Abstract
Lifelong dietary restriction (DR) is known to have many potential beneficial effects on brain function as well as delaying the onset of neurological diseases. In the present investigation, the effect of late-onset short-term intermittent fasting dietary restriction (IF-DR) regimen was studied on motor coordination and cognitive ability of ageing male rats. These animals were further used to estimate protein carbonyl content and mitochondrial complex I–IV activity in different regions of brain and peripheral organs, and the degree of age-related impairment and reversion by late-onset short-term IF-DR was compared with their levels in 3-month-old young rats. The results of improvement in motor coordination by rotarod test and cognitive skills by Morris water maze in IF-DR rats were found to be positively correlated with the decline in the oxidative molecular damage to proteins and enhanced mitochondrial complex IV activity in different regions of ageing brain as well as peripheral organs. The work was further extended to study the expression of synaptic plasticity-related proteins, such as synaptophysin, calcineurin and CaM kinase II to explore the molecular basis of IF-DR regimen to improve cognitive function. These results suggest that even late-onset short-term IF-DR regimen have the potential to retard age-associated detrimental effects, such as cognitive and motor performance as well as oxidative molecular damage to proteins.
Keywords: Intermittent fasting–dietary restriction (IF-DR), Ageing, Synaptic plasticity, Mitochondrial electron transport chain (ETC), Morris water maze (MWM), Protein carbonyl content
Introduction
Dietary restriction (DR) has become one of the most powerful tool and active area of research in realm of biogerontology, which tends to reestablish the disrupted homeostasis and results in the adaptive process called hormesis or preconditioning (Mattson 2008). Several DR regimens have been shown to extend lifespan, with the two most commonly used protocols being intermittent (every other day) feeding and paired feeding that employs feed pellets containing 30–40% less calories than pellets in control diet (Mattson 2003). Various reports justify the status of DR regimens in the yeast Saccharomyces cerevisiae (Lin et al. 2000), mice and rats (Weindruch 1996) and humans (Roth et al. 2002). DR has been shown to slow down the onset of neurodegenerative diseases with increased resistance of neurons to dysfunction and degeneration in experimental models of Alzheimer, Parkinson and stroke disorders (Mattson 2003; Maswood et al. 2004; Love 2005), cancer (Hursting et al. 2009), kidney diseases (Keenan et al. 2000) and cardiovascular diseases (Ripple et al. 2009).
Ageing of mammalian species, including humans and rats, is associated with significant accumulation of oxidised protein reactive carbonyl derivatives, measured as biomarkers of protein oxidation (Chevion et al. 2000; Grune et al. 2001). The protein oxidation may be associated with reactive oxygen species (ROS) generation by electron transport chain (ETC) in mitochondria, which results in the decline in oxidative phosphorylation with age (Liu et al. 2002; Kaur et al. 2008). The oxidative damage found to be associated with life expectancy is suggested to be markedly inhibited by long-term calorie restriction (CR) in skeletal muscle (Zainal et al. 2000) and liver (Chaudhuri et al. 2006). In the preservation of enzymatic activities and functions by CR, retardation in deterioration of ETC chain by reducing free radical leak has also been reported (Olgun et al. 2002).
Increased oxidative damage with altered activity of mitochondrial ETC (Forster et al. 1996; Navarro et al. 2004, 2005) and altered energy homeostasis leads to neuronal damage associated with impaired learning, memory and motor skills (Albers and Beal 2000; Keller et al. 2005), and deterioration of synaptic function (Lesne et al. 2006). The procedure used widely to assess learning, memory and motor coordination in aged rats is the spatial discrimination version of the Morris water maze (MWM) and rotarod test (Monville et al. 2006). The hippocampal neurogenesis is observed to decline with advancing age (Lemaire et al. 2000), altering synaptic proteins, phosphatases and kinases expression in the brain. Learning and memory are important aspects of cognitive function, and synaptic plasticity seems to play an essential role in this process. Synaptophysin is used as an index of synaptic number and density, and decreased expression of this marker reflects a decrease in neurotransmission affecting spatial memory (Liu et al. 2005). Ca2+-dependent synaptic plasticity is also regulated by serine/threonine protein phosphatase Calcineurin (CaN) and protein kinase CaMkinase (CaM) (Klee et al. 1998). CaN acts as a critical link between Ca2+ regulation, synaptic plasticity, cell survival and cognition (Mansuy 2003), and alters hippocampal synaptic plasticity in aged rats (Jouvenceau and Dutar 2006). Activation of CaM plays a key role in synapse formation, neurotransmitter release, neuroplasticity, learning and memory (Soderling 1993; Giese et al. 1998).
Many previous studies report that lifelong DR initiated in early adulthood is the only established means of extending lifespan and delay the onset of age associated diseases. On the other hand, some recent studies reported that DR initiated even in late age for a limited time span also have beneficial effects (Goto 2006, 2007; Kaur et al. 2008; Sharma et al. 2010). The present study was aimed to test the hypothesis whether short-term late-onset IF-DR regimen has the potential to improve age associated loss of cognitive and motor functions as well as the underlying region-specific oxidative molecular damage and synaptic plasticity-associated proteins in Wistar strain male rats. A positive correlation was observed between the improvement in the cognitive function, motor coordination and protein carbonylation and mitochondrial ETC activity as well as synaptic proteins expression in 24-month-old rats on IF-DR regimen as compared to their ad libitum (OAL) fed counterparts. Three-month-old young rats were used as positive controls.
Experimental procedures
The primary antibodies used for the immunohistochemistry and Western blot analysis were monoclonal mouse anti-synaptophysin (clone SVP-38), anti-calcineurin α (clone CN-A1) and anti-CaMKinase IIα (6G9) (Sigma). The secondary antibodies used were anti-mouse and anti-rabbit IgG (whole molecule) biotin conjugated, extravidin peroxidase and antimouse IgG or anti-rabbit IgG-peroxidase conjugated. Other chemicals were procured from Sigma (St. Louis, MO, USA) and local sources available commercially.
Experimental animals
Wistar strain male albino rats in the age group of 3 and 24 months were used for these experiments. These animals have maximum lifespan of 30–36 months (Altun et al. 2007); thus, the 24-month-old rats had completed nearly 70% of their lifespan. Animal care and procedures were followed in accordance with the guidelines of Institutional Animal Ethical Committee. The paradigm of DR involved periodic fasting, in which 21-month-old Wistar strain male albino rats (ODR) were deprived of food for a full day, every other day and were fed ad libitum on the intervening day for 3 months. Rat feed pallets were provided or removed at 10 a.m. every day. Another group of rats of similar age was fed ad libitum (OAL) and used as control. Young adult rats of 3 months of age were taken as positive controls. Normal balanced diet used to feed these rats had ingredients, such as crude proteins (21%), carbohydrates (∼60%), crude fats (5%), fibre (5%) and minerals and vitamins in suitable proportions. Water was available ad libitum to all the animals. The body weights were recorded every 15th day in old AL and DR rats. Blood glucose level was determined once a month. The behavioural experiments were performed between 8 a.m. to 10 a.m. on the day when the rats were put on fasting so the animals were well fed at the time of these behavioural tests. These rats were put on fasting and feeding at 10 a.m. every day. The ODR animals were harvested at the end of fed day (day of having free access to food). Both the ODR and AL group animals were fasted overnight, i.e. these animals did not have access to food for 8–10 h before killing.
Rotarod test
The apparatus was a motor-driven treadmill (Rotamex-5; Columbus Instruments) that consisted of a 7.0 × 9.5 cm spindle diameter with a fall height of 44.5 cm from the centre. On the first day, the rats were habituated to the rotarod for 180s at a constant speed of 10 rpm. Testing was completed in five trials during next ten days (trial on alternate day) with fixed speed of 15 rpm. Rats were tested for a maximum of 300s in each trial. The number of falls and the time spent on the rotating rod were recorded for each trial and averaged for each group.
Morris water maze test
After 3 months of IF-DR, MWM procedure used to test spatial memory was performed. Escape latency, i.e. time to reach the hidden platform, was recorded for five consecutive days as per procedure described earlier (Pathan et al. 2006). Briefly, on five consecutive days, OAL and ODR rats were given two acquisition trials per day with an intertrial interval of 2 h. The other parameters, like time spent in the quadrant and number of platform crossing, were also recorded. Rats were allowed a maximum of 60 s to locate the platform and permitted to stay on it for a maximum of 30 s. Rats that failed to locate the platform were placed on the platform by the experimenter for a maximum of 30 s.
Determination of protein carbonyl content
After completion of 3 months of IF-DR and performing behavioural tests, these OAL and IF-DR rats along with 3-month-old young rats were used to measure protein carbonyl content and mitochondrial ETC complex I–IV from different brain regions, such as piriform cortex, hippocampus and hypothalamus, and peripheral organs, such as kidney, liver and heart, according to protocol of Levine et al. using the 2,4-dinitrophenylhydrazine (DNPH) procedure (Levine et al. 1990). The difference in absorbance between the DNPH- and HCl-treated samples was determined at 366 nm, and the results are expressed as nanomoles of carbonyl groups per milligram of protein using the extinction coefficient of 22.0 mM−1 cm−1 for aliphatic hydrazones.
Mitochondrial ETC complex assays
Mitochondrial fraction was prepared from brain regions and peripheral organs by the modified method of Griffths (1989). Total protein content of mitochondrial fraction was estimated, and equal amount of sample was used for ETC activity measurement as also explained by Lopez-Torres et al. (2002). DPNH-coenzyme Q reductase (complex I) activity was estimated as per the method of Hatefi and Rieske (1967). Succinate dehydrogenase coenzyme Q reductase (complex II) activity was measured according to the method of Ziegler and Rieske (1967). Coenzyme Q cytochrome-C reductase (complex III) activity was assayed by the method described by Rieske (1967). Cytochrome-C oxidase (complex IV) assay was done as per the method of Yonetani (1967), using reduced cytochrome c as substrate, which was freshly prepared by the addition of a few grains of sodium dithionite to a 1% solution in 10 mM phosphate buffer, pH 7.0 (Wharton and Tzagoloff 1967).
Immunohistochemical staining of synaptic proteins
Brains were perfused transcardially with 4% paraformaldehyde in phosphate-buffered saline (PBS, 0.1 M) and cryopreserved in 10–30% sucrose, and frozen brain sections were further processed for immunostaining (Kaur et al. 2008). After permeabilisation step, brain sections were treated with hydrogen peroxide (H2O2) for 15 min followed by three washes again and incubation with blocking solution (5% normal goat serum in 0.3% PBST). Sections were incubated with mouse monoclonal anti-synaptophysin (syn), anti-calcineurin (CaN) and anti-CaMkinase II (CaM) (1:500 each) in 0.1% Triton-X100 and 1% bovine serum albumin in PBS for 60 h at 4°C; specificity of staining was determined by negative staining control procedures without adding primary antibody. Washings were followed by incubation in anti-mouse IgG biotin-conjugated secondary antibody (1:500) in 0.1% PBST for 2 h. Sections after washing were incubated for 2 h with extravidin peroxidase (1:500) in 0.1% PBST and stained with DAB (0.2 mg/ml) and H2O2 (0.02%) for 30 min. After rinsing, sections were dehydrated in ascending alcohol series and mounted with DPX.
Data analysis
The stained sections were examined using a Nikon Brightfield Microscope E600. Images were captured using a Cool Snap ProTM CCD camera (Media Cybernetics), and the pictures were analysed using Image Pro-plusTM software version 4.5.1 from the Media Cybernetics. The intensity of immunoreactivity was quantified in randomly selected fields in each section using the count/size command. Five consecutive sections each from five to six animals in all groups were used for data analysis. An area of interest was selected and placed within region on each section, and density measurement was made for staining intensity of all synaptic proteins. All the slides were coded for their group assignment, and code was not broken until the intensity was measured. An examiner blind to the group assignment of each animal did density measurement.
Western blot hybridisation
Different brain regions were dissected, and 5% homogenate was prepared in 50 mM Tris–HCl (pH 7.4) containing 1 mM EDTA (Sigma) and protease inhibitor cocktail (Sigma) and centrifuged for 5 min at 10,000 rpm. The protein samples (30 μg) separated on a sodium dodecyl sulphate polyacrylamide gel were electroblotted onto a polyvinylidene fluoride membrane (Millipore) using a semidry Novablot system (Amersham Pharmacia) at 25 V for 3 h. Immunoassays were performed with anti-Syn (1:5,000), anti-CaN (1:7,000) and anti-CaM (1:7,000) in TBS-T for overnight at 4°C. The immunocomplexes formed were visualised with anti-mouse IgG or anti-rabbit IgG peroxidiase conjugated (1:7,000) and developed using enhanced chemiluminescence (ECL Plus) Western blot detection system and exposed to Hyper film ECL. The films were then developed, and the antibody-labelling intensity was analysed using Gel documentation system (AlphaEaseTM, Alpha Innotech Corporation).
Complementary DNA synthesis and RT-PCR
RNA (5 μg) was isolated using Trizol reagent (Sigma) and was reverse transcribed using 200 U of Mo-MLV reverse transcriptase, 20 U of RNase inhibitor, 0.6 mM of dNTP and 250 ng random primers per reaction, and the mixture was heated at 65°C for 5 min followed by chilling on ice. Buffer (4 μl), DTT (2 μl) and reverse transcriptase (1 μl) were mixed and incubated for 5–10 min at 25°C. Reaction was carried out at 37°C for 1 h. PCR complementary DNA preparations were used to detect Syn, CaN and CaM gene expression using a TECHNE PCR machine. The reactions mixture (50 μl) contained 20 mM Tris–HCl, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTP, 2 U of Taq DNA polymerase and 50 pmol of 5′ and 3′ primers for Syn (Liu et al. 2005), CaN (Jabr et al. 2007) and CaM (Wang et al. 2008). After initial denaturation for 3 min at 94°C, 35 cycles of amplification (at 94°C for 40 s, 55°C for 45 s and 72°C for 1 min) were performed, followed by a 10-min extension at 72°C. PCR products were electrophoresed on agarose gel with 0.2 μg/ml ethidium bromide and photographed under UV transillumination. The expression values of all markers were quantified by semiquantitative reverse transcription PCR (RT-PCR) analysis, using actin messenger RNA (mRNA) as an internal standard.
Statistical analysis
Values are expressed as mean ± SEM. A one-way analysis of variance (ANOVA) was used to compare the results in different groups of rats. When ANOVA detected a difference, these sets of young, old AL and old DR rats were subjected to post hoc comparison using the Bonferroni’s test (SigmaStat 2.03) for pairwise multiple comparisons to determine the statistical significance, which was assumed to be different when the comparison showed a significance level of p < 0.05.
Results
IF-DR is a simple and effective regimen to decrease calorie intake. Old rats on IF-DR regimen showed 23% reduction in bodyweight as compared to their age matched AL counterparts. Rats placed on IF-DR regimen were observed to binge on food on feeding day, and they almost consumed 30–40% more food on feeding day as compared to OAL rats. Therefore, the average food intake per day of IF-DR group was estimated to be around 25–30% less as compared to OAL animals. The average blood glucose level was 118 mg/dl, which was reduced in IF-DR rats to 79.23 mg/dl. Physiological parameters, such as body temperature and blood pressure, have been documented earlier to decrease under similar experimental conditions (Weindruch 1996; Mattson 2003).
Amelioration of the age-associated impairment in motor coordination and learning and memory function by IF-DR
With the fixed speed rotarod protocol used, the young rats rapidly acquired the necessary skilled behaviour on the rotating rod to prevent a fall and were able to stay on the rod for the maximum time at 15 rpm (Fig. 1a, b). OAL rats showed an increase in the number of falls from rod across trials. The results presented in Fig. 1a, b further suggest the improved performance by ODR rats on rotarod. Not only the number of falls was lowered, but also the time spent by IF-DR rats on rotating rod was significantly higher, indicating acquisition of the skilled behaviour. Next, we measured performance on MWM task in young adult, OAL and ODR rats. All animals learned the task and became more accurate in locating the escape platform with trials, but the mean for escape latency to platform remained significantly greater in OAL rats over trial time as compared to young animals (Fig. 1c, d). On the other hand, ODR rats showed lower escape latency in comparison to OAL rats on days 4 and 5 of trials, whereas they showed higher escape latency on day 2, and no difference was observed between ODR and OAL animals on day 3. During the probe trial in which the escape platform was removed, rats on IF-DR regimen showed improvement in time spent in target quadrant (Fig. 1e), which was significantly higher in ODR rats as compared to OAL rats. Moreover, the numbers of platform crossings by ODR rats was higher as compared to OAL group, but the change was not statistically significant (Fig. 1f). These animals were further used for estimation of protein carbonyl content and mitochondrial ETC complex I–IV activity to correlate cognitive impairment and motor deficits with degree of protein oxidative damage and mitochondrial dysfunction.
Fig. 1.
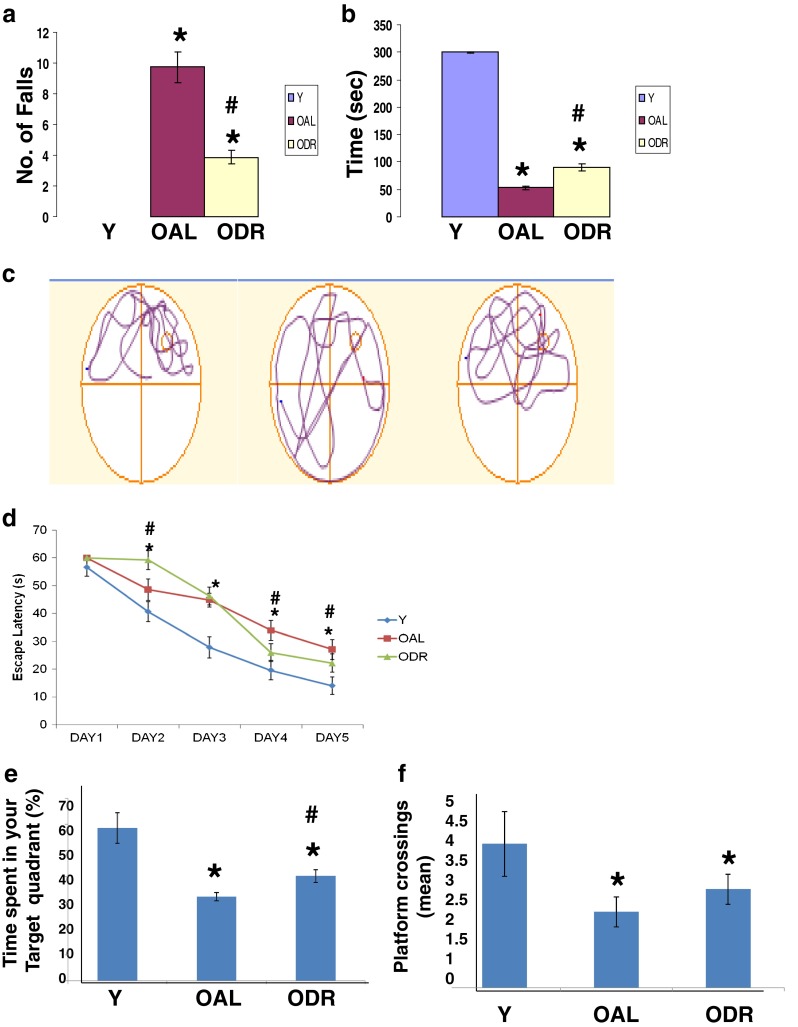
Old intermittent fasting–dietary restricted (ODR) rats performed better than their respective OAL (old ad libitum fed) groups as assessed by latency to fall from the rotarod (a) and time spent on the rotarod (b). Morris water maze performance during training and testing is shown in c–f. OAL rats show significantly longer latencies to find the platform compared with ODR (d), and they failed to swim in the correct quadrant during trial sessions (e, f). Comparatively, ODR spent significantly higher time in target quadrant as compared to OAL rats (e). ODR rats also had more platform crossings than OAL animals, but the change was not significant (f). Values are mean ± SEM of five experiments (n = 20) from 3 months young adult, 24-month-old AL and DR rats. *p < 0.05 young vs old AL and DR rats, #p < 0.05 old AL vs old DR rats, Bonferroni’s test after one-way ANOVA
Attenuation of age-related increase in protein carbonylation by late-onset IF-DR
The protein carbonyl content of OAL was significantly higher as compared to young animals in cortex, hippocampus and hypothalamus regions of the brain (p < 0.05). In comparison, the protein carbonyl content was significantly lower in ODR rats as compared to OAL group (p < 0.05) but higher as compared to young rats. Peripheral organs also showed enhanced protein carbonylation (p < 0.05) in aged rats, but in comparison to brain, IF-DR was less effective in reducing the damage to proteins in liver, heart and kidney. Results are presented in Fig. 2a.
Fig. 2.

Effects of age and IF-DR on carbonyl content (nmol carbonyls/mg protein) of aged rats. a Average carbonyl content of age matched old ad libitum fed (OAL) and old intermittent fasting–dietary restricted (ODR) are shown in comparison to their young counterparts. b–e Specific activity of mitochondrial complex I (DPNH-coenzyme Q reductase), complex II (succinate dehydrogenase coenzyme Q reductase), complex III (coenzyme Q cytochrome C reductase) and complex IV (cytochrome C oxidase) from different brain regions such as pyriform cortex (PC), hippocampus (HIP) and hypothalamus (HYP) and peripheral organs, kidney (K), liver (L) and heart (H) from young, OAL and ODR rats. Values are mean ± SEM of five experiments (n = 3–6) from 3-month young adult, 24-month-old AL and DR rats. *p < 0.05 young vs old AL and DR rats, #p < 0.05 old AL vs old DR rats, Bonferroni’s test after one-way ANOVA
Effect of IF-DR on mitochondrial ETC complexes
The activity of mitochondrial ETC complexes I–IV showed marked reduction in OAL rats as compared to their young counterparts. Complexes I and IV, which play regulatory role, showed more pronounced decrease of 36–61% (complex I) and 24–50% (complex IV) as compared to the activity in young animals. Old rats on IF-DR regimen showed partial recovery in ETC complex activity (Fig. 2). A significant decline in the specific activity of complex I was observed in the OAL rats as compared to young group (p < 0.05), whereas IF-DR rats did not show any beneficial effect on the complex I activity from both brain regions as well as peripheral organs (Fig. 2b). A decline in the specific activity of complex II was observed from OAL rats (Fig. 2c), which was partially recovered in ODR rats. Complex III showed the highest activity in brain regions as well as peripheral organs from young rats (Fig. 2d); however, no significant change was observed in complex III activity in OAL and ODR rats from cortex and hippocampus, whereas hypothalamus region and peripheral organs showed significant decline in activity of complex III both in OAL and ODR rats (p < 0.05). The specific activity of complex IV showed a significant decline in all brain areas and peripheral organs in OAL rats (Fig. 2e) as compared to the young group (p < 0.05), which was significantly restored by IF-DR regimen as compared to young rats (p < 0.05; Fig. 2e).
IF-DR-attenuated age-related impairment of synaptic proteins
To further understand the molecular mechanisms that may underlie the beneficial role of DR in later life, the expression of some markers of brain plasticity like Syn, CaN and CaM were studied in young, OAL and ODR rats in discrete brain regions. In OAL animals, Syn and CaM expressions were significantly decreased in CA3 and DG region of hippocampus, piriform cortex and hypothalamus as compared with young rats (Fig. 3), whereas their expression in IF-DR rats was partially restored (p < 0.05). These results were further strengthened by immunoblots and mRNA expression study (Figs. 4, 5 and 6).
Fig. 3.

Representative immunohistochemical images for synaptophysin DAB staining in CA1, CA3 and dentate gyrus (DG) of hippocampus and piriform cortex (PC) and median eminence (ME) region of hypothalamus of young (Y), old ad libitum fed (OAL) and old intermittent fasting dietary restricted (ODR) rats. The intense staining was observed in CA1, CA3 and DG regions of hippocampus and PC of brain in old DR rats. Histograms represent percent change in staining intensity taking intensity in 3-month-old young rats as 100%. Values are mean ± SEM of five experiments (n = 3–5) from 3-month young adult, 24-month-old AL and DR rats. *p < 0.05 young vs old AL and DR rats, #p < 0.05 old AL vs old DR rats, Bonferroni’s test after one-way ANOVA
Fig. 4.

Representative immunohistochemical images for calcineurin DAB staining in CA1, CA3 and dentate gyrus (DG) of hippocampus and piriform cortex (PC) and median eminence (ME) region of hypothalamus of young (Y), old ad libitum fed (OAL) and old intermittent fasting dietary restricted (ODR) rats. The intense staining was observed in CA1, CA3 and DG regions of hippocampus and PC of brain in old DR rats. Histograms represent percent change in staining intensity taking intensity in 3-month-old young rats as 100%. Values are mean ± SEM of five experiments (n = 3–5) from 3 months young adult, 24-month-old AL and DR rats. *p < 0.05 young vs old AL and DR rats, #p < 0.05 old AL vs old DR rats, Bonferroni’s test after one-way ANOVA
Fig. 5.

Representative immunohistochemical images for CaMKinase DAB staining in CA1, CA3 and dentate gyrus (DG) of hippocampus and piriform cortex (PC) and median eminence (ME) region of hypothalamus of young (Y), old ad libitum fed (OAL) and old intermittent fasting dietary restricted (ODR) rats. The intense staining was observed in CA1, CA3 and DG regions of hippocampus and PC of brain in old DR rats. Histograms represent percent change in staining intensity taking intensity in 3-month-old young rats as 100%. Values are mean ± SEM of five experiments (n = 3–5) from 3-month young adult, 24-month-old AL and DR rats. *p < 0.05 young vs old AL and DR rats, #p < 0.05 old AL vs old DR rats, Bonferroni’s test after one-way ANOVA
Fig. 6.

Representative Western blot and RT-PCR images for synaptophysin (Syn), calcineurin (CaN) and CaMKinae II (CaM) in pyriform cortex (PC), hippocampus (HIP), hypothalamus (HYP) regions of young adult, old AL and old DR rats (n = 3 for each group). Quantitative densitometric analysis for synaptic markers is shown in histograms. *p < 0.05 young vs old AL and DR rats, #p < 0.05 old AL vs old DR rats, Bonferroni’s test after one-way ANOVA
On the other hand, diffused CaN immunostaining was seen in the CA3 and DG regions of hippocampus from young rats, which was significantly increased in OAL rat (p < 0.05). IF-DR rats showed lower CaN expression (p < 0.05) as compared to OAL rats (Fig. 4). Further immunoblotting and RT-PCR data also revealed significant restoration by inhibiting CaN expression especially in hippocampus and pyriform cortex (p < 0.05).
Discussion
The present results reveal the potential beneficial effects of late-onset short-term IF-DR regimen on markers of oxidative stress and mitochondrial dysfunction from discrete brain regions and peripheral organs. Furthermore, we observed improved expression of synaptic proteins and amelioration of age-dependent loss of cognitive and motor skills in animals on IF-DR as compared to AL group. IF-DR regimen seems more feasible option for humans as compared to lifelong DR on the calorie deprivation, which is extremely difficult to adopt over lifetime. The current study examined the effects of late-onset short-term intermittent fasting regimen in ageing animals. An interesting finding of this study is that even late-onset DR delays age-associated decline in motor skills and spatial memory. Although the fasting period was followed by ad libitum feeding period (during which these animals ate 30–40% more food as compared to AL rats), still the overall food intake per day and the body weight of animals on IF-DR regimen were significantly lower than ad libitum fed rats The current results may suggest that IF-DR regimen can contribute to healthy ageing by enhancing cellular resistance to disease. Anson et al (2003) reported that the beneficial effects of IF regimen on glucose regulation and neuronal resistance to injury exceeded from caloric restriction over a period of 20 weeks initiated in 2-month-old C57BL/6 mice. In their study, average food intake in ad libitum fed and IF animals was not different; thus, they suggested that the potential beneficial effects of IF may be independent of caloric intake in these mice. Similarly, previous studies from our lab and others have reported that IF regimen provides neuroprotective and cardioprotective effects in rats against excitotoxic and ischemic injuries (Ahmet et al. 2005; Sharma et al. 2010; reviewed by Martin et al. 2006; Kumar et al. 2010). On the other hand, Yanai et al. (2004) observed some negative effects of long-term caloric restriction on cognitive ability of middle age and old rats and proposed that low blood glucose may be responsible for poor performance of these food restricted animals. Similarly, Carter et al. (2009) reported that male F344xBN rats on CR showed increased physical activity, which may be considered as a confounding factor in interpreting the results of dietary/caloric restriction regimens. Since the behavioural experiments in the current study were performed between 8 a.m. to 10.00 a.m. in the morning before the rats were put on fasting, the animals were well fed at the time of these behavioural tests.
The present findings suggest that IF-DR regimen has protective adaptive response, as observed for the motor, learning and memory skills using rotarod and MWM tests. There was significant improvement in mean time spent on rod and number of falls in ODR rats, indicating their better grip and ability to move on a rotating rod for longer, thus suggesting that ODR rats established better equilibrium and acquired skilled behaviour more rapidly than the OAL rats. The correction of grip strength and time spent on rotating rod may be correlated to a decrease in the body weight of rats on IF-DR regimen. These results are supported by previous findings that CR improves motor capability and memory in rodents and humans (Markowska and Savonenko 2002; Adams et al. 2008; Witte et al. 2009) and in amyotrophic sclerosis (Hamadeh et al. 2005), which may be due to improved body functions and metabolic status. The performance of OAL and ODR rats in MWM, a test predictive of hippocampal neurogenesis (Drapeau et al. 2003), was poor compared to young rats, but the escape latency and the swimming distance in the ODR rats were comparatively less as compared to OAL rats. The OAL rats swam relatively longer before locating escape platform, whereas ODR rats swam relatively faster and had more number of platform crossings, indicating more time spent in the respective quadrant. CR rats have also been shown to exhibit similar improvements in age-related hippocampus deficits and learning memory tasks (Okada et al. 2003; Hashimoto and Watanabe 2005; reviewed by Mattson 2010).
The highest protein carbonyl content was seen in OAL rats, and IF-DR regimen slowed down the protein carbonylation from 341% to 182% though still higher than the 3-month-old young animals (taken as 100%). A decline in protein oxidation in ODR rats may be an indication of improved cellular functions showing more efficient protein degradation. The potential beneficial effect of IF-DR on carbonyl levels may be due to low ROS generation and enhanced antioxidant activity (Sharma et al. 2010, 2010). It has been earlier shown that CR can induce a condition of elevated cellular processes by virtue of lowering steady state levels of oxidative damage (Forster et al. 2000). The nature of mechanisms that retard protein oxidation with DR seems to be tissue specific as IF-DR did not impart same level of protection in peripheral organs, which may be due to their increased protein alteration and aggregation. Similar recent studies have also suggested that age-associated increase in oxidative damage in mice and rats can be reduced by relatively short-term DR initiated in old age (Goto 2006; Goto et al. 2007).
Furthermore, we observed age-associated decline in mitochondrial electron chain complexes I–IV in different brain regions as well as in peripheral organs with complexes I and IV showing more pronounced reduction. These observations are supported by our previous study (Sandhu and Kaur 2003) and strongly overlap with the pattern of mitochondrial DNA (mtDNA) damage observed in discrete brain regions with ageing (Filburn et al. 1996 and reviewed in Mattson et al. 1999; Lopez-Torres et al. 2002). Seven out of the 41 subunits of complex I are encoded by mtDNA, whereas all four subunits of complex II are of nuclear origin. On the other hand, complex III has 10 subunits of nuclear origin and one of mitochondrial and complex IV consists of 13 subunits, three of which are encoded by mtDNA. This may also explain the higher degree of decline in complex I activity as compared to complex IV and their relative preservation by IF-DR in old age. The relationship of mitochondrial activity to oxidative stress is critically related with ageing as explained by positive feedback loop, suggesting that free radicals are mainly generated in mitochondria (Sandhu and Kaur 2002; Olgun et al. 2002; Aksenov et al. 2010). Moreover, the effect of IF-DR regimen on complex I–IV activities varied in different post-mitotic and mitotic tissues. No significant change was observed in complex II and III activity in ODR as compared to OAL rats though there was age-related decline in their activities in all brain regions and peripheral organs.
Marginal decline in complex I activity in cortex, hippocampus and kidneys tissues from ODR rats may be explained by low metabolic rate allowing bypass of electrons and hence low ROS production analogous to what happens during CR (Gredilla et al. 2001a, b; Ayala et al. 2007). The possible mechanism responsible for such change may be related to degree of electronic reduction of complex I free radical generation with reduction in mtDNA damage, indicating an adaptive preconditioning response (hormesis) induced as mild stress with IF-DR regimen. IF-DR had potentially beneficial effects on complex IV in all brain regions and peripheral organs. The age-associated decrease in the complex IV activity could lead to accumulation of electrons in the upstream complexes, and IF-DR seems to effectively restore the functioning of the complex IV. The increase in activity of complex IV also suggests better performance of this enzyme as final electron acceptor and thus decreasing ROS generation in animals on IF-DR regimen. If the mitochondrial dysfunction and oxidative damage is one of the possible mechanisms responsible for age-related loss in functional capacity and hippocampus dysfunction (Navarro et al. 2008), then late-onset short-term DR may be expected to delay or reduce the accumulation of such damage and may explain the difference in the mortality observed between the two groups, i.e. OAL and ODR. Similar study on age-related decline in activity and function of complexes I to IV reported earlier (Feuers 1998) showed marginal increase in activity of complex IV with CR and lower total respiratory rate, thus suggesting that DR may retard age-associated mitochondrial respiratory function impairment by preserving the enzymatic activities and their function.
Further age-related memory deficits are associated with Ca2+ dysregulation and decline in synaptic plasticity (Djordjevic et al. 2010). The present data show that the ODR rats have better cognitive skills and also exhibit pronounced increase in levels of Syn. Syn being a synaptic density marker could be a reflection of recovery of loss of synapse density and improved synaptic plasticity in ODR rats. The increase in Syn has been interpreted as increase in the presynaptic terminals and neurotransmission. Our findings are consistent with the previous reports that CR restores the age-related decline in Syn levels (King and Arendash 2002; Rutten et al. 2005; Adams et al. 2008; Djordjevic et al. 2010). Davies et al. (2003) reported that Syn immunogold labelling and density of particles per pre-synaptic bouton of synapses decrease in DG region of the hippocampus of aged rats by 50% than in younger rats. IF-DR induced increase in Syn in DG and CA3 region of hippocampus and cortex region in brain may lead to reduced hippocampus stress and enhanced synaptic plasticity and neurogenesis preventing continuous decline in synaptic functionality as also reported by Djordjevic et al. (2010). Previous recent reports in literature suggest that altered energy metabolism and oxidative stress negatively affect synaptic plasticity and cognitive ability (Scheff et al. 2005; Wu et al. 2006). Moreover, mitochondrial bioenergetic dysfunction, ROS overproduction and protein oxidation adversely affects synaptic plasticity and neuronal loss (Pandya et al. 2007). Previous study from our lab reported that IF-DR regimen reverses the age-related impairments in neuronal plasticity marker NCAM (Kaur et al. 2008). Post-synaptic NCAM expression is involved in activity-dependent strengthening of synaptic connections and is also used as a quantitative marker in synaptic remodelling, which increases synaptic transmission, presynaptic function and improved memory function (Cambon et al. 2004; Dityatev et al. 2000; Cremer et al. 1998).
Ageing is associated with altered balance of protein kinase/phosphatase activities, which results in memory deficits (Hsu et al. 2002). Decreased CaN levels as well as increased CaM expression were seen in the hippocampus of aged rats on IF-DR regimen. The present data elucidates the potential beneficial effect of IF-DR regimen on the expression of synaptic proteins regulating calcium homeostasis and other regulatory mechanisms not only at their translational expression levels but at transcriptional regulation as well. CR has been reported to improve Ca2+ homeostasis impairments as well as Ca2+-dependent synaptic plasticity (Toescu et al. 2004). The present data suggest that even short-term late onset IF-DR regimen can efficiently shift the balance between the postsynaptic CaM and CaN levels, which are important regulators of synaptic transmission and strength. IF-DR regimen may also be used as a synergistic approach along with pharmacological strategy to ameliorate cognitive deficits in neurodegenerative diseases (Dineley et al. 2007). Recently, Harvie et al. (2011) compared the potential beneficial effects of intermittent continuous energy (IER) with continuous energy restriction (CER) in young overweight women subjects and found that IER is as effective as CER for health biomarkers, such as weight loss, insulin sensitivity, leptin level reduction, lipid profile, etc.
The search for interventions to improve or slow down the age-associated decline in cognitive abilities has interested human beings forever. Here, we provide a scientific evidence for cognition and motor coordination enhancing properties of late-onset short-term intermittent fasting DR regimen in ageing rats. These observations further suggest that the improvement in physical activity and memory task by IF-DR in ageing rats is possibly due to prevention/slowing of deterioration of impairments in synaptic functions as well as mitochondrial ROS generation. In addition, it is suggested that simultaneous focus on “biological” and “behavioral” mechanisms that contribute to the potential beneficial effects of DR regimens may have greater clinical applications as also suggested by Carter et al. (2009). Also as per the study of Harvie et al. (2011) in young overweight women, intermittent energy restriction may be recommended as an alternate regimen to continuous energy restriction for weight loss and reducing disease risk. Several reports in literature have described the potential beneficial effects of dietary restriction (recently reviewed by Mattson 2008; Calabrese and Mattson 2011). We hope the current study further helps in raising awareness that even late-onset and short-term DR intervention is beneficial and prevents/slows the age-associated brain function impairments.
Acknowledgements
This grant was funded by Indian Council of Medical Research (ICMR) under the National Task Force Project—an initiative on ageing research. Rumani Singh and Sandeep Sharma are thankful to ICMR for the research fellowship grant during entire course of study.
References
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle D, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112(20):3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Aksenov V, Long J, Lokuge S, Foster JA, Liu J, Rollo CD. Dietary amelioration of locomotor, neurotransmitter and mitochondrial aging. Exp Biol Med. 2010;235:66–76. doi: 10.1258/ebm.2009.009219. [DOI] [PubMed] [Google Scholar]
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl. 2000;59:133–154. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- Altun M, Bergman E, Edström E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiol Behav. 2007;92(5):911–923. doi: 10.1016/j.physbeh.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100(10):6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala V, Naudı A, Sanz A, Caro P, Portero-Otin M, Barja G, Pamplona R. Dietary protein restriction decreases oxidative protein damage, peroxidizability index, and mitochondrial complex I content in rat liver. J Gerontol A Biol sci Med Sci. 2007;62:352–360. doi: 10.1093/gerona/62.4.352. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal. 2011;5(1):25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon K, Hansen SM, Venero C, Herrero AI, Skibo G, Berezin V, Bock E, Sandi C. A synthetic neural cell adhesion molecule mimetic peptide pomotes synaptogenesis, enhances presynaptic function and facilitates memory consolidation. J Neurosci. 2004;24:4197–4204. doi: 10.1523/JNEUROSCI.0436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SC, Leeuwenburgh C, Daniels M, Foster CT. Influence of calorie restriction on measures of age related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64:850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri AR, Waal E, Pierce A, Remmen VA, Ward FW, Richardson F. Detection of protein carbonyls in aging liver tissue: a fluorescence-based proteomic approach. Mech Ageing Dev. 2006;127:849–861. doi: 10.1016/j.mad.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res. 2000;33:S99–S108. [PubMed] [Google Scholar]
- Cremer H, Genevieve C, Carleton A, Gordis C, Vincent JD, Lledo PM. Long term but not short term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule deficient mice. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AH, Kelly A, Dhanrajan TM, Lynch MA, Rodrıguez JJ, Stewart GM. Synaptophysin immunogold labelling of synapses decreases in dentate gyrus of the hippocampus of aged rats. Brain Res. 2003;986:191–195. doi: 10.1016/S0006-8993(03)03251-7. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Hogan D, Zhang WR, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007;88:217–224. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Schachner M. Synaptic strength as a function of post versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron. 2000;26:207–217. doi: 10.1016/S0896-6273(00)81151-4. [DOI] [PubMed] [Google Scholar]
- Djordjevic MA, Perovic M, Tesic V, Tanic N, Rakic L, Ruzdijic S, Kanazir S. Long-term dietary restriction modulates the level of presynaptic proteins in the cortex and hippocampus of the aging rat. Neurochem Int. 2010;56(2):250–255. doi: 10.1016/j.neuint.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuers RJ. The effects of dietary restriction on mitochondrial dysfunction in aging. Ann N Y Acad Sci. 1998;854:192–201. doi: 10.1111/j.1749-6632.1998.tb09902.x. [DOI] [PubMed] [Google Scholar]
- Filburn CR, Edris W, Tamatani M, Hogue B, Kudryashova I, Hansford RG. Mitochondrial electron transport chain activities and DNA deletions in regions of the rat brain. Mech Ageing Dev. 1996;87(1):35–46. doi: 10.1016/0047-6374(96)01696-X. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Sohal BH, Sohal RS. Reversible effects of long-term caloric restriction on protein oxidative damage. J Gerontol A Biol Sci Med Sci. 2000;55:B522–B529. doi: 10.1093/gerona/55.11.B522. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Goto S. Health span extension by later-life caloric or dietary restriction: a view based on rodent studies. Biogerontology. 2006;7:135–138. doi: 10.1007/s10522-006-9011-4. [DOI] [PubMed] [Google Scholar]
- Goto S, Takahashi R, Radak Z, Sharma R. Beneficial biochemical outcomes of late-onset dietary restriction in rodents. Ann N Y Acad Sci. 2007;1100:431–441. doi: 10.1196/annals.1395.048. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G, Lopez-Torres M. Effect of short-term caloric restriction on H2O2 production and oxidative DNA damage in rat liver mitochondria and location of the free radical source. J Bioenerg Biomembr. 2001;33:279–287. doi: 10.1023/A:1010603206190. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Sanz A, Lopez-Torres M, Barja G. Calorie restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:U481–U496. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- Griffths D. Clarification and extraction. In: Harris ELV, Angal S, editors. Protein purification methods a practical approach. Oxford: IRL; 1989. pp. 91–97. [Google Scholar]
- Grune T, Shringarpure R, Sitte N, Davies K. Age-related changes in protein oxidation and proteolysis in mammalian cells. J Gerontol A Biol Sci Med Sci. 2001;56A:B459–B467. doi: 10.1093/gerona/56.11.B459. [DOI] [PubMed] [Google Scholar]
- Hamadeh MJ, Rodriguez MC, Kaczor JJ, Tarnopolsky MA. Caloric restriction transiently improves motor performance but hastens clinical onset of disease in the Cu/Zn-superoxide dismutase mutant G93A mouse. Muscle Nerve. 2005;31:214–220. doi: 10.1002/mus.20255. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35(5):714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Watanabe S. Chronic food restriction enhances memory in mice—analysis with matched drive levels. Neuroreport. 2005;16:1129–1133. doi: 10.1097/00001756-200507130-00019. [DOI] [PubMed] [Google Scholar]
- Hatefi Y, Rieske JS. The preparation and properties of DPNH-cytochrome C reductase (Complex I of respiratory chain) In: Estabrook RW, Pullman ME, editors. Methods in enzymology, vol 10. New York: Academic; 1967. pp. 235–239. [Google Scholar]
- Hsu KS, Huang CC, Liang YC, Wu HM, Chen YL, Lo SW, Ho WC. Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus. 2002;12:787–802. doi: 10.1002/hipo.10032. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2009;31:83–89. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- Jabr RI, Wilson JA, Riddervold M, Jenkins AH, Perrino BA, Clapp LH. Nuclear translocation of calcineurin Aα but not calcineurin Aβ by platelet-derived growth factor in rat aortic smooth muscle. Am J Physiol Cell Physiol. 2007;292:C2213–C2225. doi: 10.1152/ajpcell.00139.2005. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Dutar P. A role for the protein phosphatase 2B in altered hippocampal synaptic plasticity in the aged rat. J Physiol Paris. 2006;99:154–161. doi: 10.1016/j.jphysparis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Kaur M, Sharma S, Kaur G. Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology. 2008;9:441–454. doi: 10.1007/s10522-008-9168-0. [DOI] [PubMed] [Google Scholar]
- Keenan KP, Coleman JB, McCoy CL, Hoe CM, Soper KA, Laroque P. Chronic nephropathy in ad libitum overfed Sprague–Dawley rats and its early attenuation by increasing degrees of dietary (caloric) restriction to control growth. Toxicol Pathol. 2000;28:788–798. doi: 10.1177/019262330002800604. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW. Maintained synaptophysin immunoreactivity in Tg2576 transgenic mice during aging: correlations with cognitive impairment. Brain Res. 2002;926:58–68. doi: 10.1016/S0006-8993(01)03294-2. [DOI] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Kumar S, Parkash J, Kataria H, Kaur G. Interactive effect of excitotoxic injury and dietary restriction on neurogenesis and neurotrophic factors in adult male rat brain. Neurosci Res. 2010;65:367–374. doi: 10.1016/j.neures.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Liu HX, Zhang JJ, Zheng P, Zhang Y. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res. 2005;139:169–177. doi: 10.1016/j.molbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres M, Gredilla R, Sanz A, Barja G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Rad Biol and Med. 2002;32:882–889. doi: 10.1016/S0891-5849(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Love R. Calorie restriction may be neuroprotective in AD and PD. Lancet Neurol. 2005;4:84. doi: 10.1016/S1474-4422(05)00985-3. [DOI] [PubMed] [Google Scholar]
- Mansuy MI. Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun. 2003;311:1195–1208. doi: 10.1016/j.bbrc.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol Aging. 2002;23:75–86. doi: 10.1016/S0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5(3):332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc Natl Acad Sci USA. 2004;101:17887–17888. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Gene–diet interactions in brain aging and neurodegenerative disorders. Ann Intern Med. 2003;139:441–444. doi: 10.7326/0003-4819-139-5_part_2-200309021-00012. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7(1):43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. The impact of dietary energy intake on cognitive aging. Front Aging Neurosci. 2010;2:1–12. doi: 10.3389/neuro.24.005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann N Y Acad Sci. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- Monville C, Torres ME, Dunnett BS. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods. 2006;158:219–223. doi: 10.1016/j.jneumeth.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Lopez-Cepero MJ, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Sanchez-Pino MJ, Gonzalez H, Bandez MJ, Boveris AD, Boveris A. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1329–R1399. doi: 10.1152/ajpregu.00834.2004. [DOI] [PubMed] [Google Scholar]
- Navarro A, Lopez-Cepero JM, Bandez MJ, Sanchez-Pino MJ, Gomez C, Cadenas E, Boveris A. Hippocampal mitochondrial dysfunction in rat aging. Am J Physiol Regul Integr Comp Physiol. 2008;294:R501–R509. doi: 10.1152/ajpregu.00492.2007. [DOI] [PubMed] [Google Scholar]
- Okada M, Nakanishi H, Amamoto T, Urae R, Ando S, Yazawa K, Fujiwara M. How does prolonged caloric restriction ameliorate age-related impairment of long-term potentiation in the hippocampus? Brain Res Mol Brain Res. 2003;111:175–181. doi: 10.1016/S0169-328X(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Olgun A, Akman S, Serdar AM, Kutluay T. Oxidative phosphorylation enzyme complexes in caloric restriction. Exp Gerontol. 2002;37:639–645. doi: 10.1016/S0531-5565(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J. Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- Pathan AR, Viswanad B, Sonkusare SK, Ramarao P. Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci. 2006;79(23):2209–2216. doi: 10.1016/j.lfs.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Rieske JS. Preparation and properties of reduced coenzyme Q cytochrome C reductase (complex III of the respiratory brain) In: Estabrook RW, Pullman ME, editors. Methods in Enzymology, vol 10. New York: Academic Press; 1967. pp. 239–245. [Google Scholar]
- Ripple MJ, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Müller C, de Jong M, van IJcken W, IJzermans JN, Hoeijmakers JH, de Bruin RW. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2009;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Rutten BP, Vander Kolk NM, Zandvoort V, Bayer MA, Steinbusch TA, Schmitz C. Age-related loss of synaptophysin immunoreactive presynaptic boutons within the hippocampus of APP751SL, PS1M146L, and APP751SL/PS1M146L transgenic mice. Am J Pathol. 2005;167:161–173. doi: 10.1016/S0002-9440(10)62963-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu SK, Kaur G. Alterations in oxidative stress scavenger system in aging rat brain and lymphocytes. Biogeronotology. 2002;3(3):161–173. doi: 10.1023/A:1015643107449. [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Kaur G. Mitochondrial electron transport chain complexes in aging rat brain and lymphocytes. Biogerontology. 2003;4:19–29. doi: 10.1023/A:1022473219044. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Hicks RR, Baldwin SA, Robinson S, Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- Sharma S, Singh R, Kaur M, Kaur G. Late-onset dietary restriction compensates for age-related increase in oxidative stress and alterations of HSP 70 and synapsin1 protein levels in male Wistar rats. Biogerontology. 2010;11:197–209. doi: 10.1007/s10522-009-9240-4. [DOI] [PubMed] [Google Scholar]
- Soderling TR. Calcium/calmodulin-dependent protein kinase II: role in learning and memory. Mol Cell Biochem. 1993;127–128:93–101. doi: 10.1007/BF01076760. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Wang P, Wang WP, Sun-Zhang WHX, Yan-Lou FYH. Impaired spatial learning related with decreased expression of calcium/calmodulin-dependent protein kinase IIα and cAMP-response element binding protein in the pentylenetetrazol-kindled rats. Brain Res. 2008;1238:108–117. doi: 10.1016/j.brainres.2008.07.103. [DOI] [PubMed] [Google Scholar]
- Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol. 1996;24:742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- Wharton DC, Tzagoloff A. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 1967;10:245–250. doi: 10.1016/0076-6879(67)10048-7. [DOI] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Floela A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci USA. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinnilla F. Dietary curcumin counteracts the outcome of tramatic brain injury on oxidative stress, synaptic plasticity and cognition. Exp Neurol. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Yanai S, Okaichi Y, Okaichi H. Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol Aging. 2004;25(3):325–332. doi: 10.1016/S0197-4580(03)00115-5. [DOI] [PubMed] [Google Scholar]
- Yonetani T. Cytochrome oxidase: beef heart. In: Estabrook RW, Pullman ME, editors. Methods in enzymology, vol 10. New York: Academic; 1967. pp. 332–335. [Google Scholar]
- Zainal TA, Oberley TD, Allison DB, Szweda LI, Weindruch R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB J. 2000;14:1825–1836. doi: 10.1096/fj.99-0881com. [DOI] [PubMed] [Google Scholar]
- Ziegler D, Rieske JS. Preparation and properties of succinate dehydrogenase coenzyme Q reductase (complex II) In: Estabrook RW, Pullman ME, editors. Methods in enzymology, vol 10. New York: Academic; 1967. pp. 231–235. [Google Scholar]


