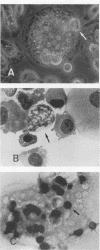
Abstract
The production of mature monocytes/macrophages is regulated by a group of hematopoietic growth factors, or colony-stimulating factors (CSF). We investigated the in vitro effect of human hematopoietic growth factors on human blood monocyte/macrophage differentiation and proliferation in short- and long-term in vitro cultures. The addition of macrophage CSF, granulocyte-macrophage CSF, and granulocyte CSF and interleukin-6 and interleukin-3 growth factors to monocyte/macrophage cultures induced morphological changes in cultured cells, including enhancement of cell growth and the formation of multinucleated giant cells, spindle-like cells, and fibroblast-like cells. In addition, CD4 and HLA-DR antigen expression was down regulated by the addition of growth factors without a change in the expression of other surface antigens, including CD3, CD11B, CD14, CD15, NK H1, and B1. The proliferating cell nuclear antigen was not detected in growth factor-treated nonadherent monocytes/macrophages in long-term cultures. Bromodeoxyuridine was incorporated in the adherent monocytes/macrophages, and intense staining in the small rounded cells which occur above the adherent cells in these cultures was observed after a 72-h pulse, indicating that monocytes/macrophages are slowly dividing cells.
Full text
PDF
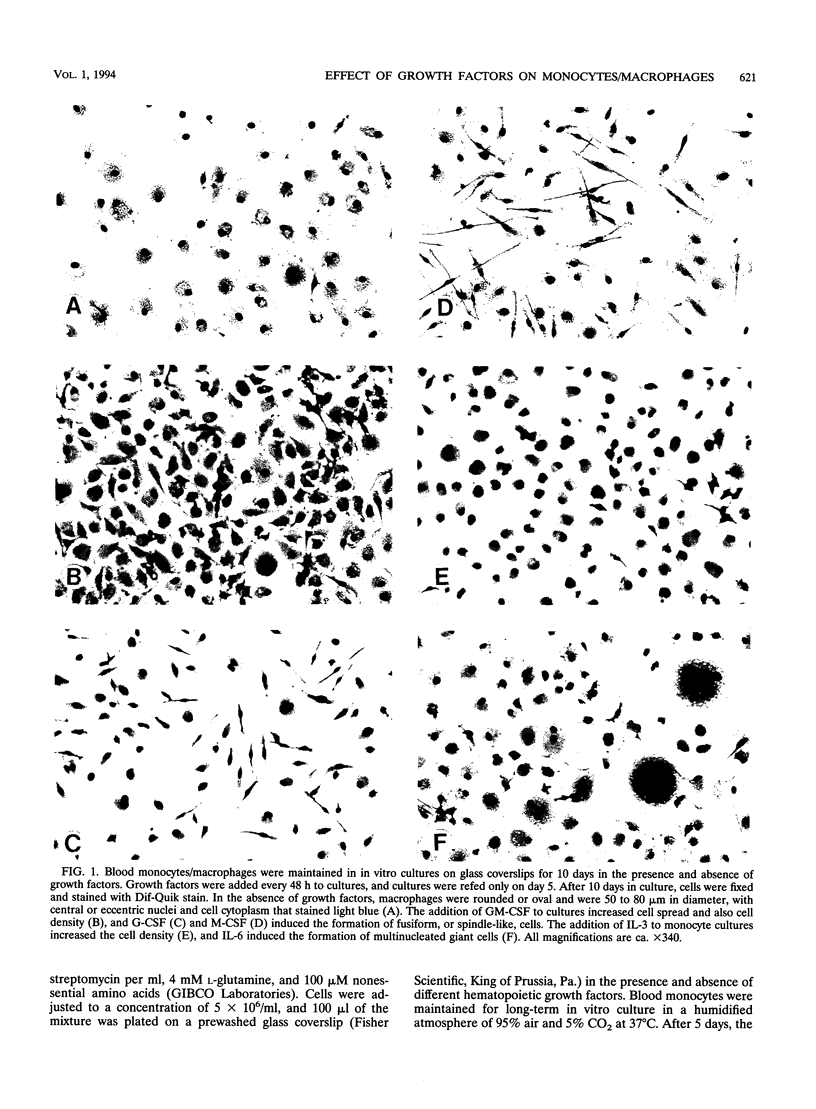

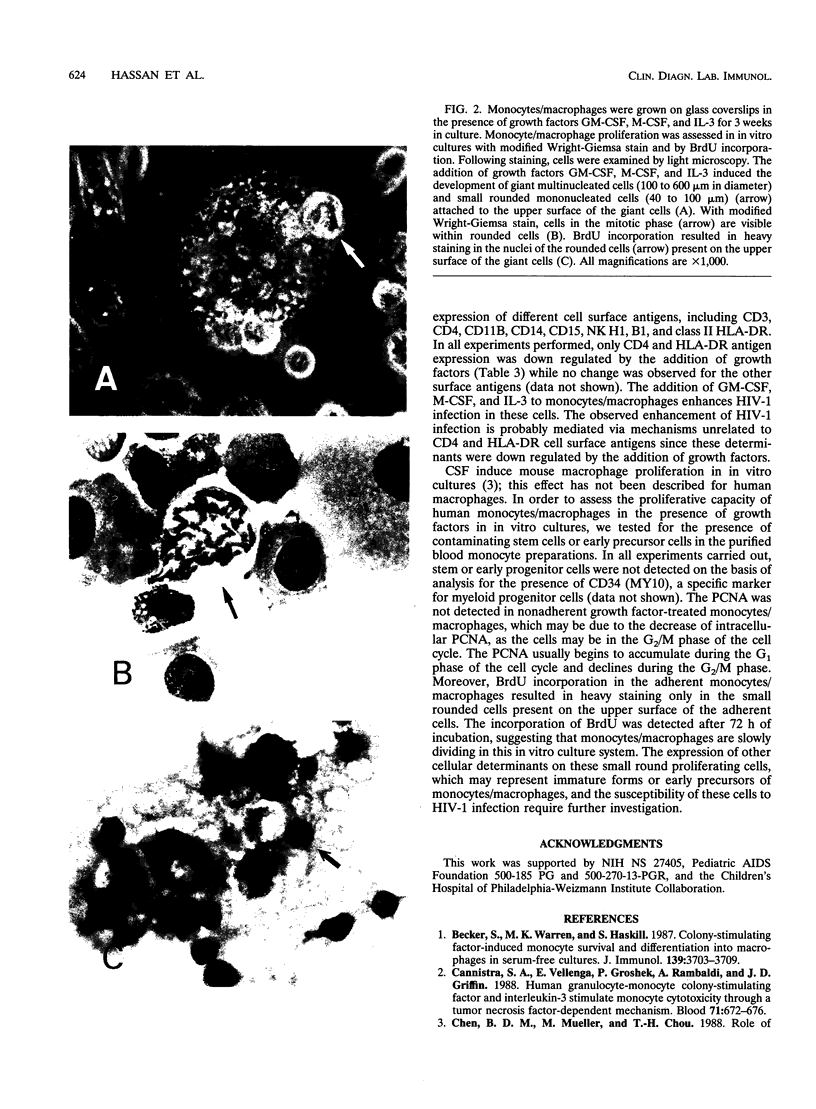

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker S., Warren M. K., Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol. 1987 Dec 1;139(11):3703–3709. [PubMed] [Google Scholar]
- Cannistra S. A., Vellenga E., Groshek P., Rambaldi A., Griffin J. D. Human granulocyte-monocyte colony-stimulating factor and interleukin 3 stimulate monocyte cytotoxicity through a tumor necrosis factor-dependent mechanism. Blood. 1988 Mar;71(3):672–676. [PubMed] [Google Scholar]
- Chen B. D., Mueller M., Chou T. H. Role of granulocyte/macrophage colony-stimulating factor in the regulation of murine alveolar macrophage proliferation and differentiation. J Immunol. 1988 Jul 1;141(1):139–144. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Hassan N. F., Campbell D. E., Douglas S. D. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986 Dec 24;95(2):273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Hassan N. F., Cutilli J. R., Douglas S. D. Isolation of highly purified human blood monocytes for in vitro HIV-1 infection studies of monocyte/macrophages. J Immunol Methods. 1990 Jul 3;130(2):283–285. doi: 10.1016/0022-1759(90)90058-4. [DOI] [PubMed] [Google Scholar]
- Hassan N. F., Kamani N., Meszaros M. M., Douglas S. D. Induction of multinucleated giant cell formation from human blood-derived monocytes by phorbol myristate acetate in in vitro culture. J Immunol. 1989 Oct 1;143(7):2179–2184. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Koyanagi Y., O'Brien W. A., Zhao J. Q., Golde D. W., Gasson J. C., Chen I. S. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988 Sep 23;241(4873):1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- McInnes A., Rennick D. M. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988 Feb 1;167(2):598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. E. Cytokines and retroviruses. Clin Immunol Immunopathol. 1992 Jul;64(1):23–27. doi: 10.1016/0090-1229(92)90054-r. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Burgess A. W., Johnson G. R., Nicola N. A., Nice E. C., DeLamarter J., Thatcher D. R., Mermod J. J. In vitro actions on hemopoietic cells of recombinant murine GM-CSF purified after production in Escherichia coli: comparison with purified native GM-CSF. J Cell Physiol. 1986 Sep;128(3):421–431. doi: 10.1002/jcp.1041280311. [DOI] [PubMed] [Google Scholar]
- Orentas R. J., Reinlib L., Hildreth J. E. Anti-class II MHC antibody induces multinucleated giant cell formation from peripheral blood monocytes. J Leukoc Biol. 1992 Mar;51(3):199–209. doi: 10.1002/jlb.51.3.199. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Warren M. K., Vogel S. N. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J Immunol. 1985 Feb;134(2):982–989. [PubMed] [Google Scholar]