Abstract
Context
Secondary analyses of two randomized controlled trials (RCTs) and supportive epidemiologic and preclinical indicated the potential of selenium and vitamin E for preventing prostate cancer.
Objective
To determine whether selenium or vitamin E or both could prevent prostate cancer with little or no toxicity in relatively healthy men.
Design, Setting, and Participants
Randomization of a planned 32,400 men to selenium, vitamin E, selenium plus vitamin E, and placebo in a double-blinded fashion. Participants were recruited and followed in community practices, local hospitals and HMOs, and tertiary cancer centers in the United States, Canada and Puerto Rico. Baseline eligibility included 50 years or older (African American) or 55 years or older (all others), a serum prostate-specific antigen (PSA) ≤ 4 ng/mL, and a digital rectal examination (DRE) not suspicious for prostate cancer. Between 2001 and 2004, 35,533 men (10% more than planned because of a faster-than-expected accrual rate) were randomly assigned to the four study arms, which were well balanced with respect to all potentially important risk factors.
Interventions
Oral selenium (200 µg/day from L-selenomethionine) and matched vitamin E placebo, vitamin E (400 IU/day of all rac-α-tocopheryl acetate) and matched selenium placebo, or the two combined or placebo plus placebo for a planned minimum of 7 and maximum of 12 years.
Main Outcome Measures
Prostate cancer (as determined by routine community diagnostic standards) and prespecified secondary outcomes including lung, colorectal and overall cancer.
Results
Study supplements were discontinued at the recommendation of the Data and Safety Monitoring Committee at a planned 7-year interim analysis because the evidence convincingly demonstrated no benefit from either study agent (p < 0.0001) and no possibility of a benefit to the planned degree with additional follow-up. As of October 23, 2008, median overall follow-up was 5.46 years (range, 4.17 and 7.33). Hazard ratios (number of prostate cancers, 99% confidence intervals [CIs]) for prostate cancer were 1.13 for vitamin E (n=473; CI, 0.91–1.41), 1.04 for selenium (n=432; CI, 0.83–1.30), and 1.05 for the combination (n=437; CI, 0.83–1.31) compared with placebo (n=416). There were no significant differences (all p-values > 0.15) in any prespecified cancer endpoints. There were nonsignificant increased risks of prostate cancer in the vitamin E arm (p=0.06; relative risk [RR]=1.13; 99% CI, 0l95–1.35) and of Type 2 diabetes mellitus in the selenium arm (p=0.16; RR=1.07; 99% CI, 0.94–1.22), but they were not observed in the combination arm.
Conclusion
Selenium or vitamin E, alone or in combination, did not prevent prostate cancer in this population at the doses and formulations used.
Prostate cancer mortality in the United States has declined in recent years, but this cancer remains the most common non-skin epithelial malignancy in U.S. men, with 186,320 new cases and 28,660 deaths (the second leading cause of cancer death) estimated for 2008.1 An effective prevention strategy for prostate cancer would have substantial public health benefits, including the potential to reduce the incidence of biologically indolent prostate cancer, which is significantly over-detected by widespread screening with prostate-specific antigen (PSA) and for which most newly diagnosed men still undergo curative-intent therapy involving substantial morbidity despite surgical and other advances.2–6
Important secondary results of two randomized controlled trials (RCTs)—the Nutritional Prevention of Cancer (NPC) study and Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study—showed prostate-cancer risk reductions of 63% for selenized yeast and 32% for alpha-tocopherol (or vitamin E).7–10. In addition, a large-scale RCT involving several different regimens found that a combination of selenium, vitamin E and beta-carotene reduced overall cancer mortality.11 These clinical data, supported by epidemiological and preclinical data,12–19 led to the design of the Selenium and Vitamin E Cancer Prevention Trial (SELECT).20
Investigators from major cooperative groups of the U.S. National Cancer Institute (NCI) and U.S. Department of Veterans Affairs utilized the Prostate Cancer Prevention Trial (PCPT) accrual infrastructure (200 clinical sites, 18,882 randomized men) in designing and activating SELECT. We report here the effects of selenium and vitamin E, alone or in combination, on the risk of prostate cancer and secondary endpoints in SELECT.
METHODS
Study Design
SELECT is a phase III randomized, placebo-controlled trial of selenium (200 µg/day from L-selenomethionine) and/or vitamin E (400 IU/day of all rac-α-tocopheryl acetate) supplementation (planned minimum of 7 years and maximum of 12 years) for prostate cancer prevention. The major eligibility requirements included age of ≥ 50 years for African American men and ≥ 55 years for all other men, no prior prostate-cancer diagnosis, ≤ 4 ng/mL of PSA in serum, and a digital rectal examination (DRE) not suspicious for cancer. No current use of anticoagulant therapy other than ≤ 175 mg/day of acetasalicylic acid or ≤ 81 mg/day of acetasalicylic acid with clopidogrel bisulfate, no history of hemorrhagic stroke, and normal blood pressure were also required because of anti-platelet effects of vitamin E and related findings of the ATBC Study. Participant characteristics were based on self-report including self-identification of race and ethnicity as defined by the US Census Bureau. We collected race and ethnicity data mainly for the generalizability of trial results. All potentially eligible men were required to provide written informed consent before being allowed to participate in the trial. Baseline blood and toenail specimens and a five-year blood sample were collected for future biologic studies. Prostate tissue samples collected during the trial were submitted for confirmation by central pathology review (no samples were collected at baseline). Participants had clinic visits once every 6 months throughout the trial: adherence and adverse events were monitored every 6 months, and a limited physical examination including assessments of blood pressure, weight and smoking status was conducted annually. Prespecified adverse events known to be associated with vitamin E or selenium were graded according to the NCI Common Toxicity Criteria.
Although eligible PSA and DRE results were required at study entry, annual prostate cancer screening with PSA and DRE was not mandatory since the benefits of this screening were under debate when the trial opened and community screening standards were expected to change during the trial. Participants were recommended during annual clinic visits to undergo a PSA test and DRE according to the standard of care at their study sites and the participant’s wishes. A formal pre-randomization period (28–90 days; no placebo run-in capsules) gave potential participants time to decide if they would agree to stop disallowed over-the-counter supplements of selenium or vitamin E throughout the study and to demonstrate—by returning for randomization—their willingness to adhere to the trial. Other adherence measures included offering each participant a free multivitamin containing no selenium or vitamin E and assessing serum levels of vitamin E and selenium in all participants at a subset of study sites (22 sites representing 7.8% of the trial population). These sites were chosen a priori to be representative of the broad range of sites in the trial. The local Institutional Review Board of each study site approved the study for activation and reviewed its progress annually. The trial was activated in July 2001 and follow-up ended on October 23, 2008.
Endpoint Assessment
Participants reported prostate cancers to the study site staff. Study staff obtained medical records supporting the diagnosis and abstracted the diagnostic method and clinical stage. Tissue and the corresponding pathology report were sent to the central pathology laboratory for confirmation. Gleason Score was based on central pathology review.
Men were asked at their first 6-month clinic visit to report new events since entering the trial and thereafter to report new events since their last visit. Cardiac-event data were collected in detail from the trial beginning (2001); data on diabetes were added through self-reported glitazone-medication use (beginning in 2003) and diagnosis of diabetes (beginning in late 2005), which was initially asked retroactive to randomization date and then reported at interval visits thereafter. A general question regarding any events considered severe or life-threatening (Grade 3 or 4), regardless of attribution to the study supplements, was also asked. A Social Security Death Index search was conducted in July 2008 for participants who had a last contact date greater than 18 months prior to the search. Other specifically queried events (known at study inception to be related to either of the study supplements) included alopecia, dermatitis, fatigue, halitosis, nail changes, and nausea.
Statistical Analysis
The primary endpoint was prostate cancer incidence as determined by routine clinical management. Cancers that were not confirmed centrally were included in the analysis. SELECT was designed as a four arm trial with five pre-specified comparisons: selenium versus placebo, vitamin E versus placebo, combination versus placebo, selenium versus the combination, and vitamin E versus the combination. With a sample size of 32,400 men, using a 1-sided alpha-level of .005 (equivalent to a 2-sided alpha level of 0.01), there was 96% power to detect a 25% reduction in prostate cancer for either of the single agents (versus placebo), 89% power to detect a 25% reduction for the combination (versus an active single agent) and greater than 99% power to detect a 44% reduction of the combination (versus placebo).
Design assumptions were based on the PCPT, ATBC and NPC trials. The details of the statistical design have been described elsewhere.20 Important elements included (1) constant accrual over five years; (2) prostate cancer incidence in the placebo arm based on PCPT for the first three years and the 1995 Puget Sound SEER registry afterwards; (3) adherence to the study supplements, which was assumed to decrease over the course of the trial with a five-year rate of 68% and 12-year rate of 51%; (4) a constant 10% drop-in rate, defined as participants on placebo who are taking active supplementation off-study; (5) loss-to-follow-up of 0.5% per year; and (6) deaths estimated from PCPT for years 1–3 and from the 1995 US standard rates of men of age 63 and all races for year 4 onward. The sample size was calculated to be 32,400 men. Under the assumed conditions, the required median time under observation was estimated to be 8.8 years.
The primary analysis consisted of the five pre-specified comparisons detailed above. These comparisons allowed for a meaningful analysis of the study results whether or not an interaction between vitamin E and selenium occurred. Each individual test was conducted at a 1-sided 0.5% level, (equivalent to a 2-sided 1% level) using a Bonferroni factor of five to preserve an overall 1-sided level of 2.5% (equivalent to a 2-sided 5% alpha-level).
An independent Data and Safety Monitoring Committee (DSMC) met yearly and reviewed data on safety, adherence and diagnosis of prostate cancer. In addition to the final analysis, interim analyses were planned for years 5, 7, 9, 10 and 11 after the first participant was randomized; the percentages of the expected total number of prostate cancer events on the placebo arm at each interval were 14%, 35%, 61%, 74% and 88%, respectively. Each interim analysis resulted in recommendations which could have included modifications to the study including termination of accrual, modifications to data collection or early reporting of results. Recommendations were made to the Steering Committee which makes the final decisions. The interim analyses tested the null hypothesis at a one-sided .0005 level (equivalent to a two-sided .001 level) using the proportional hazards regression model. In addition, the alternative hypothesis of a 25% reduction in prostate cancer incidence was tested at a one-sided level of .0005 (equivalent to a two-sided .001 level) using an extension of the proportional hazards regression model that allows for testing a relative risk not equal to 1. The purpose of the second analysis was to allow for the study to stop if it was determined that the expected reduction in prostate cancer would not be seen. The frequencies of the number of cardiac events and cases of diabetes were tested with a chi-square test and were not corrected for multiple comparisons. For cardiac event and diabetes analyses, we did not capture the report of the date of the event, which thus was not incorporated into the analysis.
Participants were randomized in a randomized block scheme, where the block was the study site. This insured a balance of the four treatment groups within each study site. All analyses were performed using an intent-to-treat analysis in which men were classified according to the arm to which they were randomized. All men were followed until death or loss to follow-up. For cancer endpoints, men were censored at the time of their last follow-up or death. The analysis did not incorporate adjustments for baseline covariates. Data were analyzed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina).
Supplement Quality Control/Quality Assurance
The Pharmacy Coordinating Center received the study supplements for bottling as finished capsules in shipments containing lots of active capsules along with the appropriate matching placebo. As required by current good manufacturing practice (cGMP; 21 Code of Federal Regulations—Parts 210 and 211), each lot of capsules was quarantined upon receipt until testing was performed to assure that capsules labeled “active” by the manufacturer contained the appropriate active agent and that capsules labeled as placebo did not contain an active agent. In addition, each time the capsules were bottled, production-run-verification testing was performed to assure that bottles labeled as an active agent or placebo contained the appropriate material. To assure that the quality of the blind was maintained, capsules received in each subsequent lot were compared with the previous lot and with matching capsules in the current shipment for their characteristics of weight, shape and size, color and external marking, odor, and comparability of contents of opened capsules. Whether participants guessed or had an external validation of whether or not he was getting the active agent or placebo was not assessed.
RESULTS
On September 15, 2008, the independent DSMC met, reviewed data from the second formal interim analysis, and recommended the discontinuation of study supplements because the alternative hypothesis of no evidence of benefit from either study agent was convincingly demonstrated (p < 0.0001) and there was no possibility of a benefit to the planned degree with additional follow-up. The supplements were discontinued, and the data presented in this report are current as of October 23, 2008.
Participants
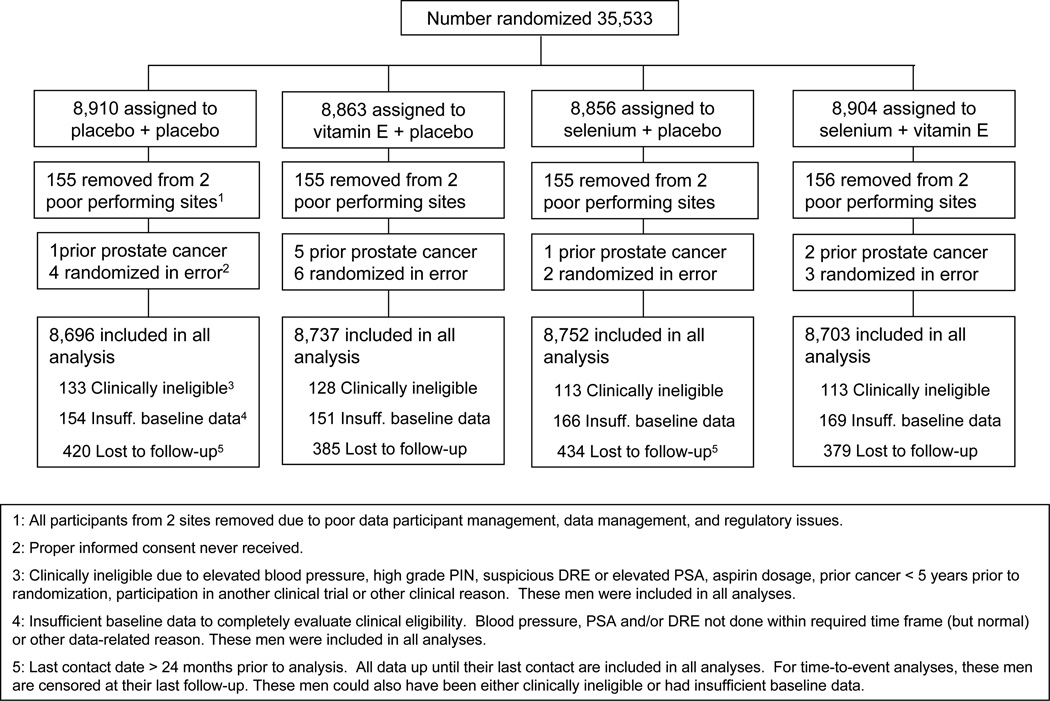
35,533 Men were accrued and randomly assigned at 427 participating sites in the United States, Canada and Puerto Rico between August 22, 2001, and June 24, 2004. Figure 1 presents the SELECT randomization scheme including participants who were excluded from analyses; all 621 participants at two study sites were removed from the analysis because of severe problems (that were detected early on) including poor data and participant management and regulatory issues. These participants differed substantially from the rest of the SELECT population in being from sites in the South of the U.S., 99% African-American, younger (median age 57), and of a lower education level (67% had less than a high school education) and in having lower PSA levels (57% < 1.0 ng/ml) and a higher prevalence of current smokers (33%). An additional 10 participants were removed because they were found to have had prostate cancer at randomization, and 15 were removed because their informed consent was never received. More men were accrued (35,533 in 3 years) than initially planned (32,400 in 5 years) mainly because of a far faster-than-expected accrual rate and the administrative time it takes to close down accrual.
Figure 1.
Trial Flow of Randomizations, Exclusions, Lost to Follow-up, and Participants Included in Analysis by Arm
The baseline characteristics of SELECT participants by each of the four arms (placebo, vitamin E, selenium and combined selenium and vitamin E) are presented in Table 1; all potentially important risk factors were well balanced among the arms. 2.6% Of SELECT men were former PCPT men randomized to finasteride; 4.7% of the non-PCPT participants reported use of finasteride (n=1592) or Propecia (n=76).
Table 1.
Baseline Participant Characteristics
| PLACEBO (n=8,696) |
VITAMIN E (n=8,737) |
SELENIUM (n=8,752) |
COMBINATION (n=8,703) |
|||||
|---|---|---|---|---|---|---|---|---|
| AGE | ||||||||
| Median | 62.6 | 62.3 | 62.6 | 62.4 | ||||
| 25th percentile | 58.1 | 58.0 | 58.2 | 58.1 | ||||
| 75th percentile | 67.8 | 67.8 | 68.0 | 67.8 | ||||
| AGE GROUP | ||||||||
| 50 – 54 years | 355 | 4% | 402 | 5% | 337 | 4% | 385 | 4% |
| 55 – 64 years | 5,078 | 58% | 5,143 | 59% | 5,076 | 58% | 5,052 | 58% |
| 65 – 74 years | 2,702 | 31% | 2,641 | 30% | 2,733 | 31% | 2,731 | 31% |
| 75+ years | 561 | 6% | 551 | 6% | 606 | 7% | 535 | 6% |
| RACE | ||||||||
| White | 6,863 | 79% | 6,890 | 79% | 6,942 | 79% | 6,874 | 79% |
| African American | 1,078 | 12% | 1,107 | 13% | 1,053 | 12% | 1,076 | 12% |
| Hispanic (Non-African. American) | 492 | 6% | 477 | 5% | 481 | 5% | 484 | 6% |
| Hispanic (African. American) | 76 | 1% | 103 | 1% | 86 | 1% | 95 | 1% |
| Other¥ | 187 | 2% | 160 | 2% | 190 | 2% | 174 | 2% |
| EDUCATION (HIGHEST LEVEL) | ||||||||
| High school grad or GED or less | 1,993 | 23% | 1,875 | 22% | 1,917 | 22% | 1,898 | 22% |
| Some college/Vocational school | 2,291 | 26% | 2,387 | 27% | 2,327 | 27% | 2,349 | 27% |
| College graduate or greater | 4,317 | 50% | 4,394 | 51% | 4,430 | 51% | 4,371 | 50% |
| Unknown/Missing | 95 | <1% | 81 | < 1% | 78 | <1% | 75 | < 1% |
| PSA (ng/ml) | ||||||||
| 0.1 – 1.0 | 4,122 | 47% | 4,208 | 48% | 4,218 | 48% | 4,213 | 48% |
| 1.1 – 2.0 | 2,728 | 31% | 2,653 | 30% | 2,661 | 30% | 2,666 | 31% |
| 2.1 – 3.0 | 1,168 | 13% | 1,228 | 14% | 1,211 | 14% | 1,149 | 13% |
| 3.1 – 4.0 | 666 | 8% | 634 | 7% | 652 | 7% | 659 | 8% |
| >4.0 | 5 | 0% | 3 | 0% | 2 | 0% | 1 | 0% |
| Unknown/Missing | 7 | 0% | 11 | 0% | 8 | 0% | 15 | 0% |
| SMOKING STATUS | ||||||||
| Never | 3,682 | 42% | 3,752 | 43% | 3,780 | 43% | 3,666 | 42% |
| Current | 655 | 8% | 659 | 8% | 631 | 7% | 670 | 8% |
| Former | 4,208 | 48% | 4,194 | 48% | 4,214 | 48% | 4,242 | 49% |
| Ever (Unknown status) | 63 | 1% | 55 | 1% | 61 | 1% | 56 | 1% |
| Unknown | 88 | 1% | 77 | 1% | 66 | 1% | 69 | 1% |
Other races include Asian (n=420), Native American (n=99), Pacific Islander (n=39), multiple (n=34) and unknown (n=119)
The median overall follow-up was 5.46 years (range, 4.17 and 7. 33 years). The percentages of participants with a recent last-contact date were over 88% within 7 months and 92% within 13 months of the SELECT data analysis. Loss to follow-up, defined as having a last contact date over 24 months prior to analysis, involved 5.1% of participants, which was slightly higher than had been estimated for the trial design (3.5% at 7 years after trial activation).
Adherence to both study agents as determined by pill count was similar across all study arms, and averaged 83% at year 1 and 65% at year 5. Adherence to at least one of the two agents was 87% at year 1 and 72% at year 5 (the design-estimated adherence rates were 90% at year 1 and 68% at year 5). Bioadherence was measured in a subset of participants by serum levels of selenium and cholesterol-adjusted alpha- and gamma-tocopherol and showed a good separation in agent serum levels between the arms (Table 2). The drop-in rate was assessed by a direct question to the participants about taking either of the supplements. Positive responses were 3.1% or less for vitamin E and 1.8% or less for selenium in each year (well below the design drop-in estimate of 10%). Prostate tissue samples were sent to the central pathology laboratory for confirmation in 86% of cases. The central laboratory agreed with the clinical site’s prostate cancer diagnosis in 99% of these cases.
Table 2.
Adherence to Study Supplements: Pill Counts and Bioadherence
| PLACEBO | VITAMIN E ALONE | SELENIUM ALONE | COMBINATION | |||||
|---|---|---|---|---|---|---|---|---|
| PILL COUNTS* | % | Range¥ | % | Range | % | Range | % | Range |
| SELENIUM/MATCHING PLACEBO | ||||||||
| YEAR 1 | 85% | (76%, 85%) | 85% | (77%, 85%) | 84% | (76%, 84%) | 85% | (77%, 84%) |
| YEAR 2 | 81% | (72%, 81%) | 80% | (72%, 81%) | 79% | (71%, 80%) | 80% | (72%, 80%) |
| YEAR 3 | 76% | (68%, 77%) | 77% | (69%, 77%) | 75% | (68%, 76%) | 76% | (69%, 77%) |
| YEAR 4 | 69% | (65%, 73%) | 73% | (66%, 74%) | 71% | (64%, 72%) | 72% | (65%, 74%) |
| YEAR 5 | 69% | (63%, 71%) | 71% | (64%, 73%) | 69% | (62%, 70%) | 70% | (64%, 71%) |
| VITAMIN E/MATCHING PLACEBO | ||||||||
| YEAR 1 | 85% | (76%, 85%) | 85% | (77%, 85%) | 85% | (76%, 85%) | 85% | (77%, 85%) |
| YEAR 2 | 80% | (71%, 80%) | 80% | (71%, 80%) | 79% | (70%, 79%) | 79% | (71%, 80%) |
| YEAR 3 | 75% | (67%, 75%) | 75% | (67%, 76%) | 74% | (67%, 75%) | 76% | (69%, 77%) |
| YEAR 4 | 70% | (63%, 72%) | 70% | (63%, 72%) | 69% | (62%, 71%) | 70% | (63%, 72%) |
| YEAR 5 | 67% | (61%, 69%) | 69% | (62%, 71%) | 67% | (61%, 69%) | 68% | (61%, 70%) |
|
PLACEBO (n=285) |
VITAMIN E ALONE (n=290) |
SELENIUM ALONE (n=277) |
COMBINATION (n=257) |
|||||
| SERUM SELENIUM (ug/L) | ||||||||
| MEDIAN (INTERQUARTILE RANGE) | ||||||||
| Baseline | 137.6 | 124.7 – 151.8 | 135.9 | 122.4 – 148.4 | 135.0 | 123.4 – 145.9 | 136.4 | 122.9 – 150.0 |
| 6-mo visit | 137.4 | 123.3 – 152.0 | 138.4 | 124.1 – 154.0 | 223.4 | 198.6 – 251.8 | 227.0 | 199.4 – 251.2 |
| 1st annual visit | 138.1 | 125.2 – 152.2 | 137.7 | 124.1 – 150.4 | 232.4 | 204.2 – 261.4 | 228.5 | 205.5 – 258.1 |
| 2nd annual visit | 132.0 | 120.8 – 143.1 | 129.8 | 120.1 – 139.9 | 228.0 | 206.3 – 256.9 | 220.7 | 194.0 – 249.5 |
| 4th annual visit† | 140.1 | 124.3 – 150.8 | 143.8 | 126.2 – 158.6 | 251.6 | 218.7 – 275.0 | 253.1 | 210.5 – 283.0 |
| CHOLESTEROL-ADJUSTED ALPHA-TOCOPHEROL (ug/mL) | ||||||||
| MEDIAN (INTERQUARTILE RANGE) | ||||||||
| Baseline | 12.45 | 10.70 – 14.95 | 12.79 | 10.69 – 15.37 | 12.58 | 10.43 – 14.75 | 12.20 | 10.12 – 15.35 |
| 6-mo visit | 11.68 | 10.09 – 13.61 | 18.14 | 15.21 – 22.45 | 11.62 | 10.10 – 13.44 | 17.90 | 15.11 – 20.84 |
| 1st annual visit | 11.68 | 10.24 – 13.44 | 18.50 | 15.08 – 22.46 | 11.69 | 10.10 – 13.03 | 18.04 | 14.77 – 22.35 |
| 2nd annual visit | 12.13 | 10.80 – 13.72 | 18.35 | 15.13 – 22.85 | 11.80 | 10.57 – 13.58 | 18.44 | 15.32 – 22.89 |
| 4th annual visit† | 12.09 | 9.95 – 14.41 | 16.57 | 13.86 – 22.61 | 12.03 | 9.57 – 13.53 | 17.87 | 14.68 – 22.31 |
| CHOLESTEROL-ADJUSTED GAMMA-TOCOPHEROL (ug/mL) | ||||||||
| MEDIAN (INTERQUARTILE RANGE) | ||||||||
| Baseline | 1.31 | 0.83 – 2.01 | 1.43 | 0.89 – 2.21 | 1.50 | 0.96 – 2.21 | 1.44 | 0.96 – 2.02 |
| 6-mo visit | 1.50 | 1.07 – 1.97 | 0.78 | 0.51 – 1.12 | 1.64 | 1.22 – 2.29 | 0.74 | 0.48 – 1.11 |
| 1st annual visit | 1.53 | 1.09 – 2.05 | 0.75 | 0.52 – 1.16 | 1.69 | 1.27 – 2.33 | 0.70 | 0.48 – 1.04 |
| 2nd annual visit | 1.57 | 1.13 – 2.13 | 0.74 | 0.49 – 1.08 | 1.76 | 1.26 – 2.43 | 0.66 | 0.50 – 1.03 |
| 4th annual visit† | 1.69 | 1.14 – 2.29 | 0.80 | 0.50 – 1.23 | 1.90 | 1.48 – 2.70 | 0.69 | 0.47 – 1.07 |
Percent of men at least adherent defined as taking at least 80% of their Study Supplements. Denominators decrease over time reflecting the varying amounts of follow-up.
The ranges are estimates for those with missing data assuming those missing were either all not adherent (low estimate) or all adherent (high estimate).
N’s for 4th annual visit are 79, 78, 72 and 71
Prostate Cancer
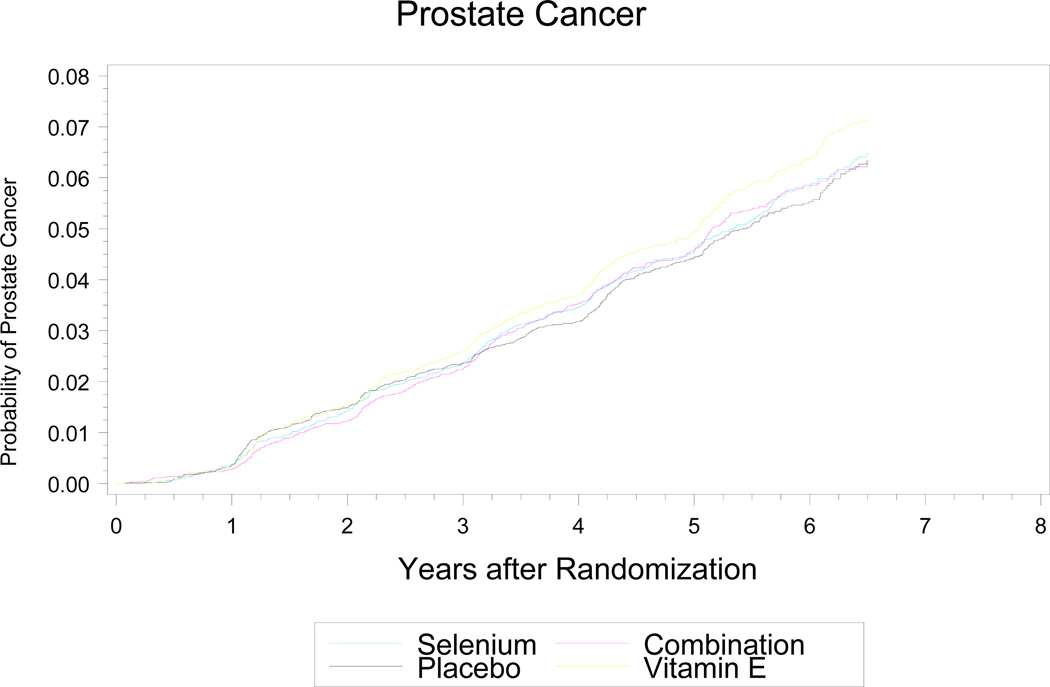
As shown in Table 3 and Fig. 2, there were no statistically significant differences in the absolute numbers (nor 5-year [median follow-up] incidence rates) of prostate cancer diagnoses between the four arms: placebo, 416 cases (5-year rate of 4.43%); selenium, 432 cases (4.56%); vitamin E, 473 cases (4.93%); combination, 437 cases (4.56%). There was a statistically non-significant increase (p = 0.06; HR=1.13; 99% CI, 0.95–1.35; 95% CI, 0.99–1.29) in prostate cancer incidence in the vitamin E-alone arm (versus placebo), but not in the combination arm (p=0.52; HR=1.05; 99% CI, 0.88–1.25; 95% CI, 0.91 – 1.20). The DSMC had some concern over this non-significant increase in prostate cancer and a nonsignificant increase in diabetes mellitus associated with selenium (see below). The test of the alternate hypothesis of a 25% reduction in prostate cancer was rejected at the p < 0.0001 level for both single agents, indicating that within the timeframe of the trial, the chance of finding a 25% reduction could be excluded. The 99.0% CIs around the hazard ratios were 0.87–1.24 for selenium, 0.95–1.35 for vitamin E, and 0.88–1.25 for the combination. The vast majority of prostate cancers diagnosed during the trial were early stage and low grade, and cancer stage and grade were similar across all arms (Table 3). The percentage of patients who had an annual PSA and DRE and the biopsy rate were similarly high across all arms, indicating that the prostate cancer findings were not due to screening-associated detection bias. Over 95% of prostate cancers were diagnosed by biopsy, the triggers for which (based on PSA and other factors) are shown in Table 3 and were similar across all arms. The overall rate of prostate cancer in the entire cohort was slightly higher than what was estimated for the placebo cohort at study inception.
Table 3.
Local Clinically Diagnosed Prostate Cancers
| PLACEBO (n=8,696) |
VITAMIN E (n=8,737) |
SELENIUM (n=8,752) |
COMBINATION (n=8,703) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| TOTAL PROSTATE CANCERS DIAGNOSED BY SITE |
416 | 473 | 432 | 437 | ||||
| METHOD OF DIAGNOSOS | ||||||||
| Prostate biopsy | 404 | 97% | 458 | 97% | 419 | 97% | 420 | 96% |
| Other/Unknown | 12 | 3% | 15 | 3% | 13 | 3% | 17 | 4% |
| TOTAL PROSTATE BIOPSIES | 1,020 | 1,011 | 982 | 997 | ||||
| PSA TESTS* | ||||||||
| Year 1 | 6,708 | 83% | 6,876 | 84% | 6,807 | 84% | 6,838 | 84% |
| Year 2 | 6,641 | 86% | 6,652 | 85% | 6,635 | 85% | 6,673 | 86% |
| Year 3 | 6,284 | 85% | 6,334 | 85% | 6,376 | 85% | 6,349 | 85% |
| Year 4 | 6,043 | 85% | 6,087 | 84% | 6,065 | 85% | 6,045 | 84% |
| Year 5 | 4,265 | 84% | 4,246 | 84% | 4,271 | 84% | 4,257 | 84% |
| DRE TESTS* | ||||||||
| Year 1 | 5,766 | 72% | 5,936 | 73% | 5,870 | 72% | 5,833 | 72% |
| Year 2 | 5,567 | 72% | 5,563 | 72% | 5,561 | 72% | 5,591 | 72% |
| Year 3 | 5,180 | 70% | 5,188 | 70% | 5,198 | 70% | 5,190 | 70% |
| Year 4 | 4,862 | 69% | 4,823 | 67% | 4,878 | 69% | 4,878 | 68% |
| Year 5 | 3,420 | 68% | 3,418 | 68% | 3,397 | 68% | 3,425 | 68% |
| BIOPSY PROMPT (Positive biopsies) | ||||||||
| Increased PSA | 259 | 64% | 324 | 71% | 296 | 71% | 263 | 63% |
| PSA (ng/ml) prompting biopsy Median |
4.60 | 4.60 | 4.83 | 4.70 | ||||
| 25th percentile | 4.00 | 3.99 | 4.05 | 4.00 | ||||
| 75th percentile | 5.50 | 5.60 | 5.70 | 5.60 | ||||
| PSA Velocity | 112 | 3% | 10 | 2% | 13 | 3% | 16 | 4% |
| Abnormal DRE | 66 | 16% | 58 | 13% | 46 | 11% | 56 | 13% |
| Increased PSA/PSA Velocity + Abnormal DRE | 55 | 14% | 49 | 11% | 56 | 13% | 72 | 17% |
| T-STAGE | ||||||||
| T1a–c | 278 | 70% | 343 | 75% | 301 | 73% | 286 | 69% |
| T2a–b | 122 | 30% | 114 | 25% | 108 | 26% | 128 | 31% |
| T3a–b | 0 | 0% | 2 | 0% | 5 | 1% | 3 | 1% |
| TX/Not staged | 16 | 14 | 18 | 20 | ||||
| N-STAGE | ||||||||
| N0 | 109 | 100% | 127 | 100% | 125 | 99% | 117 | 100% |
| N1 | 0 | 0% | 0 | 0% | 1 | 1% | 0 | 0% |
| NX/Not staged | 307 | 346 | 306 | 320 | ||||
| M-STAGE | ||||||||
| M0 | 124 | 100% | 134 | 99% | 122 | 96% | 119 | 98% |
| M1a–b | 0 | 0% | 2 | 1% | 5 | 4% | 2 | 2% |
| MX/Not staged | 284 | 331 | 285 | 304 | ||||
| GLEASON SCORE | ||||||||
| Number graded by central lab | 365 | 396 | 361 | 365 | ||||
| 2 – 6 | 240 | 66% | 249 | 63% | 217 | 60% | 220 | 60% |
| 7 (3+4) | 80 | 22% | 97 | 24% | 105 | 29% | 91 | 25% |
| 7 (4+3) | 21 | 6% | 27 | 7% | 19 | 5% | 24 | 7% |
| 8 – 10 | 24 | 7% | 23 | 6% | 20 | 5% | 30 | 8% |
Percents are based on alive participants who are prostate-cancer free and for whom the form was submitted.
Figure 2.
Cumulative Incidence of Prostate Cancers and Number of Prostate Cancers Detected Each Year by Arm. The curve is truncated at 6.5 years.
Secondary Outcomes
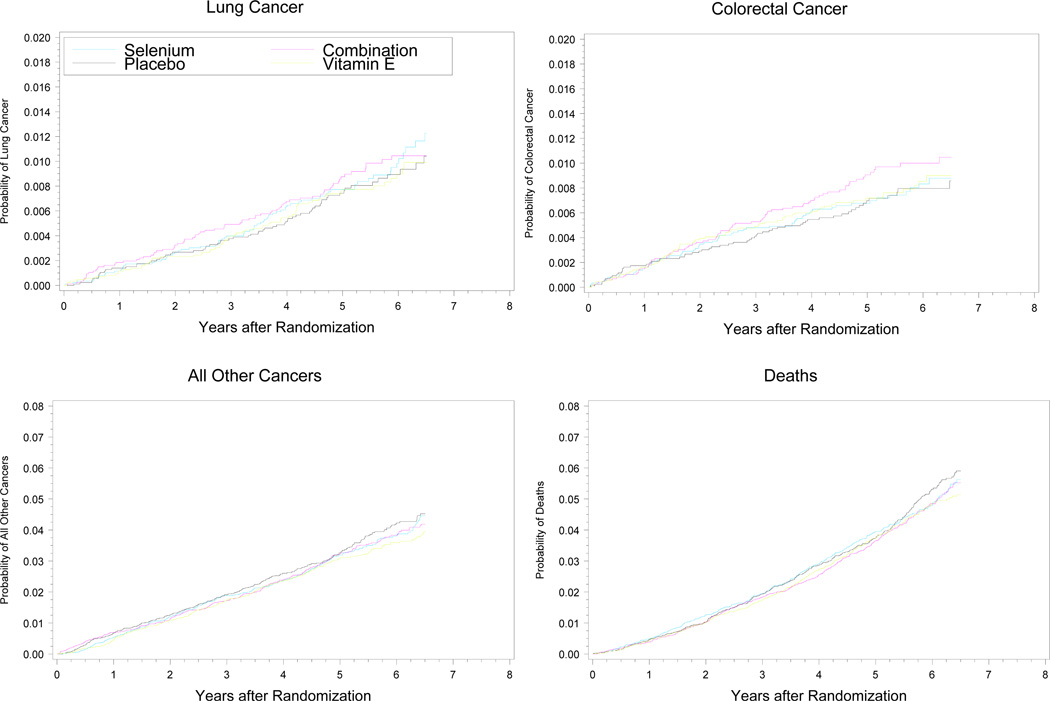
There were no significant differences (all p-values > 0.15) in any prespecified secondary cancer endpoints (Fig. 3 and Table 4). At five years, the cumulative death rate on the placebo arm was 38 deaths/1,000 participants (95% CI, 34/1000–42/1000); the estimated rate at trial inception was 48/1,000. The numbers of deaths from any cause were similar across the four arms: 382 on placebo; 358 on vitamin E; 378 on selenium; and 359 on the combination.
Figure 3.
Cumulative Incidence of Lung Cancers, Colorectal Cancer, All Other Primary Cancers and Deaths by Arm. All curves are truncated at 6.5 years.
Note: Cumulative incidence curves. All curves truncated at 6.5 years.
Table 4.
Secondary Outcomes Including Diagnosis of Other Cancers, Diabetes, Cardiovascular Events and Deaths
| PLACEBO (n=8,696) |
VITAMIN E (n=8,737) |
SELENIUM (n=8,752) |
COMBINATION (n=8,703) |
|||||
|---|---|---|---|---|---|---|---|---|
| CANCERS | N | HR Ω | N | HRM (99% CI)Ω | N | HR (99% CI) Ω | N | HR (99% CI) Ω |
| Any cancer (including prostate) Δ | 824 | 1.00 | 856 | 1.03 (0.91 – 1.17) | 837 | 1.01 (0.89 – 1.15) | 846 | 1.02 (0.90 – 1.16) |
| Lung | 67 | 1.00 | 67 | 1.00 (0.64 – 1.55) | 75 | 1.12 (0.73 – 1.72) | 78 | 1.16 (0.76 – 1.78) |
| Colorectal | 60 | 1.00 | 66 | 1.09 (0.69 – 1.73) | 63 | 1.05 (0.66 – 1.67) | 77 | 1.28 (0.82 – 2.00) |
| Other primary cancer* | 306 | 1.00 | 274 | 0.89 (0.72 – 1.10) | 292 | 0.95 (0.77 – 1.17) | 289 | 0.94 (0.76 – 1.16) |
| N | RR Ω | N | RR (99% CI) Ω | N | RR (99% CI) Ω | N | RR (99% CI) Ω | |
| DIABETES† | 669 | 1.00 | 700 | 1.04 (0.91 – 1.18) | 724 | 1.07 (0.94 – 1.22) | 660 | 0.97 (0.85 – 1.11) |
| CARDIOVASCULAR EVENTS | N | RR Ω | N | RR (99% CI) Ω | N | RR (99% CI) Ω | N | RR (99% CI) Ω |
| Any event (including death) | 1,050 | 1.00 | 1,034 | 0.98 (0.88 – 1.09) | 1,080 | 1.02 (0.92 – 1.13) | 1,041 | 0.99 (0.89 – 1.10) |
| Non-fatal strokes | ||||||||
| Hemorrhagic | 11 | 1.00 | 7 | 0.63 (0.18 – 2.20) | 11 | 0.99 (0.33 – 2.98) | 12 | 1.09 (0.37 – 3.19) |
| Ischemic | 56 | 1.00 | 49 | 0.87 (0.51 – 1.44) | 51 | 0.90 (0.55 – 1.49) | 67 | 1.20 (0.75 – 1.90) |
| NOS€ | 25 | 1.00 | 14 | 0.56 (0.2 – 1.32) | 11 | 0.44 (0.17 – 1.11) | 20 | 0.80 (0.37 – 1.73) |
| Other non-fatal CV Event (worst grade) ¥ | ||||||||
| Grade 3 | 626 | 1.00 | 642 | 1.02 (0.89 – 1.17) | 685 | 1.09 (0.95 – 1.25) | 624 | 1.00 (0.87 – 1.15) |
| Grade 4 | 190 | 1.00 | 203 | 1.06 (0.82 – 1.38) | 193 | 1.01 (0.78 – 1.31) | 201 | 1.06 (0.82 – 1.37) |
| N | HR Ω | N | HR (99% CI)Ω | N | HR (99% CI) Ω | N | HR (99% CI) Ω | |
| DEATHS | 382 | 1.00 | 358 | 0.93 (0.77 – 1.13) | 378 | 0.99 (0.82 – 1.19) | 359 | 0.94 (0.77 – 1.13) |
| Cancer | 125 | 1.00 | 106 | 0.84 (0.60 – 1.18) | 128 | 1.02 (0.74 – 1.41) | 117 | 0.93 (0.67 – 1.30) |
| Prostate | 0 | 1.00 | 0 | N/A | 1 | N/A | 0 | N/A |
| Lung | 41 | 1.00 | 38 | 0.92 (0.52 – 1.65) | 45 | 1.10 (0.63 – 1.91) | 39 | 0.95 (0.53 – 1.69) |
| Colorectal | 10 | 1.00 | 13 | 1.30 (0.44 – 3.83) | 10 | 1.00 (0.32 – 3.16) | 15 | 1.49 (0.52 – 4.28) |
| Other primary cancer* | 74 | 1.00 | 55 | 0.74 (0.47 – 1.17) | 72 | 0.97 (0.63 – 1.49) | 63 | 0.85 (0.55 – 1.32) |
| Cardiovascular | 142 | 1.00 | 119 | 0.84 (0.61 – 1.15) | 129 | 0.91 (0.66 – 1.24) | 117 | 0.82 (0.60 – 1.13) |
| Hemorrhagic stroke | 8 | 1.00 | 9 | 1.12 (0.32 – 3.92) | 9 | 1.12 (0.32 – 3.93) | 12 | 1.49 (0.46 – 4.84) |
| Other CV | 134 | 1.00 | 110 | 0.82 (0.59 – 1.14) | 120 | 0.89 (0.65 – 1.24) | 105 | 0.78 (0.56 – 1.09) |
| Other | 115 | 1.00 | 133 | 1.15 (0.83 – 1.60) | 121 | 1.05 (0.75 – 1.47) | 125 | 1.08 (0.78 – 1.51) |
Participants that had more than one cancer placebo (n=25), vitamin E (n=24), selenium (n=25) and combination (n=36)
Excluding basal cell and squamous cell skin cancers
Based on self report or reported use of diabetes medications of the glitazone class; excludes prevalent cases at randomization
Not specified as to whether an ischemic or hemorrhagic stroke
Compared to placebo; RR = relative risk, HR = Hazard ratio.
Excluding deaths
The study agents had no significant effects on the overall incidence of cardiac events (Table 4). A statistically non-significant increase in Type 2 diabetes mellitus (DM; diagnosed after randomization) occurred in the selenium-alone arm versus placebo—724 (10.0%; 99% CI, 9.1%–11.0%) versus 669 (9.3%; 99% CI 8.5%–10.2%), respectively (RR=1.07, p = 0.16). The number (percentage) of cases of DM was 700 (9.7%; 99% CI 8.8%–10.6%) on vitamin E and 660 (9.1%; 99% CI 8.2%–10.0%) on the combination; p-values of these figures compared with placebo DM were 0.47 (vitamin E) and 0.61 (combination). Data on known, clinically less-significant side effects of the study agents (alopecia, dermatitis, halitosis, nail changes, fatigue and nausea) are presented in Table 5.
Table 5.
Adverse Events Known to be Associated with the Study Supplements
| PLACEBO (n=8,696) |
VITAMIN E (n=8,737) |
SELENIUM (n=8,752) |
COMBINATION (n=8,703) |
|||||
|---|---|---|---|---|---|---|---|---|
| EVENT* | N | RR Ω | N | RR (99% CI)Ω | N | RR (99% CI) Ω | N | RR (99% CI) Ω |
| Alopecia £ | 206 | 1.00 | 220 | 1.06 (0.83 – 1.36) | 265 | 1.28 (1.01 – 1.62)¥ | 238 | 1.15 (0.91 – 1.47) |
| Dermatitis | ||||||||
| Grades 1–2 | 516 | 1.00 | 591 | 1.14 (0.98 – 1.32) | 605 | 1.17 (1.00 – 1.35) ¥ | 554 | 1.07 (0.92 – 1.25) |
| Grade 3–4 | 8 | 1.00 | 12 | 1.49 (0.46 – 4.83) | 14 | 1.73 (0.55 – 5.44) | 16 | 2.00 (0.66 – 6.09) |
| Halitosis£ | 427 | 1.00 | 493 | 1.15 (0.97 – 1.36) | 503 | 1.17 0.99 – 1.38) | 531 | 1.24 (1.06 – 1.46) |
| Nail changes£ | 1035 | 1.00 | 1041 | 1.00 (0.90 – 1.11) | 1087 | 1.04 (0.94 – 1.16) | 1075 | 1.04 (0.93 – 1.15) |
| Fatigue | ||||||||
| Grades 1–2 | 586 | 1.00 | 604 | 1.03 (0.89 – 1.19) | 645 | 1.09 (0.95 – 1.26) | 612 | 1.04 (0.90 – 1.20) |
| Grade 3–4 | 24 | 1.00 | 29 | 1.20 (0.59 – 2.45) | 21 | 0.87 (0.40 – 1.88) | 20 | 0.83 (0.38 – 1.81) |
| Nausea | ||||||||
| Grades 1–2 | 203 | 1.00 | 191 | 0.94 (0.72 – 1.21) | 244 | 1.19 (0.94 – 1.52) | 202 | 0.99 (0.77 – 1.28) |
| Grade 3 | 9 | 1.00 | 3 | 0.33 (0.06 – 1.84) | 9 | 0.99 (0.30 – 3.34) | 8 | 0.89 (0.25 – 3.10) |
Maximum grade experienced by a participant; NCI Common Toxicity Criteria version 2.0 for alopecia, nail changes, fatigue and nausea. Halitosis and dermatitis were defined in the study protocol . Generally, grade 1 = mild, grade 2=moderate, grade 3 = severe and grade 4 = life-threatening.
Only defined for Grades 1 – 2
Relative Risk compared to placebo.
p < .01
COMMENT
In this trial, neither 200 µg of selenomethionine or 400 IU of synthetic DL of alpha-tocopherol, given orally alone or combined for a median of 5.5 years had significant effects on the primary or secondary endpoints. A non-significant increased incidence of prostate cancer (p=0.06) was observed in the vitamin E arm but not in the combination arm. The trial supplements were discontinued early--in year 7 of the overall 12-year study--in accordance with a unanimous recommendation of the DSMC stating that, based on the evidence to date from the 7-year planned interim analyses, there was no evidence of a benefit from either study agent and no possibility of a benefit to the planned degree with additional follow-up. Sensitivity analyses suggested that the prespecified 25% risk reduction was extremely unlikely to be reached for either agent even with additional exposure. The statistical assumptions made in SELECT involving accrual rate, study supplement adherence and drop-in rates, prostate cancer incidence, death rate, and loss to follow-up were accurate and gave the trial significant power to detect the estimated preventive effects. Furthermore, the large sample size, inclusion of a substantial proportion of non-Caucasians, and equal distribution of known risk factors across all trial arms make the conclusions drawn from SELECT especially robust and generalizable.
Why were selenium and vitamin E ineffective in preventing prostate cancer in SELECT despite strong secondary evidence suggesting efficacy?7, 8 Considering selenium first, the secondary reduction in prostate cancer incidence in the NPC study could have been subject to limitations inherent in secondary analyses, such as chance findings due to multiple testing, especially since the overall NPC sample size was relatively small (1312 men and women versus 29,133 men in the ATBC Study). Second, the formulation (high-selenium yeast) given in the NPC trial may have been more active than the l-selenomethionine given in SELECT (both trials gave an equivalent selenium dose). In designing SELECT, we very carefully evaluated the choice of l-selenomethionine versus high-selenium yeast (and other formulations),20 and our rationale for selecting l-selenomethionine included the following considerations: selenomethionine was the major component of apparently active high-selenium yeast; evidence indicated substantial batch-to-batch variations in specific organoselenium compounds in samples of NPC yeast, making it unlikely that we could duplicate the selenium yeast formulation used in the NPC study; potential genotoxicity of highly active inorganic selenium compounds, such as selenite, made them potentially unsuitable for long-term prevention; lowering (versus selenomethionine) of overall body selenium stores with selenite, which is neither absorbed nor retained well; practical and safety concerns over newer selenium compounds, such as monomethylated forms (e.g., lacking availability, investigational new drug certification, and clinical data); and in vitro data indicating that selenomethionine was more effective than were other formulations in suppressing malignant and not normal prostate cells.15 Despite this careful rationale, it is impossible to know now whether or not selenized yeast would have been more active than was l-selenomethionine in SELECT. Last, the earlier trial was conducted in men chosen for deficient levels of selenium, finding that selenium was most preventive in the men with the lowest baseline selenium levels;9 SELECT men generally were replete in selenium at baseline, with median serum selenium levels of 135 ng/mL versus 113 ng/mL in NPC. The NPC cut-point for the lowest two tertiles was 121.6 units; 78% of SELECT men were above this level. The NPC trial found a non-significant increase in overall cancer rate in its highest tertile (hazard ratio=1.20, 95% CI, 0.77–1.86.)21
There are potential reasons why vitamin E did not prevent prostate cancer in SELECT. First, the high dose (400 IU/day) of the α-tocopherol form of vitamin E in SELECT may have been less effective than a lower dose such as the eight-fold lower 50 mg/day (roughly equivalent to 50 IU/day) that produced the earlier positive secondary findings in the ATBC Study.7 (The vitamin E formulation, synthetic all rac-α-tocopheryl acetate, was the same in SELECT and the ATBC Study.) A secondary analysis of the HOPE trial found that a relatively high dose of natural vitamin E did not reduce prostate cancer incidence.22 Achieving higher plasma or tissue levels of α-tocopherol within the physiological range, such as through a 50 mg/day supplement, may have some prostate cancer (or other) preventive effect such as cell proliferation or tumor growth inhibition.23 Furthermore, high pharmacologic doses of α-tocopherol may have an adverse impact on cytochrome p450-enzyme and other regulatory mechanisms24 that a lower dose would not have. It is also possible (but not certain) that the known effect of α-tocopherol in suppressing potentially beneficial plasma γ-tocopherol levels would have been less with the lower than higher dose of α-tocopherol.20 Nevertheless, men on vitamin E with the highest baseline (and thus total) serum vitamin E levels in the ATBC Study had the highest reduction in prostate and lung cancer,25 which supported our choice of the higher dose. A higher dose also was associated with potential benefits such as reductions in aging-related Alzheimer’s disease and macular degeneration. Second, several studies have suggested that vitamin E is more protective against prostate cancer in smokers, and less than 60% of SELECT men were current or former smokers (whereas all men in the ATBC Study were smokers). For example, observational analyses in a trial-based cohort of the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO), a trial of screening versus standard health care routines, there was a 71% reduction in the incidence of advanced prostate cancer associated with supplemental vitamin E use in current and recent smokers.26 A subgroup analysis of current and former smokers in SELECT, however, did not show a smoking-related benefit: 4.6% (223/4863; placebo) versus 4.8% (232/4853; vitamin E alone). As with selenium in the NPC study, vitamin E effects on prostate cancer incidence in the ATBC Study could have been due to chance findings in secondary analyses.
Selenium was not associated with significant effects on cardiac events, lung cancer, other cancers, or overall mortality in SELECT. One safety concern with selenium is a potential association with increased risk for Type 2 DM, for which there are mixed data from prior studies.27, 28 A recent analysis of the NPC population showed a significant increase in Type 2 DM (by self-report and medical records), largely limited to the top tertile of plasma selenium levels at baseline.29 In SELECT, a non-significant increase in risk (RR=1.07, p=0.16) of DM (compared with placebo) was observed in the selenium arm but not in the combination arm. Concerns about the safety of vitamin E supplementation arose during SELECT. One meta-analysis30 found that vitamin E at doses of ≥ 400 IU/day increased all-cause mortality, and another31 found evidence that vitamin E supplementation, alone or in combination with other anti-oxidants, may increase mortality. Neither study is directly relevant to the doses and population studied in SELECT; many studies included in these meta-analyses were in patients with serious disease, and the finding of increased mortality was driven by studies using doses far higher than 400 IU/day. In more relevant, placebo-controlled trials completed in healthy men and women, there were no associations of vitamin E supplementation with increased risks of either cardiovascular disease or overall mortality.32 SELECT results support the safety of vitamin E at 400 IU/day in healthy men, as there were no increases in either cardiovascular disease or total mortality in the vitamin E arms.
The 35,533 randomized men of SELECT were needed because of the robust statistical design accommodating four study arms with five primary comparisons; this large trial population made SELECT the largest cancer chemoprevention trial ever conducted. African American men have among the highest prostate cancer risks in the world, and SELECT had the highest participation of African Americans (13%) of any large-scale cancer chemoprevention trial to date. The statistical rigor of the trial was matched by the rigor of its implementation. Features of this implementation included the SELECT Workbench, a secure Web site administered by the SELECT Statistical Center and used by study-site staff and investigators. The Workbench is used to access participant and site-specific reports, the study protocol, and a detailed Study Manual and to submit data using Web-based forms. Form submission included detailed edit checks and a tracking system to identify all expected forms. Training and monitoring consisted of semiannual workshops, Quality Assurance audits at least once every 3 years, and mentoring by trained Statistical Center staff and experienced Clinical Research Associates. SELECT also maintains a public Web site initially designed to recruit participants and later used to promote participant adherence and to keep SELECT in the public’s eye.20
Potential limitations of SELECT include that it did not test different formulations or doses of selenium and vitamin E and that it did not definitively assess results in subgroups of men who may have responded differently than did the overall population. Because of active annual screening with PSA and DRE and early detection, SELECT could not assess effects in reducing advanced or fatal prostate cancer, which recent data suggest may be a potential benefit of vitamin E and selenium18, 26, 33–35 SELECT also could not assess intervention effects in a population deficient in vitamin E and/or selenium (since our trial population was well-nourished at baseline) or in current smokers (since they represented only 7%–8% of the SELECT population—a substantial difference from the ATBC Study in predominantly heavy smokers).
Cancer chemoprevention is an important approach for reducing cancer burden.36 Several RCTs have demonstrated significant cancer or premalignancy risk reductions in the breast, colon-rectum, prostate and stomach.37–43 Prostate cancer is a particularly attractive target for chemoprevention because of its clinical ubiquity, high or substantial treatment-associated morbidity, and stepwise molecular pathogenesis. In the large-scale PCPT, which was reported two years after SELECT was activated, finasteride produced a 25% relative reduction in the 7-year period prevalence of prostate cancer (versus placebo),42 and recent data suggest that finasteride reduces the risk of clinically significant disease and may not induce high-grade cancers despite initial concerns to the contrary44–48 SELECT now has definitively demonstrated that selenium, vitamin E or the two combined (at the tested doses and formulations) did not prevent prostate cancer in the generally healthy, heterogeneous population of men in SELECT. These data underscore the prudence that is needed in considering recommendations to use agents for the prevention or control of disease in the absence of convincing clinical trial results. These findings also compel the medical research community to continue the search for new, effective agents for prostate cancer prevention.
Acknowledgments
We owe an incalculable debt of gratitude to the 35,533 men and many principal investigators and clinical research associates at our 427 clinical sites, whose participation in SELECT has written an important chapter in the history of cancer prevention. We thank the many personnel of the Southwest Oncology Group (SWOG; the coordinating group of this Intergroup trial), whose tireless efforts allowed SELECT to successfully complete the test of its primary hypotheses.
Role of the Sponsor: The U.S. NCI was involved in the design and conduct of the study; the analysis and interpretation of the data; and the preparation, review and approval of the manuscript.
Funding Support: This investigation was supported in part by Public Health Service Cooperative Agreement grant CA37429 awarded by the National Cancer Institute (NCI), National Institutes of Health (NIH), Department of Health and Human Services DHHS, and in part by the National Center for Complementary and Alternative Medicine (NIH). Study agents and packaging were provided by Perrigo Company (Allegan, MI), Sabinsa Corporation (Piscataway, NJ), Tishcon Corporation (Westbury, NY) and DSM Nutritional Products, Inc (Parsipanny, NJ).
Footnotes
Address reprint requests to: Southwest Oncology Group (S-0000), Group Chair’s Office, 24 Frank Lloyd Wright Dr., Ste. A3400, P.O. Box 483, Ann Arbor, MI 48106
Author Contributions:
Study concept and design: Lippman, Klein, Goodman, Thompson, Ford, Parnes, Minasian, Gaziano, Probstfield, Santella, Kristal, Albanes, Taylor, Ganz, Crowley, Coltman.
Acquisition of data: Cook, Parsons, Bearden, Claudio, Winquist, Probstfield, Hartline, Crowley, Goodman, Darke, Arnold.
Analysis and interpretation of data: Lippman, Klein, Goodman, Thompson, Ford, Parnes, Minasian, Gaziano, Kristal, Darke, Arnold, Crowley, Coltman, Baker.
Drafting of manuscript: Lippman, Klein, Goodman, Minasian, Kristal, Crowley, Baker, Coltman.
Critical revision of the manuscript for important intellectual content: Lippman, Klein, P Goodman, Lucia, Thompson, Ford, Parnes, Minasian, Gaziano, Hartline, Parsons, Bearden, Crawford, G Goodman, Claudio, Winquist, Cook, Karp, Walther, Lieber, Kristal, Darke, Arnold, Ganz, Santella, Albanes, Taylor, Probstfield, Jagpal, Crowley, Meyskens, Baker, Coltman.
Statistical analysis: Goodman, Darke, Arnold, Crowley.
Obtained funding: Lippman, Klein, Coltman, Crowley, Gaziano.
Administrative, technical or material support: Lippman, Klein, Goodman, Hartline, Probstfield, Crowley, Coltman, Baker, Ford, Minasian.
Study supervision: Lippman, Klein, Coltman, Goodman, Hartline, Minasian, Gaziano, Ford, Thompson.
Financial Disclosures: Dr. Gaziano reported receiving investigator-initiated research funding from the National Institutes of Health, the Veterans Administration, Veroscience, Amgen and BASF Corporation, and research support in the form of study agents and packaging from BASF Corporation, Wyeth Pharmaceutical and DSM Nutritional Products Inc (formerly Roche Vitamins); he also reported serving as a consultant or receiving honoraria from Bayer AG and Pfizer, and serving as an expert witness for Merck. Dr. Karp reported that he is Principal Investigator for the Eastern Cooperative Oncology Group (ECOG) E5597 Intergroup Study of Selenium Only vs. Placebo in Resected Stage I Lung Cancer. Dr. Lucia reported that he serves as a consultant for GlaxoSmithKline and Veridex, and is a member of the Advisory Board for GenProbe. Dr. Meyskens reported that he is Co-founder of Cancer Prevention Pharmaceuticals. Dr. Parsons reported that he receives grant support from the National Cancer Institute and the Department of Defense. Dr. Thompson reported that he serves as a consultant for Veridex and Mission Pharmacal (with fees paid to University of Texas HSC at San Antonio).
Access to Data: SELECT lead statistician Phyllis J. Goodman, MS, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. She is independent of any commercial funder.
Active SELECT Clinical Sites with ≥ 100 Participants as of October 23, 2008: San Diego, U of CA: J. Kellogg Parsons, Principal Investigator (PI) [1,743 men]; Upstate Carolina CCOP: Jay Bearden III, PI (1,201 men); London Regional Cancer Program, London Health Sciences Centre: Joseph L. Chin and Eric Winquist, PIs (981 men); University of Colorado: E. David Crawford, PI (964 men); Swedish Medical Ctr: Gary E. Goodman, PI (934 men); VAMC Jesse Brown: Thomas E. Lad, PI (749 men); Harbor-UCLA: Rowan T. Chlebowski, PI (629 men); Le Centre de Recherche: Yves Fradet, PI (628 men); Altamira Family Med: Jaime Claudio, PI (610 men); Mayo – Rochester: Michael M. Lieber, PI (606 men); Capital Region Prostate Centre: Gary Steinhoff, PI (543 men); Vancouver Hospital: Mark FitzGerald, PI (423 men); Rush University Medical Center: Steven K. Rothschild, PI (385 men); MD Anderson Cancer Center: Elise D. Cook, PI (381 men); VAMC San Juan: Luis Baez, PI (359 men); SUNY Stony Brook: Iris A. Granek, PI (358); Sherbrooke University Hospital: Abdenour Nabid, PI (348 men); George Washington University: Richard J. Katz, PI (342 men); William Beaumont Hospital: David A. Decker, PI (321 men); Wilford Hall Medical Center: Kyle J. Weld, PI (309 men); Cascadia Cancer Prevention at St. Joseph Hospital: Frank E. James, PI (299 men); Dayton CCOP: Lawrence J. Litscher, PI (296 men); Grand Rapids CCOP: Marianne K. Lange, PI (287 men) ; VAMC Minneapolis: Timothy J. Wilt, PI (270 men); Carle Cancer Center CCOP: David L. Graham, PI (253 men); LDS Hospital: Scott Chidester, PI (250 men); University of Mississippi: Charles R. Pound, PI (238 men); Greenville CCOP: Jeffrey K. Giguere, PI (230 men); Metro-Minnesota CCOP: Alice C. Shapiro, PI (229 men); VAMC Cleveland: Donald R. Bodner, PI (227 men); Wichita CCOP: Shaker R. Dakhil, PI (219 men); Arizona Cancer Center: Frederick R. Ahmann, PI (219 men); Marshfield Clinic: Matthias Weiss, PI (215 men); University of Iowa Hospital: Richard D. Williams, PI (207 men); Baptist Hospital East: Kerry Short, PI (202 men); Downstate Medical Center: Richard J. Macchia, PI (197 men); Kalamazoo CCOP: Raymond S. Lord III, PI (181 men); Southern Nevada CCOP: John A. Ellerton, PI (173 men); Sunnybrook Health Science Center: Laurence Klotz, MD, PI (171 men); Missouri Baptist Medical Center: Paul K. Schultz, PI (170 men); Geisinger Clinic: Albert M. Bernath, PI (165 men); VAMC Kansas City: Peter J. Van Veldhuizen Jr., PI (163 men); Orocovis Med Ctr: Jose S. Aponte, PI (163 men); Sutter Health Cancer Research Group-Eastern Division: Vincent Caggiano, PI (160 men); VAMC Washington, DC: Steven H. Krasnow, PI (154 men); Bay Area CCOP: Norman R. Cohen, PI (153 men); Sentara Cancer Institute: Robert W. Given, PI (152 men); VAMC Fargo: William K. Becker, PI (151 men); Medical College of Wisconsin: Robert F. Donnell, PI (149 men); VAMC Houston: Teresa G. Hayes, PI (146 men); Baptist Regional Cancer Insitute: Neil Abramson, PI (136 men); Mount Sinai CCOP: Rogerio C. Lilenbaum, PI (134 men); Methodist Hospitals of Dallas: John V. Cox, PI (133 men); Miguel Sosa Padilla: Miguel Sosa-Padilla, PI (133 men); Kaiser Permanente: Nagendra R. Tirumali, PI (132 men); Duluth CCOP: Steven A. Kuross, PI (131 men); Stormont-Vail Health Care: Stanley J. Vogel, PI (130 men); Decatur Memorial Hospital: James L. Wade III, PI (126 men); VAMC Puget Sound: Daniel W. Lin, PI (124 men); VAMC Boston: Mary T. Brophy, PI (122 men); Scott & White CCOP: Scott Coffield, PI (119 men); Schiffler Cancer Center: Gregory S. Merrick, PI (116 men); MeritCare Hospital CCOP: Preston D. Steen, PI (115 men); Gaston Memorial Hospital: Steven W. Yates, PI (114 men); VAMC Phoenix: James V. Felicetta, PI (113 men); Lehigh Valley Hospital: Gregory R. Harper, PI (113 men); Cancer Resource Ctr: Sushil S. Lacy, PI (112 men); Holy Cross Hospital: Leonard J. Seigel, PI (112 men); Cleveland Clinic: Eric A. Klein, PI (111 men); Walter Reed AMC: Rob Dean, PI (111 men); Kaiser Permanente-GA: Joshua I. Barzilay, PI (110 men); Columbia River CCOP: Keith S. Lanier, PI (110 men); Oregon Health & Science University: Mark G. Garzotto,PI (110 men); H Lee Moffitt Cancer Center: Julio M. Pow-Sang, PI (110 men); McGill University Health Center: Simon Tanguay, PI (110 men); Vanderbilt University: Michael S. Cookson, PI (109 men); St Luke's Mountain State Tumor Institute: Thomas M. Beck, PI (107 men); Washington University: Robert L. Grubb III, PI (107 men); VAMC Southern Arizona: Maria C. Bishop, PI (106 men); Andres Grillasca: Luis Baez, PI (106 men); VAMC Hines: Nirmala Bhoopalam, PI (102 men); University of Oklahoma: Daniel J. Culkin, PI(102 men); Kaiser Permanente-Oakland: Louis Fehrenbacher, PI (100 men); St Vincent Hospital: Thomas J. Saphner, PI (100 men).
Intergroup Participants: Eastern Cooperative Oncology Group (ECOG): Chief Liaison (CL), D Karp; Cancer and Leukemia Group B (CALGB): CL, P Walther; North Central Cancer Treatment Group (NCCTG): CL, M Lieber; Radiation Therapy Oncology Group (RTOG): CL, F Khuri; and Veterans Affairs Cooperative Studies Program (VACSP): CL, M Gaziano.
SELECT Steering Committee: Gary E. Goodman, M.D., Philip R. Taylor, M.D., Sc.D., Powel H. Brown, M.D., Ph.D., Paul Godley, M.D., Ph.D., Charles Bennett, M.D., Ph.D., Michael M. Lieber, M.D., Lewis Musgrove, Ellen Richmond, M.S., R.N., Alan R. Kristal, Dr.P.H., Julia E. Vertrees, Pharm.D., Regina M. Santella, Ph.D., M. Scott Lucia, M.D., Demetrius Albanes, M.D., Patricia A. Ganz, M.D., Jeffrey L. Probstfield, M.D., Neil E. Fleshner, M.D., M.P.H., Isaac J. Powell, M.D., T. J. Jagpal, CCRP, William R. Markesbery, M.D., William Christen, Sc.D., Patricia A. Cassano, Ph.D., M. Peter Lance, M.D., Carolyn J. Hoban, D.Sc., Marjorie A. Godfrey, Abbie L. Brown, Dana B. Sparks, M.A.T., Elaine Armstrong, M.S, Frank L. Meyskens, Jr., M.D., Cathy Tangen, Dr.P.H., Garnet L. Anderson, Ph.D., Amy Darke, M.S., Katie Arnold, M.S., Karen Anderson, Monica Yee, Scott M. Lippman, M.D., Eric A. Klein, M.D., Phyllis J. Goodman, M.S., Ian M. Thompson, M.D., Leslie G. Ford, M.D., Howard L. Parnes, M.D., J. Michael Gaziano, M.D., M.P.H., Lori Minasian, M.D., Jo Ann L. Hartline, M.P.H., J. Kellogg Parsons, M.D., M.H.S., James D. Bearden, III, M.D., Jaime Claudio, M.D., Elise D. Cook, M.D., Laurence H. Baker, D.O., John J. Crowley, Ph.D., Charles A. Coltman, Jr., M.D.
SELECT Committees and Subcommittees: Recruitment and Adherence Committee: J. L. Probstfield (Chair); Minority and Medically Underserved Subcommittee: E. D. Cook (Chair); Health-related Quality of Life Committee: C. M. Moinpour and P. A. Ganz (Co-Chairs); Pathology and Biomarkers Committee: M. S. Lucia (Chair); Molecular Epidemiology Committee: R. M. Santella (Chair); Diet and Nutrition Committee: A. R. Kristal (Chair); Site Coordinators Committee: T. J. Jagpal (Chair).
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Harlan SR, Cooperberg MR, Elkin EP, Lubeck DP, Meng MV, Mehta SS, et al. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. J Urol. 2003;170(5):1804–1807. doi: 10.1097/01.ju.0000091641.34674.11. [DOI] [PubMed] [Google Scholar]
- 3.Potosky AL, Legler J, Albertsen PC, Stanford JL, Gilliland FD, Hamilton AS, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92(19):1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 4.Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. Jama. 2000;283(3):354–360. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–S19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman RM, Gilliland FD, Penson DF, Stone SN, Hunt WC, Potosky AL. Cross-sectional and longitudinal comparisons of health-related quality of life between patients with prostate carcinoma and matched controls. Cancer. 2004;101(9):2011–2019. doi: 10.1002/cncr.20608. [DOI] [PubMed] [Google Scholar]
- 7.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 8.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276(24):1957–1963. [PubMed] [Google Scholar]
- 9.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91(7):608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 10.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90(6):440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 11.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85(18):1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 12.Fleshner N, Fair WR, Huryk R, Heston WD. Vitamin E inhibits the high-fat diet promoted growth of established human prostate LNCaP tumors in nude mice. J Urol. 1999;161(5):1651–1654. [PubMed] [Google Scholar]
- 13.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60(11):2882–2886. [PubMed] [Google Scholar]
- 14.Jiang C, Wang Z, Ganther H, Lu J. Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001;61(7):3062–3070. [PubMed] [Google Scholar]
- 15.Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev. 2000;9(11):1171–1182. [PubMed] [Google Scholar]
- 16.Redman C, Scott JA, Baines AT, Basye JL, Clark LC, Calley C, et al. Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998;125(1–2):103–110. doi: 10.1016/s0304-3835(97)00497-7. [DOI] [PubMed] [Google Scholar]
- 17.Taylor PR, Albanes D. Selenium, vitamin E, and prostate cancer--ready for prime time? J Natl Cancer Inst. 1998;90(16):1184–1185. doi: 10.1093/jnci/90.16.1184. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizawa K, Willett WC, Morris SJ, Stampfer MJ, Spiegelman D, Rimm EB, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998;90(16):1219–1224. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- 19.Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001;61(19):7071–7078. [PubMed] [Google Scholar]
- 20.Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM, Jr, Kristal AR, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97(2):94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 21.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, Fischbach LA, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11(7):630–639. [PubMed] [Google Scholar]
- 22.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 23.Venkateswaran V, Fleshner NE, Klotz LH. Modulation of cell proliferation and cell cycle regulators by vitamin E in human prostate carcinoma cell lines. J Urol. 2002;168(4 Pt 1):1578–1582. doi: 10.1016/S0022-5347(05)64524-7. [DOI] [PubMed] [Google Scholar]
- 24.Traber MG. How much vitamin E? Just enough! Am J Clin Nutr. 2006;84(5):959–960. doi: 10.1093/ajcn/84.5.959. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein SJ, Wright ME, Pietinen P, King I, Tan C, Taylor PR, et al. Serum alpha-tocopherol and gamma-tocopherol in relation to prostate cancer risk in a prospective study. J Natl Cancer Inst. 2005;97(5):396–399. doi: 10.1093/jnci/dji045. [DOI] [PubMed] [Google Scholar]
- 26.Kirsh VA, Hayes RB, Mayne ST, Chatterjee N, Subar AF, Dixon LB, et al. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. J Natl Cancer Inst. 2006;98(4):245–254. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]
- 27.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S adults. Diabetes Care. 2007;30(4):829–834. doi: 10.2337/dc06-1726. [DOI] [PubMed] [Google Scholar]
- 28.Rajpathak S, Rimm E, Morris JS, Hu F. Toenail selenium and cardiovascular disease in men with diabetes. J Am Coll Nutr. 2005;24(4):250–256. doi: 10.1080/07315724.2005.10719472. [DOI] [PubMed] [Google Scholar]
- 29.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(4):217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 30.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 31.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 32.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. Jama. 2008;300(18):2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee IM, Gaziano JM, Buring JE. Vitamin E in the prevention of prostate cancer: where are we today? J Natl Cancer Inst. 2006;98(4):225–227. doi: 10.1093/jnci/djj066. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Stampfer MJ, Giovannucci EL, Morris JS, Willett WC, Gaziano JM, et al. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst. 2004;96(9):696–703. doi: 10.1093/jnci/djh125. [DOI] [PubMed] [Google Scholar]
- 35.Taylor PR, Parnes HL, Lippman SM. Science peels the onion of selenium effects on prostate carcinogenesis. J Natl Cancer Inst. 2004;96(9):645–647. doi: 10.1093/jnci/djh147. [DOI] [PubMed] [Google Scholar]
- 36.Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: progress and promise. J Natl Cancer Inst. 1998;90(20):1514–1528. doi: 10.1093/jnci/90.20.1514. [DOI] [PubMed] [Google Scholar]
- 37.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 38.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 39.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369(9573):1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 40.Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 41.Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine Plus Sulindac for the Prevention of Sporadic Colorectal Adenomas: A Randomized Placebo-Controlled, Double-Blind Trial. Cancer Prev Res Phila Pa. 2008;1:9–11. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 43.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. Jama. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 44.Scardino PT. The prevention of prostate cancer--the dilemma continues. N Engl J Med. 2003;349(3):297–299. doi: 10.1056/NEJMe038109. [DOI] [PubMed] [Google Scholar]
- 45.Logothetis CJ, Schellhammer PF. High-Grade Prostate Cancer and the Prostate Cancer Prevention Trial. Cancer Prev Res. 2008;1(3):151–152. doi: 10.1158/1940-6207.CAPR-08-0085. [DOI] [PubMed] [Google Scholar]
- 46.Lucia MS, Darke AK, Goodman PJ, La Rosa FG, Parnes HL, Ford LG, et al. Pathologic Characteristics of Cancers Detected in the Prostate Cancer Prevention Trial: Implications for Prostate Cancer Detection and Chemoprevention. Cancer Prev Res. 2008;1(3):167–173. doi: 10.1158/1940-6207.CAPR-08-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucia MS, Epstein JI, Goodman PJ, Darke AK, Reuter VE, Civantos F, et al. Finasteride and high-grade prostate cancer in the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007;99(18):1375–1383. doi: 10.1093/jnci/djm117. [DOI] [PubMed] [Google Scholar]
- 48.Redman MW, Tangen CM, Goodman PJ, Lucia MS, Coltman CA, Jr, Thompson IM. Finasteride Does Not Increase the Risk of High-Grade Prostate Cancer: A Bias-Adjusted Modeling Approach. Cancer Prev Res. 2008;1(3):174–181. doi: 10.1158/1940-6207.CAPR-08-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]