Abstract
The retinal pigment epithelial (RPE) cell is a potent regulatory cell within the retina. It helps to maintain normal retinal activity, and following gamma interferon (IFN-gamma) exposure, it may express major histocompatibility complex class II molecules and function as an antigen-presenting cell. Since interleukin-1 (IL-1) and IL-6 are potent cytokines observed in ocular inflammatory processes, we initiated studies to evaluate conditions which enable RPE cells to produce these cytokines. Cultures of human RPE cells from two eye donors were established and characterized, and enzyme immunoassays were employed to screen for IL-1 and IL-6 production. Treatment of RPE cells with lipopolysaccharide (LPS) or recombinant tumor necrosis factor alpha, IL-1, or IFN-gamma resulted in a significant level of secretion of IL-6. In contrast, treatment with recombinant epidermal growth factor, basic fibroblast growth factor, platelet-derived growth factor, or transforming growth factor alpha, or LPS can dramatically augment the secretion of IL-6 by RPE cells. Thus, these inflammatory mediators can act alone or synergistically with IFN-gamma to activate RPE cells and dramatically increase the expression and secretion of IL-6. In contrast, IL-1 was not detected following stimulation with any of the above-mentioned cytokines or LPS. Characterization of IL-6 protein production by RPE cells revealed that 98% of the protein is promptly secreted by the cell, its induction is dependent upon the time and concentration of the stimulant, and the continuous presence of the stimulant is required for IL-6 production. Moreover, Western blot (immunoblot) analysis of secreted proteins revealed that IL-6 was produced in multiple molecular forms.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

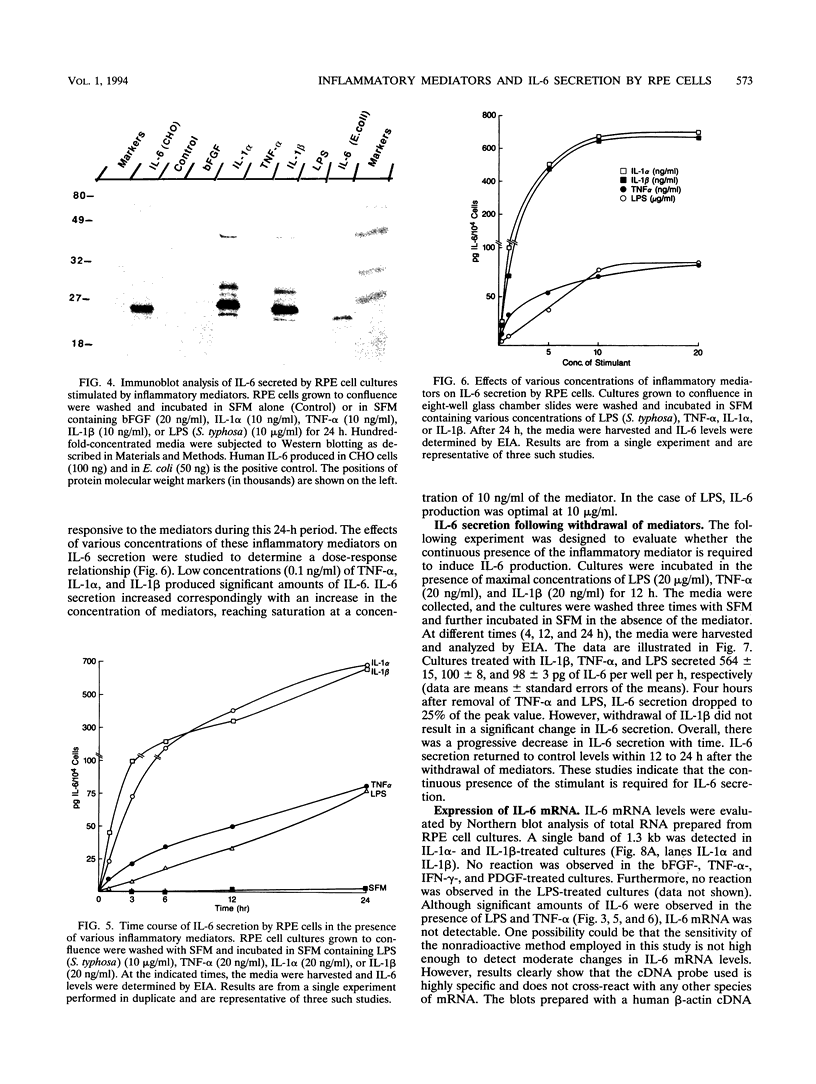
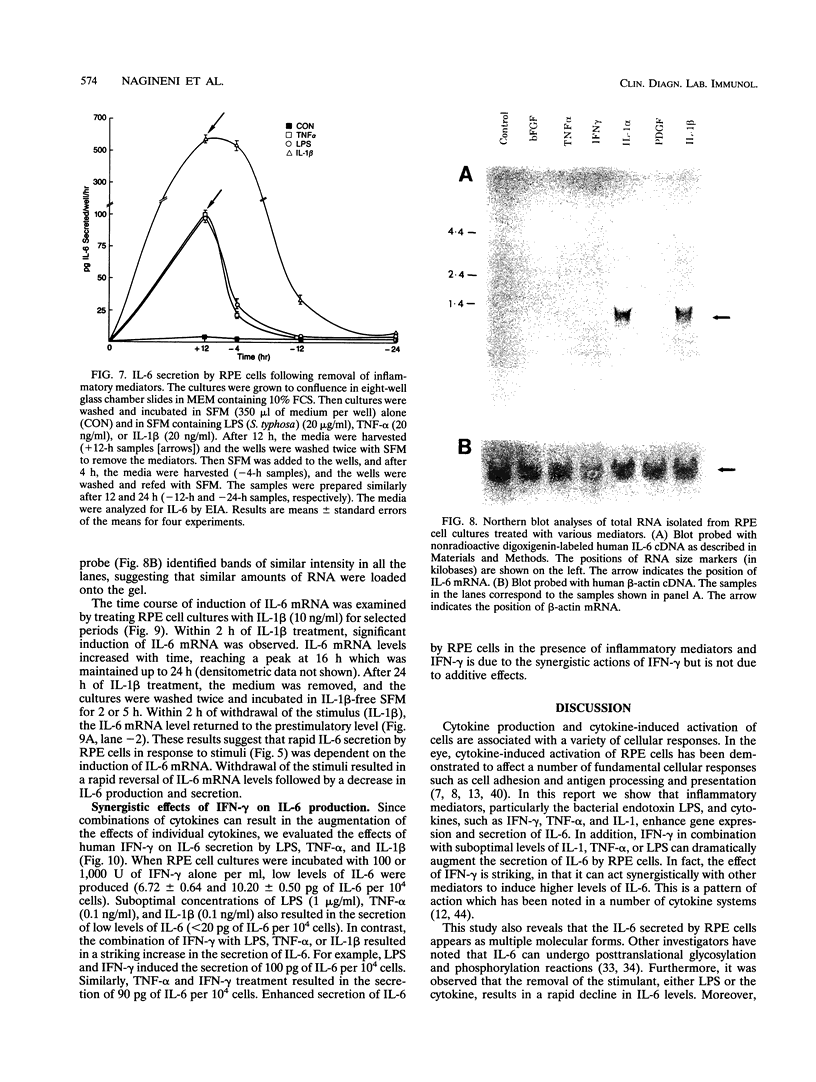
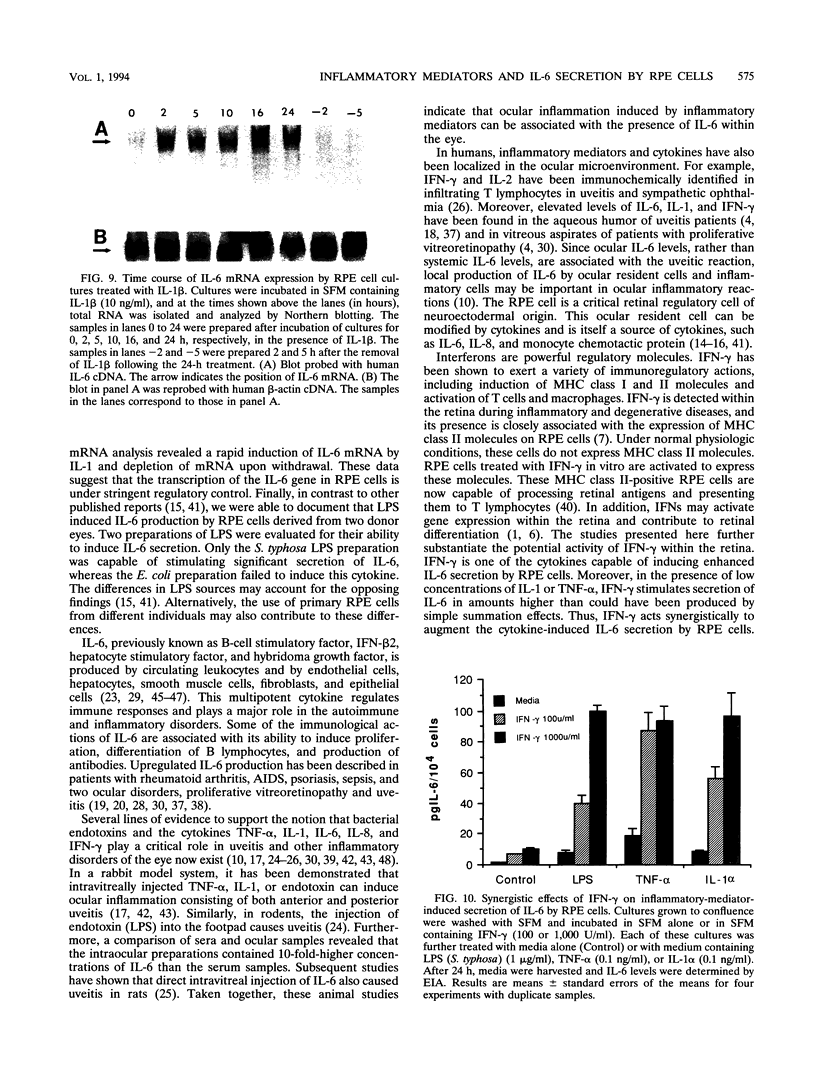


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barez S., Boumpas D. T., Percopo C. M., Anastassiou E. D., Hooks J. J., Detrick B. Modulation of major histocompatibility complex class 1 genes in human retinoblastoma cells by interferons. Invest Ophthalmol Vis Sci. 1993 Aug;34(9):2613–2621. [PubMed] [Google Scholar]
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1985 Dec;26(12):1659–1694. [PubMed] [Google Scholar]
- Chan C. C., Detrick B., Nussenblatt R. B., Palestine A. G., Fujikawa L. S., Hooks J. J. HLA-DR antigens on retinal pigment epithelial cells from patients with uveitis. Arch Ophthalmol. 1986 May;104(5):725–729. doi: 10.1001/archopht.1986.01050170115034. [DOI] [PubMed] [Google Scholar]
- De Vos A. F., Hoekzema R., Kijlstra A. Cytokines and uveitis, a review. Curr Eye Res. 1992 Jun;11(6):581–597. doi: 10.3109/02713689209001814. [DOI] [PubMed] [Google Scholar]
- Deschênes J., Char D. H., Kaleta S. Activated T lymphocytes in uveitis. Br J Ophthalmol. 1988 Feb;72(2):83–87. doi: 10.1136/bjo.72.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrick B., Evans C. H., Chader G., Percopo C. M., Hooks J. J. Cytokine-induced modulation of cellular proteins in retinoblastoma. Analysis by flow cytometry. Invest Ophthalmol Vis Sci. 1991 May;32(6):1714–1722. [PubMed] [Google Scholar]
- Detrick B., Newsome D. A., Percopo C. M., Hooks J. J. Class II antigen expression and gamma interferon modulation of monocytes and retinal pigment epithelial cells from patients with retinitis pigmentosa. Clin Immunol Immunopathol. 1985 Aug;36(2):201–211. doi: 10.1016/0090-1229(85)90121-7. [DOI] [PubMed] [Google Scholar]
- Detrick B., Rodrigues M., Chan C. C., Tso M. O., Hooks J. J. Expression of HLA-DR antigen on retinal pigment epithelial cells in retinitis pigmentosa. Am J Ophthalmol. 1986 May 15;101(5):584–590. doi: 10.1016/0002-9394(86)90949-9. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Lentz V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL-6 production and stabilize IL-6 messenger RNA. J Immunol. 1990 Jul 1;145(1):161–166. [PubMed] [Google Scholar]
- Elner S. G., Elner V. M., Pavilack M. A., Todd R. F., 3rd, Mayo-Bond L., Franklin W. A., Strieter R. M., Kunkel S. L., Huber A. R. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Invest. 1992 Feb;66(2):200–211. [PubMed] [Google Scholar]
- Elner S. G., Strieter R. M., Elner V. M., Rollins B. J., Del Monte M. A., Kunkel S. L. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991 Jun;64(6):819–825. [PubMed] [Google Scholar]
- Elner V. M., Scales W., Elner S. G., Danforth J., Kunkel S. L., Strieter R. M. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Exp Eye Res. 1992 Mar;54(3):361–368. doi: 10.1016/0014-4835(92)90048-w. [DOI] [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Elner S. G., Baggiolini M., Lindley I., Kunkel S. L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990 Apr;136(4):745–750. [PMC free article] [PubMed] [Google Scholar]
- Fleisher L. N., Ferrell J. B., McGahan M. C. Synergistic uveitic effects of tumor necrosis factor-alpha and interleukin-1 beta. Invest Ophthalmol Vis Sci. 1992 Jun;33(7):2120–2127. [PubMed] [Google Scholar]
- Franks W. A., Limb G. A., Stanford M. R., Ogilvie J., Wolstencroft R. A., Chignell A. H., Dumonde D. C. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11 (Suppl):187–191. doi: 10.3109/02713689208999531. [DOI] [PubMed] [Google Scholar]
- Grossman R. M., Krueger J., Yourish D., Granelli-Piperno A., Murphy D. P., May L. T., Kupper T. S., Sehgal P. B., Gottlieb A. B. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerne P. A., Zuraw B. L., Vaughan J. H., Carson D. A., Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989 Feb;83(2):585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel C. P., Tsilou E., Harris E., Pfeffer B. A., Hooks J. J., Detrick B., Redmond T. M. A developmentally regulated microsomal protein specific for the pigment epithelium of the vertebrate retina. J Neurosci Res. 1993 Mar 1;34(4):414–425. doi: 10.1002/jnr.490340406. [DOI] [PubMed] [Google Scholar]
- Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992 Jan;62(1 Pt 2):S60–S65. doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Hoekzema R., Murray P. I., van Haren M. A., Helle M., Kijlstra A. Analysis of interleukin-6 in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1991 Jan;32(1):88–95. [PubMed] [Google Scholar]
- Hoekzema R., Verhagen C., van Haren M., Kijlstra A. Endotoxin-induced uveitis in the rat. The significance of intraocular interleukin-6. Invest Ophthalmol Vis Sci. 1992 Mar;33(3):532–539. [PubMed] [Google Scholar]
- Hooks J. J., Chan C. C., Detrick B. Identification of the lymphokines, interferon-gamma and interleukin-2, in inflammatory eye diseases. Invest Ophthalmol Vis Sci. 1988 Sep;29(9):1444–1451. [PubMed] [Google Scholar]
- Hooks J. J., Detrick B., Percopo C., Hamel C., Siraganian R. P. Development and characterization of monoclonal antibodies directed against the retinal pigment epithelial cell. Invest Ophthalmol Vis Sci. 1989 Oct;30(10):2106–2113. [PubMed] [Google Scholar]
- Houssiau F. A., Devogelaer J. P., Van Damme J., de Deuxchaisnes C. N., Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988 Jun;31(6):784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Limb G. A., Little B. C., Meager A., Ogilvie J. A., Wolstencroft R. A., Franks W. A., Chignell A. H., Dumonde D. C. Cytokines in proliferative vitreoretinopathy. Eye (Lond) 1991;5(Pt 6):686–693. doi: 10.1038/eye.1991.126. [DOI] [PubMed] [Google Scholar]
- Machemer R., Laqua H. Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation). Am J Ophthalmol. 1975 Jul;80(1):1–23. doi: 10.1016/0002-9394(75)90862-4. [DOI] [PubMed] [Google Scholar]
- May L. T., Ghrayeb J., Santhanam U., Tatter S. B., Sthoeger Z., Helfgott D. C., Chiorazzi N., Grieninger G., Sehgal P. B. Synthesis and secretion of multiple forms of beta 2-interferon/B-cell differentiation factor 2/hepatocyte-stimulating factor by human fibroblasts and monocytes. J Biol Chem. 1988 Jun 5;263(16):7760–7766. [PubMed] [Google Scholar]
- May L. T., Santhanam U., Tatter S. B., Ghrayeb J., Sehgal P. B. Multiple forms of human interleukin-6. Phosphoglycoproteins secreted by many different tissues. Ann N Y Acad Sci. 1989;557:114–121. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Murray P. I., Hoekzema R., van Haren M. A., de Hon F. D., Kijlstra A. Aqueous humor interleukin-6 levels in uveitis. Invest Ophthalmol Vis Sci. 1990 May;31(5):917–920. [PubMed] [Google Scholar]
- Nishimura T., Goodnight R., Prendergast R. A., Ryan S. J. Activated macrophages in experimental subretinal neovascularization. Ophthalmologica. 1990;200(1):39–44. doi: 10.1159/000310075. [DOI] [PubMed] [Google Scholar]
- Percopo C. M., Hooks J. J., Shinohara T., Caspi R., Detrick B. Cytokine-mediated activation of a neuronal retinal resident cell provokes antigen presentation. J Immunol. 1990 Dec 15;145(12):4101–4107. [PubMed] [Google Scholar]
- Planck S. R., Dang T. T., Graves D., Tara D., Ansel J. C., Rosenbaum J. T. Retinal pigment epithelial cells secrete interleukin-6 in response to interleukin-1. Invest Ophthalmol Vis Sci. 1992 Jan;33(1):78–82. [PubMed] [Google Scholar]
- Rosenbaum J. T., Howes E. L., Jr, Rubin R. M., Samples J. R. Ocular inflammatory effects of intravitreally-injected tumor necrosis factor. Am J Pathol. 1988 Oct;133(1):47–53. [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. T., Samples J. R., Hefeneider S. H., Howes E. L., Jr Ocular inflammatory effects of intravitreal interleukin 1. Arch Ophthalmol. 1987 Aug;105(8):1117–1120. doi: 10.1001/archopht.1987.01060080119040. [DOI] [PubMed] [Google Scholar]
- Sanceau J., Beranger F., Gaudelet C., Wietzerbin J. IFN-gamma is an essential cosignal for triggering IFN-beta 2/BSF-2/IL-6 gene expression in human monocytic cell lines. Ann N Y Acad Sci. 1989;557:130-41, discussion 141-3. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Wakefield D., Lloyd A. The role of cytokines in the pathogenesis of inflammatory eye disease. Cytokine. 1992 Jan;4(1):1–5. doi: 10.1016/1043-4666(92)90028-p. [DOI] [PubMed] [Google Scholar]
- Whitcup S. M., Chan C. C., Li Q., Nussenblatt R. B. Expression of cell adhesion molecules in posterior uveitis. Arch Ophthalmol. 1992 May;110(5):662–666. doi: 10.1001/archopht.1992.01080170084029. [DOI] [PubMed] [Google Scholar]
- Wolvekamp M. C., Marquet R. L. Interleukin-6: historical background, genetics and biological significance. Immunol Lett. 1990 Mar-Apr;24(1):1–9. doi: 10.1016/0165-2478(90)90028-o. [DOI] [PubMed] [Google Scholar]
- Young R. W., Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969 Aug;42(2):392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987 Mar-Apr;31(5):291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- de Boer J. H., van Haren M. A., de Vries-Knoppert W. A., Baarsma G. S., de Jong P. V., Postema F. J., Rademakers A. J., Kijlstra A. Analysis of IL-6 levels in human vitreous fluid obtained from uveitis patients, patients with proliferative intraocular disorders and eye bank eyes. Curr Eye Res. 1992;11 (Suppl):181–186. doi: 10.3109/02713689208999530. [DOI] [PubMed] [Google Scholar]
- di Giovine F. S., Duff G. W. Interleukin 1: the first interleukin. Immunol Today. 1990 Jan;11(1):13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]