Abstract
Lactococcus lactis, a food-grade nonpathogenic lactic acid bacterium, is a good candidate for the production of heterologous proteins of therapeutic interest. We examined host factors that affect secretion of heterologous proteins in L. lactis. Random insertional mutagenesis was performed with L. lactis strain MG1363 carrying a staphylococcal nuclease (Nuc) reporter cassette in its chromosome. This cassette encodes a fusion protein between the signal peptide of the Usp45 lactococcal protein and the mature moiety of a truncated form of Nuc (NucT). The Nuc secretion efficiency (secreted NucT versus total NucT) from this construct is low in L. lactis (∼40%). Twenty mutants affected in NucT production and/or in secretion capacity were selected and identified. In these mutants, several independent insertions mapped in the dltA gene (involved in d-alanine transfer in lipoteichoic acids) and resulted in a NucT secretion defect. Characterization of the dltA mutant phenotype with respect to NucT secretion revealed that it is involved in a late secretion stage by causing mature NucT entrapment at the cell surface.
Lactococcus lactis is widely used in the food industry and is considered a good candidate for production of heterologous proteins for developing nutraceuticals or new live vaccine strategies. Numerous genetic tools have been developed for gene expression and protein secretion in L. lactis (14), and many heterologous proteins have already been produced in L. lactis, including bacterial and viral antigens (3, 39, 48) and enzymes and cytokines (2, 43). Recently, efficient food-grade production systems were developed in L. lactis (19, 42). However, so far there have been few analyses of lactococcal host factors involved in heterologous protein production and secretion machinery.
Factors that affect secretion efficiency (secreted NucT versus total NucT) include elements of the secretion machinery itself, as well as factors involved in protein stability or degradation and folding. Furthermore, conditions that alter the microenvironment at the cell surface may affect protein folding and release into the culture medium (29, 45). For the L. lactis secretion machinery, knowledge of the lactococcal genetic apparatus required for secretion is essentially limited to genome sequence information derived from L. lactis strain IL1403 (7). Several sec genes were revealed by sequence homology (7). Other putative and/or unidentified lactococcal genes may also be involved in the secretion process. It is notable that SecDF, which is involved in the late steps of translocation, is absent in L. lactis. Also, L. lactis has only a single signal peptidase for nonlipoproteins, compared to six such enzymes for the grampositive paradigm organism Bacillus subtilis (46). The unique signal peptidase and the absence of SecDF are potential bottlenecks when an L. lactis strain is engineered for high-level secretion. L. lactis encodes a unique cell surface protease, HtrA. An htrA mutant stabilizes production of several heterologous exported proteins or fusion proteins in L. lactis (31, 36).
We developed a novel mutagenesis screening procedure based on levels of secretion of the staphylococcal nuclease (Nuc) (41) to identify accessory genes involved in secretion. Nuc has been used as a secretion reporter in L. lactis and other lactic acid bacteria (15, 26–28). The strain developed for this mutagenesis procedure carries a single copy of the stable expression cassette integrated in the chromosome. This work is the first random insertional mutagenesis analysis reported in which accessory genes involved in the production and secretion of heterologous proteins in L. lactis were identified. In the mutants isolated by this novel screening method, several independent insertions were mapped to the dltA gene and resulted in a NucT secretion defect. Characterization of dltA inactivation demonstrated that cell wall modifications affect cell viability and late-stage protein secretion.
MATERIALS AND METHODS
Strains, plasmids, media, and growth conditions
L. lactis subsp. cremoris MG1363 (17) and Escherichia coli TG1 (18) [supE hsd-5 thi Δ(lac-proAB) F′(traD6 proAB+ lacIq lacZΔM15)] and TG1rep (containing a chromosomal copy of the pWV01 repA gene; kindly provided by K. Leenhouts) were used as bacterial hosts. The plasmids used are listed in Table 1. E. coli was grown on Luria-Bertani medium (40) and incubated at 37°C with vigorous shaking. L. lactis was grown in M17 medium (44) containing 0.5% glucose (GM17) or on brain heart infusion (Difco) when Nuc detection was required. Most L. lactis cultures were incubated at 30°C; the only exceptions were cultures used during pGhost9:ISS1-mediated mutagenesis (see below). Antibiotics were added at the following concentrations: erythromycin, 150 μg/ml for E. coli 5 and 2.5 μg/ml for plasmid and chromosome-encoded resistance in L. lactis, respectively; ampicillin, 100 μg/ml for E. coli; and chloramphenicol, 5 μg/ml for L. lactis. Induction with nisin (Sigma) was performed at a concentration of 1 ng/ml for 1 h with cultures at an optical density at 600 nm (OD600) of ∼0.5.
TABLE 1.
Plasmids used
| Plasmid (replicon) | Characteristics | Reference |
|---|---|---|
| pBS:UNuc4 (ColE1) | Apr, gene expressed from Pusp encodes SPUsp:NucTprecursor | 28 |
| pBS:his (ColE1) | Apr, fragment of MG1363 his operon | 12, 13 |
| pBS:his:uspnucT:his (ColE1) | Apr, contains Pusp-SPUsp:nucT in the his operon | This study |
| pGHost9 (pWV01, repTs) | Emr, nonreplicative in L. lactis at 37°C | 30 |
| pGHost9:ISSI (pWV01, repTs) | Emr, lactococcal ISSl, nonreplicative in L. lactis at 37°C | 30 |
| pGHost9:his:uspnucT:his (pWV01, repTs) | Emr, contains Pusp-SPUsp:nucT in the his operon | This study |
| pSEC:NucT (pWV01) | Cmr, contains Pnis-SPUsp:nucT | 28 |
| pIL:nisRK (pAMβ1) | Emr, contains nisRK nisin regulatory genes | 23 |
DNA manipulations.
The general procedures for DNA manipulation were performed as described previously (40), with the following modifications. Chromosomal DNA extraction from L. lactis involved addition of lysozyme (10 mg/ml) to prepare protoplasts and then addition of proteinase K (0.8 mg/ml) to eliminate mature Nuc forms associated with protoplasts prior to cell lysis. For plasmid DNA extraction (4) from L. lactis, TES (25% sucrose, 1 mM EDTA, 50 mM Tris-HCl [pH 8]) containing lysozyme (10 mg/ml) was added for 10 min at 37°C to prepare protoplasts. Enzymes were used as recommended by the suppliers. Electroporation of L. lactis was performed as described previously (25), and transformants were plated on GM17 agar plates containing the required antibiotic.
Preparation of protein extracts, Western blotting, and immunodetection.
Cellular and supernatant protein fractions of L. lactis were prepared and concentrated as described previously (27). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroblotting onto polyvinylidene difluoride membranes (Millipore), and immunoblotting were performed as described previously (40) or according to the suppliers' recommendations. Antibodies against Nuc, raised in rabbits (Eurogentec), were used at a dilution of 1:1,000. Immunodetection was performed with protein G-horseradish peroxidase conjugate (Bio-Rad) and a chemiluminescence kit (Dupont-NEN) used as recommended by suppliers. After enhanced chemiluminescence detection, different nonsaturated film exposures were scanned (Scanjet 7400C; Hewlett-Packard). Amounts of mature NucT and preNucT were estimated with the ImageQuant program. For liberation of cell-associated NucT, 2-ml portions of overnight cultures were pelleted, and the cells were washed with TES and incubated for 15 min on ice with 10% trichloroacetic acid in order to stop cellular metabolism instantaneously. (Note that milder treatments, such as the use of glucose analogs, were not rapid enough to block metabolism and the secretion process.) The cells were then washed with TES, treated or not treated with 10 mg of lysozyme per ml (5 min at 37°C) for partial digestion of the cell wall, and washed with TES. Cell wall-associated Nuc was released by incubation in 1 ml of glycine-NaOH buffer (pH 9 or 10) (wash buffer) for 15 min at room temperature with gentle shaking. The proteins present in the cell and wash buffer were treated as described previously (27). The nisin MIC (i.e., the lowest nisin concentration that inhibited bacterial growth) was determined as follows. An overnight culture was diluted 200-fold in fresh GM17 and incubated for 90 min at 30°C, and different amounts of nisin (ranging from 10 to 100 ng/ml) were added. The OD600 of overnight cultures were then determined. Preparation of protein samples and adjustment of the concentration to an OD600 of 1 were performed as described previously (27).
Construction of the L. lactis strain used for random insertional mutagenesis.
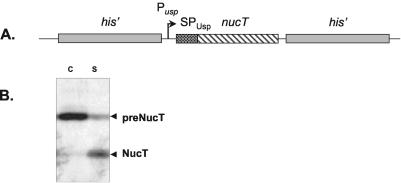
To screen for L. lactis mutants affected in Nuc secretion, we constructed an MG1363 derivative strain carrying a nuc cassette on the chromosome. It has been reported previously that native Nuc is efficiently secreted via the signal peptide of the lactococcal protein Usp45 (SPUsp45) (28). In contrast, deletion of 17 of the 21 amino acids of the Nuc propeptide (referred to as NucT) results in impaired secretion. For this construct, the majority of the precursor remains cell associated (28). The strategy used to obtain the L. lactis strain used for mutagenesis is as follows. The uspnucT cassette (containing the usp45 promoter [Pusp] and SPUsp45) was isolated on a BamHI/EcoRI fragment from pBS:UNuc4 (28) (Table 1). This cassette was blunted and cloned into SnaBI-linearized pBS:his (13), resulting in pBS:his:uspnucT:his (with his and uspnucT in the same orientation). In this plasmid, the uspnucT cassette is flanked by a 1.2-kb fragment (5′ end) and a 1.1-kb fragment (3′ end) of the inactive histidine biosynthesis (his) operon (12) (Fig. 1A). The his:uspnucT:his cassette was then isolated on an EcoRI fragment, cloned into EcoRI-digested pGhost9 (30), and established in E. coli TG1rep. The resulting plasmid, pGhost9:his:uspnucT:his (Nuc+ Emr), was then established in wild-type L. lactis subsp. cremoris MG1363. In spite of the lack of a complete genome sequence for MG1363, we used this strain instead of L. lactis subsp. lactis IL1403, which contains six prophages inducible by various stresses (9a). Furthermore, A. Bolotin confirmed that the genes which we studied were quite similar in these two L. lactis model strains (unpublished data). Stable integration of the uspnucT expression cassette by a double-crossover recombination event into the L. lactis chromosome was performed as described previously (5). The resulting strain, MG[uspnucT], was verified by Southern blotting by using a 32P radiolabel (Ready-to-Go kit; Pharmacia Biochemicals) and a DNA fragment encoding uspnucT as the probe. Growth was not affected by the uspnucT integration (data not shown). NucT expressed from the chromosome had a secretion efficiency (as evaluated by Western blot analysis) comparable to the secretion efficiency when NucT was expressed from a plasmid (28). NucT was poorly secreted from MG[uspnucT]; the majority of SPUsp45:NucT was found in the precursor form in the cell fraction (Fig. 1B).
FIG. 1.
Structure and secretion phenotype of the L. lactis strain carrying the uspnucT expression cassette. (A) nucT was fused to the Usp45 promoter (Pusp) and the signal peptide (SPUsp). The uspnucT expression cassette was integrated by double crossover in the middle of the his operon in MG1363. (B) Secretion profile for NucT in MG[uspnucT] as analyzed by Western blotting with Nuc-specific antibodies. Protein samples in cell (lane C) and supernatant (lane S) fractions were prepared from an overnight culture of MG[uspnucT]. The majority of NucT remained in the cell fraction in the precursor form (preNucT), whereas only about 40% was processed to the mature form (NucT) and released into the culture medium.
Mutagenesis and screening conditions.
Plasmid pGhost9:ISS1 was introduced into MG1363[uspnucT]. Mutagenesis with pGhost9:ISS1 was performed essentially as described previously (30). Cells were plated in order to obtain about 300 CFU/petri plate. The plates were overlaid with TBD agar to reveal halos caused by Nuc enzymatic activity (26). From the colonies screened, mutants having significantly larger or smaller halos were selected for further examination.
Determination of the pGhost9:ISS1 integration loci in MG[uspnucT] and pGhost9:ISS1 excision procedure.
Plasmid pGhost9:ISS1 contains unique sites adjacent to the ISS1 element (EcoRI and HindIII) that were used after transposition to clone chromosomal DNA flanking the pGhost9:ISS1 insertion site (referred to below as junctions) (30). Chromosomal junctions were sequenced (with an ABI Prism 310 genetic analyzer) by using primers present on the ISS1 element and directed towards the cloned chromosomal junction (pEco for the EcoRI junction [5′-ATAGTTCATTGATATATCCTCGCT]; pHind for the HindIII junction [5′-TCGGTATCTACTGAGATTAAGGTC]). Genes that were mutated in L. lactis subsp. cremoris MG1363 were identified by comparison of the sequenced junction with the closely related L. lactis subsp. lactis IL-1403 genome sequence (7; http://spock.jouy.inra.fr). The number of pGhost9:ISS1 integrations into the chromosome was determined by Southern blotting by using a 32P-radiolabeled ISS1 fragment as a probe.
Excision of pGhost9:ISS1 from the initial integration locus resulted in a stable ISS1 insertional mutant that grew at 30°C without selective pressure (30). Stable erythromycin-sensitive excision derivatives of the candidate secretion mutants were first tested to determine their Nuc phenotypes by depositing 5-μl portions of culture supernatants on TBD agar medium to detect Nuc activity. They were confirmed by Western blot analysis and Nuc immunodetection.
RESULTS
Mutagenesis and identification of transposition loci.
Random mutagenesis was performed on the MG[uspnucT] strain by using plasmid pGhost9:ISS1. Colonies were screened by the Nuc plate assay for increased or decreased amounts of secreted NucT. Of 35,000 mutant clones, 200 were selected after the first screening step. As the halo size for Nuc activity may vary widely with the colony size, mutant colonies were patched onto brain heart infusion agar medium and subjected to a second screening step. After this step, 34 of the 200 initial mutants exhibited a halo size significantly different from that of the parent, strain MG[uspnucT]. These 34 mutants were analyzed further by Western blotting and Nuc immunodetection by using overnight cultures, and the secretion profiles were compared to that of MG[uspnucT]; 20 mutants exhibited significantly different NucT secretion patterns. Junctions of these mutants were cloned, and sequences of the interrupted genes were determined by sequence comparison with the IL1403 genome sequence (7). The secretion phenotypes (as determined by Western analysis) and the genes identified by sequence similarity are listed in Table 2. For the parental strain used as a control, an index value of 1 was assigned for the amounts of preNucT and mature NucT. For each mutant, the amounts of preNucT and mature NucT were estimated as described in Materials and Methods and compared to the amounts obtained for the parental strain (Table 2). Furthermore, the secretion efficiency of NucT (secreted NucT/total Nuc forms detected) was determined. In addition, the growth rates of all 20 mutants were determined (Table 2). For six mutants, no sequence similarity with the reference IL1403 genome sequence or with the public databases was found. Eight integrations occurred in putative open reading frames having unknown functions, and three independent integrations occurred in ybdD; the ybdD mutants contained increased amounts of the precursor preNucT and of NucT (S. Nouaille, E. Morello, Y. Le Loir, J. Commissaire, A. Bolotin, A. Gruss, and P. Langella, unpublished data). Four mutations occurred in genes encoding putative components of the phosphotransferase systems in E. coli; three of the mutations were in the mannose-specific phosphotransferase system, and one was in the cellobiose-specific phosphotransferase system. These four mutations led to slightly improved secretion efficiency, which may have been due to changes in metabolism rather than to direct involvement of the genes in the secretion process. Mutant 35 produced increased amounts of both preNucT and NucT and resulted from double integration of pGhost9:ISS1; one of the insertions was in a gene similar to rgrA encoding the repressor of the trehalose operon in B. subtilis. The other integration locus was not identified. Two mutants (mutants 3 and 26) were affected in genes involved in nucleic acid metabolism. The effect of an ssbA mutation (mutant 3) on NucT secretion might have been indirect and may have resulted from a general defect in DNA metabolism. The effect of a vacB2 mutation (mutant 26) on NucT may have been linked to an increased half-life of mRNAs (for nucT or other genes involved in the secretion process) in the cell. Five independent pGhost9:ISS1 integrations mapped in the dltA gene, the first gene of the dlt operon, which comprises four genes (dltA, dltB, dltC, and dltD) that catalyze the incorporation of d-alanine residues into lipoteichoic acids (LTA) (see below) involved in the net global charge of the bacterial surface. In this work, we focused on analysis of the influence of the dlt operon on protein secretion in L. lactis. The growth curves, generation times, and final pH and biomass values for the three dltA single-insertion mutants were the same as those for the parental strain (data not shown).
TABLE 2.
Genes identified by sequence similarity and secretion phenotype
| Mutant | Genea | Gene functionb | preNucTc | NucTc | Secretion efficiencyf | Growth rate (h−1) |
|---|---|---|---|---|---|---|
| VEL11461 | uspnucT | Staphylococcal nuclease reporter gene | 1 | 1 | 30 | 2.5 |
| 2d | dltA | LTA d-alanylation | 1 | 0.6 | 26 | 2.4 |
| 5e | dltAe | LTA d-alanylation | 1 | 0.7 | 30 | 2 |
| 7 | dltA | LTA d-alanylation | 1 | 0.7 | 25 | 1.8 |
| 12 | dltA | LTA d-alanylation | 1 | 0.6 | 18 | 1.8 |
| 17d | dltA | LTA d-alanylation | 1 | 1.1 | 34 | 2.3 |
| 15 | celB | Cellobiose-specific PTS system component IIC | 0.4 | 1.2 | 58 | 2 |
| 19 | ptnC | Mannose-specific PTS system component IIC | 0.6 | 1.7 | 54 | 2.1 |
| 18 | ptnD | Mannose-specific PTS system component IID | 0.6 | 1.3 | 54 | 2.1 |
| 25 | ptnD | Mannose-specific PTS system component IID | 0.9 | 1.8 | 44 | 1.7 |
| 35d | rgrA | Trehalose operon repressor | 1.4 | 1.6 | 36 | 2.2 |
| 3 | ssbA | DNA recombination and repair | 1.2 | 0.7 | 24 | 1.8 |
| 26 | vacB2 | RNase | 0.7 | 2.5 | 58 | 1.5 |
| 8 | ybdD | Unknown | 1.3 | 2.7 | 44 | 2.7 |
| 27 | ybdD | Unknown | 1.2 | 2.6 | 47 | 2.3 |
| 32 | ybdD | Unknown | 1.2 | 2.4 | 46 | 2.5 |
| 33 | yccF | Unknown | 1.3 | 0.4 | 43 | 2.5 |
| 16 | ypcH | Unknown | 0.5 | 1.5 | 61 | 2.4 |
| 11 | yqjB | Unknown | 1 | 1.6 | 44 | 2.1 |
| 24 | ythA | Unknown | 1.2 | 1.3 | 29 | 2.2 |
| 31 | yuaE | Unknown | 0.4 | 1.1 | 61 | 1.7 |
Gene in which pGhost9:ISS1 integration occurred.
Gene function as determined by sequence similarity (7).
The phenotype of each mutant was confirmed by Western blot analysis by determining the amount of the precursor form detected inside the cells or the amount of the mature NucT form secreted into the culture medium in comparison with the MG [uspnucT] secretion profile. The index 1 was assigned to preNucT and NucT productions in VEL11461.
Two pGhost9:ISS1 plasmids were integrated into the chromosome.
pGhost9:ISS1 integration occurred in the thiE-dltA intergenic region.
Secretion efficiency, secreted NucT versus total NucT detected.
These results show that the screening procedure in which the NucT reporter was used was effective for identification of 20 putative secretion mutants in L. lactis.
Effects of dltA disruption on NucT secretion.
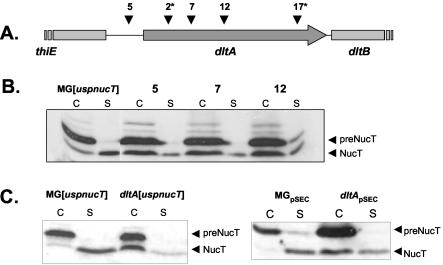
The dlt operon is required for d-alanylation of LTA in various gram-positive bacteria (1, 8, 10, 32, 34, 35). One of the five dltA insertions (Fig. 2A) occurred just upstream of the dltA start codon, and four occurred in the dltA coding sequence. In the latter cases, two mutants experienced double integration events; the second integration loci were not identified. However, the Nuc phenotype of these mutants was similar to that of the dltA single mutants, suggesting that a dltA mutation is responsible for the secretion phenotype. Only the three mutants with a single integration event in dltA (mutants 2, 7, and 12) were analyzed further. All of these mutants were impaired for NucT secretion, as determined by the Nuc plate assay, suggesting that the dlt operon is relevant in secretion. NucT secretion in these mutants was analyzed by Western blotting by using anti-Nuc antibodies and was compared to NucT secretion in the MG[uspnucT] parental strain (Fig. 2B). All three mutants displayed similar NucT secretion profiles: (i) the amounts of cell-associated preNucT in the cell fraction were the same as the amount in MG[uspnucT]; (ii) the amounts of the secreted mature form in the supernatant were reduced, consistent with observations made with the Nuc plate assay; and (iii) strikingly, the major part of mature NucT was cell associated (Fig. 2B). As the dltA mutant phenotypes were similar, we selected dltA mutant 7, containing the most 5′-proximal pGhost9:ISS1 insertion, for further characterization. The pGhost9:ISS1 excision mutant, dltA[uspnucT], exhibited the same phenotype as the initial mutant 7 (data not shown). Comparison of Nuc secretion in dltA[uspnucT] and MG[uspnucT] at 30°C without erythromycin showed that the initial Nuc phenotype was due neither to high temperature (37.5°C) nor to erythromycin selection (Fig. 2C, left panel).
FIG. 2.
dltA mutations and phenotypes of the L. lactis mutants. (A) Positions of ISS1 insertions in the five L. lactis dltA mutants. Four independent pGhost9:ISS1 insertions occurred in the coding sequence of dltA at nucleotide positions 369 (mutant 2), 450 (mutant 7), 649 (mutant 12), and 1215 (mutant 17) with respect to the ATG start codon. One integration event occurred 141 nucleotides upstream of the dltA ATG start codon (mutant 5). An asterisk indicates a mutant in which another pGhost9:ISS1 integration occurred. (B) Comparison of Nuc secretion profiles in dltA and MG[uspnucT] strains. At 37.5°C, the three single dltA mutants exhibited the same Nuc phenotype. There was no difference in the amounts of preNucT observed in the mutants and the parental strain. A reduced amount of NucT was detected in the culture medium of all mutants, whereas the majority of NucT was detected in the cell fraction. (C, left panel) NucT secretion profile of dltA[uspnucT] was conserved when the organism was grown at 30°C without erythromycin. (C, right panel) Secretion phenotype of L. lactis dltA[uspnucT] overproducing NucT. NucT secretion was analyzed by Western blotting by using Nuc-specific antibodies after 1 h of nisin induction of MG[uspnucT](pSEC:NucT/pIL:nisRK) and dltA[uspnucT](pSEC:NucT/pIL:nisRK). Overexpression of NucT did not alter the secretion profile in the parental strain or in the dltA mutant. C, cell fraction; S, supernatant fraction; MGpSEC, MG[uspnucT](pSEC:NucT/pIL:nisRK); dltApSEC, dltA[uspnucT](pSEC:NucT/pIL:nisRK).
In dltA[uspnucT], the majority of mature NucT was cell associated. This phenotype differs from the phenotype of the parent, MG[uspnucT], in which all of the mature NucT was efficiently released into the supernatant (Fig. 2C). These results show that ISS1 integration in dltA led to modification of the distribution of mature NucT between the cell and supernatant fractions. In the case of dltA, the maturation efficiency was identical to that of the parental strain, but the sensu stricto secretion efficiency was lower because mature NucT was retained at the cell surface instead of being secreted into the culture medium.
Effects of the dltA mutation on growth, survival, and nisin sensitivity.
dltA inactivation affects the charge balance of LTA at the cell surface. It reportedly influences viability, cell shape, autolytic enzyme concentration within the cell envelope, and sensitivity to cationic antimicrobial peptides, as previously reported for other gram-positive species, such as B. subtilis, Streptococcus gordonii, Streptococcus agalactiae, Staphylococcus aureus, and Listeria monocytogenes (1, 10, 35, 38, 47). Light microscopy of the MG[uspnucT] and dltA[uspnucT] strains did not reveal differences in morphology or chain length in exponential- or stationary-phase cells (data not shown). A zymogram analysis performed with supernatant and cell fractions did not reveal any differences in quantity, localization, or processing of the major autolysin AcmA (9) between the two strains, in accordance with the similar chain lengths observed (data not shown).
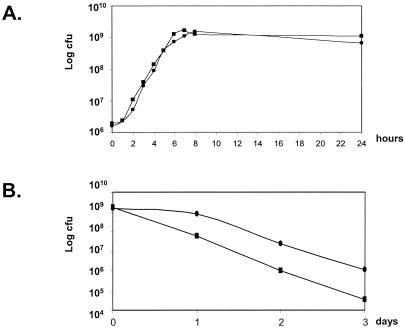
The growth rates and long-term survival of the MG[uspnucT]and dltA[uspnucT] strains were determined in GM17 at 30°C by plate counting. No significant difference was observed between MG[uspnucT] growth and dltA[uspnucT] growth (Fig. 3A). Long-term survival was estimated on days 1, 2, and 3 after cultures reached the stationary growth phase (Fig. 3B). The dltA[uspnucT] viability in stationary-phase cultures was reduced compared to that of MG[uspnucT]. After 3 days, there was a 35-fold difference in the survival of the two strains (1.15 × 106 CFU ml−1for MG[uspnucT] versus 3.3 × 104 CFU ml−1for dltA[uspnucT]). This loss of viability is less drastic than that reported for other gram-positive dltA mutants (47). As LTA is more negatively charged in a dltA mutant than in a wild-type strain, dltA mutants are expected to be more sensitive to cationic antimicrobial peptides (such as nisin) present in the medium. To test this, the MIC of nisin was determined for both strains and was found to be 60 ng/ml for MG[uspnucT] and 35 ng/ml for dltA[uspnucT]. The difference between the nisin MIC for the dltA mutant of L. lactis and the wild type is similar to the difference that was recently observed for a dltA mutant of S. agalactiae and the wild type (38). The nisin MIC were evaluated for dltA mutants of several other gram-positive species, and the values were 2- to 4,000-fold less than the values for the wild-type strains (1, 35, 38). These results may indicate that other charged moieties are present on the cell envelope, which compensate for the negative charges associated with the dltA mutation.
FIG. 3.
(A) Growth of L. lactis MG[uspnucT] and dltA[uspnucT]. MG[uspnucT] (•) and dltA[uspnucT] (▪) were grown in GM17. Viable cell counts (CFU per milliliter) were determined by plating on GM17 agar. (B) Long-term survival of L. lactis MG[uspnucT] and dltA[uspnucT]. On days 1, 2, and 3 after cultures reached the stationary growth phase, viable cell counts (CFU per milliliter) were determined by plating on GM17 agar.
Altogether, these results show that the L. lactis dltA mutant is not affected in growth but is slightly affected in long-term survival and in sensitivity to nisin compared to the wild-type strain.
Effect of the dltA mutation when Nuc is overproduced.
Nuc secretion phenotypes described above were obtained for a strain expressing a single chromosomal copy of uspnucT. Overexpression of NucT was achieved by introducing pSEC:NucT into dltA[uspnucT] (28); in this construct, uspnucT expression is under control of the nisin-inducible PnisA promoter and is regulated by nisRK expressed from plasmid pIL:nisRK (23). MG[uspnucT] containing the same two plasmids was used as a control. The NucT distribution in the parental MG[uspnucT] strain was similar regardless of overproduction of uspnucT. Similarly, when there was high-level NucT expression in the dltA background, the NucT mature form remained mainly associated with the cell fraction (Fig. 2C, right panel). This result showed that high-level NucT production did not alter the distribution of mature NucT in dltA[uspnucT], and the majority of the mature form was cell associated.
NucT is trapped in the peptidoglycan of the dltA mutant by electrostatic interactions and a peptidoglycan network.
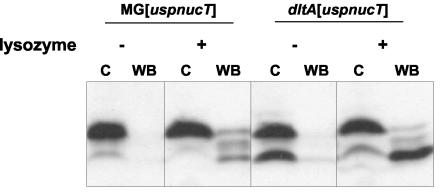
Previous work with gram-positive bacteria demonstrated that the absence of d-alanylation of LTA leads to a negatively charged cell surface (11, 35). The NucT isoelectric point is 9.48, while the pH of MG[uspnucT] overnight GM17 culture medium is around 5.7. Since at this pH NucT has a global positive net charge, there were electrostatic interactions between the positively charged NucT and the negatively charged LTA of dltA[uspnucT], as expected. This could explain the accumulation of the NucT mature form in the dltA[uspnucT] cell fraction. We tried to release NucT from the cell surface by washing the cell pellets with buffers containing high Ca2+ concentrations in order to displace the charge equilibrium by charge competition between Ca2+ ions and the positively charged NucT interacting with the modified cell wall. This treatment did not result in any release of mature NucT from the surface of the mutant, suggesting that it was not able to disrupt established interactions between NucT and the cell surface (data not shown). We then modified the net charge of NucT itself. Cells of MG[uspnucT] and dltA[uspnucT] were harvested after overnight growth and washed with buffers at pH 9 (less than the isoelectric point of NucT) and pH 10 (more than the isoelectric point of NucT). In the pH 10 buffer, NucT was negatively charged and was expected to be associated with the cell surface in MG[uspnucT], whereas it was released from the cell surface of dltA[uspnucT]. In the pH 9 buffer, NucT was expected to remain associated. Neither pH 9 treatment nor pH 10 treatment resulted in release of mature NucT from the cell fraction (data not shown). This suggests that electrostatic interactions are not solely responsible for the dltA phenotype and/or that the peptidoglycan network is somehow involved in NucT association with the cell. To examine these possibilities, cells were treated with lysozyme (10 mg/ml, 5 min, 37°C) to disrupt the peptidoglycan network prior to washes at pH 9 or 10. The combination of lysozyme treatment and washing at pH 9 did not lead to release of NucT from the cell fraction (data not shown). In contrast, the combination of lysozyme treatment and washing at pH 10 resulted in clear and efficient release of NucT into the wash buffer (Fig. 4). These results show that (i) NucT found in the cell fraction matures correctly but remains in the peptidoglycan and (ii) NucT-cell association involves both electrostatic interactions and physical entrapment in the peptidoglycan network.
FIG. 4.
Release of cell-associated mature NucT from L. lactis. Cells of MG[uspnucT] and dltA[uspnucT] overnight cultures were harvested, treated with lysozyme (+) or not treated (−), and washed in pH 10 wash buffer. Protein samples prepared from washed cells (lanes C) and wash buffer (lanes WB) were analyzed by Western blotting by using Nuc-specific antibodies.
DISCUSSION
In the present study, we developed a system to identify new host factors involved in production and secretion of heterologous proteins in L. lactis. To our knowledge, previous use of random mutagenesis to examine secretion in gram-positive bacteria was limited to two studies, both with B. subtilis (22, 24). In those studies, mutagenesis was performed with chemical mutagenic agents and a plasmid-borne reporter protein. This strategy allowed isolation of secretory mutants, but identification of the mutation was difficult, as the secretion phenotypes might have resulted from combinations of mutations affecting either the secretion machinery or the plasmid. To circumvent these problems, in our system we combined the pGhost9:ISS1 plasmid designed for random insertional mutagenesis (30) and the MG[uspnucT] strain, in which the NucT secretion reporter is expressed chromosomally. The use of a stably integrated expression cassette avoided selection of false positives related to plasmid maintenance. In contrast to chemically induced mutations, the pGhost9:ISS1 insertion locus can be directly identified.
Screening of 35,000 clones revealed 20 confirmed integration mutants that were affected in NucT secretion. As ISS1 insertion is reportedly random (30), the number of mutants screened theoretically corresponds to complete coverage of the L. lactis genome (∼2.37 Mbp [7]). In theory, pGhost9:ISS1 integration results in gene inactivation. Consequently, interruption of secretion genes, such as secY, secE, or secA, which are essential for cell viability in E. coli and B. subtilis, was not expected. Similarly, mutations that severely affect cell growth or that produce a growth phase-dependent phenotype might not be selected. However, screening did not reveal any Nuc− mutant resulting from inactivation of the uspnucT cassette, suggesting that pGhost9:ISS1 insertional transposition is not totally random. A similar mutagenesis coupled to the use of a robot to screen a higher number of secretion mutants will be performed. Among the 20 mutants identified, we focused on characterization of dltA and ybdD mutants (Nouaille et al., unpublished), which were inactivated several times independently. Among the other mutants, hypersecreting mutant 26, inactivated in vacB2, which encodes one of the five putative RNases detected in the L. lactis genome (7), is a promising candidate, and further characterization is in progress. Targeted RNase inactivation could be a way to increase heterologous protein production and secretion in this organism. Six of the remaining mutated genes lack homology with known genes.
Five independent insertions occurred in dltA, the first gene of the dltABCD operon, which is required for d-alanylation of LTA, showing its involvement in the NucT secretion process. Mutations in the dlt operon have been studied in other gram-positive bacteria, including S. aureus (20, 35), B. subtilis (21, 47), L. monocytogenes (1), Lactobacillus casei (33), S. gordonii (10), S. agalactiae (37, 38), and Streptococcus mutans (8). In all cases, dlt inactivation totally eliminates d-alanylation of LTA and the cell surface acquires negative charges which might affect the release of proteins with surface-exposed positively charges residues into the growth medium (6). Other reported phenotypes associated with dlt mutations are different in different species. In general, changes in cell morphology and septation and increased sensitivity to cationic antimicrobial peptides were observed. However, in L. lactis, the dltA mutation had no detectable effect on chain length and cell morphology. In previous work the researchers reported a modification in chain length and a lower growth rate in L. lactis dltD mutants (16). As DltD is involved in the last step of d-alanylation, the accumulation of intermediate products could be more deleterious than the complete operon impairment that results from dltA inactivation. The less pronounced phenotypes resulting from the dltA mutation in L. lactis than from the mutations observed in other gram-positive bacteria could reflect differences in cell wall composition.
NucT produced in the dltA mutant was found to be predominantly associated with the cell fraction. We believe that the altered distribution of NucT (which has a net positive charge) in the dltA context is likely due to electrostatic interactions with negatively charged LTA, leading to entrapment in the peptidoglycan. In B. subtilis, mutations in dlt genes result in stabilization of recombinant anthrax protective antigen rPA, a highly labile protein (45). Thwaite et al. suggested that anionic polymers in a dlt mutant provide a microenvironment that favors rPA folding. Our results for L. lactis show that the anionic LTA present in the dltA mutant interact with and accumulate positively charged secreted NucT. The L. lactis dltA mutant is now being tested to determine its ability to produce heterologous proteins prone to misfolding and degradation in a wild-type context. Thus, despite its isolation as a down-secretion mutant, the L. lactis dltA mutant could be a useful host for secretion of labile heterologous proteins that are concentrated in the peptidoglycan fraction.
Acknowledgments
We are grateful to Pascal Hols, Vincent Juillard, Saulius Kulakauskas, and Isabelle Poquet for helpful discussions during the course of this work. We thank Romain Briandet for the welcome in his laboratory, and Elodie Poumerol, Tatiana Rochat, and Eric Morello for technical assistance.
S.N. is a recipient of a MENRT grant from the French government. We acknowledge funding from the French Embassy in Denmark that enabled part of this work to be performed.
REFERENCES
- 1.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of d-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez-Humaran, L. G., P. Langella, N. G. Cortes-Perez, A. Gruss, R. S. Tamez-Guerra, S. C. Oliveira, C. O. S., R. Montes de Oca-Luna, and Y. Le Loir. 2003. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect. Immun. 71:1887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez-Humaran, L. G., P. Langella, A. Miyoshi, A. Gruss, R. T. Guerra, R. Montes de Oca-Luna, and Y. Le Loir. 2002. Production of human papillomavirus type 16 E7 protein in Lactococcus lactis. Appl. Environ. Microbiol. 68:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolhuis, A., H. Tjalsma, H. E. Smith, A. de Jong, R. Meima, G. Venema, S. Bron, and J. M. van Dijl. 1999. Evaluation of bottlenecks in the late stages of protein secretion in Bacillus subtilis. Appl. Environ. Microbiol. 65:2934-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd, D. A., D. G. Cvitkovitch, A. S. Bleiweis, M. Y. Kiriukhin, D. V. Debabov, F. C. Neuhaus, and I. R. Hamilton. 2000. Defects in d-alanyl-lipoteichoic acid synthesis in Streptococcus mutans results in acid sensitivity. J. Bacteriol. 182:6055-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buist, G., J. Kok, K. J. Leenhouts, M. Dabrowska, G. Venema, and A. J. Haandrikman. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 177:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Chopin, A., A. Bolotin, A. Sorokin, S. D. Ehrlich, and M. C. Chopin. 2001. Analysis of six prophages in Lactococcus lactis IL1403: different genetic structure of temperate and virulent phage populations. Nucleic Acids Res. 29:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemans, D. L., P. E. Kolenbrander, D. V. Debabov, Q. Zhang, R. D. Lunsford, H. Sakone, C. J. Whittaker, M. P. Heaton, and F. C. Neuhaus. 1999. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect. Immun. 67:2464-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debabov, D. V., M. Y. Kiriukhin, and F. C. Neuhaus. 2000. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J. Bacteriol. 182:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delorme, C., J. J. Godon, S. D. Ehrlich, and P. Renault. 1993. Gene inactivation in Lactococcus lactis: histidine biosynthesis. J. Bacteriol. 175:4391-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delorme, C., J. J. Godon, S. D. Ehrlich, and P. Renault. 1994. Mosaic structure of large regions of the Lactococcus lactis subsp. cremoris chromosome. Microbiology 140:3053-3060. [DOI] [PubMed] [Google Scholar]
- 14.de Vos, W. M. 1999. Gene expression systems for lactic acid bacteria. Curr. Opin. Microbiol. 2:289-295. [DOI] [PubMed] [Google Scholar]
- 15.Dieye, Y., S. Usai, F. Clier, A. Gruss, and J. C. Piard. 2001. Design of a protein-targeting system for lactic acid bacteria. J. Bacteriol. 183:4157-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duwat, P., A. Cochu, S. D. Ehrlich, and A. Gruss. 1997. Characterization of Lactococcus lactis UV-sensitive mutants obtained by ISS1 transposition. J. Bacteriol. 179:4473-4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson, T. J. 1984. Ph.D. thesis. University of Cambridge, Cambridge, England.
- 19.Glenting, J., S. M. Madsen, A. Vrang, A. Fomsgaard, and H. Israelsen. 2002. A plasmid selection system in Lactococcus lactis and its use for gene expression in L. lactis and human kidney fibroblasts. Appl. Environ. Microbiol. 68:5051-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross, M., S. E. Cramton, F. Gotz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyyrylainen, H. L., M. Vitikainen, J. Thwaite, H. Wu, M. Sarvas, C. R. Harwood, V. P. Kontinen, and K. Stephenson. 2000. d-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J. Biol. Chem. 275:26696-26703. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs, M. F., J. B. Andersen, T. V. Borchert, and V. P. Kontinen. 1995. Identification of a Bacillus subtilis secretion mutant using a beta-galactosidase screening procedure. Microbiology 141:1771-1779. [DOI] [PubMed] [Google Scholar]
- 23.Kleerebezem, M., M. M. Beerthuyzen, E. E. Vaughan, W. M. de Vos, and O. P. Kuipers. 1997. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl. Environ. Microbiol. 63:4581-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontinen, V. P., and M. Sarvas. 1988. Mutants of Bacillus subtilis defective in protein export. J. Gen. Microbiol. 134:2333-2344. [DOI] [PubMed] [Google Scholar]
- 25.Langella, P., Y. Le Loir, S. D. Ehrlich, and A. Gruss. 1993. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J. Bacteriol. 175:5806-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1994. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J. Bacteriol. 176:5135-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J. Bacteriol. 180:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Loir, Y., S. Nouaille, J. Commissaire, L. Bretigny, A. Gruss, and P. Langella. 2001. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl. Environ. Microbiol. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leloup, L., A. Haddaoui el, R. Chambert, and M. F. Petit-Glatron. 1997. Characterization of the rate-limiting step of the secretion of Bacillus subtilis alpha-amylase overproduced during the exponential phase of growth. Microbiology 143:3295-3303. [DOI] [PubMed] [Google Scholar]
- 30.Maguin, E., H. Prevost, S. D. Ehrlich, and A. Gruss. 1996. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J. Bacteriol. 178:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi, A., I. Poquet, V. Azevedo, J. Commissaire, L. Bermudez-Humaran, E. Domakova, Y. Le Loir, S. C. Oliveira, A. Gruss, and P. Langella. 2002. Controlled production of stable heterologous proteins in Lactococcus lactis. Appl. Environ. Microbiol. 68:3141-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuhaus, F. C., M. P. Heaton, D. V. Debabov, and Q. Zhang. 1996. The dlt operon in the biosynthesis of d-alanyl-lipoteichoic acid in Lactobacillus casei. Microb. Drug Resist. 2:77-84. [DOI] [PubMed] [Google Scholar]
- 33.Ntamere, A. S., D. J. Taron, and F. C. Neuhaus. 1987. Assembly of d-alanyl-lipoteichoic acid in Lactobacillus casei: mutants deficient in the d-alanyl ester content of this amphiphile. J. Bacteriol. 169:1702-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perego, M., P. Glaser, A. Minutello, M. A. Strauch, K. Leopold, and W. Fischer. 1995. Incorporation of d-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 270:15598-15606. [DOI] [PubMed] [Google Scholar]
- 35.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 36.Poquet, I., V. Saint, E. Seznec, N. Simoes, A. Bolotin, and A. Gruss. 2000. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 35:1042-1051. [DOI] [PubMed] [Google Scholar]
- 37.Poyart, C., M. C. Lamy, C. Boumaila, F. Fiedler, and P. Trieu-Cuot. 2001. Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183:6324-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in d-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 49:1615-1625. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro, L. A., V. Azevedo, Y. Le Loir, S. C. Oliveira, Y. Dieye, J. C. Piard, A. Gruss, and P. Langella. 2002. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 68:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 41.Shortle, D. 1983. A genetic system for analysis of staphylococcal nuclease. Gene 22:181-189. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen, K. I., R. Larsen, A. Kibenich, M. P. Junge, and E. Johansen. 2000. A food-grade cloning system for industrial strains of Lactococcus lactis. Appl. Environ. Microbiol. 66:1253-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 44.Terzaghi, B., and W. E. Sandine. 1975. Improved medium for lactic acid streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thwaite, J. E., L. W. Baillie, N. M. Carter, K. Stephenson, M. Rees, C. R. Harwood, and P. T. Emmerson. 2002. Optimization of the cell wall microenvironment allows increased production of recombinant Bacillus anthracis protective antigen from B. subtilis. Appl. Environ. Microbiol. 68:227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tjalsma, H., A. Bolhuis, J. D. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wecke, J., M. Perego, and W. Fischer. 1996. d-Alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb. Drug Resist. 2:123-129. [DOI] [PubMed] [Google Scholar]
- 48.Wells, J. M., P. W. Wilson, P. M. Norton, M. J. Gasson, and R. W. Le Page. 1993. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol. Microbiol. 8:1155-1162. [DOI] [PubMed] [Google Scholar]