Abstract
Soil nitrogen (N) budgets are used in a global, distributed flow-path model with 0.5° × 0.5° resolution, representing denitrification and N2O emissions from soils, groundwater and riparian zones for the period 1900–2000 and scenarios for the period 2000–2050 based on the Millennium Ecosystem Assessment. Total agricultural and natural N inputs from N fertilizers, animal manure, biological N2 fixation and atmospheric N deposition increased from 155 to 345 Tg N yr−1 (Tg = teragram; 1 Tg = 1012 g) between 1900 and 2000. Depending on the scenario, inputs are estimated to further increase to 408–510 Tg N yr−1 by 2050. In the period 1900–2000, the soil N budget surplus (inputs minus withdrawal by plants) increased from 118 to 202 Tg yr−1, and this may remain stable or further increase to 275 Tg yr−1 by 2050, depending on the scenario. N2 production from denitrification increased from 52 to 96 Tg yr−1 between 1900 and 2000, and N2O–N emissions from 10 to 12 Tg N yr−1. The scenarios foresee a further increase to 142 Tg N2–N and 16 Tg N2O–N yr−1 by 2050. Our results indicate that riparian buffer zones are an important source of N2O contributing an estimated 0.9 Tg N2O–N yr−1 in 2000. Soils are key sites for denitrification and are much more important than groundwater and riparian zones in controlling the N flow to rivers and the oceans.
Keywords: denitrification, global change, groundwater, nitrous oxide, riparian zone, soil
1. Introduction
The global nitrogen (N) cycle is driven by the fixation of inert atmospheric molecular nitrogen (N2) and the formation of reactive bio-available N compounds, such as nitrate (NO3−), ammonium (NH4+) and N-oxides. Humans have accelerated this cycle through fertilizer production and use, and fossil fuel combustion [1]. Denitrification is the microbial process that removes NO3−, anaerobically reducing it to nitrite (NO2−), nitric oxide (NO), the greenhouse gas nitrous oxide (N2O) and N2. In agricultural soils, NO3− mainly originates from fertilizers, animal manure, crop residues and soil organic matter. The organic substrates have to be mineralized first to ammonium by heterotrophic micro-organisms, while nitrifying bacteria subsequently oxidize the NH4+ to NO2− and NO3−. In natural ecosystems, the main N sources are biological N2 fixation and atmospheric deposition. In terrestrial ecosystems, denitrification occurs mainly in soils, groundwater and riparian zones. Other human-managed systems where denitrification occurs include manure storage systems, wastewater treatment plants, bioreactors and constructed or reclaimed wetlands.
Denitrification is an important process because it leads to significant N losses from agricultural systems, while, by converting NO3− and NO2− into gaseous N2, N2O and NOx (NO + NO2), denitrification reduces the problem of a global N overload on the hydrological system. In doing so, denitrification transfers this overload to the atmosphere, where N2O and NOx can have negative environmental impacts.
As denitrification is an anaerobic process, oxygen (O2) is the most important regulator [2]. Nitrate is the electron acceptor and source of N, and C serves as the electron donor and energy source for heterotrophic denitrifying bacteria. Soil pH has a marked effect on denitrification, with lower rates under acid than under slightly alkaline conditions [3]. Denitrification has an optimum temperature range of 25–30°C [4]. Temperature also controls decomposition and nitrification rates [2], and therefore regulates the availability of O2, NO3− and organic C. Soil denitrification and N2O production and consumption are extremely variable in time and space [5] owing to variability of the major regulators.
Groundwater discharge to surface waters generally consists of a mixture of water from different parts of the aquifer, characterized by a wide range of travel times. The NO3− concentration in groundwater depends on the time of infiltration into the saturated zone, the NO3− concentration of that water and the denitrification loss during its transport [6]. As O2 becomes limiting in the saturated zone, heterotrophic micro-organisms increasingly switch to NO3− as an electron acceptor for the oxidation of organic C. This organic C may be present in either dissolved form or as part of the sediment matrix. If pyrite is present in subsurface sediments, denitrification coupled to pyrite oxidation may become the dominant removal process [7]. Pyrite is a common iron disulfide mineral, and is often found in association with unconsolidated marine deposits in the terrestrial subsurface and in association with coal and shale deposits.
Riparian areas are wetlands located at the interface between the terrestrial and aquatic components of the landscape, which, owing to their position, contribute to the control of nutrient and energy fluxes towards surface waters. Riparian denitrification is an important mechanism to remove N in subsurface run-off and shallow groundwater moving from uplands towards streams [8]. Similar to soils, N2O emission from riparian zones is caused by an imbalance between N2O production and consumption. These imbalances increase at high concentrations of O2 and NO3− in the soil and at low pH [9]. Although limited in surface area, riparian zones have been identified as hot spots for denitrification and N2O emission especially along lower-order streams [10].
Both rates of denitrification and N2O emissions [11] have changed as a result of human intervention in the global N cycle. Future population growth and economic development may lead to further acceleration of the global N cycle. Quantifying where, when and how much denitrification occurs on the basis of measurements alone is virtually impossible [12]. Models have, therefore, become essential tools. In this paper, we address the possible change of terrestrial denitrification and N2O emissions from soils, groundwater and riparian zones under past and future climate change, increasing food and energy production and agricultural intensification, using a simulation model. The term denitrification is used to indicate total nitrate reduction to N2, N2O and NO. Details on the data, models and scenarios used and part of the results are provided in the electronic supplementary material.
2. Estimating global terrestrial denitrification and nitrous oxide emissions
(a). Data used
Spatially explicit soil budgets at 0.5° × 0.5° resolution were used for 1900, 1950, 1970 and 2000 [11]. Projections for the period 2000–2050 [13] were based on the four Millennium Ecosystem Assessment (MEA) scenarios implemented with the spatially explicit Integrated Model to Assess the Global Environment (IMAGE) ([14]; electronic supplementary material, S2 and S3). All data, including land use, soil N budgets [13], climate and the spatial run-off data developed for the MEA scenarios [15] form the basis of the calculations presented here.
The four MEA scenarios are Global Orchestration (GO), Order from Strength (OS), Technogarden (TG) and Adapting Mosaic (AM). They differ in the assumed population growth, economic and industrial development, and human diets, leading to varying greenhouse gas emission pathways and climate change. GO portrays a globally connected society that focuses on global trade and economic liberalization, and takes a reactive approach to ecosystem problems, but also takes strong steps to reduce poverty and inequality and to invest in public goods, such as infrastructure and education. By contrast, OS is a regionalized and fragmented world, concerned with security and protection, emphasizing regional markets, paying little attention to public goods and taking a reactive approach to ecosystem problems. TG is a globally connected world relying strongly on environmentally sound technology, using managed or engineered ecosystems to deliver ecosystem services and taking a proactive approach to environmental problems. In AM, regional ecosystems are the focus, and local, proactive management of ecosystems is based on simple technologies. More details on the scenarios including the assumptions on N management in agriculture are provided in the electronic supplementary material S3 and table S1.
The annual soil N budget includes the N inputs and outputs for 0.5° × 0.5° grid cells for agricultural and natural land. N inputs include biological N2 fixation (Nfix), atmospheric N deposition (Ndep), application of synthetic N fertilizer (Nfert) and animal manure (Nman). Outputs in the soil N budget include ammonia (NH3) volatilization (Nvol), N removal from the field through crop harvesting, hay and grass cutting and grass consumed by grazing animals (Nwithdr). The soil N budget (Nbudget) was calculated as follows:
| 2.1 |
The soil N budget ignores N accumulation in soil organic matter where there is a positive budget (surplus), and also ignores N supply from soil organic matter decomposition in case of a negative budget (deficit). With no accumulation, N surpluses, therefore, represent a potential loss (by denitrification, surface run-off and leaching). Table 1 lists the global N input and output terms for agricultural land and land under natural vegetation for the different years and scenarios.
Table 1.
N input and output terms for agricultural (agr.) land and natural (nat.) ecosystems for 1900, 1950, 1970, 2000 [11] and for 2030 and 2050 for the four Millennium Ecosystem Assessment scenarios [13].
| year/scenario | agriculture |
natural ecosystems |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| fertilizer (Nfert) | manurea (Nman) | biological fixation (Nfix) | deposition (Ndep) | total N inputs | N withdrawal (Nwithdr) | NH3–N emission (Nvol) | soil N budget agr. (Nbudget) | biological fixation (Nfix) | deposition (Ndep) | soil N budget nat. (Nbudget) | |
| N (1012 g yr−1) | |||||||||||
| 1900 | 1 | 36 | 14 | 6 | 56 | 34 | 6 | 16 | 75 | 24 | 99 |
| 1950 | 4 | 52 | 23 | 13 | 93 | 52 | 10 | 30 | 63 | 23 | 86 |
| 1970 | 29 | 76 | 30 | 26 | 161 | 78 | 18 | 66 | 57 | 31 | 88 |
| 2000 | 83 | 101 | 39 | 36 | 258 | 112 | 33 | 113 | 53 | 33 | 86 |
| 2050GO | 119 | 190 | 55 | 49 | 412 | 202 | 59 | 152 | 52 | 46 | 98 |
| 2050TG | 98 | 155 | 57 | 33 | 343 | 182 | 48 | 113 | 46 | 26 | 72 |
| 2050AM | 75 | 144 | 55 | 44 | 318 | 169 | 42 | 108 | 51 | 39 | 90 |
| 2050OS | 109 | 163 | 56 | 51 | 378 | 179 | 51 | 147 | 49 | 39 | 88 |
aExcluding manure N that ends outside the agricultural system, such as manure stored in lagoons or used as fuel or building material (in 2000 this was 11 Tg N).
Compared with earlier work [13], we assumed lower biological N2-fixation rates in natural ecosystems, based on Vitousek et al. [16], who estimated that N2 fixation was only 58 Tg yr−1 (Tg = teragram; 1 Tg = 1012 g) in pre-industrial times. The estimates used to calculate N fixation in natural ecosystems are based on the medium estimate for area coverage of leguminous plants and free-living N-fixing bacteria [17]. Here, we used the low estimate for the areal coverage, and this reduces global N fixation for the year 1900 from 143 to 75 Tg N yr−1 and for the year 2000 from 101 to 53 Tg N yr−1.
(b). Computing denitrification
The model used to simulate N flows from the soil via leaching through groundwater systems and riparian zones, and via surface run-off to surface water is a modification of the conceptual model of Van Drecht et al. [18]. Details on the model are given in the electronic supplementary material, S4–S8. Here, a brief summary is provided.
Annual soil denitrification is calculated as a fraction of the surplus of the soil N budget corrected for surface run-off (see the electronic supplementary material, equations S8 and S9), based on temperature, the residence time of water and NO3− in the soil, soil texture, soil drainage and soil organic C. Leaching of NO3− from the root zone (1 m thick) to groundwater is the soil N budget minus soil denitrification and surface run-off (see the electronic supplementary material, equation S14).
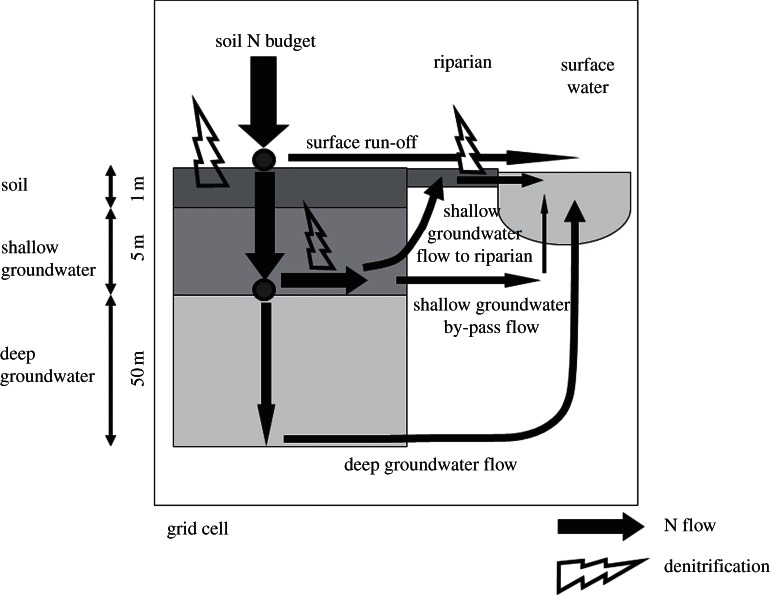
Two groundwater subsystems are distinguished (figure 1). The shallow groundwater system represents the upper metres of the saturated zone (typically 5 m) and is characterized by short residence times before water enters local surface water at short distances, or infiltrates the deep groundwater system.
Figure 1.
Global model with 0.5 × 0.5° resolution applied in this study, representing denitrification in soils, groundwater and riparian zones. Not all grid cells include all compartments, depending on the presence of shallow groundwater, deep groundwater and surface water.
A deep system with a thickness of 50 m is defined where a deeper groundwater flow is present (see the electronic supplementary material, S7). This deep groundwater system has longer residence times than the shallow system, as water flows to greater depths and drains to larger rivers at greater distances. We assume that denitrification is negligible in the deep groundwater system, and the modelled NO3− outflow from deep groundwater is thus a maximum estimate.
Riparian zones generally represent a small area of the drainage basin. However, they are critical control points for groundwater N fluxes within the watershed [19]. In our approach, all shallow groundwater (if present) in a grid cell flows towards streams (figure 1). As small streams have the largest riparian ecotone length within drainage networks, these riparian zones are considered more important for groundwater N processing than those bordering larger water bodies. We ignored surface water bodies, such as lakes or larger streams, where shallow groundwater by-passes riparian zones or riparian zones are less reactive. The calculation of denitrification in riparian zones is similar to that in soils with two differences. First, a biologically active layer with a thickness of 0.3 m is assumed, as riparian zones show strong vertical gradients. Denitrification rates are high in this topsoil owing to the high organic matter contents. Second, we included the effect of pH on denitrification rates and the gaseous end-products (see the electronic supplementary material, figure S1a).
(c). Computing nitrous oxide emissions
Nitrous oxide emission from soils under natural vegetation and from agricultural land are calculated with regression models (see the electronic supplementary material, S6). N2O production in groundwater is calculated using the Intergovernmental Panel on Climate Change (IPCC) emission factor (0.25% of the N leached from the root zone; [20]).
Our approach for riparian areas (see the electronic supplementary material, S8) includes corrections for the observed inhibition of denitrification at low soil pH, and for high N2O fractions when denitrification is inhibited [9]. With this conceptual approach, the fraction of N2O in total denitrification is high when conditions limit denitrification, and low when conditions are optimal (see the electronic supplementary material, figure S1b). Field measurements show a wide range of N2O emissions from riparian areas indicating that these can be both sources and sinks for N2O. In general, fluxes from riparian areas are low compared with those from agricultural soils. Fractions of N2O relative to the total denitrification end product (N2 + N2O) range from 0.3 up to 73 per cent (see the electronic supplementary material, table S4).
(d). Sensitivity analysis
The sensitivity of the model was investigated using Latin hypercube sampling, with uncertainty ranges for 17 parameters, and expressed using the standardized regression coefficient (SRC), to compare model output of 10 variables (more details are in the electronic supplementary material, S10 and table S5).
3. Results
(a). Period 1900–2000
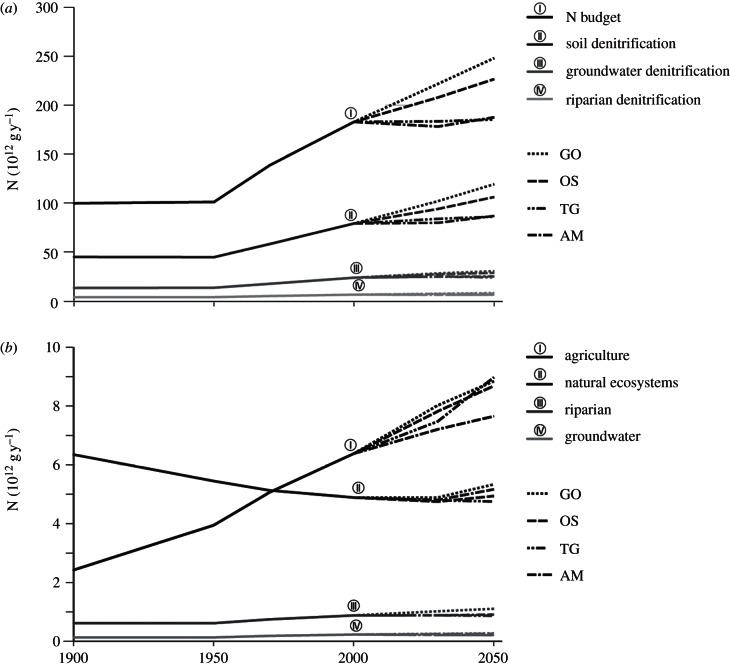
The global soil N budget has increased from 118 to 202 Tg yr−1 between 1900 and 2000. Hereafter, we exclude arid regions, where denitrification is assumed to be negligible (see the electronic supplementary material, S5). Between 1900 and 2000, the global N budget thus calculated increased from 100 to 183 Tg yr−1 (figure 2a). This increase is primarily the result of an increasing agricultural N budget, from 16 to 113 Tg yr−1, and a decreasing N budget for soils under natural vegetation (from 99 to 86 Tg yr−1), caused by land-use change. There are large differences between different countries. Soil N budgets increased rapidly after 1950 for industrialized countries, while rapid increases in developing countries like China and India started only from the 1970s. Small positive or even negative soil N budgets were recorded for many developing countries in Africa, Asia and South America.
Figure 2.
Global soil N budget (excluding arid regions) and (a) denitrification (N2, NO and N2O) and (b) N2O emission for soils, groundwater and riparian zones for 1900–2000, and for 2000–2050 for the four MEA scenarios.
Between 1900 and 2000, there has been an increase of global N fertilizer use from practically zero to more than 80 Tg yr−1, N excretion by animals (from 36 to 101 Tg yr−1) and biological N2 fixation in agriculture (14–39 Tg N yr−1). These changes in agriculture led to increasing emissions of NH3 (6–33 Tg N yr−1). Together with increasing NOx emissions (from 7 to 38 Tg yr−1 according to representative concentration pathways, http://www.iiasa.ac.at/web-apps/tnt/RcpDb) owing to expanding fossil fuel use and industrial production, this caused rapidly increasing atmospheric N deposition (from 6 to 36 Tg yr−1 for agricultural land, and from 24 to 33 Tg yr−1 for natural ecosystems) between 1900 and 2000 (table 1).
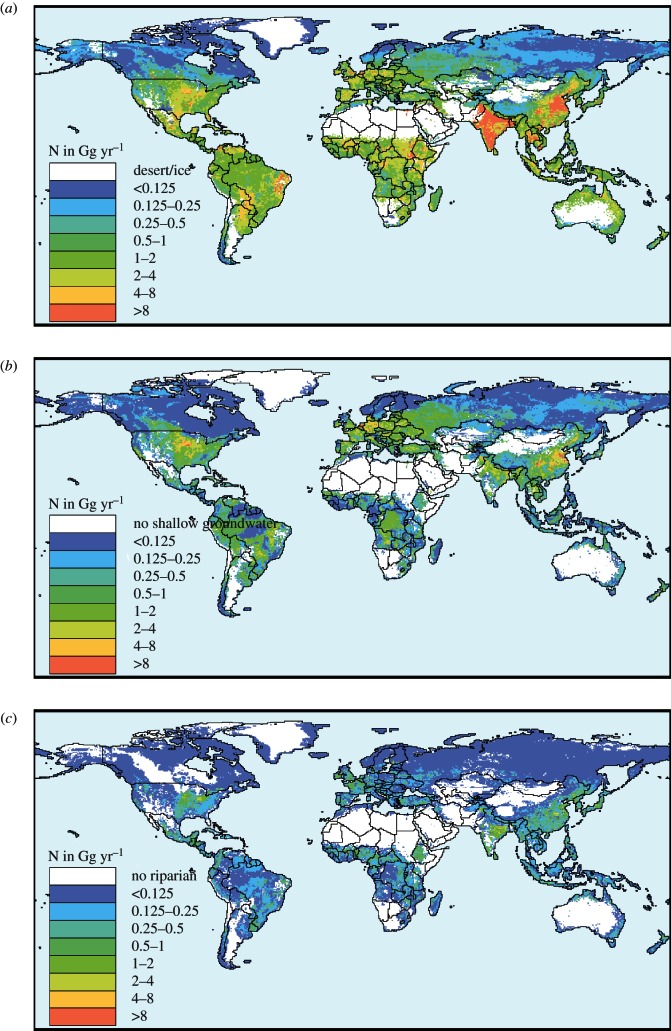
Soil denitrification largely follows the trend in the N budgets. Annual global soil denitrification increased from 45 to 79 Tg N yr−1 between 1900 and 2000 (figure 2a), which is the result of a rapid increase in denitrification in agricultural soils (from 9 to 51 Tg N yr−1), and a decrease in denitrification in soils under natural vegetation (from 36 to 28 Tg N yr−1) as a result of forest conversion to agriculture. Spatial variability is large (figure 3).
Figure 3.
Denitrification (N2, NO and N2O) computed for the year 2000 for (a) soils excluding arid regions, (b) groundwater and (c) riparian zones. Denitrification is denoted as total N in Gg (109 g) per grid cell, because the location of outgassing for groundwater and riparian zones is not known.
Between 1900 and 2000, the calculated transfer of N from soils to groundwater (51–54% of the soil N budget surplus), and denitrification in groundwater (23–24% of the N load from soil leaching) and riparian zones (11–13% of N load from shallow groundwater) were all relatively stable. The river N load increased from 38 to 65 Tg N yr−1 between 1900 and 2000. Long-term storage of N in groundwater increased from 0 to 10 Tg N yr−1 between 1900 and 2000 (figure 4), and cumulative N storage in deep groundwater between 1900 and 2000 amounted to around 376 Tg. This has long-lasting effects. A calculation with zero N inputs after 2000 yields an outflow from deep and shallow groundwater of 5.6 Tg N yr−1 in 2030 and 2.5 Tg N yr−1 in 2050 using climate and land cover data for the GO scenario.
Figure 4.
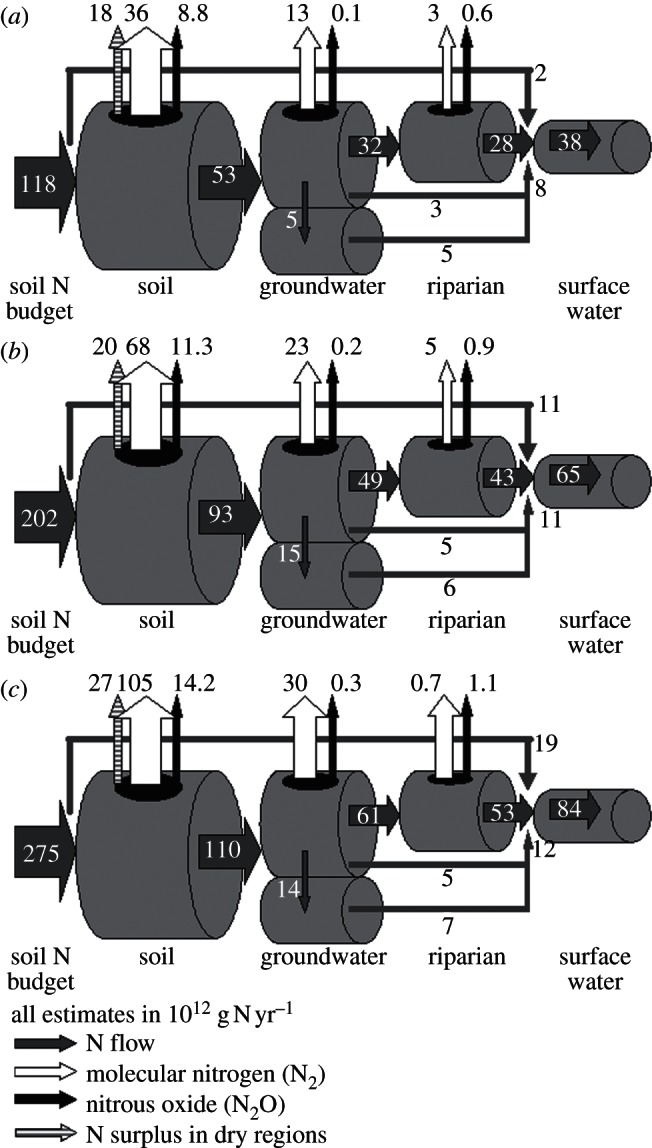
Scheme presenting the global N flows for (a) 1900, (b) 2000 and (c) 2050 for the Global Orchestration (GO) scenario through global soils, groundwater, riparian zones to surface water and associated N2 and N2O production in each compartment. The sum of N2 and N2O equals total denitrification. The imbalance between deep groundwater inflow and outflow is caused by (temporary) storage.
Between 1900 and 2000, N2O emissions from soils under natural vegetation decreased (from 6.3 to 4.9 Tg N2O–N yr−1), whereas N2O emissions from agricultural fields rapidly increased (from 2.4 to 6.4 Tg N2O–N yr−1; figure 2b). Emissions from groundwater and riparian zones changed accordingly. The overall spatial distributions of N2O emissions from groundwater and riparian areas are similar (see the electronic supplementary material, figure S3).
Global annual N2O emissions from all sources (including indirect emissions from N deposition in natural ecosystems, industry and energy-related emissions, biomass burning and oceans) increased from around 13 to 17 Tg N2O–N yr−1 between 1900 and 2000 (see the electronic supplementary material, table S7).
(b). Period 2000–2050
The four MEA scenarios portray different futures, with the annual global N budget increasing from 202 Tg yr−1 in 2000 to 275 Tg yr−1 in 2050 in the GO scenario and to 251 Tg yr−1 in the OS scenario. The environment-oriented scenarios (TG and AM) show slightly decreasing trends in N budgets (figure 2b). The N budget for land under natural vegetation reflects the result of forest conversion to agricultural land, particularly in the TG scenario, and increasing N inputs from atmospheric deposition (especially in the GO scenario; table 1).
The scenarios GO and OS show a rapid increase in soil denitrification, primarily owing to increasing denitrification in agricultural soils with large N surpluses. The TG and AM scenarios predict a stabilization, which reflects the balance between an increase of denitrification in agriculture and a decrease in natural ecosystems.
The transfer of N from soils to groundwater is expected to decrease from 51 to 44 per cent by 2050, groundwater denitrification will increase from 25 to 27–29% of N leaching, while riparian denitrification will stabilize at 13 per cent of the N inflow from groundwater in the GO scenario between 2000 and 2050. The overall N retention of the terrestrial system shows a slight increase, from 51–54% to 57 per cent of the soil N budget in 2050, for the GO scenario, primarily owing to the increase in soil denitrification. With the rapid increase of the global soil N budget, the annual N flow to rivers is, therefore, increasing rapidly, from 64 Tg N yr−1 in 2000 to 84 Tg N yr−1 in 2050 (GO scenario; figure 4).
The trends in groundwater and soil denitrification differ (figure 2a). For example, in the GO scenario, soil denitrification increases by 51 per cent between 2000 and 2050, whereas groundwater denitrification increases by 29 per cent. In the OS scenario there is also a difference (34% increase in soil and 21% increase in groundwater denitrification). The TG and AM scenarios show a small increase of soil and groundwater denitrification after 2000 (figure 2a). Long-term storage of N in groundwater ranges from 3 under AM and TG to 7 Tg N yr−1 under the GO scenario in 2050 (not shown).
Similar to the trends in soil and groundwater denitrification, the TG and AM scenarios predict a slight increase in riparian denitrification, while a larger increase is expected under the GO and OS scenarios (8 Tg N yr−1 in 2050; figure 2a).
The scenarios show increases in global N2O emissions from agricultural soils from 6.4 Tg N2O–N yr−1 in 2000 to 7.7 (AM scenario) and 9.0 Tg N2O–N yr−1 (TG, with large areas of energy crops, see the electronic supplementary material, table S1; figure 2b). Global N2O emissions from all sources increase from 16.5 Tg N2O–N yr−1 in 2000 to 18.0 (AM) and 19.7 (GO) Tg N2O–N yr−1 in 2050 (see the electronic supplementary material, table S7).
(c). Model sensitivity
Here, we discuss the SRC (see the electronic supplementary material, table S6), concentrating on SRC values exceeding 0.2 (i.e. a contribution of 0.22 = 0.04 or about 4% to the variation of global results). Results for a similar analysis on the regional or smaller scale or a different year would yield different results, depending on the N balances and hydrological and geographical setting including climate.
Temperature, net precipitation (or run-off) and the soil N budget for natural ecosystems, cropland and grassland are important determinants for almost all global model variables, including the outflow to the rivers. The direction of the effect is variable, e.g. precipitation increase reduces soil denitrification, while temperature has a positive effect. N in surface run-off is most strongly determined by the various parameters that determine run-off and the N therein, with a strong effect of land use. For global soil denitrification, the N budget in cropland (SRC = 0.60) has the largest contribution, followed by the N budget in natural ecosystems (0.47) and temperature (0.50). N leaching has a similar sensitivity, but here, the N budget in natural ecosystems (0.67) has the largest influence on variation, followed by N budget in cropland (0.54), and temperature (−0.43).
The outflow from shallow groundwater is most sensitive to variation of the thickness of the shallow layer (SRC = −0.65), the N budget in natural ecosystems (SRC = 0.50), net precipitation (0.31) and the N budget of cropland (0.25). The outflow from the deep groundwater system is sensitive to the half-life of nitrate (0.66), the thickness of the deep layer (−0.46) and the water partitioning to the deep layer (0.27). The N inflow and bypass flow for riparian zones have sensitivities comparable with those of the N outflow from shallow groundwater.
4. Discussion
Our results indicate that soil denitrification is the major terrestrial N removal process, thus confirming results from other studies [21]. Denitrification in groundwater (23–25% of the N leaching from soils) and riparian areas (11–13% of N inflow from groundwater) is, here, estimated to be much less than in soils (42–45% of the soil N budget). This is because the denitrification potential in groundwater is strongly limited by the availability of electron donors. The denitrification potential in riparian areas is limited by their relatively small area, by the geohydrological setting, that determines the residence time and contact with organic-rich riparian soils.
Our global soil denitrification estimates (58–79 Tg N yr−1 for 1970–2000) are at the low end of the range of estimates from literature (table 2). The main reasons for our low estimate are the revised estimates for biological N2 fixation in natural ecosystems (§2(a)), and the arid regions, where we calculated a N surplus of about 20 Tg N yr−1 owing to our estimation of minimal denitrification under these conditions (see figure 4 and electronic supplementary material, S5).
Table 2.
Global estimates of terrestrial denitrification.
| total | soil | groundwater | riparian zone | |
|---|---|---|---|---|
| N (Tg yr−1) |
||||
| total | ||||
| Emery et al. [22] | 69 | |||
| Gruber & Sarmiento [23] | 175–202 | |||
| Codispoti et al. [24] | 255–450 | |||
| Søderlund & Svensson [25] | 108–160 | |||
| Tiedje [2]a | 105–185 | |||
| Van Drecht et al. [18]; Seitzinger et al. [21] | 124 | 44 | ||
| Canfield et al. [26] | 100 | |||
| Galloway et al. [1]a (year ∼2000) | 125 | |||
| Galloway et al. [1]a (year 2050) | 173 | |||
| this study (year 2000) | 109 (101–118)b | 79 (72–85)b | 24 (19–29)b | 6 (5–9)b |
| this study (year 2050) | 110–158c | 80–119c | 24–30c | 6–8c |
| agriculture | ||||
| Hofstra & Bouwman [26] | 22–87 | |||
| this study (year 2000) | 51 | |||
aAs estimates are based on mass balance considerations, these numbers probably represent total terrestrial denitrification.
bStandard model and range from sensitivity analysis (electronic supplementary material, table S5 and S6).
cLowest (TG) and highest (GO) scenario.
Modelled long-term storage of N in deep groundwater systems is consistent with observations in intensively managed agricultural land in, for example, China and France [27,28]. More generally, contamination of groundwater with nitrate is a wide-spread phenomenon [29], indicating that the removal rate of reactive N via denitrification has not kept pace with accelerated N cycling during the last decades.
Any global estimate of N2O emissions is fraught with potential errors, and the global estimates based on different methods for croplands range from 2.1 to 3.2 Tg N2O–N yr−1. The estimates presented in this study are within the range of uncertainty of the various models used (see the electronic supplementary material, table S7). The results of our model indicate that riparian areas may be an important global source of N2O, but to our knowledge no other global estimates for riparian zones are available for comparison. Regarding the N2O fraction of the N inflow, our estimate of 0.02 is within the range of estimates in the literature (see the electronic supplementary material, table S4).
We discuss some aspects of the uncertainties in our soil N budgets, denitrification in soil, groundwater and riparian zones and N2O emissions. Comparison of different datasets on global spatially explicit soil N budgets showed that there are important differences in the spatial allocation of biological N2 fixation, atmospheric N deposition and the output terms crop N withdrawal and NH3 volatilization [30]. Furthermore, uncertainty analysis within the agricultural system showed that livestock N excretion rates and NH3 emission rates from animal houses and storage systems are consistently the most important parameters in most parts of the world [31].
The assumption of a static soil N pool is important in all landscape components, as illustrated by the sensitivity to variation in the N budget (see the electronic supplementary material, table S6). Accounting for the current N release from soil organic matter loss of 25 Tg yr−1 owing to deforestation [32] would increase the estimated global N budget and thus, total denitrification by 12 per cent.
One of the largest uncertainties in estimating the N cycle is the amount of reactive N that is converted to N2 in the process of denitrification [1]. As the concentration of N2, the largest component of the atmosphere, cannot be used to derive N2 emissions in a top-down approach, the only methods to estimate global denitrification are the upscaling of field measurements and the application of denitrification models. Most field methods for determining denitrification rates have serious potential flaws, and measured denitrification rates are, therefore, only proxies for actual denitrification rates ([33]; electronic supplementary material, S1). Available methods have various problems; they change substrate availability or disturb the physical conditions of the process, lack sensitivity or are time consuming and expensive. The measurement of denitrification is also difficult owing to the high spatial and temporal variations in terrestrial environments. Mass balance and stoichiometric approaches seem most suitable to constrain denitrification estimates at larger spatial scales [33].
Models for estimating soil denitrification range from the scale of micro-sites and microbial dynamics to simplified factorial approaches used at global scales [12,34]. Not surprisingly, model comparison reveals strong differences in the results, indicating considerable shortcomings in current knowledge of scaling effects on soil denitrification and its controlling variables in models.
Compared with other soil denitrification models [34], the model applied in this paper is simple, computing denitrification based on soil N budgets as a function of temperature, soil water, organic matter, texture and drainage. Apart from uncertainties in soil N budgets, the soil denitrification model is most sensitive to the N availability (soil N budget), water flux and temperature (see the electronic supplementary material, table S6).
In groundwater, the continued availability of electron donors for denitrification will depend on its source. If dissolved organic carbon (DOC) leaches from soils in significant amounts, there will be a continuous supply of electron donors with the incoming NO3−. The importance of DOC as an electron donor in aquifers depends on the subsurface setting [35]. Where solid phase components, such as organic matter and pyrite in the aquifer matrix are dominant [36], the electron donors are not replenished and can gradually be depleted with time [7]. Recent studies confirm the important role of pyrite for NO3− removal in groundwater [35].
Owing to lack of information on the availability of organic matter and pyrite, the conceptual model approach used in this paper applies lithology as a proxy for denitrification potential in groundwater, represented by the half-life of NO3−. The travel time determines the disappearance of NO3− and the outflow to surface water or riparian areas. The model assumption of no denitrification in deep groundwater may be incorrect if NO3− moves to deep groundwater in sedimentary deposits containing reactive organic matter and pyrite. However, the sensitivity of modelled river loading due to variation of the half-life of nitrate in deep groundwater is relatively small (see the electronic supplementary material, table S6).
Riparian processing is a spatially explicit phenomenon based on the interaction of NO3− in groundwater with the biologically active zone of the riparian area [37]. There are large uncertainties in our estimates of the by-pass flow (which can occur when groundwater intersects with the ground surface and emerges as a ‘seep’), and deep flow below the microbially active portion of soil that emerges below the river, i.e. underflow. By-pass flow through highly permeable coarse-grained sediments such as gravel and sand can also significantly reduce the denitrification capacity owing to shorter residence time and low organic matter contents. Effective nitrate removal in riparian zones of more than 90 per cent is predominantly found in glaciated areas where permeable surface soils are underlain with an impermeable layer at a depth of 1–4 m [37].
Modelling riparian processes requires spatial information on their hydrogeological conditions. Even detailed maps (1 : 10 000 to 1 : 20 000) to determine the topography of riparian areas have limitations [19], and it is impossible to know the location of riparian areas at the 0.5° × 0.5° resolution of this study. With all the simplifications in the simulation of the N inflow to riparian areas and denitrification, the model results are a first attempt to quantify global denitrification and N2O emissions, showing strong sensitivity to total water flow, soil N budget and N inflow from shallow aquifers (see the electronic supplementary material, table S6).
Turning to N2O emissions, we see several causes of uncertainty. The fraction of N lost as N2O from nitrification, nitrifier denitrification or denitrification often shows a strong nonlinear relationship with the controlling factors, which makes upscaling difficult. In order to estimate direct N2O emissions, measurement data at the field scale have been used to derive relationships between controlling factors and N2O emissions with statistical techniques [38,39], neural networks [40] or using process-based models [41]. Contrary to N2, the emissions of N2O at larger scales can be evaluated with top-down (inverse) modelling of N2O emissions [42].
The statistical approach used here to estimate N2O emission from soils [39] has a model uncertainty of −40 to +70 per cent. This uncertainty does not include the potential errors related to the upscaling and the spatial data used that are difficult to quantify. Our estimate for N2O emissions from groundwater under agricultural soils is lower than that based on the IPCC method [20], primarily because our simulated N leaching of N inputs for 2000 is smaller (24%) than that proposed by the IPCC (30%). The IPCC emission factor approach represents the combined emission from groundwater and riparian zones, although recent studies [43] suggest that the N2O production in groundwater alone may be close to the IPCC value. In addition, the IPCC approach represents the N2O emission from N during its travel time in groundwater; this is not consistent with the denitrification and N2O emission estimates for soil and riparian zones, which represent a specific year. However, with half-life values of 2–5 years, and assuming that the year-to-year change of the N inflow to groundwater is small, this error is acceptable when compared with all other uncertainties.
Predictions of N2O emissions from riparian zones are complex, as denitrification controls both the production and the consumption of this greenhouse gas. Uncertainty exists on how N2O transported by shallow groundwater through the subsoil contributes to the surface N2O emissions from riparian zones. As groundwater comes to the soil surface in these zones, outgassing of N2O can be significant. However, soil conditions in riparian zones can be favourable for denitrifier activity, and significant N2O consumption may occur in the topsoil [44]. N2O can also result from nitrification or nitrifier denitrification [45], particularly at the upland edges of the riparian zones where soil conditions are oxic and ammonium concentrations high owing to direct agricultural inputs. Furthermore, soil acidification from nitrifier activity may lead to an increase in N2O emission by reducing denitrifier N2O consumption.
5. Concluding remarks
Despite large uncertainties in our model approach, we conclude that N removal by denitrification as a fraction of the N inflow has not changed in the continuum formed by soil–groundwater–riparian zones during the twentieth century. This implies that the transfer of N from land to surface water has increased roughly at the same rate as the increase in the N budget.
In terrestrial systems, denitrification in soils is the primary N removal process, followed by groundwater and riparian zones. Our model shows that the removal of reactive N via denitrification from terrestrial and aquatic systems has not kept pace with accelerated N inputs into agriculture.
Our attempt to simulate riparian denitrification and N2O emission is the first ever done, and indicates that riparian zones may be an important landscape element for N removal at the global level; given the conditions in many riparian areas, with incomplete denitrification accompanied by high fractional N2O production, global N2O emissions from riparian areas may exceed those from groundwater.
The scenarios indicate that the gradual increase of reactive N transfer to surface waters and the atmosphere will continue in the coming decades, unless drastic improvements in agricultural management occur. This may have important consequences for biogeochemistry in river networks and coastal seas, inducing a series of processes, such as stimulation of plant growth, algal blooms, decomposition, burial and hypoxia.
Acknowledgements
A.F.B. and A.H.W.B. gratefully acknowledge financial support from the Global Environment Facility (GEF), United Nations Environment Programme (UNEP), Intergovernmental Oceanographic Commission of the UNESCO (IOC/UNESCO) and other partners through the UNEP/GEF project ‘Global foundations for reducing nutrient enrichment and oxygen depletion from land-based pollution in support of global nutrient cycle’.
References
- 1.Galloway JN, et al. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226 10.1007/s10533-004-0370-0 (doi:10.1007/s10533-004-0370-0) [DOI] [Google Scholar]
- 2.Tiedje JM. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In Biology of anaerobic microorganisms (ed. Zehnder AJB.), pp. 179–244 New York, NY: Wiley and Sons [Google Scholar]
- 3.Yamulki S, Harrison RM, Goulding KWT, Webster CP. 1997. N2O, NO and NO2 fluxes from a grassland: effect of soil pH. Soil Biol. Biochem. 29, 1199–1208 10.1016/S0038-0717(97)00032-1 (doi:10.1016/S0038-0717(97)00032-1) [DOI] [Google Scholar]
- 4.Saad ALO, Conrad R. 1993. Temperature dependence of nitrification, denitrification and turnover of nitric oxide in different soils. Biol. Fertil. Soils 15, 21–27 10.1007/BF00336283 (doi:10.1007/BF00336283) [DOI] [Google Scholar]
- 5.McClain ME. 2003. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6, 301–312 10.1007/s10021-003-0161-9 (doi:10.1007/s10021-003-0161-9) [DOI] [Google Scholar]
- 6.Böhlke J-K, Wanty R, Tuttle M, Delin G, Landon M. 2002. Denitrification in the recharge area and discharge area of a transient agricultural nitrate plume in a glacial outwash sand aquifer, Minnesota. Water Resour. Res. 38, 10-1 to 10-26 10.1029/2001WR000663 (doi:10.1029/2001WR000663) [DOI] [Google Scholar]
- 7.Zhang Y-C, Slomp CP, Broers HP, Passier HF, Van Cappellen P. 2009. Denitrification coupled to pyrite oxidation and changes in groundwater quality in a shallow sandy aquifer. Geochim. Cosmochim. Acta 73, 6716–6726 10.1016/j.gca.2009.08.026 (doi:10.1016/j.gca.2009.08.026) [DOI] [Google Scholar]
- 8.Mayer PM, Reynolds SK, Jr, McCutchen MD, Canfield TJ. 2007. Meta-analysis of nitrogen removal in riparian buffers. J. Environ. Qual. 36, 1172–1180 10.2134/jeq2006.0462 (doi:10.2134/jeq2006.0462) [DOI] [PubMed] [Google Scholar]
- 9.Van den Heuvel RN, Bakker SE, Jetten MSM, Hefting MM. 2011. Decreased N2O reduction by low soil pH causes high N2O emissions in a riparian ecosystem. Geobiology 9, 294–300 10.1111/j.1472-4669.2011.00276.x (doi:10.1111/j.1472-4669.2011.00276.x) [DOI] [PubMed] [Google Scholar]
- 10.Groffman PM, Gold AJ, Jacinthe P-A. 1998. Nitrous oxide production in riparian zones and groundwater. Nutr. Cycl. Agroecosyst. 52, 179–186 10.1023/A:1009719923861 (doi:10.1023/A:1009719923861) [DOI] [Google Scholar]
- 11.Bouwman AF, Klein Goldewijk K, Van der Hoek KW, Beusen AHW, Van Vuuren DP, Willems WJ, Rufinoe MC, Stehfest E. 2011. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl Acad. Sci. USA. 10.1073/pnas.1012878108 (doi:10.1073/pnas.1012878108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer EW, Alexander RB, Parton WJ, Li C, Butterbach-Bahl K, Donner SD, Skaggs RW, Del Grosso SJ. 2006. Modeling denitrification in terrestrial and aquatic ecosystems at regional scales. Ecol. Appl. 16, 2123–2142 10.1890/1051-0761(2006)016[2123:MDITAA]2.0.CO;2 (doi:10.1890/1051-0761(2006)016[2123:MDITAA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 13.Bouwman AF, Beusen AHW, Billen G. 2009. Human alteration of the global nitrogen and phosphorus soil balances for the period 1970–2050. Glob. Biogeochem. Cycles 23, GB0A04. 10.1029/2009GB003576 (doi:10.1029/2009GB003576) [DOI] [Google Scholar]
- 14.Bouwman AF, Kram T, Klein Goldewijk K. (eds). 2006. Integrated modelling of global environmental change. An overview of IMAGE 2.4. Publication 500110002/2006 Bilthoven, The Netherlands: Netherlands Environmental Assessment Agency [Google Scholar]
- 15.Fekete BM, Wisser D, Kroeze C, Mayorga E, Bouwman AF, Wollheim WM, Vörösmarty CJ. 2011. Millennium ecosystem assessment scenario drivers (1970–2050): climate and hydrological alterations. Glob. Biogeochem. Cycles 24, GB0A12. 10.1029/2009GB003593 (doi:10.1029/2009GB003593) [DOI] [Google Scholar]
- 16.Vitousek PM, Menge DNL, Reed SC, Cleveland CC. 2013. Biological nitrogen fixation: rates, patterns, and ecological controls in terrestrial ecosystems. Phil. Trans. R. Soc. B 368, 20130119. 10.1098/rstb.2013.0119 (doi:10.1098/rstb.2013.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleveland CC, et al. 1999. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 13, 623–645 10.1029/1999GB900014 (doi:10.1029/1999GB900014) [DOI] [Google Scholar]
- 18.Van Drecht G, Bouwman AF, Knoop JM, Beusen AHW, Meinardi CR. 2003. Global modeling of the fate of nitrogen from point and nonpoint sources in soils, groundwater and surface water. Glob. Biogeochem. Cycles 17, 1115. 10.129/2003GB002060 (doi:10.129/2003GB002060) [DOI] [Google Scholar]
- 19.Vidon PG, Hill AR. 2006. A landscape-based approach to estimate riparian hydrological and nitrate removal functions. J. Am. Water Res. Assoc. 42, 1099–1112 10.1111/j.1752-1688.2006.tb04516.x (doi:10.1111/j.1752-1688.2006.tb04516.x) [DOI] [Google Scholar]
- 20.IPCC 2006. IPCC guidelines for national greenhouse gas inventories. Hayama, Japan: IPCC NGGIP Programme, IPCC-TSU/IGES; (Published by the Institute for Global Environmental Strategies (IGES), Hayama, Japan on behalf of the IPCC) [Google Scholar]
- 21.Seitzinger SP, Harrison JA, Böhlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Drecht GV. 2006. Denitrification across landscapes and waterscapes: a synthesis. Ecol. Appl. 16, 2064–2090 10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2 (doi:10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 22.Emery KO, Orr WL, Rittenberg SC. 1995. Nutrient budgets in the ocean. In Essays in the natural sciences in honor of Captain Alan Hancock, pp. 299–310 Los Angeles, CA: University of Southern California Press [Google Scholar]
- 23.Gruber N, Sarmiento JL. 1997. Global patterns of marine nitrogen fixation and denitrification. Glob. Biogeochem. Cycles 11, 235–266 10.1029/97GB00077 (doi:10.1029/97GB00077) [DOI] [Google Scholar]
- 24.Codispoti LA, Brandes JA, Christensen JP, Devol AH, Naqvi SWA, Pearl HW, Yoshinari T. 2001. The oceanic fixed nitrogen and nitrous oxide budgets: moving targets as we enter the Anthropocene. Sci. Mar. 65, 85–105 [Google Scholar]
- 25.Søderlund R, Svensson BH. 1976. The global nitrogen cycle. In Nitrogen, phosphorus and sulphur—global cycles. SCOPE, vol. 7 (eds Svensson BH, Soderlund R.), pp. 23–73 Stockholm, Sweden: Ecological Bulletin [Google Scholar]
- 26.Canfield DE, Glazer AN, Falkowski PG. 2010. The evolution and future of earth's nitrogen cycle. Science 330, 192–196 10.1126/science.1186120 (doi:10.1126/science.1186120) [DOI] [PubMed] [Google Scholar]
- 27.Hofstra N, Bouwman AF. 2005. Denitrification in agricultural soils: summarizing published data and estimating global annual rates. Nutr. Cycl. Agroecosyst. 72, 267–278 10.1007/s10705-005-3109-y (doi:10.1007/s10705-005-3109-y) [DOI] [Google Scholar]
- 28.Ju X, Liu X, Zhang F, Roelcke M. 2004. Nitrogen fertilization, soil nitrate accumulation, and policy recommendations. Ambio 33, 300–305 [DOI] [PubMed] [Google Scholar]
- 29.Ducharne A. 2007. Long term prospective of the Seine River system: confronting climatic and direct anthropogenic changes. Sci. Total Environ. 375, 292–311 10.1016/j.scitotenv.2006.12.011 (doi:10.1016/j.scitotenv.2006.12.011) [DOI] [PubMed] [Google Scholar]
- 30.Griffioen J, Brunt R, Vasak S, Van der Gun J. 2005. A global inventory of groundwater quality: first results. In Bringing groundwater quality research to the watershed scale, vol. 297 (ed. Thomson NR.), pp. 3–10 Wallingford, UK: International Association of Hydrological Sciences (IAHS) [Google Scholar]
- 31.Van Drecht G, Bouwman AF, Boyer EW, Green P, Siebert S. 2005. A comparison of global spatial distributions of nitrogen inputs for nonpoint sources and effects on river nitrogen export. Glob. Biogeochem. Cycles 19, GB4S06. 10.1029/2005GB002454 (doi:10.1029/2005GB002454) [DOI] [Google Scholar]
- 32.Beusen AHW, Bouwman AF, Heuberger PSC, Van Drecht G, Van Der Hoek KW. 2008. Bottom-up uncertainty estimates of global ammonia emissions from global agricultural production systems. Atmos. Environ. 42, 6067–6077 10.1016/j.atmosenv.2008.03.044 (doi:10.1016/j.atmosenv.2008.03.044) [DOI] [Google Scholar]
- 33.Bodirsky BL, Popp A, Weindl I, Dietrich JP, Rolinski S, Scheiffele L, Schmitz C, Lotze-Campen H. 2012. Current state and future scenarios of the global agricultural nitrogen cycle. Biogeosciences 9, 4169–4197 10.5194/bg-9-4169-2012 (doi:10.5194/bg-9-4169-2012) [DOI] [Google Scholar]
- 34.Groffman PM, et al. 2006. Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol. Appl. 16, 2091–2122 10.1890/1051-0761(2006)016[2091:MFMDDA]2.0.CO;2 (doi:10.1890/1051-0761(2006)016[2091:MFMDDA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 35.Heinen M. 2006. Simplified denitrification models: overview and properties. Geoderma 133, 444–463 10.1016/j.geoderma.2005.06.010 (doi:10.1016/j.geoderma.2005.06.010) [DOI] [Google Scholar]
- 36.Rivett MO, Buss SR, Morgan P, Smith JWN, Bemment CD. 2008. Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res. 42, 4215–4232 10.1016/j.watres.2008.07.020 (doi:10.1016/j.watres.2008.07.020) [DOI] [PubMed] [Google Scholar]
- 37.Postma D, Boesen C, Kristiansen H, Larsen F. 1991. Nitrate reduction in an unconfined sandy aquifer: water chemistry, reduction processes, and geochemical modeling. Water Resour. Res. 27, 2027–2045 10.1029/91WR00989 (doi:10.1029/91WR00989) [DOI] [Google Scholar]
- 38.Hill AR. 1996. Nitrate removal in stream riparian zones. J. Environ. Qual. 25, 743–755 10.2134/jeq1996.00472425002500040014x (doi:10.2134/jeq1996.00472425002500040014x) [DOI] [Google Scholar]
- 39.Stehfest E, Bouwman AF. 2006. N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 74, 207–228 10.1007/s10705-006-9000-7 (doi:10.1007/s10705–006–9000–7) [DOI] [Google Scholar]
- 40.Bouwman AF, Boumans LJM, Batjes NH. 2002. Modeling global annual N2O and NO emissions from fertilized fields. Glob. Biogeochem. Cycles 16, 1080. 10.29/2001GB001812 (doi:10.29/2001GB001812) [DOI] [Google Scholar]
- 41.Ryan M, Müller C, Di HJ, Cameron KC. 2004. The use of artificial neural networks (ANNs) to simulate N2O emissions from a temperate grassland ecosystem. Ecol. Model. 175, 189–194 10.1016/j.ecolmodel.2003.10.010 (doi:10.1016/j.ecolmodel.2003.10.010) [DOI] [Google Scholar]
- 42.Farquharson R, Baldock J. 2008. Concepts in modelling N2O emissions from land use. Plant Soil 309, 147–167 10.1007/s11104-007-9485-0 (doi:10.1007/s11104-007-9485-0) [DOI] [Google Scholar]
- 43.Corazza M, et al. 2011. Inverse modelling of European N2O emissions: assimilating observations from different networks. Atmos. Chem. Phys. 11, 2381–2398 10.5194/acp-11-2381-2011 (doi:10.5194/acp-11-2381-2011) [DOI] [Google Scholar]
- 44.Vilain G, Garnier J, Tallec G, Tournebize J. 2011. Indirect N2O emissions from shallow groundwater in an agricultural catchment (Seine Basin, France). Biogeochemistry 111, 253–271 [Google Scholar]
- 45.DeSimone J, Macrae ML, Bourbonniere RA. 2010. Spatial variability in surface N2O fluxes across a riparian zone and relationships with soil environmental conditions and nutrient supply. Agric. Ecosyst. Environ. 138, 1–9 10.1016/j.agee.2010.03.007 (doi:10.1016/j.agee.2010.03.007) [DOI] [Google Scholar]
- 46.Wrage N, Velthof GL, Van Beusichem ML, Oenema O. 2001. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 33, 1723–1732 10.1016/S0038-0717(01)00096-7 (doi:10.1016/S0038-0717(01)00096-7) [DOI] [Google Scholar]