Abstract
The introduction of the excimer laser for keratorefractive surgery in the 1990s permanently reshaped the treatment landscape for correcting refractive errors, such as myopia, hyperopia, and astigmatism. Until that point, these treatments had relied on less predictable techniques, such as radial keratotomy and automated lamellar keratectomy. In recent years, other new technologies, along with increased understanding of the basic science of refractive errors, higher-order aberrations, biomechanics, and the biology of corneal wound healing, have allowed for a reduction in the surgical complications of keratorefractive surgery. Novel technologies, such as eye tracking, anterior segment imaging, the femtosecond laser, and asphericity-optimized and wavefront-guided custom laser in situ keratomileusis, have assisted refractive surgeons in achieving greater predictability of their laser vision correction procedures. Understanding the cascade of events involved in the corneal wound healing process and examination of how corneal wound healing influences corneal biomechanics and optics are crucial to improve the efficacy and safety of laser vision correction.
Keywords: angiogenesis, corneal wound healing, laser vision correction, metalloproteinases, refractive outcomes
Corneal surgery modifies the corneal curvature. The most common laser refractive procedures performed today include ablative photorefractive keratectomy (PRK) and laser in situ keratomileusis (LASIK). Research over the last decade has helped to better elucidate the biological aspects of corneal wound healing; we now know that the maintenance of corneal avascularity is an active process involving a balance between the activities of pro-angiogenic and anti-angiogenic factors.1,2 In 1995, the excimer laser was approved by the United States Food and Drug Administration (FDA) for use in the correction of mild-to-moderate myopia.3 In the 2 decades since then, refractive surgery has undergone considerable progress and development, particularly in terms of eye-tracking systems, anterior segment imaging, the introduction of the femtosecond laser, asphericity-optimized, wavefront-guided custom laser vision correction (LVC) and an improved understanding of the basic science of refractive errors, higher-order aberrations (HOAs), and biomechanics and the biology of corneal wound healing. Such advances have assisted in refractive surgeons achieving better outcomes in terms of the safety and predictability of their LVC procedures. However, despite these advances, limitations exist and complications do occur, particularly related to the somewhat unpredictable nature of corneal wound healing and the biomechanical response to surgery. These issues can lead to postoperative refractive surprises, discrepancies between attempted and achieved visual outcomes, and biomechanical and wound healing problems.
In this review, we describe the interactions between matrix metalloproteinases (MMPs; MMP-2, -7, and -14), pro-angiogenic factors, anti-angiogenic factors, and other regulatory proteins during corneal wound healing and summarize the current understanding of major wound healing pathways that contribute to the corneal response to LVC.
CONVENTIONAL VERSUS CUSTOM EXCIMER LASER SURGERY
Several effective options for laser refractive surgery are available to patients, which provide the opportunity to meet more of the needs of an individual patient. This review focuses on mainstream excimer LVC procedures, which include lamellar procedures, such as LASIK, and procedures involving surface ablation [ie, PRK, laser epithelial keratomileusis (LASEK), and Epi-LASIK]. Basic knowledge, surgical issues, and complications will be discussed.
Laser In Situ Keratomileusis
LASIK is a lamellar laser refractive surgery in which the excimer laser ablation is performed under a partial-thickness lamellar corneal flap. Until recently, this lamellar flap was generated using a microkeratome with an oscillating blade to shave a 100 to 200 μm corneal flap, ranging in size from 9 to 10.5 mm. This is done after immobilization of the eye by the positioning of a suction ring.4 Once the flap is created, it is folded back to expose the corneal stroma. The excimer laser is then focused and centered over the pupil, with the patient looking at the fixation light, and a preprogrammed excimer ablation of the stroma is performed. After the laser ablation is complete, the flap is reflected onto the treated corneal stromal bed. The refractive outcomes after uncomplicated LASIK are relatively stable several years after surgery. The flap perimeter and interface undergo slow wound healing, which allows for early and stable refractive corrections (Fig. 1). However, LASIK is not without complications. The creation of the lamellar flap during the LASIK procedure increases the risk of intraoperative and postoperative complications. The complications of LASIK are listed in Table 1.4
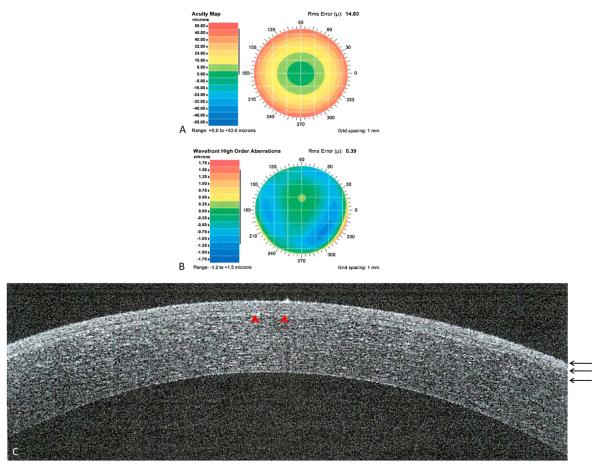
FIGURE 1.
Custom LASIK after 1 year. Preoperative acuity map of patient with manifest refraction of −10.71 +1.36 × 81 (A). Preoperative wavefront high-order aberrations of a patient with defocus of 14.8, astigmatism of 0.90, coma of 0.205, trefoil of 0.10, and spherical aberration of 0.27 (B). Spectral-domain optical coherence tomography showing a cross-section of the cornea. Red arrow-heads showing the LASIK flap (C). Three black arrows showing the epithelium, Bowman’s membrane, and stroma, respectively.
TABLE 1.
LASIK Complications
| Complication | FDA Study* | Non-FDA Study† |
|---|---|---|
| Epithelial defect, % | 0.50 | 5.0–22.6 |
| Lamellar keratitis, % | 0.99 | 0.2–3.2 |
| Haze, % | 0.57 | 1.8–6.2 |
| Flap folds, % | 1.00 | 0.2–1.1 |
| Thin flap, % | 0.42 | 0.08–0.75 |
| Free-cap, % | 3.40 | 0.08–1.00 |
| Irregular flap, % | n/a | 0.09–0.2 |
| Buttonholed flap, % | n/a | 0.13–0.56 |
| Incomplete flap, % | n/a | 0.23–0.75 |
| Dislodged flap, % | 0.22 | 1.1–2.0 |
| Epithelial ingrowth, % | 0.14 | 0.33–9.1 |
| Debris, % | 3.31 | 1.9–100 |
| Infectious keratitis, % | 0.0 | 0–0.2 |
| Post-LASIK ectasia, % | 0.0 | 0.2 |
Includes studies of wavefront-guided LASIK for myopia, LASIK for myopia, and LASIK for hyperopia.
Includes complications reported in studies of 1000 people or more and in reports focusing on specific complications. Data are presented as the proportion of patients affected. Modified with permission from Sakimoto et al.4
n/a, not available.
There have been a number of technological advancements to overcome the difficulties associated with intraoperative flap and microkeratome-related complications. The femtosecond laser is one such technology. It was developed as a replacement of the microkeratome and allowed surgeons to customize the thickness and diameter of the lamellar corneal flap within the corneal stroma, providing more accuracy in flap thickness than with previous methods. Unlike mechanical microkeratomes, which can have variable flap thickness, the femtosecond laser minimizes irregular flap thickness and epithelial injury as it etches a lamellar flap at a desired corneal depth.5 Furthermore, there are potential biomechanical and histopathological advantages with femtosecond laser flap creation. In LASIK, a larger flap is often desired (up to 9 or 10 mm in diameter), particularly in high myopes and in patients with large pupils to compensate for any decentration. With the femtosecond laser, a smaller flap is possible if centered over the optical zone. Overall, the femtosecond laser has been reported to minimize aberrations and to be less dependent on corneal curvature,6 but it comes with increased cost, surgical time and risk of diffuse lamellar keratitis. The latter can be reduced, however, with intensive perioperative topical corticosteroids.4
Irrespective of whether the LASIK flap is cut with a microkeratome or a femtosecond laser, there will still be corneal flap-related complications.6 In a meta-analysis of 7 randomized controlled trials, femtosecond LASIK did not have an advantage in efficacy (uncorrected visual acuity ≥20/20), accuracy [±0.50 diopter (D), mean spherical equivalent (SE)], and safety (loss of ≥2 lines of corrected visual acuity) over the mechanical microtome, but total aberrations and spherical aberrations were reported to be statistically significantly lower with the femtosecond laser.6
KERATOREFRACTIVE SURGERY (LASIK AND PRK) AND CORNEAL ECTASIA
Not every patient is a candidate for keratorefractive surgery. A history of keratoconus (KC) is a contraindication to having LVC. KC is an ectatic corneal disorder in which the cornea progressively thins and steepens to produce myopia, irregular astigmatism, and, eventually, loss of best-corrected visual acuity.7 It has an incidence of approximately 1 in 2000.8 Progressive thinning and steepening of the cornea may also occur after LASIK. This condition (commonly referred to as post-LASIK ectasia) is similar to KC and has an estimated average time of onset of 12 to 24 months after LASIK.9 Although post-LASIK ectasia is statistically rare (Table 1), it is a serious vision-threatening complication.10 Ectatic corneal disorders (including both KC and post-LASIK ectasia) were reported to be the second most common indications for keratoplasty in the United States, where they accounted for about 15% of all corneal transplants performed.11 Recent developments in understanding the causes and risk factors for post-LASIK ectasia and improvements in topography technologies are helping to identify patients who are at risk of developing this condition.
The risk of developing post-LASIK ectasia increases in patients with pre-existing KC, deep flaps, high myopia, and deep laser ablation.10 However, none of these risk factors can definitively predict the development of ectasia as it can potentially develop in eyes with no identifiable risk factors.12 KC itself can be challenging to diagnose, especially in patients with coexistent Fuchs endothelial dystrophy, because these patients tend to have thick corneas,13 whereas the classical KC patient has a thin cornea. It is imperative to properly screen patients at the preoperative stage by measurement of corneal thickness and examination of the endothelium and ocular surface to rule out these corneal abnormalities.
Automated corneal topographic analysis, which can measure the corneal curvature at multiple points across the cornea, has become the standard of care in the preoperative evaluation of all refractive patients. The corneal map identifies the overall corneal shape and irregularities. Ectatic disorders, such as KC and pellucid marginal degeneration (PMD), can be identified by careful analysis of these maps. Furthermore, a predisposition for these disorders, forme fruste KC, which is a contraindication to undergoing LASIK, can be identified using topography, even when there is no evidence of manifest KC by slit-lamp biomicroscopic examination. Also, patients found to have asymmetrical inferior corneal steepening or bow-tie pattern on topography may have a greater risk of post-LASIK ectasia.
It is also essential to examine the peripheral cornea and limbus carefully, particularly in patients with against-the-rule astigmatism, to avoid operating on ectatic disorders, such as PMD.10 PMD is a progressive noninflammatory ectatic condition, characterized by thinning of the inferior peripheral cornea in a crescent-shaped pattern, concentric to the inferior limbus. PMD is known to be associated with a “crab-claw” pattern on corneal topography (Fig. 2).14 Even in patients with no other clinical signs, this inferior crab-claw pattern accompanied by central flattening (“blue spot”) is at risk for the development of PMD even if there are no clinical signs of it. It is strongly recommended that LASIK should be avoided in eyes exhibiting this topographic pattern.10 Proper screening can help to decrease the risk of ectasia, even in eyes with high myopic errors.15
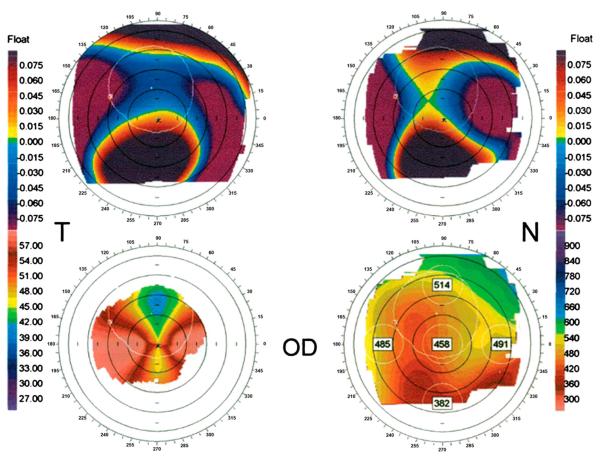
FIGURE 2.
Claw-shaped topography patterns in patients with PMD. Orbscan II topographic data of a representative patient with PMD. (Upper left) Anterior elevation float. (Upper right) Posterior elevation float. (Lower left) Mean keratometric axial power map showing superior flattening and horizontal steepening. (Lower right) Pachymetry map. Note the thin pachymetry in the inferior corneal periphery. Reprinted with permission from Lee et al.14
SURFACE ABLATION PROCEDURES (PRK, LASEK, AND EPI-LASIK)
Three types of excimer laser surface ablation procedures, which differ in the way the epithelium layer is handled, are currently used: PRK, LASEK, and Epi-LASEK. Surface ablation procedures are not associated with flap-related complications because, unlike in LASIK, there is no lamellar flap. However, patients undergoing surface ablation procedures experience more pain and have a slower visual recovery than patients undergoing LASIK. A surface ablation procedure may be considered in patients with thin corneas and low-to-moderate myopia and myopic astigmatism. Surface ablation is also a popular choice for patients who are predisposed to trauma, such as military personnel and athletes, given the absence of a stromal corneal flap.
PRK has a long track record; it was the most commonly performed surgical procedure before the introduction of LASIK in the mid-1990s. The surgical techniques and procedures for PRK involve epithelial removal before excimer ablation, and so the cornea is more likely than LASIK to retain its biomechanical strength. In PRK, a large epithelial defect occurs and healing proceeds through migration and division of the surrounding epithelium.16 Figure 3 shows a cross-section of the cornea immediately after PRK, showing the ablated Bowman’s layer. Although PRK is relatively safe and effective, the ablation of Bowman’s layer and the anterior stroma and the subsequent epithelial–stromal interactions lead to a wound healing response that can cause greater stromal haze and scarring than that in LASIK, the risk of which is higher in patients with high myopia. Furthermore, the presence of a large epithelial defect may explain why there is a greater likelihood for patients undergoing PRK to experience postoperative pain and why the rate of visual recovery is often slower than with LASIK. The average LASIK patient has improved vision 2 to 3 days after surgery and experiences little or no pain; patients who have surface ablation tend to have episodes of moderate-to-severe discomfort for 1 to 4 days after the procedure and usually do not achieve vision similar to LASIK patients for several weeks—a major limiting factor of PRK. Improved long-term wound healing results of PRK have been shown, especially with the application of topical mitomycin-C (Fig. 4).
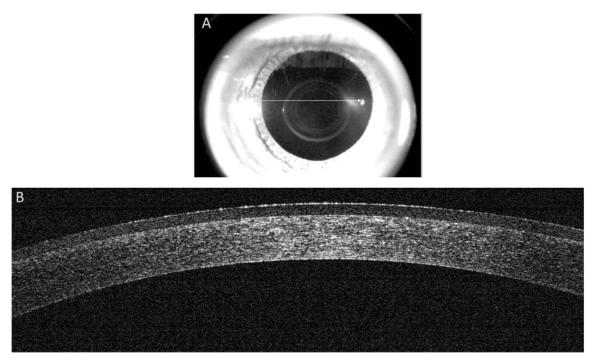
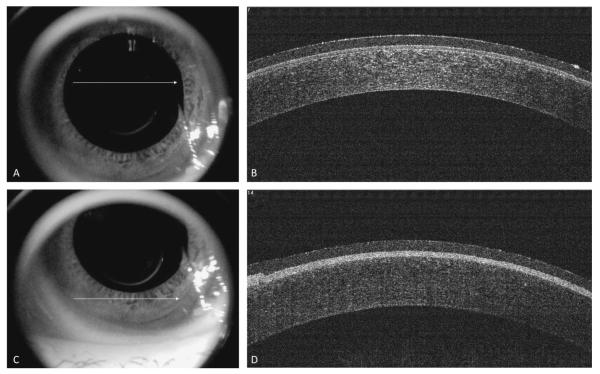
FIGURE 3.
Spectral-domain optical coherence tomography immediately after PRK. Image taken from the center of the cornea (white arrow) after application of a therapeutic nonrefractive soft contact lens (A). Spectral-domain optical coherence tomography showing a cross-section of the cornea (B). Note central corneal thinning and absent Bowman’s layer (and absent epithelium) under the contact lens in the treatment zone.
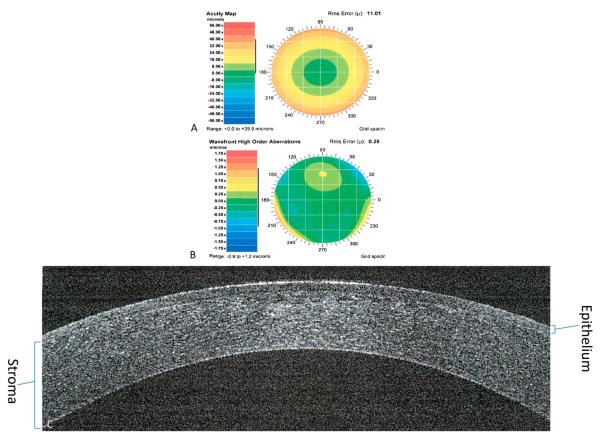
FIGURE 4.
One year after custom PRK. Preoperative acuity map of a patient with manifest refraction of 11.54 + 1.81 × 96 (A). Preoperative wavefront high-order aberrations of a patient with defocus of 10.96, astigmatism of 0.96, coma of 0.16, trefoil of 0.14, and spherical aberration of 0.046 (B). Spectral-domain optical coherence tomography showing a cross-section of the cornea showing the healed epithelium (C).
LASEK is an extension of PRK and may combine some of the advantages of both PRK and LASIK while avoiding some disadvantages of both these procedures. LASEK avoids all the flap-related complications and reduces the risk of keratectasia associated with LASIK. Furthermore, it has been reported that the pain is less and recovery times are faster than with PRK.17
In LASEK, instead of debriding the patient’s epithelium, the epithelium is lifted and reflected as an entire sheet after being loosened with warm alcohol. If deemed “healthy,” this original epithelial sheet is then repositioned onto the stromal bed to its original position after laser ablation using preplaced corneal surface markers for guidance.
Epi-LASIK is a newer technique, initially described by Pallikaris et al,18 where an epithelial sheet is created mechanically using an epikeratome, which eliminates the need to use alcohol. A variety of epikeratomes have been developed for this purpose, differing from LASIK keratomes, in that the blade and angle of cutting are optimized for subepithelial dissection without disrupting the stroma. Using spectral-domain optical coherence tomography, one can visualize the repositioned epithelium in the treatment zone immediately after Epi-LASIK (Fig. 5).
FIGURE 5.
Spectral-domain optical coherence tomography immediately after Epi-LASIK. Image taken from the center of the cornea (white arrow) after application of a therapeutic nonrefractive soft contact lens (A), with its corresponding cross-section of the cornea (B). Image taken from the inferior edge of the cornea (white arrow) after application of a therapeutic nonrefractive soft contact lens (C), with its corresponding cross-section of the cornea (D).
Comparisons of different types of surface ablation treatments show that there are minimal demonstrable differences in their refractive and visual outcomes. In addition, available data suggest that surface ablation achieves results similar to LASIK.4,19 In a retrospective matched case–control study of 2257 eyes that underwent LASEK or LASIK for the treatment of low-to-moderate myopia, we found no statistically significant difference between outcomes in terms of their safety, effectiveness, and predictability.19
MONOVISION KERATOREFRACTIVE SURGERY FOR RESBYOPIA
Presbyopia, the age-related changes of the lens and ciliary muscles that result in a diminished ability to accommodate and focus on near-range objects, has traditionally been treated by corrective lenses, such as reading glasses or bifocals. Monovision is a method of presbyopic correction, whereby the dominant eye is usually corrected for distance vision and the nondominant eye corrected for near. This approach has been used successfully with contact lens correction and in refractive surgical corrections for pre-presbyopic and presbyopic patients.
The criteria for monovision typically involve correcting the dominant eye for distance (made emmetropic), and the nondominant eye is corrected for near vision (either made or left myopic by 0.75 D or 1.75 D). Instances of crossed monovision (dominant eye corrected for near vision and the non-dominant eye for distance vision) also occur and vary by patient preference.20
Although monovision can occur as an undesirable “refractive surprise” of laser refractive surgery, elective intentional monovision refractive surgery is becoming more popular for patients in the presbyopic age group. Monovision was initially used “off label,” but it was approved by the FDA for the correction of presbyopia in 2007.20 However, monovision depends not only on the ability of the laser to accurately reach the target refraction but also on the ability of the patient to adjust to anisometropia and the postoperative decrease in stereopsis. In a retrospective observational study of 145 consecutive laser refractive surgery patients, we found that 42 patients had an outcome of monovision (defined as a distance vision SE of −0.50 to +0.5 D, near vision SE of −3.75 to −1.00 D, and anisometropia of 1.00 D or greater), including 18 patients (43%) with crossed monovision (where the dominant eye was corrected for near). The overall patient satisfaction rate (as recorded in the medical records) with monovision was 88%, which is higher than that previously reported for patients having contact lens monovision (76%).20,21
The most important factor for obtaining good outcomes with monovision is proper selection of patients and adequate counseling before the procedure. Some patients cannot tolerate monovision; thus, a trial of contact lenses to induce monovision before the surgery can sometimes help with decision making. If the patient is not satisfied with the monovision result, the patient may consider undergoing further laser retreatment of the undercorrected eye, which would “balance the eyes.” The patient would need to be prepared to use reading glasses after surgery.
CUSTOM LASER SURGERY
Conventional ablations make use of data obtained during manifest and cycloplegic refractions. The ablation profile contains a spherical component and an astigmatic component when applicable. Although the profiles incorporate additional nomogram adjustments and additional components for blending the transition zone between the treated and untreated stroma, they essentially offer similar treatment to glasses. In comparison, customized laser surgery attempts to optimize the eye’s optical system using a variety of spherical, cylindrical, aspheric, and asymmetrical treatments based on an individual eye’s functional, anatomical, and optical aspects as well as patient needs and preferences.
Adaptive optics and wavefront sensors were introduced to identify and correct low- and high-order aberrations in the human eye. Today, a large proportion of refractive surgeons use wavefront-guided, wavefront-optimized, and Q-factor profile-guided ablations. Wavefront-guided treatments allow optical aberrations beyond spherical and cylindrical errors to be corrected, representing a major advancement in laser refractive surgery. Wavefront aberrometers capture data that describe the optical aberrations of a patient’s eye as an optical system that takes into account factors beyond corneal irregularities.4
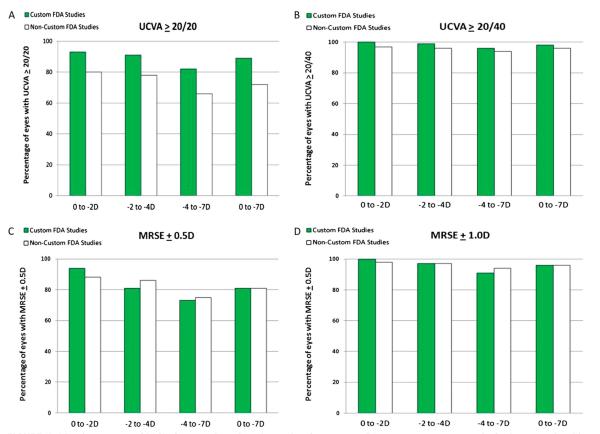
Despite the advantages of wavefront-customized ablation, there still remain a number of challenges that need to be addressed in refractive surgery. Repeat preoperative measurements are sometimes needed to compensate for eye drift. Furthermore, wound healing and unexpected biomechanical responses to surgery affect the accuracy of customized treatments and produce variable responses. Ablation-induced steepening and thickening in the mid-periphery of the cornea may also increase HOAs. Sakimoto et al4 have reviewed and compared FDA clinical outcomes (uncorrected visual acuity and SE) of conventional and wavefront-guided LASIK for myopia. A marked difference in uncorrected visual acuities of ≥20/20 was seen between wavefront-guided and conventional treatments. In wavefront-guided LASIK, 89% of patients achieved this level of vision as compared with 72% of patients who underwent conventional treatment (grouped data 0 to −7 D; Fig. 6). These differences were not seen with the 20/40 or better acuity outcome. Furthermore, 95% of patients up to −2 D and 91% in the range of −2 to −4 D achieve acuity of ≥20/20 (Fig. 6).4 However, some caution needs to be exercised in comparing data between wavefront-guided and conventional treatments because the FDA data for conventional treatments were gathered with earlier generation laser machines.
FIGURE 6.
Visual outcomes of LASIK for myopia at 3 to 6 months after surgery. For every assessment, results for low myopia with error 0 to −2 D, −2 to −4 D, −4 to −7 D, and grouped data (0 to −7 D) are presented for custom and noncustom FDA studies. UCVA, uncorrected visual acuity; MRSE, manifest refraction spherical equivalents. Modified with permission from Sakimoto et al.4
CORNEAL WOUND HEALING AFTER REFRACTIVE SURGERY
Corneal wound healing is a major contributor to the success of traditional excimer laser ablation procedures and custom Q-linked or wavefront-guided corneal ablations used to optimize visual outcomes. Biological differences in wound healing responses are thought to be a major factor limiting the predictability of refractive surgery in some patients (over-correction, undercorrection and regression, and induction of irregular astigmatism). In addition, wound healing may contribute to some of the complications of PRK, LASEK, or LASIK, including haze formation,1,2 which are more common after surface ablation than LASIK, particularly in deeper ablations for higher degrees of myopia.
CORNEAL SHAPE AFTER WOUND HEALING
Evaluation of corneal shape is an important, if not essential, component of the preoperative assessment of refractive surgery candidates. As discussed, the development of automated corneal topographic analysis has made a considerable difference to the assessment of patients and planning of their surgery, and such measurements on keratometric power (and others such as pachymetry and refractive status) should be obtained as accurately as possible before refractive surgery. The introduction of better ways of mapping corneal shape has also helped to further understand the importance that corneal asphericity has on visual performance.
For a given value of corneal asphericity (Q value), an increase in apical radius (surface flattening) results in a decrease in the distance from the best-fit sphere (BFS). With increasing prolateness (negative surface asphericity), the opposite is true and the BFS increases. For a corneal surface with an apical radius of 7.75 mm, changing the asphericity from −0.1 to −0.5 results in a 4-fold increase in distance from BFS.15
Q-values matter in terms of visual outcome, and having a negative Q is better than a positive one.15 Based solely on mathematical theory, a patient who has a negative Q (prolate cornea) would be more likely to obtain a better visual outcome after refractive surgery; however, in practice, the clinical evidence suggests that this is not always true, in part because of the variability in corneal wound healing.
A common question asked by refractive surgeons is what do you do with a patient with a thin cornea where there is a risk of biomechanical change? It is imperative to measure corneal thickness before refractive surgery, but in terms of the definitive minimum safe stromal bed thickness, controversy exists. In essence, it is a trade-off between the benefits of reducing HOA with a deeper ablation versus the effects of inducing further biomechanical changes during wound healing. In a thin cornea, one must avoid going deep into the cornea and induce corneal biomechanical changes. In a high myope undergoing LASIK, it would be sensible to opt for a small-diameter flap, to limit the depth of ablation, and accept that there will be HOA.22 Conversely, in a low myope with a thick cornea, there is a definite benefit from providing a wide-diameter treatment to minimize HOA.
CORNEAL ANGIOGENIC PRIVILEGE: INSIGHT INTO CORNEAL AVASCULARITY DURING
WOUND HEALING AFTER KERATOREFRACTIVE SURGERY The cornea is a highly specialized transparent avascular tissue with a smooth, convex outer surface and concave inner surface, of which the main function is to refract and transmit light. Approximately 1 mm thick peripherally and 0.5 mm centrally, the cornea comprises 5 histological layers, including an outer stratified squamous nonkeratinized epithelium (which provides a smooth refractive surface and acts as a barrier against microorganisms), Bowman’s layer (a narrow acellular layer with uncertain functions), an inner connective tissue stroma (accounting for 90% of the corneal thickness and containing keratinocytes), and a cuboidal endothelium innermost layer with its basement membrane (Descemet’s membrane). The endothelium has a vital role in maintaining dehydration of the cornea via an adenosine triphosphate–driven sodium/potassium pump—essential for transparency.23
The normal cornea is unique, in that it is entirely devoid of blood and lymphatic vessels, and this “angiogenic privilege” is essential for proper transparency and function of the cornea.2,23 The cornea with its natural avascularity has, therefore, been used as an in vivo model for angiogenic and anti-angiogenic molecules.
Studies over the last decade have focused on understanding the mechanisms that maintain corneal avascularity under homeostatic conditions and in avascular wound healing. These studies have suggested that the maintenance of corneal avascularity depends on a fine balance between the production of angiogenic and antiangiogenic factors (rather than the compactness of corneal collagen and the presence of a limbal stem cell barrier).2,3,24
THE BALANCE OF ANGIOGENIC AND ANTI-ANGIOGENIC FACTORS IN THE CORNEA
Experimental studies have demonstrated that corneal avascularity is an active process, rather than a passive one, involving the production of anti-angiogenic factors, which counterbalance the pro-angiogenic factors that are upregulated during wound healing. This balance between angiogenic and anti-angiogenic factors in the corneal epithelium has an important role in the avascularity of the cornea and its angiogenic privilege.
Corneal neovascularization, when it occurs as a result of trauma, infection, and inflammatory or degenerative disorders, involves the invasion of blood (hemangiogenesis) and lymphatic (lymphangiogenesis) vessels; however, more often, corneal wound healing occurs in the absence of neovascularization. In this situation, the balance between angiogenic factors, such as fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF), and anti-angiogenic molecules, such as angiostatin, endostain, and pigment epithelium–derived factor, is tilted toward angiogenesis (Table 2).2
TABLE 2.
Balance Between Corneal Pro-angiogenic and Anti-angiogenic Factors
| Corneal Pro-angiogenic Factors | Corneal Anti-angiogenic Factors |
|---|---|
| VEGF-A | Sflt-1 |
| bFGF | Angiostatin |
| PDGF | Endostatin |
| aFGF | Neostatin-7 and -14 |
| NRP-1 | PEDF |
| Ang1 and 2 | Restin |
| IL-8 | Prolactin |
| IL-1β | SPARC |
| HGF | TSP-1 and -2 |
| PDGFR | Calreticulen |
| TGF-β | TIMP |
| Endoglin | |
| Plasminogen activator | |
| Galectin-3 | |
| Ephrin-B2 |
Modified with permission from Ellenberg et al.1
PEDF, pigment epithelium–derived factor; PDGF, platelet-derived growth factor PDGFR, platelet-derived growth factor receptor; aFGF, acidic fibroblast growth factor; NRP-1, neuropilin 1; IL, interleukin; HGF, hepatocyte growth factor; TGF-B, transforming growth factor beta; Sflt-1, soluble fms-like tyrosine kinase-1; SPARC, secreted protein, acidic, and rich in cysteine; TSP, thrombospondin; TIMP, tissue inhibitor of metalloproteinase 1.
ROLE OF ANGIOGENIC AND ANTI-ANGIOGENIC FACTORS IN CORNEAL WOUND HEALING
Several anti-angiogenic factors have been characterized in the corneal epithelium that are thought to have an important role in maintaining avascular wound healing. Some of these inhibit corneal neovascularization [eg, soluble vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-3, and neostatins], whereas others regulate corneal neovascularization through proteolytic action (MMP-2, -7, and -14).3,24,25 The corneal epithelium expresses soluble forms of VEGF-1 and ectopic VEGFR-3, which act as endogenous VEGF traps, therefore serving as a control mechanism for the pro-angiogenic properties of the VEGF family. On suppression of this endogenous VEGF trap in mice, using RNA interference or Cre-lox–mediated gene disruption, mice develop extensive corneal neovascularization. Furthermore, it has been shown that human corneas that have neovascularization have a greatly reduced expression of soluble VEGFR-1 with less VEGF bound to VEGFR-1.25
After corneal injury, MMPs are also upregulated in the cornea.26 MMPs are another class of molecules that regulate corneal avascularity. MMP-7 (matrilysin) appears to protect against neovascularization.27 A different MMP, MMP-14, is upregulated in the cornea in response to injury. MMP-14 is constitutively expressed in the corneal basal epithelium and corneal stromal keratocytes, and it has been shown to have both anti-angiogenic and pro-angiogenic properties in the cornea.3
The pro-angiogenic role of MMP-14 has been well documented in MMP-14 knockout mice that exhibit impaired bFGF-induced corneal neovascularization.28 MMP-14 cleaves proMMP-2 to active MMP-2 (gelatinase A), which is pro-angiogenic.29 It also cleaves the anti-angiogenic molecule decorin, pigment epithelium–derived factor, EphB1, and EphB4.2,30 This causes extracellular matrix cleavage and enhanced vascular endothelial cell proliferation, migration, and tube formation. MMP-14 also enables VEGF production (Fig. 7).
FIGURE 7.
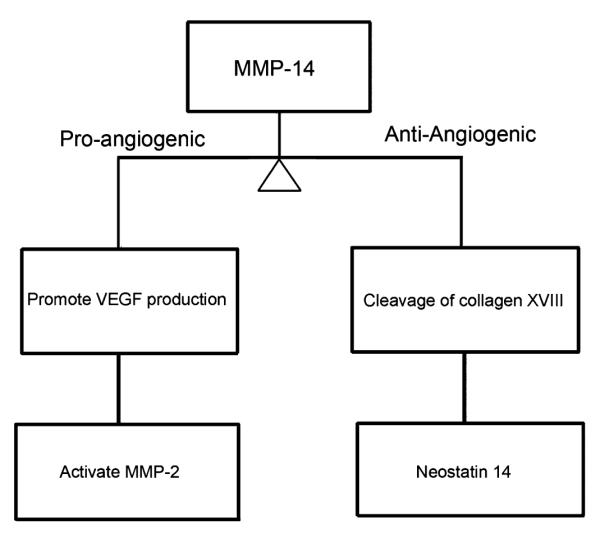
Pro-angiogenic MT1–MMP pathways in the cornea.
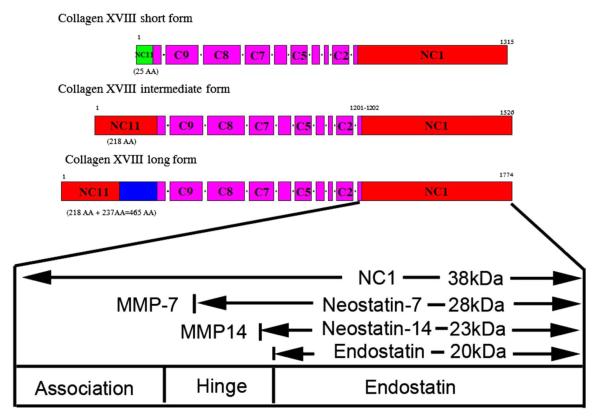
At the same time, there is an opposite effect happening, as MMP-14 also has an anti-angiogenic role: MMP-14 generates potent anti-angiogenic molecules, angiostatin and neostatin-14, by the cleavage of plasminogen and collagen XVIII, with the latter expressed in the cornea, ciliary body, retina, and lens.31,32 Neostatin-7 is generated by the effect of MMP-7 (Fig. 8), and it has been shown to prevent bFGF-induced corneal neovascularization.31,33,34
FIGURE 8.
Structure of mouse collagen XVIII. Modified with permission from Chang et al.31
Corneal avascularity not only occurs as a result of the upregulation of anti-angiogenic factors but also from the downregulation of pro-angiogenic factors.3 In a healthy cornea, the upregulation of anti-angiogenic factors tilts the balance toward vessel regression and new vessels do not enter the cornea, which maintains its avascularity. A role for the corneal epithelium in the maintenance of corneal angiogenic privilege has been suggested but not fully elucidated.
Acknowledgments
Supported by National Institutes of Health grants EY10101 (D.T.A.), EY01792 (D.T.A.), and EY021886 (J.H.C.) and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ellenberg D, Azar DT, Hallak JA, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration [Accessed May 30, 2012];Devices@FDA [Online] 1995 Available at: http://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?search_term=LZS%20or%20LASIK.
- 4.Sakimoto T, Rosenblatt MI, Azar DT. Laser eye surgery for refractive errors. Lancet. 2006;367:1432–1447. doi: 10.1016/S0140-6736(06)68275-5. [DOI] [PubMed] [Google Scholar]
- 5.Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:804–811. doi: 10.1016/j.jcrs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZH, Jin HY, Suo Y, et al. Femtosecond laser versus mechanical microkeratome laser in situ keratomileusis for myopia: metaanalysis of randomized controlled trials. J Cataract Refract Surg. 2011;37:2151–2159. doi: 10.1016/j.jcrs.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–273. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- 9.Rad AS, Jabbarvand M, Saifi N. Progressive keratectasia after laser in situ keratomileusis. J Refract Surg. 2004;20(5 suppl):s718–s722. doi: 10.3928/1081-597X-20040903-18. [DOI] [PubMed] [Google Scholar]
- 10.Binder PS. Ectasia after in situ keratomileusis. J Cataract Refract Surg. 2003;29:2419–2429. doi: 10.1016/j.jcrs.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Eye Bank Association of America . 2004 Eye Banking Statistical Report. Eye Bank Association of America; Washington, DC: 2004. [Google Scholar]
- 12.Randleman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267–275. doi: 10.1016/S0161-6420(02)01727-X. [DOI] [PubMed] [Google Scholar]
- 13.Jurkunas U, Azar DT. Potential complications of ocular surgery in patients with coexistent keratoconus and Fuchs’ endothelial dystrophy. Ophthalmology. 2006;113:2187–2197. doi: 10.1016/j.ophtha.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Lee BW, Jurkunas UV, Harissi-Dagler M, et al. Ectatic disorders associated with a claw-shaped pattern on corneal topography. Am J Ophthalmol. 2007;144:154–156. doi: 10.1016/j.ajo.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Gatinel D, Malet J, Hoang-Xuan T, et al. Corneal elevation topography: best fit sphere, elevation distance, asphericity, toricity, and clinical implications. Cornea. 2011;30:508–515. doi: 10.1097/ICO.0b013e3181fb4fa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taneri S, Feit R, Azar DT. Safety, efficacy, and stability indices of LASEK correction in moderate myopia and astigmatism. J Cataract Refract Surg. 2004;30:2130–2137. doi: 10.1016/j.jcrs.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 17.Lee JB, Seong GJ, Lee JH, et al. Comparison of laser epithelial keratomileusis and photorefractive keratectomy for low to moderate myopia. J Cataract Refract Surg. 2001;27:565–570. doi: 10.1016/s0886-3350(00)00880-4. [DOI] [PubMed] [Google Scholar]
- 18.Pallikaris IG, Naoumidi II, Kalyvianaki MI, et al. Epi-LASIK: comparative histological evaluation of mechanical and alcohol-assisted epithelial separation. J Cataract Refract Surg. 2003;29:1496–1501. doi: 10.1016/s0886-3350(03)00348-1. [DOI] [PubMed] [Google Scholar]
- 19.Tobaigy FM, Ghanem RC, Sayegh RR, et al. A control-matched comparison of laser epithelial keratomileusis and laser in situ keratomileusis for low to moderate myopia. Am J Ophthalmol. 2006;142:901–908. doi: 10.1016/j.ajo.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Jain S, Arora I, Azar DT. Success of monovision in presbyopes: review of the literature and potential applications to refractive surgery. Surv Ophthalmol. 1996;40:491–499. doi: 10.1016/s0039-6257(96)82015-7. [DOI] [PubMed] [Google Scholar]
- 21.Jain S, Ou R, Azar DT. Monovision outcomes in presbyopic individuals after refractive surgery. Ophthalmology. 2001;108:1430–1433. doi: 10.1016/s0161-6420(01)00647-9. [DOI] [PubMed] [Google Scholar]
- 22.Gatinel D, Malet J, Hoang-Xuan T, et al. Analysis of customized corneal ablations: theoretical limitations of increasing negative asphericity. Invest Ophthalmol Vis Sci. 2002;43:941–948. [PubMed] [Google Scholar]
- 23.Gipson I, Joyce N, Zieske J. The anatomy and cell biology of the cornea, superficial limbus and conjunctiva. In: Foster SC, Azar DT, Dohlman CH, editors. Smolin and Thoft’s the Cornea: Scientific Foundations and Clinical Practice of Ophthalmology. WB Saunders; Philadelphia, PA: 2005. pp. 612–629. [Google Scholar]
- 24.Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50–57. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- 25.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakimoto T, Shoji J, Yamada A, et al. Upregulation of matrix metalloproteinase in tear fluid of patients with recurrent corneal erosion. Jpn J Ophthalmol. 2007;51:343–346. doi: 10.1007/s10384-007-0455-0. [DOI] [PubMed] [Google Scholar]
- 27.Kure T, Chang JH, Kato T, et al. Corneal neovascularization after excimer keratectomy wounds in matrilysin-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:137–144. doi: 10.1167/iovs.01-1058. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Apte SS, Soininen R, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato T, Kure T, Chang JH, et al. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508:187–190. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- 30.Mimura T, Han KY, Onguchi T, et al. MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009;46:541–550. doi: 10.1159/000226222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang JH, Javier JA, Chang GY, et al. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 32.Kato T, Chang JH, Azar DT. Expression of type XVIII collagen during healing of corneal incisions and keratectomy wounds. Invest Ophthalmol Vis Sci. 2003;44:78–85. doi: 10.1167/iovs.01-1257. [DOI] [PubMed] [Google Scholar]
- 33.Lin HC, Chang JH, Jain S, et al. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- 34.Kojima T, Azar DT, Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582:2515–2520. doi: 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]