Abstract
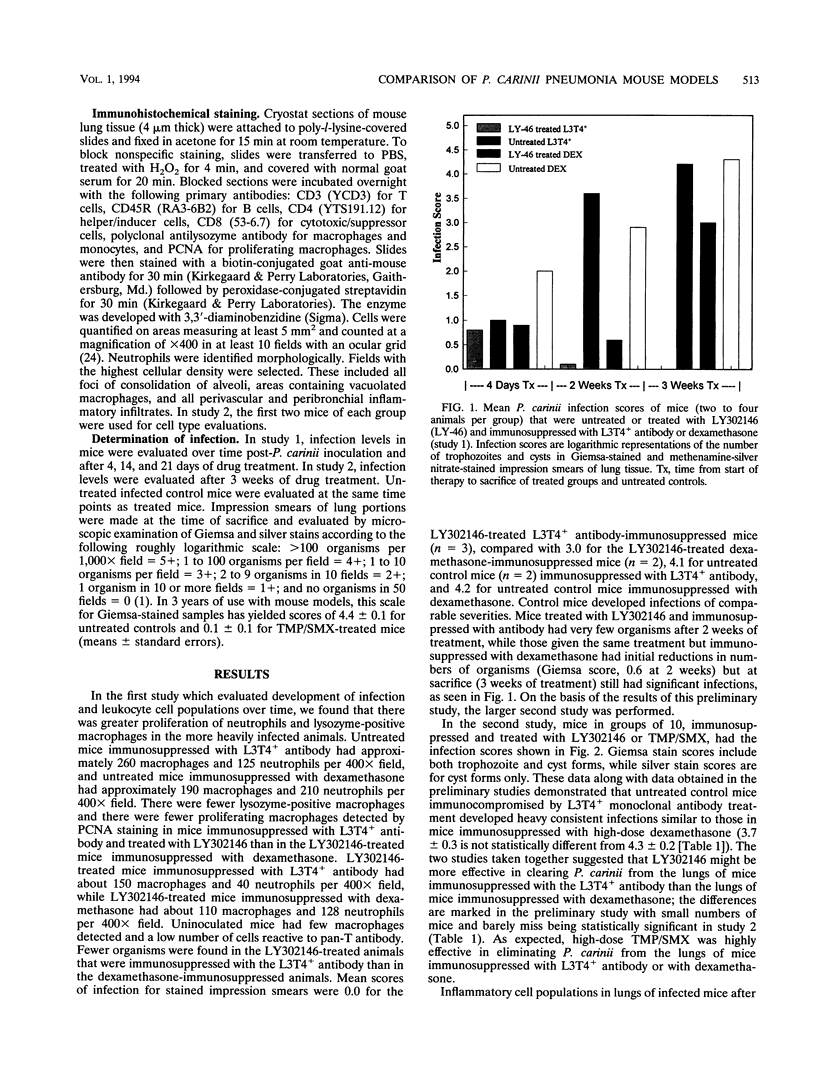
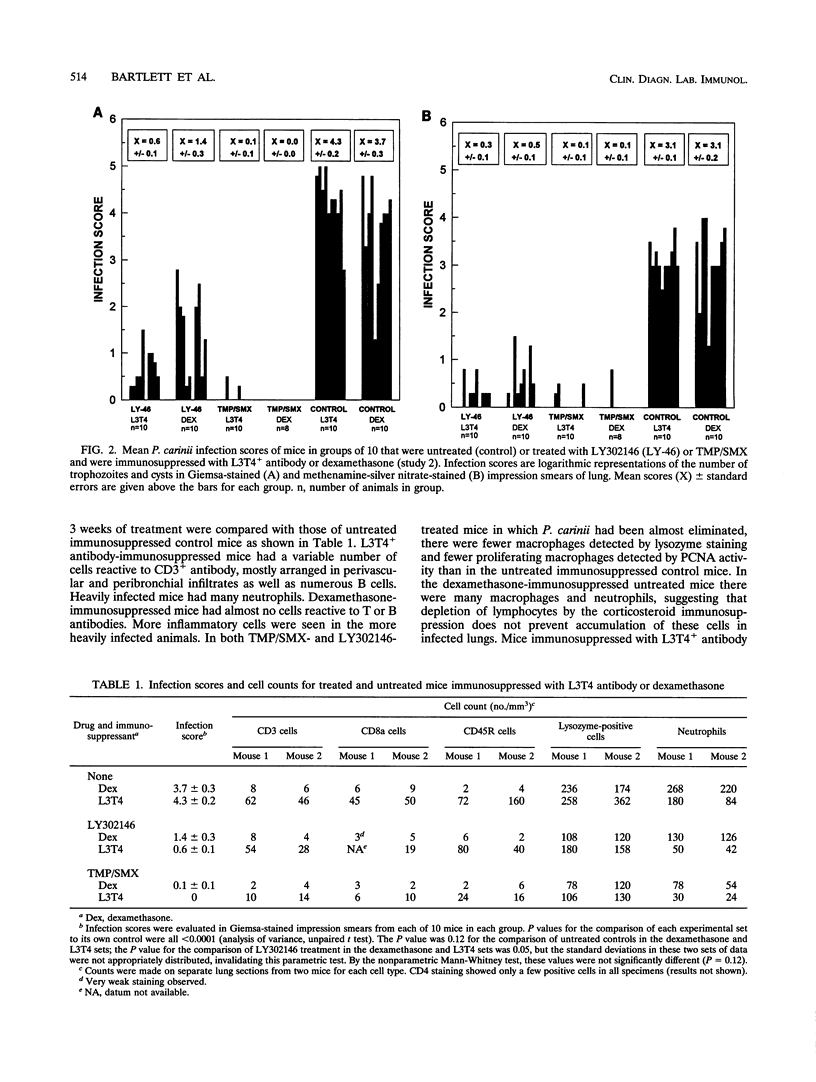
An immunologically immunosuppressed mouse model of Pneumocystis carinii pneumonia using antibody developed by Dialynas et al. (Immunol. Rev. 74:29-55, 1983) directed to L3T4+ T cells (referred to as L3T4+ antibody) was compared with a corticosteroid-immunosuppressed mouse model. Corticosteroid- or L3T4+ antibody-immunosuppressed BALB/c mice transtracheally inoculated with P. carinii developed severe infections within 5 weeks after inoculation and responded to treatments with an echinocandin B analog, LY302146, or trimethoprim plus sulfamethoxazole so that they had decreased numbers of P. carinii cysts and trophozoites. LY302146 appeared to be more effective in L3T4+ antibody-immunosuppressed mice than in dexamethasone-immunosuppressed mice. Leukocyte populations in lungs of both mouse models during development of infection and during treatment were compared by using immune cell-specific staining. Lungs of L3T4+ antibody-immunosuppressed mice had many more cells detected with pan-B antibody and pan-T antibody than dexamethasone-immunosuppressed mice and the lungs of successfully treated mice had about the same numbers of macrophages as those of nonimmunosuppressed uninfected mice. The immunologically immunosuppressed model will allow study of cytokines and other immune modulators alone and in combination with drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett M. S., Fishman J. A., Durkin M. M., Queener S. F., Smith J. W. Pneumocystis carinii: improved models to study efficacy of drugs for treatment or prophylaxis of Pneumocystis pneumonia in the rat (Rattus spp.). Exp Parasitol. 1990 Jan;70(1):100–106. doi: 10.1016/0014-4894(90)90089-u. [DOI] [PubMed] [Google Scholar]
- Bartlett M. S., Fishman J. A., Queener S. F., Durkin M. M., Jay M. A., Smith J. W. New rat model of Pneumocystis carinii infection. J Clin Microbiol. 1988 Jun;26(6):1100–1102. doi: 10.1128/jcm.26.6.1100-1102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett M. S., Queener S. F., Durkin M. M., Shaw M. A., Smith J. W. Inoculated mouse model of Pneumocystis carinii infection. Diagn Microbiol Infect Dis. 1992 Feb;15(2):129–134. doi: 10.1016/0732-8893(92)90036-s. [DOI] [PubMed] [Google Scholar]
- Bartlett M. S., Queener S. F., Tidwell R. R., Milhous W. K., Berman J. D., Ellis W. Y., Smith J. W. 8-Aminoquinolines from Walter Reed Army Institute for Research for treatment and prophylaxis of Pneumocystis pneumonia in rat models. Antimicrob Agents Chemother. 1991 Feb;35(2):277–282. doi: 10.1128/aac.35.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. M., Liggitt H. D., Brunette E. N., Fuchs H. J., Shellito J. E., Debs R. J. Reduction in intensity of Pneumocystis carinii pneumonia in mice by aerosol administration of gamma interferon. Infect Immun. 1991 Nov;59(11):3859–3862. doi: 10.1128/iai.59.11.3859-3862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan C. J., Current W. L. Improved rat model of Pneumocystis carinii pneumonia: induced laboratory infections in Pneumocystis-free animals. Infect Immun. 1992 Apr;60(4):1589–1597. doi: 10.1128/iai.60.4.1589-1597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T., Zhang J., Kaselis M., Giuntoli D., Stringer S. L., Stringer J. R. Evidence for two genetic variants of Pneumocystis carinii coinfecting laboratory rats. J Clin Microbiol. 1993 May;31(5):1217–1223. doi: 10.1128/jcm.31.5.1217-1223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Ershler W. B., Klopp R. G., Moore A. L., Krauss S. L., Ranges G. Increased susceptibility to inoculated Lewis lung carcinoma (3LL) but unaltered tumor growth in mice treated with monoclonal antibody to L3T4 on mouse T-helper cells. Cancer Invest. 1989;7(4):339–343. doi: 10.3109/07357908909039860. [DOI] [PubMed] [Google Scholar]
- Frenkel J. K., Good J. T., Shultz J. A. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966 Oct;15(10):1559–1577. [PubMed] [Google Scholar]
- Furney S. K., Roberts A. D., Orme I. M. Effect of rifabutin on disseminated Mycobacterium avium infections in thymectomized, CD4 T-cell-deficient mice. Antimicrob Agents Chemother. 1990 Sep;34(9):1629–1632. doi: 10.1128/aac.34.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A. M., Bullock W. E., Taylor C. L., Deepe G. S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988 Jul;56(7):1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman J., Wang Y. M., Teschler H., Kienast K., Brockmeyer N., Costabel U. Phenotypic analysis of bronchoalveolar lavage lymphocytes from acquired immunodeficiency patients with and without Pneumocystis carinii pneumonia. Acta Cytol. 1992 Nov-Dec;36(6):900–904. [PubMed] [Google Scholar]
- Harmsen A. G., Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990 Sep 1;172(3):937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., Gray V. L., Gutteridge W. E., Latter V. S., Pudney M. Efficacy of a hydroxynaphthoquinone, 566C80, in experimental Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1990 Feb;34(2):225–228. doi: 10.1128/aac.34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes W. T., McNabb P. C., Makres T. D., Feldman S. Efficacy of trimethoprim and sulfamethoxazole in the prevention and treatment of Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1974 Mar;5(3):289–293. doi: 10.1128/aac.5.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. K., Hall J. E., Allen M. A., Morrison S. D., Ohemeng K. A., Reddy V. V., Geratz J. D., Tidwell R. R. Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1990 Jun;34(6):1026–1030. doi: 10.1128/aac.34.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. A., Masur H. Prophylaxis of Pneumocystis carinii pneumonia: an update. J Infect Dis. 1989 Nov;160(5):882–886. doi: 10.1093/infdis/160.5.882. [DOI] [PubMed] [Google Scholar]
- Orazi A., Cattoretti G., Schiró R., Siena S., Bregni M., Di Nicola M., Gianni A. M. Recombinant human interleukin-3 and recombinant human granulocyte-macrophage colony-stimulating factor administered in vivo after high-dose cyclophosphamide cancer chemotherapy: effect on hematopoiesis and microenvironment in human bone marrow. Blood. 1992 May 15;79(10):2610–2619. [PubMed] [Google Scholar]
- Pesanti E. L., Cox C. Metabolic and synthetic activities of Pneumocystis carinii in vitro. Infect Immun. 1981 Dec;34(3):908–914. doi: 10.1128/iai.34.3.908-914.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles M. A., McFadden D. C., Pittarelli L. A., Schmatz D. M. Mouse model for Pneumocystis carinii pneumonia that uses natural transmission to initiate infection. Infect Immun. 1992 Apr;60(4):1397–1400. doi: 10.1128/iai.60.4.1397-1400.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellito J., Suzara V. V., Blumenfeld W., Beck J. M., Steger H. J., Ermak T. H. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest. 1990 May;85(5):1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota T., Yamada M., Yoshida Y. Morphology, development and behavior of Pneumocystis carinii observed by light-microscopy in nude mice. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Aug;262(2):230–239. doi: 10.1016/s0176-6724(86)80024-4. [DOI] [PubMed] [Google Scholar]
- Stull S. J., Kyriakos M., Sharp G. C., Braley-Mullen H. Prevention and reversal of experimental autoimmune thyroiditis (EAT) in mice by administration of anti-L3T4 monoclonal antibody at different stages of disease development. Cell Immunol. 1988 Nov;117(1):188–198. doi: 10.1016/0008-8749(88)90087-1. [DOI] [PubMed] [Google Scholar]
- Sundberg J. P., Burnstein T., Shultz L. D., Bedigian H. Identification of Pneumocystis carinii in immunodeficient mice. Lab Anim Sci. 1989 May;39(3):213–218. [PubMed] [Google Scholar]
- Ueda K., Goto Y., Yamazaki S., Fujiwara K. Chronic fatal pneumocystosis in nude mice. Jpn J Exp Med. 1977 Dec;47(6):475–482. [PubMed] [Google Scholar]
- Vestbo J., Nielsen T. L., Junge J., Lundgren J. D. Amount of Pneumocystis carinii and degree of acute lung inflammation in HIV-associated P carinii pneumonia. Chest. 1993 Jul;104(1):109–113. doi: 10.1378/chest.104.1.109. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Foy J., Linke M. J., Cushion M. T. Cationic antitrypanosomal and other antimicrobial agents in the therapy of experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1988 Jun;32(6):896–905. doi: 10.1128/aac.32.6.896. [DOI] [PMC free article] [PubMed] [Google Scholar]


