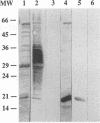
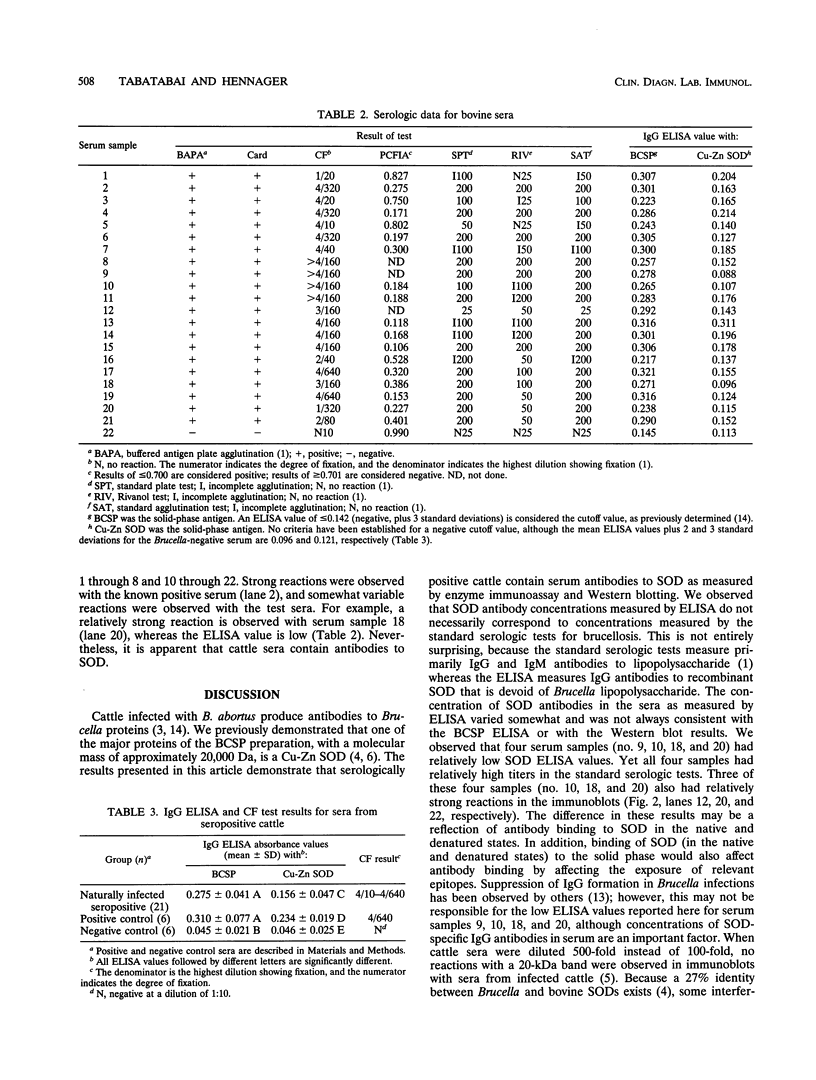
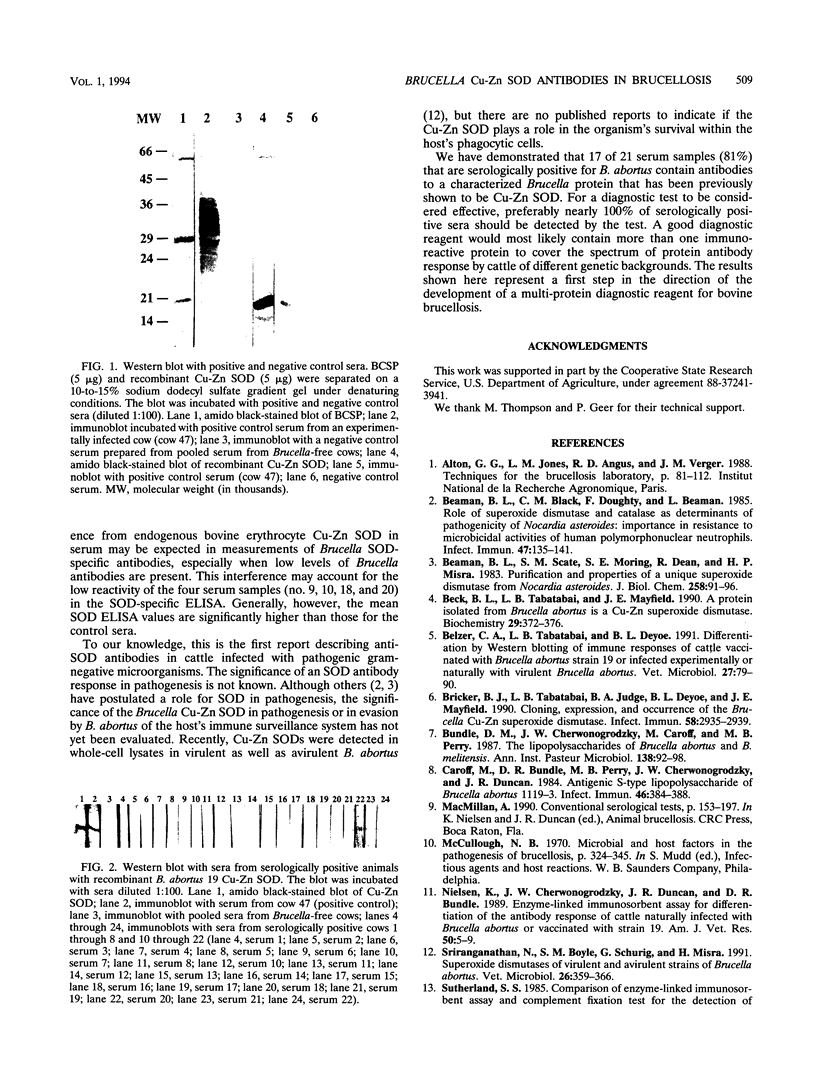
Abstract
In this study, we demonstrated by a Cu-Zn superoxide dismutase-specific enzyme-linked immunoassay that cattle that are serologically positive for Brucella abortus have serum immunoglobulin G antibodies to B. abortus Cu-Zn superoxide dismutase. The specificity of the antibody reactivity was confirmed by Western blot (immunoblot) analysis with B. abortus salt-extractable proteins containing native Cu-Zn superoxide dismutase and with recombinant B. abortus Cu-Zn superoxide dismutase. The results represent a first step in the direction of the development of a multiprotein diagnostic reagent for bovine brucellosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Scates S. M., Moring S. E., Deem R., Misra H. P. Purification and properties of a unique superoxide dismutase from Nocardia asteroides. J Biol Chem. 1983 Jan 10;258(1):91–96. [PubMed] [Google Scholar]
- Beck B. L., Tabatabai L. B., Mayfield J. E. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry. 1990 Jan 16;29(2):372–376. doi: 10.1021/bi00454a010. [DOI] [PubMed] [Google Scholar]
- Belzer C. A., Tabatabai L. B., Deyoe B. L. Differentiation by western blotting of immune responses of cattle vaccinated with Brucella abortus strain 19 or infected experimentally or naturally with virulent Brucella abortus. Vet Microbiol. 1991 Mar;27(1):79–90. doi: 10.1016/0378-1135(91)90064-m. [DOI] [PubMed] [Google Scholar]
- Bricker B. J., Tabatabai L. B., Judge B. A., Deyoe B. L., Mayfield J. E. Cloning, expression, and occurrence of the Brucella Cu-Zn superoxide dismutase. Infect Immun. 1990 Sep;58(9):2935–2939. doi: 10.1128/iai.58.9.2935-2939.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Caroff M., Perry M. B. The lipopolysaccharides of Brucella abortus and B. melitensis. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):92–98. doi: 10.1016/0769-2609(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K., Cherwonogrodzky J. W., Duncan J. R., Bundle D. R. Enzyme-linked immunosorbent assay for differentiation of the antibody response of cattle naturally infected with Brucella abortus or vaccinated with strain 19. Am J Vet Res. 1989 Jan;50(1):5–9. [PubMed] [Google Scholar]
- Sriranganathan N., Boyle S. M., Schurig G., Misra H. Superoxide dismutases of virulent and avirulent strains of Brucella abortus. Vet Microbiol. 1991 Feb 15;26(4):359–366. doi: 10.1016/0378-1135(91)90029-f. [DOI] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L., Ritchie A. E. Isolation and characterization of toxic fractions from Brucella abortus. Infect Immun. 1979 Nov;26(2):668–679. doi: 10.1128/iai.26.2.668-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L. Specific enzyme-linked immunosorbent assay for detection of bovine antibody to Brucella abortus. J Clin Microbiol. 1984 Aug;20(2):209–213. doi: 10.1128/jcm.20.2.209-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]