Abstract
Isolation-reared male rodents show abnormal behaviors such as hyperlocomotion, aggressive behaviors, deficits of prepulse inhibition, and depression- and anxiety-like behaviors, but the neurochemical mechanism for the effects of psychological stress in these animals is not fully understood. This study examined the effects of social interactions between isolation-reared mice and intruder mice on brain monoaminergic systems. A cage was divided into two compartments by a mesh partition to prevent direct physical interactions. The 20-min encounter with an intruder elicited a restless and hyperexcitable state (hyperactivity) in male, but not in female, isolation-reared mice, whereas encounters with a sleeping intruder or a novel object did not. Although the encounter did not affect prefrontal neuronal-activity-marker c-Fos expression, dopamine (DA) levels, or serotonin (5-HT) levels in male group-reared mice or female isolation-reared mice, it increased prefrontal c-Fos expression, DA levels, and 5-HT levels in male isolation-reared mice. Furthermore, encounter-induced increases in c-Fos expression in the dorsal raphe nucleus and ventral tegmental area, but not in the nucleus accumbens shell, were much greater in isolation-reared than group-reared male mice. A 5-HT1A receptor agonist, a metabotropic glutamate 2/3 receptor agonist, and a gamma-aminobutyric acid A receptor agonist attenuated isolation-induced aggressive behaviors and encounter-induced hyperactivity, c-Fos expression in the prefrontal cortex and dorsal raphe nucleus, and increases in prefrontal 5-HT levels. These findings suggest that the prefrontal DA and 5-HT systems are activated by encounter stimulation in male isolation-reared mice, and the encounter-induced activation of 5-HT system triggers the induction of some abnormal behaviors in male isolation-reared mice. Furthermore, this study implies that the encounter stimulation-induced signal has a pharmacological significance.
Keywords: encounter stimulation, isolation rearing, prefrontal cortex, c-Fos, dopamine (DA), serotonin (5-HT)
INTRODUCTION
Being raised in social isolation from early in life causes abnormal behaviors in adulthood, such as hyperlocomotion, aggressive behavior, deficits of prepulse inhibition, cognitive impairments, decreased social contact, and depression- and anxiety-like behavior in rodents (Ago et al, 2007, 2008; Fone and Porkess, 2008; Lukkes et al, 2009a, 2009b; Zhao et al, 2009). Post-weaning social isolation is a useful model to study the effects of adverse early-life experiences on behavior and the neural mechanisms associated with isolation-induced changes in mood and anxiety (Lapiz et al, 2003; Lukkes et al, 2009c). Previous pharmacological studies have shown that serotonin 1A (5-HT1A) receptor agonists and metabotropic glutamate 2/3 (mGlu2/3) receptor agonists reverse isolation-rearing-induced abnormal behaviors (Ago et al, 2012; Jones et al, 2011; Maisonnette et al, 1993; Sakaue et al, 2001, 2003; Sánchez et al, 1993), but the exact neurochemical mechanisms are not known. There have been several studies on the neurochemical effects of isolation rearing, particularly focusing on the monoaminergic systems. These studies suggest that isolation rearing affects dopamine (DA) and 5-HT turnover in the brain regions, including the prefrontal cortex and nucleus accumbens (Eells et al, 2006; Heidbreder et al, 2000). Furthermore, some physical stressors or aversive stimuli cause a variety of changes in brain monoaminergic systems in isolation-reared rodents (Ago et al, 2002; Cabib et al, 2002; Fulford and Marsden, 1998a, 1998b, 2007; Hall et al, 1998; Jones et al, 1992; for reviews, see Hall, 1998; Hall and Perona, 2012). However, the effects of social stress on brain monoaminergic systems in isolation-reared animals remain to be investigated.
As aggressive behaviors and social interaction deficits in isolation-reared rodents are induced by exposure to an intruder, it is likely that encounter with an intruder may produce neurobiological changes responsible for abnormal behaviors. There have been several neurochemical studies on resident–intruder interactions. In resident–intruder tests, van Erp and Miczek (2000) reported that encounters with the intruder increased prefrontal and accumbal DA and decreased cortical 5-HT levels in the resident aggressive rats. Anstrom et al (2009), using fast-scan cyclic voltammetry and multiunit recording techniques, reported that an aggressive encounter increases phasic DA transmission in the mesolimbic pathway in defeated rats. These neurochemical changes were observed both during and after the encounters, and it is likely that the changes are related to both psychological and physical stress. However, the neurochemical changes responsible for abnormal behaviors in aggressive rodents are not known.
In this study, we aimed to identify the primary neurochemical changes for induction of abnormal behaviors in isolation-reared mice. To address this issue, we used a cage that was divided into two compartments (large and small) by a mesh partition, and we examined the effects of intruder encounters on c-Fos expression and the levels of DA and 5-HT in the brains of resident mice reared in a group or in isolation. We used c-Fos expression to measure regional changes in brain activity (Koda et al, 2010; Kovács, 2008). As a control, we further examined female isolation-reared mice, because they do not develop hyper-aggression, unlike male isolation-reared mice (Pinna et al, 2005, 2008).
MATERIALS AND METHODS
Animals and Drugs
All animal studies were approved by the Animal Care and Use Committee of the Graduate School of Pharmaceutical Sciences, Osaka University. All experimental procedures were conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Every effort was made to minimize animal suffering, and to reduce the number of animals used. The ddY outbred male or female mice (SHIMIZU Laboratory Supplies, Kyoto, Japan) were weaned at 3 weeks of age and divided equally and concurrently into isolation- and group-housed conditions. The isolated male and female mice were individually housed for 6 weeks in wire-topped opaque polypropylene cages (24 × 17 × 12 cm3), whereas control male mice were housed in groups (5–6 per cage) in the same-sized wire-topped clear plastic cages (Ago et al, 2007, 2008; Koda et al, 2008). All mice were housed under a standard 12-h light/dark cycle (lights on at 0800 hours) at a constant temperature of 22±1 °C, with free access to food and water throughout the experiments. All experiments were conducted on 9-week-old male or female mice. The isolated male ddY-strain mice showed abnormal behaviors (Ago et al, 2007, 2008, 2012; Sakaue et al, 2001, 2003) consistent with those in C57BL/6J or ICR strain mice (Ibi et al, 2008; Koike et al, 2009; Varty et al, 2006; Zhang et al, 2012). We used 100 mice for behavioral analyses (Figures 1 (n=30), 4a (n=20) and 5 (n=50)), 92 mice for c-Fos immunochemistry (Figures 2 (n=32) and 6 (n=60)), and 180 mice for microdialysis experiments (Figures 3 (n=60), 4b (n=20), 7 (n=50) and 8 (n=50)) in single use for each purpose. The drugs osemozotan (Mitsubishi Pharma, Yokohama, Japan), diazepam (Sigma, St Louis, MO, USA), LY379268 (Tocris Cookson, Bristol, UK), and pentobarbital (Nacalai Tesque, Kyoto, Japan) were used in this study. Osemozotan and diazepam were suspended in 0.5% w/v carboxymethylcellulose. LY379268 was dissolved in saline (0.9% solution of NaCl). Pentobarbital was dissolved in sterile water containing 10% ethanol. The drugs were injected intraperitoneally in a volume of 10 ml/kg. Mice were treated with a single injection of diazepam (1 mg/kg), osemozotan (0.3 mg/kg), or vehicle 30 min before the encounter with an unfamiliar male intruder (Figures 5, 6, 7, 8). LY379268 (1 mg/kg) or saline was also given as a single-dose injection 60 min before the encounter (Figures 5, 6, 7, 8). These doses of the drugs used here were determined by referring to previous studies: the doses potently inhibited isolation rearing-induced aggressive behavior, hyperactivity, deficits of prepulse inhibition, or cognitive impairments (Jones et al, 2011; Sakaue et al, 2001, 2003).
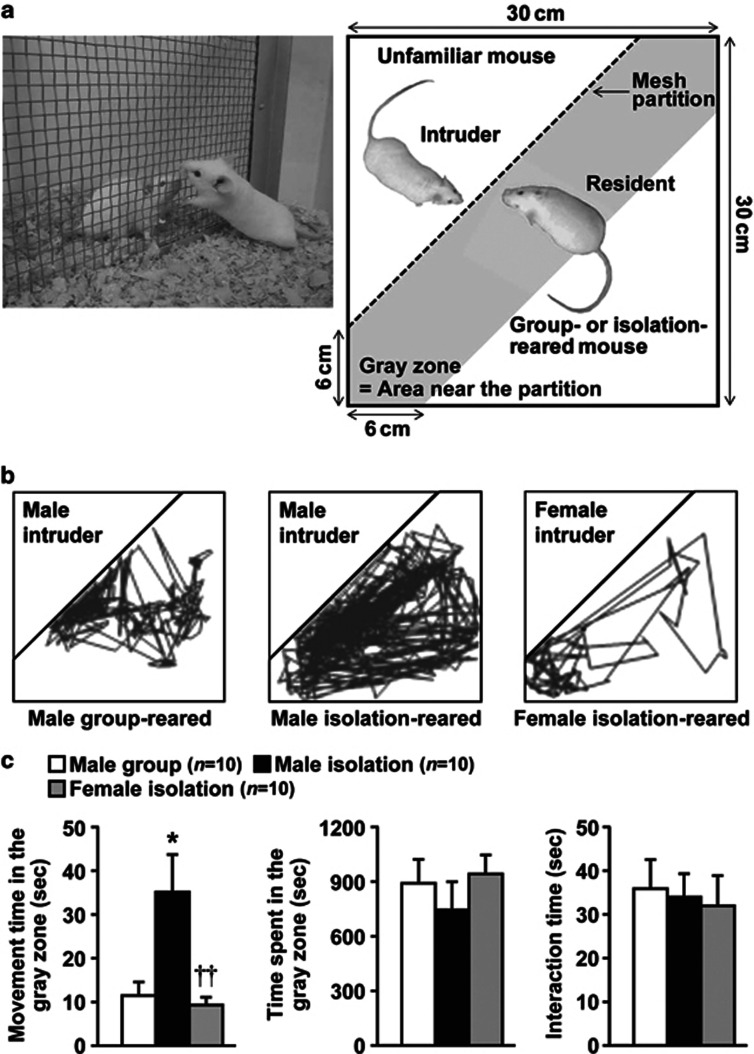
Figure 1.
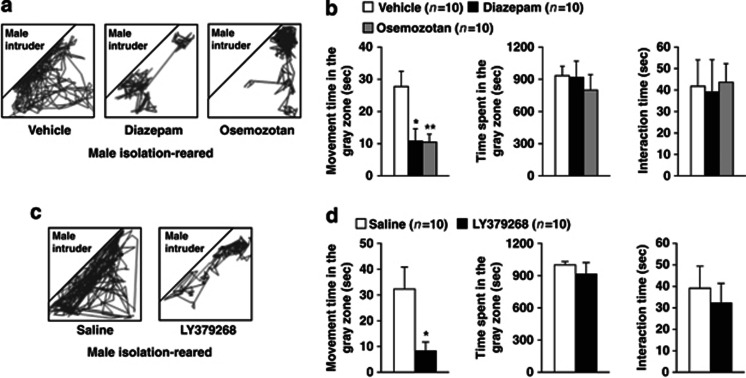
Effects of encounter stimulation on the behavior of mice. (a) Experimental design for the social encounter paradigm. A group- or isolation-reared mouse was individually placed into the large compartment of a novel clear Plexiglas cage divided by a mesh partition. An unfamiliar mouse was introduced into the unoccupied small compartment for 20 min. The gray zone was defined as the area near the partition. (b) Representative locomotor paths from resident male group-reared and isolation-reared mice and female isolation-reared mice during the 20-min encounters with the same sex intruders. (c) The locomotor activity and total time spent in the gray zone by the resident mice and the total time the resident and intruder mice spent interacting were analyzed according to the description in the Materials and Methods. The data are expressed as the mean±SEM of 10 mice/group. *p<0.05, compared with the male group-reared mice. ††p<0.01, compared with the male isolation-reared mice.
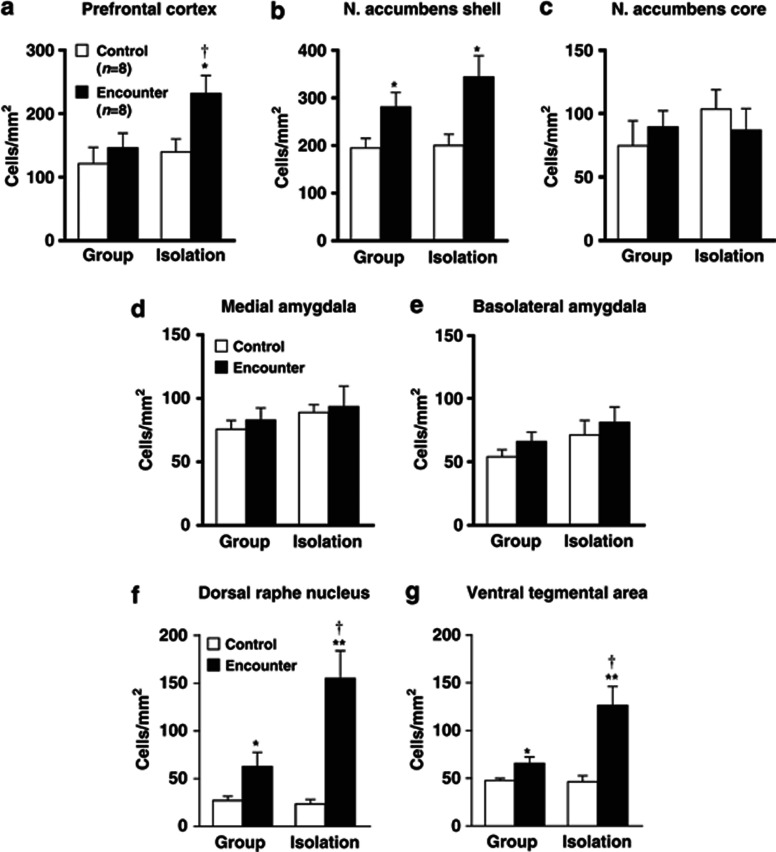
Figure 2.
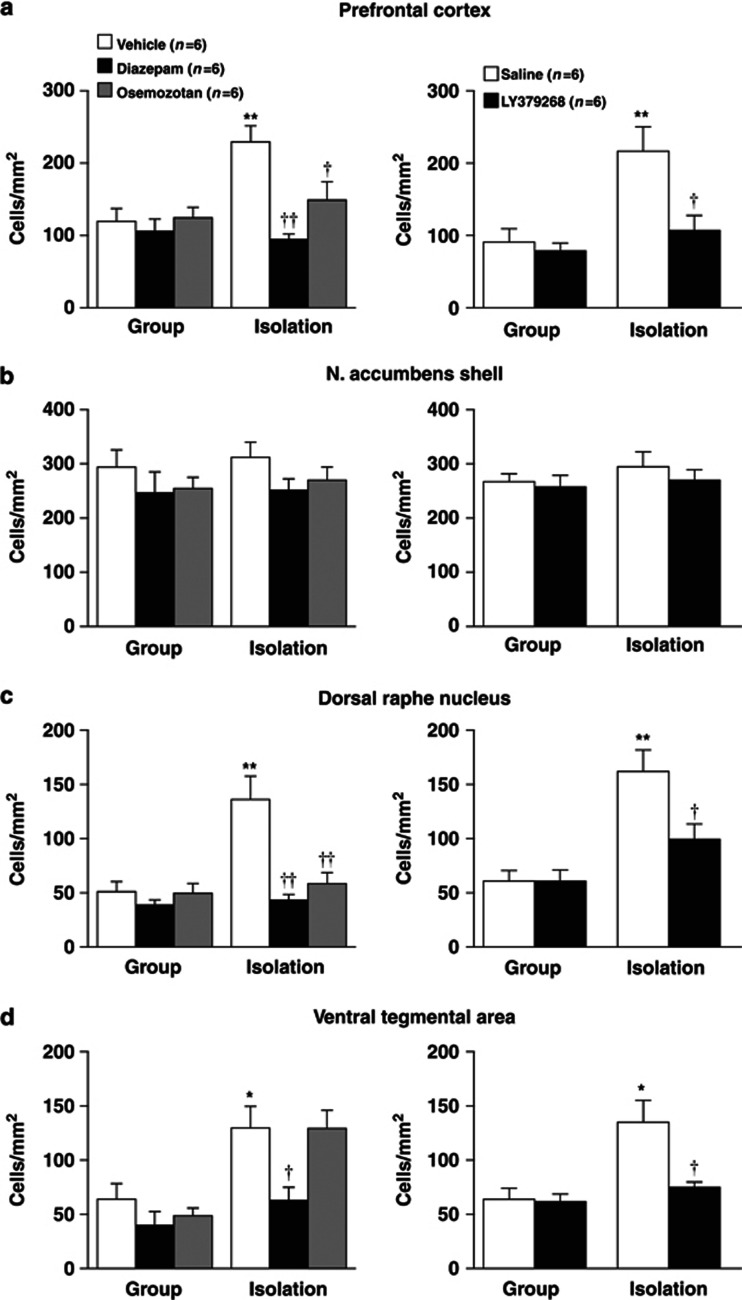
Effects of encounter stimulation on c-Fos expression in the prefrontal cortex (a), nucleus accumbens shell (b) and core (c), the medial (d) and basolateral (e) amygdala, dorsal raphe nucleus (f), and ventral tegmental area (g) of mice. Immunohistochemical localization of the neuronal-activity-marker c-Fos in the male group- and isolation-reared mice was determined 2 h after the encounter. Mice that were not exposed to an intruder were used as controls. The data are expressed as the mean±SEM of 8 mice/group. *p<0.05, **p<0.01, compared with the control. †p<0.05, compared with the encounter stress-challenged group-reared mice.
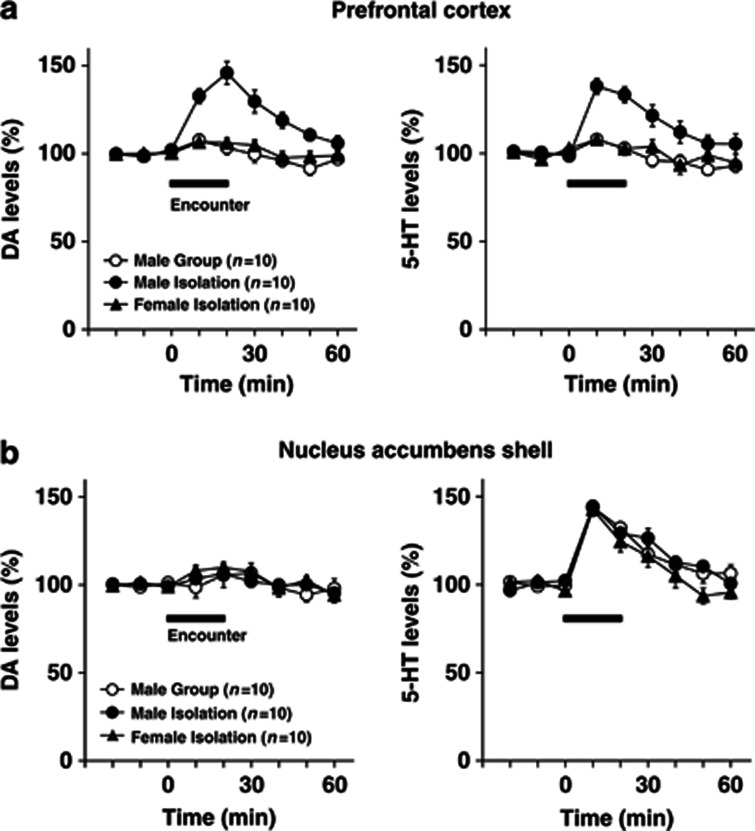
Figure 3.
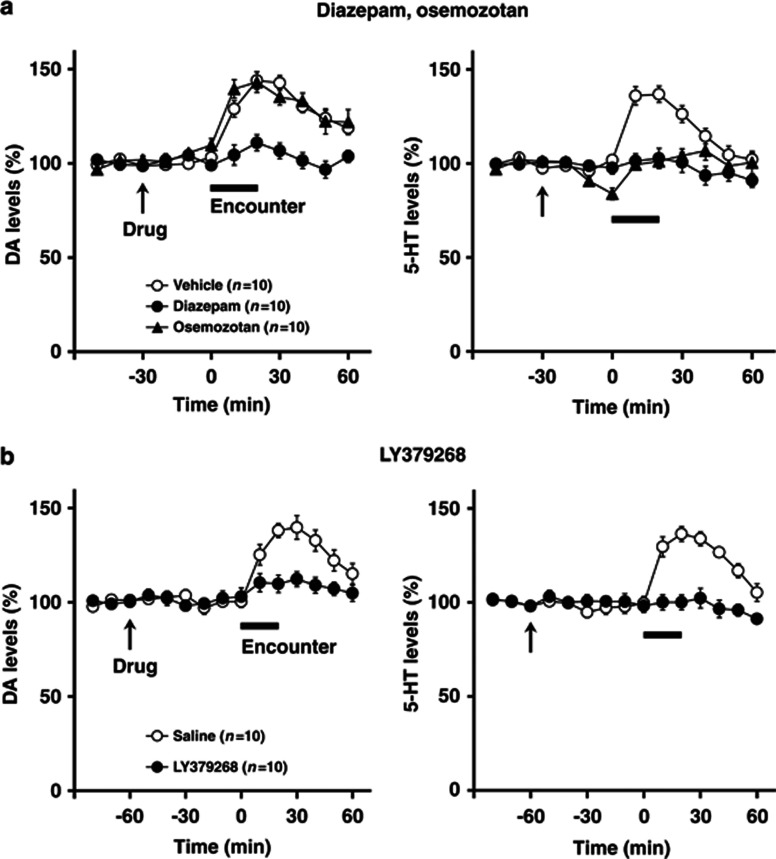
Effects of encounter stimulation on extracellular levels of DA and 5-HT in the prefrontal cortex (a) and nucleus accumbens shell (b) of mice. A male group- or isolation-reared mouse or female isolation-reared mouse was placed in a novel clear Plexiglas cage divided into two compartments by a mesh partition. After a 3-h habituation period, an unfamiliar intruder of the same sex as the resident was placed into the unoccupied compartment and the mice were allowed to interact for 20 min, as indicated by the horizontal bar. The data are expressed as the mean±SEM of 10 mice/group.
Social Encounter Stimulation and Behavioral Analysis
A 9-week-old male group-reared, male isolation-reared, or female isolation-reared mouse was placed in the large compartment of a novel clear Plexiglas cage (30 × 30 × 35 cm3) divided by a mesh partition from a smaller compartment, under an ambient lighting of 100 lux (Figure 1a left). This allowed the animal to see, hear, and smell, but not to contact the neighbor physically. After a 3-h habituation period, an unfamiliar 9-week-old ddY mouse was introduced into the unoccupied small compartment as an intruder (male resident vs male intruder, and female resident vs female intruder). The resident and intruder mice were allowed to interact through the partition for 20 min, and then the intruder mouse was removed. To characterize the encounter stimulation, we also examined the effects of the introduction of a male intruder mouse under pentobarbital (40 mg/kg) anesthesia or a novel object (golf ball) for 20 min. The behaviors of the resident mouse were videotaped, and its locomotor path was automatically analyzed off-line using the ANY-maze video tracking software (Stoelting Company, Wood Dale, IL). The total time spent and locomotor activity (movement time) near the partition of the resident mouse (Figure 1a right; gray zone) was used to estimate the behavioral reactivity of mice to the intruder. The interaction times between the resident and intruder mice (time spent smelling, putting one or two paws on the partition, and putting noses into the holes toward the intruder) were also analyzed.
c-Fos Immunohistochemistry
The neuronal-activity-marker c-Fos was determined 2 h after the encounter in the brains of male group- and isolation-reared mice, as described previously (Ago et al, 2011; Koda et al, 2010). Mice that were not exposed to an intruder were used as controls (Figure 2). Each group- or isolation-reared mouse was deeply anesthetized with pentobarbital and perfused transcardially with saline, followed by a solution of 4% paraformaldehyde in phosphate-buffered saline (PBS). The brain was fixed with 4% paraformaldehyde in PBS over 1 day and then transferred to 20% sucrose in PBS for 2 days. Serial 20-μm-thick coronal sections containing the prefrontal cortex (+2.0 through +1.8 mm with respect to bregma), nucleus accumbens (+1.1 through+0.9 mm with respect to bregma), amygdala (−1.3 through −1.5 mm with respect to bregma), ventral tegmental area (−2.8 through −3.0 mm with respect to bregma), and dorsal raphe nucleus (−4.4 through −4.6 mm with respect to bregma) were cut using a cryostat microtome at −20 °C. The free-floating sections were preincubated for 30 min in 0.3% hydrogen peroxide in PBS to remove endogenous peroxidase activity. The sections were washed in PBS and then incubated with anti-c-Fos rabbit polyclonal primary antibodies (1 : 1000 dilution; sc-52, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at room temperature. Subsequently, the primary incubation sections were washed in PBS and incubated in a secondary antibody solution containing biotinylated anti-rabbit IgG (1 : 500 dilution; Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. The sections were then incubated with avidin–biotin–horseradish peroxidase complex (Vectastain ABC kit; Vector Laboratories) for 30 min at room temperature. Brown cytosolic products were obtained by reaction with 3,3′-diaminobenzidine (Histofine SAB-PO (M) kit, Nichirei Bioscience, Tokyo, Japan). Sections were then mounted, dehydrated, and cover slipped. Four independent sections per animal containing the prefrontal cortex, nucleus accumbens, amygdala, dorsal raphe nucleus, or ventral tegmental area were selected. Then, c-Fos-positive nuclei were counted manually by experienced observers blinded to the rearing and encounter conditions of the animals, under bright-field illumination using a Keyence microscope (BIOREVO, BZ-9000) (Ago et al, 2011; Koda et al, 2010). The number of c-Fos-positive nuclei in each section was determined in a 500 × 500 μm2 area in the left and right hemispheres and averaged using an ImageJ 1.41 software package (NIH, Bethesda, MD, USA). The mean of this average across four sections was then calculated for each subject.
In Vivo Microdialysis
Each mouse was anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and stereotaxically implanted with a guide cannula (one site per animal) for a dialysis probe (Eicom Corp., Kyoto, Japan) targeting the prefrontal cortex (A +1.9 mm, L −0.5 mm, V −0.8 mm, from the bregma and skull) or nucleus accumbens shell (A +1.2 mm, L −0.5 mm, V −4.0 mm) (Franklin and Paxinos, 1997). The cannula was cemented in place with dental acrylic, and the animal was kept warm and allowed to recover from anesthesia. Postoperative analgesia was a single injection of buprenorphine (0.1 mg/kg, i.p.; Ago et al, 2008, 2011). The active probe membranes were 3- and 1-mm long in the prefrontal cortex and nucleus accumbens, respectively. Two days after the surgery, the probe was perfused with Ringer's solution (147.2 mM NaCl, 4.0 mM KCl, and 2.2 mM CaCl2, pH 6.0; Fuso Pharmaceutical Industries, Osaka, Japan) at a constant flow rate of 2 μl/min. A stabilization period of 3 h, which was identical to the 3-h habituation period in the clear Plexiglas cage described above, was established before the onset of the experiment. Microdialysis samples (20 μl) were collected every 10 min and injected immediately onto an HPLC column for simultaneous assay of DA and 5-HT, as previously reported (Ago et al, 2006, 2008). After the experiments, Evans Blue dye was microinjected through the cannula to histologically verify the position of the probe. Only data from animals with correct probe placements were used in the analysis.
Statistics
All results are presented as the mean±SEM. For the microdialysis experiments, the data were calculated as the percentage of change from basal dialysate concentrations, with 100% defined as the average of three samples before drug administration. These data were analyzed using two-way ANOVA for rearing/sex or treatment as the inter-subject factor and repeated measures with time as the intra-subject factor. For the c-Fos expression experiments, the data were analyzed using two-way ANOVA, followed by the Tukey–Kramer post-hoc test. For the behavioral experiments, the data were analyzed using Student's t-test or one-way ANOVA, followed by the Tukey–Kramer post hoc test. Statistical analyses were performed using the software package, Statview 5.0J, for Apple Macintosh (SAS Institute, Cary, NC). A P-value of <0.05 was considered statistically significant.
RESULTS
Effects of Encounter Stimulation on the Behavior of Mice
We first examined the effects of encounter stimulation on the behavior of mice. A group-reared male, isolation-reared male, or isolation-reared female mouse was placed in a large compartment of a novel clear Plexiglas cage divided by a mesh partition, allowing the animal to see, hear, and smell but not to contact the neighbor physically (Figure 1a). After a habituation period, an unfamiliar intruder mouse was introduced into the unoccupied small compartment. Figure 1b shows the representative locomotor paths of the resident mice during the 20-min encounters. These locomotor patterns indicate that male isolation-reared mice were restless and hyperexcitable during the encounters, compared with male group-reared mice and female isolation-reared mice. Locomotor activities (movement time) of male isolation-reared mice in the area near the partition (Figure 1a, gray zone) were significantly higher than those of male group-reared mice and female isolation-reared mice (F(2,27)=7.2, p<0.01; Figure 1c). Conversely, the total time spent in the gray zone by the resident mice (F(2,27)=0.6, p>0.05) and the total time the resident and intruder mice spent interacting (F(2,27)=0.1, p>0.05) did not differ between male group- and isolation-reared mice and female isolation-reared mice (Figure 1c).
Effects of Encounter Stimulation on Neuronal Activity
The neuronal-activity-marker c-Fos was determined 2 h after the social encounters in the prefrontal cortex, nucleus accumbens, amygdala, dorsal raphe nucleus, and ventral tegmental area of male group- and isolation-reared mice (Figure 2). Encounter stimulation caused a significant increase in the number of c-Fos-positive nuclei in the prefrontal cortex of male isolation-reared but not of male group-reared mice (F(1,28)=5.4, p<0.05; Figure 2a). Encounter stress increased c-Fos expression in the nucleus accumbens shell (F(1,28)=12.4, p<0.01; Figure 2b), dorsal raphe nucleus (F(1,28)=25.5, p<0.0001; Figure 2f), and ventral tegmental area (F(1,28)=19.9, p<0.001; Figure 2g) of both male group- and isolation-reared mice. The increase in c-Fos expression in the dorsal raphe nucleus (F(1,28)=8.4, p<0.01) and the ventral tegmental area (F(1,28)=7.8, p<0.01), but not in the nucleus accumbens shell (F(1,28)=0.8, p>0.05), of male isolation-reared mice was significantly higher than that in male group-reared mice. That is, the responses of the prefrontal cortex, dorsal raphe nucleus, and ventral tegmental area to encounter stimulation were higher in isolation-reared than in group-reared mice. Encounter stimulation did not affect c-Fos expression in the nucleus accumbens core or the central or basolateral nucleus of the amygdala (Figure 2c–e).
Effects of Encounter Stimulation on Extracellular DA and 5-HT Levels in the Prefrontal Cortex and Nucleus Accumbens Shell of Mice
We next examined the effects of social encounters on extracellular DA and 5-HT levels in the prefrontal cortex (Figure 3a) and nucleus accumbens shell (Figure 3b). The basal extracellular levels of DA and 5-HT in the prefrontal cortex and nucleus accumbens shell did not differ significantly between male group- and isolation-reared mice and female isolation-reared mice: the DA levels in the prefrontal cortex (the mean±SEM, n=10) were 0.38±0.07 (male group), 0.39±0.05 (male isolation), and 0.43±0.03 pg/20 μl (female isolation); the 5-HT levels in the prefrontal cortex (the mean±SEM, n=10) were 0.78±0.04 (male group), 0.72±0.08 (male isolation), and 0.76±0.13 pg/20 μl (female isolation); the DA levels in the nucleus accumbens shell (the mean±SEM, n=10) were 0.40±0.04 (male group), 0.39±0.07 (male isolation), and 0.39±0.07 pg/20 μl (female isolation); the 5-HT levels in the nucleus accumbens shell (the mean±SEM, n=10) were 0.32±0.08 (male group), 0.30±0.05 (male isolation), and 0.36±0.03 pg/20 μl (female isolation).
Encounter stress increased extracellular DA and 5-HT levels in the prefrontal cortex of male isolation-reared, but not of male group-reared or female isolation-reared, mice (significant interaction between the time and rearing/sex (F(10,135)=4.3, p<0.001 for DA; F(10,135)=3.4, p<0.001 for 5-HT); Figure 3a). Conversely, encounter stress increased extracellular 5-HT, but not DA, levels in the nucleus accumbens shell of all mice to a similar degree, as there was no significant difference in encounter-stress-induced 5-HT increases between the three groups (F(10,135)=1.0, p>0.05; Figure 3b).
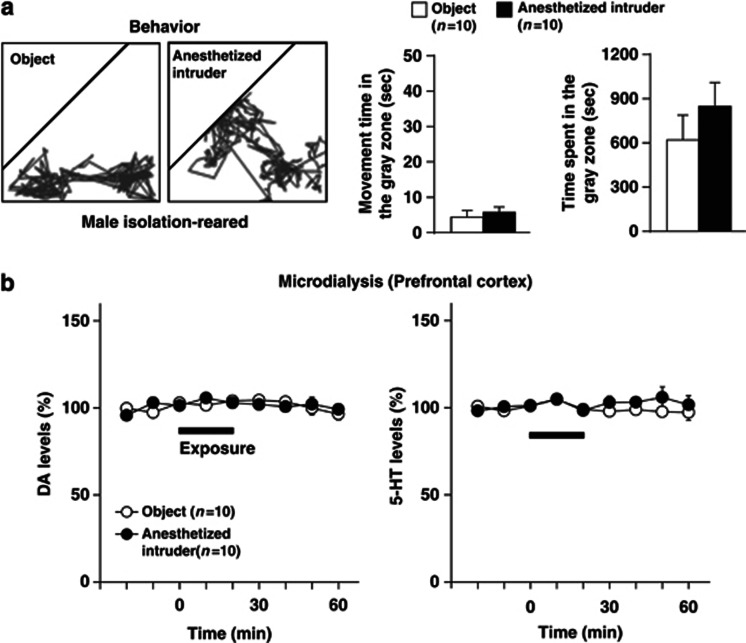
To characterize the encounter stress, we compared the effects of an encounter with a male intruder mouse with the introduction of a male intruder mouse under pentobarbital (40 mg/kg) anesthesia or a novel object (golf ball) for 20 min on the behaviors (Figure 4a) and prefrontal monoamine levels (Figure 4b) of the resident male isolation-reared mouse. In contrast to encounter with a conscious intruder mouse, male isolation-reared mice exhibited less activity and spent the most time in the area near the partition (gray zone) during either comparison condition. In addition, exposure to an anesthetized mouse or exposure to a novel object did not affect extracellular DA and 5-HT levels in the prefrontal cortex.
Figure 4.
Effects of exposure to an anesthetized intruder or a novel object on behaviors (a) and extracellular levels of DA and 5-HT in the prefrontal cortex (b) of male isolation-reared mice. A male isolation-reared mouse was placed in a novel clear Plexiglas cage divided into two compartments by a mesh partition. After a 3-h habituation period, a pentobarbital-anesthetized (40 mg/kg, i.p.) male mouse or a novel object (golf ball) was placed into the unoccupied compartment for 20 min. (a) Representative locomotor paths of the resident mice during the 20-min exposure are shown in left panels. The locomotor activity of and total time spent in the gray zone by the resident mice during the exposure are shown in right panels. (b) The horizontal bars indicate the period of exposure to an anesthetized intruder or a novel object. The data are expressed as the mean±SEM of 10 mice/group.
Effects of Diazepam, Osemozotan, and LY379268 on Encounter-Induced Behavioral Changes in Male Isolation-Reared Mice
Encounter stimulation induced hyperactivity in male isolation-reared mice, suggesting that this hyperactivity is a behavioral response to an emotional state, such as anxiety or fear. Previous studies have shown that gamma-aminobutyric acid A (GABAA) receptor agonists, 5-HT1A receptor agonists, and mGlu2/3 receptor agonists attenuate isolation-rearing-induced aggressive behaviors and/or anxiety-like behaviors (Ago et al, 2012; Da Silva et al, 1996; Maisonnette et al, 1993; Sakaue et al, 2001; Sánchez et al, 1993; Wongwitdecha and Marsden, 1996). Thus, we next examined the effects of the GABAA receptor agonist diazepam, the 5-HT1A receptor agonist osemozotan, and the mGlu2/3 receptor agonist LY379268 on encounter-induced behavioral changes in male isolation-reared mice (Figure 5). Figure 5a and c show representative locomotor paths of drug-treated male isolation-reared mice during the 20-min encounters. Diazepam (1 mg/kg, i.p.), osemozotan (0.3 mg/kg, i.p.) (F(2,27)=6.7, p<0.01), and LY379268 (1 mg/kg, i.p.) (p<0.05 by Student's t-test) inhibited encounter-induced hyperactivity. None of these drugs affected the total time spent in the gray zone or the total time the resident and intruder mice spent interacting (Figure 5b and d).
Figure 5.
Effects of diazepam, osemozotan (upper panels), and LY379268 (lower panels) on encounter-induced behavioral changes in male isolation-reared mice. A male isolation-reared mouse was placed into the large compartment of a novel clear Plexiglas cage divided by a mesh partition. After a 3-h habituation period, the resident mice were intraperitoneally injected with vehicle, diazepam (1 mg/kg), or osemozotan (0.3 mg/kg) 30 min before the encounter with an unfamiliar male intruder for 20 min. Saline or LY379268 (1 mg/kg) was intraperitoneally injected 60 min before the encounter. (a, c) Representative locomotor paths of the resident mice during the 20-min encounters are shown. (b, d) The locomotor activity of and total time spent in the gray zone by the resident mice and the total time the resident and intruder mice spent interacting were analyzed according to the description in the Materials and Methods. The data are expressed as the mean±SEM of 10 mice/group. *p<0.05, **p<0.01, compared with the vehicle or saline-treated mice.
Effects of Diazepam, Osemozotan, and LY379268 on Encounter-Induced c-Fos Expression in the Brain
We examined the effects of diazepam, osemozotan, and LY379268 on encounter-stress-induced c-Fos expression in the prefrontal cortex, nucleus accumbens shell, dorsal raphe nucleus, and ventral tegmental area of male group- and isolation-reared mice (Figure 6). The increases in prefrontal c-Fos expression during encounter stress were blocked by diazepam (1 mg/kg, i.p.), osemozotan (0.3 mg/kg, i.p.; significant interaction between the rearing and treatment: F(2,30)=5.8, p<0.01), and LY379268 (1 mg/kg, i.p.; F(1,20)=4.7, p<0.05) in male isolation-reared mice (Figure 6a). None of the drugs affected prefrontal c-Fos expression in male group-reared mice. Conversely, the increases in accumbal c-Fos expression during encounter stress were not blocked by diazepam, osemozotan, or LY379268 in either group (Figure 6b). All three drugs inhibited encounter-stress-induced increases in c-Fos expression in the dorsal raphe nucleus of male isolation-reared, but not of group-reared, mice (F(2,30)=7.8, p<0.01 for diazepam and osemozotan; F(1,20)=4.8, p<0.05 for LY379268; Figure 6c). Diazepam and LY379268, but not osemozotan, inhibited encounter-stress-induced increases in c-Fos expression in the ventral tegmental area of male isolation-reared mice (Figure 6d).
Figure 6.
Effects of diazepam, osemozotan (left panels), and LY379268 (right panels) on encounter-induced c-Fos expression in the prefrontal cortex (a), nucleus accumbens shell (b), dorsal raphe nucleus (c), and ventral tegmental area (d) of male group- and isolation-reared mice. A mouse was placed into the large compartment of a novel clear Plexiglas cage divided by a mesh partition. After a 3-h habituation period, the resident mice were intraperitoneally injected with vehicle, diazepam (1 mg/kg), or osemozotan (0.3 mg/kg) 30 min before the encounter with an unfamiliar male intruder for 20 min. Saline or LY379268 (1 mg/kg) was intraperitoneally injected 60 min before the encounter. Immunohistochemical localization of the neuronal-activity-marker c-Fos was determined 2 h after the encounter. The data are expressed as the mean±SEM of 6 mice/group. *p<0.05, **p<0.01, compared with the vehicle or saline-treated group-reared mice. †p<0.05, ††p<0.01, compared with the vehicle or saline-treated isolation-reared mice.
Effects of Diazepam, Osemozotan, and LY379268 on Encounter-Induced Increases in Prefrontal DA and 5-HT and Accumbal 5-HT Levels in Male Isolation-Reared Mice
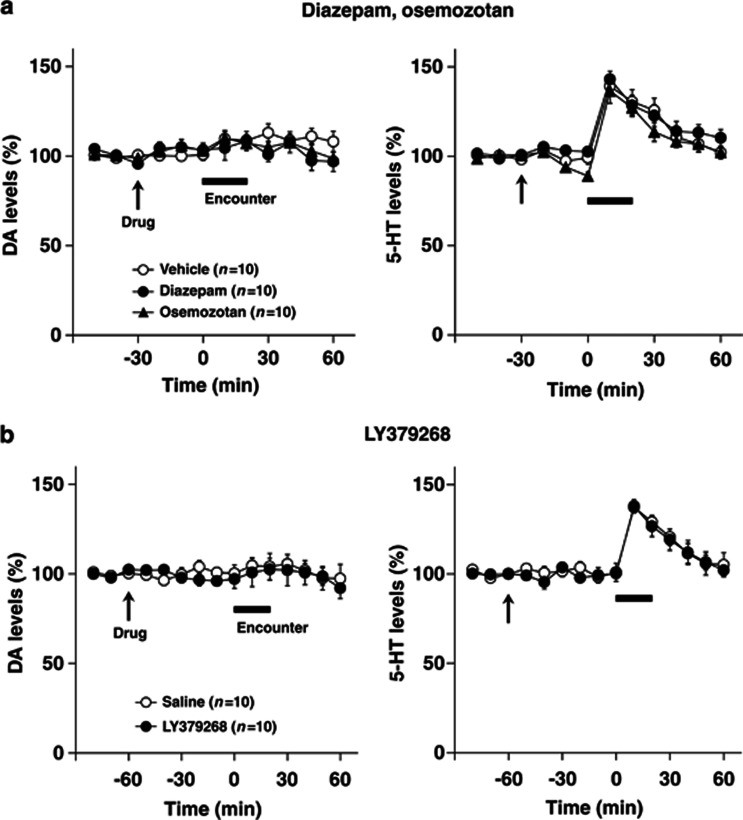
To investigate further the possible involvement of encounter-induced neurochemical signal in induction of abnormal behaviors in male isolation-reared mice, we examined the effects of diazepam, osemozotan, and LY379268 on encounter-induced increases in prefrontal DA and 5-HT levels (Figure 7) and accumbal 5-HT levels (Figure 8), respectively. In the prefrontal cortex, both diazepam (1 mg/kg, i.p.) and LY379268 (1 mg/kg, i.p.) blocked the increases in DA (F(5,90)=2.4, p<0.05 for diazepam; F(5,90)=3.8, p<0.01 for LY379268) and 5-HT (F(5,90)=5.4, p<0.001 for diazepam; F(5,90)=3.2, p<0.01 for LY379268) (from 0 to 60 min) levels seen in male isolation-reared mice during encounter stress. By contrast, osemozotan (0.3 mg/kg, i.p.) blocked encounter-stress-induced increases in 5-HT (F(5,90)=8.6, p<0.001), but not DA (F(5,90)=1.2, p>0.05), levels (from 0 to 60 min) in male isolation-reared mice. Basal extracellular levels of DA and 5-HT were not affected by either diazepam (from −30 to 0 min) or LY379268 (from −60 to 0 min), whereas basal extracellular levels of 5-HT (F(2,36)=11.8, p<0.001), but not DA (from −30 to 0 min), were slightly decreased by osemozotan. In contrast to the prefrontal cortex, diazepam, osemozotan, and LY379268 did not affect encounter-stress-induced increases in extracellular 5-HT levels in the nucleus accumbens shell of male isolation-reared mice (from 0 to 60 min; Figure 8). Basal extracellular levels of 5-HT were not affected by either diazepam (from −30 to 0 min) or LY379268 (from −60 to 0 min), whereas those of 5-HT were slightly decreased by osemozotan in male isolation-reared mice (F(2,36)=3.5, p<0.05; from −30 to 0 min). Basal extracellular levels of DA were not affected by any of the drugs tested.
Figure 7.
Effects of diazepam and osemozotan (a), and LY379268 (b)on encounter-induced increases in DA and 5-HT levels in the prefrontal cortex of male isolation-reared mice. A male isolation-reared mouse was placed into the large compartment of a novel clear Plexiglas cage divided by a mesh partition. After a 3-h habituation period, the resident mice were intraperitoneally injected with vehicle, diazepam (1 mg/kg), or osemozotan (0.3 mg/kg) 30 min before the encounter (a) and were intraperitoneally injected with saline or LY379268 (1 mg/kg) 60 min before the encounter (b) (arrow). After the drug treatment, an unfamiliar male intruder was placed into the unoccupied compartment and the mice were allowed to interact for 20 min, as indicated by the horizontal bar. The data are expressed as the mean±SEM of 10 mice/group.
Figure 8.
Effects of diazepam and osemozotan (a), and LY379268 (b) on encounter-induced increases in extracellular 5-HT levels in the nucleus accumbens shell of male isolation-reared mice. A male isolation-reared mouse was placed into the large compartment of a novel clear Plexiglas cage divided by a mesh partition. After a 3-h habituation period, the resident mice were intraperitoneally injected with vehicle, diazepam (1 mg/kg), or osemozotan (0.3 mg/kg) 30 min before the encounter (a) and were intraperitoneally injected with saline or LY379268 (1 mg/kg) 60 min before the encounter (b) (arrow). After the drug treatment, an unfamiliar male intruder was placed into the unoccupied compartment and the mice were allowed to interact for 20 min, as indicated by the horizontal bar. The data are expressed as the mean±SEM of 10 mice/group.
DISCUSSION
The present study examined whether encounter stimulation produces neurochemical changes that induce abnormal behaviors in isolation-reared mice. Previous studies in resident–intruder tests have shown that DA and 5-HT systems are involved in aggressive and defeat behaviors during the encounter (Anstrom et al, 2009; Ferrari et al, 2003; Tidey and Miczek, 1996; van Erp and Miczek, 2000). It appears that the effects of the encounter in resident-intruder tests are related to both psychological and physical stress. In this study, we designed the encounter stimulation to occur through a mesh partition. This experimental procedure avoids aggressive contact (ie, biting attacks, wrestling, lateral threats) from isolation-reared mice to an intruder mouse. However, the mice were able to see, hear, and smell their neighbor. Thus, a social encounter with an unfamiliar conspecific through a mesh partition is considered a form of psychological stress, and it is useful for studies of the neurochemical changes that may be involved in the abnormal behaviors of isolation-reared animals (Ago et al, 2012; Fone and Porkess, 2008; Lukkes et al, 2009a, 2009b; Pinna et al, 2008).
In this study, the total time that the resident mice spent near the partition and the total time the resident and intruder mice spent interacting did not differ between the male group-reared, female isolation-reared, and male isolation-reared mice (Figure 1c). This suggests that the attention of the resident mice to the intruder is similar between these three groups. Under the conditions used, we found that encounter stimulation increased locomotor activity in male isolation-reared mice but not in male group-reared or female isolation-reared mice (Figure 1b and c). Furthermore, we demonstrated that the encounter with the sleeping intruder or exposure to a novel object did not increase locomotor activity in male isolation-reared mice (Figure 4a). As social isolation does not induce aggressive behavior in female mice (Pinna et al, 2003, 2005, 2008), the present findings indicate that encounter-induced behavioral responses to emotional states, such as anxiety or fear, are observed selectively in male isolation-reared mice.
As the neural circuits that include the nucleus accumbens, amygdala, and prefrontal cortex are known to have crucial roles in emotions, motivation, and reward (Cardinal et al, 2002; Carlezon and Thomas, 2009; Ishikawa et al, 2008; Kalivas and Nakamura, 1999), we examined the effects of encounter stimulation on c-Fos expression in these brain regions. Encounter stimulation increased c-Fos expression in the nucleus accumbens shell of group- and isolation-reared mice to a similar degree. Activation of nucleus accumbens increases exploratory and social play behaviors in rats (Falowski et al, 2011; Trezza et al, 2011; van Kuyck et al, 2007). Thus, enhanced neuronal activity in the nucleus accumbens shell may contribute to social interactions in male group- and isolation-reared mice. It should be noted that encounter stimulation increased prefrontal c-Fos expression in male isolation-reared mice, whereas it did not affect the c-Fos expression in group-reared mice. Furthermore, isolation-reared male mice showed enhanced c-Fos expression in response to encounter stimulation in the ventral tegmental area and dorsal raphe nucleus. The prefrontal cortex is innervated by dopaminergic fibers from the ventral tegmental area and serotonergic fibers from dorsal raphe nucleus. Thus, it is likely that increased prefrontal neuronal activity via activation of the dorsal raphe nucleus and ventral tegmental area triggers abnormal behaviors in male isolation-reared mice.
In agreement with the results of c-Fos expression, encounter stimulation increased extracellular DA and 5-HT levels in the prefrontal cortex of male isolation-reared, but not male group-reared or female isolation-reared, mice. We found that an intruder anesthetized with pentobarbital or exposure to a novel object did not affect extracellular DA and 5-HT levels in the prefrontal cortex of male isolation-reared mice. This suggests that interactions between the resident mouse and an intruder mouse are necessary for these increases in prefrontal DA and 5-HT in male isolation-reared mice. Previous studies have shown that prefrontal DA levels are higher during or after an aggressive encounter in both attacking and defending rats (Anstrom et al, 2009; Tidey and Miczek, 1996; van Erp and Miczek, 2000). Furthermore, exposure to tests of ‘anxiety', such as the social interaction test, causes an increase in hippocampal 5-HT release in rats from the more anxious Wistar strain, but not in rats from the less anxious Sprague–Dawley strain (Rex et al, 2004). These studies suggest that neurochemical changes are involved in encounter-induced behaviors, although these neurochemical changes may be induced by both psychological and physical stress. Under these circumstances, we performed pharmacological experiments to examine whether encounter-induced increases in prefrontal DA and 5-HT release are indeed the neurochemical basis for induction of abnormal behaviors in male isolation-reared mice.
In these experiments, we used the GABAA receptor agonist diazepam, the 5-HT1A receptor agonist osemozotan, and the mGlu2/3 receptor agonist LY379268. Diazepam is a potent anxiolytic (do-Rego et al, 2006) and attenuates aggressive behavior (Sakaue et al, 2001; Wongwitdecha and Marsden, 1996) and anxiety-like behavior in the elevated plus-maze (Da Silva et al, 1996) in isolation-reared rodents. Osemozotan and mGlu2/3 receptor agonists also inhibit aggressive behavior and anxiety-like behavior in the elevated plus-maze, and they reverse the deficits of prepulse inhibition in isolation-reared animals (Ago et al, 2012; Maisonnette et al, 1993; Sakaue et al, 2001, 2003; Sánchez et al, 1993). The present study demonstrated that encounter-induced hyperactivity and increased prefrontal 5-HT levels were blocked by diazepam, osemozotan, and LY379268. In contrast, encounter-induced increases in prefrontal DA levels were blocked by diazepam and LY379268, but not osemozotan. Consistent with these findings, encounter-induced increases in c-Fos expression in the prefrontal cortex and dorsal raphe nucleus were also blocked by all the three drugs in male isolation-reared mice. Concerning the mechanisms for the effects of the drugs, serotonergic neurons in the dorsal raphe nucleus are regulated not only by presynaptic 5-HT1A receptors but also by inhibitory GABA and excitatory glutamate neurons (Celada et al, 2001; Liu et al, 2000), and mGlu2/3 receptor agonists may inhibit glutamate release (Cartmell and Schoepp, 2000). In the ventral tegmental area, dopaminergic neurons are regulated by the inhibitory and excitatory neurons (Geisler and Wise, 2008). Then, it is conceivable that diazepam and LY379268 inhibit prefrontal 5-HT and DA release and osemozotan inhibits prefrontal 5-HT release. Furthermore, we found that encounter stimulation increased extracellular 5-HT, but not DA, levels in the nucleus accumbens shell of all the three groups. However, none of the drugs affected encounter-induced 5-HT release or c-Fos expression in the nucleus accumbens shell of male isolation-reared mice. Taken together, it is likely that prefrontal 5-HT release is the signal that induces abnormal behaviors in response to encounter stimulation in male isolation-reared mice.
In conclusion, this study demonstrates that male isolation-reared mice show enhanced encounter-induced increases in c-Fos expression in the prefrontal cortex, dorsal raphe nucleus, and ventral tegmental area. Consistent with these observations, microdialysis showed that the encounter stimulation increased prefrontal DA and 5-HT levels in male isolation-reared mice. In addition, we found that the increased prefrontal 5-HT levels were blocked by diazepam, osemozotan, and LY379268, whereas increased prefrontal DA levels were only blocked by diazepam and LY379268. Taken together, it is likely that encounter stimulation specifically activates prefrontal DA and 5-HT systems, and that the 5-HT system has a key role in the encounter-induced behaviors in male isolation-reared mice. We also speculate that the activation of prefrontal 5-HT system triggers the induction of some abnormal behaviors in isolation-reared mice under conditions of psychological stress, such as anxiety-related tests and a startle test (prepulse inhibition). As isolation-reared rodents are considered to be a model of schizophrenia, anxiety, or mood disorders, the present findings imply that serotonergic projections from the dorsal raphe to the frontal cortex are a potential target for treatment of psychiatric disorders.
Acknowledgments
This study was supported, in part, by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (from the Ministry of Education, Culture, Sports, Science and Technology of Japan).
All authors declare no conflict of interest.
References
- Ago Y, Araki R, Yano K, Kawasaki T, Chaki S, Nakazato A, et al. The selective metabotropic glutamate 2/3 receptor agonist MGS0028 reverses isolation rearing-induced abnormal behaviors in mice. J Pharmacol Sci. 2012;118:295–298. doi: 10.1254/jphs.11200sc. [DOI] [PubMed] [Google Scholar]
- Ago Y, Arikawa S, Yata M, Yano K, Abe M, Takuma K, et al. Antidepressant-like effects of the glucocorticoid receptor antagonist RU-43044 are associated with changes in prefrontal dopamine in mouse models of depression. Neuropharmacology. 2008;55:1355–1363. doi: 10.1016/j.neuropharm.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Uda M, Kajii Y, Abe M, Baba A, et al. Attenuation by the 5-HT1A receptor agonist osemozotan of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacology. 2006;51:914–922. doi: 10.1016/j.neuropharm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Ago Y, Sakaue M, Baba A, Matsuda T. Selective reduction by isolation rearing of 5-HT1A receptor-mediated dopamine release in vivo in the frontal cortex of mice. J Neurochem. 2002;83:353–359. doi: 10.1046/j.1471-4159.2002.01128.x. [DOI] [PubMed] [Google Scholar]
- Ago Y, Takahashi K, Nakamura S, Hashimoto H, Baba A, Matsuda T. Anxiety-like and exploratory behaviors of isolation-reared mice in the staircase test. J Pharmacol Sci. 2007;104:153–158. doi: 10.1254/jphs.fp0070325. [DOI] [PubMed] [Google Scholar]
- Ago Y, Yano K, Hiramatsu N, Takuma K, Matsuda T. Fluvoxamine enhances prefrontal dopaminergic neurotransmission in adrenalectomized/castrated mice via both 5-HT reuptake inhibition and σ1 receptor activation. Psychopharmacology (Berl) 2011;217:377–386. doi: 10.1007/s00213-011-2293-5. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Ventura R, Puglisi-Allegra S. Opposite imbalances between mesocortical and mesoaccumbens dopamine responses to stress by the same genotype depending on living conditions. Behav Brain Res. 2002;129:179–185. doi: 10.1016/s0166-4328(01)00339-4. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carlezon JrWA, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56 (Suppl 1:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva NL, Ferreira VM, Carobrez Ade P, Morato GS. Individual housing from rearing modifies the performance of young rats on the elevated plus-maze apparatus. Physiol Behav. 1996;60:1391–1396. doi: 10.1016/s0031-9384(96)00254-5. [DOI] [PubMed] [Google Scholar]
- do-Rego JC, Viana AF, Le Maître E, Deniel A, Rates SM, Leroux-Nicollet I, et al. Comparisons between anxiety tests for selection of anxious and non anxious mice. Behav Brain Res. 2006;169:282–288. doi: 10.1016/j.bbr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Eells JB, Misler JA, Nikodem VM. Early postnatal isolation reduces dopamine levels, elevates dopamine turnover and specifically disrupts prepulse inhibition in Nurr1-null heterozygous mice. Neuroscience. 2006;140:1117–1126. doi: 10.1016/j.neuroscience.2005.12.065. [DOI] [PubMed] [Google Scholar]
- Falowski SM, Sharan A, Reyes BA, Sikkema C, Szot P, Van Bockstaele EJ. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery. 2011;69:1281–1290. doi: 10.1227/NEU.0b013e3182237346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA, USA; 1997. [Google Scholar]
- Fulford AJ, Marsden CA. Effect of isolation-rearing on conditioned dopamine release in vivo in the nucleus accumbens of the rat. J Neurochem. 1998a;70:384–390. doi: 10.1046/j.1471-4159.1998.70010384.x. [DOI] [PubMed] [Google Scholar]
- Fulford AJ, Marsden CA. Conditioned release of 5-hydroxytryptamine in vivo in the nucleus accumbens following isolation-rearing in the rat. Neuroscience. 1998b;83:481–487. doi: 10.1016/s0306-4522(97)00423-5. [DOI] [PubMed] [Google Scholar]
- Fulford AJ, Marsden CA. An intact dopaminergic system is required for context-conditioned release of 5-HT in the nucleus accumbens of postweaning isolation-reared rats. Neuroscience. 2007;149:392–400. doi: 10.1016/j.neuroscience.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Hall FS, Perona MT. Have studies of the developmental regulation of behavioral phenotypes revealed the mechanisms of gene-environment interactions. Physiol Behav. 2012;107:623–640. doi: 10.1016/j.physbeh.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, et al. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, et al. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155:573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Brown AM, Auer DP, Fone KC. The mGluR2/3 agonist LY379268 reverses post-weaning social isolation-induced recognition memory deficits in the rat. Psychopharmacology (Berl) 2011;214:269–283. doi: 10.1007/s00213-010-1931-7. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T. Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem. 2010;114:259–270. doi: 10.1111/j.1471-4159.2010.06750.x. [DOI] [PubMed] [Google Scholar]
- Koda K, Ago Y, Kawasaki T, Hashimoto H, Baba A, Matsuda T. Galantamine and donepezil differently affect isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2008;196:293–301. doi: 10.1007/s00213-007-0962-1. [DOI] [PubMed] [Google Scholar]
- Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, et al. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res. 2009;202:114–121. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Kovács KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33:13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Lukkes J, Vuong S, Scholl J, Oliver H, Forster G. Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J Neurosci. 2009b;29:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav. 2009a;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL.2009cConsequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents Front Behav Neurosci 3Article18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonnette S, Morato S, Brandão ML. Role of resocialization and of 5-HT1A receptor activation on the anxiogenic effects induced by isolation in the elevated plus-maze test. Physiol Behav. 1993;54:753–758. doi: 10.1016/0031-9384(93)90087-v. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press: Washington, DC, USA; 1996. [Google Scholar]
- Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. Proc Natl Acad Sci USA. 2005;102:2135–2140. doi: 10.1073/pnas.0409643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Gustedt C, Beckett S, Fink H. Anxiolytic-like profile in Wistar, but not Sprague-Dawley rats in the social interaction test. Psychopharmacology (Berl) 2004;177:23–34. doi: 10.1007/s00213-004-1914-7. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Baba A, Matsuda T. The 5-HT1A receptor agonist MKC-242 reverses isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2003;170:73–79. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Murakami C, Sowa C, Sakamoto Y, Koyama Y, et al. Involvement of benzodiazepine binding sites in an antiaggressive effect by 5-HT1A receptor activation in isolated mice. Eur J Pharmacol. 2001;432:163–166. doi: 10.1016/s0014-2999(01)01511-4. [DOI] [PubMed] [Google Scholar]
- Sánchez C, Arnt J, Hyttel J, Moltzen EK. The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology (Berl) 1993;110:53–59. doi: 10.1007/BF02246950. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci. 2011;31:6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuyck K, Gabriëls L, Cosyns P, Arckens L, Sturm V, Rasmussen S, et al. Behavioural and physiological effects of electrical stimulation in the nucleus accumbens: a review. Acta Neurochir Suppl. 2007;97 (Pt 2:375–391. doi: 10.1007/978-3-211-33081-4_43. [DOI] [PubMed] [Google Scholar]
- Varty GB, Powell SB, Lehmann-Masten V, Buell MR, Geyer MA. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behav Brain Res. 2006;169:162–167. doi: 10.1016/j.bbr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zu X, Luo W, Yang H, Luo G, Zhang M, et al. Social isolation produces anxiety-like behaviors and changes PSD-95 levels in the forebrain. Neurosci Lett. 2012;514:27–30. doi: 10.1016/j.neulet.2012.02.043. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N, et al. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1173–1177. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]