Abstract
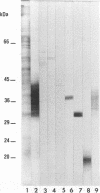
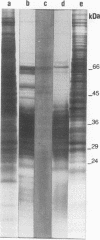
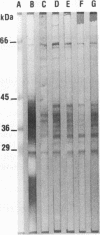
The antigen complex of A60 of Mycobacterium bovis BCG was analyzed by different immunological techniques to assess its relevance to tuberculosis and the involvement of its components in the immune reactions elicited in humans by tuberculous infection. A60 is composed of about 30 components, of which 8 were identified by available monoclonal antibodies (lipoarabinomannan, a glycolipid, and proteins of 65, 40, 38, 35, 19, and 14 kDa). The majority (87.5%) of anti-mycobacterial antibodies in sera from tuberculosis patients was directed against A60. Western blot (immunoblot) analysis indicated that the majority of the highly antigenic proteins present in mycobacterial homogenates were components of the A60 complex. A small percentage (7.8%) of A60 epitopes proved to be species specific. Thus, A60 proteins of 66, 41, 38, 37, 35, 34, 32, and 22 kDa were found to contain B-cell epitopes specific for M. bovis and not shared by Mycobacterium leprae oR Mycobacterium avium.
Full text
PDF
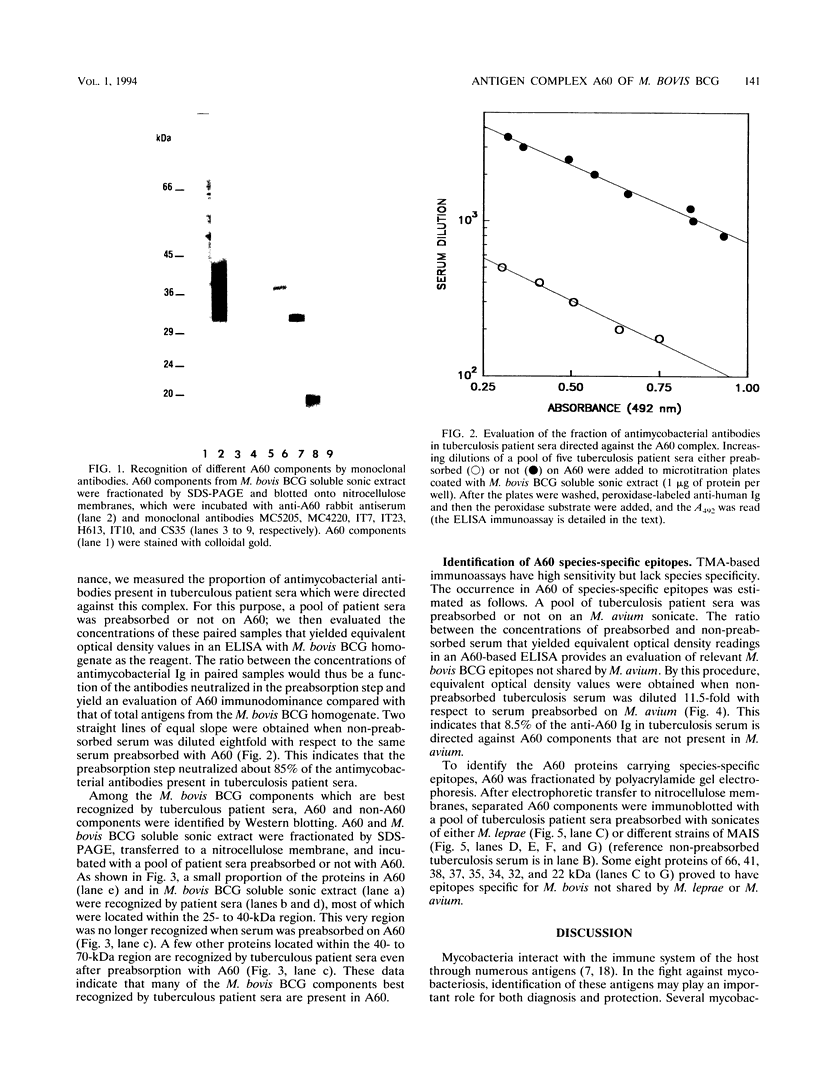
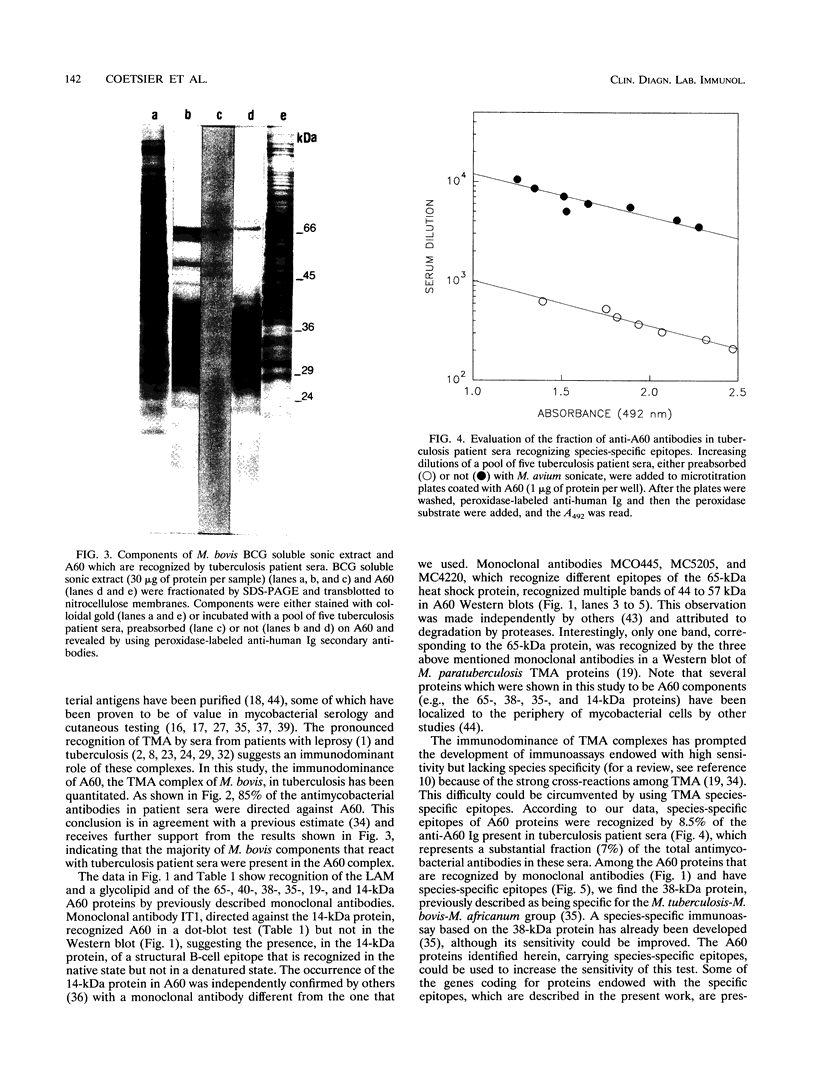


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H., Harboe M., Closs O., Godal T. BCG antibody profiles in tuberculoid and lepromatous leprosy. Infect Immun. 1974 May;9(5):952–958. doi: 10.1128/iai.9.5.952-958.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baelden M. C., Vanderelst B., Dieng M., Prignot J., Cocito C. Serological analysis of human tuberculosis by an ELISA with mycobacterial antigen 60. Scand J Infect Dis. 1990;22(1):63–73. doi: 10.3109/00365549009023121. [DOI] [PubMed] [Google Scholar]
- Benoit C., Beschin A., Desmecht M., Dekeyser P., Cocito C. Delayed hypersensitivity reactions by the mycobacterial antigen A60 and cutaneous testing in tuberculosis. Med Microbiol Immunol. 1989;178(2):105–112. doi: 10.1007/BF00203306. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruneteau M., Perret J., Vanlinden F., Michel G., Cocito C. Composition and immunogenicity of the polysaccharide components of the thermostable macromolecular antigen group of mycobacterial antigens. Med Microbiol Immunol. 1992;181(1):13–23. doi: 10.1007/BF00193392. [DOI] [PubMed] [Google Scholar]
- Carlucci S., Beschin A., Tuosto L., Ameglio F., Gandolfo G. M., Cocito C., Fiorucci F., Saltini C., Piccolella E. Mycobacterial antigen complex A60-specific T-cell repertoire during the course of pulmonary tuberculosis. Infect Immun. 1993 Feb;61(2):439–447. doi: 10.1128/iai.61.2.439-447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparas S. D. The immunology of mycobacterial infections. Crit Rev Microbiol. 1982;9(2):139–197. doi: 10.3109/10408418209104488. [DOI] [PubMed] [Google Scholar]
- Charpin D., Herbault H., Gevaudan M. J., Saadjian M., de Micco P., Arnaud A., Vervloet D., Charpin J. Value of ELISA using A60 antigen in the diagnosis of active pulmonary tuberculosis. Am Rev Respir Dis. 1990 Aug;142(2):380–384. doi: 10.1164/ajrccm/142.2.380. [DOI] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Cocito C. G. Properties of the mycobacterial antigen complex A60 and its applications to the diagnosis and prognosis of tuberculosis. Chest. 1991 Dec;100(6):1687–1693. doi: 10.1378/chest.100.6.1687. [DOI] [PubMed] [Google Scholar]
- Cocito C., Baelden M. C., Benoit C. Immunological properties of antigen 60 of BCG. Induction of humoral and cellular immune reactions. Scand J Immunol. 1987 Jun;25(6):579–585. doi: 10.1111/j.1365-3083.1987.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Cocito C., Vanlinden F. Metabolism of the TMA group of antigens during the growth cycle of mycobacteria. Med Microbiol Immunol. 1988;177(6):357–367. doi: 10.1007/BF02389908. [DOI] [PubMed] [Google Scholar]
- Cocito C., Vanlinden F. Preparation and properties of antigen 60 from Mycobacterium bovis BCG. Clin Exp Immunol. 1986 Nov;66(2):262–272. [PMC free article] [PubMed] [Google Scholar]
- Cocito C., Vanlinden F. Subcellular localisation and sedimentation behaviour of antigen 60 from Mycobacterium bovis BCG. Med Microbiol Immunol. 1988;177(1):15–25. doi: 10.1007/BF00190307. [DOI] [PubMed] [Google Scholar]
- Damiani G., Biano A., Beltrame A., Vismara D., Mezzopreti M. F., Colizzi V., Young D. B., Bloom B. R. Generation and characterization of monoclonal antibodies to 28-, 35-, and 65-kilodalton proteins of Mycobacterium tuberculosis. Infect Immun. 1988 May;56(5):1281–1287. doi: 10.1128/iai.56.5.1281-1287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Debanne S. M. The serodiagnosis of tuberculosis and other mycobacterial diseases by enzyme-linked immunosorbent assay. Am Rev Respir Dis. 1987 May;135(5):1137–1151. doi: 10.1164/arrd.1987.135.5.1137. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Debanne S. M., van der Kuyp F. Enzyme-linked immunosorbent assay using Mycobacterium tuberculosis antigen 5 and PPD for the serodiagnosis of tuberculosis. Chest. 1985 Sep;88(3):388–392. doi: 10.1378/chest.88.3.388. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kesel M., Gilot P., Coene M., Cocito C. Composition and immunological properties of the protein fraction of A36, a major antigen complex of Mycobacterium paratuberculosis. Scand J Immunol. 1992 Aug;36(2):201–212. doi: 10.1111/j.1365-3083.1992.tb03092.x. [DOI] [PubMed] [Google Scholar]
- Fabre I., L'Homme O., Bruneteau M., Michel G., Cocito C. Chemical composition of antigen 60 from Mycobacterium bovis BCG. Scand J Immunol. 1986 Nov;24(5):591–602. doi: 10.1111/j.1365-3083.1986.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Fadda G., Grillo R., Ginesu F., Santoru L., Zanetti S., Dettori G. Serodiagnosis and follow up of patients with pulmonary tuberculosis by enzyme-linked immunosorbent assay. Eur J Epidemiol. 1992 Jan;8(1):81–87. doi: 10.1007/BF03334976. [DOI] [PubMed] [Google Scholar]
- Gevaudan M. J., Bollet C., Charpin D., Mallet M. N., De Micco P. Serological response of tuberculosis patients to antigen 60 of BCG. Eur J Epidemiol. 1992 Sep;8(5):666–676. doi: 10.1007/BF00145382. [DOI] [PubMed] [Google Scholar]
- Gilot P., Cocito C. Comparative analysis of three sensitins used in cutaneous testing for tuberculosis and paratuberculosis in cattle. FEMS Microbiol Lett. 1993 Jul 1;110(3):307–311. doi: 10.1111/j.1574-6968.1993.tb06340.x. [DOI] [PubMed] [Google Scholar]
- Gilot P., De Kesel M., Coene M., Cocito C. Induction of cellular immune reactions by A36, an antigen complex of Mycobacterium paratuberculosis: comparison of A36 and johnin components. Scand J Immunol. 1992 Dec;36(6):811–821. doi: 10.1111/j.1365-3083.1992.tb03143.x. [DOI] [PubMed] [Google Scholar]
- Gilot P., De Kesel M., Machtelinckx L., Coene M., Cocito C. Isolation and sequencing of the gene coding for an antigenic 34-kilodalton protein of Mycobacterium paratuberculosis. J Bacteriol. 1993 Aug;175(15):4930–4935. doi: 10.1128/jb.175.15.4930-4935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange J. M. The humoral immune response in tuberculosis: its nature, biological role and diagnostic usefulness. Adv Tuberc Res. 1984;21:1–78. [PubMed] [Google Scholar]
- Gulletta E., Del Pezzo M., Sanduzzi A., Bariffi F., Covelli I. Serodiagnosis survey of tuberculosis by a new ELISA method. Eur J Epidemiol. 1988 Sep;4(3):331–334. doi: 10.1007/BF00148920. [DOI] [PubMed] [Google Scholar]
- Gunnarsson E., Fodstad F. H. Analysis of antigens in Mycobacterium paratubercuolsis. Acta Vet Scand. 1979;20(2):200–215. doi: 10.1186/BF03546612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjorvatn B., Bjune G. Antibodies against BCG antigen 60 in mycobacterial infection. Br Med J. 1977 Aug 13;2(6084):430–433. doi: 10.1136/bmj.2.6084.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Closs O., Bjorvatn B., Kronvall G., Axelsen N. H. Antibody response in rabbits to immunization with Mycobacterium leprae. Infect Immun. 1977 Dec;18(3):792–805. doi: 10.1128/iai.18.3.792-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Closs O., Svindahl K., Deverill J. Production and assay of antibodies against one antigenic component of Mycobacterium bovis BCG. Infect Immun. 1977 May;16(2):662–672. doi: 10.1128/iai.16.2.662-672.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Wiker H. G. The 38-kDa protein of Mycobacterium tuberculosis: a review. J Infect Dis. 1992 Oct;166(4):874–884. doi: 10.1093/infdis/166.4.874. [DOI] [PubMed] [Google Scholar]
- Hubbard R. D., Flory C. M., Collins F. M., Cocito C. Immunization of mice with the antigen A60 of Mycobacterium bovis BCG. Clin Exp Immunol. 1992 Apr;88(1):129–131. doi: 10.1111/j.1365-2249.1992.tb03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanolkar-Young S., Kolk A. H., Andersen A. B., Bennedsen J., Brennan P. J., Rivoire B., Kuijper S., McAdam K. P., Abe C., Batra H. V. Results of the third immunology of leprosy/immunology of tuberculosis antimycobacterial monoclonal antibody workshop. Infect Immun. 1992 Sep;60(9):3925–3927. doi: 10.1128/iai.60.9.3925-3927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melsom R., Naafs B., Harboe M., Closs O. Antibody activity against Mycobacterium leprae antigen 7 during the first year of DDS treatment in lepromatous (BL-LL) leprosy. Lepr Rev. 1978 Mar;49(1):17–29. [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop on monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1985 May;48(2):603–605. doi: 10.1128/iai.48.2.603-605.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara D., Mezzopreti M. F., Gilardini Montani M. S., Gilardini M. S., Del Porto P., Lombardi G., Piccolella E., Damiani G., Rappuoli R., Colizzi V. Identification of a 35-kilodalton Mycobacterium tuberculosis protein containing B- and T-cell epitopes. Infect Immun. 1990 Jan;58(1):245–251. doi: 10.1128/iai.58.1.245-251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Bennedsen J., Closs O. The antigens of Mycobacterium tuberculosis, H37Rv, studied by crossed immunoelectrophoresis. Comparison with a reference system for Mycobacterium bovis, BCG. Scand J Immunol. 1988 Feb;27(2):223–239. doi: 10.1111/j.1365-3083.1988.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]