoyasu T, Tokumitsu A, Morioka M, Yamamoto T (2002) The ATP-dependent lon protease of Salmonella enterica serovar Typhimurium regulates invasion and expression of genes carried on Salmonella pathogenicity island 1. J Bacteriol 184: 224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leyman B, Boyen F, Van Parys A, Verbrugghe E, Haesebrouck F, et al. (2012) Tackling the issue of environmental survival of live Salmonella Typhimurium vaccines: deletion of the lon gene. Res Vet Sci 93: 1168–72. [DOI] [PubMed] [Google Scholar]
- 27. Matsuda K, Chaudhari AA, Kim SW, Lee KM, Lee JH (2010) Physiology, pathogenicity and immunogenicity of lon and/or cpxR deleted mutants of Salmonella Gallinarum as vaccine candidates for fowl typhoid. Vet Res 41: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nandre RM, Chaudhari AA, Matsuda K, Lee JH (2011) Immunogenicity of a Salmonella Enteritidis mutant as vaccine candidate and its protective efficacy against salmonellosis in chickens. Vet Immunol Immunopathol 144: 299–311. [DOI] [PubMed] [Google Scholar]
- 29. Slattery A, Victorsen AH, Brown A, Hillman K, Phillips GJ (2013) Isolation of Highly Persistent Mutants of Salmonella enterica Serovar Typhimurium Reveals a New Toxin-Antitoxin Module. J Bacteriol 195: 647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia-Calderon CB, Casadesus J, Ramos-Morales F (2007) Rcs and PhoPQ regulatory overlap in the control of Salmonella enterica virulence. J Bacteriol 189: 6635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Q, Zhao Y, McClelland M, Harshey RM (2007) The RcsCDB signaling system and swarming motility in Salmonella enterica serovar typhimurium: dual regulation of flagellar and SPI-2 virulence genes. J Bacteriol 189: 8447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, et al. (2005) Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A 102: 9247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karasova D, Sebkova A, Havlickova H, Sisak F, Volf J, et al. (2010) Influence of 5 major Salmonella pathogenicity islands on NK cell depletion in mice infected with Salmonella enterica serovar Enteritidis. BMC Microbiol 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97: 6640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]