Abstract
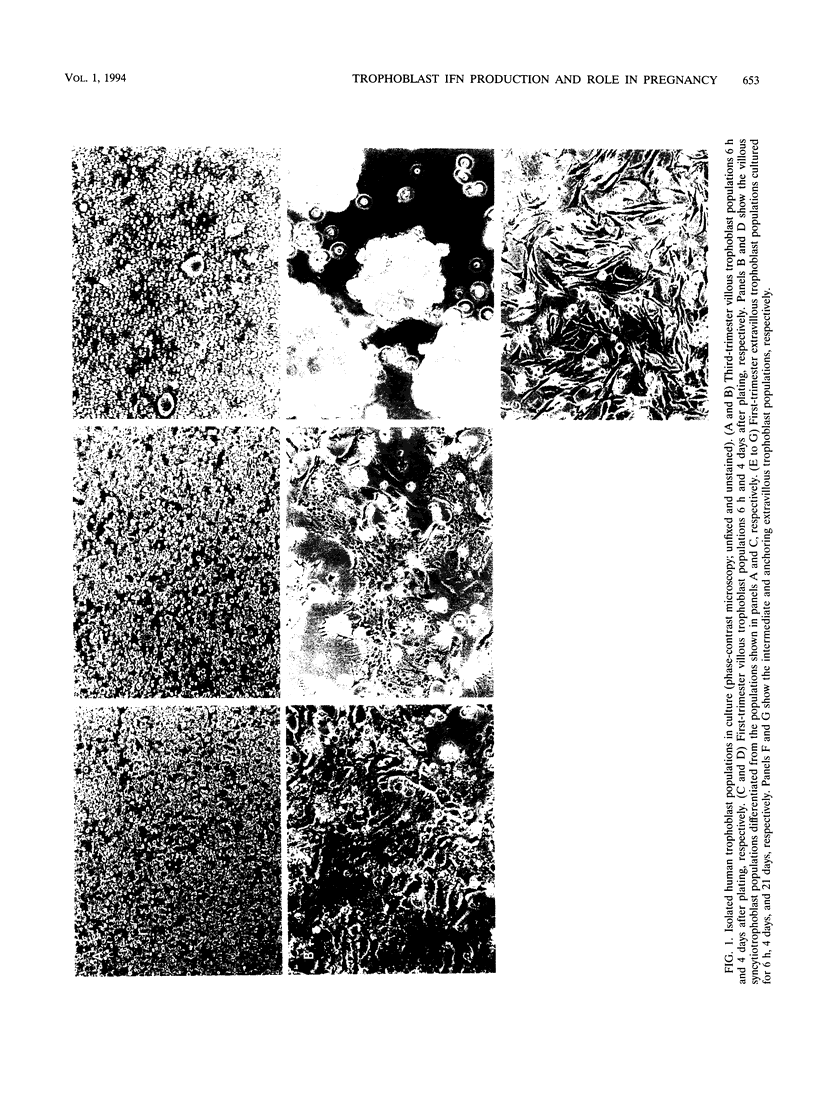
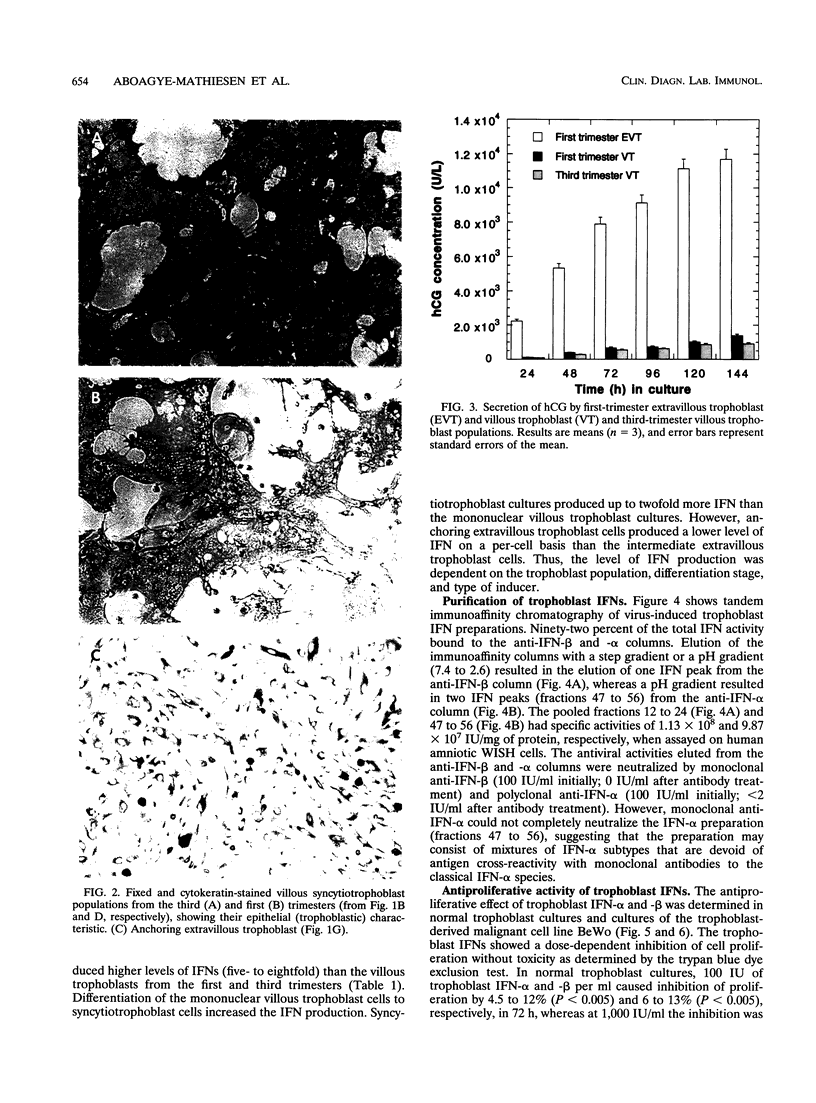
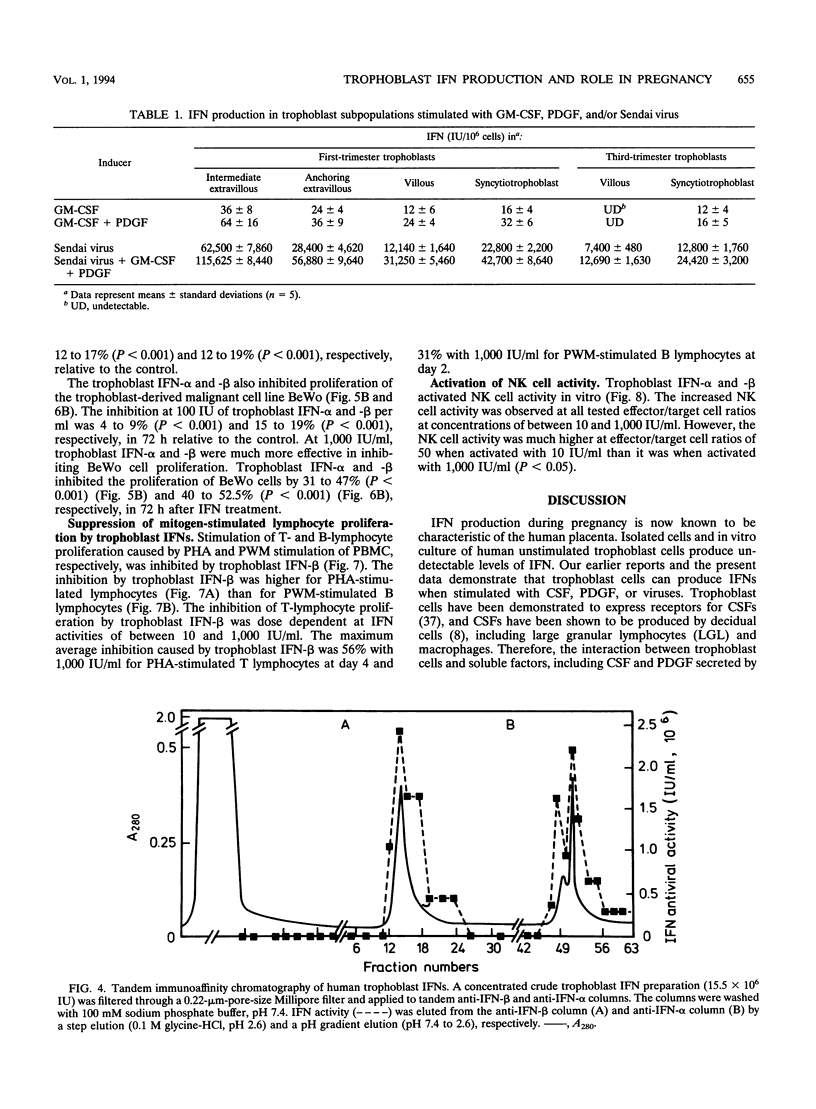
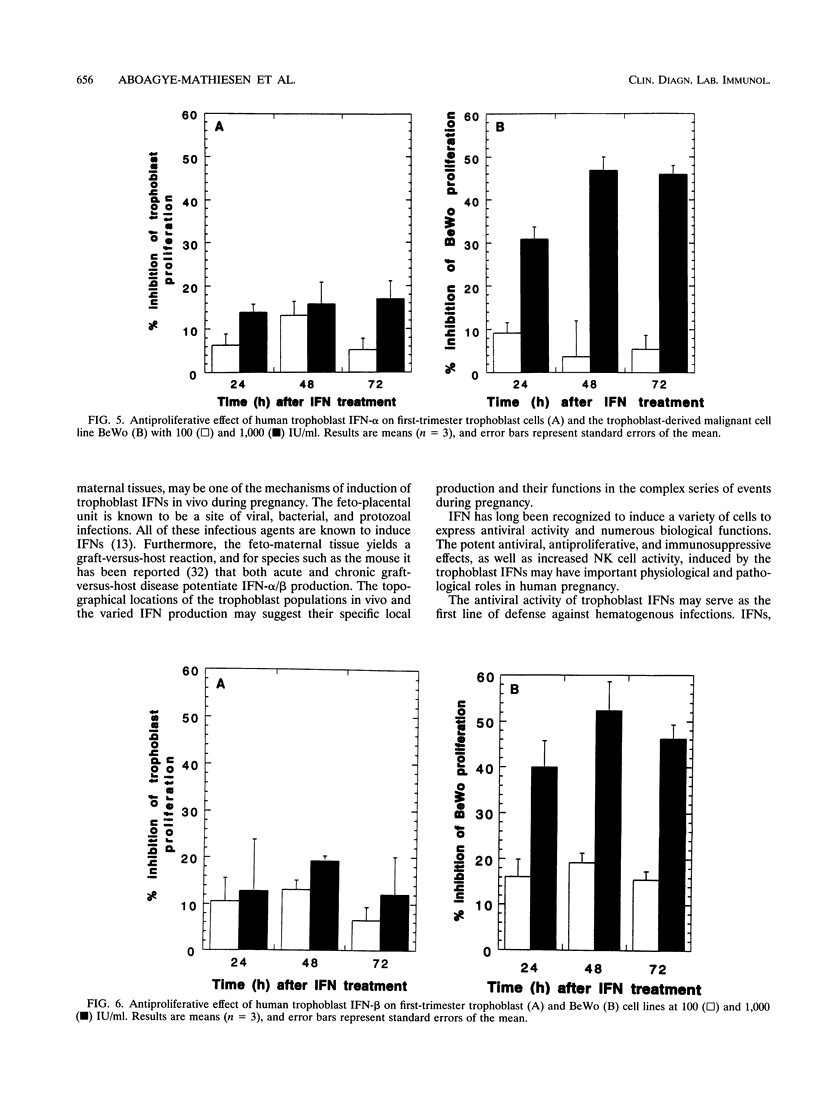
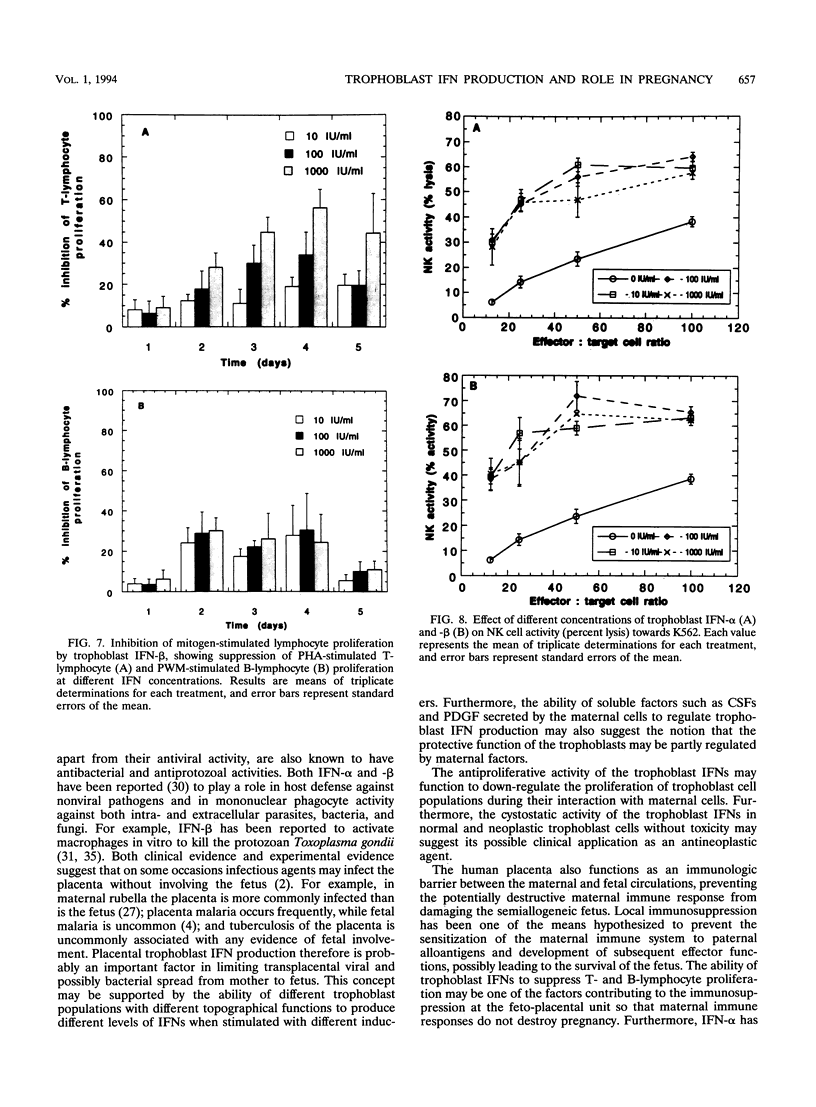
The human cytotrophoblasts are the first fetal cells to arise during embryogenesis and are the progenitor cells to villous (noninvasive), syncytiotrophoblast (noninvasive), "intermediate" extravillous (invasive), and "anchoring" extravillous (invasive) trophoblast subpopulations. These trophoblast subpopulations were isolated from first- and third-trimester placentae and were stimulated with Sendai virus, granulocyte-macrophage colony-stimulating factors (GM-CSF), and platelet-derived growth factor (PDGF) to produce interferons (IFNs). GM-CSF and PDGF induced very low levels of IFN in first-trimester extravillous and villous trophoblast subpopulations. Highly proliferating and invasive intermediate extravillous trophoblast cultures produced five- to eightfold more IFNs than villous trophoblast cultures and two- to fivefold more IFN than the syncytiotrophoblast cultures when stimulated with Sendai virus. Syncytiotrophoblast cultures produced higher levels of IFNs (up to twofold) than villous trophoblast cultures when stimulated with the same virus. Pretreatment of first-trimester extravillous and villous trophoblast cultures with GM-CSF and PDGF followed by infection with Sendai virus resulted in greater IFN production than when the cultures were stimulated with virus alone. The levels of IFN produced were dependent on the type of trophoblast, the type of inducer, and the stage of differentiation of the trophoblasts. The purified trophoblast IFNs have potent antiviral activities when assayed on human amniotic WISH cells, and they inhibited proliferation of normal trophoblasts and trophoblast-derived malignant cells in vitro without any toxicity. Furthermore, the trophoblast IFNs activated NK cell activity and suppressed mitogen-stimulated lymphocyte proliferation at concentrations of between 10 and 1,000 IU/ml. The possible functions of the trophoblast IFNs during pregnancy are discussed with respect to human placental and fetal protection and development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboagye-Mathiesen G., Tóth F. D., Juhl C., Nørskov-Lauritsen N., Petersen P. M., Zachar V., Ebbesen P. Characterization of Sendai virus-induced human placental trophoblast interferons. J Gen Virol. 1991 Aug;72(Pt 8):1871–1876. doi: 10.1099/0022-1317-72-8-1871. [DOI] [PubMed] [Google Scholar]
- COVELL G. Congenital malaria. Trop Dis Bull. 1950 Dec;47(12):1147–1167. [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T., Lindahl P., Gresser I. Inhibitory effect of interferon preparations and inducers on the multiplication of transplanted allogeneic spleen cells and syngeneic bone marrow cells. Nat New Biol. 1973 Apr 4;242(118):152–153. doi: 10.1038/newbio242152a0. [DOI] [PubMed] [Google Scholar]
- Douglas G. C., King B. F. Isolation of pure villous cytotrophoblast from term human placenta using immunomagnetic microspheres. J Immunol Methods. 1989 May 12;119(2):259–268. doi: 10.1016/0022-1759(89)90405-5. [DOI] [PubMed] [Google Scholar]
- Duc-Goiran P., Robert-Galliot B., Lopez J., Chany C. Unusual apparently constitutive interferons and antagonists in human placental blood. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5010–5014. doi: 10.1073/pnas.82.15.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley D. J., Mitchell M. D., Creighton K., Branch D. W. Lymphokine production during term human pregnancy: differences between peripheral leukocytes and decidual cells. Am J Obstet Gynecol. 1990 Dec;163(6 Pt 1):1890–1893. doi: 10.1016/0002-9378(90)90769-4. [DOI] [PubMed] [Google Scholar]
- Flint A. P., Lamming G. E., Stewart H. J. A role for interferons in the maternal recognition of pregnancy. Mol Cell Endocrinol. 1988 Aug;58(2-3):109–111. doi: 10.1016/0303-7207(88)90144-x. [DOI] [PubMed] [Google Scholar]
- Genbacev O., Schubach S. A., Miller R. K. Villous culture of first trimester human placenta--model to study extravillous trophoblast (EVT) differentiation. Placenta. 1992 Sep-Oct;13(5):439–461. doi: 10.1016/0143-4004(92)90051-t. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Ellis D. A., Black P. H., Monaco A. P., Wood M. L. Immunosuppressive effects of an interferon preparation in vivo. Transplantation. 1974 Feb;17(2):234–236. doi: 10.1097/00007890-197402000-00014. [DOI] [PubMed] [Google Scholar]
- Hoshina M., Boothby M., Boime I. Cytological localization of chorionic gonadotropin alpha and placental lactogen mRNAs during development of the human placenta. J Cell Biol. 1982 Apr;93(1):190–198. doi: 10.1083/jcb.93.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshina M., Boothby M., Hussa R., Pattillo R., Camel H. M., Boime I. Linkage of human chorionic gonadotrophin and placental lactogen biosynthesis to trophoblast differentiation and tumorigenesis. Placenta. 1985 Mar-Apr;6(2):163–172. doi: 10.1016/s0143-4004(85)80066-7. [DOI] [PubMed] [Google Scholar]
- Howatson A. G., Farquharson M., Meager A., McNicol A. M., Foulis A. K. Localization of alpha-interferon in the human feto-placental unit. J Endocrinol. 1988 Dec;119(3):531–534. doi: 10.1677/joe.0.1190531. [DOI] [PubMed] [Google Scholar]
- Hsi B. L., Hunt J. S., Atkinson J. P. Differential expression of complement regulatory proteins on subpopulations of human trophoblast cells. J Reprod Immunol. 1991 Apr;19(3):209–223. doi: 10.1016/0165-0378(91)90036-p. [DOI] [PubMed] [Google Scholar]
- Imakawa K., Hansen T. R., Malathy P. V., Anthony R. V., Polites H. G., Marotti K. R., Roberts R. M. Molecular cloning and characterization of complementary deoxyribonucleic acids corresponding to bovine trophoblast protein-1: a comparison with ovine trophoblast protein-1 and bovine interferon-alpha II. Mol Endocrinol. 1989 Jan;3(1):127–139. doi: 10.1210/mend-3-1-127. [DOI] [PubMed] [Google Scholar]
- Imakawa K., Helmer S. D., Nephew K. P., Meka C. S., Christenson R. K. A novel role for GM-CSF: enhancement of pregnancy specific interferon production, ovine trophoblast protein-1. Endocrinology. 1993 Apr;132(4):1869–1871. doi: 10.1210/endo.132.4.7681767. [DOI] [PubMed] [Google Scholar]
- Jondal M., Spine C., Targan S. Human spontaneous killer cells selective for tumour-derived target cells. Nature. 1978 Mar 2;272(5648):62–64. doi: 10.1038/272062a0. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986 Apr;118(4):1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Lebon P., Girard S., Thépot F., Chany C. The presence of alpha-interferon in human amniotic fluid. J Gen Virol. 1982 Apr;59(Pt 2):393–396. doi: 10.1099/0022-1317-59-2-393. [DOI] [PubMed] [Google Scholar]
- Lefèvre F., Boulay V. A novel and atypical type one interferon gene expressed by trophoblast during early pregnancy. J Biol Chem. 1993 Sep 15;268(26):19760–19768. [PubMed] [Google Scholar]
- Loke Y. W., Burland K. Human trophoblast cells cultured in modified medium and supported by extracellular matrix. Placenta. 1988 Mar-Apr;9(2):173–182. doi: 10.1016/0143-4004(88)90015-x. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Pathogenesis of viral infections of the fetus. Prog Med Virol. 1968;10:194–237. [PubMed] [Google Scholar]
- Mobraaten L. E., De Maeyer E., De Maeyer-Guignard J. Prolongation of allograft survival in mice by inducers of interferon. Transplantation. 1973 Nov;16(5):415–420. doi: 10.1097/00007890-197311000-00005. [DOI] [PubMed] [Google Scholar]
- Morrish D. W., Shaw A. R., Seehafer J., Bhardwaj D., Paras M. T. Preparation of fibroblast-free cytotrophoblast cultures utilizing differential expression of the CD9 antigen. In Vitro Cell Dev Biol. 1991 Apr;27A(4):303–306. doi: 10.1007/BF02630907. [DOI] [PubMed] [Google Scholar]
- Murray H. W. The interferons, macrophage activation, and host defense against nonviral pathogens. J Interferon Res. 1992 Oct;12(5):319–322. doi: 10.1089/jir.1992.12.319. [DOI] [PubMed] [Google Scholar]
- Orellana M. A., Suzuki Y., Araujo F., Remington J. S. Role of beta interferon in resistance to Toxoplasma gondii infection. Infect Immun. 1991 Sep;59(9):3287–3290. doi: 10.1128/iai.59.9.3287-3290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. E., Klimpel G. R. Interferon alpha/beta synthesis during acute graft-versus-host disease. Transplantation. 1987 Mar;43(3):412–416. doi: 10.1097/00007890-198703000-00018. [DOI] [PubMed] [Google Scholar]
- Ritson A., Bulmer J. N. Endometrial granulocytes in human decidua react with a natural-killer (NK) cell marker, NKH1. Immunology. 1987 Oct;62(2):329–331. [PMC free article] [PubMed] [Google Scholar]
- Roberts R. M., Imakawa K., Niwano Y., Kazemi M., Malathy P. V., Hansen T. R., Glass A. A., Kronenberg L. H. Interferon production by the preimplantation sheep embryo. J Interferon Res. 1989 Apr;9(2):175–187. doi: 10.1089/jir.1989.9.175. [DOI] [PubMed] [Google Scholar]
- Schmitz J. L., Carlin J. M., Borden E. C., Byrne G. I. Beta interferon inhibits Toxoplasma gondii growth in human monocyte-derived macrophages. Infect Immun. 1989 Oct;57(10):3254–3256. doi: 10.1128/iai.57.10.3254-3256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth F. D., Nørskov-Lauritsen N., Juhl C. B., Aboagye-Mathiesen G., Ebbesen P. Interferon production by cultured human trophoblasts and choriocarcinoma cell lines induced by Sendai virus. J Gen Virol. 1990 Dec;71(Pt 12):3067–3069. doi: 10.1099/0022-1317-71-12-3067. [DOI] [PubMed] [Google Scholar]
- Uzumaki H., Okabe T., Sasaki N., Hagiwara K., Takaku F., Tobita M., Yasukawa K., Ito S., Umezawa Y. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9323–9326. doi: 10.1073/pnas.86.23.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui J., Garcia-Lloret M., Brown A. J., Berdan R. C., Morrish D. W., Wegmann T. G., Guilbert L. J. Functional, long-term cultures of human term trophoblasts purified by column-elimination of CD9 expressing cells. Placenta. 1994 Apr;15(3):231–246. doi: 10.1016/0143-4004(94)90015-9. [DOI] [PubMed] [Google Scholar]