Abstract
Background and Purpose
The α7 nicotinic ACh receptor subtype is abundantly expressed in the CNS and in the periphery. Recent evidence suggests that α7 nicotinic ACh receptor (nAChR) subtypes, which can be activated by an endogenous cholinergic tone comprising ACh and the α7 agonist choline, play an important role in chronic pain and inflammation. In this study, we evaluated whether type II α7 positive allosteric modulator PNU-120596 induces antinociception on its own and in combination with choline in the formalin pain model.
Experimental Approach
We assessed the effects of PNU-120596 and choline and the nature of their interactions in the formalin test using an isobolographic analysis. In addition, we evaluated the interaction of PNU-120596 with PHA-54613, an exogenous selective α7 nAChR agonist, in the formalin test. Finally, we assessed the interaction between PNU-120596 and nicotine using acute thermal pain, locomotor activity, body temperature and convulsing activity tests in mice.
Key Results
We found that PNU-120596 dose-dependently attenuated nociceptive behaviour in the formalin test after systemic administration in mice. In addition, mixtures of PNU-120596 and choline synergistically reduced formalin-induced pain. PNU-120596 enhanced the effects of nicotine and α7 agonist PHA-543613 in the same test. In contrast, PNU-120596 failed to enhance nicotine-induced convulsions, hypomotility and antinociception in acute pain models. Surprisingly, it enhanced nicotine-induced hypothermia via activation of α7 nAChRs.
Conclusions and Implications
Our results demonstrate that type II α7 positive allosteric modulators produce antinociceptive effects in the formalin test through a synergistic interaction with the endogenous α7 agonist choline.
Keywords: positive allosteric modulators, nicotinic agonists, 7 nicotinic ACh receptors, formalin test
Introduction
Recent studies have confirmed the therapeutic potential of targeting α7 nicotinic ACh receptors (nAChRs) for the treatment of schizophrenia and Alzheimer's disease (Mansvelder et al., 2006; Dziewczapolski et al., 2009; Wang et al., 2009; Thomsen et al., 2010). α7 nAChRs have also been increasingly considered a potential target in the search for anti-inflammatory and analgesic drugs (Buccafusco et al., 2005; Vincler, 2005; Wang et al., 2005; Bertrand and Gopalakrishnan, 2007; Marrero and Bencherif, 2009; Haydar and Dunlop, 2010). Indeed, α7 nAChR agonists possess antinociceptive and anti-inflammatory properties in rodent models of chronic neuropathic pain and inflammation (Damaj et al., 2000; Decker et al., 2001; Hama and Menzaghi, 2001; Hamurtekin and Gurun, 2006; de Jonge and Ulloa, 2007; Medhurst et al., 2008). However, there are still a number of uncertainties in the development of α7 nicotinic agonists for the treatment of pain, including receptor selectivity (namely cross-reactivity with 5HT3 receptors, which have high homology with α7 nAChRs) and possible adverse effects. In addition, some α7 agonists have been shown to promote long-term receptor desensitization and/or inactivation in vitro, suggesting possible adaptations to their effects after chronic use (Papke et al., 2009; Sattelle et al., 2009). Given these challenges to the development of direct agonists, α7 nAChR allosteric modulators present an attractive and versatile alternative.
Positive allosteric modulators (PAMs) can reinforce endogenous cholinergic neurotransmission without directly activating the receptor and are thought to exert their effects in the presence of endogenous neurotransmitter only, giving them the ability to specifically (spatially and temporally) amplify neurotransmission. Also, PAMs may cause less excessive receptor activation than that caused by direct agonists (Christopoulos, 2002). These notions generated substantial interest in the development of various selective α7 nAChR PAMs (Bertrand and Gopalakrishnan, 2007; Moaddel et al., 2007; Faghih et al., 2008).
These α7 nAChR PAMs have been classified as either type I, such as NS1738, or type II, such as PNU-120596, on the basis of a difference in their effect on receptor desensitization (Bertrand and Gopalakrishnan, 2007; Timmermann et al., 2007; Collins et al., 2011). Type I PAMs predominantly increase the apparent peak current with little effect on desensitization kinetics, whereas type II PAMs increase the apparent peak current and evoke a distinct, slowly decaying current (Hurst et al., 2005). Moreover, the type II α7 nAChR PAM PNU-120596 can efficaciously reactivate desensitized α7 nAChRs (Hurst et al., 2005; Gronlien et al., 2007). Animal studies have found that type II PAMs, including PNU-120596, enhance memory and cognition in vivo (Gronlien et al., 2007; Thomsen et al., 2010; Williams et al., 2011); however, their effects on pain and inflammation are just starting to emerge (Munro et al., 2012).
Endogenous cholinergic neurotransmission, which is mediated by ACh and choline (Zhu et al., 2009), is a strong candidate for enhancement by α7 nAChR PAMs. While α7 PAMs were reported to enhance the actions of ACh in α7 nAChRs expressing systems in vitro (Bertrand and Gopalakrishnan, 2007), the rapid clearance of ACh by AChsterases in the synaptic cleft makes choline, an ACh precursor and metabolite, a more likely candidate (Alkondon and Albuquerque, 2006). Indeed, choline is a selective endogenous agonist for α7 nAChRs in the CNS (Alkondon et al., 1997; González-Rubio et al., 2006). Furthermore, PNU-120596 was recently reported to enhance choline's effects on native and expressed α7 nAChRs (Gusev and Uteshev, 2010; Kalappa et al., 2010). In addition, centrally administered exogenous choline has been known to produce α7 nAChR-mediated antinociception in various animal models of pain (Damaj et al., 2000; Wang et al., 2005). Therefore, in this study, we used choline to investigate the effects of PNU-120596 on an endogenous α7 agonist in a mouse model of pain. Choline is a charged cation and cannot easily pass through the blood–brain barrier (BBB) (Allen and Lockman, 2003), so the interaction with α7 PAMs was evaluated by intrathecally administering choline.
The present study investigated the effects of PNU-120596 administered alone or in combination with nicotinic agonists in the mouse formalin test, a model of persistent pain (Hunskaar and Hole, 1987). Studies were designed to test the working hypotheses that (i) PNU-120596 administered alone would produce antinociception, and (ii) PNU-120596 would synergistically enhance the effects of the α7 nAChR agonist choline. In particular, we assessed the nature of the interaction between PNU-120596 and choline in the formalin test by performing an isobolographic analysis (Wessinger, 1986; Ossipov et al., 1990; Tallarida, 2000; Tallarida, 2006; Negus et al., 2008). In addition, we evaluated the interaction of PNU-120596 with PHA-543613, an exogenous, selective α7 nAChR agonist, in the formalin test. Finally, considering that PNU-120596's potentiation of nicotine's adverse effects might be a clinical concern for smokers and patients undergoing α7 nAChR agonist-based nicotine-replacement therapy (Brunzell and McIntosh, 2012), we assessed the interaction between PNU-120596 and nicotine using acute thermal pain (tail-flick and hot-plate tests), locomotor activity, body temperature and convulsing activity tests in mice.
Materials and methods
Subjects
Male adult Institute for Cancer Research mice obtained from Harlan Laboratories (Indianapolis, IN, USA) were used throughout the study. Mice null for the α7 (Jackson Laboratories, Bar Harbor, ME, USA) subunits and their wild-type littermates were bred in an animal care facility at Virginia Commonwealth University. For all experiments, mice were backcrossed at least 8 to 10 generations. Mutant and wild types were obtained from crossing heterozygote mice. This breeding scheme controlled for any irregularities that might occur with crossing solely mutant animals. Mice had free access to food and water and were housed in groups of five in a 21-C humidity-controlled animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. The rooms were on a 12-h light/dark cycle (lights on at 0700 h). Mice were 8–10 weeks of age and weighed approximately 20–25 g at the start of all the experiments. All experiments were performed during the light cycle (between 0700 and 1900 h), and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institute of Health guide for the Care and Use of Laboratory animals. Results of the studies were reported in accordance with the ARRIVE guidelines for reporting experiments involving animals.
Drugs
(-)-Nicotine hydrogen tartrate salt [(-)-1-methyl-2-(3-pyridyl) pyrrolidine (-)-bitartrate salt] was purchased from Sigma-Aldrich (St. Louis, MO, USA). Methyllycaconitine citrate (MLA) and choline were purchased from RBI (Natick, MA, USA). PNU-120596 [1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl), PHA-543613 [N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide] (selective α7 agonist) and morphine were obtained from the Drug Supply Program of the National Institute on Drug Abuse (Rockville, MD, USA). All drugs except for PNU-120596 were dissolved in physiological saline (0.9% sodium chloride) and injected subcutaneously at a total volume of 1 mL per 100 g body weight, unless noted otherwise. PNU-120596 was dissolved in a mixture of 1:1:18 vehicle [1 volume ethanol : 1 volume Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ, USA): 18 volumes distilled water] and administered via i.p. injection. All doses are expressed as the free base of the drug. The drug/molecular target nomenclature conforms to British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2011).
Drug interactions in the formalin test
The formalin test was assessed as described by Dubuisson and Dennis (1977) and adapted to mice. Briefly, the test was carried out in an open Plexiglas cage, with a mirror placed at a 45-degree angle behind the cage to allow for an unobstructed view of the paws. Mice were allowed to acclimate for 15 min in the test cage before formalin injection. Each animal was injected with 20 μL of 2.5% formalin in the intraplantar (i.pl.) region of the right hindpaw. Mice were then observed (two at a time) at 0–5 min (Phase 1) and at 20–45 min (Phase 2) post-formalin, and the amount of time spent licking the injected paw was recorded with a digital stopwatch. The period between the two phases of nociceptive responding is generally considered to be a phase of weak activity.
Interactions between PNU-120596 and choline in the formalin test were studied using an experimental design that facilitated isobolographic data presentation and dose-addition analysis (Tallarida, 2000; Negus et al., 2008; see Data Analysis). Dose–response curves for PNU-120596 and choline were obtained using at least six animals per dose. Mice were given i.p. injections of either vehicle or PNU-120596 (0.1, 0.32, 1.0, 3.2 or 10 mg·kg−1) and 15 min later, were given an i.pl. injection of formalin. Similarly, separate groups of mice were given intrathecal (i.t) injections of vehicle or choline (1, 3.2, 10, 32 or 100 μg·mouse−1) and 5 min later were given an i.pl. injection of formalin. Subsequently, fixed proportions of PNU-120596 in combination with choline were examined, and the proportion of drugs in the combination was determined by their relative potency in phase II of the formalin test (the only phase in which both drugs were active at the 50% effect level). Specifically, relative potency was defined as phase II ED50 choline (μg i.t.) ÷ phase II ED50 PNU-120596 (mg·kg−1 i.p.) = 7.2 (see Table 1). The proportion of choline to PNU-120596 in the combination was then set to this relative potency. A dose-effect curve for the combination was then determined, such that the proportion of choline to PNU-120596 was held constant at 1:7.2 PNU-120596/choline, and doses of both drugs increased from 0.32 mg·kg−1 PNU-120596 +2.32 μg choline to 10 mg·kg−1 PNU-120596 + 72 μg choline. In the drug combination experiments, mice received one injection of each drug: PNU-120596 (i.p.) was administered first followed 15 min later by a choline (i.t.) injection. Formalin (i.pl.) was administered 5 min after the choline injection. For ED50 values estimation, data were expressed as % MPE = (licking time of vehicle − licking time of test drug) / (licking time of vehicle) × 100.
Table 1.
Fixed combinations of PNU-120596 and choline produced synergistic antinociceptive effects in the formalin test are presented in the first column
| Treatment (alone or mixture) [PNU: mg·kg−1 | choline: μg·mouse−1] | Mixture ratio | Theoretical ED50 [Zadd (95% CL)] | Experimental ED50 [Zmix (95% CL)] |
|---|---|---|---|
| PNU alone | – | – | 2.61 (2.13–3.19) |
| Choline alone | – | – | 18.90 (14.79–24.17) |
| PNU-120596 (in PNU-120596 + choline mixture) | – | – | 0.47 (0.28–0.78) |
| Choline (in PNU-120596 + choline mixture) | – | – | 3.44 (2.36–4.99) |
| PNU-120596 + choline | 1:7.2 | 10.76 (8.71–13.18) | 3.92 (2.70–5.68) |
| 0.32 + 2.32 | |||
| 1.0 + 7.2 | |||
| 3.2 + 23.2 | |||
| 10 + 72 |
Predicted additive ED50 values (Zadd) and experimentally determined ED50 (Zmix) values for the interaction of different doses of PNU-120596 (0.32, 1.0, 3.2 and 10 mg·kg−1, i.p.) and choline (2.32, 7.2, 23.2 and 72 μg·mouse−1, i.t.) are presented in the following columns.
For the interaction studies of PNU-120596 with other agonists, the drug was administered via i.p., whereas nicotine, PHA-543613 and morphine were given via s.c. injection. Nicotine was given 5 min after PNU-120596 treatment. PHA-543613 and morphine were given 15 min after PNU-120596 treatment.
Effects of PNU-120596 on nicotine's acute pharmacological effects in the mouse
The effect of PNU-120596 on nicotine's actions in the mouse was assessed measuring various pharmacological responses of nicotine after acute injection. Mice were pretreated with i.p. PNU-120596 followed by an injection of nicotine 15 min later. Animals were assessed at optimal times after nicotine injection in the different tests.
Tail-flick test
The antinociceptive effects of drugs were assessed by the tail-flick method of D'Amour and Smith (1941), as modified by Dewey et al. (1970). Briefly, each animal was restrained before treatment, and baseline reaction time was measured by focusing a beam of light on the distal one-third portion of the animals' tail. A control response was determined for each mouse before treatment, and test latency was determined after drug administration. Mice with baseline latencies of less than 2 s or more than 4 s were excluded from the study. To minimize tissue damage, a maximum latency of 10 s was imposed. Antinociceptive response was calculated as a percent maximum possible effect (%MPE), where %MPE = [(test value − control value) / (cut-off (10 s) − control value)] × 100. Groups of six animals were used for each dose and for each treatment. Interaction studies were carried out by pretreating the mice with either vehicle or PNU-120596 15 min before vehicle or nicotine (0.5 and 2.5 mg·kg−1 s.c.) and animals were tested 5 min later.
Hot-plate test
The antinociceptive effects of drugs were also assessed by hot-plate method. Mice were placed into a 10-cm wide glass cylinder on a hot-plate (Thermojust Apparatus, Columbus, OH) maintained at 55°C. The device was connected to a manually operated timer that recorded the amount of time the mouse spent on the heated surface before showing signs of nociception (e.g. jumping, paw licks). Two control latencies at least 10 min apart were determined for each mouse. Mice with baseline latencies of less than 8 s or more than 12 s were excluded from the study. To avoid tissue damage, the hot-plate would automatically disengage after 40 s. Antinociceptive response was calculated as a percentage of maximum possible effect (% MPE), where %MPE = {[test value − control] / [cut-off time (40 s) − control] × 100}. The reaction time was recorded when the animal jumped or licked its paws. Interaction studies were carried out by pretreating the mice with either vehicle or PNU-120596 15 min before vehicle or nicotine (0.5 and 2.5 mg·kg−1 s.c.) and animals were tested 5 min later.
Body temperature measure
Rectal temperature was measured by a thermistor probe (inserted 24 mm) and digital thermometer (YSI Inc., Yellow Springs, OH). In this set of experiments readings were taken just before and 30 min after the last injection. In the first experiment, mice were pretreated with an s.c injection of either vehicle or nicotine (0.5, 1 or 2.5 mg·kg−1). PNU-120596 was given i.p. 15 min before nicotine treatment. In the second experiment, mice were pretreated with either vehicle or different doses of PNU-120596 (1, 4 or 8 mg·kg−1, i.p.), and 15 min later they received an s.c injection of vehicle or nicotine 0.5 mg·kg−1. In the third experiment, mice were pretreated 15 min with either vehicle or PNU-120596 and tested 15 min after a second injection of either vehicle or PHA-543613. Antagonism studies were carried out by pretreating the mice with either vehicle or MLA (10 mg·kg−1) followed 10 min later by vehicle or PNU-120596 (8 mg·kg−1, i.p.) and 25 min later by 0.5 mg·kg−1 nicotine. The difference in rectal temperature before and after treatment was calculated for each mouse. The ambient temperature of the laboratory varied from 21 to 24°C from day to day.
Locomotor activity test
Mice were placed into individual Omnitech photocell activity cages (28 × 16.5 cm) (Columbus, OH, USA) after the pretreatment of either i.p. vehicle or PNU-120596 (8 mg·kg−1, i.p.) and an s.c. injection of either vehicle or nicotine (0.1, 0.5 and 1 mg·kg−1). The initial pretreatment, of PNU-120596 or vehicle, was given 15 min before the second pretreatment, of either vehicle or nicotine, which was administered 5 min before the test. Interruptions of the photocell beams (two banks of eight cells each) were then recorded for the next 30 min. Data are expressed as the number of photocell interruptions.
Seizure testing
After an s.c. pretreatment injection of either vehicle or nicotine (3, 5 and 9 mg·kg−1), each animal was immediately placed in a 30 × 3 × 30 cm3 Plexiglas cage for 3 min, after which, mice were observed for 7 min. The interaction study between PNU-120596 and nicotine was carried out by pretreating the mice with PNU-120596 (8 mg·kg−1, i.p.) 15 min before nicotine. The occurrence or absence of a clonic seizure was noted for each animal over a 7-min time period after the s.c. administration of nicotine. This time was chosen because seizures occur very quickly after nicotine administration. The percentage of animals exhibiting a seizure was calculated.
Intrathecal injections
Injections were performed free-hand between the fifth and sixth lumbar vertebra in unanaesthetized male mice according to the method of Hylden and Wilcox (1980). The injection was performed using a 30-G needle attached to a glass microsyringe. The injection volume in all cases was 5 μL. The accurate placement of the needle was evidenced by a quick ‘flick’ of the mouse's tail.
Statistical analysis
ED50 analysis
Results are expressed as mean ± standard error of the mean (SEM) or as ED50 values with 95% confidence limits. The data obtained were analyzed using GraphPad® software program. Statistical analysis was done by one-way analysis of variance test anova followed by the Tukey test. P-values less than 0.05 (P < 0.05) were considered significant. The ED50 values with 95% confidence intervals were calculated using standard linear regression analysis of the dose–response curve for each drug alone or in combination. To determine the ED50 values, the antinociceptive activity (reduction in paw licking) was calculated as a percentage of the maximum possible effect (%MPE) using the following equation: %MPE = 100 * [(mean paw licking in control group) − (mean paw licking in drug(s) treated group)] / mean of paw licking in control group).
Dose-addition analysis
Interactions between PNU-120596 and choline within phase II of the formalin test were assessed using both graphical and statistical approaches to dose-addition analysis (Wessinger, 1986; Tallarida, 2000) as described previously (Negus et al., 2008). Graphically, mean ED50 values for PNU-120596 administered either alone or as part of a mixture were plotted as a function of the ED50 value for choline administered alone or as part of a mixture. This data presentation format is known as an isobologram, and the line in an isobologram that connects the data points for each drug alone shows predicted data points for drug mixtures assuming dose additivity. Points that fall above the line of additivity (away from the origin) are suggestive of sub-additivity, whereas points that fall below the line (towards the origin) are suggestive of super-additivity.
Statistical evaluation of drug interactions was accomplished by comparing the experimentally determined ED50 values for the mixture (Zmix) with predicted additive ED50 values (Zadd) as described by Tallarida (Tallarida, 2000). Zmix values were determined empirically as described earlier. Zadd values were calculated from the equation Zadd = fA + (1 − f)B, where A was the ED50 for PNU-120596 alone; B was the ED50 for choline alone; and f was the fractional multiplier of A in the computation of the additive total dose. Any choice of f is related to the proportion of drug A (ρA) in a mixture according to the equation ρA = fA/Zadd, and under the conditions used here wherein the proportion of drugs in the combination was equal to their relative potency, f = 0.5. Zmix and Zadd values were considered to be significantly different if 95% confidence limits did not overlap.
Results
Dose–response analysis of PNU-120596 and choline alone and their combinations in the formalin test
PNU-120596 (i.p) and choline (i.t.) dose-dependently reduced formalin nociceptive behaviours in both phase I and II. Figure 1A and B shows the dose–response curves for the antinociceptive effects of PNU-120596 in phase I [F(5,49) = 14.57, P < 0.0001] and phase II [F(5,49) = 54.43, P < 0.0001] and choline phase I [F(5,49) = 21.16, P < 0.0001] and phase II [F(5,49) = 64,91, P < 0.0001] alone in mice. The ED50 values and 95% confidence limit for PNU-120596 and choline in phase II were 2.61 (2.13–3.19) and 18.90 (14.79–24.17) mg·kg−1 (μg·mouse−1) respectively as shown in Table 1.
Figure 1.
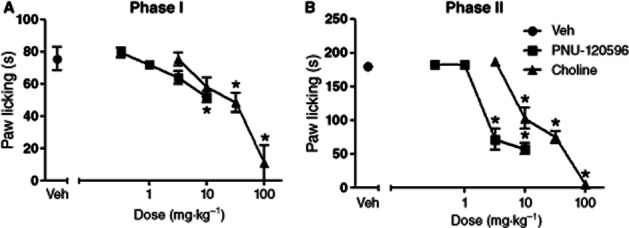
Effects of α7 type II PAM PNU-120596 and α7 nicotinic agonist choline in the mouse formalin test. Dose–response curves of PNU-120596 and choline on (A) phase I and (B) phase II in the formalin test. Mice were treated with either vehicle or PNU-120596 (15 min, i.p.) or vehicle or choline (5 min, i.t.). Testing occurred immediately after the formalin injection. Each point represents the mean ± SEM of total time spent licking for six to eight mice per group and *denotes P < 0.05 versus vehicle. Veh, vehicle.
Shown in Figure 2A and B is the combination of PNU-120596 and choline, with the dose of PNU-120596 plotted on the abscissa. The dose–response curve of PNU-120596 alone is plotted in this graph for comparison. The same data are also plotted in Figure 2C and D, with the dose of choline plotted on the abscissa. The dose–response curve of choline alone is included in this graph for comparison. The dose–effect curve for the PNU-120596 + choline mixture was shifted to left of the dose–effect curves for either drug alone. The interaction between PNU-120596 (i.p. 15 min before the formalin injection) and choline (i.t. 5 min before the formalin injection) was assessed by isobolographic analysis. Isobolographic analysis suggests that a synergistic interaction occurs between PNU-120596 and choline because the experimental ED50 is located below the additive dose line. The synergistic interaction between PNU-120596 and choline was confirmed by statistical comparison of the predicted additive ED50 values (Z add) and experimentally derived ED50 values (Zmix) as shown in Table 1.
Figure 2.
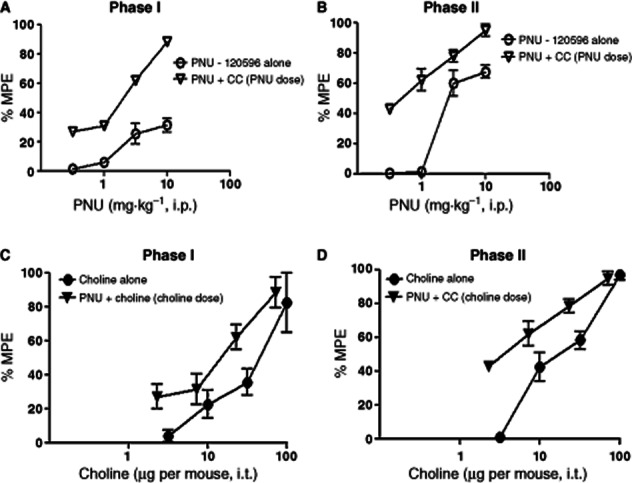
Effects of the interaction of PNU-120596 with choline in the formalin test, with respect to each drugs dose. Dose–response curves for PNU alone and in combination with choline in (A) phase I and (B) phase II in the formalin test, plotted against the doses of PNU-120596. Mice treated with either vehicle or PNU-120596 15 min before vehicle or choline, which was given 5 min before formalin (i.p.). Dose–response curves for choline alone and in combination with PNU-120596 in (C) phase I and (D) phase II in the formalin test, plotted against choline doses. Testing occurred immediately after the formalin injection. Each point represents the mean ± SEM of total time spent licking for six to eight mice per group and *denotes P < 0.05 versus vehicle. Veh, vehicle.
PNU-120596 enhances PHA-543613's effects in the formalin test
Because PNU-120596 was shown to enhance the antinociceptive effect of choline, we evaluated if it also enhances the effect of an exogenous selective α7 nAChR agonist, such as PHA-54613. Therefore, we tested PNU-120596 and PHA-54613 for their effects, separately and in combination, in the formalin test. Low doses of PNU-120596 (0.5 mg·kg−1 i.p.) and PHA-543613 (1.0 mg·kg−1 i.p.), both administered alone and given 15 min before formalin, did not produce significant antinociceptive effects in phases I [F(2,18) = 2.449, P = 0.1146] and II [F(2,17) = 0.4704, P = 0.6327] of the formalin test. On the other hand, pretreatment with PNU-120596 significantly potentiated (Figure 3) the effects of PHA-543613 effects in both phase I [F(3,22) = 23.72, P < 0.0001] and phase II [F(3,22) = 9.468, P = 0.0003] of the formalin test (Figure 4A & B).
Figure 3.
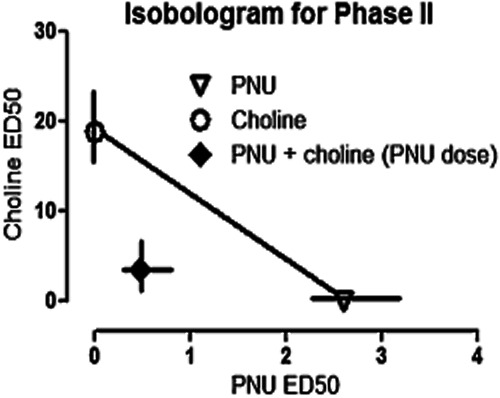
ED50 isobologram for the interaction of PNU-120596 and choline in the formalin test. The ED50 values for PNU-120596 and choline are depicted on the x- and y-axes, respectively. The experimental ED50 values of mixtures of PNU-120596 and choline at fixed-ratio combinations of 1:7.2, with 95% confidence intervals, were significantly below the theoretical isobole of additivity (see Table 1), indicating a synergistic interaction. Each point represents the mean ± SEM for 6 animals. *P < 0.05, compared with control mice given vehicle. Zmix corresponds to experimental ED50 ± SEM of the combination with 95% confidence limits.
Figure 4.
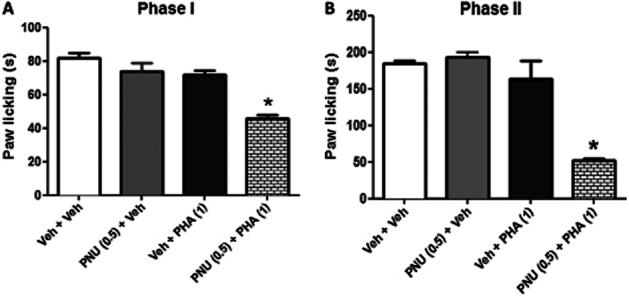
PNU-120596 enhances PHA-543613's effects in the formalin test. Mice received vehicle or PNU-120596 (0.5 mg·kg−1, i.p.) and 15 min later PHA-54613 (0.5 mg·kg−1, s.c.). They were tested in the formalin test 15 min later. Paw-licking time was measured in both (A) phase I and (B) phase II of the test. Data are expressed as mean ± SEM; n = 6 mice per group. *P < 0.05 versus control group.
PNU-120596 enhances nicotine's effects in the formalin test
To further explore the interaction between PNU-120596 and nicotinic agonists, the effects of PNU-120596 on nicotine's antinociception in the formalin test were also studied. As shown in Figure 5A and B, neither PNU-120596 (0.5 mg·kg−1 i.p.), administered 15 min before formalin, nor nicotine (0.05 mg·kg−1 s.c.), administered 5 min before formalin, induced significant antinociceptive effects in phase I [F(3,21) = 0.610, P = 0.3218] or II [F(3,21) = 1.029, P = 0.3746] in the formalin test. However, the pretreatment of mice with PNU-120596 significantly enhanced nicotine's effects in phases I [F(3,28) = 13.59, P < 0.0001] and II [F(3,25) = 34.64, P < 0.0001] of the test (Figure 5A & B).
Figure 5.
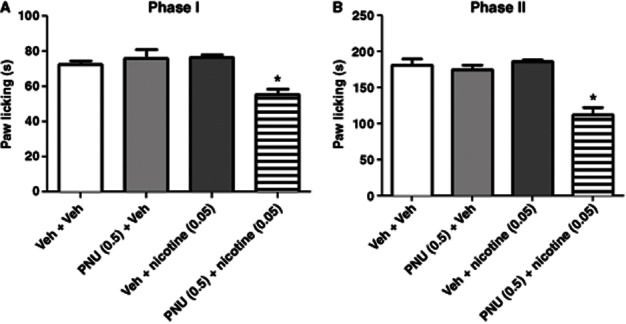
PNU-120596 enhances nicotine's effects in the formalin test. Mice received vehicle or PNU-120596 (0.5 mg·kg−1, i.p.) and 10 min later nicotine (0.05 mg·kg−1, s.c.). They were tested in the formalin test 5 min later. Paw-licking time was measured in both (A) phase I and (B) phase II of the test. Data are expressed as mean ± SEM; n = 6 mice per group. *P < 0.05 versus control group.
PNU-120596 failed to enhance morphine's effects in the formalin test
Considering the implication of μ-opioid receptors in nociception, we investigated the interaction between PNU-120596 and morphine in the formalin test. Neither a single pretreatment (15 min) injection of PNU-120596 (1 mg·kg−1, i.p.) nor a pretreatment (10 min) injection of morphine (0.5 mg·kg−1, s.c.) produced significant antinociceptive effects in formalin test phases I [F(2,13) = 0.5056, P = 0.6145] and II [F(2,13) = 0.5056, P = 0.6145], compared with the vehicle group. To then assess the effect of PNU-120596 on morphine, mice were injected with PNU-120596 (1 mg·kg−1, i.p.) 15 min before they were injected with morphine (0.5 mg·kg−1, s.c.), which was given 15 min before the formalin injection (i.pl.). As shown in Figure 6A and B, the interaction of ineffective doses of PNU-120596 and morphine did not produce antinociceptive effects in either phase I [F(3,19) = 0.3993, P = 0.7551] or phase II [F(3,19) = 0.09381, P = 0.9625] of the formalin test.
Figure 6.
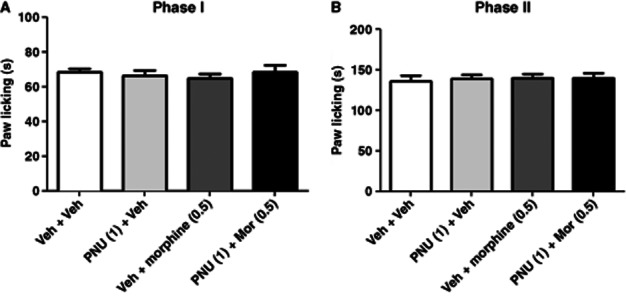
PNU-120596 did not enhance morphine's effects in the formalin test. Mice received vehicle or PNU-120596 (0.5 mg·kg−1, i.p.) and 15 min later morphine (0.5 mg·kg−1, s.c.). They were tested in the formalin test 15 min later. Paw-licking time was measured in both (A) phase I and (B) phase II of the test. Data are expressed as mean ± SEM; n = 6 mice per group. *P < 0.05 versus control group.
The effects of PNU-120596 on nicotine-induced antinociception, decrease in locomotion, seizures and hypothermia
To further assess the interaction between PNU-120596 and nicotine, we determined the effects of PNU-120596 on various pharmacological responses after the acute injection of nicotine in mice.
We first assessed the effects of PNU-120596 on nicotine-induced antinociception in acute thermal pain models using the tail-flick and hot-plate tests. Neither a 15-min pretreatment of PNU-120596 (8 mg·kg−1) nor a 5-min pretreatment of nicotine (0.5 mg·kg−1) showed significant antinociceptive effects in the tail-flick [F(3,22) = 0.5612, P = 0.6463] and hot-plate [F(3,20) = 2.000, P = 0.1465] tests when compared with vehicle. In contrast, a 5-min pretreatment of a higher dose of nicotine (2.5 mg·kg−1) induced significant antinociceptive effects in the tail-flick [F(5,32) = 16.91, P < 0.0001] and hot-plate tests [F(5,30) = 15.83, P < 0.0001] when compared with vehicle (Figure 7A & B). To determine whether PNU-120596 enhanced or blocked the antinociceptive effect of nicotine (0.5 and 2.5 mg·kg−1 s.c), mice were first pretreated (15 min) with PNU-120596 (8 mg·kg−1 i.p.) and then pretreated (5 min) with nicotine (0.5 or 2.5 mg·kg−1 s.c). Results shown in Figure 7A and B demonstrate that the pretreatment with PNU-120596 failed to significantly alter nicotine's responses in the tail-flick and hot-plate test.
Figure 7.
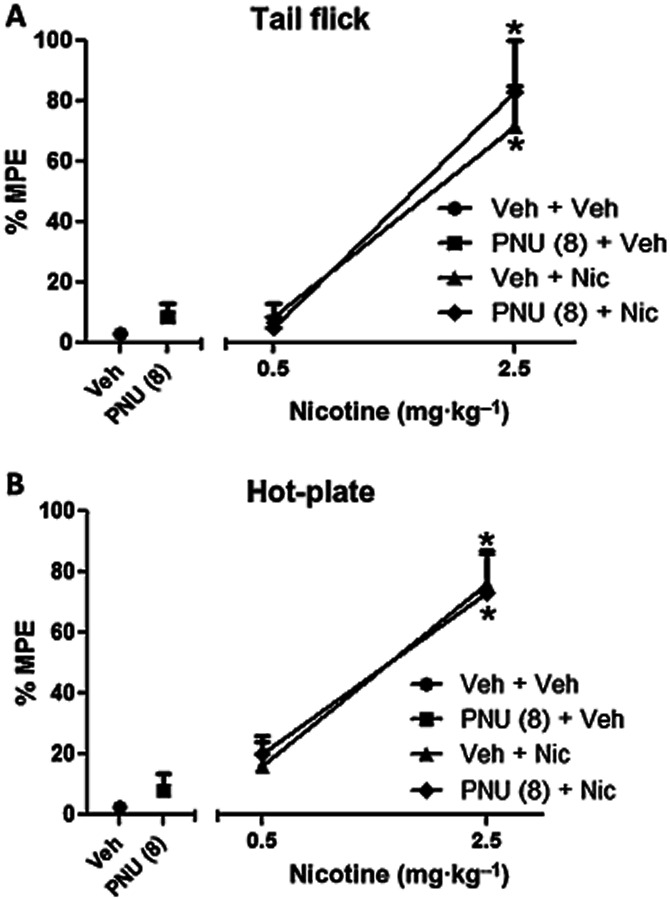
PNU-120596 did not enhance nicotine's antinociception in tail-flick and hot-plate tests after acute administration. Effects of PNU-120596 on nicotine in the (A) tail-flick and (B) hot-plate tests. Mice received vehicle or PNU-120596 (8 mg·kg−1, i.p.) and 15 min later nicotine (0.5 or 2.5 mg·kg−1, s.c.). They were tested in the tail-flick and hot-plate tests 5 min later. Each point represents the mean ± SEM of total time spent licking for six mice per group and * denotes P < 0.05 versus vehicle. Veh = vehicle.
We then determined the effects of PNU-120596 on the nicotine-induced decrease in locomotor activity in mice. A pretreatment of PNU-120596 (8 mg·kg−1) did not induce a significant shift in the nicotine dose–response curve compared with the vehicle-treated group [ED50 (±CL) values are 0.23 (0.07–0.73) and 0.33 (0.1–0.94) mg·kg−1 for vehicle and PNU-120596-treated groups, respectively] (Figure 8A) (Table 1). A similar lack of significant effects of PNU-120596 on nicotine-induced seizures (Figure 8B) was also observed [ED50 (±CL) values are 6.4 (4.3–9.5) and 7.1 (6.4–7.7) mg·kg−1 for vehicle and PNU-120596-treated groups, respectively] (Table 2).
Figure 8.
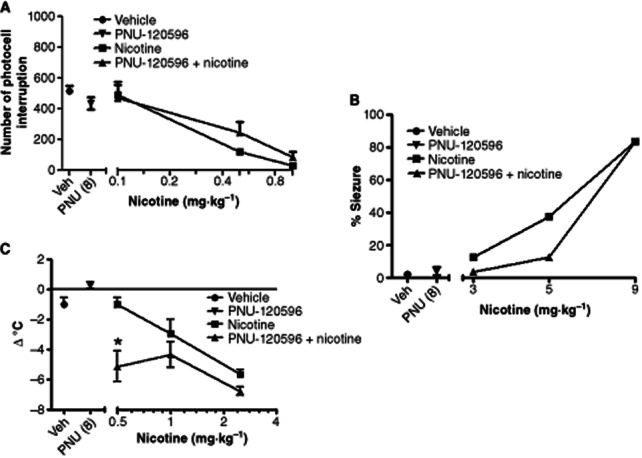
Effects of PNU-120596 on nicotine-induced locomotor activity depression, seizure occurrence and hypothermia. The effects of PNU-120596 (8 mg·kg−1, i.p.) on nicotine's actions on (A) locomotor activity, (B) seizure occurrence and (C) body temperature measure in mice. Animals were treated with vehicle or PNU-120596 15 min before nicotine. Changes in locomotor activity and seizure occurrence were measured 5 min later. Changes in body temperature were measured 30 min later. Each point represents the mean ± SEM of six mice. The asterisks denote the significance levels when compared with the control group: *P < 0.05.
Table 2.
Summary of the potencies of various acute nicotine responses after pretreatment with PNU-120596
| Response | Vehicle | PNU-120596 |
|---|---|---|
| Spontaneous activity | 0.23 (0.07–0.73) | 0.33 (0.1–0.94) |
| Seizures | 6.40 (4.3–9.5) | 7.10 (6.4–7.7) |
| Hypothermia | 0.47 (0.28–0.78) | 0.18 (0.09–0.35) |
Potency is expressed as ED50 ± confidence limits (mg·kg). Each group contained 6 mice.
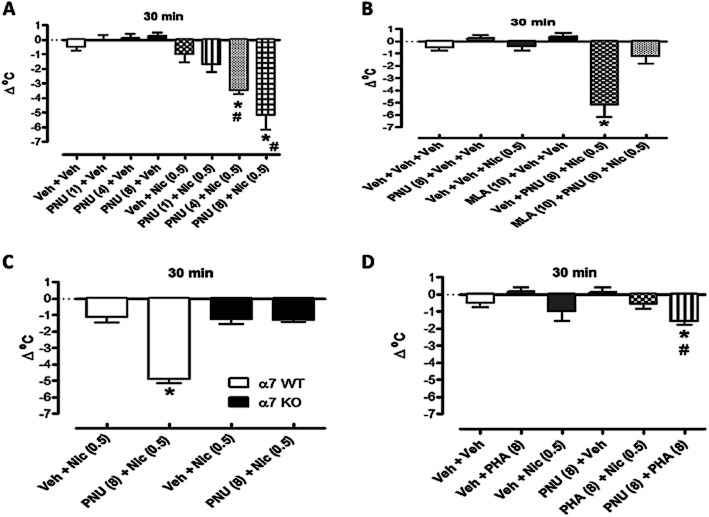
We finally investigated the effects of PNU-120596 on nicotine-induced hypothermia. While a pretreatment of PNU-120596 (8 mg·kg−1) did not produce a significant leftward shift in the dose–response curve for nicotine-induced hypothermia [ED50 (±CL) values are 0.47 (0.28–0.78) and 0.18 (0.09–0.35) mg·kg−1 for vehicle and PNU-120596-treated groups, respectively] (Table 2), significant interaction with the low dose of nicotine was found. As shown in Figure 8C, PNU-120596 (8 mg·kg−1 i.p.), administered 15 min before 0.5 mg·kg−1 of nicotine, induced a significant hypothermia. The enhancement of nicotine's effects disappeared at the higher doses of nicotine. On its own PNU-120596 (8 mg·kg−1 i.p.) did not induce a significant change in the body temperature of mice compared with vehicle (Figure 8C).
Characterization of nicotine-induced hypothermia in the presence of PNU-120596
The effect of PNU-120596 on nicotine-induced hypothermia was dose-dependent. On their own, PNU-120596-, nicotine- and vehicle-pretreament groups did not induce significant changes in body temperature [F(4,21) = 2.059, P = 0.1226]. In contrast, PNU-120596 dose-dependently (1, 4 and 8 mg·kg−1 i.p.) enhanced nicotine-induced hypothermia [F(7,37) = 17.57, P < 0.0001] (Figure 9A).
Figure 9.
PNU-120596 enhanced the effect of nicotine-induced hypothermia. (A) Effects of different doses of PNU-120596 (1, 4 and 8 mg·kg−1, i.p.) on nicotine-induced hypothermia (0.5 mg·kg−1, s.c.). Mice were treated with either vehicle or PNU-120596 15 min before vehicle or nicotine. Body temperatures were measured 30 min after nicotine. (B) The effects of MLA on PNU-120596 enhanced nicotine-induced hypothermia. Mice were treated with vehicle or MLA (10 mg·kg−1, s.c.) 15 min before PNU-120596 (8 mg·kg−1, s.c.). Mice were then injected with nicotine (0.5 mg·kg−1, s.c.) 15 min after PNU-120596 or vehicle. Changes in body temperature were measured 30 min later. (C) The effects of PNU-120596 (8 mg·kg−1, i.p.) on nicotine-induced hypothermia in α7 WT and α7 knockout (KO) mice. Mice were treated with either vehicle or PNU-120596 15 min before vehicle or nicotine (0.5 mg·kg−1, s.c.). Body temperatures were measured 30 min after nicotine. (D) Interaction of PHA-543613 (8 mg·kg−1, s.c.) with nicotine (0.5 mg·kg−1, s.c.) or PNU-120596 (8 mg·kg−1, i.p.). Mice were treated with vehicle, PNU-120596 or PHA-543613 15 min before vehicle, PHA-543613 or nicotine. Body temperatures were measured 30 min after the first drug. Each point represents the mean ± SEM of six mice. The asterisks denote the significance levels when compared with the control group: *P < 0.05.
The enhancement of nicotine's hypothermic effects by PNU-120596 was mediated by α7 nAChRs. Indeed, MLA, an α7 nicotinic antagonist, at a dose of 10 mg·kg−1, s.c. given 5 min before, blocked the effects of PNU-120596 (8 mg·kg−1 i.p.) on nicotine-induced hypothermia (0.5 mg·kg−1 s.c.) (Figure 9B) [F(6,32) = 14.34, P < 0.0001]. On their own, PNU-120596-, nicotine-, vehicle- or MLA-pretreatments did not induce significant changes in body temperature in mice [F(3,18) = 2.828, P = 0.0677]. Furthermore, PNU-120596's enhancement of nicotine-induced hypothermia observed in α7 WT mice was not evident in α7 KO mice (Figure 9C).
In contrast to the α7 PAM PNU-120596, the α7 nAChR agonist PHA-543613 (8 mg·kg−1 s.c.) failed to enhance the effects of nicotine (0.5 mg·kg−1 s.c.) on body temperature [F(3,18) = 2.317, P = 0.1100] (Figure 9C). However, the combination of PNU-120596 and PHA-543613, produced significantly different hypothermic effects [F(5,29) = 5.054, P = 0.0019], suggesting that PNU-120596 enhances the effect of PHA-543613 on body temperature (Figure 9D).
Discussion
In the present study, we first evaluated the interaction between the α7 type II PAM PNU-120596 and various nicotinic agonists in the mouse formalin test, a model of persistent pain. Using an isobolographic analysis, we were able to show synergism between PNU-120596 and choline in this model. In addition, PNU-120596 enhances the effects of another selective α7 agonist (PHA-543613) and nicotine in the formalin test. In contrast, PNU-120596 did not alter the effects of morphine in either phase of the formalin test, implying that opioid receptors were not involved in PNU-120596's enhancement effect in this test. Furthermore, PNU-120596 failed to alter nicotine-induced seizures, decrease in motor activity and antinociception in acute thermal pain tests. Surprisingly, PNU-120596 did significantly enhance nicotine-induced hypothermia via α7 nAChRs.
The antinociceptive effects of PNU-120596 in the formalin test is in line with its anti-inflammatory and anti-hyperalgesic properties recently reported in rats after systemic administration in the carrageenan and complete Freund's adjuvant tests (Munro et al., 2012). Collectively, these antinociceptive properties of PNU-120596 suggest an enhancement of endogenous α7 nAChR-mediated mechanisms. While it has been shown that α7 PAMs act by enhancing the actions of ACh (Bertrand and Gopalakrishnan, 2007), its rapid clearance by AChsterases in the synaptic cleft, makes it an unlikely candidate for the effects of PNU-120596. Our data showing the ability of systemic PNU-120596 to enhance spinal choline's antinociceptive in the formalin test suggest that endogenous choline in the CNS may play a more important role. Still, the relative contribution of choline and ACh in the effects of PNU-120596 at peripheral sites (immune cells for example) is not clear.
Isobolographic analysis indicated a synergistic antinociceptive interaction between PNU-120596 and choline in the formalin test. These data suggest that PNU-120596's antinociceptive effects could be mediated in part by neuronal endogenous choline. Indeed, choline is present in the CSF at a much lower concentration (∼10 μM) relative to its potency (EC50 ∼ 0.5–1.5 mM) as an α7 agonist (Alkondon et al., 1997; Papke and Porter, 2002). Hence, endogenous choline may not be effective in activating native α7* nAChRs in the absence of a PAM because of choline's low potency and thus narrow therapeutic significance. Indeed, cholinergic synaptic inputs were not observed in the absence of exogenous nicotinic agonists and innate α7* nAChRs were not actively sustained or desensitized by the low physiological concentrations of choline (Uteshev et al., 2003). However, recently, Kalappa et al. (2010) and Gusev and Uteshev (2010) showed that, in the presence of PNU-120596, physiological concentrations of choline become effective in the activation of native functional α7* nAChRs in hippocampal CA1 pyramidal neurons. Interestingly, exogenous choline's antinociceptive effect involves the modulation of α7 nAChRs in a variety of pain models (Damaj et al., 2000; Wang et al., 2005; Hamurtekin and Gurun, 2006).
Our results with the α7 nAChR PAM are in line with α4β2 PAMs recently reported. NS-9283, an α4β2* selective PAM, enhanced the effects of an α4β2 agonist (ABT-594) in rodent inflammatory and chronic neuropathic pain models (Lee et al., 2011; Zhu et al., 2011). In contrast to PNU-120596, the α4β2 PAM failed to produce an antinociceptive effect in these models on its own. These differences suggest that in contrast to α7 nAChRs, tonic endogenous anti-inflammatory and antinociceptive mechanisms mediated by α4β2* nAChRs are not evident.
Because PNU-120596 enhanced the antinociceptive effects of nicotine in the tonic pain model, we subsequently evaluated the interaction between PNU-120596 and nicotine in thermal acute pain models (tail-flick and hot-plate tests). Surprisingly, PNU-120596 did not enhance or block nicotine's effect in either test. There are several factors that may account for this variability such as the difference between the nociceptive response of the acute thermal pain models, which is based on a short, high-intensity stimulus generating a brief pain, and the nociceptive responses of chronic pain models, such as the formalin test, which involves a continued pain generated by injured tissue caused by the i.pl. injection of formalin (Tjolsen et al., 1992). Thus, PNU-120596's modulation of nociception for pain elicited by short-lasting stimuli and for pain elicited by long-lasting stimuli may differ.
PNU-120596's enhancement of the effects of nicotine in the formalin test led us to evaluate if it would potentiate other nicotinic responses, in particular those associated with CNS-related adverse effects. Our results show PNU-120596 at 8 mg·kg−1 did not significantly alter nicotine-induced locomotor depression. In addition, the administration of PNU-120596 (8 mg·kg−1) did not enhance nicotine-induced seizures, a response partially mediated by α7 nAChRs (Stitzel et al., 1998; Damaj et al., 1999; Stitzel et al., 2000; Tritto et al., 2004). It is possible that the mediation of seizure sensitivity is more closely related to a direct activation of α7 nAChRs rather than allosteric activation. On its own, PNU-120596 (8 mg·kg−1) failed either to alter locomotor activity of mice or to induce seizures or convulsions in these animals.
Interestingly, PNU-120596 dose-dependently enhanced hypothermia induced by the lowest dose (0.5 mg·kg−1) of nicotine. This enhancement disappeared at higher doses of the drug. The α7 antagonist MLA blocked PNU-120596's enhancement of nicotine-induced hypothermia, suggesting the plausible involvement of α7 nAChRs. In addition, in the presence of PNU-120596, mice were more sensitive to the effects of α7 agonist PHA-543613 (8 mg·kg−1) on body temperature. These findings were surprising because nicotine-induced hypothermia is mediated by β2-, β4-, α4-, α5-, but not α7-containing nAChRs (Tritto et al. 2004; Sack et al., 2005; Tapper et al., 2007; Jackson et al., 2010; Ortiz et al., 2012). The enhancement of nicotine's hypothermic effects seems to require positive allosteric modulation, because the α7 direct agonist PHA-543613, failed to alter this nicotinic response. The potentiation of nicotine's effects might therefore be the result of a higher calcium influx due to a prolonged receptor activation by PNU-120596. Higher calcium influx has been demonstrated to induce hypothermia in murine hippocampal neurons (Warren et al., 2012). However, mechanisms behind PNU-120596 and nicotine interaction on body temperature are not clear. It has been suggested that nicotine produces hypothermia by interacting with presynaptic nicotinic receptors to release ACh, which acts on muscarinic receptors (Gordon, 1994; Overstreet et al., 1998). Furthermore, dopaminergic and opiate mechanisms were reported to mediate the effects of nicotine on the body temperature (Zarrindast and Abolfathi-Araghi, 1992; Zarrindast and Tabatabai, 1992; Sakoori and Murphy, 2009).
Overall, our results demonstrate that the interaction between PNU-120596 and choline has a synergistic, antinociceptive effect in the formalin test. The in vivo studies presented here provide support for the further study of synergistic antinociceptive mechanisms for the interactions between PNU-120596 and α7 agonists.
Glossary
- %MPE
percent maximum possible effect
- i.pl
intraplantar injection
- i.t
intrathecal injection
- nAChR(s)
nicotinic ACh receptor(s)
- PAMs
positive allosteric modulators
- PNS
peripheral nervous system
Conflict of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Subtype-specific inhibition of nicotinic acetylcholine receptors bycholine: a regulatory pathway. J Pharmacol Exp Ther. 2006;318:268–275. doi: 10.1124/jpet.106.103135. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Allen DD, Lockman PR. The blood-brain barrier choline transporter as a brain drug delivery vector. Life Sci. 2003;73:1609–1615. doi: 10.1016/s0024-3205(03)00504-6. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Letchworth SR, Bencherif M, Lippiello PM. Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol Sci. 2005;26:352–360. doi: 10.1016/j.tips.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- Collins T, Young GT, Millar NS. Competitive binding at a nicotinic receptor transmembrane site of two α7-selective positive allosteric modulators with differing effects on agonist-evoked desensitization. Neuropharmacology. 2011;61:1306–1313. doi: 10.1016/j.neuropharm.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Aceto MD, Martin BR. Pharmacological characterization of nicotine-induced seizures in mice. J Pharmacol Exp Ther. 1999;291:1284–1291. [PubMed] [Google Scholar]
- Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Decker MW, Meyer MD, Sullivan JP. The therapeutic potential of nicotinic acetylcholine receptor agonists for pain control. Expert Opin Investig Drugs. 2001;10:1819–1830. doi: 10.1517/13543784.10.10.1819. [DOI] [PubMed] [Google Scholar]
- Dewey WL, Harris LS, Howes JF, Nuite JA. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J Pharmacol Exp Ther. 1970;175:435–442. [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the alpha7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer's disease. J Neurosci. 2009;29:8805–8815. doi: 10.1523/JNEUROSCI.6159-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghih R, Gopalakrishnan M, Briggs CA. Allosteric modulators of the alpha7 nicotinic acetylcholine receptor. J Med Chem. 2008;51:701–712. doi: 10.1021/jm070256g. [DOI] [PubMed] [Google Scholar]
- González-Rubio JM, Rojo J, Tapia L, Maneu V, Mulet J, Valor LM, et al. Activation and blockade by choline of bovine alpha7 and alpha3beta4 nicotinic receptors expressed in oocytes. Eur J Pharmacol. 2006;535:53–60. doi: 10.1016/j.ejphar.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. 24-Hour control of body temperature in the rat: II. Diisopropyl fluorophosphate-induced hypothermia and hyperthermia. Pharmacol Biochem Behav. 1994;49:747–754. doi: 10.1016/0091-3057(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Gronlien JH, Håkerud M, Ween H, Thorin-hagene K, Briggs CA, Gopalakrishnan M, et al. Distinct profiles of α7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- Gusev AG, Uteshev VV. Physiological concentrations of choline activate native α7-containing nicotinic acetylcholine receptors in the presence of PNU-120596 [1-(5-chloro-2,4-dimethoxyphenyl)-3-5-methylisoxazol-3-yl)-urea] Pharmacology. 2010;332:588–598. doi: 10.1124/jpet.109.162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Menzaghi F. Antagonist of nicotinic acetylcholine receptors (nAChR) enhances formalin-induced nociception in rats: tonic role of nAChRs in the control of pain following injury. Brain Res. 2001;888:102–106. doi: 10.1016/s0006-8993(00)03022-5. [DOI] [PubMed] [Google Scholar]
- Hamurtekin E, Gurun MS. The antinociceptive effects of centrally administered CDP-choline on acute pain models in rats: the involvement of cholinergic system. Brain Res. 2006;1117:92–100. doi: 10.1016/j.brainres.2006.07.118. [DOI] [PubMed] [Google Scholar]
- Haydar SN, Dunlop J. Neuronal nicotinic acetylcholine receptors – targets for the development of drugs to treat cognitive impairment associated with schizophrenia and Alzheimer's disease. Curr Top Med Chem. 2010;10:144–152. doi: 10.2174/156802610790410983. [DOI] [PubMed] [Google Scholar]
- Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajo M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor?: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalappa BI, Gusev AG, Uteshev VV. Activation of functional α7-containing nAChRs in hippocampal CA1 pyramidal neurons by physiological levels of choline in the presence of PNU-120596. PLoS ONE. 2010;5:e13964. doi: 10.1371/journal.pone.0013964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Zhu C, Malysz J, Campbell T, Shaughnessy T, Honore P, et al. α4β2 Neuronal nicotinic receptor positive allosteric modulation: an approach for improving the therapeutic index of α4β2 nAChR agonists in pain. Biochem Pharmacol. 2011;82:959–966. doi: 10.1016/j.bcp.2011.06.044. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Bencherif M. Convergence of alpha 7 nictonic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT 3 and NF-kappaB. Brain Res. 2009;1256:1–7. doi: 10.1016/j.brainres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, et al. Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain. 2008;7:580–587. doi: 10.1016/j.jpain.2008.01.336. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Jozwiak K, Wainer IW. Allosteric modifiers of neuronal nicotinic acetylcholine receptors: new methods, new opportunities. Med Res Rev. 2007;27:723–753. doi: 10.1002/med.20091. [DOI] [PubMed] [Google Scholar]
- Munro G, Hansen RR, Erichsen HK, Timmermann DB, Christensen JK, Hansen HH. The α7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br J Pharmacol. 2012;167:421–435. doi: 10.1111/j.1476-5381.2012.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Schrode K, Stevenson GW. Mu/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol. 2008;16:386–399. doi: 10.1037/a0013088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz NC, O'Neill HC, Marks MJ, Grady SR. Varenicline blocks β2*-nAChR-mediated response and activates β4*-nAChR-mediated responses in mice in vivo. Nicotine Tob Res. 2012;14:711–719. doi: 10.1093/ntr/ntr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Harris S, Lloyd P, Messineo E. An isobolographic analysis of the antinociceptive effect of systemically and intrathecally administered combinations of clonidine and opiates. J Pharmacol Exp Ther. 1990;255:1107–1116. [PubMed] [Google Scholar]
- Overstreet DH, Daws LC, Schiller GD, Orbach J, Janowsky DS. Cholinergic/serotonergic interactions in hypothermia: implications for rat models of depression. Pharmacol Biochem Behav. 1998;59:777–785. doi: 10.1016/s0091-3057(97)00514-5. [DOI] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther. 2009;329:791–807. doi: 10.1124/jpet.108.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Porter JK. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R, Gochberg-Sarver A, Rozovsky U, Kedmi M, Rosner S, Orr-Urtreger A. Lower core body temperature and attenuated nicotine-induced hypothermic response in mice lacking the beta4 neuronal nicotinic acetylcholine receptor subunit. Brain Res Bull. 2005;66:30–36. doi: 10.1016/j.brainresbull.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Enhanced nicotine sensitivity in nociceptin/orphanin FQ receptor knockout mice. Neuropharmacology. 2009;56:896–904. doi: 10.1016/j.neuropharm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Sattelle DB, Buckingham SD, Akamatsu M, Matsuda K, Pienaar IS, Jones AK, et al. Comparative pharmacology and computational modelling yield insights into allosteric modulation of human alpha7 nicotinic acetylcholine receptors. Biochem Pharmacol. 2009;78:836–843. doi: 10.1016/j.bcp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Stitzel JA, Blanchette JM, Collins AC. Sensitivity to the seizure-inducing effects of nicotine is associated with strain-specific variants of the alpha 5 and alpha 7 nicotinic receptor subunit genes. J Pharmacol Exp Ther. 1998;284:1104–1111. [PubMed] [Google Scholar]
- Stitzel JA, Jimenez M, Marks MJ, Tritto T, Collins AC. Potential role of the alpha4 and alpha6 nicotinic receptor subunits in regulating nicotine-induced seizures. J Pharmacol Exp Ther. 2000;293:67–74. [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Analysis. Boca Raton, FL: Chapman & Hall/CRC Press; 2000. [Google Scholar]
- Tallarida RJ. An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Marks MJ, Lester HA. Nicotine responses in hypersensitive and knockout alpha 4 mice account for tolerance to both hypothermia and locomotor suppression in wild-type mice. Physiol Genomics. 2007;31:422–428. doi: 10.1152/physiolgenomics.00063.2007. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–343. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Grønlien JH, Kohlhaas KL, Nielsen EØ, Dam E, Jørgensen TD, et al. An allosteric modulator of the α7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Tritto T, MecCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, et al. Null mutant analysis of responses to nicotine: deletion of β2 nicotinic acetylcholine receptor subunit but not α7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob Res. 2004;6:145–158. doi: 10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through alpha 7 nicotinic receptors. J Neurophysiol. 2003;89:1797–1806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- Vincler M. Neuronal nicotinic receptors as targets for novel analgesics. Expert Opin Investig Drugs. 2005;14:1191–1198. doi: 10.1517/13543784.14.10.1191. [DOI] [PubMed] [Google Scholar]
- Wang HY, Stucky A, Liu J, Shen C, Trocme-Thibierge C, Morain P. Dissociating beta-amyloid from alpha 7 nicotinic acetylcholine receptor by a novel therapeutic agent, S 24795, normalizes alpha 7 nicotinic acetylcholine and NMDA receptor function in Alzheimer's disease brain. J Neurosci. 2009;29:10961–10973. doi: 10.1523/JNEUROSCI.6088-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Su DM, Wang RH, Liu Y, Wang H. Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience. 2005;132:49–56. doi: 10.1016/j.neuroscience.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Warren DE, Bickler PE, Clark JP, Gregersen M, Brosnan H, McKleroy W, et al. Hypothermia and rewarming injury in hippocampal neurons involve intracellular Ca (2+) and glutamate excitotoxicity. Neuroscience. 2012;207:316–325. doi: 10.1016/j.neuroscience.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Wessinger WD. Approaches to the study of drug interactions in behavioral pharmacology. Neurosci Biobehav Rev. 1986;10:103–113. doi: 10.1016/0149-7634(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol. 2011;82:915–930. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Abolfathi-Araghi F. Effects of bupropion on core body temperature of mice. Psychopharmacology (Berl) 1992;106:248–252. doi: 10.1007/BF02801980. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Tabatabai SA. Involvement of dopamine receptor subtypes in mouse thermoregulation. Psychopharmacology (Berl) 1992;107:341–346. doi: 10.1007/BF02245159. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Chin CL, Rustay NR, Zhong C, Mikusa J, Chandran P, et al. Potentiation of analgesic efficacy but not side effects: co-administration of an α4β2 neuronal nicotinic acetylcholine receptor agonist and its positive allosteric modulator in experimental models of pain in rats. Biochem Pharmacol. 2011;82:967–976. doi: 10.1016/j.bcp.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Zhu W, An Y, Zheng J, Tang L, Zhang W, Jin L, et al. A new microdialysis-electrochemical device for in vivo simultaneous determination of acetylcholine and choline in rat brain treated with N-methyl-(R)-salsolinol. Biosens Bioelectron. 2009;24:3594–3599. doi: 10.1016/j.bios.2009.05.023. [DOI] [PubMed] [Google Scholar]