Background: We showed that nuclear tyrosine phosphorylation is involved in chromatin structural changes.
Results: Several tyrosine kinases phosphorylate KAP1 at Tyr-449, Tyr-458, and Tyr-517 in the nucleus, resulting in a decrease of KAP1 association with heterochromatin.
Conclusion: Tyrosine phosphorylation of KAP1 by nucleus-localized tyrosine kinases, including Src, involves heterochromatin structural changes.
Significance: These findings provide a new insight into nuclear tyrosine phosphorylation signals.
Keywords: Heterochromatin, Nucleus, Protein Phosphorylation, Protein-Protein Interactions, Tyrosine Protein Kinase (Tyrosine Kinase), HP1, KAP1
Abstract
Protein tyrosine phosphorylation regulates a wide range of cellular processes at the plasma membrane. Recently, we showed that nuclear tyrosine phosphorylation by Src family kinases (SFKs) induces chromatin structural changes. In this study, we identify KRAB-associated protein 1 (KAP1/TIF1β/TRIM28), a component of heterochromatin, as a nuclear tyrosine-phosphorylated protein. Tyrosine phosphorylation of KAP1 is induced by several tyrosine kinases, such as Src, Lyn, Abl, and Brk. Among SFKs, Src strongly induces tyrosine phosphorylation of KAP1. Nucleus-targeted Lyn potentiates tyrosine phosphorylation of KAP1 compared with intact Lyn, but neither intact Fyn nor nucleus-targeted Fyn phosphorylates KAP1. Substitution of the three tyrosine residues Tyr-449/Tyr-458/Tyr-517, located close to the HP1 binding-motif, into phenylalanine ablates tyrosine phosphorylation of KAP1. Immunostaining and chromatin fractionation show that Src and Lyn decrease the association of KAP1 with heterochromatin in a kinase activity-dependent manner. KAP1 knockdown impairs the association of HP1α with heterochromatin, because HP1α associates with KAP1 in heterochromatin. Intriguingly, tyrosine phosphorylation of KAP1 decreases the association of HP1α with heterochromatin, which is inhibited by replacement of endogenous KAP1 with its phenylalanine mutant (KAP1-Y449F/Y458F/Y517F, KAP1–3YF). In DNA damage, KAP1–3YF repressed transcription of p21. These results suggest that nucleus-localized tyrosine kinases, including SFKs, phosphorylate KAP1 at Tyr-449/Tyr-458/Tyr-517 and inhibit the association of KAP1 and HP1α with heterochromatin.
Introduction
Protein tyrosine phosphorylation is one of the key post-translational modifications that controls a wide variety of cellular events, such as cell proliferation, differentiation, gene transcription, and cell adhesion (1, 2). Although it is well known that tyrosine phosphorylation is important for signal transduction at the plasma membrane, the role of nuclear tyrosine phosphorylation in nuclear events is poorly understood (3, 4).
Human genome encodes 518 protein kinases, which consist of 428 serine and threonine kinases and 90 tyrosine kinases (5). Receptor-type tyrosine kinases transmit signals from the extracellular milieu to the inside of the cell. On the other hand, the function of non-receptor-type tyrosine kinases is influenced by their intracellular localization (6). Src family kinases (SFKs),3 which belong to a family of non-receptor-type tyrosine kinases, consist of proto-oncogene products and structurally related proteins, such as c-Src, Lyn, and Fyn (7, 8). Recently, we showed that SFKs are imported into and rapidly exported from the nucleus (9, 10). Brk (breast tumor kinase), an Src-related kinase, is localized to the cytoplasm and the nucleus (11). The proto-oncogene product c-Abl, a non-receptor-type tyrosine kinase, can shuttle between the cytoplasm and the nucleus (12). ErbB4, a member of ErbB receptor tyrosine kinases, is cleaved upon ligand stimulation, and the intracellular domain is released into the cytoplasm and the nucleus (13).
Recently, we reported that nucleus-localized tyrosine kinases, such as SFK, Abl, Chk, and ErbB4, play roles in the regulation of chromatin structure and histone modifications through tyrosine phosphorylation (10, 14–17). To substantiate the involvement of nuclear tyrosine kinases in chromatin structural changes, we constructed mutants with a nuclear localization signal (NLS). Compared with the intact form of tyrosine kinases, NLS-SFK, NLS-c-Abl, and NLS-4ICD (NLS-tagged ErbB4 intracellular domain) have strong effects on chromatin structural changes (10, 16, 17). NLS-tagged tyrosine kinases help us to understand the relationships between tyrosine kinases and chromatin structural changes. However, it is still unclear how chromatin structure is regulated through tyrosine phosphorylation.
In this study, we sought to identify nuclear tyrosine-phosphorylated proteins that are related to chromatin structural changes and found a candidate protein called KRAB-associated protein 1 (KAP1/TIF1β/Trim28). We revealed that three tyrosine residues of KAP1 are the common tyrosine phosphorylation sites for various tyrosine kinases localizing in the nucleus. We further showed that tyrosine phosphorylation of KAP1 decreases the amounts of chromatin-bound KAP1 and HP1α. In addition, tyrosine phosphorylation of KAP1 is involved in transcription of p21 upon DNA damage.
EXPERIMENTAL PROCEDURES
Plasmids
Intact c-Src, c-Src (c-Src-HA), and NLS-Src were constructed from cDNA encoding human wild-type Src (18) (provided by D. J. Fujita) as described (10, 19). Intact Lyn, Lyn (Lyn-HA), Lyn-K275A (Lyn(KD)-HA, KD indicating kinase-dead), NLS-Lyn, and NLS-Lyn-K275A (NLS-Lyn(KD)) were constructed from cDNA encoding human wild-type Lyn (20) (provided by T. Yamamoto) as described (10, 19). To construct pOZ-NLS-Lyn, cDNA encoding human wild-type Lyn was subcloned into the XhoI-NotI site of the pOZ-FLAG-HA-N vector (21) (provided by A. Iwama). NLS was inserted between the HA epitope and Lyn. The sequence GGL was inserted between the HA epitope and the NLS. The sequence LDPAQWRPRDPLCWTRPAAPKLSPRAGN was inserted between the NLS and Lyn. pOZ-NLS-Lyn-K275R/Y508F(KD), in which the ATP binding site and the negative regulatory site were mutated, was created by PCR using pOZ-NLS-Lyn as a template. Intact Fyn and NLS-Fyn were constructed from cDNA encoding human wild-type Fyn (22) (provided by T. Yamamoto) as described (10, 19). NLS-4ICD was constructed from cDNA encoding human ErbB4 CYT-1 (23) (provided by S. Yokoyama) as described (17). NLS-c-Abl was constructed from cDNA encoding human wild-type c-Abl-1b (24) (provided by E. Canaani) as described (16). NLS-Syk was constructed from cDNA encoding human wild-type Syk (25) (provided by E. A. Clark) as described (10). cDNA encoding human Brk (Open Biosystems) was subcloned into the pcDNA4/TO-FH vector (16). Myc-JMJD2a was constructed from cDNA containing human wild-type JMJD2a and subcloned into the Myc-pcDNA3 vector. Wild-type KAP1 (FLAG-KAP1-wt), FLAG-KAP1(N1)(1–469), and FLAG-KAP1(C)(469–835) were gifts from H. Ariga (26). FLAG-KAP1(N2)(1–380) was constructed from FLAG-KAP1(N1)(1–469) by digesting with BamHI, blunting, and ligation. The Tyr → Phe mutants of KAP1 were created by PCR using KAP1-wt as a template with the primers (supplemental Table S1).
RNA Interference
Knockdown of KAP1 was performed with short hairpin RNA (shRNA) for silencing KAP1 (5′- gcatgaaccccttgtgctg-3′) (27). The nucleotides for shRNA were annealed and subcloned into the BglII-XbaI site of the pENTR4/H1 vector (provided by H. Miyoshi) (28, 29). To establish a KAP1-stable knockdown cell line, HeLa S3 cells were co-transfected with pENTR4/H1/shKAP1 and a plasmid containing the hygromycin-resistant gene and selected in 250 μg/ml hygromycin. To replace endogenous KAP1 with KAP1-wt or KAP1–3YF mutant, KAP1 knockdown cells were transfected with knockdown-resistant KAP1-wt or KAP1–3YF, and cell clones expressing KAP1-wt or KAP1–3YF were selected in 300 μg/ml G418. shRNA-resistant KAP1 constructs were made by mutation of the shRNA target site by PCR using KAP1 as a template with the sense primer 5′- acacaagcatgaaccactagtactgttttgtgagagctgtgatactc-3′ and the antisense primer 5′-ctctcacaaaacagtactagtggttcatgcttgtgtacgttgcaata-3′.
Antibodies
The following antibodies were used: Tyr(P) (4G10 and polyclonal antibody; Upstate Biotechnology, Inc.; provided by T. Tamura and T. Yoshimoto (30)), Lyn (Lyn9; Wako Pure Chemical Industries (Osaka, Japan) and Lyn44 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA)), FLAG (M2 and polyclonal antibody; Sigma), HA (Y11; Santa Cruz Biotechnology, Inc.), actin (clone C4; CHEMICON International), α-tubulin (MCA78G; Serotec), Src phosphorylated on Tyr-416 (Cell Signaling Technology), KAP1 (ab10484; Abcam and Bethyl Laboratories), HP1α (05-689; Millipore), histone H3 trimethylated on lysine 9 (H3K9me3) (ab8898; Abcam), Syk (4D10; Santa Cruz Biotechnology, Inc.), Brk (C-18; Santa Cruz Biotechnology, Inc.), Abl (8E9; BD Pharmingen), Fyn (Fyn3; Santa Cruz Biotechnology, Inc.), Src (GD11; Upstate Biotechnology, Inc.), Ku70 (C-19; Santa Cruz Biotechnology, Inc.), ErbB4 (C-18; Santa Cruz Biotechnology, Inc.), Chk2 (DCS-273; Medical and Biological Laboratories), Chk2-pT68 (Cell Signaling Technology), KAP1-Ser(P)-473 (BioLegend), KAP1-Ser(P)-824 (Bethyl Laboratories), and lamin A/C (N-18; Santa Cruz Biotechnology, Inc.). Horseradish peroxidase (HRP)-F(ab′)2 secondary antibodies were purchased from Amersham Biosciences. FITC-IgG, TRITC-IgG, and Alexa Fluor 488-, Alexa Fluor 546-, and Alexa Fluor 647-labeled IgG secondary antibodies were purchased from BioSource International, Sigma-Aldrich, and Invitrogen.
Cells and Transfection
Cells were cultured in Iscove's modified DME medium containing 5% bovine serum (COS-1 and HeLa S3 cells; Japanese Collection of Research Bioresources, Osaka). Cells seeded in a 35-mm (60-mm) culture dish were transiently transfected with 1 μg (3 μg) of plasmid DNA using 5 μg (15 μg) of linear polyethyleneimine (25 kDa) (Polyscience, Inc.) (31) or Lipofectamine 2000 (Invitrogen). To generate HeLa S3 cells stably expressing NLS-Lyn or NLS-Lyn(KD), standard transfection and retroviral production procedures were used (21). The infected cells expressing CD25 were enriched by cell sorting. To stimulate tyrosine phosphorylation in HeLa S3/NLS-Lyn, cells were treated with 0.5 mm sodium orthovanadate (Na3VO4) for 1.5 h. A HeLa S3 cell clone expressing v-Src was generated in an inducible manner (HeLa S3/TR/v-Src). To induce DNA damage responses, cells were treated with 100 ng/ml Adriamycin (ADR) for 1 h. To inhibit NLS-Lyn-mediated tyrosine phosphorylation, cells were treated with 10 μm PP2 (Sigma) or 5–10 μm SU6656 (Sigma).
Western Blotting and Immunoprecipitation
Cell lysates were prepared in SDS-PAGE sample buffer or Triton X-100 lysis buffer (30 mm HEPES, pH 7.4, 100 mm NaCl, 0.5% Triton X-100, 4 mm EDTA, 10 mm NaF, 50 μg/ml aprotinin, 100 μm leupeptin, 25 μm pepstatin A, and 2 mm PMSF) and subjected to SDS-PAGE and electrotransferred onto polyvinylidene difluoride membranes (Millipore). Immunodetection was performed by enhanced chemiluminescence (Millipore), as described (32–34). Results were analyzed using a ChemiDoc XRS-Plus image analyzer (Bio-Rad). Immunoprecipitation was performed using antibody-precoated protein G beads, as described (9, 29, 33, 35). The intensity of chemiluminescence was measured using Quantity One software (Bio-Rad). Relative amounts of KAP1, HP1α, and histone H3K9me3 represent means ± S.D. from three independent experiments. Asterisks indicate the significant differences (*, p < 0.05; **, p < 0.01) calculated by Student's t test (Fig. 3, E and L). Composite figures were prepared using the GNU Image Manipulation Program version 2.6.2 software (GIMP) and Illustrator version 14.0 software (Adobe).
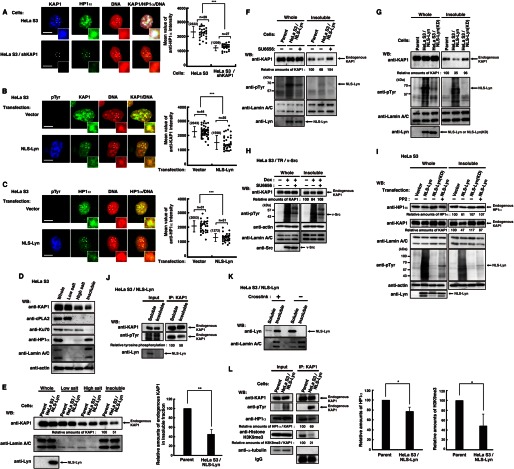
FIGURE 3.
Decrease in the levels of KAP1 association with heterochromatin induced by nuclear tyrosine phosphorylation. A, parental HeLa S3 cells or HeLa S3/shKAP1 cells were extracted, fixed, and triply stained with the indicated antibodies and PI. The plot represents the mean intensity of anti-HP1α staining in each cell. B and C, HeLa S3 cells transfected with vector or NLS-Lyn were cultured for 24 h. Cells were extracted, fixed, and triply stained with the indicated antibodies and PI. The plot represents the mean fluorescence intensity of anti-KAP1 (B) or anti-HP1α (C) staining in each cell. D, HeLa S3 cells were subjected to subcellular fractionation. Immunoblotting (WB) was performed with the indicated antibodies. E, parental HeLa S3 or HeLa S3/NLS-Lyn cells treated with 0.5 mm Na3VO4 were cultured for 1.5 h. Cells were subjected to subcellular fractionation. Immunoblotting was performed with the indicated antibodies. Relative amounts of KAP1 were quantitated. Error bars, S.D. F–I, cells were subjected to subcellular fractionation. Immunoblotting was performed with the indicated antibodies. F, parental HeLa S3 or HeLa S3/NLS-Lyn cells treated with SU6656 or DMSO for 3 h were cultured in the presence of 0.5 mm Na3VO4 during the last 1.5 h. G, parental HeLa S3, HeLa S3/NLS-Lyn, or HeLa S3/NLS-Lyn(KD) cells treated with 0.5 mm Na3VO4 were cultured for 1.5 h. H, HeLa S3/TR/v-Src cells treated with or without doxycycline (Dox) were cultured for 24 h in the presence of DMSO or SU6656 during the last 12 h. I, HeLa S3 cells transfected with the indicated plasmids were cultured for 36 h in the presence or absence of 10 μm PP2 during the last 24 h. J, HeLa S3/NLS-Lyn cells treated with 0.5 mm Na3VO4 were cultured for 1.5 h. Cells were subjected to subcellular fractionation. The insoluble fraction was suspended in high salt buffer and solubilized by sonication. Endogenous KAP1 was immunoprecipitated (IP) with anti-KAP1 antibody from the soluble and the insoluble fractions. Immunoblotting was performed with the indicated antibodies. K, HeLa S3/NLS-Lyn cells were cross-linked in medium containing 1% paraformaldehyde and incubated at 4 °C for 15 min. The cross-linking reaction was stopped by adding glycine to 0.125 m. Cells were subjected to subcellular fractionation. Immunoblotting was performed with the indicated antibodies. L, parental HeLa S3 or HeLa S3/NLS-Lyn cells treated with 0.5 mm Na3VO4 were cultured for 1.5 h and extracted by low salt buffer. The resultant insoluble fraction was suspended in high salt buffer, solubilized by sonication. Endogenous KAP1 was immunoprecipitated with anti-KAP1 antibody. Immunoblotting was performed with the indicated antibodies. Relative amounts of HP1α and histone H3K9me3 were quantitated.
Subcellular Fractionation
Cell pellets were washed with phosphate-buffered saline (PBS) and resuspended in low salt buffer (10 mm HEPES, pH 7.4, 10 mm KCl, 0.1% Triton X-100, 0.34 m sucrose, 10% glycerol, 1.7 mm MgCl2, 10 mm NaF, 4 mm β-glycerophosphate, 2 mm Na3VO4, 50 μg/ml aprotinin, 100 μm leupeptin, 25 μm pepstatin A, and 2 mm PMSF), and the cells were kept on ice for 10 min. Cytosolic proteins were separated from nuclei by centrifugation at 2,000 × g for 5 min. Isolated nuclei were lysed in high salt buffer (50 mm HEPES, pH 7.4, 300 mm KCl, 1.0% Triton X-100, 20% glycerol, 50 mm NaF, 10 mm β-glycerophosphate, 10 mm Na3VO4, 1 mm EDTA, 50 μg/ml aprotinin, 100 μm leupeptin, 25 μm pepstatin A, and 2 mm PMSF). After a 20-min incubation on ice, soluble nuclear proteins were separated from chromatin by centrifugation at 17,900 × g for 10 min. The resulting chromatin fraction was once washed with high salt buffer, solubilized in SDS sample buffer, and sheared by sonication (36–38).
Immunofluorescence
Confocal and differential interference-contrast images were obtained using a Fluoview Fv500 confocal laser-scanning microscope with a 40 × 1.00 or a 60 × 1.00 numerical aperture water immersion objective (Olympus, Tokyo), as described (15, 16, 39). One planar (xy) section slice (0.6- or 2.0-μm thickness) was shown in all experiments. For HP1α and KAP1 staining, cells were extracted with high salt buffer for 3 min on ice and fixed in 100% methanol for 5 min at −20 °C. Cells were permeabilized in PBS containing 0.2% Triton X-100 and 3% bovine serum albumin at room temperature (40). Cells were subsequently reacted with appropriate primary antibodies for 1 h, washed with PBS containing 0.1% saponin, and stained with FITC-, TRITC-, Alexa Fluor 488-, Alexa Fluor 546-, or Alexa Fluor 647-conjugated secondary antibodies for 1 h. For DNA staining, cells were treated with 200 μg/ml RNase A for 1 h and with 20 μg/ml propidium iodide (PI) or 200 nm TOPRO-3 for 30 min and mounted with Prolong AntifadeTM reagent (Molecular Probes, Inc.). The resulting red emission of TOPRO-3 and Alexa Fluor 647 are pseudocolored blue. Composite figures were prepared using GIMP version 2.6.2 and Illustrator version 14.0. For quantitation of amounts of KAP1 and HP1α, confocal images were obtained using a Fluoview Fv500 confocal laser-scanning microscope, and then fluorescence intensities of immunostaining were measured using ImageJ software (National Institutes of Health). Bars represent means ± S.D. from a representative experiment. Numbers in parentheses indicate mean values, and asterisks indicate significant differences (**, p < 0.01; ***, p < 0.001) calculated by Student's t test. Scale bars are 10 μm (Figs. 3 (A–C), 4 (B–E), and 5 (B–D)) and 20 μm (Figs. 1A, 4A, and 5A). Because two or three independent experiments gave similar results, a representative experiment is shown.
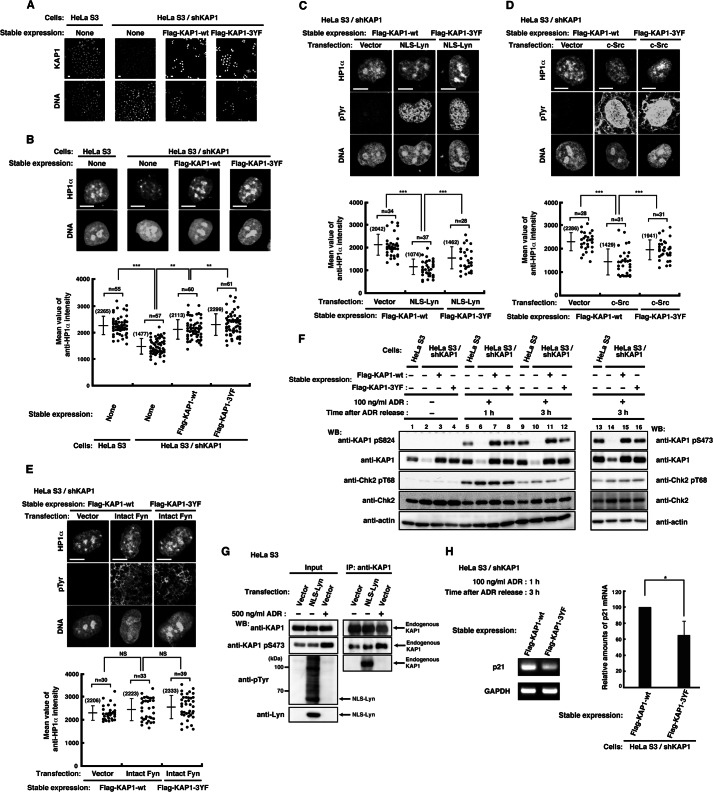
FIGURE 4.
Effect of tyrosine phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 on the association of HP1α with chromatin. A, parental HeLa S3 cells or HeLa S3/shKAP1 cells in which endogenous KAP1 had been knocked down and replaced with KAP1-wt or KAP1–3YF were fixed and doubly stained with anti-KAP1 antibody and PI. B, cells were extracted, fixed, and doubly stained with anti-HP1α antibody and PI. The plot represents the mean intensity of anti-HP1α staining in each cell. C–E, HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF were transfected with the indicated plasmids and cultured for 36 h. Cells were extracted, fixed, and triply stained with the indicated antibodies and TOPRO-3. The plot represents the mean intensity of anti-HP1α staining in each cell. F, HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF were treated with 100 ng/ml ADR for 1 h and lysed at the indicated times after the removal of ADR. Immunoblotting (WB) was performed with the indicated antibodies. G, HeLa S3 cells transfected with vector or NLS-Lyn were cultured for 24 h. Cells treated with DMSO or 500 ng/ml ADR for 1 h were cultured for 3 h after the removal of ADR or DMSO. Endogenous KAP1 was immunoprecipitated (IP) with anti-KAP1 antibody. Immunoblotting was performed with the indicated antibodies. H, cells were treated as described in F. The levels of p21 expression were assessed by semiquantitative RT-PCR, and the amounts of p21 product were quantitated by measuring band intensities and normalizing to the levels of GAPDH. Error bar, S.D.
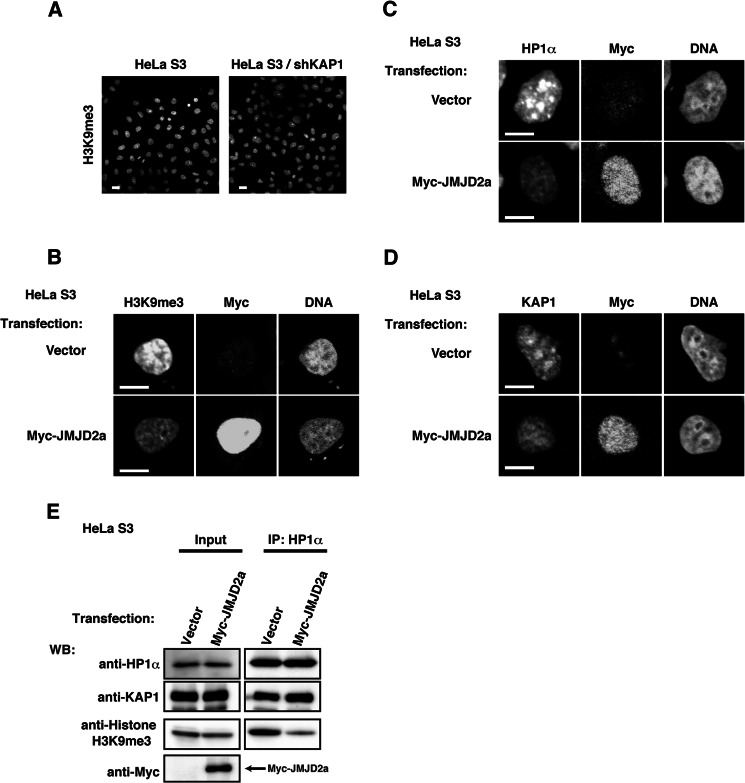
FIGURE 5.
Association of HP1α and KAP1 with heterochromatin through H3K9me3. A, parental HeLa S3 cells or HeLa S3/shKAP1 cells were fixed and stained with anti-H3K9me3 antibody. B–E, HeLa S3 cells transfected with vector or Myc-JMJD2a were cultured for 36 h. B, cells were fixed and triply stained with the indicated antibodies and TOPRO3. C and D, cells were extracted, fixed, and triply stained with the indicated antibodies and TOPRO3. E, cells were suspended in high salt buffer and solubilized by sonication (insoluble fraction). HP1α was immunoprecipitated (IP) with anti-HP1α antibody. Immunoblotting (WB) was performed with the indicated antibodies.
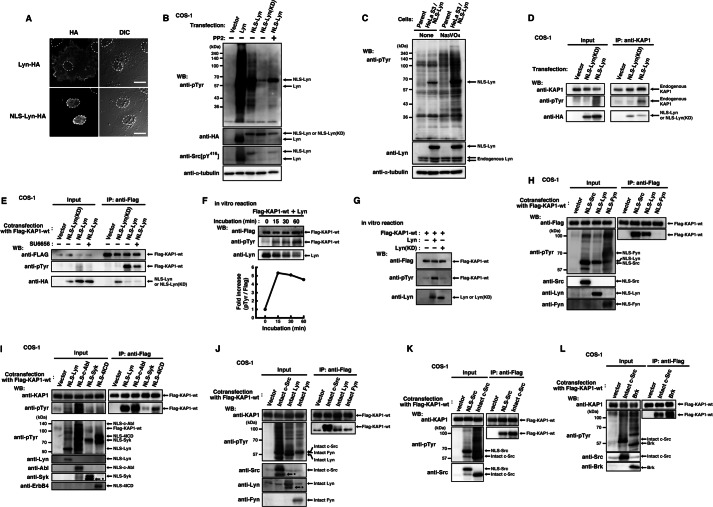
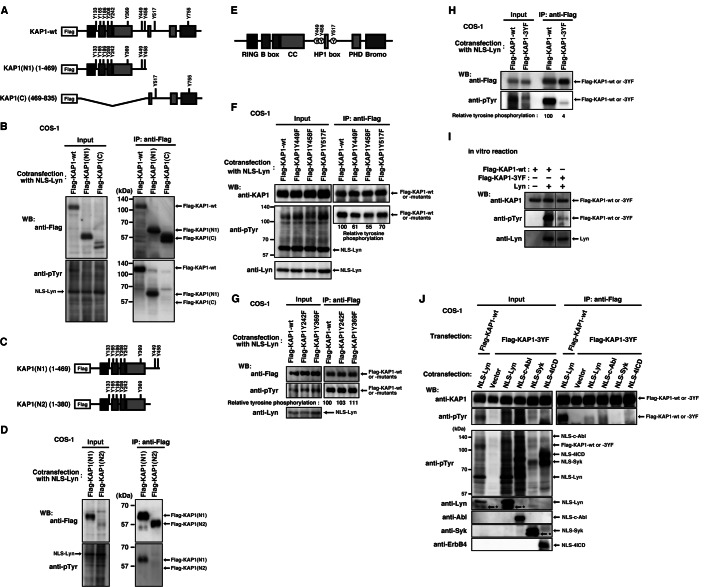
FIGURE 1.
Tyrosine phosphorylation of KAP1 induced by nuclear tyrosine kinases. A, COS-1 cells transfected with Lyn-HA or NLS-Lyn-HA were cultured for 24 h. Cells were fixed and stained with anti-HA antibody. The areas of nuclei are marked by dotted lines. DIC, differential interference-contrast. B, COS-1 cells transfected with the indicated plasmids were cultured for 24 h in the presence or absence of 10 μm PP2 during the last 12 h. Immunoblotting (Western blot; WB) was performed with the indicated antibodies. C, parental HeLa S3 or HeLa S3/NLS-Lyn cells treated with or without 0.5 mm sodium orthovanadate (Na3VO4) were cultured for 1.5 h. Immunoblotting was performed with the indicated antibodies. D, COS-1 cells transfected with the indicated plasmids were cultured for 24 h. Endogenous KAP1 was immunoprecipitated (IP) with anti-KAP1 antibody. Immunoblotting was performed with the indicated antibodies. E, COS-1 cells cotransfected with KAP1-wt plus the indicated plasmids were cultured for 24 h in the presence of 10 μm SU6656 or DMSO (dimethyl sulfoxide, solvent control) during the last 3 h. KAP1-wt was immunoprecipitated with anti-FLAG antibody. Immunoblotting was performed with the indicated antibodies. F, KAP1-wt immunoprecipitates were incubated with Lyn immunoprecipitates in reaction buffer. Samples were taken at the indicated times. Immunoblotting was performed for FLAG, Tyr(P), and Lyn. The levels of tyrosine phosphorylation at each incubation time (15, 30, and 60 min) relative to that of the control (0 min) are shown. G, KAP1-wt immunoprecipitates were incubated with Lyn or Lyn(KD) immunoprecipitates for 30 min in reaction buffer. Immunoblotting was performed with the indicated antibodies. H–L, COS-1 cells cotransfected with KAP1-wt plus the indicated plasmids were cultured for 24 h. KAP1-wt was immunoprecipitated with anti-FLAG antibody. Immunoblotting was performed with the indicated antibodies. Asterisks show degradation products.
In Vitro Kinase Assay
Lysates were prepared in Triton X-100 lysis buffer. Immunoprecipitation was performed using anti-FLAG or anti-HA antibody-precoated protein G beads. Immunodetection was performed as reported (41). In vitro kinase assays were performed as described (14, 32, 35, 42). In brief, Lyn was immunoprecipitated with anti-HA antibody from Triton X-100 lysates of COS-1 cells transfected with Lyn (Lyn-HA) or Lyn(KD) (Lyn(KD)-HA). After washing, equal amounts of each immunoprecipitate were reacted with FLAG peptide-eluted FLAG-KAP1 in kinase buffer (40 mm HEPES, pH 7.4, 0.1% Triton X-100, 5 mm MnCl2, 5 mm MgCl2, 1 mm Na3VO4) containing 100 μm unlabeled ATP at 30 °C for the indicated periods. Phosphorylated bands were immunodetected with anti-Tyr(P) antibody, and the intensity of chemiluminescence was measured using Quantity One software (Bio-Rad). Composite figures were prepared using GIMP version 2.6.2 and Illustrator version 14.0.
Identification of p110 by Peptide Mapping
Parental HeLa S3 or HeLa S3/NLS-Lyn cells were treated with 0.5 mm Na3VO4 for 1.5 h and lysed with SDS-lysis buffer (100 mm Tris, pH 6.8, 3% SDS, 20% glycerol, 10 mm Na3VO4). Cell lysates were boiled at 95 °C for 5 min and sonicated. To dilute SDS to a concentration of 0.1%, wash buffer (30 mm HEPES, pH 7.4, 300 mm NaCl, 1.0% Triton X-100) was added before immunoprecipitation. Tyrosine-phosphorylated proteins were collected on anti-Tyr(P) antibody-precoated protein G beads from cell lysates. After extensively washing the beads with wash buffer, the immune pellets were analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. The protein band corresponding to p110 was cut out and digested with trypsin (Trypsin Gold; Promega). After the digestion, molecular mass analysis of trypsin fragments was performed by LC/MS/MS. Identification of the protein was carried out by comparison between the molecular weights determined by LC/MS/MS and theoretical masses.
Semiquantitative RT-PCR
Total RNAs were isolated from cells with the TRIzol reagent (Invitrogen), and cDNAs were synthesized from 1 μg of each RNA preparation using the PrimeScript RT reagent kit (TakaraBio, Shiga) as described (16). To avoid saturation of PCR products, conditions of PCR were optimized before semiquantitative RT-PCR was carried out. The primers used for PCR are as follows: p21, 5′-actctcagggtcgaaaacgg-3′ (sense) and 5′-cttcctgtgggcggattagg-3′ (antisense); glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-accacagtccatgccatcac-3′ (sense) and 5′-tccaccaccctgttgctgta-3′ (antisense) (16, 17). The sizes of PCR products are 104 bp for p21 and 452 bp for GAPDH. Amplification was carried out using an MJ mini thermal cycler (Bio-Rad) with Ex TaqDNA polymerase (TakaraBio) under the following conditions: for p21, initial heating at 94 °C for 2 min, followed by 27 cycles of denaturation at 94 °C for 30 s, annealing at 63 °C for 30 s, and extension at 72 °C for 1 min; for GAPDH, initial heating at 94 °C for 2 min, followed by 25 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 1 min. The products of RT-PCR were electrophoresed on a 2.5% agarose gel containing ethidium bromide. The density of each amplified fragment was quantitated using a ChemiDoc XRSPlus image analyzer and Quantity One software. The relative amount of p21 represents the mean ± S.D. from three independent experiments. An asterisk indicates the significant difference (*, p < 0.05) calculated by Student's t test (Fig. 4H).
RESULTS
Tyrosine Phosphorylation of KAP1
To identify tyrosine-phosphorylated proteins in the nucleus, we established cell lines expressing Lyn tyrosine kinase tagged with a nuclear localization signal (NLS-Lyn). Affinity-purified proteins using anti-Tyr(P) antibody were excised from an SDS-polyacrylamide gel and subjected to proteolytic cleavage followed by LC/MS/MS. The peptide derived from p110 exhibited 100% identity with the amino acid sequence of human KAP1 (Fig. 1, A–C, and supplemental Table S2), a component of chromatin structure (43).
To verify tyrosine phosphorylation of KAP1, we transfected COS-1 cells with NLS-Lyn or NLS-Lyn(KD) and immunoprecipitated endogenous KAP1 from cell lysates. Endogenous KAP1 was tyrosine-phosphorylated by NLS-Lyn but not NLS-Lyn(KD) (Fig. 1D). Furthermore, cells were cotransfected with KAP1-wt plus NLS-Lyn or KAP1-wt plus NLS-Lyn(KD) and cultured in the presence or absence of the SFK inhibitor SU6656. Expressed KAP1-wt was also tyrosine-phosphorylated by NLS-Lyn but not NLS-Lyn(KD), and treatment with SU6656 inhibited tyrosine phosphorylation of KAP1-wt (Fig. 1E). These results suggest that tyrosine phosphorylation of KAP1 is induced by NLS-Lyn. Furthermore, we tested whether Lyn directly phosphorylated KAP1 at tyrosine residues. KAP1-wt, Lyn, and Lyn(KD) were immunoprecipitated from respective transfected cells and subjected to in vitro kinase assays (Fig. 1, F and G). Tyrosine phosphorylation of KAP1-wt by Lyn was observed in a kinase activity-dependent manner, suggesting that Lyn directly phosphorylates KAP1 at tyrosine residues.
To examine whether the other SFK members were able to phosphorylate KAP1 at tyrosine residues, we cotransfected cells with KAP1-wt in conjunction with NLS-Lyn, NLS-Src, or NLS-Fyn, which are all localized to the nucleus (10) (Fig. 1A). Unlike NLS-Fyn, NLS-Src and NLS-Lyn were able to phosphorylate KAP1 at tyrosine residues (Fig. 1H). Previously, we showed a strong relationship between various tyrosine kinases and chromatin structural changes (10, 16, 17). Next, we examined whether the other families of tyrosine kinases were able to phosphorylate KAP1. NLS-c-Abl, NLS-Syk, and NLS-4ICD were found to phosphorylate KAP1-wt at tyrosine residues, irrespective of various levels of tyrosine phosphorylation of KAP1-wt (Fig. 1I).
Next, to examine the effect of the NLS sequence per se on KAP1 phosphorylation, we cotransfected cells with KAP1-wt in conjunction with intact c-Src, intact Lyn, or intact Fyn. Like NLS-Src, intact c-Src strongly phosphorylated KAP1-wt at tyrosine residues (Fig. 1, H and J–L). Intact Lyn also phosphorylated KAP1-wt at tyrosine residues despite the low levels of tyrosine phosphorylation (Fig. 1J). The levels of tyrosine phosphorylation of KAP1 were increased when NLS-Lyn was used in place of intact Lyn (Fig. 1, compare H with J). However, neither NLS-Fyn nor intact Fyn phosphorylated KAP1-wt at tyrosine residues (Fig. 1, H and J). Brk, a non-receptor-type tyrosine kinase that localizes in the nucleus, also phosphorylated KAP1-wt at tyrosine residues (Fig. 1L). These results suggest that KAP1 is a substrate for various tyrosine kinases that localize in the nucleus, and their nuclear localization is critical for tyrosine phosphorylation of KAP1.
Tyrosine Phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517
To determine the tyrosine phosphorylation sites of KAP1, we constructed several deletion mutants of KAP1 (Fig. 2, A and C). Cells were cotransfected with NLS-Lyn plus KAP1-wt or KAP1 deletion mutants, and we found that NLS-Lyn induced tyrosine phosphorylation of KAP1(N1)(1–469) but not KAP1(C)(469–835) and KAP1(N2)(1–380) (Fig. 2, B and D). In addition, phospho-Tyr-517 on KAP1 was detected by mass spectrometry (supplemental Table S2). Given that KAP1 has only two tyrosine residues at positions 449 and 458 (Tyr-449 and Tyr-458) in the region between 380 and 469, we constructed KAP1 mutants, which are mutated at Tyr-449, Tyr-458, and Tyr-517 to phenylalanine (KAP1-Y449F, KAP1-Y458F, KAP1-Y517F, and KAP1–3YF) (Fig. 2E). Cotransfection of cells with NLS-Lyn plus KAP1-wt or NLS-Lyn plus KAP1 mutants showed that Tyr-449, Tyr-458, and Tyr-517 were phosphorylated to a similar extent (Fig. 2, F and G). Importantly, we found that tyrosine phosphorylation of KAP1–3YF was barely detected (Fig. 2H). Similar results were obtained with in vitro kinase assays (Fig. 2I). These results suggest that Tyr-449, Tyr-458, and Tyr-517 are the major tyrosine phosphorylation sites of KAP1.
FIGURE 2.
Tyrosine phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517. A, C, E, a schematic representation of KAP1. RING, RING finger domain; B box, B box zinc fingers 1 and 2; CC, coiled-coil domain; HP1 box, heterochromatin protein 1 box; PHD, plant homeodomain; Bromo, Bromo domain. B, D, and F–H, COS-1 cells cotransfected with NLS-Lyn plus the indicated plasmids were cultured for 24 h. KAP1-wt and its mutants were immunoprecipitated (IP) with anti-FLAG antibody. Immunoblotting (WB) was performed with the indicated antibodies. I, KAP1-wt or KAP1–3YF immunoprecipitates were incubated with Lyn immunoprecipitates, as described in the legend to Fig. 1G. Immunoblotting was performed with the indicated antibodies. J, COS-1 cells cotransfected with KAP1-wt plus NLS-Lyn or KAP1–3YF plus the indicated plasmids were cultured for 24 h. KAP1-wt and KAP1–3YF were immunoprecipitated with anti-FLAG antibody. Immunoblotting was performed with the indicated antibodies. Asterisks show degradation products.
Next, we examined whether various tyrosine kinases phosphorylated the same tyrosine residues of KAP1. In fact, we found that NLS-Lyn, NLS-c-Abl, NLS-Syk, and NLS-4ICD did not induce tyrosine phosphorylation of KAP1–3YF in cells cotransfected with KAP1–3YF plus each tyrosine kinase (Fig. 2J; see Fig. 1I), indicating that Tyr-449, Tyr-458, and Tyr-517 are the common tyrosine phosphorylation sites of KAP1 for various tyrosine kinases.
Inhibitory Role of Tyrosine Phosphorylation in Recruitment of KAP1 and HP1α to Chromatin
KAP1 binds to chromatin and acts as a scaffold for the assembly of heterochromatin proteins, including heterochromatin protein 1α (HP1α), that participate in heterochromatin formation (44–46). To examine the state of chromatin-bound proteins, cells were extracted with high salt buffer before fixation and immunostained for KAP1 and HP1α. Consistent with previous studies (37, 47), immunostaining showed that both endogenous KAP1 and HP1α were strongly stained within heterochromatic condensed chromatin, compared with decondensed chromatin (Fig. 3A). Moreover, we established KAP1 knockdown cell lines (HeLa S3/shKAP1) and immunostained for HP1α. KAP1 knockdown was found to decrease the levels of chromatin-bound HP1α (Figs. 3A and 4B). These results substantiate the co-association of KAP1 and HP1α within heterochromatin.
To examine the effect of nuclear tyrosine phosphorylation on KAP1 binding to chromatin, we transfected HeLa S3 cells with NLS-Lyn and immunostained for chromatin-bound KAP1. Intriguingly, the levels of chromatin-bound KAP1 were decreased upon NLS-Lyn expression (Fig. 3B). Furthermore, we immunostained for chromatin-bound HP1α and found that upon NLS-Lyn expression, the levels of chromatin-bound HP1α were decreased within heterochromatic condensed chromatin as well as those of chromatin-bound KAP1 (Fig. 3, B and C). These results suggest that nuclear tyrosine phosphorylation inhibits recruitment of KAP1 and HP1α to heterochromatin.
Effect of Tyrosine Phosphorylation on the Association of KAP1 with Heterochromatin
To examine whether nuclear tyrosine phosphorylation inhibited KAP1 association with heterochromatin, cells were subfractionated into the low salt soluble fraction, the high salt soluble fraction, and the insoluble fraction (see “Experimental Procedures”). The low salt soluble fraction contained the cytosolic protein cPLA2, and the high salt soluble fraction contained the chromatin-binding protein Ku70 (Fig. 3D). The insoluble fraction, which we focused on, contained the nuclear matrix protein lamin A/C and the heterochromatin protein HP1α. KAP1 was distributed into the low salt soluble fraction, the high salt soluble fraction, and the insoluble fraction (Fig. 3D), confirming that KAP1 is a component of heterochromatin, but not all of KAP1 associates with heterochromatin. In fact, nearly 25% of KAP1 binds to highly compacted heterochromatin (48). We subfractionated parental and NLS-Lyn-expressing cells and found that the amounts of endogenous KAP1 were decreased in the insoluble fraction upon NLS-Lyn expression, although the protein levels of KAP1 in whole cells were almost unchanged irrespective of NLS-Lyn expression (Fig. 3E; see also Fig. 3K). These results suggest that the association of KAP1 with heterochromatin is inhibited upon NLS-Lyn expression.
Next, we examined the inhibitory role of NLS-Lyn kinase activity in the association of KAP1 with heterochromatin. Treatment with SU6656 inhibited the NLS-Lyn-mediated decrease in the amounts of endogenous KAP1 in the insoluble fraction (Fig. 3F). Upon NLS-Lyn(KD) expression, the amounts of endogenous KAP1 were not decreased in the insoluble fraction (Fig. 3G). These results suggest that the tyrosine kinase activity is critical for a decrease in the levels of KAP1 association with heterochromatin.
Next we examined whether c-Src, which strongly phosphorylated KAP1 (see Fig. 1J), decreased the amounts of KAP1 in the insoluble fraction. We subfractionated HeLa S3 cells inducibly expressing v-Src, a highly activated variant of c-Src. The amounts of endogenous KAP1 were decreased in the insoluble fraction when v-Src was induced, and the decrease was inhibited by SU6656 treatment (Fig. 3H). Furthermore, we found that the amounts of HP1α were decreased in the insoluble fraction in a kinase activity-dependent manner as well as those of KAP1 (Fig. 3I). These results suggest that nuclear tyrosine phosphorylation decreases the association of KAP1 and HP1α with heterochromatin.
To examine whether tyrosine-phosphorylated KAP1 decreased its association with heterochromatin, we compared the tyrosine phosphorylation levels of KAP1 in the soluble fraction with those in the insoluble fraction. The tyrosine phosphorylation levels of endogenous KAP1 in immunoprecipitates from the soluble fraction were about 2 times as high as those from the insoluble fraction (Fig. 3, J and K). Then we examined the effect of NLS-Lyn on KAP1 association with HP1α and H3K9me3 (44, 45, 49). HP1α and H3K9me3 were coimmunoprecipitated with endogenous KAP1, and we found that the amounts of HP1α and H3K9me3 coimmunoprecipitated with KAP1 were decreased when KAP1 was tyrosine-phosphorylated (Fig. 3L). These results suggest that tyrosine phosphorylation of KAP1 impairs its association with heterochromatin.
Inhibitory Role of Tyrosine Phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 in HP1α Association with Chromatin
Tyrosine phosphorylation of KAP1 decreased the amounts of chromatin-bound KAP1 and HP1α (Fig. 3, B and C). To examine the effect of tyrosine phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 on HP1α association with chromatin, endogenous KAP1 was complemented by stable expression of shRNA-resistant KAP1-wt or KAP1–3YF in HeLa S3/shKAP1 cells. We confirmed the expression of shRNA-resistant KAP1 in these cell lines with immunostaining and Western blotting (Fig. 4, A and F). HeLa S3 cells, HeLa S3/shKAP1 cells, and HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF were immunostained for HP1α. KAP1 knockdown decreased the amounts of chromatin-bound HP1α (Fig. 4B; see also Fig. 3A). It is of note that the levels of chromatin-bound HP1α in KAP1–3YF-expressing cells were significantly increased compared with those in KAP1-wt-expressing cells (Fig. 4B). To further ascertain the effect of tyrosine phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 on HP1α association with chromatin, HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF were transfected with NLS-Lyn. The amounts of chromatin-bound HP1α were decreased in KAP1-wt-expressing cells upon NLS-Lyn expression, and the decrease was inhibited in KAP1–3YF-expressing cells (Fig. 4C). These results suggest that phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 has an inhibitory role in HP1α association with chromatin.
Next, HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF were transfected with c-Src or Fyn. c-Src, which strongly phosphorylated KAP1, induced a decrease in the amounts of chromatin-bound HP1α in KAP1-wt-expressing cells, and the decrease was inhibited in KAP1–3YF-expressing cells (Fig. 4D; see also Fig. 1J). In contrast to c-Src, Fyn, which did not phosphorylate KAP1, had no effect on HP1α association with chromatin (Fig. 4E; see also Fig. 1J). Intriguingly, tyrosine phosphorylation mediated by c-Src was detected in the nucleus, but tyrosine phosphorylation mediated by Fyn was detected largely in the cytoplasm (Fig. 4, D and E). These results suggest that c-Src decreases HP1α association with chromatin through phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 in the nucleus.
KAP1 phosphorylation at serine 824 (Ser(P)-824) by ATM and at serine 473 (Ser(P)-473) by Chk2 plays important roles in DNA damage responses (27, 37, 50), and the tyrosine kinases Lyn and c-Abl are activated in DNA damage responses (51, 52). We thus examined the effect of KAP1 tyrosine phosphorylation on KAP1-Ser(P)-824 and KAP1-Ser(P)-473. HeLa S3 cells, HeLa S3/shKAP1 cells, and HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF were treated with the DNA-damaging agent ADR. The levels of Ser(P)-824 and Ser(P)-473 of KAP1–3YF were found to be lower than those of KAP1-wt (Fig. 4F, compare lane 7 with lane 8, lane 11 with lane 12, and lane 15 with lane 16). However, the levels of KAP1-Ser(P)-473 were not changed without DNA damage, irrespective of tyrosine phosphorylation of KAP1 (Fig. 4G). These results suggest that tyrosine phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 is involved in phosphorylation of KAP1 at Ser-824 and Ser-473 in DNA damage responses.
Because phosphorylation of KAP1 at Ser-824 and Ser-473 is required for transcription of p21 in DNA damage responses (50), we analyzed the expression levels of the p21 mRNA in HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF treated with ADR. Semiquantitative RT-PCR analysis showed that the levels of p21 gene expression were decreased in KAP1–3YF expressing cells compared with KAP1-wt expressing cells (Fig. 4H). These results suggest that tyrosine phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 is involved in DNA damage responses.
DISCUSSION
In the present study, we show that KAP1 is phosphorylated at Tyr-449, Tyr-458, and Tyr-517. These three tyrosine residues are the common tyrosine phosphorylation sites of KAP1 for various tyrosine kinases that are localized within the nucleus. We further show that tyrosine phosphorylation of KAP1 decreases its association with heterochromatin, which is accompanied by a decrease in the amounts of chromatin-bound HP1α.
Previous reports showed post-translational modifications of KAP1 and its importance for proper functions. Phosphorylation of KAP1 at Ser-473 decreases its association with HP1, and phosphorylation at Ser-824 relaxes chromatin structure (27, 53). SUMOylation of KAP1 is required for gene silencing (54). Although tyrosine phosphorylation of KAP1 is reported to be induced by c-Fes tyrosine kinase (55) and PDGF stimulation (56), the major tyrosine phosphorylation sites of KAP1 have not been identified thus far, and the roles of tyrosine phosphorylation of KAP1 are largely unknown.
We have been studying nuclear tyrosine phosphorylation and the effect of nuclear tyrosine phosphorylation on chromatin structural changes. Chk tyrosine kinase localizes within the nucleus and brings about growth retardation and aberrant chromosome movement leading to multinucleation (14, 57). SFK and c-Abl tyrosine kinase localized in the nucleus induce chromatin structural changes (10, 16). In this study, to understand the regulatory mechanism of chromatin structural changes through tyrosine phosphorylation, we tried to identify nuclear tyrosine-phosphorylated proteins by mass spectrometry. Tyrosine phosphorylation induced by NLS-Lyn was barely detected without Na3VO4, an inhibitor of tyrosine phosphatases (Fig. 1C). We thus incubated NLS-Lyn-expressing cells in medium supplemented with Na3VO4 (Fig. 1C), because tyrosine phosphatases may be abundant in the nucleus (4). Taken together with the fact that Na3VO4 treatment greatly increases nuclear tyrosine phosphorylation (10), these results suggest that tyrosine-phosphorylated proteins may be rapidly dephosphorylated in the nucleus. In addition to Na3VO4 treatment, to further inactivate tyrosine phosphatase activities, cell lysates were boiled before immunoprecipitation (also see “Experimental Procedures”). These methods enabled us to identify KAP1 as a nuclear tyrosine-phosphorylated protein (supplemental Table S2). Tyrosine phosphorylation of KAP1 was induced by various tyrosine kinases (Fig. 1). In addition to the tyrosine kinases that we examined in this study, the other tyrosine kinases may also phosphorylate KAP1.
To find the tyrosine phosphorylation sites of KAP1, we tried to predict the sites with Web-based programs. NetPhos version 2.0 predicts three tyrosine phosphorylation sites, Tyr-208, Tyr-458, and Tyr-755, on KAP1. Scansite predicts the other tyrosine phosphorylation sites Tyr-242 and Tyr-517 besides Tyr-755. Although both computer programs predict Tyr-755 as a tyrosine phosphorylation site, in fact our results indicate that Tyr-755 is not the main tyrosine phosphorylation site. Phosphorylation at Tyr-755 was not detected by mass spectrometry, and KAP1(C) containing Tyr-755 was weakly phosphorylated (supplemental Table S2 and Fig. 2, A–D). In the PhosphoSitePlus proteomics database, the phosphorylation sites of KAP1 at Tyr-458 and Tyr-517 are most frequently shown in SW480 cells, Jurkat T cells, K562 cells, etc., and the phosphorylation site at Tyr-449 is also listed. As indicated in the PhosphoSitePlus database, we detected that the two tyrosine residues Tyr-458 and Tyr-517 on KAP1 are the major phosphorylation sites and that Tyr-449 is heavily phosphorylated as well (Fig. 2, F and G). We therefore conclude that the three tyrosine residues Tyr-449, Tyr-458, and Tyr-517 are the major tyrosine phosphorylation sites of KAP1 (Fig. 2).
NLS-Lyn induced tyrosine phosphorylation of KAP1 much more strongly than intact Lyn (Fig. 1, F–J), suggesting that nuclear localization is important to induce tyrosine phosphorylation of KAP1. Recently, we reported that Lyn mutants lacking the lipid modification sites are accumulated in the nucleus, compared with Lyn-wt (9). At present, myristoylation and palmitoylation are known as the major forms of lipid modification of tyrosine kinases (58). c-Src and Brk are not palmitoylated, whereas Lyn and Fyn are palmitoylated (11, 59). Intriguingly, c-Src and Brk induced high levels of tyrosine phosphorylation of KAP1, compared with Lyn and Fyn (Fig. 1, J–L). Immunostaining showed that c-Src but not Fyn induces tyrosine phosphorylation in the nucleus (Fig. 4, D and E). It is possible that palmitoylation negatively regulates nuclear localization of SFK and tyrosine phosphorylation of KAP1. Alternatively, it is conceivable that the levels of tyrosine phosphorylation of KAP1 are determined by the substrate specificity, because unlike NLS-Src and NLS-Lyn, NLS-Fyn did not phosphorylate KAP1 (Fig. 1H).
KAP1 is a component of chromatin structure (43). We found that tyrosine phosphorylation of KAP1 decreases its association with heterochromatin (Fig. 3, B and E–L), leading to an intriguing hypothesis that tyrosine phosphorylation of KAP1 takes place in highly compacted heterochromatin (the insoluble fraction), and tyrosine-phosphorylated KAP1 can be released from the insoluble fraction into the high salt soluble fraction. Previous studies showed that KAP1 directly associates with HP1α and plays important roles in chromatin binding of HP1α (43, 60). KAP1 knockdown affects the level of histone H3 Lys-9 methylation that is required for chromatin binding of HP1α (61) and compromises recruitment of HP1α to DNA damage sites (62). Although our results showed that the levels of H3K9me3 are not drastically changed, we substantiated that KAP1 knockdown decreases the amounts of chromatin-bound HP1α (Figs. 3A and 5A). When the histone demethylase JMJD2a is expressed, the levels of H3K9me3 and the amounts of chromatin-bound HP1α are drastically decreased (Fig. 5, B and C), consistent with previous studies (63). We revealed that JMJD2a expression also decreases the amounts of chromatin-bound KAP1 as well as those of HP1α (Fig. 5D), suggesting that the association of KAP1 and HP1α with chromatin is regulated by chromatin structure. However, the interaction of KAP1 and HP1 was not affected by chromatin structure (Fig. 5E). Therefore, KAP1 could strengthen the association of HP1α with heterochromatin.
The association of HP1α with heterochromatin is evidently decreased through phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 (Fig. 4, B–D). Previous studies showed that KAP1 associates with HP1α through the HP1-binding motif (PXVXL, amino acids 486–490), and the mutation of this motif to PEESL (amino acids 486–490) abolishes the interaction of KAP1 with HP1 (44, 60). The tyrosine phosphorylation sites of KAP1 identified in this study are located close to the HP1-binding motif (Fig. 2E), leading to the hypothesis that tyrosine phosphorylation of KAP1 affects HP1 binding to KAP1 (see also Fig. 3L). Although the association of KAP1 with HP1α is disturbed also by phosphorylation of KAP1 at Ser-473 (53), the level of KAP1-Ser(P)-473 was not increased by tyrosine phosphorylation of KAP1 without ADR treatment (Fig. 4G). These results suggest that the inhibitory effect of tyrosine phosphorylation of KAP1 on the association of KAP1 with heterochromatin was not due to the induction of Ser(P)-473.
Upon ADR treatment, the levels of Ser(P)-824 and Ser(P)-473 of KAP1–3YF were lower than those of KAP1-wt (Fig. 4F). Given that the levels of Ser(P)-824 and Ser(P)-473 of KAP1 with mutation in the HP1-binding motif are higher than those of wild-type KAP1 (64), we can speculate that an increase of the association of KAP1–3YF with heterochromatin and HP1α may cause a decrease in the levels of Ser(P)-824 and Ser(P)-473 on KAP1–3YF, which leads to repression of p21 (Fig. 4H).
Although Jak2 tyrosine kinase phosphorylates histone H3 at Tyr-41 and excludes HP1 from chromatin (65), we are able to present another type of evidence for HP1 exclusion from chromatin. It is known that KAP1 is a critical regulator of development and differentiation through gene silencing and heterochromatin formation (43). KAP1 knock-out embryos are arrested in their development at the early egg cylinder stage (66). KAP1 is therefore required for epigenetic stability during the mouse oocyte-to-embryo transition (67). Furthermore, association of KAP1 with HP1, which is also important for heterochromatin formation and gene silencing (46), is required for corepressor functions of KAP1 (68). Taken together, tyrosine phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 may be related to development and differentiation. Generation of KAP1–3YF knock-in mice might enable us to investigate a role of tyrosine phosphorylation of KAP1 in development and differentiation.
In conclusion, we show that Tyr-449, Tyr-458, and Tyr-517 on KAP1 are the major tyrosine phosphorylation sites for various tyrosine kinases, including SFKs. Moreover, tyrosine phosphorylation of KAP1 has inhibitory roles in its association with heterochromatin. Further studies will help us to understand the relationship between nuclear tyrosine phosphorylation and chromatin structure.
Acknowledgments
We are grateful to Dr. Hiroyoshi Ariga (Hokkaido University), Dr. Donald J. Fujita (University of Calgary), Dr. Tadashi Yamamoto (University of Tokyo), Dr. Eli Canaani (Weizmann Institute of Science), Dr. Toshiki Tamura (National Institute of Infectious Diseases), Dr. Takayuki Yoshimoto (Tokyo Medical University), Dr. Edward A. Clark (University of Washington), Dr. Atsushi Iwama (Chiba University), Dr. Shigeyuki Yokoyama (RIKEN), and Dr. Hiroyuki Miyoshi (RIKEN BRC) for invaluable plasmids and antibodies.
This work was supported in part by grants-in-aid for scientific research, the Global Center for Education and Research in Immune Regulation and Treatment (G-COE) Program, and Special Funds for Education and Research (Development of SPECT Probes for Pharmaceutical Innovation) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.

This article contains supplemental Tables S1 and S2.
- SFK
- Src family kinase
- NLS
- nuclear localization signal
- KD
- kinase-dead
- H3K9me3
- histone H3 trimethylated on lysine 9
- TRITC
- tetramethylrhodamine isothiocyanate
- ADR
- Adriamycin
- PI
- propidium iodide
- KAP1-wt
- wild-type KAP1
- KAP1–3YF
- KAP1-Y449F/Y458F/Y517F mutant.
REFERENCES
- 1. Hubbard S. R., Till J. H. (2000) Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 69, 373–398 [DOI] [PubMed] [Google Scholar]
- 2. Hunter T. (2009) Tyrosine phosphorylation. Thirty years and counting. Curr. Opin. Cell Biol. 21, 140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cans C., Mangano R., Barilá D., Neubauer G., Superti-Furga G. (2000) Nuclear tyrosine phosphorylation. The beginning of a map. Biochem. Pharmacol. 60, 1203–1215 [DOI] [PubMed] [Google Scholar]
- 4. Moorhead G. B., Trinkle-Mulcahy L., Ulke-Lemée A. (2007) Emerging roles of nuclear protein phosphatases. Nat. Rev. Mol. Cell Biol. 8, 234–244 [DOI] [PubMed] [Google Scholar]
- 5. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 6. Ullrich A., Schlessinger J. (1990) Signal transduction by receptors with tyrosine kinase activity. Cell 61, 203–212 [DOI] [PubMed] [Google Scholar]
- 7. Brown M. T., Cooper J. A. (1996) Regulation, substrates, and functions of Src. Biochim. Biophys. Acta 1287, 121–149 [DOI] [PubMed] [Google Scholar]
- 8. Thomas S. M., Brugge J. S. (1997) Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 [DOI] [PubMed] [Google Scholar]
- 9. Ikeda K., Nakayama Y., Togashi Y., Obata Y., Kuga T., Kasahara K., Fukumoto Y., Yamaguchi N. (2008) Nuclear localization of Lyn tyrosine kinase mediated by inhibition of its kinase activity. Exp. Cell Res. 314, 3392–3404 [DOI] [PubMed] [Google Scholar]
- 10. Takahashi A., Obata Y., Fukumoto Y., Nakayama Y., Kasahara K., Kuga T., Higashiyama Y., Saito T., Yokoyama K. K., Yamaguchi N. (2009) Nuclear localization of Src-family tyrosine kinases is required for growth factor-induced euchromatinization. Exp. Cell Res. 315, 1117–1141 [DOI] [PubMed] [Google Scholar]
- 11. Derry J. J., Richard S., Valderrama Carvajal H., Ye X., Vasioukhin V., Cochrane A. W., Chen T., Tyner A. L. (2000) Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol. Cell Biol. 20, 6114–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taagepera S., McDonald D., Loeb J. E., Whitaker L. L., McElroy A. K., Wang J. Y., Hope T. J. (1998) Nuclear-cytoplasmic shuttling of c-Abl tyrosine kinase. Proc. Natl. Acad. Sci. U.S.A. 95, 7457–7462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni C. Y., Murphy M. P., Golde T. E., Carpenter G. (2001) γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294, 2179–2181 [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi N., Nakayama Y., Urakami T., Suzuki S., Nakamura T., Suda T., Oku N. (2001) Overexpression of the Csk homologous kinase (Chk tyrosine kinase) induces multinucleation. A possible role for chromosome-associated Chk in chromosome dynamics. J. Cell Sci. 114, 1631–1641 [DOI] [PubMed] [Google Scholar]
- 15. Nakayama Y., Yamaguchi N. (2005) Multi-lobulation of the nucleus in prolonged S phase by nuclear expression of Chk tyrosine kinase. Exp. Cell Res. 304, 570–581 [DOI] [PubMed] [Google Scholar]
- 16. Aoyama K., Fukumoto Y., Ishibashi K., Kubota S., Morinaga T., Horiike Y., Yuki R., Takahashi A., Nakayama Y., Yamaguchi N. (2011) Nuclear c-Abl-mediated tyrosine phosphorylation induces chromatin structural changes through histone modifications that include H4K16 hypoacetylation. Exp. Cell Res. 317, 2874–2903 [DOI] [PubMed] [Google Scholar]
- 17. Ishibashi K., Fukumoto Y., Hasegawa H., Abe K., Kubota S., Aoyama K., Kubota S., Nakayama Y., Yamaguchi N. (2013) Nuclear ErbB4 signaling through H3K9me3 is antagonized by EGFR-activated c-Src. J. Cell Sci. 126, 625–637 [DOI] [PubMed] [Google Scholar]
- 18. Bjorge J. D., Bellagamba C., Cheng H. C., Tanaka A., Wang J. H., Fujita D. J. (1995) Characterization of two activated mutants of human pp60c-src that escape c-Src kinase regulation by distinct mechanisms. J. Biol. Chem. 270, 24222–24228 [DOI] [PubMed] [Google Scholar]
- 19. Sato I., Obata Y., Kasahara K., Nakayama Y., Fukumoto Y., Yamasaki T., Yokoyama K. K., Saito T., Yamaguchi N. (2009) Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J. Cell Sci. 122, 965–975 [DOI] [PubMed] [Google Scholar]
- 20. Yamanashi Y., Fukushige S., Semba K., Sukegawa J., Miyajima N., Matsubara K., Yamamoto T., Toyoshima K. (1987) The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56 lck. Mol. Cell Biol. 7, 237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakatani Y., Ogryzko V. (2003) Immunoaffinity purification of mammalian protein complexes. Methods Enzymol. 370, 430–444 [DOI] [PubMed] [Google Scholar]
- 22. Tezuka T., Umemori H., Akiyama T., Nakanishi S., Yamamoto T. (1999) PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-d-aspartate receptor subunit NR2A. Proc. Natl. Acad. Sci. U.S.A. 96, 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogiso H., Ishitani R., Nureki O., Fukai S., Yamanaka M., Kim J. H., Saito K., Sakamoto A., Inoue M., Shirouzu M., Yokoyama S. (2002) Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 110, 775–787 [DOI] [PubMed] [Google Scholar]
- 24. Shtivelman E., Lifshitz B., Gale R. P., Canaani E. (1985) Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature 315, 550–554 [DOI] [PubMed] [Google Scholar]
- 25. Law C. L., Sidorenko S. P., Chandran K. A., Draves K. E., Chan A. C., Weiss A., Edelhoff S., Disteche C. M., Clark E. A. (1994) Molecular cloning of human Syk. A B cell protein-tyrosine kinase associated with the surface immunoglobulin M-B cell receptor complex. J. Biol. Chem. 269, 12310–12319 [PubMed] [Google Scholar]
- 26. Satou A., Taira T., Iguchi-Ariga S. M., Ariga H. (2001) A novel transrepression pathway of c-Myc. Recruitment of a transcriptional corepressor complex to c-Myc by MM-1, a c-Myc-binding protein. J. Biol. Chem. 276, 46562–46567 [DOI] [PubMed] [Google Scholar]
- 27. Ziv Y., Bielopolski D., Galanty Y., Lukas C., Taya Y., Schultz D. C., Lukas J., Bekker-Jensen S., Bartek J., Shiloh Y. (2006) Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat. Cell Biol. 8, 870–876 [DOI] [PubMed] [Google Scholar]
- 28. Nakayama Y., Igarashi A., Kikuchi I., Obata Y., Fukumoto Y., Yamaguchi N. (2009) Bleomycin-induced over-replication involves sustained inhibition of mitotic entry through the ATM/ATR pathway. Exp. Cell Res. 315, 2515–2528 [DOI] [PubMed] [Google Scholar]
- 29. Obata Y., Fukumoto Y., Nakayama Y., Kuga T., Dohmae N., Yamaguchi N. (2010) The Lyn kinase C-lobe mediates Golgi export of Lyn through conformation-dependent ACSL3 association. J. Cell Sci. 123, 2649–2662 [DOI] [PubMed] [Google Scholar]
- 30. Tamura T., Kunimatsu T., Yee S. T., Igarashi O., Utsuyama M., Tanaka S., Miyazaki S.-i., Hirokawa K., Nariuchi H. (2000) Molecular mechanism of the impairment in activation signal transduction in CD4+ T cells from old mice. Int. Immunol. 12, 1205–1215 [DOI] [PubMed] [Google Scholar]
- 31. Fukumoto Y., Obata Y., Ishibashi K., Tamura N., Kikuchi I., Aoyama K., Hattori Y., Tsuda K., Nakayama Y., Yamaguchi N. (2010) Cost-effective gene transfection by DNA compaction at pH 4.0 using acidified, long shelf-life polyethylenimine. Cytotechnology 62, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kasahara K., Nakayama Y., Ikeda K., Fukushima Y., Matsuda D., Horimoto S., Yamaguchi N. (2004) Trafficking of Lyn through the Golgi caveolin involves the charged residues on αE and αI helices in the kinase domain. J. Cell Biol. 165, 641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuda D., Nakayama Y., Horimoto S., Kuga T., Ikeda K., Kasahara K., Yamaguchi N. (2006) Involvement of Golgi-associated Lyn tyrosine kinase in the translocation of annexin II to the endoplasmic reticulum under oxidative stress. Exp. Cell Res. 312, 1205–1217 [DOI] [PubMed] [Google Scholar]
- 34. Kuga T., Hoshino M., Nakayama Y., Kasahara K., Ikeda K., Obata Y., Takahashi A., Higashiyama Y., Fukumoto Y., Yamaguchi N. (2008) Role of Src-family kinases in formation of the cortical actin cap at the dorsal cell surface. Exp. Cell Res. 314, 2040–2054 [DOI] [PubMed] [Google Scholar]
- 35. Mera A., Suga M., Ando M., Suda T., Yamaguchi N. (1999) Induction of cell shape changes through activation of the interleukin-3 common α chain receptor by the RON receptor-type tyrosine kinase. J. Biol. Chem. 274, 15766–15774 [DOI] [PubMed] [Google Scholar]
- 36. Méndez J., Stillman B. (2000) Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle. Assembly of prereplication complexes in late mitosis. Mol. Cell Biol. 20, 8602–8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodarzi A. A., Noon A. T., Deckbar D., Ziv Y., Shiloh Y., Löbrich M., Jeggo P. A. (2008) ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol. Cell 31, 167–177 [DOI] [PubMed] [Google Scholar]
- 38. Herkert B., Dwertmann A., Herold S., Abed M., Naud J. F., Finkernagel F., Harms G. S., Orian A., Wanzel M., Eilers M. (2010) The Arf tumor suppressor protein inhibits Miz1 to suppress cell adhesion and induce apoptosis. J. Cell Biol. 188, 905–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakayama Y., Matsui Y., Takeda Y., Okamoto M., Abe K., Fukumoto Y., Yamaguchi N. (2012) c-Src but not Fyn promotes proper spindle orientation in early prometaphase. J. Biol. Chem. 287, 24905–24915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tada J., Omine M., Suda T., Yamaguchi N. (1999) A common signaling pathway via Syk and Lyn tyrosine kinases generated from capping of the sialomucins CD34 and CD43 in immature hematopoietic cells. Blood 93, 3723–3735 [PubMed] [Google Scholar]
- 41. Hirao A., Hamaguchi I., Suda T., Yamaguchi N. (1997) Translocation of the Csk homologous kinase (Chk/Hyl) controls activity of CD36-anchored Lyn tyrosine kinase in thrombin-stimulated platelets. EMBO J. 16, 2342–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iwama A., Yamaguchi N., Suda T. (1996) STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking sites conserved among the HGF receptor family. EMBO J. 15, 5866–5875 [PMC free article] [PubMed] [Google Scholar]
- 43. Iyengar S., Farnham P. J. (2011) KAP1 protein. An enigmatic master regulator of the genome. J. Biol. Chem. 286, 26267–26276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lechner M. S., Begg G. E., Speicher D. W., Rauscher F. J., 3rd (2000) Molecular determinants for targeting heterochromatin protein 1-mediated gene silencing. Direct chromoshadow domain-KAP-1 corepressor interaction is essential. Mol. Cell Biol. 20, 6449–6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schultz D. C., Ayyanathan K., Negorev D., Maul G. G., Rauscher F. J., 3rd (2002) SETDB1. A novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 16, 919–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng W., Ball A. R., Jr., Yokomori K. (2010) HP1. Heterochromatin binding proteins working the genome. Epigenetics 5, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lachner M., O'Carroll D., Rea S., Mechtler K., Jenuwein T. (2001) Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 [DOI] [PubMed] [Google Scholar]
- 48. Thurman R. E., Rynes E., Humbert R., Vierstra J., Maurano M. T., Haugen E., Sheffield N. C., Stergachis A. B., Wang H., Vernot B., Garg K., John S., Sandstrom R., Bates D., Boatman L., Canfield T. K., Diegel M., Dunn D., Ebersol A. K., Frum T., Giste E., Johnson A. K., Johnson E. M., Kutyavin T., Lajoie B., Lee B. K., Lee K., London D., Lotakis D., Neph S., Neri F., Nguyen E. D., Qu H., Reynolds A. P., Roach V., Safi A., Sanchez M. E., Sanyal A., Shafer A., Simon J. M., Song L., Vong S., Weaver M., Yan Y., Zhang Z., Zhang Z., Lenhard B., Tewari M., Dorschner M. O., Hansen R. S., Navas P.A., Stamatoyannopoulos G., Iyer V. R., Lieb J. D., Sunyaev S. R., Akey J. M., Sabo P. J., Kaul R., Furey T. S., Dekker J., Crawford G. E., Stamatoyannopoulos J. A. (2012) The accessible chromatin landscape of the human genome. Nature 489, 75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Noon A. T., Shibata A., Rief N., Löbrich M., Stewart G. S., Jeggo P. A., Goodarzi A. A. (2010) 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat. Cell Biol. 12, 177–184 [DOI] [PubMed] [Google Scholar]
- 50. Lee D. H., Goodarzi A. A., Adelmant G. O., Pan Y., Jeggo P. A., Marto J. A., Chowdhury D. (2012) Phosphoproteomic analysis reveals that PP4 dephosphorylates KAP-1 impacting the DNA damage response. EMBO J. 31, 2403–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kharbanda S., Saleem A., Yuan Z. M., Kraeft S., Weichselbaum R., Chen L. B., Kufe D. (1996) Nuclear signaling induced by ionizing radiation involves colocalization of the activated p56/p53lyn tyrosine kinase with p34cdc2. Cancer Res. 56, 3617–3621 [PubMed] [Google Scholar]
- 52. Baskaran R., Wood L. D., Whitaker L. L., Canman C. E., Morgan S. E., Xu Y., Barlow C., Baltimore D., Wynshaw-Boris A., Kastan M. B., Wang J. Y. (1997) Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature 387, 516–519 [DOI] [PubMed] [Google Scholar]
- 53. Chang C. W., Chou H. Y., Lin Y. S., Huang K. H., Chang C. J., Hsu T. C., Lee S. C. (2008) Phosphorylation at Ser473 regulates heterochromatin protein 1 binding and corepressor function of TIF1β/KAP1. BMC Mol. Biol. 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ivanov A. V., Peng H., Yurchenko V., Yap K. L., Negorev D. G., Schultz D. C., Psulkowski E., Fredericks W. J., White D. E., Maul G. G., Sadofsky M. J., Zhou M. M., Rauscher F. J., 3rd (2007) PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol. Cell 28, 823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Delfino F. J., Shaffer J. M., Smithgall T. E. (2006) The KRAB-associated co-repressor KAP-1 is a coiled-coil binding partner, substrate and activator of the c-Fes protein tyrosine kinase. Biochem. J. 399, 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Amanchy R., Zhong J., Hong R., Kim J. H., Gucek M., Cole R. N., Molina H., Pandey A. (2009) Identification of c-Src tyrosine kinase substrates in platelet-derived growth factor receptor signaling. Mol. Oncol. 3, 439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakayama Y., Kawana A., Igarashi A., Yamaguchi N. (2006) Involvement of the N-terminal unique domain of Chk tyrosine kinase in Chk-induced tyrosine phosphorylation in the nucleus. Exp. Cell Res. 312, 2252–2263 [DOI] [PubMed] [Google Scholar]
- 58. Resh M. D. (1999) Fatty acylation of proteins. New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451, 1–16 [DOI] [PubMed] [Google Scholar]
- 59. Resh M. D. (1994) Myristylation and palmitylation of Src family members. The fats of the matter. Cell 76, 411–413 [DOI] [PubMed] [Google Scholar]
- 60. Ryan R. F., Schultz D. C., Ayyanathan K., Singh P. B., Friedman J. R., Fredericks W. J., Rauscher F. J., 3rd (1999) KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins. A potential role for Krüppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol. Cell Biol. 19, 4366–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goodarzi A. A., Kurka T., Jeggo P. A. (2011) KAP-1 phosphorylation regulates heterochromatic CHD3 nucleosome remodeling during the DNA double strand break response. Nat. Struct. Mol. Biol. 18, 831–839 [DOI] [PubMed] [Google Scholar]
- 62. Baldeyron C., Soria G., Roche D., Cook A. J., Almouzni G. (2011) HP1α recruitment to DNA damage by p150CAF-1 promotes homologous recombination repair. J. Cell Biol. 193, 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cloos P. A., Christensen J., Agger K., Maiolica A., Rappsilber J., Antal T., Hansen K. H., Helin K. (2006) The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature 442, 307–311 [DOI] [PubMed] [Google Scholar]
- 64. White D., Rafalska-Metcalf I. U., Ivanov A. V., Corsinotti A., Peng H., Lee S. C., Trono D., Janicki S. M., Rauscher F. J., 3rd (2012) The ATM substrate KAP1 controls DNA repair in heterochromatin. Regulation by HP1 proteins and serine 473/824 phosphorylation. Mol. Cancer Res. 10, 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dawson M. A., Bannister A. J., Göttgens B., Foster S. D., Bartke T., Green A. R., Kouzarides T. (2009) JAK2 phosphorylates histone H3Y41 and excludes HP1α from chromatin. Nature 461, 819–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cammas F., Mark M., Dollé P., Dierich A., Chambon P., Losson R. (2000) Mice lacking the transcriptional corepressor TIF1β are defective in early postimplantation development. Development 127, 2955–2963 [DOI] [PubMed] [Google Scholar]
- 67. Messerschmidt D. M., de Vries W., Ito M., Solter D., Ferguson-Smith A., Knowles B. B. (2012) Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science 335, 1499–1502 [DOI] [PubMed] [Google Scholar]
- 68. Cammas F., Herzog M., Lerouge T., Chambon P., Losson R. (2004) Association of the transcriptional corepressor TIF1β with heterochromatin protein 1 (HP1). An essential role for progression through differentiation. Genes Dev. 18, 2147–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]