Abstract
The nitrogen cycle of pre-industrial ecosystems has long been remarkably closed, in spite of the high mobility of this element in the atmosphere and hydrosphere. Inter-regional and international commercial exchanges of agricultural goods, which considerably increased after the generalization of the use of synthetic nitrogen fertilizers, introduced an additional type of nitrogen mobility, which nowadays rivals the atmospheric and hydrological fluxes in intensity, and causes their enhancement at the local, regional and global scales. Eighty-five per cent of the net anthropogenic input of reactive nitrogen occurs on only 43 per cent of the land area. Modern agriculture based on the use of synthetic fertilizers and the decoupling of crop and animal production is responsible for the largest part of anthropogenic losses of reactive nitrogen to the environment. In terms of levers for better managing the nitrogen cascade, beyond technical improvement of agricultural practices tending to increase nitrogen use efficiency, or environmental engineering management measures to increase nitrogen sinks in the landscape, the need to better localize crop production and livestock breeding, on the one hand, and agriculture and food demand on the other hand, is put forward as a condition to being able to supply food to human populations while preserving environmental resources.
Keywords: nitrogen cascade, agriculture, watersheds
1. Introduction
The characteristics of the nitrogen biogeochemical cycle are unique because of both the relative scarcity of the reactive forms of this element in the biosphere and the extreme mobility of a number of these reactive forms, in either the gaseous phase (ammonia and nitrogen oxides that are easily transported in the atmosphere over long distances) or the soluble phase (the high solubility of nitrate making it easily leached from the soil profile and transported through hydrosystems). Because of its scarcity in nature, reactive nitrogen has long been the most limiting element controlling the primary productivity in terrestrial and aquatic ecosystems, which is why the nitrogen cycle of natural ecosystems has remained so remarkably closed in spite of the mobility of this element [1].
Together with the advent of the ‘cheap’ and unlimited industrial nitrogen fixation made possible by the Haber–Bosch process, a third factor of nitrogen mobility was recently added by human activity, namely the inter-regional or international commercial exchanges of agricultural goods. We will show in this paper that the nitrogen fluxes related to these exchanges today not only rival in intensity atmospheric and hydrospheric fluxes, but also are often a major cause of their enhancement at the regional and global scale.
While farms represent the smallest operational units at which decisions are taken, determining the fluxes of nitrogen associated with agriculture [2], regional watersheds, composed of a mosaic of interacting natural, semi-natural, agricultural and urban landscapes, are the most convenient units at which to describe, but also to manage, the anthropogenic alteration of the nitrogen cycle [3,4]. Indeed, drainage networks, as a water resource as well as a means of commodity transport, have historically played a prominent role in the localization of cities and the structural organization of their surrounding supplying rural hinterlands, often making watersheds a coherent territorial unit from the point of view of the distribution of human activities. Our analysis will therefore be centred on the regional watershed level.
Regional statistics provide the data required to establish coherent budgets and to feed models at the regional scale. To illustrate our concepts and approaches, we will use here published data on five well-documented, contrasted basins: the Seine and Scheldt [5–7], the Ebro [8], the Mississippi [9] and the Hong [10,11]. At the global scale, consistent data are much more difficult to obtain, although FAOSTAT [12] offers complete datasets on agriculture at the country level. Partly based on these data, the GlobalNEWS project has developed several modelling tools and approaches to calculate riverine nitrogen delivery to the coastal ocean and characterize the N metabolism of the world's 6080 watersheds [13–16]. The global descriptions, budgets and scenarios elaborated in the present paper are largely based on the same input data (see the electronic supplementary material, §S1 for details).
First, we will adopt a black box approach to statistically relate the total anthropogenic input of reactive nitrogen into watersheds to its output through the hydrosphere. Then, we will describe and analyse some of the processes involved at the local ecosystem level and try to relate them to observed features at the watershed scale using a mechanistic modelling approach. Finally, we will examine the possible levers that can reduce environmental nitrogen pollution and address the question, at both regional and global scales, of the possibility of supplying food to growing populations while preserving water resources from eutrophication and nitrate contamination.
2. A black box approach to nitrogen cycle perturbation
(a). Nitrogen inputs to watersheds
Anthropogenic reactive nitrogen input to watersheds occurs through four processes: crop N2 fixation; synthetic fertilizer application; atmospheric deposition resulting from nitrogen oxide formation by motor traffic and electricity production; and, finally, net commercial import of food and feed. The sum of these four terms defines the net anthropogenic N inputs (NANIs), expressed in kg N km−² yr−1, thus characterizing the intensity of human perturbation of the natural N cycle [17].
The quantitative value of the first three processes can be derived from the GlobalNEWS database [15,16,18]. Table 1 summarizes the values of reactive N introduced into watersheds through N2 fixation, N deposition and fertilizer use, as derived from these model data.
Table 1.
Global fluxes of reactive nitrogen introduced into watersheds, commercially exchanged between watersheds and exported by the rivers at their outlet in 1970 and 2000, as derived from the GlobalNEWS database (inputs) and model (river delivery).
| (all in Tg N yr−1) | 1970 | 2000 |
|---|---|---|
| natural N2 fixation | 102 | 99 |
| crop N2 fixation | 27 | 30 |
| fertilizer application | 27 | 80 |
| atmospheric deposition (oxidized N)a | 28 | 34 |
| total NANI | 82 | 144 |
| commercial exchange of food and feedb | 11 | 16 |
| river delivery to the sea | 37 | 43 |
aThe available data are for total (red + ox) N. The oxidized N emissions have been considered to roughly amount to half this total.
bThe sum of the difference (autotrophy−heterotrophy = export (or import) of agricultural goods) for all the watersheds is close to zero, indicating the internal consistency of the data (sum of imports = sum of exports). The total commercial exchange is calculated as half the sum of the absolute value of this difference for all watersheds.
A value close to 100 Tg N yr−1 is estimated for atmospheric nitrogen fixation in natural areas. This is at the highest side of the estimations of terrestrial pristine N2 fixation compiled by Vitousek et al. [19], who argue that the pre-industrial value might be even lower, by a factor of two. Together, biological N2 fixation by crops and fertilizer application today globally exceeds this natural biological nitrogen fixation, as underlined by several authors [20,21]. Atmospheric deposition, excluding the internal recycling of ammonia volatilized and re-deposited within a short distance, mainly comes from nitrogen oxide emission through high-temperature combustion processes related to motor traffic and electricity generation. Globally, agriculture clearly contributes the largest share of the overall NANI.
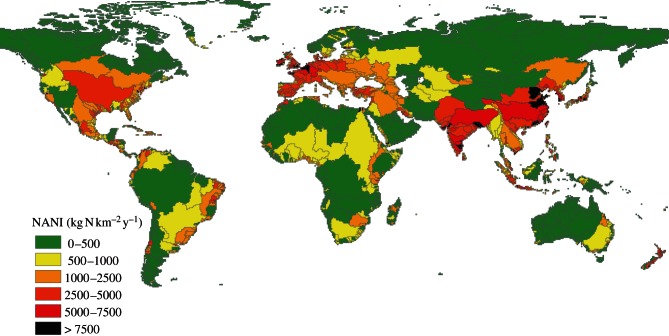
The contribution to the individual watershed N balance of food and feed commercial exchange is more delicate to assess, as international trade statistics [12] are obviously not suitable at that scale. We have introduced the concept of anthropogenic N autotrophy and heterotrophy to deal with this question [14]. The anthropogenic nitrogen autotrophy of a given territory is defined as its production of food and feed (harvested and grazed products, expressed as N content), and nitrogen heterotrophy as the N content of food and feed required to sustain the local human and livestock populations. The balance between autotrophy and heterotrophy is a measurement of the net commercial export (or import) of agricultural products, i.e. the surplus (or shortage) of agricultural production over the consumption by the local population and its livestock. Using this approach at the scale of the world watersheds makes it possible to (i) characterize their autotrophic or heterotrophic nature, hence the openness of their nitrogen cycle' (ii) assess the net flux of nitrogen introduced (or exported) as food and feed and (iii) calculate the distribution of NANI at the global scale (figure 1).
Figure 1.
Distribution of NANI at the scale of the world's watersheds. Data from GlobalNEWS.
Comparing NANI with the natural nitrogen inputs (N2 fixation in natural areas as estimated in the GlobalNEWS database) allows one to identify those watersheds where anthropogenic inputs equal or exceed natural inputs, which thus have transgressed the threshold of high perturbation of the nitrogen cycle. Collectively, these ‘N-intensive’ basins cumulate 84 per cent of the global NANI, while covering only 43 per cent of the total continental area (figure 1). Synthetic fertilizers most often are the dominant component of NANI in those basins; only a few of them are balanced in terms of autotrophy and heterotrophy (see the electronic supplementary material, §S2).
The commercial exchanges of agricultural products between watersheds contribute quite significantly to their N balance, the total flux involved at the global scale increasing from 11 to 16 Tg N yr−1 between 1970 and 2000 (table 1). For many watersheds in Europe and North America, this term represents a major component of the net anthropogenic nitrogen input, reflecting their (often recent) specialization towards either export crop production or livestock farming based on imported feed: their historical trajectory in terms of autotrophy and heterotrophy shows that the autotrophic character of some of them, such as the Seine and the Mississippi, has substantially increased over the past 50 years, as has the heterotrophic character for others such as the Scheldt and the Ebro basins (see figure 2 and electronic supplementary material, §S2).
Figure 2.
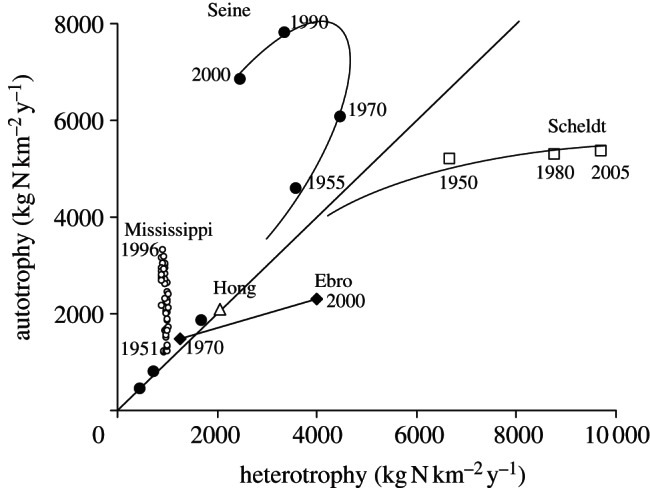
The historical trajectory of five typical watersheds in terms of autotrophy and heterotrophy (data sources: Seine and Scheldt: [5–7], Ebro: [8], Mississippi: [9], Hong: [10,11])
(b). River nitrogen export and the ‘missing’ nitrogen
At the global scale, 43 Tg N yr−1 are delivered by rivers to the coastal zone, out of 144 Tg N yr−1 entering their watersheds as NANI. In other words, around 70 per cent of the reactive nitrogen introduced by humans into the continental biosphere does not reach the coastal ocean but is retained in the landscape or eliminated by denitrification during its transfer—often designated as ‘the nitrogen cascade’—along the land–wetland–river continuum. By definition, the above-described black box modelling approach cannot tell more about the fate of this ‘missing’ nitrogen, but it allows mapping the world's watersheds according to their nitrogen delivery [16], as well as the amount of emission–retention along their N cascade. In the five above-mentioned example watersheds, the relative importance of these two NANI pathways (delivery to the sea versus emission–retention) varies a great deal: in the Ebro River basin, the largest share of introduced reactive nitrogen is retained or dissipated upstream in the watershed, with very low transfer to the river outlet; hence, there are limited problems of coastal eutrophication [8]. The same is true for the Hong basin, but most of the retention occurs downstream in the delta area [22]. By contrast, in the Seine, Scheldt and Mississippi river basins, a much higher fraction of NANI is delivered to the sea, causing severe marine eutrophication in the receiving coastal waters [23,24]. Although the processes responsible for the ‘retention’ of N along the N cascade are numerous and complex, Howarth et al. [17,25,26] have shown that their overall effect in terms of the fraction of net total nitrogen inputs delivered to the sea is highly correlated to climate, varying between 100 per cent in cold and humid regions to less than 10 per cent under arid and warm climate conditions. Billen et al. [14] have proposed a sigmoid relationship to represent the effect of specific run-off on the fraction of net total nitrogen input (NTNI = NANI + natural inputs) delivered to the sea (see the electronic supplementary material, figure S3).
3. Process analysis of the nitrogen cascade
To go beyond the black box input–output approach described earlier requires describing the mechanisms by which reactive nitrogen is channelled by anthropogenic action and natural processes into the different components of the watershed territory. Several terms of the net inputs of reactive nitrogen are functionally related to each other through the organization of agriculture. Basically, the very nature of traditional agriculture is to manage the transfer of nitrogen between the N-fixing and non-N-fixing components of the landscape mosaic in order to promote the growth of N-demanding cereal crops and to return to the soil the nutrients exported with the harvest. The spatial organization of these transfers, as well as the spatial distribution of livestock and human populations (either rural or urban), largely condition the major intentional N fluxes within and across regional watersheds. Considerable unintentional losses to the atmosphere and the hydrosphere are associated with these intended transfers. In this section, we discuss how to model these different processes.
(a). A generic model of the agro-food system
Traditional agriculture benefits from the atmospheric nitrogen fixation capacity of semi-natural systems to provide the nitrogen resources required to grow cereals. In slash-and-burn agriculture, a long forested fallow is used to restore the nutrient stocks on which crops will be cultivated for 1 or 2 years. In irrigated agriculture, in alluvial valleys or delta areas, silt deposits from seasonal flooding, extracted from a large upstream watershed, are the sources of nutrient provisioning arable lands. In many other traditional agricultural systems, livestock is the main biogeochemical agent ensuring the transfer of nitrogen from N-fixing semi-natural areas to arable land, based on the use of manure for fertilization. Crop rotations including the cultivation of legume fodder crops in alternation with cereals strongly increase the net input of reactive nitrogen through symbiotic N2 fixation and provide ample feed. In this way, livestock breeding and crop cultivation are intimately linked to each other, both functionally and geographically, which results in the balance between anthropogenic N autotrophy and heterotrophy.
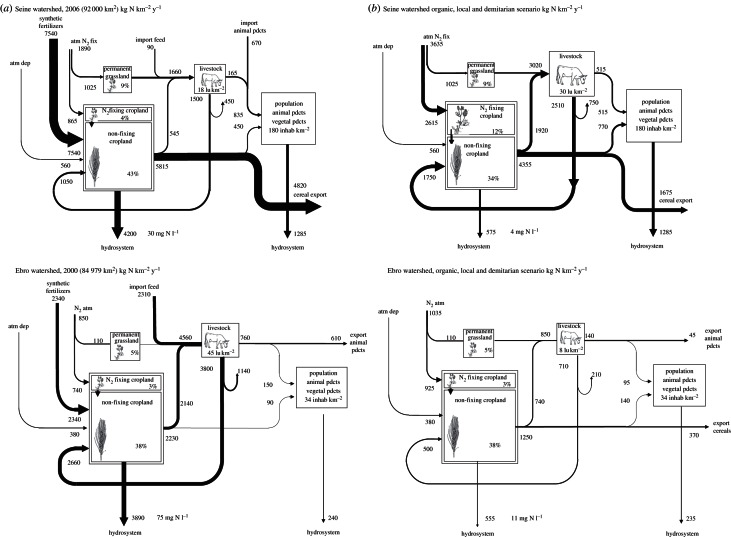
The advent of Haber–Bosch industrial N2 fixation offered the possibility of disconnecting crop cultivation and animal farming in those regions of the world where both activities used to be linked together. This resulted in the specialization of some areas in intensive crop cultivation based on synthetic N fertilization, whereas others specialized in animal farming largely based on feed imports from distant areas. The Seine and Mississippi basins illustrate the former situation, whereas the Ebro and Scheldt basins are examples of the latter, and the Hong remains a rather well-balanced basin (figure 3a and the electronic supplementary material, figure S4a). It is clear from the above discussion that territorial specialization induces fluxes in reactive nitrogen on the same order of magnitude as the other NANI components.
Figure 3.
Schematic of the N fluxes linked to agriculture in the Seine (upper panels) and Ebro (lower panels) watersheds used as examples. (a) Description of the current situation based on data from [6–8]. (b) Construction of a hypothetical organic and demitarian scenario for both basins, based on the following constraints: (i) human consumption of animal protein is set to 35–40% of the total current intake of proteins; (ii) no synthetic fertilizers are allowed; (iii) livestock is adjusted to the local requirements of animal proteins as far as the local vegetal protein production is able to satisfy the requirements of both livestock and human populations (no import of feed is allowed) and (iv) nitrate concentration of arable land subroot water is kept below the drinking water standard (11 mg N l−1). The crop production of arable land and the N soil surplus are calculated from the total N soil inputs by manure, symbiotic N2 fixation and atmospheric deposition, using the same relationship as observed in the current situation of each watershed (figure 5). See the electronic supplementary material, §S4 for further examples.
The scheme used in figure 3 to depict the organization of N flows in agriculture at the scale of the example watersheds can be generalized to any regional territory. It represents a functional model of agro-systems, linking arable land productivity, livestock and the semi-natural or managed grassland that contributes to its feeding, and finally, human food requirements.
Three crucial aspects of this model concern (1) the composition of the human diet, (2) the productivity of arable land and (3) the role of livestock.
(1) The human diet is obviously a major driver of territorial nitrogen metabolism. Both the total protein intake and the share of animal protein in the total vary greatly at the global scale, around the median value of 3.2 kg N per capita per year with 35 per cent animal protein (figure 4). The World Health Organization [27] recommends a mean per capita intake of proteins of 2.8 and 3.5 kg N per capita per year (respectively for women and men, with 55–75 kg body weight).
-
(2) The relationship between the yield of cultivated crops and total fertilization (calculated as the sum of synthetic fertilizer and manure application, biological atmospheric N fixation by legumes, and atmospheric deposition), as calculated over the long term and integrated over the total of arable land of a given territory, typically shows an asymptotic curve expressing a trend to lower N use efficiency at higher fertilization rates (see figure 5 and the electronic supplementary material, §S4, figure S6). In every case, the single parameter relationship can be fitted to the data:
where Ymax expresses the maximum yield at saturating fertilization.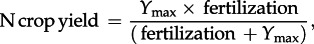
3.1 The analysis of long-term data demonstrates the robustness of this relationship for given pedo-climatic conditions. It thus provides a strong internal constraint when establishing alternative scenarios of nitrogen flows through the components of agro-food systems.
Ymax values for the different areas of the world range widely, from 1 to 500 kg N ha−1 yr−1, with a median at 40 kg N ha−1 yr−1 (see the electronic supplementary material, §S4, figure S6c), reflecting the global diversity of agriculture productivity. In a number of basins, representing 20 per cent of the global population, the current maximum yield of arable land (Ymax × arable land area), added to the production of marginal and extensive grassland, is lower than the current food and feed requirement. These basins are intrinsically dependent on long-distance trade to sustain the population, whatever the level of fertilization of arable land may be.
-
(3) Livestock in most agricultural systems play the double role of providing manure for arable land and animal protein as meat or milk for human nutrition. The conversion efficiency of feed into animal products for human nutrition, as calculated from the available FAO data, varies from 3 to 16 per cent (see the electronic supplementary material, §S4, table S1), depending on the degree of intensiveness or the share of ruminant versus monogastric animals in the livestock.
The constraints exerted by these three aspects of the agricultural model determine to what extent local agriculture can or cannot meet self-sufficiency in food and feed supply. Imports or exports of feed, animal products and cereals are adjusted to the surplus or shortage of local agricultural production with respect to local requirements.
Figure 4.

Relationship with (a) gross domestic product of per capita protein intake and (b) the fraction of animal protein in the human diet [12].
Figure 5.
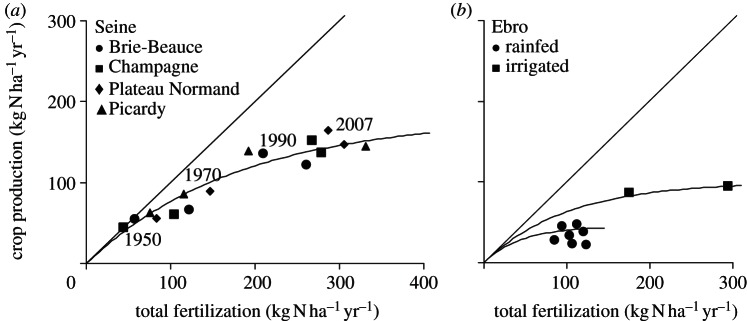
Relationship observed between N in crop production of agricultural land and total fertilization in two example watersheds, (a) the Seine and (b) the Ebro (see other examples in electronic supplementary material, §S4). In each case, the relationship is fitted by the equation N crop yield = Ymax × fertilization/(fertilization + Ymax), where Ymax expresses the maximum yield at saturating fertilization.
(b). Urban wastewater production
Because half the world's population lives in cities, the nutrients released from human excretion cannot easily be recycled back to the site where its food was produced. Although agricultural reuse of urban wastewater was once organized to a large extent [28–30], the modern trend is towards collection of wastewater in centralized sewers and release into rivers with or without treatment. At the scale of the world's watersheds, according to the GlobalNEWS database, the raw production of N in human excretion in 2000 was 19 Tg N yr−1. Taking into account recycling of sewage to agriculture (in rural areas) and wastewater treatment (in urban areas), the net input of human sewage to rivers is reduced to 6 Tg N yr−1 [31], clearly not the largest source of nitrogen contamination of river water on a global scale.
(c). Losses and retention processes throughout the drainage network
(i). Diffuse losses from agricultural soils and groundwater contamination
While forest or grassland soils are generally highly retentive with respect to nitrogen leaching, except under high nitrogen input rates, arable land is by far the largest diffuse source driving the aquatic nitrogen cascade. The potential for environmental loss of nitrogen from agricultural soils can be assessed as the difference between crop export and total fertilization (figure 5 and the electronic supplementary material, §S4). The historical trend revealed by the data is clearly an increasing surplus accompanying increasing fertilization. Arable soil N surpluses are particularly high in those basins that have specialized in livestock farming based on imported feedstuff because such specialization creates structural excess of manure. At the global scale, according to the GlobalNEWS database, the agricultural surplus is estimated at 138 Tg N yr−1.
The agricultural N surplus represents a potential for environmental loss. Part of this surplus can be temporarily stored in the soil organic nitrogen pool, a part can be emitted under gaseous forms or denitrified, and another part can be leached with infiltrating water below the root zone. Emission as ammonia might play the major role in arid systems, whereas denitrification eliminates a major fraction of excess N in waterlogged soils where anaerobic conditions prevail, such as in paddy fields. The data gathered by Hofstra & Bouwman [32] suggest that denitrification in well-drained upland arable soils eliminates roughly 20 per cent of the total fertilizer (organic and synthetic) inputs; the rest is leached out of the root zone. Data from temperate arable soils (figure 6) show that leaching out of the root zone accounts for 80 per cent of the long-term soil balance, with the remaining 20 per cent fraction stored, volatilized or denitrified. In permanent grassland, sometimes heavily fertilized with mineral nitrogen and manure, leaching is much lower than the N soil surplus below a certain threshold (figure 6), and denitrification, together with storage in the soil organic pool, is likely to be the major fate of the agricultural N surplus.
Figure 6.
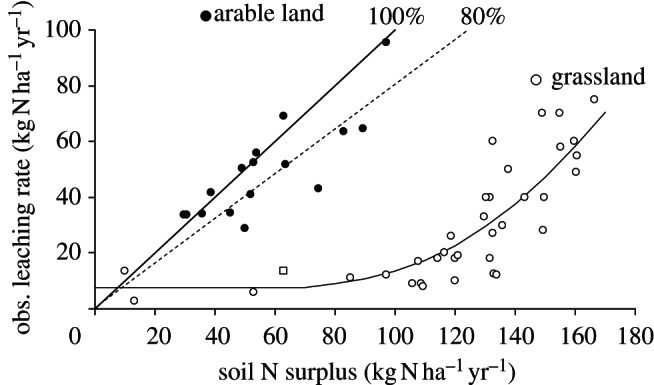
Observed leaching flux (from long-term lysimetric measurements) below the root zone as a function of the agricultural N surplus calculated from long-term data on agricultural practices in the corresponding agricultural plots. (Data from different studies in the North of France [33–39] in arable land (solid symbols), and permanent grassland (open symbols). The solid and dotted straight lines represent, respectively, 100% and 80% leaching of the surplus.
Soil leaching of nitrate from excess fertilization of agricultural soils is the primary cause of groundwater nitrate contamination. Except when they contain pyrite, large unconfined aquifers are not the site of significant denitrification, through lack of reducing agents. Because of the often very long residence time in aquifers, the nitrogen concentration may not yet be in equilibrium with the nitrate concentration in the inflowing water, as is frequently observed [40,41]. In this case, until equilibrium is reached, the aquifer temporarily stores nitrogen and releases less nitrogen than it receives.
(ii). Riparian and wetland denitrification
Before reaching the drainage network, nitrogen flows associated with surface water and groundwater from the watershed have to cross those areas forming the interface between the river and the terrestrial systems, where the groundwater table often reaches the uppermost, biogeochemically active layer of the soil. When comparing the composition of small rural river water with that of the ground- and subroot-water feeding them, much lower nitrate concentrations are typically observed in the river, as well as an isotopic composition signal typical of denitrification [42–44] (see the electronic supplementary material, §S5). Indeed, the riparian areas are known to play a major role in the nitrogen cascade, as a site for agricultural nitrate denitrification and for N2O emissions [45–48]. Exactly the same processes occur in areas of shallow groundwater, such as river flood plains and delta areas, where nitrate from agricultural soils is in contact with organic-rich alluvial material: in our conceptual model, these sites of intense denitrification and N2O emission at the interface between upland groundwater and surface water are considered as (admittedly large) riparian zones. This approach differs from that of Bouwman et al. [49], who include in their soil and groundwater compartments what we consider as hotspots of denitrification in interface areas. This simple difference in the definition of the environmental compartments explains the (only apparent) discrepancies between the two approaches.
(iii). In-stream retention processes
Contrary to the processes described so far, in-stream retention affects the nitrogen load from both point and diffuse sources. Benthic denitrification is the main process leading to nitrogen elimination during its downwards travel through the drainage network. The process is controlled by the content of sediment organic matter, the nitrate concentration in the water column and the residence time of the water masses. Typical benthic denitrification rates observed in rivers and lakes are between 5 and 50 mg N m−² h−1 [50]. Stagnant portions of the drainage network, such as ponds, lakes and reservoirs, are obviously hotspots of denitrification, because of both (i) the longer residence time of the water masses and (ii) the higher organic matter content of the sediments resulting from sedimentation of particulate organic material. Prolonged residence of water bodies in stagnant areas can lead to the elimination of a large fraction of their nitrate loading [8,51,52].
(d). Comprehensive modelling of the nitrogen cascade through the drainage network
Only a few models are available to mechanistically represent the processes involved in the N cascade from agricultural soils to the river outlet, including a description of the seasonally variable hydrological transfer through the watershed and the drainage network. SWAT [53–56] is one of them, mainly centred on the description of water and nutrient routing and transformation in the watershed controlled by plant dynamics; only mixing equations and simple parametric relationships are used for describing drainage network processes. INCA [57,58] has similar characteristics. Seneque/Riverstrahler [7,59–62] directly uses agricultural surpluses and urban point sources as input but describes the N dynamics within the drainage network in greater detail, using a kinetic formulation for each process. From these models, a detailed budget of the processes involved in reactive nitrogen elimination throughout the N cascade of individual watersheds can be calculated for individual watersheds (table 2).
Table 2.
Budget of N transfers along the cascade from agricultural soils to the river outlet in a number of regional watersheds. Data from [7–10]; see electronic supplementary material, §S5 for a graphical representation of the same data. n.a., not available.
| watershed year | Seine 2006 | Scheldt 2005 | Ebro 2000 | Mississippi 1996 | Hong 1997 |
|---|---|---|---|---|---|
| watershed area (km²) | 92 000 | 19 860 | 84 979 | 3200 | 156 450 |
| agricultural surplus (kg N km−² yr−1) | 4200 | 6915 | 3890 | 600 | 1745 |
| root-zone processes | n.a. | 400 | n.a. | n.a. | n.a. |
| groundwater storage | 700 | 400 | n.a. | n.a. | n.a. |
| riparian denitrification | 1160 | 1200 | n.a. | n.a. | 810 |
| gross urban point sources | 1285 | 3480 | 292 | 130 | 1030 |
| wastewater treatment or recycling | 735 | 2430 | 118 | 40 | 530 |
| net point sources release into rivers | 550 | 1050 | 174 | 90 | 500 |
| benthic denitrification and sedimentation | 210 | 250 | n.a. | n.a. | 250 |
| river export to the sea | 2680 | 2500 | 394 | 396 | 1100 |
4. Managing the nitrogen cascade: the levers for change
As discussed earlier, a number of watersheds in the world receive a large amount of reactive nitrogen from anthropogenic sources. By far the largest of these inputs are related to agriculture. The excess of total fertilization of agricultural soils over crop export directly threatens the quality of air as well as of ground and surface water resources. River water quality is partly protected through denitrification in soils, riparian wetlands and river-bottom sediments, but this process results in enhanced N2O emissions, and freshwater ecosystems still suffer from excess nitrate concentration, which threatens aquatic biodiversity. The nitrogen fluxes ultimately delivered at the coastal zone, even if they account for a mean of less than 30 per cent of the total N fluxes introduced into the watersheds, are still too high and locally cause severe eutrophication problems depending on the morphological and hydrological characteristics of the receiving coastal ecosystems.
(a). Technical measures to manage the nitrogen cascade
Levers for the management of the nitrogen cascade aiming to reduce environmental losses can be identified along the entire land–wetland–river continuum. These measures are ranked hereafter by increasing difficulty of implementation.
Tertiary treatment of urban wastewater is technically feasible and is rapidly being implemented in developed countries as part of the measures to combat marine eutrophication.
In the European Union, N elimination, most often through denitrification, is now compulsory for wastewater treatment plants serving more than 10 000 inhabitants. However, in addition to the fact that this measure is extremely cost-intensive, it wastes fixed nitrogen and contributes to N2O emissions [63]. Moreover, urban point sources are definitely not the major source of nitrogen contamination of river water except in very densely populated and urbanized watersheds.
Landscape management, for example, construction of artificial, or the restoration of artificial, wetlands or ponds, is very promising [64], but also contributes to N2O emissions [47]. Today, riparian and alluvial wetlands eliminate about half the diffuse nitrate pollution of river systems (see above). The protection of these areas, namely against till drainage or insulation of the river from its alluvial plains, is therefore crucial; it should be coordinated with flood control measures.
Improving agricultural practices is certainly the most cost efficient way to reduce nitrogen contamination at the source. The relationship depicted in figure 5 suggests two approaches to reducing the potential for N loss of agricultural soils: increasing N use efficiency at constant fertilization and/or reducing total fertilization. Good & Beatty [65] have recently discussed the possible technical measures that could be implemented at the global scale in this area. It is clear, however, that reducing total fertilization remains necessary in the most intensive agricultural areas of the world, which will lead to some reduction of global crop production. This therefore sets the dilemma between food production and the preservation of water resources (including drinking water production) and biodiversity: can we feed the world while preserving the environment? Three important papers have recently been published on the issue of food security and environmental conservation. Foley et al. [66] analyse the potential for improving the productivity of underperforming lands, increasing cropping efficiency, shifting diets and reducing waste. Bouwman et al. [67] examine the role of livestock in the world food system. Tilman et al. [68] shown that more efficient nutrient use and moderate agriculture intensification focused on those existing croplands that have current yields well below their potential can equitably meet the increasing food demand at the 2050 horizon with a sustainable environmental impact. A similar conclusion is reached by Mueller et al. [69].
(b). Localization of production and consumption and change in the human diet
As the above-mentioned analysis has shown that the basins with the highest disturbance of the N cycle, as judged by their NANI value (figure 1), often show a considerable imbalance between anthropogenic autotrophy and heterotrophy, we would like to explore another line of thinking, raising the question of localization of agricultural production and consumption.
There is presently a lively debate on the possibility of local sourcing of the urban food supply, with the aim of supporting local agricultural communities, increasing food security and sovereignty and restoring the link between cities and their rural hinterland. The major cause of the extension of the ‘foodprint’ of large cities lies in the specialization of some territories either in export crop production or in livestock farming based on imported feed [70–72]. This industrial livestock husbandry responds to the current global increase of animal protein consumption. However, more and more voices advocate a reduction of animal protein consumption in industrialized countries, for reasons of public health [73,74] and environmental protection [75,76], and as a condition for world food security and equity [77].
For the Seine basin, a local, organic and ‘demitarian’ [76] scenario has been constructed and analysed by Billen et al. [71] (figure 3b). It obeys the following principles: livestock is adjusted to meet the requirements in animal protein of the local population (with the assumption of a ‘demitarian’ diet, i.e. 35–40% animal proteins); the livestock is fed only with locally produced feed; no synthetic fertilizer is used and arable land is fertilized with locally N2-fixing crops and the subsequent manure and crop residues; the area of the latter is adjusted to meet the requirements of livestock; the production of non-fixing arable land (mostly cereals) is calculated from the total fertilization rate, as is the leached nitrogen surplus (using the relationship illustrated in figure 5); the cereal production supplies first and foremost the local population, whereas the excess is available for export. This scenario was demonstrated to be able to reconcile the dual function of rural areas, namely feeding the city and producing high-quality water, while allowing the Seine basin to continue exporting a large share of its cereals production, notably with a larger net export of proteins than in the current situation [71].
We have applied the same principles to the four other example watersheds (figure 3b and the electronic supplementary material, §S6). The constraints were to satisfy a per capita protein requirement of 6 kg N yr−1 with 40 per cent from animal origin for the current population of each watershed, without recourse to either synthetic fertilizers or feed import, while keeping the nitrate in arable land leaching water (surplus diluted in the infiltrated water flux) below some threshold limit (we arbitrarily choose the value of 11 mg N l−1, the WHO standard for drinking water). In all cases, the scenarios meet the new requirements of the local population and strongly reduce nitrogen contamination of the hydrosphere. Only in the case of the heavily populated Scheldt basin, import of animal products remains necessary to satisfy the human consumption of meat and milk, even greatly reduced as in this scenario. In the Hong basin, the scenario increases both the total protein consumption and the share of animal products while reducing diffuse nitrogen contamination of water resources.
(c). Application to the world's watersheds
In an attempt to evaluate the potential global effects of localizing food production and consumption at the watershed scale, the same principles were applied stepwise to the world watershed data. The detailed procedure, which is based on the information available in the GlobalNEWS database, is presented in the electronic supplementary material, §S6. In essence, for each world watershed in its current situation, it consists of optimizing fertilization of arable land in order to meet the local food and feed requirements as far as possible while maintaining the agricultural soil N surplus below some stated threshold, taking into account the current relationship between yield and fertilization. An obvious constraint is that, at the global scale, total arable land production plus production of extensive and marginal grassland should match the total food and feed requirements: the threshold soil N surplus is gradually increased until this condition is met.
The first scenario, which will be used as a reference (REF), is constructed by adjusting the use of synthetic fertilizer in each watershed, taking into account the current recorded rate of symbiotic N fixation and atmospheric deposition, as well as the available manure at the current livestock distribution. The results differ a little from the original GlobalNEWS data as nitrogen fertilizer use has been recalculated taking the earlier-mentioned constraints into consideration: the overall global N soil surplus is a little higher, for a slightly lower global use of fertilizer (table 3); these differences are not significant, however, supporting the coherency of the approach used below to establish the scenarios.
Table 3.
Global scenarios exploring an alternative organization of the world agricultural system (see electronic supplementary material, §S6 for a world map of these results).
| scenario | autotrophy (Tg N yr−1) | heterotrophy (Tg N yr−1) | human food consumption (Tg N yr−1) | % animal protein (%) | synthetic fertilizer use (Tg N yr−1) | agric. N soil surplus (Tg N yr−1) |
|---|---|---|---|---|---|---|
| GlobalNEWS data | 122 | 118 | 19 | — | 80 | 138 |
| REF | 118 | 118 | 19 | 32 | 75 | 143 |
| LOC | 86 | 86 | 19 | 32 | 53 | 93 |
| LOC-ORG | 86 | 86 | 19 | 32 | 0 | 38 |
| LOC-DIET | 97 | 97 | 26 | 35 | 42 | 79 |
| LOC-ORG-DIET | 97 | 97 | 26 | 35 | 0 | 54 |
In a second scenario (LOC), the livestock is redistributed per watershed to meet the local requirements of the current human diet. Nothing else is changed with respect to the current situation in terms of the area of arable land and permanent grassland, of the production of extensive and marginal grassland and of the efficiency of vegetal to animal protein conversion rates. The rate of synthetic fertilizer application to arable land is then calculated by watershed according to the same rule as explained earlier. The results of this scenario (table 3) reveal that less livestock would be needed for meeting the current animal protein requirements than in the current situation and that the better distribution of manure resources results in a lower level of synthetic fertilizers, with a lower agricultural N soil surplus.
Currently at the global scale, synthetic fertilizers account for 34 per cent of the total fertilization of arable land, whereas symbiotic nitrogen fixation directly accounts for only 13 per cent. Peoples et al. [78] have stressed the fact that the potential of symbiotic nitrogen fixation is currently largely underexploited, as very few countries have a fraction of arable land devoted to legume crops higher than a few per cent. Increased areas of legumes might be achieved by including more leguminous crops in rotations or by the introduction of short-duration legume green manures or ‘catch crops’. In organic farming systems in Europe, legumes can occupy as much as two-thirds of the arable area, with the succeeding cereal crop yields typically 30 per cent lower than those reached under conventional chemical fertilization [79]. These practices have several advantages because, contrary to the use of synthetic fertilizers that may be difficult to access for either geographical or economic reasons, they represent local and mostly free sources of reactive nitrogen. To evaluate the global potential in symbiotic N fixation, we have calculated an extreme scenario, starting from the above LOC scenario and replacing synthetic fertilizers with an adjusted fraction of arable land devoted to fodder legume crops (LOC-ORG scenario). As a conservative estimation, we assumed that the yield of this legume crop is limited to the current Ymax of the corresponding watershed (figure 6) and that total (shoot + root) N fixation represents 1.4 times the harvested yield [78]. The results clearly show that symbiotic N fixation has the potential to support a localized agricultural production throughout the world, with significantly lower losses of N to the environment as judged by the corresponding agricultural soil surplus (table 3).
The effect of changing the human diet at the global scale warrants a special discussion. Obviously, the debate raised above on reducing the fraction of animal protein in the human diet is only relevant for rich industrialized countries. Today, half the world's population has a diet with less than 32 per cent animal proteins. Equity would imply a global target of providing at least 35 per cent animal protein to every person on the Earth. This target would result in a higher anthropogenic heterotrophy (increasing from 86 to 97 Tg N yr−1) if the total amount of protein intake is not changed. The LOC-DIET scenario following this target shows that such improvement of the current world food situation would require less synthetic fertilizer (because of a better distribution of manure resources) and result in a lower agricultural surplus than the LOC scenario with conventional agriculture. Note also that this scenario, like the preceding ones, assumes no changes in the current conversion efficiency of vegetal to animal protein by watershed: the differences in the efficiency of livestock between the different parts of the world are considerable, and the results of the scenario depend heavily on this parameter. Bouwman et al. [67] have already discussed this issue, namely in connection with the difference between the efficiency of red and white meat production.
Finally, we tested the possibility of meeting the food requirements of the above-mentioned LOC-DIET scenario in purely organic agriculture. This scenario (LOC-ORG-DIET) is able to provide the required food with better environmental performance in terms of agricultural soil surplus than the conventional LOC-DIET scenario (table 3).
Although the scenarios explored here are simple exercises for defining the potential of some radical changes in the agro-food system at the global scale, their results are quite intriguing. They clearly show that it is possible from an agronomical and biogeochemical point of view to meet the requirements of the world population with a much lower environmental impact than those characterizing the current agricultural system. Independently of the governance issues involved, acting on the very structure of the agro-food system offers a much more efficient lever to mitigate the effects of the nitrogen cascade than many other environmental measures.
5. Conclusion
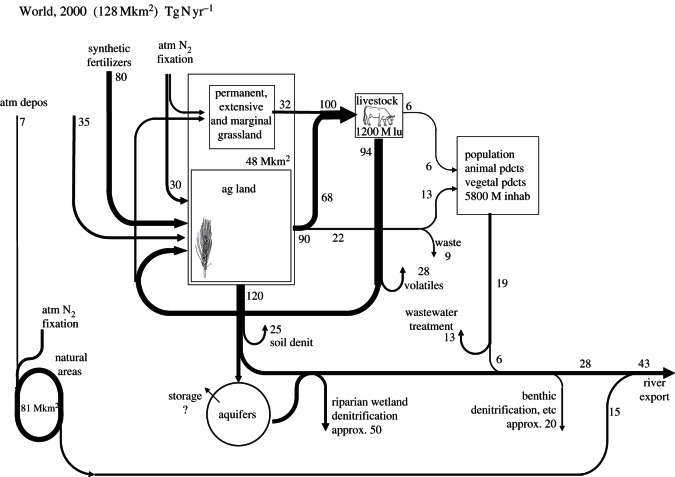
The data discussed in this paper provide a schematic of the nitrogen cascade at the global scale (figure 7), which stresses the prominent role of agriculture and livestock farming in initiating the whole chain of processes ultimately leading to atmospheric pollution and coastal marine eutrophication. Our approach conceptually differs from that of Bouwman et al. [49] in that we include riparian zones, foot slope areas, floodplains, delta areas and more generally all areas where groundwater meets the upper, biogeochemically active layer of the soil before joining the river network, as the interface between the terrestrial and surface water system. Denitrification in this interface is evaluated at about 50 Tg N yr−1, a much higher figure than the 8 Tg N yr−1 attributed by these authors to riparian strips, but which includes much of what they consider as soil and shallow groundwater denitrification. Urban point sources to surface water represent only 6 Tg N yr−1. Denitrification along the drainage network further eliminates about 20 Tg N yr−1.
Figure 7.
Representation of the major nitrogen fluxes associated with the global nitrogen cascade in 2000 (fluxes in Tg N yr−1).
The nitrogen fluxes delivered to surface water by ‘natural’ areas, although representing 81 × 106 km², i.e. 63 per cent of the global land area, are disproportionately lower than those from agricultural areas. Howarth et al. [17] estimate the N fluxes to the sea coming from land systems in the absence of anthropogenic perturbation at about 100 kg N km−2 yr−1 (range, 76–230 kg N km−² yr−1). Taking into account a mean global anthropogenic input of 350 kg N km−² yr−1 by atmospheric deposition to natural areas, 25 per cent of which reaches the river outlet, brings the N flux reaching the sea from natural areas to 15 Tg N yr−1 (range, 13–25 Tg N yr−1). On the other hand, the total N delivery to the ocean is estimated at 43 Tg N yr−1 by the GlobalNEWS model [15], thus indicating a contribution by natural areas of 35 per cent (range, 30–58%). Clearly, agricultural soil leaching today is the most significant contributor to groundwater and surface water contamination (figure 7).
Data discretization by watershed shows the very heterogeneous distribution of the factors responsible for the perturbation of the nitrogen cycle, with, as indicated earlier, a cumulated 84 per cent contribution to the global NANI by the basins covering only 43 per cent of the total continental area (figure 1). Similarly, 25 per cent of the global arable land produces 50 per cent of the global agricultural N soil surplus initiating the nitrogen cascade (see the electronic supplementary material, §S6).
Levers for change towards reducing nitrogen environmental losses exist at many stages of the nitrogen cascade, including technical measures for improving crop and livestock farming practices, for preserving or enhancing the natural filter effects of wetlands, and for reducing the discharge of urban wastewater and the emission of nitrogen oxides stemming from motor vehicle traffic and electric power generation. However, the few example scenarios analysed herein showed that structural issues related to the spatial organization of the world food system and the composition of the human diet are highly significant in determining the environmental losses of nitrogen. The challenge of providing to all humans on the planet an adequate protein diet will undoubtedly require more directly reconnecting crop production and livestock farming and localizing agricultural production and consumption. The idea that solving the problems of our hungry planet by further intensifying agriculture in the most productive regions of the world together with developing commercial trade of agricultural products [80] should be considered with great caution. On the contrary, the scenarios we have presented here suggest that reorganizing agro-food systems in order to better match the local food and feed demand by local agricultural production, and better use the potential of N2-fixing crops, would not only ‘feed the world’, but also considerably reduce, at the source, the dissipation of reactive nitrogen into the environment. Such actions, although technically very simple, go against deep and strong trends in the logic of the current organization of the agro-food system, characterized by the competition within an open and globalized market between a very large number of small, often poor producers and a small number of large organizations able to manage very large fluxes of agricultural goods [81]. Considerable governance issues are therefore involved to make these changes possible and the global food system more fair and sustainable.
Acknowledgements
Research and manuscript preparation were supported by grants from the Ville de Paris (Paris 2030, Research in Paris), the PIREN-Seine programme and the FIRE federation. Discussions with Lex Bouwman, Mark Sutton and Peter Vitousek significantly contributed to the analysis. We are grateful to Hans van Grinsven and an anonymous referee for constructive comments.
References
- 1.Galloway JN. 1998. The global nitrogen cycle: changes and consequences. Environ. Pollut. 102, 15–24 10.1016/S0269-7491(98)80010-9 (doi:10.1016/S0269-7491(98)80010-9) [DOI] [Google Scholar]
- 2.Jarvis S, Hutchings N, Brentrup F, Olesen J, van der Hoek K. 2011. Nitrogen flows in farming systems across Europe. Ch. 10. In The European nitrogen assessment: sources, effects and policy perspectives (eds Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt A, van Grinsven H, Grizzetti B.), pp. 211–228 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Billen G, Garnier J, Mouchel J-M, Silvestre M. 2007. The Seine system: introduction to a multidisciplinary approach of the functioning of a regional river system. Sci. Total Environ. 375, 1–12 10.1016/j.scitotenv.2006.12.001 (doi:10.1016/j.scitotenv.2006.12.001) [DOI] [PubMed] [Google Scholar]
- 4.Billen G, et al. 2011. Nitrogen flows from European regional watersheds to coastal marine waters. Ch. 13. In The European nitrogen assessment: sources, effects and policy perspectives (eds Sutton M, Howard C, Erisman JW, Billen G, Bleeker A, Grennfelt P, van Grinsven H, Grizzetti B.), pp. 271–297 Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Billen G, Garnier J, Rousseau V. 2005. Nutrient fluxes and water quality in the drainage network of the Scheldt basin over the last 50 years. Hydrobiologia 540, 47–67 10.1007/s10750-004-7103-1 (doi:10.1007/s10750-004-7103-1) [DOI] [Google Scholar]
- 6.Billen G, Garnier J, Nemery J, Sebilo M, Sferratore A, Benoit P, Barles S, Benoit M. 2007. A long term view of nutrient transfers through the Seine river continuum. Sci. Total Environ. 275, 80–97 10.1016/j.scitotenv.2006.12.005 (doi:10.1016/j.scitotenv.2006.12.005) [DOI] [PubMed] [Google Scholar]
- 7.Thieu V, Billen G, Garnier J. 2009. Nutrient transfer in three contrasting NW European watersheds: the Seine, Somme, Scheldt Rivers. A comparative application of the Seneque/Riverstrahler model. Water Res. 43, 1740–1748 10.1016/j.watres.2009.01.014 (doi:10.1016/j.watres.2009.01.014) [DOI] [PubMed] [Google Scholar]
- 8.Lassaletta L, Romero E, Billen G, Garnier J, García-Gómez H, Rovira JV. 2012. Spatialized N budgets in a large agricultural mediterranean watershed: high loading and low transfer. Biogeosciences 9, 57–70 10.5194/bg-9-57-2012 (doi:10.5194/bg-9-57-2012) [DOI] [Google Scholar]
- 9.McIsaac GF, David MB, Gertner GZ, Goolsby DA. 2002. Relating net nitrogen input in the Mississippi River basin to nitrate flux in the lower Mississippi River: a comparison of approaches. J. Environ. Qual. 31, 1610–1622 10.2134/jeq2002.1610 (doi:10.2134/jeq2002.1610) [DOI] [PubMed] [Google Scholar]
- 10.Le TPQ, Billen G, Garnier J, Théry S, Fézard C, Chau VM. 2005. Nutrient (N, P) budgets for the Red River basin (Vietnam and China). Glob. Biogeochem. Cycles 19, GB2022. 10.1029/2004GB002405 (doi:10.1029/2004GB002405) [DOI] [Google Scholar]
- 11.Le TPQ, Billen G, Garnier J, Théry S, Ruelland D, Nguyem XA, Chau VM. 2010. Modelling nutrient transfer in the sub-tropical Red River system (China and Vietnam): implementation of the Seneque/Riverstrahler model. J. Asian Earth Sci. 37, 259–274 10.1016/j.jseaes.2009.08.010 (doi:10.1016/j.jseaes.2009.08.010) [DOI] [Google Scholar]
- 12.FAOSTAT. 2006. Rome, Italy: FAO. See http://faostat.fao.org/
- 13.Bouwman AF, Beusen AHW, Billen G. 2009. Human alteration of the global nitrogen and phosphorus soil balances for the period 1970–2050. Glob. Biogeochem. Cycles 23, GB0A04. 10.1029/2009GB003576 (doi:10.1029/2009GB003576) [DOI] [Google Scholar]
- 14.Billen G, Beusen AHW, Bouwman AF, Garnier J. 2010. Anthropogenic nitrogen autotrophy and heterotrophy of the world's watersheds: past, present, and future trends. Glob. Biogeochem. Cycles 24, GB0A11. 10.1029/2009GB003702 (doi:10.1029/2009GB003702) [DOI] [Google Scholar]
- 15.Mayorga E, et al. 2010. Global nutrient export from watersheds (NEWS 2): model development and implementation. Environ. Model. Software 25, 837–853 10.1016/j.envsoft.2010.01.007 (doi:10.1016/j.envsoft.2010.01.007) [DOI] [Google Scholar]
- 16.Seitzinger SP, et al. 2010. Global river nutrient export: a scenario analysis of past and future trends. Glob. Biogeochem. Cycles 24, GB0A08. 10.1029/2009GB003587 (doi:10.1029/2009GB003587) [DOI] [Google Scholar]
- 17.Howarth RW, et al. 1996. Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry 35, 75–139 10.1007/BF02179825 (doi:10.1007/BF02179825) [DOI] [Google Scholar]
- 18.Vorosmarty CJ, Fekete BM, Meybeck M, Lammers RB. 2000. Geomorphometric attributes of the global system of rivers at 30-minute spatial resolution. J. Hydrol. 237, 17–39 10.1016/S0022-1694(00)00282-1 (doi:10.1016/S0022-1694(00)00282-1) [DOI] [Google Scholar]
- 19.Vitousek PM, Menge DNL, Reed SC, Cleveland CC. 2013. Biological nitrogen fixation: rates, patterns, and ecological controls in terrestrial ecosystems. Phil. Trans. R. Soc. B 368, 20130119. 10.1098/rstb.2013.0119 (doi:10.1098/rstb.2013.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galloway JN, et al. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226 10.1007/s10533-004-0370-0 (doi:10.1007/s10533-004-0370-0) [DOI] [Google Scholar]
- 21.Erisman JW, Galloway JN, Sutton MS, Klimont Z, Winiwater W. 2008. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 10.1038/ngeo325 (doi:10.1038/ngeo325) [DOI] [Google Scholar]
- 22.Luu TNM, Garnier J, Billen G, Le TPQ, Némery J, Orange D, Le LA. 2011. N, P and Si budgets for the Red River Delta (Northern Vietnam). How the delta affects river nutrient delivery to the sea. Biogeochemistry 107, 241–259 10.1007/s10533-010-9549-8 (doi:10.1007/s10533-010-9549-8) [DOI] [Google Scholar]
- 23.Lancelot C, Billen G, Sournia A, Weisse T, Colijn F, Veldhuis M, Davies A, Wassman P. 1987. Phaeocystis blooms and nutrient enrichment in the continental coastal zones of the North Sea. Ambio 16, 38–46 [Google Scholar]
- 24.Rabouille C, et al. 2008. Comparison of hypoxia among four river-dominated ocean margins: the Changjiang (Yangtze), Mississippi, Pearl, and Rhône rivers. Continental Shelf Res. 28, 1527–1537 10.1016/j.csr.2008.01.020 (doi:10.1016/j.csr.2008.01.020) [DOI] [Google Scholar]
- 25.Howarth RW, Boyer EW, Marino R, Swaney D, Jaworski N, Goodale C. 2006. The influence of climate on average nitrogen export from large watersheds in the northeastern United States. Biogeochemistry 79, 163–186 10.1007/s10533-006-9010-1 (doi:10.1007/s10533-006-9010-1) [DOI] [Google Scholar]
- 26.Howarth RW, Swaney D, Billen G, Garnier J, Hong B, Humborg C, Johnes P, Mörth CM, Marino R. 2012. Nitrogen fluxes from the landscape are controlled by net anthropogenic nitrogen inputs and by climate. Front. Ecol. Environ. 10, 37–43 10.1890/100178 (doi:10.1890/100178) [DOI] [Google Scholar]
- 27.WHO 2007. Protein and amino acid requirements in human nutrition. WHO/FAO/UNU. WHO Technical Report Series no. 935 Geneva: WHO; [PubMed] [Google Scholar]
- 28.Barles S. (ed.) 2005. L'invention des déchets urbains: France, pp. 296 Seysell, France: Champ-Vallon [Google Scholar]
- 29.Barles S. 2007. Feeding the city: food consumption and circulation of nitrogen, Paris, 1801–1914. Sci. Total Environ. 375, 48–58 10.1016/j.scitotenv.2006.12.003 (doi:10.1016/j.scitotenv.2006.12.003) [DOI] [PubMed] [Google Scholar]
- 30.Svirejeva-Hopkins A, et al. 2011. Nitrogen flows and fate in urban landscapes. In The European nitrogen assessment: sources, effects and policy perspectives, ch. 12 (eds Sutton M, Howard C, Erisman JW, Billen G, Bleeker A, Grennfelt P, van Grinsven H, Grizzetti B.), pp. 249–270 Cambridge, UK: Cambridge University Press [Google Scholar]
- 31.Van Drecht G, Bouwman AF, Harrison J, Knoop JM. 2009. Global nitrogen and phosphate in urban waste water for the period 1970–2050. Glob. Biogeochem. Cycles 23, GB003458. 10.1029/2009GB003458 (doi:10.1029/2009GB003458) [DOI] [Google Scholar]
- 32.Hofstra N, Bouwman AF. 2005. Denitrification in agricultural soils: summarizing published data and estimating global annual rates. Nutr. Cycling Agroecosyst. 72, 267–278 10.1007/s10705-005-3109-11 (doi:10.1007/s10705-005-3109-11) [DOI] [Google Scholar]
- 33.Briffaux G. 2009. Limiter le lessivage des nitrates. Essai longue durée AREP site de Thibie (Marne), Résultats acquis de 1991 à 2008. Châlons en Champagne: AREP [Google Scholar]
- 34.Chiesi F. 2011. Acquisition de données sur les pertes en azote nitrique sous différents systèmes culturaux en Champagne crayeuse. Adéquat-Environnement. Etude réalisée pour le compte de l'AESN, direction territoriale Vallée de Marne. Reims, France [Google Scholar]
- 35.Arnaud L, Baran N.2009. Détermination des vitesses de transfert de l'eau et des nitrates dans la zone non saturée de l'aquifère crayeux en Haute Normandie. Report BRGM-RP-57828-FR. Paris, France.
- 36.Billy C, Birgand F, Sebilo M, Billen G, Tournebize J, Kao C. 2009. Nitrate dynamics in artificially drained nested watersheds. Phys. Chem. Earth 36, 506–514 10.1016/j.pce.2008.09.007 (doi:10.1016/j.pce.2008.09.007) [DOI] [Google Scholar]
- 37.Vertes F, Simon JC, Laurent F, Besnard A. 2007. Prairies et qualité de l'eau. Evaluation des risques de lixiviation d'azote et optimisation des pratiques. Fourrages 192, 423–440 [Google Scholar]
- 38.Vertes F, Benoit M, Dorioz JM. 2010. Couverts herbacés pérennes et enjeux environnementaux (en particulier eutrophisation) : atouts et limites. Fourrages 202, 83–94 [Google Scholar]
- 39.Vertès F, Jeuffroy MH, Justes E, Thiébeau P, Corson M. 2010. Connaître et maximiser les bénéfices environnementaux liés à l'azote chez les légumineuses, à l’échelle de la culture, de la rotation et de l'exploitation. Innovations Agronomiques 11, 25–43 [Google Scholar]
- 40.Jackson BM, Browne CA, Butler AP, Peach D, Wade AJ, Wheater HS. 2008. Nitrate transport in chalk catchments: monitoring, modelling and policy implications. Environ. Sci. Policy 11, 125–135 10.1016/j.envsci.2007.10.006 (doi:10.1016/j.envsci.2007.10.006) [DOI] [Google Scholar]
- 41.Bouraoui F, Grizzetti B. 2011. Long term change of nutrient concentrations of rivers discharging in European seas. Sci. Total Environ. 409, 4899–4916 10.1016/j.scitotenv.2011.08.015 (doi:10.1016/j.scitotenv.2011.08.015) [DOI] [PubMed] [Google Scholar]
- 42.Billen G, Garnier J. 2000. Nitrogen transfers through the Seine drainage network: a budget based on the application of the Riverstrahler model. Hydrobiologia 410, 139–150 10.1023/A:1003838116725 (doi:10.1023/A:1003838116725) [DOI] [Google Scholar]
- 43.Curie F, Ducharne A, Bendjoudi H, Billen G. 2011. Spatialization of riparian denitrification in regional-scale watersheds: case study of the Seine river basin. Phys. Chem. Earth 36, 530–538 10.1016/j.pce.2009.02.004 (doi:10.1016/j.pce.2009.02.004) [DOI] [Google Scholar]
- 44.Sebilo M, Billen G, Grably M, Mariotti A. 2003. Isotopic composition of nitrate-nitrogen as a marker of riparian and benthic denitrification at the scale of the whole Seine River system. Biogeochemistry 63, 35–51 10.1023/A:1023362923881 (doi:10.1023/A:1023362923881) [DOI] [Google Scholar]
- 45.Haag D, Kaupenjohann M. 2001. Landscape fate of nitrate fluxes and emissions in Central Europe: a critical review of concepts, data, and models for transport and retention. Agric. Ecosyst. Environ. 86, 1–21 10.1016/S0167-8809(00)00266-8 (doi:10.1016/S0167-8809(00)00266-8) [DOI] [Google Scholar]
- 46.Haycock NE, Pinay G, Walker C. 1993. Nitrogen retention in river corridors. European perspective. Ambio 22, 340–346 [Google Scholar]
- 47.Vilain G, Garnier J, Tallec G, Cellier P. 2010. Effect of slope position and landuse on nitrous oxide (N2O) emissions (Seine Basin, France). Agric. Forest Meteorol. 150, 1192–1202 10.1016/j.agrformet.2010.05.004 (doi:10.1016/j.agrformet.2010.05.004) [DOI] [Google Scholar]
- 48.Vilain G, Garnier J, Roose-Amsaleg C, Laville P. 2012. Potential of denitrification and nitrous oxide production from agricultural soil profiles (Seine basin, France). Nutr. Cycling Agroecosyst. 92, 35–50 10.1007/s10705-011-9470-0 (doi:10.1007/s10705-011-9470-0). [DOI] [Google Scholar]
- 49.Bouwman AF, et al. 2013. Global trends and uncertainties in terrestrial denitrification and N2O emissions. Phil. Trans. R. Soc. B 368, 20130112. 10.1098/rstb.2013.0112 (doi:10.1098/rstb.2013.0112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durand P, et al. 2011. Nitrogen processes in aquatic ecosystems. Ch. 7. In The European nitrogen assessment: sources, effects and policy perspectives (eds Sutton M, Howard C, Erisman JW, Billen G, Bleeker A, Grennfelt P, van Grinsven H, Grizzetti B.), pp. 126–146 Cambridge, UK: Cambridge University Press [Google Scholar]
- 51.Garnier J, Leporcq B, Sanchez N, Philippon X. 1999. Biogeochemical mass-balances (C, N, P, Si) in three large reservoirs of the Seine basin (France). Biogeochemistry 47, 119–146 10.1007/BF00994919 (doi:10.1007/BF00994919) [DOI] [Google Scholar]
- 52.Kreiling RM, Richardson WB, Cavanaugh JC, Bartsch LA. 2011. Summer nitrate uptake and denitrification in an upper Mississippi River backwater lake: the role of rooted aquatic vegetation. Biogeochemistry 104, 309–324 10.1007/s10533-010-9503-9 (doi:10.1007/s10533-010-9503-9) [DOI] [Google Scholar]
- 53.Arnold JG, Srinivasan R, Muttiah RS, Williams JR. 1998. Large area hydrologic modeling and assessment. I. Model development. J. Am. Water Resources Assoc. 34, 73–89 10.1111/j.1752-1688.1998.tb05961.x (doi:10.1111/j.1752-1688.1998.tb05961.x) [DOI] [Google Scholar]
- 54.Arnold JG, Srinivasan R, Muttiah RS, Allen PM, Walker C. 1999. Continental scale simulation of the hydrologic balance. J. Am. Water Resources Assoc. 35, 1037–1052 10.1111/j.1752-1688.1999.tb04192.x (doi:10.1111/j.1752-1688.1999.tb04192.x) [DOI] [Google Scholar]
- 55.Neitsch SL, Arnold JG, Kiniry JR, Williams JR. 2001. Soil and water assessment tool: user manual version 2000. Temple, TX: Blackland Research Centre, Agricultural Research Service [Google Scholar]
- 56.Neitsch SL, Arnold JG, Kiniry JR, Williams JR.2005. SWAT theoretical documentation. See www:bcr.tamus.edu/swat/ .
- 57.Whitehead PG, Wilson EJ, Butterfield D. 1998. A semi-distributed nitrogen model for multiple source assessments in catchments (INCA). I. Model structure and process equations. Sci. Total Environ. 210/211, 547–558 10.1016/S0048-9697(98)00037-0 (doi:10.1016/S0048-9697(98)00037-0) [DOI] [Google Scholar]
- 58.Wade AJ, Durand P, Beaujouan V, Wessel WW, Raat KJ, Whitehead PG, Butterfield D, Rankinen K, Lepisto A. 2002. A nitrogen model for European catchments: INCA, new model structure and equations. Hydrol. Earth Syst. Sci. 6, 559–682 10.5194/hess-6-559-2002 (doi:10.5194/hess-6-559-2002) [DOI] [Google Scholar]
- 59.Billen G, Garnier J, Hanset P. 1994. Modelling phytoplankton development in whole drainage networks: the RIVERSTRAHLER model applied to the Seine river system. Hydrobiologia 289, 119–137 10.1007/BF00007414 (doi:10.1007/BF00007414) [DOI] [Google Scholar]
- 60.Garnier J, Billen G, Coste M. 1995. Seasonal succession of diatoms and Chlorophyceae in the drainage network of the river Seine: observations and modelling. Limnol. Oceanogr. 40, 750–765 10.4319/lo.1995.40.4.0750 (doi:10.4319/lo.1995.40.4.0750) [DOI] [Google Scholar]
- 61.Garnier J, Billen G, Hannon E, Fonbonne S, Videnina Y, Soulie M. 2002. Modeling transfer and retention of nutrients in the drainage network of the Danube River. Estuar. Coast. Shelf Sci. 54, 285–308 10.1006/ecss.2000.0648 (doi:10.1006/ecss.2000.0648) [DOI] [Google Scholar]
- 62.Ruelland D, Billen G, Brunstein D, Garnier J. 2007. SENEQUE 3: a GIS interface to the RIVERSTRAHLER model of the biogeochemical functioning of river systems. Sci. Total Environ. 375, 257–273 10.1016/j.scitotenv.2006.12.014 (doi:10.1016/j.scitotenv.2006.12.014) [DOI] [PubMed] [Google Scholar]
- 63.Tallec G, Garnier J, Billen G, Gousailles M. 2006. Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: effect of oxygenation level. Water Res. 40, 2972–2980 10.1016/j.watres.2006.05.037 (doi:10.1016/j.watres.2006.05.037) [DOI] [PubMed] [Google Scholar]
- 64.Passy P, Garnier J, Billen G, Fesneau C, Tournebize J. 2012. Restoration of ponds in rural landscapes: modelling the effect on nitrate contamination of surface water (the Seine watershed, France). Sci. Total Environ. 430, 280–290 10.1016/j.scitotenv.2012.04.035 (doi:10.1016/j.scitotenv.2012.04.035) [DOI] [PubMed] [Google Scholar]
- 65.Good AG, Beatty PH. 2011. Fertilizing nature: a tragedy of excess in the commons. PLoS Biol. 9, e1001124. 10.1371/journal.pbio.1001124 (doi:10.1371/journal.pbio.1001124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foley JA, et al. 2011. Solutions for a cultivated planet. Nature 478, 337–342 10.1038/nature10452 (doi:10.1038/nature10452) [DOI] [PubMed] [Google Scholar]
- 67.Bouwman AF, Goldewijk KK, Van Der Hoek KW, Beusen AHW, Van Vuuren DP, Willems J, Rufino MC, Stehfest E. 2011. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl Acad. Sci. USA (doi:10.1073/pnas.1012878108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA. 108, 20 260–20 264 10.1073/pnas.1116437108 (doi:10.1073/pnas.1116437108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mueller ND, Gerber JS, Johnston M, Ray DK, Ramankutty N, Foley JA. 2012. Closing yield gaps through nutrient and water management. Nature 490, 254–257 10.1038/nature11420 (doi:10.1038/nature11420) [DOI] [PubMed] [Google Scholar]
- 70.Billen G, Barles S, Chatzimpiros P, Garnier J. 2011. Grain, meat and vegetables to feed Paris: where did and do they come from? Localising Paris food supply areas from the eighteenth to the twenty-first century. Regional Environ. Change 12, 325–335 10.1007/s10113-011-0244-7 (doi:10.1007/s10113-011-0244-7) [DOI] [Google Scholar]
- 71.Billen G, Garnier J, Silvestre M, Thieu V, Barles S, Chatzimpiros P. 2012. Localising the nitrogen imprint of Paris food supply: the potential of organic farming and changes in human diet. Biogeosciences 9, 607–616 10.5194/bg-9-607-2012 (doi:10.5194/bg-9-607-2012) [DOI] [Google Scholar]
- 72.Weis F, Leip A. 2012. Greenhouse gas emissions from the EU livestock sector: a life cycle assessment carried out with the CAPRI model. Agric. Ecosyst. Environ. 149, 124–134 10.1016/j.agee.2011.12.015 (doi:10.1016/j.agee.2011.12.015) [DOI] [Google Scholar]
- 73.Lloyd-Williams F, Mwatsama M, Birt C. 2008. Estimating the cardiovascular mortality burden attributable to the common agricultural policy on dietary saturated fats. Bull. World Health Organ. 86, 535–545 10.2471/BLT.08.053728 (doi:10.2471/BLT.08.053728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Norat T, Bingham S, Ferrari P. 2005. Meat, fish and colorectal cancer risks: the European prospective investigation into cancer and nutrition. J. Nat. Cancer Inst. 97, 906–916 10.1093/jnci/dji164 (doi:10.1093/jnci/dji164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stehfest E, Bouwman L, van Vuuren D, den Elzen M, Eickhout B, Kabat P. 2009. Climate benefits of changing diet. Clim. Change. 95, 83–102 10.1007/s10584-008-9534-6 (doi:10.1007/s10584-008-9534-6) [DOI] [Google Scholar]
- 76.Nitrogen in Europe. 2009 The Barsac declaration. See http://www.nine-esf.org/barsac-declaration . [Google Scholar]
- 77.Paillard S, Treyer S, Dorin B. 2010. Agrimonde: scenarios et défis pour nourrir le monde en 2050. Paris: Quae [Google Scholar]
- 78.Peoples M, et al. 2009. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 48, 1–17 10.1007/BF03179980 (doi:10.1007/BF03179980) [DOI] [Google Scholar]
- 79.Seufert V, Ramankutty N, Foley JA. 2012. Comparing the yields of organic and conventional agriculture. Nature 485, 229–232 10.1038/nature11069 (doi:10.1038/nature11069) [DOI] [PubMed] [Google Scholar]
- 80.European Fertilizer Manufacturers' Association. 2009 Modern agriculture feeds the world. See http://www.fertilizerseurope.com/site/index.php?id=387 . [Google Scholar]
- 81.Mazoyer M. 2001. Protecting small farmers and the rural poor in the context of globalization. Rome, Italy: FAO [Google Scholar]