Abstract
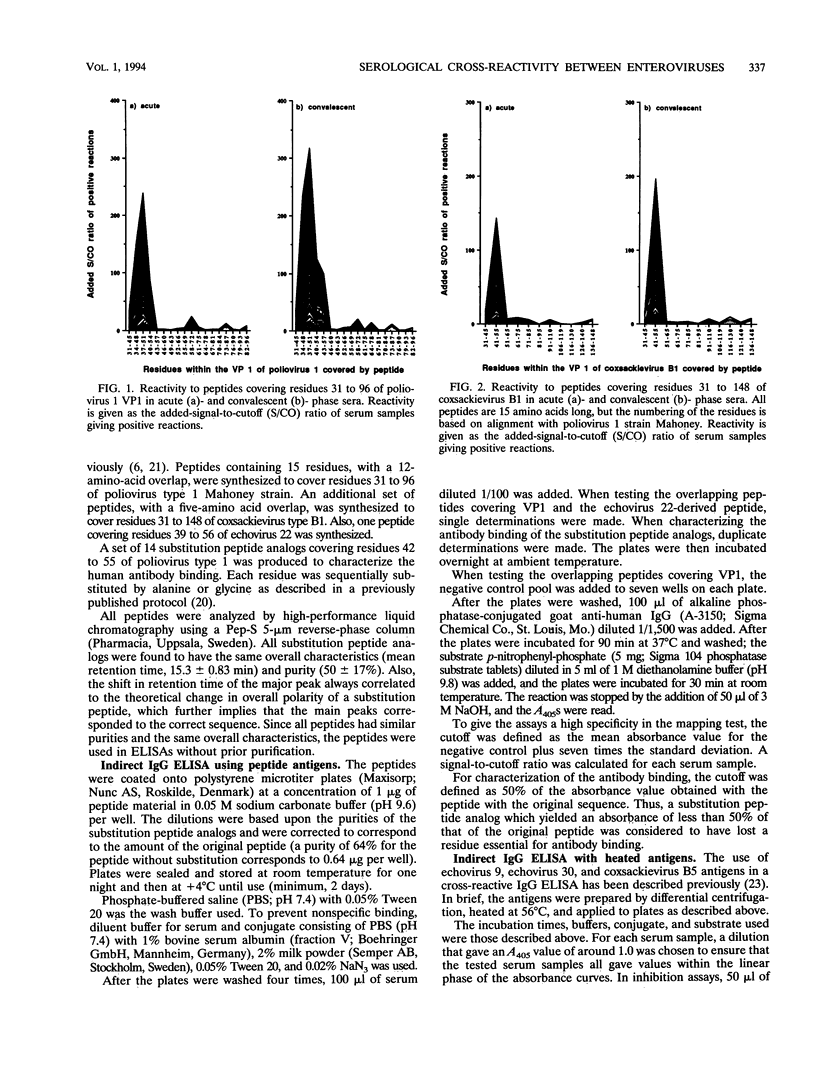
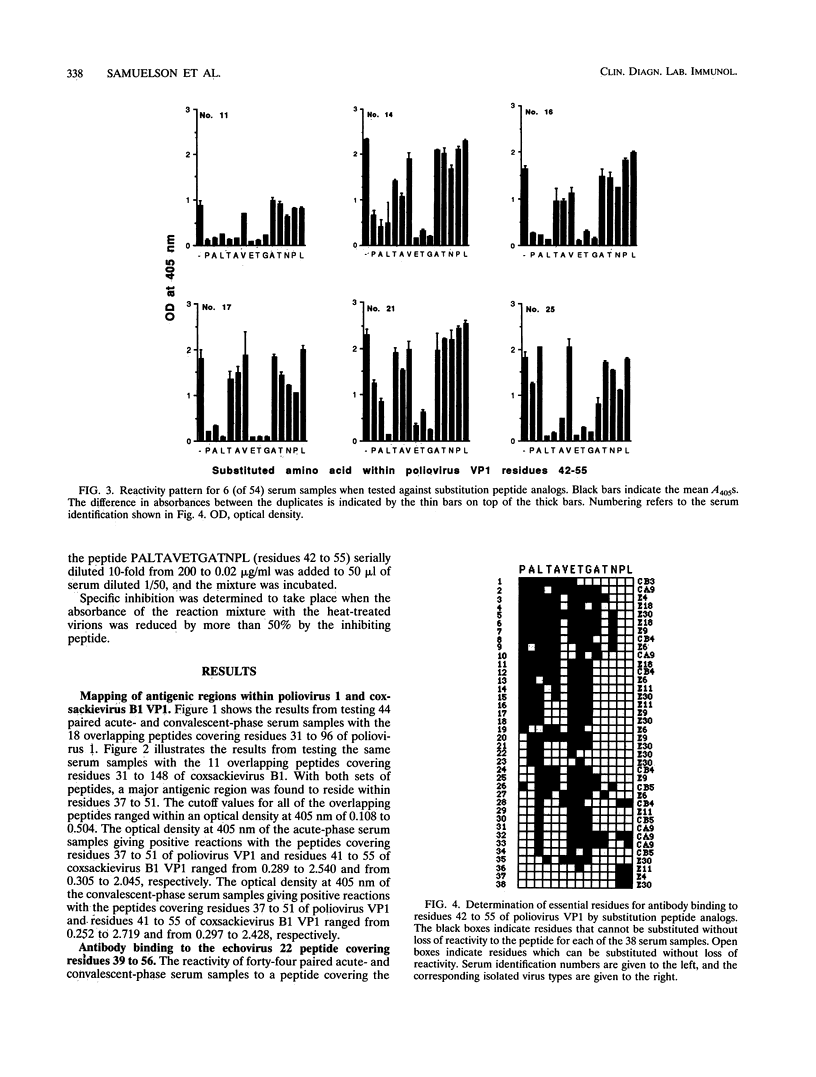
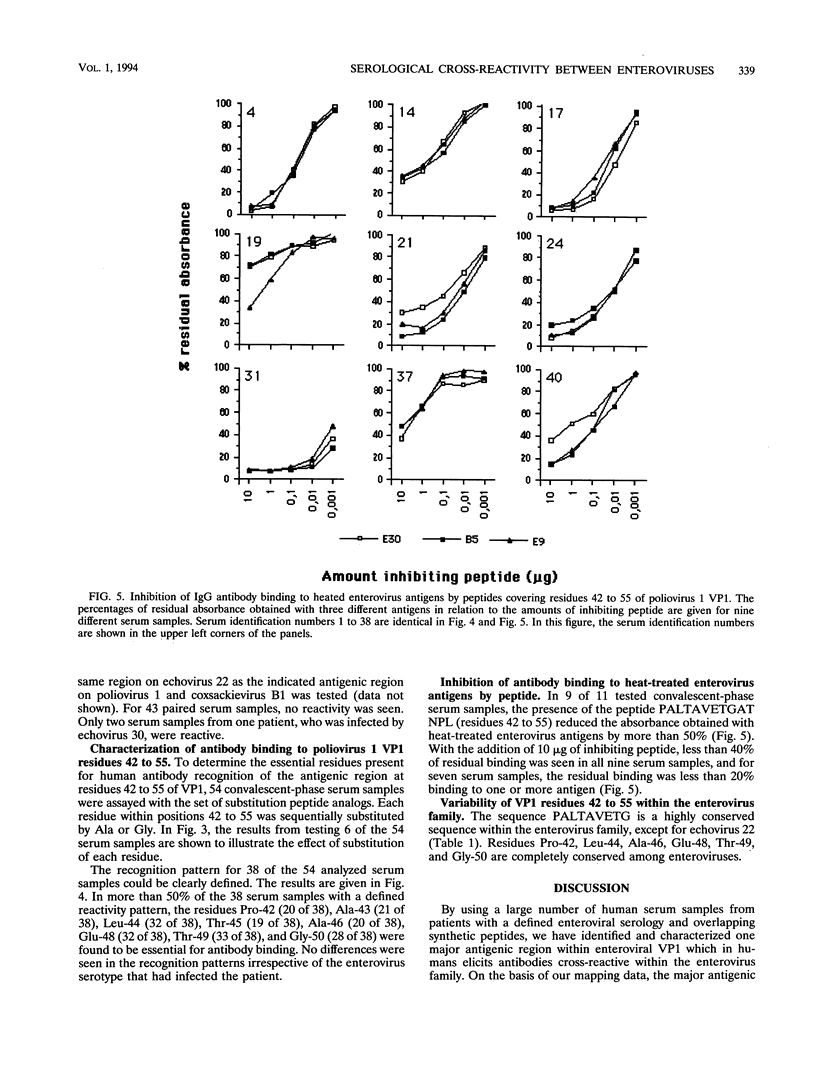
The recognition sites for human antibodies which are cross-reactive between different types of enteroviruses were determined and characterized. Serum samples obtained from 58 patients with culture-confirmed enteroviral infections were analyzed in enzyme immunoassays against two sets of overlapping synthetic peptides covering residues 31 to 96 of poliovirus 1 VP1 (Mahoney strain) and residues 31 to 148 of coxsackievirus B1 VP1 (position based on alignment with poliovirus 1 VP1, Mahoney strain). A major antigenic region eliciting cross-reactive antibodies could be located to residues 37 to 51 of VP1. Furthermore, a single peptide covering residues 42 to 55 almost completely inhibited the binding of human antibodies to heat-inactivated enteroviruses, indicating that residues 42 to 55 of VP1 contain a major region eliciting cross-reactive antibodies. By using peptide analogs in which each residue within positions 42 to 55 of VP1 was sequentially substituted by Ala or Gly, we were able to determine the most essential residues for human antibody binding in 38 of the convalescent-phase patient serum samples. In a majority of the serum samples, the most essential residues for antibody binding were found to be Pro-42, Ala-43, Leu-44, Thr-45, Ala-46, Glu-48, Thr-49, and Gly-50. All of these residues are conserved, according to known enterovirus sequences, with the divergent echovirus 22 excepted. In conclusion, we could demonstrate that the essential residues for binding of cross-reactive antibodies are well conserved within the enterovirus family. These findings provide a molecular basis for the observed antibody cross-reactivity within the enterovirus group.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cello J., Samuelson A., Stålhandske P., Svennerholm B., Jeansson S., Forsgren M. Identification of group-common linear epitopes in structural and nonstructural proteins of enteroviruses by using synthetic peptides. J Clin Microbiol. 1993 Apr;31(4):911–916. doi: 10.1128/jcm.31.4.911-916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. H., Auvinen P., Hyypiä T., Stanway G. The nucleotide sequence of coxsackievirus A9; implications for receptor binding and enterovirus classification. J Gen Virol. 1989 Dec;70(Pt 12):3269–3280. doi: 10.1099/0022-1317-70-12-3269. [DOI] [PubMed] [Google Scholar]
- Ehrnst A., Eriksson M. Epidemiological features of type 22 echovirus infection. Scand J Infect Dis. 1993;25(3):275–281. doi: 10.3109/00365549309008499. [DOI] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovi T., Roivainen M. Peptide antisera targeted to a conserved sequence in poliovirus capsid VP1 cross-react widely with members of the genus Enterovirus. J Clin Microbiol. 1993 May;31(5):1083–1087. doi: 10.1128/jcm.31.5.1083-1087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. J., North C., Minor P. D., Stanway G. The complete nucleotide sequence of coxsackievirus A21. J Gen Virol. 1989 Nov;70(Pt 11):2943–2952. doi: 10.1099/0022-1317-70-11-2943. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Horsnell C., Maaronen M., Khan M., Kalkkinen N., Auvinen P., Kinnunen L., Stanway G. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8847–8851. doi: 10.1073/pnas.89.18.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka N., Kuge S., Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987 Jan;156(1):64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Jenkins O., Booth J. D., Minor P. D., Almond J. W. The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the Picornaviridae. J Gen Virol. 1987 Jul;68(Pt 7):1835–1848. doi: 10.1099/0022-1317-68-7-1835. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Ferguson M., Evans D. M., Almond J. W., Icenogle J. P. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol. 1986 Jul;67(Pt 7):1283–1291. doi: 10.1099/0022-1317-67-7-1283. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page G. S., Mosser A. G., Hogle J. M., Filman D. J., Rueckert R. R., Chow M. Three-dimensional structure of poliovirus serotype 1 neutralizing determinants. J Virol. 1988 May;62(5):1781–1794. doi: 10.1128/jvi.62.5.1781-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Oh C. K., Cunningham L. L., Calenoff M., Jubelt B. Localization of genomic regions specific for the attenuated, mouse-adapted poliovirus type 2 strain W-2. J Gen Virol. 1990 Jan;71(Pt 1):43–52. doi: 10.1099/0022-1317-71-1-43. [DOI] [PubMed] [Google Scholar]
- Roivainen M., Närvänen A., Korkolainen M., Huhtala M. L., Hovi T. Antigenic regions of poliovirus type 3/Sabin capsid proteins recognized by human sera in the peptide scanning technique. Virology. 1991 Jan;180(1):99–107. doi: 10.1016/0042-6822(91)90013-2. [DOI] [PubMed] [Google Scholar]
- Ryan M. D., Jenkins O., Hughes P. J., Brown A., Knowles N. J., Booth D., Minor P. D., Almond J. W. The complete nucleotide sequence of enterovirus type 70: relationships with other members of the picornaviridae. J Gen Virol. 1990 Oct;71(Pt 10):2291–2299. doi: 10.1099/0022-1317-71-10-2291. [DOI] [PubMed] [Google Scholar]
- Stanway G., Cann A. J., Hauptmann R., Hughes P., Clarke L. D., Mountford R. C., Minor P. D., Schild G. C., Almond J. W. The nucleotide sequence of poliovirus type 3 leon 12 a1b: comparison with poliovirus type 1. Nucleic Acids Res. 1983 Aug 25;11(16):5629–5643. doi: 10.1093/nar/11.16.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supanaranond K., Takeda N., Yamazaki S. The complete nucleotide sequence of a variant of Coxsackievirus A24, an agent causing acute hemorrhagic conjunctivitis. Virus Genes. 1992 Apr;6(2):149–158. doi: 10.1007/BF01703064. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Pushko P., Berzinsh I., Bichko V., Sillekens P., Noah M., Pumpens P., Grens E., Wahren B., Magnius L. O. Immunochemical structure of the carboxy-terminal part of hepatitis B e antigen: identification of internal and surface-exposed sequences. J Gen Virol. 1993 Jul;74(Pt 7):1335–1340. doi: 10.1099/0022-1317-74-7-1335. [DOI] [PubMed] [Google Scholar]
- Sällberg M., Rudén U., Magnius L. O., Norrby E., Wahren B. Rapid "tea-bag" peptide synthesis using 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids applied for antigenic mapping of viral proteins. Immunol Lett. 1991 Sep;30(1):59–68. doi: 10.1016/0165-2478(91)90090-w. [DOI] [PubMed] [Google Scholar]
- Wiegers K., Uhlig H., Dernick R. N-AgIB of poliovirus type 1: a discontinuous epitope formed by two loops of VP1 comprising residues 96-104 and 141-152. Virology. 1989 Jun;170(2):583–586. doi: 10.1016/0042-6822(89)90452-2. [DOI] [PubMed] [Google Scholar]


