Abstract
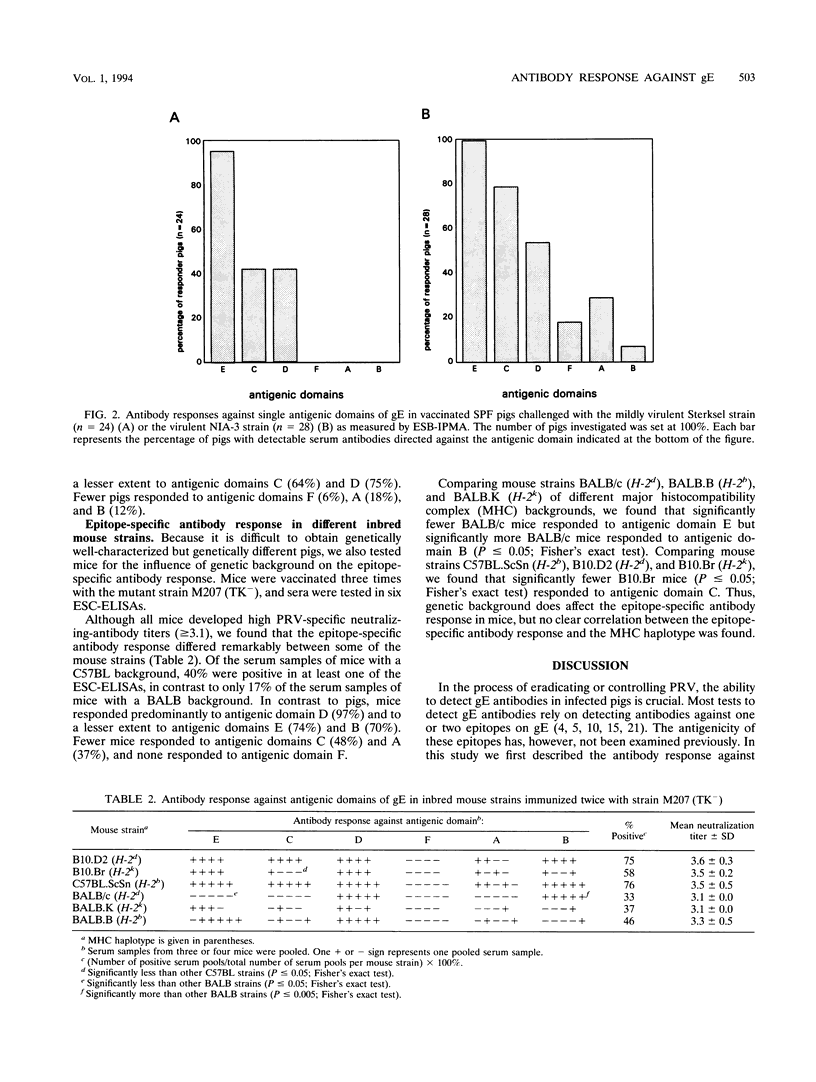
In this study we investigated the epitope-specific antibody response against glycoprotein E (gE) of pseudorabies virus. Epitope-specific antibody responses were investigated by enzyme-linked immunoperoxidase monolayer assays. In a vaccinated crossbred pig population, most pigs responded to antigenic domain E and to a lesser degree to antigenic domains C and D. Only few pigs responded to antigenic domains F, A, and B. Using vaccinated pigs, we investigated the influence of two different pseudorabies virus strains and the genetic background of the host on the epitope-specific antibody response. More pigs infected with the virulent NIA-3 strain had a detectable antibody response against antigenic domains C, F and A than did pigs infected with the mildly virulent Sterksel strain (P < or = 0.05; Fisher's exact test). No differences in the epitope-specific antibody responses of two genetically different pig breeding lines were observed (P > or = 0.1; Fisher's exact test). In both breeding lines the incidence of the epitope-specific antibody response was comparable to that in the crossbred pig population. In addition, we studied the epitope-specific antibody responses were strikingly different and indicated that genetic background influenced the epitope-specific antibody response. Of the serum samples of mice with C57BL and a BALB background, 40 and 17% respectively, were positive in the one of the epitope-specific immunoassays. In contrast to pigs, mice responded predominantly to antigenic domain D and to a lesser degree to antigenic domains E and B. Only few mice had a detectable antibody response against antigenic domains C and A, and none had a detectable antibody response against antigenic domain F.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z., Smith J. A. A proposal for the nomenclature of antigenic sites in peptides and proteins. Immunochemistry. 1978 Aug;15(8):609–610. doi: 10.1016/0161-5890(78)90016-0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., DeMarchi J. M., Lomniczi B., Kaplan A. S. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology. 1986 Oct 30;154(2):325–334. doi: 10.1016/0042-6822(86)90458-7. [DOI] [PubMed] [Google Scholar]
- Bitsch V., Eskildsen M. A comparative examination of swine sera for antibody to Aujeszky virus with the conventional and a modified virus-serum neutralization test and a modified direct complement fixation test. Acta Vet Scand. 1976;17(2):142–152. doi: 10.1186/BF03547923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T. M., Bäckström B. T., Prestidge R. L., Love S. G., Harding D. R., Watson J. D. Immune responses to the 18-kDa protein of Mycobacterium leprae. Similar B cell epitopes but different T cell epitopes seen by inbred strains of mice. J Immunol. 1991 Mar 15;146(6):1934–1940. [PubMed] [Google Scholar]
- Eloit M., Fargeaud D., Vannier P., Toma B. Development of an ELISA to differentiate between animals either vaccinated with or infected by Aujeszky's disease virus. Vet Rec. 1989 Jan 28;124(4):91–94. doi: 10.1136/vr.124.4.91. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Meloen R. H., Gielkens A. L., Van Oirschot J. T. Epitope analysis of glycoprotein I of pseudorabies virus. J Gen Virol. 1990 Apr;71(Pt 4):881–887. doi: 10.1099/0022-1317-71-4-881. [DOI] [PubMed] [Google Scholar]
- Katz J. B., Pederson J. C. Analysis of glycoprotein I (gI) negative and aberrant pseudorabies viral diagnostic isolates. Am J Vet Res. 1992 Dec;53(12):2259–2263. [PubMed] [Google Scholar]
- Kennedy M. W., McIntosh A. E., Blair A. J., McLaughlin D. MHC (RT1) restriction of the antibody repertoire to infection with the nematode Nippostrongylus brasiliensis in the rat. Immunology. 1990 Nov;71(3):317–322. [PMC free article] [PubMed] [Google Scholar]
- McFerran J. B., Dow C. Studies on immunisation of pigs with the Bartha strain of Aujeszky's disease virus. Res Vet Sci. 1975 Jul;19(1):17–22. [PubMed] [Google Scholar]
- Mellencamp M. W., Pfeiffer N. E., Suiter B. T., Harness J. R., Beckenhauer W. H. Identification of pseudorabies virus-exposed swine with a gI glycoprotein enzyme-linked immunosorbent assay. J Clin Microbiol. 1989 Oct;27(10):2208–2213. doi: 10.1128/jcm.27.10.2208-2213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moormann R. J., de Rover T., Briaire J., Peeters B. P., Gielkens A. L., van Oirschot J. T. Inactivation of the thymidine kinase gene of a gI deletion mutant of pseudorabies virus generates a safe but still highly immunogenic vaccine strain. J Gen Virol. 1990 Jul;71(Pt 7):1591–1595. doi: 10.1099/0022-1317-71-7-1591. [DOI] [PubMed] [Google Scholar]
- Peeters B., de Wind N., Hooisma M., Wagenaar F., Gielkens A., Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992 Feb;66(2):894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oirschot J. T., Daus F., Kimman T. G., van Zaane D. Antibody response to glycoprotein I in maternally immune pigs exposed to a mildly virulent strain of pseudorabies virus. Am J Vet Res. 1991 Nov;52(11):1788–1793. [PubMed] [Google Scholar]
- van Oirschot J. T., Gielkens A. L., Moormann R. J., Berns A. J. Marker vaccines, virus protein-specific antibody assays and the control of Aujeszky's disease. Vet Microbiol. 1990 Jun;23(1-4):85–101. doi: 10.1016/0378-1135(90)90139-m. [DOI] [PubMed] [Google Scholar]
- van Oirschot J. T. Induction of antibodies to glycoprotein I in pigs exposed to different doses of a mildly virulent strain of Aujeszky's disease virus. Vet Rec. 1988 Jun 18;122(25):599–603. doi: 10.1136/vr.122.25.599. [DOI] [PubMed] [Google Scholar]
- van Oirschot J. T., Rziha H. J., Moonen P. J., Pol J. M., van Zaane D. Differentiation of serum antibodies from pigs vaccinated or infected with Aujeszky's disease virus by a competitive enzyme immunoassay. J Gen Virol. 1986 Jun;67(Pt 6):1179–1182. doi: 10.1099/0022-1317-67-6-1179. [DOI] [PubMed] [Google Scholar]
- van Zijl M., Quint W., Briaire J., de Rover T., Gielkens A., Berns A. Regeneration of herpesviruses from molecularly cloned subgenomic fragments. J Virol. 1988 Jun;62(6):2191–2195. doi: 10.1128/jvi.62.6.2191-2195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


