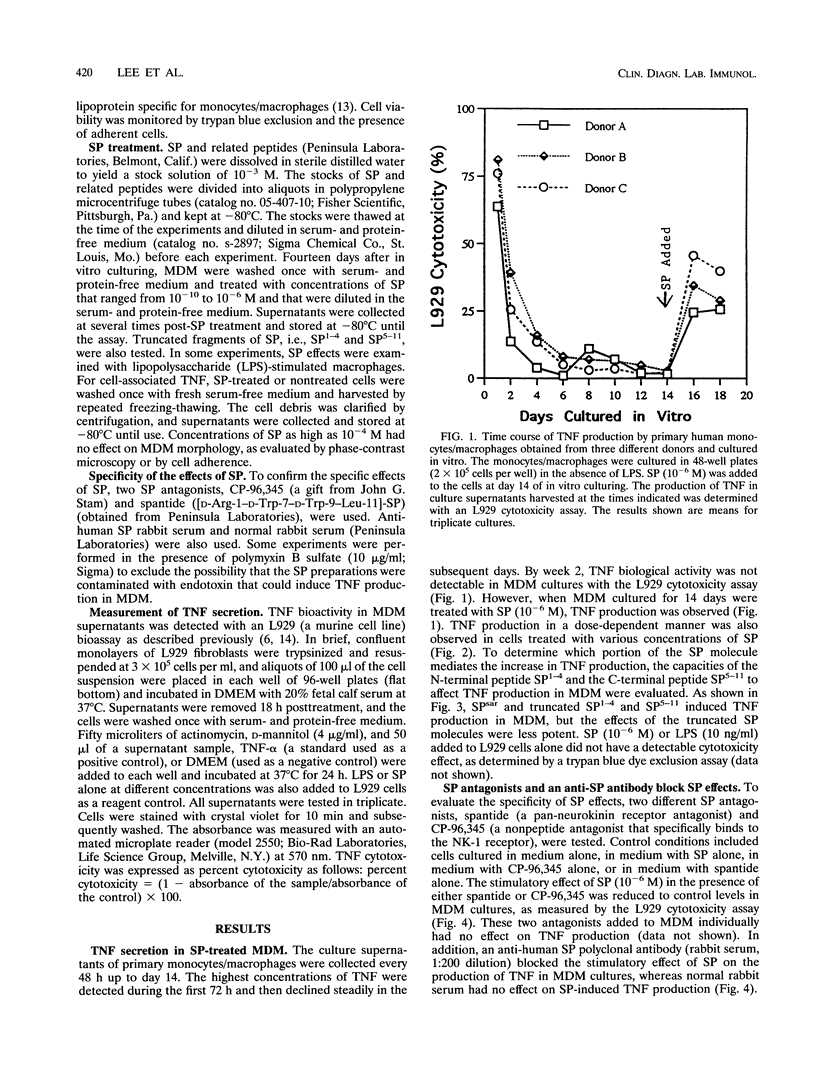
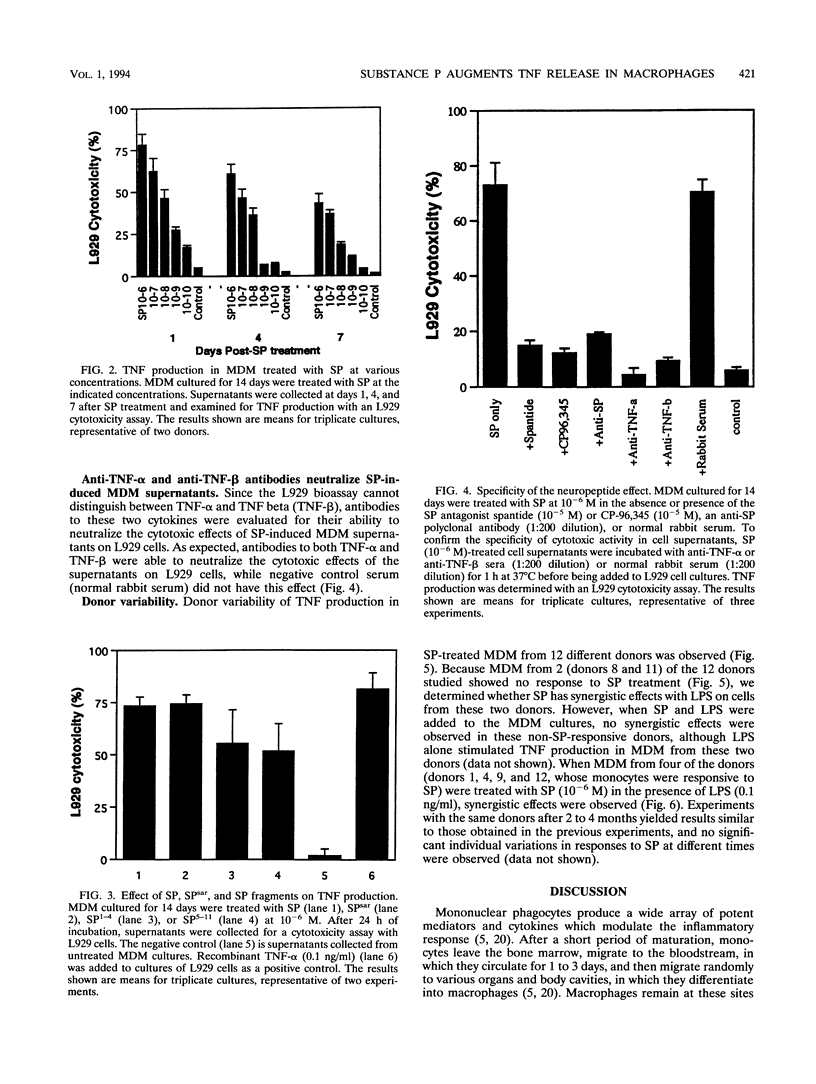
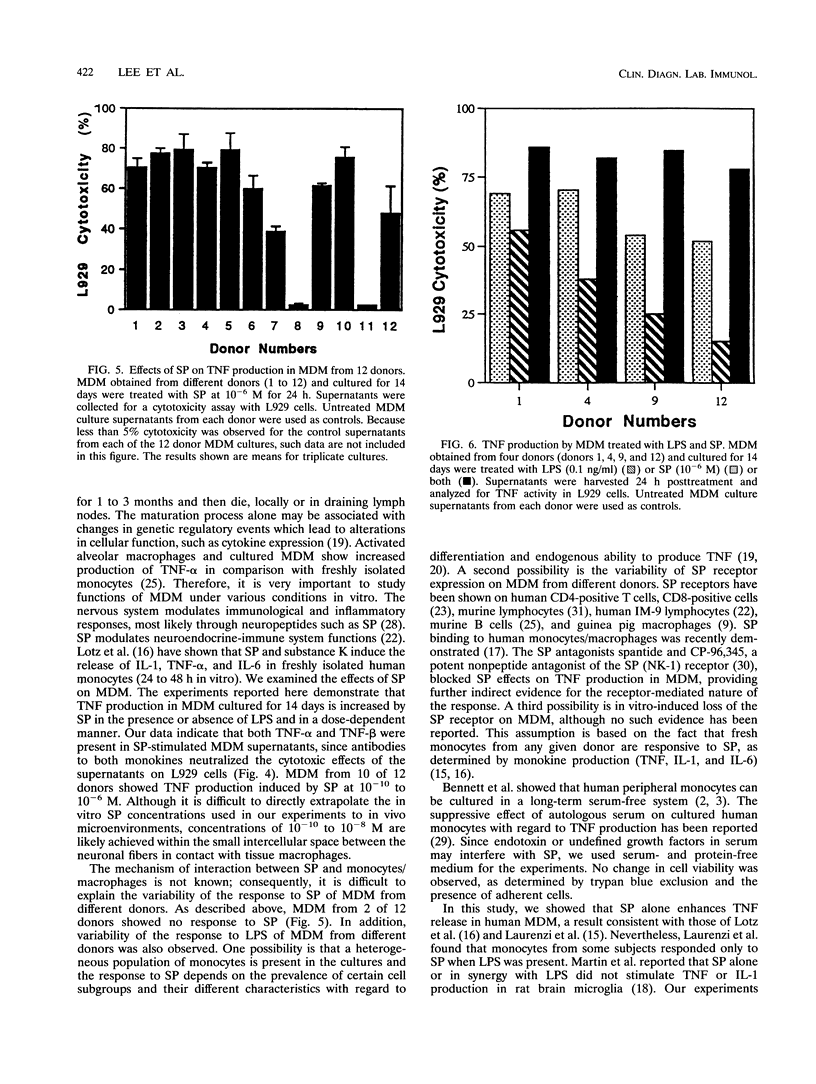
Abstract
Substance P (SP) is an undecapeptide that has the amino sequence Arg-Pro-Lys-Pro-Gin-Gln-Phe-Phe-Gly-Leu-Met-NH2 and that belongs to a family of structurally related peptides known as tachykinins, the latter are widely distributed in the central nervous system. SP is involved in the biological activities of cells in the immune system, including the induction of cytokines in immune cells. We have investigated the effects of SP on constitutive and/or lipopolysaccharide (LPS)-induced expression of tumor necrosis factor (TNF) in cultured blood monocyte-derived macrophages (MDM). Cells cultured in vitro for 14 days were treated with SP at various concentrations (10(-10) to 10(-6M) in the presence of LPS before culture supernatants were harvested. TNF bioactivity in culture supernatants was measured with L929 cell line MDM from 10 of 12 donors treated with a SP alone showed increased TNF production. SP and LPS also interacted in a synergistic fashion in upregulating TNF production in MDM from responders. The stimulatory effect of SP was inhibited by two SP antagonists, spantide ([D-Arg-1-D-Trp-7-D-Trp-7-D-Trp-9-leu-11]-SP) and CP-96,345 (a nonpeptide antagonist of the SP receptor). In addition, an anti SP polyclonal antibody blocked the SP effect on TNF production in cultured MDM, further indicating the specificity of these effects. These results demonstrate that SP is an important regulator of monokine production by human monocytes/macrophages.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett S., Por S. B., Cooley M. A., Breit S. N. In vitro replication dynamics of human culture-derived macrophages in a long term serum-free system. J Immunol. 1993 Mar 15;150(6):2364–2371. [PubMed] [Google Scholar]
- Bennett S., Por S. B., Stanley E. R., Breit S. N. Monocyte proliferation in a cytokine-free, serum-free system. J Immunol Methods. 1992 Aug 30;153(1-2):201–212. doi: 10.1016/0022-1759(92)90323-l. [DOI] [PubMed] [Google Scholar]
- Beutler B. The tumor necrosis factors: cachectin and lymphotoxin. Hosp Pract (Off Ed) 1990 Feb 15;25(2):45–56. doi: 10.1080/21548331.1990.11703910. [DOI] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Heininger K., Schäfer B., Toyka K. V. Substance P and astrocytes: stimulation of the cyclooxygenase pathway of arachidonic acid metabolism. FASEB J. 1988 Jan;2(1):48–51. doi: 10.1096/fasebj.2.1.2446942. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Toyka K. V. Activation of macrophages by substance P: induction of oxidative burst and thromboxane release. Eur J Pharmacol. 1983 May 6;89(3-4):301–305. doi: 10.1016/0014-2999(83)90511-3. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Wolters K., Toyka K. V. Substance P: binding properties and studies on cellular responses in guinea pig macrophages. J Immunol. 1986 May 15;136(10):3856–3863. [PubMed] [Google Scholar]
- Hassan N. F., Campbell D. E., Douglas S. D. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986 Dec 24;95(2):273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Hassan N. F., Cutilli J. R., Douglas S. D. Isolation of highly purified human blood monocytes for in vitro HIV-1 infection studies of monocyte/macrophages. J Immunol Methods. 1990 Jul 3;130(2):283–285. doi: 10.1016/0022-1759(90)90058-4. [DOI] [PubMed] [Google Scholar]
- Ho W. Z., Lioy J., Song L., Cutilli J. R., Polin R. A., Douglas S. D. Infection of cord blood monocyte-derived macrophages with human immunodeficiency virus type 1. J Virol. 1992 Jan;66(1):573–579. doi: 10.1128/jvi.66.1.573-579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenzi M. A., Persson M. A., Dalsgaard C. J., Haegerstrand A. The neuropeptide substance P stimulates production of interleukin 1 in human blood monocytes: activated cells are preferentially influenced by the neuropeptide. Scand J Immunol. 1990 Apr;31(4):529–533. doi: 10.1111/j.1365-3083.1990.tb02801.x. [DOI] [PubMed] [Google Scholar]
- Lotz M., Vaughan J. H., Carson D. A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988 Sep 2;241(4870):1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Lucey D. R., Novak J. M., Polonis V. R., Liu Y., Gartner S. Characterization of substance P binding to human monocytes/macrophages. Clin Diagn Lab Immunol. 1994 May;1(3):330–335. doi: 10.1128/cdli.1.3.330-335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. C., Anton P. A., Gornbein J. A., Shanahan F., Merrill J. E. Production of interleukin-1 by microglia in response to substance P: role for a non-classical NK-1 receptor. J Neuroimmunol. 1993 Jan;42(1):53–60. doi: 10.1016/0165-5728(93)90212-h. [DOI] [PubMed] [Google Scholar]
- Mayernik D. G., Ul-Haq A., Rinehart J. J. Differentiation-associated alteration in human monocyte-macrophage accessory cell function. J Immunol. 1983 May;130(5):2156–2160. [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan D. G., Brewster D. R., Goetzl E. J. Stereospecific receptors for substance P on cultured human IM-9 lymphoblasts. J Immunol. 1984 Dec;133(6):3260–3265. [PubMed] [Google Scholar]
- Payan D. G., Brewster D. R., Missirian-Bastian A., Goetzl E. J. Substance P recognition by a subset of human T lymphocytes. J Clin Invest. 1984 Oct;74(4):1532–1539. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payan D. G., McGillis J. P., Renold F. K., Mitsuhashi M., Goetzl E. J. Neuropeptide modulation of leukocyte function. Ann N Y Acad Sci. 1987;496:182–191. doi: 10.1111/j.1749-6632.1987.tb35764.x. [DOI] [PubMed] [Google Scholar]
- Payan D. G. Neuropeptides and inflammation: the role of substance P. Annu Rev Med. 1989;40:341–352. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- Rich E. A., Panuska J. R., Wallis R. S., Wolf C. B., Leonard M. L., Ellner J. J. Dyscoordinate expression of tumor necrosis factor-alpha by human blood monocytes and alveolar macrophages. Am Rev Respir Dis. 1989 Apr;139(4):1010–1016. doi: 10.1164/ajrccm/139.4.1010. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Immunopathogenesis of HIV infection. FASEB J. 1991 Jul;5(10):2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Wahl S. M., Pert C. B. Substance P receptor-mediated chemotaxis of human monocytes. Peptides. 1985;6 (Suppl 2):107–111. doi: 10.1016/0196-9781(85)90142-1. [DOI] [PubMed] [Google Scholar]
- Scicchitano R., Biennenstock J., Stanisz A. M. In vivo immunomodulation by the neuropeptide substance P. Immunology. 1988 Apr;63(4):733–735. [PMC free article] [PubMed] [Google Scholar]
- Snider R. M., Constantine J. W., Lowe J. A., 3rd, Longo K. P., Lebel W. S., Woody H. A., Drozda S. E., Desai M. C., Vinick F. J., Spencer R. W. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991 Jan 25;251(4992):435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- Stanisz A. M., Scicchitano R., Dazin P., Bienenstock J., Payan D. G. Distribution of substance P receptors on murine spleen and Peyer's patch T and B cells. J Immunol. 1987 Aug 1;139(3):749–754. [PubMed] [Google Scholar]


