Abstract
Homologous tissues, such as adipose tissue, may be an interesting source of acellular scaffolds, maintaining a complex physiological three-dimensional (3D) structure, to be recellularized with autologous cells. The aim of the present work is to evaluate the possibility of obtaining homologous acellular scaffolds from decellularization of the omentum, which is known to have a complex vascular network. Adult rat and human omenta were treated with an adapted decellularization protocol involving mechanical rupture (freeze-thaw cycles), enzymatic digestion (trypsin, lipase, deoxyribonuclease, ribonuclease) and lipid extraction (2-propanol). Histological staining confirmed the effectiveness of decellularization, resulting in cell-free scaffolds with no residual cells in the matrix. The complex 3D networks of collagen (azan-Mallory), elastic fibers (Van Gieson), reticular fibers and glycosaminoglycans (PAS) were maintained, whereas Oil Red and Sudan stains showed the loss of lipids in the decellularized tissue. The vascular structures in the tissue were still visible, with preservation of collagen and elastic wall components and loss of endothelial (anti-CD31 and -CD34 immunohistochemistry) and smooth muscle (anti-alpha smooth muscle actin) cells. Fat-rich and well vascularized omental tissue may be decellularized to obtain complex 3D scaffolds preserving tissue architecture potentially suitable for recellularization. Further analyses are necessary to verify the possibility of recolonization of the scaffold by adipose-derived stem cells in vitro and then in vivo after re implantation, as already known for homologus implants in regenerative processes.
Key words: omentum, scaffold, decellularization, adipose tissue engineering, regenerative medicine, microvascularization
Introduction
In reconstructive surgery and regenerative medicine, tissue implants are useful in treating post-operative, congenital or post-traumatic loss of subcutaneous fat layers, which may result in scar tissue formation, deformity, and loss of function. Autologous, allogenic or alloplastic materials may be used for soft tissue augmentation.1 Many kinds of scaffolds have been developed, both naturally derived, like collagen, 2 fibrin,3 derivatives of hyaluronan4 and silk protein,5 and synthetic, like polylactic-coglycolic acid,6,7 polyglycolic acid8 and polyethylene glycol diacrylate.9 These matrices are usually seeded with human- or animal-derived primary cells or cell lines to evaluate the proliferative and differentiative responses in vitro and in vivo.10 Scaffolds must satisfy specific conditions, such as mechanical or material properties which make them suitable for incorporation in the host site: for example, they should be capable of mimicking native tissues into which they are placed in and minimizing any inflammation events or scar tissue formation.10
Synthetic implants are more frequently associated with immune rejection, allergic reactions, or implant migration and resorption, with consequent failure to integrate into the host tissues. For these reasons, tissue-engineered adipose substitutes, promoting regeneration rather than repair, have been developed, although it is not simple to maintain physiologic irroration after tissue re-implant.1,10
The greater omentum has been employed in various surgical procedures, because of its rich vascular supply with high capacity for absorption, pronounced angiogenic activity supporting hypoxic/ischemic tissues, innate immune function, ability to adhere to local structures, and high concentration of growth factors and factors promoting hemostasis.11–15 In particular, in hypoxic and traumatic conditions, omental adipocytes have been observed to increase the expression of angiogenic factors, mainly vascular endothelial growth factor (VEGF).16–19
The aim of the present work is to evaluate the effects of a decellularization protocol involving mechanical, enzymatic and lipid extractive mechanisms on the tissue architecture of rat and human omentum, to develop a novel scaffold suitable for autologous re-cellularization and implant in reconstructive surgery.
Materials and Methods
Male and female Sprague-Dawley rats were used according to the Italian Public Health Office regulations and European Communities Council Directive of 24 November 1986 (86/609/EEC). After an abdominal cut the greater omentum was resected and washed in sterile fresh cation-free phosphate buffered saline (PBS, Sigma-Aldrich, Milan, Italy) supplemented with 20 mg/mL bovine serum albumin (BSA, Sigma-Aldrich) within 2 h of extraction. Samples of human omentum were collected from autopsies of adult subjects. Samples were delivered to the laboratory on ice for processing within 2 h in sterile fresh PBS supplemented with 20 mg/mL BSA.
Decellularization of omentum
Briefly, adult rat and human omenta were treated with the following 5-day decellularization protocol involving mechanical rupture, polar solvent extraction, and enzymatic digestion. The protocol was mainly derived from previous studies on decellularization of placenta20 and adipose tissue10 and differs from methods proposed in a recent patent for the use of decellularized omentum matrix.21 Optimal incubation times in the different decellularization solutions were identified through intermediate morphologic analyses and DNA quantifications. The differences in incubation times between rats and humans mainly rely on tissue size, although the human omentum also showed higher tissue density and fat content than rat omentum. The following protocol refers to decellularization of samples of human omentum of 2×2 cm. Incubation times in the case of decellularization of rat samples (about 1×1 cm in size) were about 50% shorter.
Within 2 h of sampling, all pieces first underwent three freeze-thaw cycles (from −80°C to 37°C) in Freezing Buffer solution, containing 10 mM Tris base (Sigma-Aldrich) and 5 mM ethylenediaminetetraacetic acid (EDTA, Sigma Aldrich) at pH 8.0. The tissues were then transferred to Enzymatic Solution #1, composed of 0.25% trypsin and 0.1% EDTA (Invitrogen, Milan, Italy) and incubated for 16 h at 37°C, with shaking to separate cells. The following day, the tissues were treated with a polar-solvent extraction solution, composed of 2-propanol 99.9% (Sigma-Aldrich) for 48 h with shaking, to remove any lipid components. Subsequently, samples were rinsed 3 times for 30 min in Rinsing Buffer Solution, made up of 8 g/L NaCl, 200 mg/L KCl, 1 g/L Na2HPO4, and 200 mg/L KH2PO4 (all reagents from Sigma-Aldrich) at pH 8.0, to remove any 2-propanol residues, and underwent then further Enzymatic Solution #1 digestion for 6 h at 37°C with shaking. Following 3 more wash cycles in Rinsing Buffer Solution, the tissues were incubated with Enzymatic Solution #2, containing 55 mM Na2HPO4, 17 mM KH2PO4, 4.9 mM MgSO4*7H2O, 15,000 U deoxyribonuclease I type II (from bovine pancreas), 12.5 mg ribonuclease type IIIA (from bovine pancreas), 2000 U lipase type VI-S (from porcine pancreas) (all reagents from Sigma-Aldrich) for 16 h. At the end of digestion #2, treated tissues were rinsed 3 times in Rinsing Buffer Solution and subjected to a final polar solvent extraction for 6 h. At the end of processing, the decellularized matrix was rinsed three times in Rinsing Buffer Solution, three times in 70% ethanol, and then stored in sterile PBS supplemented with 1% antibioticantimycotic solution (ABAM, A5955, Sigma-Aldrich) at 4°C.
All decellularization solutions were supplemented with 1% ABAM. Moreover, all decellularization solutions with the exception of Enzymatic Solution #1 were also supplemented with 1% phenylmethanesulphonylfluoride (PMSF, Sigma-Aldrich).
Histological and immunohistochemical analysis
Samples of human and rat omentum before and after decellularization were fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin and cut into 3-µm thick sections. In particular, representative samples were taken from various regions of the decellularized scaffold, including the most central regions and the external surfaces. The following histochemical stainings were performed: Hematoxylin and Eosin (H&E), azan-Mallory, Van Gieson, PAS. Samples were also frozen in OCT compound for Oil Red, Sudan and 4',6-diamidino-2-phenylindole (DAPI) staining.
Immunohistochemical examinations were carried out with the following antibodies: anti-CD31 (Monoclonal Mouse Anti-Human CD31, Endothelial Cell, Code M0823, Dako, Milan, Italy); anti-CD34 (Monoclonal Mouse Anti-Human CD34 Class II, Code M7165, Dako); antialpha-smooth muscle actin (Monoclonal Mouse Anti-alpha-smooth muscle actin, mouse IgG2a kappa, clone 1A4, Code No. M0851, Dako). For anti-CD31 and -CD34 immunohistochemistry, unmasking was performed with 10 mM sodium citrate buffer, pH 6.0, at 90°C for 30 min. No antigen unmasking was necessary for anti-alpha-smooth muscle actin immunohistochemistry. Sections were incubated in 0.3% hydrogen peroxide for 10 min at room temperature, to prevent endogenous peroxidase activity, and then in blocking serum for 30 min at room temperature. As regards primary antibody incubation, both anti-CD31 and -CD34 antibodies were diluted 1:50 in blocking serum [0.04% bovine serum albumin (A2153, Sigma-Aldrich) and 0.5% normal goat serum (X0907, Dako Corporation, Carpinteria, CA, USA) in PBS] and incubated for 1 h at room temperature. Antialpha-smooth muscle actin antibody was diluted 1:100 in blocking serum and incubated for 1 h at room temperature. Sections were then washed three times for 5 min in PBS and incubated with ImmPRESS Universal Antibody (anti-mouse Ig/anti-rabbit Ig, peroxidase) Polymer Detection Kit (catalog no. MP-7500; Vector Laboratories, Burlingame, CA, USA) for 30 min. Lastly, sections were developed in 3,3 -diaminobenzidine (DAB, Sigma-Aldrich) and counterstained with hematoxylin.
DAPI staining, DNA extraction and quantification
Sections were fixed in acetone for 15 min at room temperature, washed in PBS for 10 min, incubated for 1 min in DAPI diluted 1:500 in methanol, washed twice in PBS, covered, and then examined under a fluorescent microscope. Some samples of human and rat omentum, before and after decellularization, were also frozen on dry ice and stored at −80°C for DNA extraction and quantification. Genomic DNA was extracted with the DNeasy® Blood & Tissue Kit (QIAGEN, Milan, Italy). Briefly, tissue was cut into small pieces, placed in a 1.5-mL microcentrifuge tube, and incubated with 20 µL of proteinase K for 56°C until completely lysed. To collect genomic DNA, 200 µL of first Buffer AL are added and then 200 µL ethanol (96–100%). The mixture was pipetted into a DNeasy Mini spin column placed in a 2-mL collection tube and centrifuged at 8000 rpm for 1 min; the flow-through was discarded. To wash DNA, spin column was filled in with 500 µL of Buffer AW1, centrifuged for 1 min at 8000 rpm, added with 500 µL of Buffer AW2, and centrifuged for 3 min at 14,000 rpm. The DNA was eluted by adding 200 µL of Elution Buffer and centrifuged for 1 min at 8000 rpm.
Total DNA concentration was measured with a spectrophotometer (NANODROP 1000, Techno Scientific, CELBIO, Milan, Italy) by reading the optical density (O.D.) at λ=260 nm, corresponding to the maximum absorption of nitrogenous bases.
Results
From a macroscopic point of view, the decellularization protocol, mainly during 2-isopropanol incubation, caused the loss of the lipid components of the samples, which passed toward the upper phase of the solution in the form of oil drops. As a result of lipid loss, omental samples changed color, from yellow to whitish. The decellularized samples preserved their volume, and did not show rupture of the tissue, although a slight reduction in consistency was observed.
Both in rat and human samples, histological stains showed loss of the cellular omental components and preservation of the 3D architecture of connective fibers (Figures 1 and 2). In particular, azan-Mallory, and van Gieson showed the persistence of collagen and elastic fibers, respectively, around the spaces previously occupied by adipocytes. Azan-Mallory identified many blue collagen fibers, which appeared to be packed densely and arranged in ordered bundles in the bulk of the material. Van Gieson showed elastic fibers organized in a quite complex 3D network surrounding adipocytes. PAS staining also showed the persistence of reticular fibers and glycosaminoglycans, which colored violet by this staining. Oil Red and Sudan stains also confirmed the loss of adipocytes and lipids from omental tissue after decellularization (Figure 3).
Figure 1.
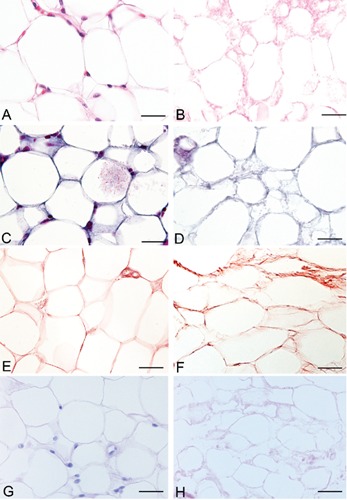
Representative sections of rat omentum, before (A, C, E, G) and after (B, D, F, H) decellularization protocol, showing persistence of three-dimensional organization of collagen (C–D), elastic (E–F), reticular fibers and glycosaminoglycans (G–H) (A–B: Hematoxylin & Eosin; C–D: azan Mallory; E–F: Van Gieson; G–H: PAS). Scale bars: A–H, 23.8 µm.
Figure 2.
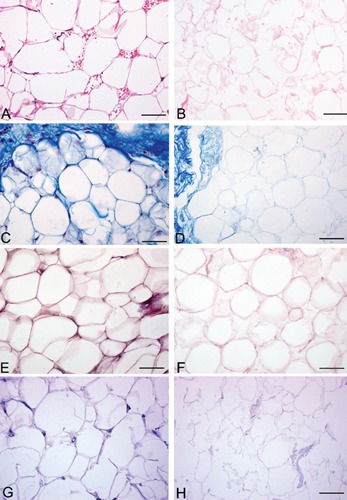
Representative sections of human omentum, before (A, C, E, G) and after (B, D, F, H) decellularization protocol, showing persistence of three-dimensional organization of collagen (CD), elastic (E–F), reticular fibers and glycosaminoglycans (G–H) (A–B, Hematoxylin&Eosin; C–D, azan-Mallory; E–F, Van Gieson; G–H, PAS). Scale bars: A–H, 37.5 µm.
Figure 3.
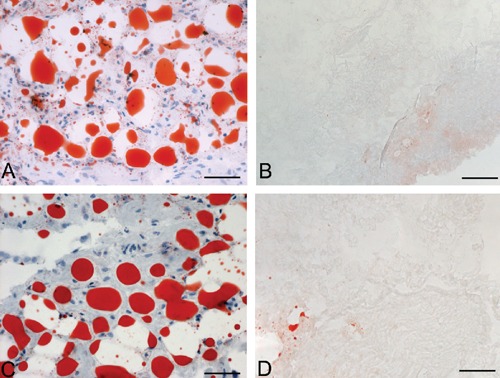
Sudan-stained (A–B) and Oil Red-stained (C–D) sections of human omentum, before (A, C) and after (B, D) decellularization, showing loss of lipids after protocol. Scale bars: A–D, 75 µm.
Analysis of the vascular components also showed that decellularization preserved vessel profiles, the lumens remaining visible with preservation of collagen and elastic fibers in the vessel walls in sections stained with azan-Mallory and van Gieson (Figures 4 and 5). Conversely, anti-CD31 and -CD34 immunohistochemical analyses showed the complete loss of immunostained endothelial cells (Figure 6). Anti-Smooth Muscle Actin immunohistochemistry also showed the absence of smooth muscle cells in vessel profiles exposed to decellularization (Figure 7).
Figure 4.
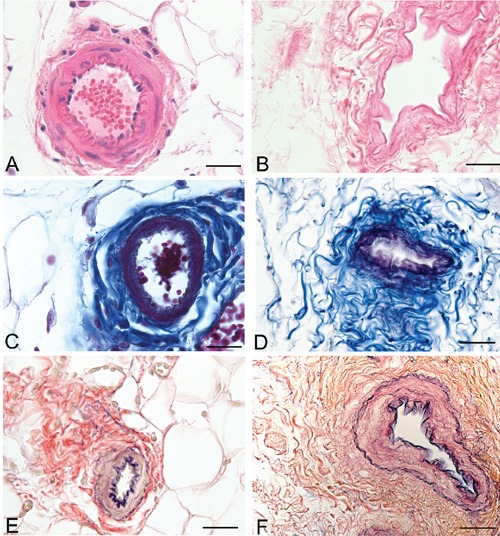
Representative sections of rat omentum blood vessels, before (A, C, E) and after (B, D, F) decellularization protocol, showing persistence of collagen (C–D) and elastic (E–F) fibers of vessel walls (A–B, Hematoxylin & Eosin; C–D, azan-Mallory; E–F, Van Gieson). Scale bars: A–F, 37.5 µm.
Figure 5.
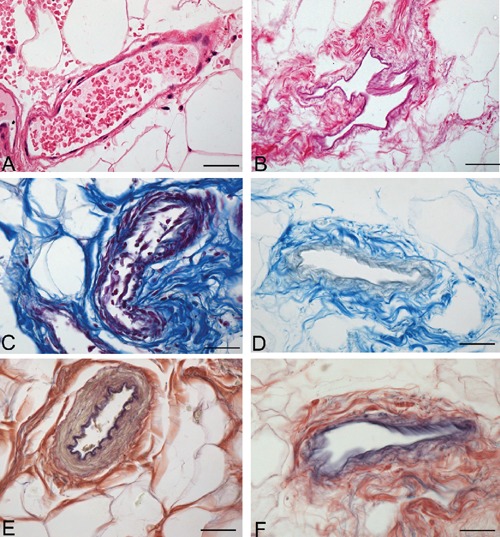
Representative sections of human omentum blood vessels, before (A, C, E) and after (B, D, F) decellularization protocol, showing persistence of collagen (C–D) and elastic (E–F) fibers of vessel walls (A–B, Hematoxylin & Eosin; C–D, azan Mallory; EF, Van Gieson). Scale bars: A–F, 37.5 µm.
Figure 6.
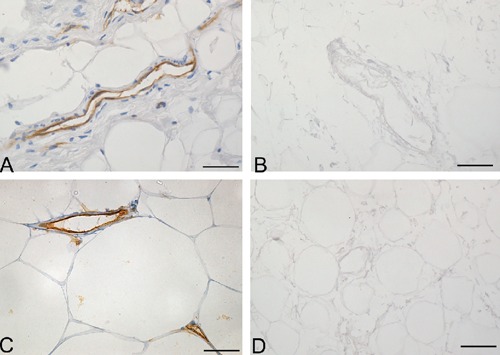
Anti-CD31 (A–B) and -CD34 (C–D) immunohistochemical analyses of human omentum, before (A, C) and after (B, D) decellularization protocol, showing loss of immunostained endothelial cells in decellularized samples. Scale bars: A–D, 37.5 µm.
Figure 7.
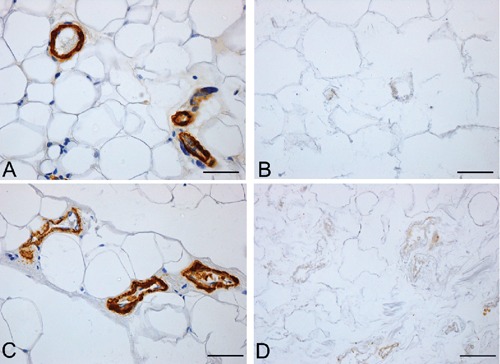
Anti-Smooth Muscle Actin immunohistochemistry of rat (A–B) and human (C–D) omentum, before (A, C) and after (B, D) decellularization protocol, showing loss of immunostained muscle cells in decellularized samples. Scale bars: A–D, 37.5 µm.
DAPI staining demonstrated the removal of nuclear material in decellularized omentum (Figure 8). Quantitative evaluation of DNA by spectrophotometry demonstrated <50 ng dsDNA per mg dry weight in all trials, these values being considered the minimal ones to achieve optimal decellularization.22–24
Figure 8.
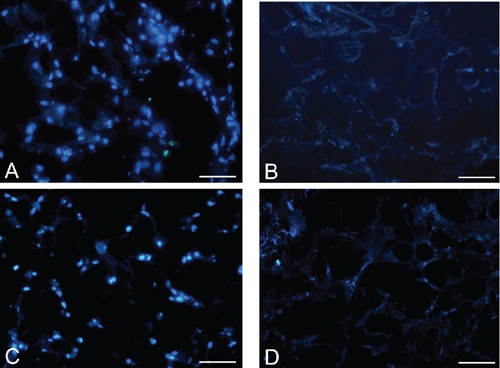
DAPI staining of rat (A–B) and human (C–D) omentum, before (A, C) and after (B, D) decellularization protocol, showing loss of nuclear material in decellularized samples. Scale bars: A–D, 75 µm.
Discussion
Omentum has frequently been used, as a peduncled or free flap, in several kinds of surgery, thanks to its angiogenic and regenerative properties. For instance, it has been used to cover colonic/colorectal, esophageal/esophagogastric and tracheal/bronchial anastomoses, to manage perforated gastric/duodenal ulcers and to reduce bleeding in hepatectomy or pancreaticoduodenectomy. 25–31 In pelvic irradiation, omental flap transpositions have also been used to exclude and protect the small bowel and bladder from the radiation field.32,33 Laparotomically or laparoscopically harvested omental flaps have been used for reconstructive surgery of superficial extra-abdominal structures. For instance, they have been used to treat large scalp defects,34 sternal wound infections after sternotomy for coronary artery bypass grafting,35 scrotal reconstruction,36–38 soft tissue augmentation of breasts39 and facial contour defects.40,41 In rats, fragmented omental tissues, with or without cotransplantation with preadipocytes, have been proved potentially useful for soft tissue augmentation.42 In pigs, omentum has also been wrapped around a scaffold made up of small intestinal submucosa matrix seeded with autologous urothelium and smooth muscle cell primary cultures, in order to develop engineered bladders43 and neoureters.44 In these latter cases, omentum provided autologous support for the growth and differentiation of the seeded cells, functioning as an in vivo bioreactor. A patent has also been published about methods for extracting fat from omentum and for applying decellularized omentum for tissue engineering.21 In this study, we demonstrated the possibility of decellularizing human omentum in order to obtain a biologic scaffold, with preservation of the three-dimensional structure of the extracellular matrix.
Several biological scaffolds derived from decellularization of human or animal materials have been approved for use in human patients. As regards allogenic scaffolds, it is worth mentioning products derived from decellularization of human dermis (AlloDerm®, LifeCell, Corp; AxisTM dermis, Mentor; etc.), fascia lata (AlloPatch®, Musculoskeletal Transplant Foundation; FasLata®, Bard) or pericardium (IOPatchTM, IOP Inc.) Decellularized placental matrix has also been proposed as a tissue-engineered adipose substitute. 20,45,46 In particular, the complex vascular network of the human placenta has been suggested to be useful for revascularization. Xenogenic biological scaffolds derived from porcine mesothelium (Meso BioMatrix™, Kensey Nash Corp.) are also commercially available. Conversely, apart from the above patent,21 biological scaffolds obtained by decellularization of human or animal omentum have not yet been considered in the scientific literature and are not commercially available. In particular, allogenic omentum could be obtained from both living and deceased donors. It could be re-cellularized in vitro through seeding of autologous adipose-derived stem cells and then implanted for soft tissue reconstruction.
Many decellularization protocols have been developed to obtain biologic scaffolds composed of extracellular matrix.24,47 Decellularization should ensure the removal of all residual cellular material and preserve the native architecture, ultrastructure and composition of the extracellular matrix. Residual cellular material may induce adverse host responses after implantation.23,48 Conversely, a preserved extracellular matrix may induce cell mitogenesis, chemotaxis and differentiation, favoring a constructive host tissue remodeling response.24,49,50 The integrity of the material should avoid any phenomena of degradation after tissue reconstruction and must promote tissue regeneration. Decellularized biological scaffolds have the advantage of possessing an extracellular matrix rich in the cell signaling components essential for cell adhesion, migration, proliferation and differentiation.51 Decellularization protocols may involve physical, chemical and/or biologic methods. The main physical methods are direct pressure, agitation, sonication, or snap freezing. Chemical agents include acids and bases, hypotonic and hypertonic solutions, detergents and alcohols. Enzymes usually used in decellularization are nucleases, collagenase, trypsin, lipase and others. Non-enzymatic biologic agents are EDTA and EGTA. The patent by Yang et al.21 involved a first phase of fat removal involving dehydration solvents (methanol, ethanol, isopropanol or propanol) and extraction solvents, both polar (acetone, dioxane or acetonitrile) and non-polar (hexane, xylene, benzene, toluene or ethyl acetate). After rehydration, decellularization phases involved non-ionic detergents, such as TRITON®X-100, metal salts (magnesium chloride, phosphate, acetate or citrate), and an enzyme solution with endonuclease. In the present work, we applied a different decellularization protocol involving multiple methods, i.e., freeze-thaw cycles, agitation, EDTA, trypsin, deoxyribonuclease, ribonuclease, lipase, 2-propanol and ethanol. The use of various methods increases the effectiveness of decellularization by means of different mechanisms. For instance, freezing is useful for disrupting cellular membranes and causing cell lysis, but must be integrated by methods to remove the products of cell lysis. In this sense, EDTA and trypsin are usually used together (in our decellularization protocol, enzymatic solution #1) to remove cellular materials. EDTA disrupts cell adhesion to the extracellular matrix by chelating divalent cations (Ca2+ and Mg2+) which intervene in cell attachment to collagen and fibronectin. Trypsin cleaves the peptide bonds on the carbon side of arginine and lysine when the next residue is not proline.47 Obviously, trypsin/EDTA exposure must not be too prolonged, in order to prevent excessive reduction in laminin, fibronectin and elastin contents.47 However, decellularization protocols involving trypsin/EDTA have been reported to allow endothelial cell growth in vitro.52,53 Ribonuclease and deoxyribonuclease are necessary to degrade RNA and DNA, and their presence in biological scaffolds is directly correlated with adverse host reactions.22,23
The absence of nucleic material in decellularized tissues was qualitatively verified in sections stained with H&E and DAPI. Quantitative evaluation of DNA was also performed. This analysis demonstrated <50 ng dsDNA per mg dry weight in all trials, according to the minimal criteria sufficient to achieve optimal decellularization.22–24 Obviously, in decellularization of omentum, the main components to be removed are lipids. In our protocol, lipase was the main agent used for delipidation. However, it has been stressed in the literature that lipase, if used alone, is usually not sufficient to remove all lipids and that the use of alcohols is also necessary.10,54 The decellularization protocol used in this study showed satisfactory lipid removal, as demonstrated in sections stained with Sudan and Oil Red.
Apart from the above agents used for decellularization, it is worth noting the role played by protease inhibitors and antibiotics in ensuring optimal decellularization. Due to cell lysis, many proteases are released and may damage the native ultrastructure of the extracellular matrix, so that protease inhibitors, such as PMSF used in the present protocol, are usually added. Antibiotics and antimycotics were also used to minimize microbial contamination. However, it has been stressed in the literature that antibiotics/antimycotics remaining in the scaffold after decellularization may increase the complexity of regulatory approval, the biological scaffold being considered a drug rather than a medical device.47
A further step of the present work will be recellularization of the scaffold with autologous stem cells before implant. In the patent by Yang et al.,21 decellularized omentum was proposed to be co-cultured with human kidney derived cells, urothelial cells, or endothelial cells in a tubular omentum matrix. However, adipose tissue is also a rich source of stem cells, the so-called adipose-derived stem cells, which have the capacity to differentiate along the adipogenic,55 chondrogenic, 56 myogenic,57 osteogenic,58 neurogenic,59 endothelial,60–63 and smooth muscle64 lineages. In our opinion, this kind of cells could be the most suitable ones for recellularization process. Autologous adipose-derived stem cells may easily be derived from liposuctioned fat, in which their frequency is much higher than in bone marrow.65 Structural studies have also stressed that some adipose tissue depots, such as the trochanteric fat pad, are particularly suited for regenerative procedures due to higher dissociability of the adipose elements and richness in stem cells.66,67 After a first in vitro recellularization phase, further cell invasion will derive in vivo after implantation, although these aspects must be addressed in future works. Omentum is frequently used in surgery, due to its elevated vascularization. The decellularization protocol used in the present work allowed vessel profiles to be preserved after removal of endothelial and smooth muscle cells, suggesting the possibility of re-cellularizing the omental vascular network with autologous adipose-derived stem cells or also with blood-derived endothelial progenitor cells. The complex microvascularisation of omentum may increase the potential of this tissue in reconstructive surgery, also with respect to autologous transplantation of other kinds of adipose tissue. Adipocytes in omentum are characterized by their high production of growth and angiogenic factors,16 which may also be preserved (at least partly) in decellularized scaffolds, favoring engraftment, proliferation and differentiation of stem cells. After decellularization, the same extracellular matrix may preserve components essential for cell adhesion, migration, proliferation and differentiation.51
In conclusion, in this study we present a decellularization protocol which proved effective in removing cell material and maintaining the extracellular matrix architecture of omentum, stressing the potential of this tissue for developing a new allogenic biological scaffold to be recellularized with autologous stem cells. Such a scaffold may be a viable alternative in various fields of reconstructive surgery and regenerative medicine.
References
- 1.Flynn LE, Prestwich GD, Semple JL, Woodhouse KA. Proliferation and differentiation of adipose-derived stem cells on naturally derived scaffolds. Biomaterials. 2008;29:1862–71. doi: 10.1016/j.biomaterials.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 2.Gentleman E, Nauman EA, Livesay GA, Dee KC. Collagen composite biomaterials resist contraction while allowing development of adipocytic soft tissue in vitro. Tissue Eng. 2006;12:1639–49. doi: 10.1089/ten.2006.12.1639. [DOI] [PubMed] [Google Scholar]
- 3.Cho SW, Kim SS, Rhie JW, Cho HM, Choi CY, Kim BS. Engineering of volumestable adipose tissues. Biomaterials. 2005;26:3577–85. doi: 10.1016/j.biomaterials.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Hemmrich K, von Heimburg D, Rendchen R, Di Bartolo C, Milella E, Pallua N. Implantation of preadipocyte-loaded hyaluronic acid-based scaffolds into nude mice to evaluate potential for soft tissue engineering. Biomaterials. 2005;26:7025–37. doi: 10.1016/j.biomaterials.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 5.Kang JH, Gimble JM, Kaplan DL. In vitro 3D model for human vascularized adipose tissue. Tissue Eng Part A. 2009;15:2227–36. doi: 10.1089/ten.tea.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrick CW. Breast tissue engineering. Annu Rev Biomed Eng. 2004;6:109–30. doi: 10.1146/annurev.bioeng.6.040803.140032. [DOI] [PubMed] [Google Scholar]
- 7.Neubauer M, Hacker M, Bauer-Kreisel P, Weiser B, Fischbach C, Schulz MB. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng. 2005;11:1840–51. doi: 10.1089/ten.2005.11.1840. [DOI] [PubMed] [Google Scholar]
- 8.Fischbach C, Spruss T, Weiser B, Neubauer M, Becker C, Hacker M. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res. 2004;300:54–64. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Alhadlaq A, Tang M, Mao JJ. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng. 2005;11:556–66. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 10.Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715–24. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Cartier R, Brunette I, Hashimoto K, Bourne WM, Schaff HV. Angiogenic factor: a possible mechanism for neovascularization produced by omental pedicles. J Thorac Cardiovasc Surg. 1990;99:264–8. [PubMed] [Google Scholar]
- 12.Saltz R, Stowers R, Smith M, Gadacz TR. Laparoscopically harvested omental free flap to cover a large soft tissue defect. Ann Surg. 1993;217:542–6. doi: 10.1097/00000658-199305010-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micheau P. The greater omentum. Its role in reconstructive plastic surgery. Ann Chir Plast Esthet. 1995;40:192–207. [PubMed] [Google Scholar]
- 14.Ignjatovic M, Pervulov S, Cuk V, Kostic Z, Minic L. Early angiogenic capabilities of the transposed omental flap after omentomyelopexy. Acta Chir Iugosl. 2001;48:41–3. [PubMed] [Google Scholar]
- 15.Costa SS, Blotta RM, Mariano MB, Meurer L, Edelweiss MI. Laparoscopic treatment of Poland's syndrome using the omentum flap technique. Clinics. 2010;65:401–6. doi: 10.1590/S1807-59322010000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum mediated angiogenesis. J Surg Res. 1997;67:147–54. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 17.Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3/F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27:1187–95. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- 18.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–34. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 19.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr. 2008;100:227–35. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 20.Flynn L, Semple JL, Woodhouse KA. Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A. 2006;79:359–69. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 21.Yang C, John TM, Gosiewska A, Buensuceso CS, Colter DC, Seyda A, et al. European Patent Specification (2012), EP 2 229 191B1. United States Patent Application Publication (2009), Pub. No. US 2009/0163990 A1; Decellularized omentum matrix and uses thereof. [Google Scholar]
- 22.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61–7. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–30. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller LC, Abendstein B, Salzer GM. Use of the greater omentum for treatment and prophylaxis of anastomotic and stump dehiscence in major airway surgery. Thorac Cardiovasc Surg. 1992;40:323–5. doi: 10.1055/s-2007-1020173. [DOI] [PubMed] [Google Scholar]
- 26.Darzi A, Cheshire NJ, Somers SS, Super PA, Guillou PJ, Monson JR. Laparoscopic omental patch repair of perforated duodenal ulcer with an automated stapler. Br J Surg. 1993;80:1552–1552. doi: 10.1002/bjs.1800801221. [DOI] [PubMed] [Google Scholar]
- 27.Merad F, Hay JM, Fingerhut A, Flamant Y, Molkhou JM, Laborde Y. Omentoplasty in the prevention of anastomotic leakage after colonic or rectal resection: a prospective randomized study in 712 patients. French Associations for Surgical Research. Ann Surg. 1998;227:179–86. doi: 10.1097/00000658-199802000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tocchi A, Mazzoni G, Lepre L, Costa G, Liotta G, Agostini N, et al. Prospective evaluation of omentoplasty in preventing leakage of colorectal anastomosis. Dis Colon Rectum. 2000;43:951–5. doi: 10.1007/BF02237357. [DOI] [PubMed] [Google Scholar]
- 29.Maeda A, Ebata T, Kanemoto H, Matsunaga K, Bando E, Yamaguchi S, et al. Omental flap in pancreaticoduodenectomy for protection of splanchnic vessels. World J Surg. 2005;29:1122–6. doi: 10.1007/s00268-005-7900-3. [DOI] [PubMed] [Google Scholar]
- 30.Bhat MA, Dar MA, Lone GN, Dar AM. Use of pedicled omentum in esophagogastric anastomosis for prevention of anastomotic leak. Ann Thorac Surg. 2006;82:1857–62. doi: 10.1016/j.athoracsur.2006.05.101. [DOI] [PubMed] [Google Scholar]
- 31.Collins D, Hogan AM, O'Shea D, Winter DC. The omentum: anatomical, metabolic, and surgical aspects. J Gastrointest Surg. 2009;13:1138–46. doi: 10.1007/s11605-009-0855-1. [DOI] [PubMed] [Google Scholar]
- 32.Russ JE, Smoron GL, Gagnon JD. Omental transposition flap in colorectal carcinoma: adjunctive use in prevention and treatment of radiation complications. Int J Radiat Oncol Biol Phys. 1984;10:55–62. doi: 10.1016/0360-3016(84)90412-7. [DOI] [PubMed] [Google Scholar]
- 33.Kim TH, Kim DY, Jung KH, Hong YS, Kim SY, Park JW, et al. The role of omental flap transposition in patients with locoregional recurrent rectal cancer treated with reirradiation. J Surg Oncol. 2010;102:789–95. doi: 10.1002/jso.21737. [DOI] [PubMed] [Google Scholar]
- 34.McLean DH, Buncke HJ., Jr. Autotransplant of omentum to a large scalp defect, with microsurgical revascularization. Plast Reconstr Surg. 1972;49:268–74. doi: 10.1097/00006534-197203000-00005. [DOI] [PubMed] [Google Scholar]
- 35.van Wingerden JJ, Coret ME, van Nieuwenhoven CA, Totté ER. The laparoscopically harvested omental flap for deep sternal wound infection. Eur J Cardio-thorac Surg. 2010;37:87–92. doi: 10.1016/j.ejcts.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Kamei Y, Aoyama H, Yokoo K, Fujii K, Kondo C, Sato T, et al. Composite gastric seromuscular and omental pedicle flap for urethral and scrotal reconstruction after Fournier's gangrene. Ann Plast Surg. 1994;33:565–8. doi: 10.1097/00000637-199411000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Isken T, Alagoz S, Unal C, Onyedi M, Utkan Z, Dillioglugil O. A new technique for reconstruction of the avulsed scrotum: the ‘3D- prefabricated omental flap’. J Plast Reconstr Aesthet Surg. 2009;62:410–2. doi: 10.1016/j.bjps.2008.04.065. [DOI] [PubMed] [Google Scholar]
- 38.Ng D, Tang CB, Kadirkamanathan SS, Tare M. Scrotal reconstruction with a free greater omental flap: A case report. Microsurgery. 2010;30:410–3. doi: 10.1002/micr.20763. [DOI] [PubMed] [Google Scholar]
- 39.Cothier-Savey I, Tamtawi B, Dohnt F, Raulo Y, Baruch J. Immediate breast reconstruction using a laparoscopically harvested omental flap. Plast Reconstr Surg. 2001;107:1156–65. doi: 10.1097/00006534-200104150-00009. [DOI] [PubMed] [Google Scholar]
- 40.Walkinshaw M, Caffee HH, Wolfe SA. Vascularized omentum for facial contour restoration. Ann Plast Surg. 1983;10:292–300. doi: 10.1097/00000637-198304000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Losken A, Carlson GW, Culbertson JH, Scott Hultman C, Kumar AV, Jones GE, et al. Omental free flap reconstruction in complex head and neck deformities. Head Neck. 2002;24:326–31. doi: 10.1002/hed.10082. [DOI] [PubMed] [Google Scholar]
- 42.Masuda T, Furue M, Matsuda T. Novel strategy for soft tissue augmentation based on transplantation of fragmented omentum and preadipocytes. Tissue Eng. 2004;10:1672–83. doi: 10.1089/ten.2004.10.1672. [DOI] [PubMed] [Google Scholar]
- 43.Baumert H, Simon P, Hekmati M, Fromont G, Levy M, Balaton A, et al. Development of a seeded scaffold in the great omentum: feasibility of an in vivo bioreactor for bladder tissue engineering. Eur Urol. 2007A;52:884–90. doi: 10.1016/j.eururo.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 44.Baumert H, Mansouri D, Fromont G, Hekmati M, Simon P, Massoud W, et al. Terminal urothelium differentiation of engineered neoureter after in vivo maturation in the "omental bioreactor". Eur Urol. 2007B;52:1492–8. doi: 10.1016/j.eururo.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 45.Flynn L, Woodhouse KA. Adipose tissue engineering with cells in engineered matrices. Organogenesis. 2008;4:228–35. doi: 10.4161/org.4.4.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering in vivo with adipose-derived stem cells on naturally derived scaffolds. J Biomed Mater Res A. 2009;89:929–41. doi: 10.1002/jbm.a.32044. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–91. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;16:1075–82. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yow K-H, Ingram J, Korossis SA, Ingham E, Homer-Vanniasinkam S. Tissue engineering of vascular conduits. Br J Surg. 2006;93:652–61. doi: 10.1002/bjs.5343. [DOI] [PubMed] [Google Scholar]
- 52.Schenke-Layland K, Vasilevski O, Opitz F, König K, Riemann I, Halbhuber KJ, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol. 2003;143:201–8. doi: 10.1016/j.jsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger-de Groot AC, DeRuiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27:566–71. doi: 10.1016/j.ejcts.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 54.Brown BN, Freund JM, Han L, Rubin JP, Reing JE, Jeffries EM, et al. Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng Part C Methods. 2011;17:411–21. doi: 10.1089/ten.tec.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 56.Nathan S, Das De S, Thambyah A, Fen C, Goh J, Lee EH. Cell-based therapy in the repair of osteochondral defects: a novel use for adipose tissue. Tissue Eng. 2003;9:733–44. doi: 10.1089/107632703768247412. [DOI] [PubMed] [Google Scholar]
- 57.Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–37. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 58.Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–9. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 59.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–63. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 61.Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–55. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 62.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–9. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 63.DiMuzio P, Tulenko T. Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells. J Vasc Surg. 2007;45:A99–103. doi: 10.1016/j.jvs.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodríguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–72. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–9. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 66.Sbarbati A, Accorsi D, Benati D, Marchetti L, Orsini G, Rigotti G, et al. Subcutaneous adipose tissue classification. Eur J Histochem. 2010;54:e48–e48. doi: 10.4081/ejh.2010.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panettiere P, Accorsi D, Marchetti L, Minicozzi AM, Orsini G, Bernardi P, et al. The trochanteric fat pad. Eur J Histochem. 2011;55:e16–e16. doi: 10.4081/ejh.2011.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]


