Aflatoxins, a group of polyketide-derived furanocoumarins (Fig. 1), are the most toxic and carcinogenic compounds among the known mycotoxins. Among the at least 16 structurally related aflatoxins characterized, however, there are only four major aflatoxins, B1, B2, G1, and G2 (AFB1, AFG1, AFB2, and AFG2), that contaminate agricultural commodities and pose a potential risk to livestock and human health (2, 8, 13, 33, 34, 52, 62, 81). Aspergillus flavus produces AFB1 and AFB2. Aspergillus parasiticus produces AFB1, AFG1, AFB2, and AFG2. Some other species that produce aflatoxins are Aspergillus nomius, Aspergillus pseudotamarii (51), Aspergillus bombycis (82), Aspergillus ochraceoroseus (60; J. C. Frisvad and R. A. Samson, Abstr. 10th Int. Congr. Mycol., p. 24, 2002), and Emericella venezuelensis (M. Klich, personal communication). Aflatoxins were discovered in A. flavus (hence the name “a-fla-toxin”) about 40 years ago after an outbreak of Turkey X disease in England (60). Other significant members of the aflatoxin family, M1 and M2, are oxidative forms of aflatoxin B1 modified in the digestive tract of some animals and isolated from milk, urine, and feces (14). Of the four aflatoxins, aflatoxin B1 is the most potent hepatocarcinogenic compound. There has been very detailed research on the natural occurrence, identification, characterization, biosynthesis, and genetic regulation of aflatoxins, as well as on the prevention and control of aflatoxin contamination of food and feed. Aflatoxin biosynthesis has been proposed to involve at least 23 enzymatic reactions. Thus far, at least 15 structurally well-defined aflatoxin intermediates have been identified in the aflatoxin biosynthetic pathway (reviewed in references 8, 14, 15, 35, 73, 80, 94, 120, and 123). It has been demonstrated that 25 identified genes clustered within a 70-kb DNA region in the chromosome are involved in aflatoxin biosynthesis (94, 114). Here, we propose a new naming scheme that follows the naming convention in Aspergillus. These genes and their enzymes involved in the aflatoxin biosynthetic pathway are reviewed. Sterigmatocystin (ST) and dihydrosterigmatocystin (DHST), produced by certain strains of Aspergillus nidulans (37), are the penultimate precursors of aflatoxins. The homologous genes of ST synthesis in A. nidulans and their involvement in the biochemical pathway common to aflatoxins and ST are discussed.
FIG. 1.
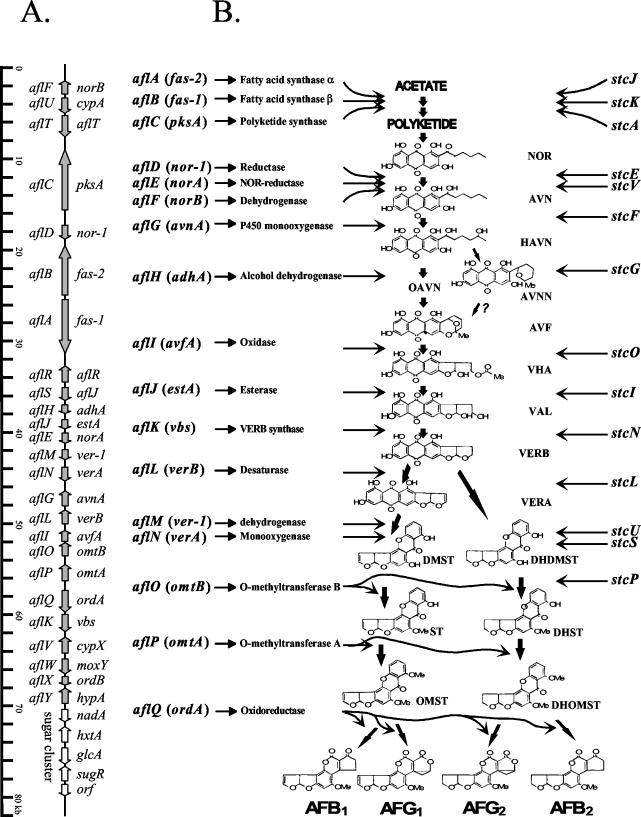
Clustered genes (A) and the aflatoxin biosynthetic pathway (B). The generally accepted pathway for aflatoxin and ST biosynthesis is presented in panel B. The corresponding genes and their enzymes involved in each bioconversion step are shown in panel A. The vertical line represents the 82-kb aflatoxin biosynthetic pathway gene cluster and sugar utilization gene cluster in A. parasiticus and A. flavus. The new gene names are given on the left of the vertical line and the old gene names are given on the right. Arrows along the vertical line indicate the direction of gene transcription. The ruler at far left indicates the relative sizes of these genes in kilobases. The ST biosynthetic pathway genes in A. nidulans are indicated at the right of panel B. Arrows in panel B indicate the connections from the genes to the enzymes they encode, from the enzymes to the bioconversion steps they are involved in, and from the intermediates to the products in the aflatoxin bioconversion steps. Abbreviations: NOR, norsolorinic acid; AVN, averantin; HAVN, 5′-hydroxyaverantin; OAVN, oxoaverantin; AVNN, averufanin; AVF, averufin; VHA, versiconal hemiacetal acetate; VAL, versiconal; VERB, versicolorin B; VERA, versicolorin A; DMST, demethylsterigmatocystin; DHDMST, dihydrodemethylsterigmatocystin; ST, sterigmatocystin; DHST, dihydrosterigmatocystin; OMST, O-methylsterigmatocystin; DHOMST, dihydro-O-methylsterigmatocystin; AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2.
AFLATOXIN PATHWAY GENE CLUSTER
The completed 70-kb DNA sequence containing the 25 genes or open reading frames (ORFs) represents a well-defined aflatoxin pathway gene cluster (Fig. 1). On average, about 2.8 kb of chromosomal DNA contains one gene. Among these genes are large ones of about 5 to 7 kb each, encoding the fatty acid synthase (FAS) alpha (5.8 kb) and beta (5.1 kb) subunits (FASα and FASβ) and polyketide synthase (PKS; 6.6 kb). Excluding these three large genes, the average size of the other 22 genes is about 2 kb. In the 5′ end of the cluster sequence, an approximately 2-kb DNA region with no identifiable ORF was located. This sequence presumably marks the end of this cluster in this orientation. The 3′ end of this gene cluster is delineated by a well-defined sugar utilization gene cluster consisting of four genes (33). The 82,081-bp fully annotated DNA sequence in A. parasiticus containing the aflatoxin pathway gene cluster and the sugar utilization gene cluster has been submitted to the GenBank database (nucleotide sequence accession number AY371490).
NEW NAMING SCHEME FOR THE AFLATOXIN PATHWAY GENES
The first aflatoxin biosynthesis gene cloned was nor-1 in A. parasiticus (23). The name of this gene, like those of many other genes in the pathway, is based on the substrate converted by the gene product. The genes named according to substrates include nor-1 (norsolorinic acid [NOR]), norA (NOR), norB (NOR), avnA (averantin [AVN]), avfA (averufin [AVF]), ver-1 (versicolorin A [VERA]), verA (VERA), and verB (versicolorin B [VERB]). Other genes were named according to their enzymatic functions. These include fas-2 (FAS alpha subunit), fas-1 (FAS beta subunit), pksA or pksL1 (PKS), adhA (alcohol dehydrogenase), estA (esterase), vbs (VERB synthase), dmtA (mt-I) (O-methyltransferase I) or omtB (O-methyltransferase B), omtA (O-methyltransferase A), ordA (oxidoreductase A), cypA (cytochrome P450 monooxygenase), cypX (cytochrome P450 monooxygenase), and moxY (monooxygenase). fas-1 was initially named uvm8 since it was identified through UV mutation. The fas-2 and fas-1 genes were also named hexA and hexB for the hexanoate synthase alpha and beta subunits, respectively (GenBank accession no. AF391094). The aflR regulatory gene was initially named afl-2 in A. flavus (79) and apa-2 in A. parasiticus (24). This regulatory gene was later named aflR in both A. flavus and A. parasiticus as well as in A. nidulans for its function as a transcription activator. Another gene was demonstrated to be somehow involved in regulation and was named aflJ (72). For consistency and uniformity with the functions of the genes in the aflatoxin biosynthetic pathway, we institute here a consensus for gene naming in Aspergillus (4, 36). The three-letter code “afl” is used to represent aflatoxin pathway genes. A capital letter in alphabetical order from “A” to “Y” represents each individual gene confirmed to be or potentially involved in aflatoxin biosynthesis, e.g., aflA to aflY for all of the 25 genes and ORFs (Fig. 1) (Table 1). Those genes whose pathway involvement has already been characterized and confirmed or proposed on the basis of homologies to known genes in aflatoxin or ST synthesis are designated aflA to aflQ from the initial conversion of fatty acids to the final products, aflatoxins. aflR (retains the same name) and aflS (aflJ) are named for transcription regulators. Those genes whose pathway involvements are ambiguous or remain unclear at this point are designated aflT (retains the same name), aflU (cypA), aflV (cypX), aflW (moxY), aflX (ordB), and aflY (hypA) (Table 1). We promote the use of this conventional naming system in the future. We also encourage the use of both the new name and the old name in parentheses, such as aflA (fas-2), the first time a gene designation appears in manuscripts to assist in understanding gene function.
TABLE 1.
Aflatoxin pathway cluster genes
| Gene | Original name and other names used (accession no.)a | ST gene homologb | Enzyme or product | Function in the pathwaye |
|---|---|---|---|---|
| aflA | fas-2 (hexA) (AF391094) | stcJ | FAS alpha subunit | Acetate → polyketide |
| aflB | fas-1 (hexB) (AF391094), uvm8, fas1, fas-1A (L48183) | stcK | FAS beta subunit | Acetate → polyketide |
| aflC | pksA (Z47198), pksL1 (L42765, L42766) | stcA | PKS | Acetate → polyketide |
| aflD | nor-1 (L27801) | stcE | Reductase | NOR → AVN |
| aflEc | norA, aad (U24698), adh-2 in A. flavus (U32377) | stcV | NOR reductase/dehydrogenase | NOR → AVN |
| aflFc | norB | Dehydrogenase | NOR → AVN | |
| aflG | avnA (U62774), ord-1 (L40839) | stcF | P450 monooxygenase | AVN → HAVN |
| aflH | adhA (U76621) | stcG | Alcohol dehydrogenase | HAVN → AVF or AVNN |
| aflI | avfA (AF154050), ord-2 (L40840) (AF159789 in A. flavus) | stcO | Oxidase | AVF → VHA |
| aflJ | estA (AF417002) | stcI | Esterase | VHA → VAL |
| aflK | vbs (AF169016, U51327) | stcN | VERB synthase | VAL → VERB |
| aflL | verB (AF106958) (AF106959 and AF106960 in A. flavus) | stcL | Desaturase | VERB → VERA |
| aflM | ver-1 (M91369) | stcU | Dehydrogenase/ketoreductase | VERA → DMST |
| aflNc | verA | stcS (verA) | Monooxygenase | VERA → DMST |
| aflO | dmtA (mt-I) (AB022905, AB022906), omtB (AF154050) (AF159789 in A. flavus) | stcP | O-Methyltransferase I or O-methyltransferase B | DMST → ST, DHDMST → DHST |
| aflP | omtA (L25834), omt-1 cDNA (L22091), (L25836 in A. flavus) | O-Methyltransferase A or O-methyltransferase II | ST → OMST, DHST → DHOMST | |
| aflQ | ordA (AF017151, AF169016), A. flavus ord-1 (U81806, U81807) | Oxidoreductase/P450 monooxygenase | OMST → AFB1 and AFG1, DHOMST → AFB2 and AFG2 | |
| aflR | aflR (L26222), apa-2 (L22177), afl-2 (AF427616, AF441429) | aflR | Transcription activator | Pathway regulator |
| aflS | aflJ (AF002660) (AF077975 in A. flavus) | Unnamed | Transcription enhancer | Pathway regulator |
| aflT | aflT (AF268071) | Transmembrane protein | Unassigned | |
| aflU | cypA | P450 monooxygenase | Unassigned | |
| aflV | cypX (AF169016) | stcB | P450 monooxygenase | Unassigned |
| aflW | moxY (AF169016) | stcW | Monooxygenase | Unassigned |
| aflX | ordB | stcQ | Monooxygenase/oxidase | Unassigned |
| aflY | hypA | Hypothetical protein | Unassigned | |
| aflR2d | aflR2 (AF452809) | Second copy | Transcription activator | |
| aflS2 | aflJ2 (AF452809, AF295204) | Second copy | Transcription enhancer | |
| aflH2 | adhA2 (AF452809) | Second copy | Alcohol dehydrogenase | |
| aflJ2 | estA2 (AF452809) | Second copy | Esterase | |
| aflE2 | norA2 (AF452809) | Second copy | Dehydrogenase (early terminated) | |
| aflM2 | ver-1B (AF452809) | Second copy | Dehydrogenase (missing N terminal) | |
| aflO2 | omtB2 (AF452809) | Second copy | Methyltransferase B (missing N terminal) |
The accession number of the complete 82,081-bp aflatoxin gene cluster, including a sugar utilization gene cluster, in A. parasiticus is AY391490 and updates the sequences of the underlined accession numbers. The genes and their accession numbers are from A. parasiticus unless otherwise noted.
The accession number of the ST gene cluster in A. nidulans is U34740, and the corresponding contig number is 1.132 (from 183018 to 242843) in the Whitehead database.
The placements of aflE (norA), aflF (norB), and aflN (verA) in the pathway were based on their homologies to aflatoxin or ST genes and their functions have not been experimentally confirmed.
The aflR2, aflS2, aflH2, aflJ2, aflE2, aflM2, and aflO2 genes are partially duplicated cluster genes (second copy) in A. parasiticus, and their functions and chromosomal locations in the genome have not yet been clarified.
Arrows signify conversion.
The names of some of the duplicated aflatoxin genes (21, 30, 64) in A. parasiticus include the numeral “2,” indicating second copy, such as aflR2, aflS2 (aflJ2), aflH2 (adhA2), aflJ2 (estA2), aflE2 (norA2), aflM2 (ver1B), and aflO2 (omtB2). The genes in this partial duplicated cluster are likely to be nonfunctional, possibly because of chromosomal location (31; J. Yu, unpublished observation). Recent evidence (J. E. Linz, unpublished observation) shows that the aflM2 (ver1B) gene is expressed, but its translation remains to be investigated.
GENES IN AFLATOXIN BIOSYNTHESIS
Aflatoxins are polyketide-derived secondary metabolites produced via the following conversion path: acetate → polyketide → anthraquinones → xanthones → aflatoxins (3, 6, 11, 15, 102, 120). The genes involved in the major conversion steps from early precursors to aflatoxins and their functions are discussed below. The gene homologs in A. nidulans involved in the biosynthesis of ST are compared and discussed in Table 2.
TABLE 2.
Aflatoxin cluster genes and their ST gene homologs
| Gene (old name) | No. of aad | Introns | TCGN5CGA position(s) (deviation)a | ST gene homolog | No. of aa for homolog | Introns in homolog | % aa identity (% of homologous aa) |
|---|---|---|---|---|---|---|---|
| aflA (fas-2) | 1,671 | 2 | −503 (TCGN5CGG) | stcJ | 1,559 | 1 | 48 (62) |
| aflB (fas-1) | 1,888 | 3 | −209 (CCGN5CGA) | stcK | 1,914 | 3 | 45 (61) |
| aflC (pksA) | 2,109 | 5 | −469, −118 | stcA (wA) | 2,181 | 2 | 60 (72) |
| aflD (nor-1) | 271 | 3 | −98 | stcE | 260 | 3 | 56 (73) |
| aflE (norA) | 388 | 1 | −150 | stcV | 387 | 2 | 66 (82) |
| aflF (norB) | 382 | 0 | −113 | ||||
| aflG (avnA) | 495 | 2 | −171, −110 (TCGN5CGG) | stcF | 506 | 1 | 71 (84) |
| aflH (adhA) | 278 | 0 | −149 | stcG | ? | ? | ? |
| aflI (avfA) | 285 | 0 | Follow omtB?b | stcO | 290 (297)c | 0 | 55 (66) |
| aflJ (estA) | 314 | 1 | −144, −99 | stcI | 286 | 0 | 50 (64) |
| aflK (vbs) | 643 | 1 | −147 | stcN | ? | ? | ? |
| aflL (verB) | 500 | 1 | −86 | stcL | 500 | 1 | 82 (89) |
| aflM (ver-1) | 262 | 2 | −182, −149 | stcU (verA) | 264 | 2 | 90 (96) |
| aflN (verA) | 492 | 1 | −238 | stcS | 505 | 0 | 64 (75) |
| aflO (omtB) | 386 | 3 | −216 | stcP | 208 (379)c | 3 | 75 (85) |
| aflP (omtA) | 418 | 4 | −174 | ||||
| aflQ (ordA) | 528 | 6 | −114 | ||||
| aflR (aflR) | 444 | 0 | −120, −249 (TTAGGCCTAA) | aflR | 433 | 0 | 33 (45) |
| aflS (aflJ) | 438 | 2 | −74 (CCGN5CGA) | Unnamed | |||
| aflT (aflT) | 514 | 5 | −320 (TCGN5CGC) | ||||
| aflU (cypA) | 498 | 4 | −176 | ||||
| aflV (cypX) | 508 | 2 | −137 | stcB | 435 | 3 | 61 (73) |
| aflW (moxY) | 481 | 0 | −188, −170 | stcW | 488 | 3 | 69 (80) |
| aflX (ordB) | 266 | 0 | −145 | stcQ | 274 | 0 | 54 (68) |
| aflY (hypA) | 495 | 2 | −124 |
TCGN5CGA is the AflR-binding motif, and deviations from this typical motif are given. Confirmed AflR-binding sites (44) are underlined.
No TCGN5CGA motif has been identified in the aflI (avfA) promoter region. It is likely cotranscribed with the alfO (omtB) gene.
Based on a recent study of A. parasiticus (117), the correct number of amino acids in ST genes is suggested in parentheses.
aa, amino acids.
aflA (fas-2), aflB (fas-1), and aflC (pksA) are involved in the conversion of acetate to NOR.
Molecular evidence has demonstrated that two FASs and a PKS are involved in the synthesis of a polyketide from the primary metabolite, acetate (16, 92). By complementation of an aflatoxin-blocked UV mutant, UVM8, Mahanti et al. (67) identified a gene initially named uvm8 which is required for NOR biosynthesis and aflatoxin production in A. parasiticus. The predicted amino acid sequence of uvm8 shares high degrees of similarity (67%) and identity (48%) to the beta subunit of FASs (FAS1) from Saccharomyces cerevisiae (96, 97). Complementation, metabolite feeding, and gene disruption experiments performed by Mahanti et al. (67) showed that the 7.5-kb transcript of the uvm8 gene encodes one subunit of a novel FAS directly involved in the formation of the polyketide backbone prior to the conversion to the next stable metabolite, NOR, in aflatoxin synthesis. Because of its presumed function, the uvm8 gene was renamed fas-1A or fas-1 for the FAS beta subunit in aflatoxin biosynthesis. Additional sequence analyses of a cosmid clone found another FAS gene, fas-2A, encoding the alpha subunit of FAS (67). These names, fas-1A and fas-2A, were then simplified to fas-1 and fas-2 for the aflatoxin pathway gene cluster encoding FAS-1 (FASβ) and FAS-2 (FASα), respectively (80). The fas-2 and fas-1 genes were also named hexA and hexB for the hexanoate synthase alpha and beta subunits, respectively (GenBank accession no. AF391094). Brown et al. (16) proposed the involvement of FAS in ST biosynthesis in A. nidulans. They identified two genes, stcJ and stcK, encoding FASα and FASβ subunits (FAS-2 and FAS-1) in the ST cluster required for ST synthesis that are homologous to fas-2 and fas-1, respectively. For consistency in nomenclature, these two genes, fas-2 and fas-1, encoding the FAS alpha and beta subunits, have been designated aflA and aflB, respectively.
Watanabe et al. (99) reported the role of the FASs as well as the presence of a PKS in aflatoxin biosynthesis. Chang et al. (25) cloned the pksA gene encoding the PKS from A. parasiticus. Trail et al. (97) demonstrated, by using knockout experiments, that pksA is important for aflatoxin biosynthesis. The pksA gene is weakly homologous to a PKS-encoding gene (wA) that was identified earlier in A. nidulans and involved in spore pigmentation (70). Feng and Leonard (45) also isolated a PKS gene, which they named pksL1, from A. parasiticus. Disruption of the pksL1 gene produced neither aflatoxins nor any aflatoxin intermediates. pksL1 was found to be identical to pksA, and they are most likely the same gene. Yu and Leonard (125) isolated a PKS gene, pksST, from A. nidulans. Its nucleotide sequence is identical to that of stcA from A. nidulans (17). However, no significant nucleotide sequence homology was found between wA (70) and stcA (pksST). The predicted amino acid sequences of these PKSs contain four conserved domains typical of other known PKS proteins: β-ketoacyl synthase, acyltransferase, acyl carrier protein, and thioesterase. The PKS gene from A. parasiticus (pksA or pksL1) was designated pksA (114) in the aflatoxin pathway gene cluster and its homolog in A. nidulans was designated stcA (17). Watanabe and Townsend (100) partially purified the roughly 1,400-kDa PKS NorS from A. parasiticus. NOR is the first stable intermediate in the pathway (1, 7, 76, 77). The conversion of noranthrone to NOR is poorly defined, but it has been proposed to occur via a noranthrone oxidase (98), a monooxygenase (12), or spontaneously (41). The pksA gene for this PKS is here renamed aflC.
aflD (nor-1), aflE (norA), and aflF (norB) are involved in the conversion of NOR to AVN.
By use of NOR-accumulating mutants, it was demonstrated by Papa (76, 77) in A. flavus and by Bennett (1) and Detroy et al. (40) in A. parasiticus that NOR is an intermediate in the aflatoxin biosynthetic pathway (7). It was found that the NOR-accumulating mutants are always leaky and that aflatoxin biosynthesis is not completely blocked (40). NOR is converted to AVN by a reductase/dehydrogenase enzyme, and this reaction is reversible depending on NADP(H) or NAD(H) (3, 12, 41, 106). Chang et al. (23) cloned the nor-1 gene that complemented a NOR-accumulating mutant of A. parasiticus. It was demonstrated that this gene encoded a ketoreductase that was capable of converting NOR to AVN (90, 95). Cary et al. (19) cloned another possible allele of the NOR reductase gene, norA, which had about 70% homology to aryl-alcohol dehydrogenases. The norA gene may be involved in the conversion of NOR to AVN (19). However, deletion of norA did not impair the ability to convert NOR to AVN (20). This might be due to the presence of aflD (nor-1), aflE2 (norA2), and other NOR reductase genes in the genome. The norA gene had no significant homology to the nor-1 gene at either the DNA or amino acid level. An additional gene, norB, was identified in the aflatoxin gene cluster and was found to have no significant homology at the DNA level to either nor-1 or norA. However, the homology to the norA protein at the amino acid level was as high as 68%. Attempts to delete the norB gene failed to generate mutants lacking aflatoxin production (Yu, unpublished). This might be due to the presence of the other two NOR reductase genes, nor-1 and norA. The nor-1 and norA gene homologs in A. nidulans are stcE and stcV, respectively. However, no norB gene homolog was identified in the ST gene cluster (17). The norA and norB genes were found in the Aspergillus flavus EST database to be expressed under aflatoxin-supportive medium conditions, indicating possible functional involvement in aflatoxin synthesis (Yu, unpublished). The enzymatic function and coordinated genetic regulation of the three genes are to be studied further. The nor-1, norA, and norB genes are renamed aflD, aflE, and aflF, respectively.
aflG (avnA) gene encodes a cytochrome P450 monooxygenase that converts AVN to HAVN.
Evidence that 5′-hydroxyaverantin (HAVN) is an intermediate in aflatoxin biosynthesis has been reported (5, 6). Yabe et al. (106) demonstrated for the first time that HAVN is a precursor of aflatoxins and that AVN is successively converted to HAVN and finally AVF by a microsome and cytosol enzyme, respectively. Yu et al. (116) cloned and characterized a gene that encoded a cytochrome P450 monooxygenase (originally reported as ord-1 [114]). Gene disruption and substrate feeding studies (116) have demonstrated that HAVN and possibly an additional compound are the intermediate products in the conversion of AVN to AVF. This avnA gene is here renamed aflG.
aflH (adhA) is involved in conversion of HAVN to AVF.
In the scheme proposed by Yabe et al. (106, 108), averufanin (AVNN) was considered to be a shunt metabolite and not an aflatoxin intermediate. Bhatnagar et al. (12) proposed that both AVN and AVNN were intermediates in the pathway from NOR to AVF on the basis of radiolabeling experiments. However, Chang et al. (29) cloned a gene, adhA, which encodes an alcohol dehydrogenase. Disruption of adhA resulted in accumulation of HAVN in the fungal mycelia. These results suggested that HAVN is converted to AVF by the enzyme encoded by adhA (29). Sakuno et al. (85) recently succeeded in characterizing two cytosolic enzymes and an intermediate, 5′-oxoaverantin (OAVN), involved in this pathway from HAVN to AVF. The enzyme that converts HAVN to OAVN is consistent with the protein encoded by adhA (85). The gene for the second enzyme has yet to be identified. The adhA gene is here renamed aflH.
aflI (avfA) encodes an oxidase for conversion of AVF to VHA.
The conversion from AVF to versiconal hemiacetal acetate (VHA) is thought to involve an oxidase (12). Yu et al. (119) cloned a gene, avfA, from A. parasiticus, an A. flavus AVF-accumulating strain, and an Aspergillus sojae strain. Gene complementation experiments using the AVF-accumulating mutant strain demonstrated that the avfA gene encodes an enzyme (oxidase) that is necessary for the conversion of AVF to VHA. The avfA gene is here renamed aflI. A more recent study (111) identified an additional stable intermediate, hydroxyversicolorone (93), between AVF and VHA. In addition, several metabolites, versicolorone (9), versicolorol, versiconol acetate (VOAc), and versiconol (VOH), were found to be transiently accumulating as well (111). These metabolites might be involved in the shunt steps from hydroxyversicolorone to VHA and to versiconal (VAL). No genes responsible for these biological steps have thus far been identified in the aflatoxin cluster.
aflJ (estA) is involved in the conversion of VHA to VAL.
Evidence for the involvement of an esterase in the conversion of VHA to VAL in aflatoxin biosynthesis was found when A. parasiticus was treated with the organophosphorus pesticide dichlorvos (5, 48, 86, 105, 106, 112). The esterase was purified from A. parasiticus (50, 61), and the gene for an esterase, estA, was cloned (124). On the basis of its homology to stcI in the ST gene cluster in A. nidulans (17), this enzyme was proposed to be involved in the conversion of VHA to VAL in aflatoxin synthesis. Gene disruption demonstrated that aflI (estA) is directly involved in the conversion of VHA to VAL and of VOAc to VOH in a separate conversion scheme of VHA to VOAc to VOH to VAL (P.-K. Chang et al., submitted for publication).
aflK (vbs) is involved in the conversion of VAL to VERB.
Conversion of VAL to VERB or versicolorin C in A. parasiticus has been shown to involve a versiconal cyclase (66). Yabe and Hamasaki (107) provided enzymatic evidence for the conversion. Silva et al. (88), Silva and Townsend (87), and McGuire et al. (71) cloned and demonstrated the function of the VERB synthase gene, vbs, for the conversion of VHA to VERB in A. parasiticus. This is a key step in aflatoxin formation since it closes the bisfuran ring of aflatoxin; this ring is required for binding to DNA and gives aflatoxin its mode of action as a mutagen. The vbs gene is here renamed aflK.
aflL (verB) is involved in the conversion of VERB to VERA.
Yabe and Hamasaki (107) have demonstrated that, in the aflatoxin biosynthetic pathway, the formation of VERA from VERB is a branch point separating biosynthesis of AFB1 and AFG1 from that of AFB2 and AFG2 (11, 13). The conversion of VERB to VERA has also been proposed and confirmed to require a desaturation of the bisfuran ring of VERB (107). Disruption of stcL in A. nidulans by Kelkar et al. (54) prevented ST synthesis and resulted in the accumulation of VERB, thereby showing that stcL encoding a P450 monooxygenase was required for the conversion. The stcL homolog, aflL (verB), from A. parasiticus and A. flavus was cloned in the aflatoxin pathway gene cluster (GenBank accession no. AF106958). The aflL gene encoding a cytochrome P450 monooxygenase/desaturase is presumed to be involved in the conversion of VERB to VERA in aflatoxin biosynthesis. The gene responsible for the conversion directly from VERB to demethyldihydrosterigmatocystin (DMDHST) and then to AFB2 and AFG2 has not been defined. It is possible that aflL is involved in conversion of both VERB to VERA and VERB to DMDHST.
aflM (ver-1) and aflN (verA) are involved in the conversion of VERA to DMST.
The ver-1 gene involved in aflatoxin synthesis was first cloned in A. parasiticus (89). This gene was shown, by complementation of a ver-1 mutant, to be required for the conversion of VERA to demethylsterigmatocystin (DMST) (65, 89). Keller et al. (56) identified a gene, stcU (formerly named verA), a homolog of ver-1, in A. nidulans that encodes a ketoreductase required for the conversion of VERA to DMST. Strains with mutations in both stcU and stcL showed accumulation of VERB only (56). Keller et al. (57) also identified stcS (formerly named verB [58]), encoding a cytochrome P450-type monooxygenase, which is involved in the conversion of VERA to DMST. Disruption of this gene resulted in the accumulation of VERA. Thus, both stcU and stcS are required for the conversion of VERA to DMST. The verA gene was recently identified in A. parasiticus SRRC 143. The ver-1 gene is here renamed aflM, and verA is renamed aflN. The aflM homolog in the ST gene cluster is stcU, and aflN is now stcS. Sequence analysis indicated that the aflN gene encodes a cytochrome P450 monooxygenase and has high homology to stcS. It is presumed that both aflM and aflN are involved in the conversion of VERA to DMST in aflatoxin biosynthesis even though no significant sequence homology between aflM and aflN at either the DNA or amino acid level has been identified. It is interesting that some degree of amino acid sequence homology (45%) has been identified between aflL and aflN, but no sequence homology has been found between aflL and aflM. The exact function of aflN is yet to be determined.
aflO (omtB, dmtA) is involved in the conversion of DMST to ST and of DMDHST to DHST.
Yabe et al. (104) demonstrated two distinct O-methyltransferase activities in A. parasiticus. The enzyme for one of the two activities is named O-methyltransferase I for the conversion of DMST to ST (104); the same enzyme is also responsible for the conversion of DMDHST to DHST (104). This O-methyltransferase has been purified and characterized (109). The gene for this O-methyltransferase in A. parasiticus was cloned by Motomura et al. (74) and was named dmtA or mt-I for O-methyltransferase I. The same gene was concurrently cloned by Yu et al. (119) in A. parasiticus, A. flavus, and A. sojae. This gene was named omtB after the cloning of the omtA gene (113, 115; see below), so the enzyme encoded by this gene was named O-methyltransferase B. The gene homolog in A. nidulans is stcP (53). Disruption of stcP (53) demonstrated the requirement of this gene for the conversion from DMST to ST. dmtA or omtB is here renamed aflO.
aflP (omtA) is involved in the conversion of ST to OMST and DMST to DHOMST.
The involvement of an O-methyltransferase in the later step of aflatoxin formation has been studied extensively (10, 11, 55, 104). Yabe et al. (104) reported two methyltransferase activities involved in aflatoxin formation in A. parasiticus, O-methyltransferase I (mentioned above) and O-methyltransferase II for the conversion of ST to O-methylsterigmatocystin (OMST) and DHST to dihydro-O-methylsterigmatocystin (DHOMST) (104). The cDNA for the gene corresponding to this activity was cloned (named omt-1) from A. parasiticus by antibody screening of a cDNA expression library (113). The enzyme was expressed in Escherichia coli, and its activity for converting ST to OMST was demonstrated by substrate feeding studies (113). The genomic DNA sequence for this gene was also cloned (named omtA) from A. parasiticus and A. flavus (115). The omt-1 or omtA gene is here renamed aflP. A gene disruption experiment by Lee et al. (63) unambiguously demonstrated the function of omtA in vivo.
aflQ (ordA) is involved in the conversion of OMST to AFB1 and AFG1 and of DMDHST to AFB2 and AFG2.
The biosynthetic relationship between B-group (AFB1 and AFB2) and G-group (AFG1 and AFG2) aflatoxins has been proposed (11, 32, 103). Enzymatic studies have demonstrated the involvement of an NADPH-dependent monooxygenase (12, 103, 110) in the conversion of OMST to AFB1 in the late stages of aflatoxin biosynthesis. Cleveland (32) demonstrated in a substrate feeding study with two A. parasiticus mutant strains that DHOMST was converted to AFB2. A cytochrome P450 monooxygenase gene, ord-1, was reported to be required for this reaction in A. flavus (83, 84). Yu et al. (117) cloned the ord-1 gene (then named ordA, now renamed aflQ), encoding a cytochrome P450 monooxygenase, from A. parasiticus and A. flavus. It has been demonstrated by expression and substrate feeding in a yeast system that this gene is responsible for the conversion of OMST to AFB1 and AFG1 and of DHOMST to AFB2 and AFG2 (117); the critical amino acids for the enzymatic activity and heme-binding motif were identified by site-directed mutagenesis. It has also been demonstrated that the synthesis of G-group toxins (AFG1 and AFG2) requires enzymes in addition to those necessary for B-group aflatoxin synthesis (110, 117).
GENES INVOLVED IN PATHWAY REGULATION
aflR is involved in transcription activation.
In both the aflatoxin and ST gene clusters, there is a positive regulatory gene, aflR (originally named afl-2 [79] and apa-2 [24]), for activating pathway gene transcription. The aflR gene encodes a sequence-specific zinc binuclear DNA-binding protein, a Gal 4-type 47-kDa polypeptide, and has been shown to be required for transcriptional activation of most, if not all, of the structural genes (24, 26, 27, 28, 42, 49, 79, 101, 126). The transcription of aflatoxin pathway genes can be activated when the AflR protein binds to the palindromic sequence 5′-TCGN5CGA-3′ (also called AflR-binding motif) in the promoter region of the structural genes (43, 44, 47) in A. parasiticus, A. flavus, and A. nidulans. The AflR-binding motifs are located from position −80 to position −600, with the majority at the −100 to −200 positions relative to the translation start site. AflR binds, in some cases, to a deviated sequence rather than the typical motif, such as in the case of aflG (avnA). When there is more than one such motif in the promoter region of a gene, only one is a preferred binding site, such as in the case of aflC (pksA [43, 44]). A. sojae, a nontoxigenic strain used in industrial fermentations, was found to contain a defective aflR gene in addition to other defects in the aflatoxin pathway structural genes (68, 69, 91). Thus, in the absence of the functional regulatory protein, no induction of aflatoxin can occur in this food grade Aspergillus.
aflS (aflJ) gene is involved in regulation of aflatoxin biosynthesis.
Adjacent to the aflR gene in the aflatoxin gene cluster, a divergently transcribed gene, aflS (originally named aflJ), was also found to be involved in the regulation of transcription (72). A recent study (22) has shown that aflS interacts with aflR but not the structural genes. In the aflS knockout mutants, the lack of aflS transcript is associated with a 5- to 20-fold reduction of expression of some aflatoxin pathway genes such as aflC (pksA), aflD (nor-1), aflM (ver-1), and aflP (omtA) and a loss of the ability to synthesize aflatoxin intermediates (72). The aflS homolog was located adjacent to the aflR gene in the ST gene cluster (U34740), but no name has yet been given to it (Daren Brown, personal communication). The exact mechanism by which aflS modulates transcription of these pathway genes in concert with aflR is presently being investigated in a USDA laboratory (Southern Regional Research Center, New Orleans, La.) by gene expression analysis using microarray technology.
CLUSTER GENES UNASSIGNED TO THE PATHWAY
Recently, additional genes have been identified in the gene cluster which are putatively involved in aflatoxin biosynthesis (Table 1). A typical AflR-binding motif was identified in the untranslated region (UTR) of all of these genes in the gene cluster, indicating that they are potential targets for AflR. In contrast, no AflR-binding motif was identified in the UTR of the four sugar utilization genes (nadA, hxtA, glcA, and sugR) that are found adjacent to the aflatoxin gene cluster (Table 2). More importantly, these new genes, like other characterized aflatoxin pathway genes, were found to be expressed under aflatoxigenic growth conditions in the Aspergillus flavus EST database, indicating possible functional involvement in aflatoxin synthesis (Yu, unpublished).
aflT.
In the aflatoxin pathway gene cluster, a gene named aflT, encoding a membrane-bound protein with homology to antibiotic efflux genes presumed to be involved in aflatoxin secretion, was discovered in A. parasiticus (P.-K. Chang et al., unpublished data). However, disruption of this gene does not affect aflatoxin formation (Chang et al., unpublished).
aflU (cypA).
aflU encodes a polypeptide of 498 amino acids. A Blast search identified significant homologies to cytochrome P450-type monooxygenase enzymes in the GenBank database. A typical heme-binding motif of cytochrome P450 monooxygenase has been identified near the C terminus. Expression studies using reverse transcriptase PCR showed that the transcript was detected only under aflatoxin-conducive conditions (81, 113) and not on nonconducive medium (peptone medium) (Yu, unpublished). These observations support the possible involvement of this gene in aflatoxin biosynthesis.
aflV (cypX).
The aflV gene encodes another cytochrome P450 monooxygenase (118) and is homologous to stcB in A. nidulans. Gene knockout experiments have been performed extensively on the aflV gene. Unfortunately, no conclusive results have been obtained. Keller et al. (59) also disrupted stcB, the aflV homolog in A. nidulans, but were unable to demonstrate a clear role in biosynthesis.
aflW (moxY).
aflW encodes a monooxygenase (118) which is homologous to stcW in ST synthesis in A. nidulans (54). As with aflV, no conclusive results regarding aflatoxin synthesis could be asserted despite studies using disruption experiments in A. parasiticus and its homolog stcW in A. nidulans.
aflX (ordB).
Adjacent to aflW, an additional gene, aflX (ordB), was found. The aflX gene encodes a polypeptide of 266 amino acids with significant homology to an oxidase in the GenBank database. At the amino acid level, the aflX gene shows 54% identity and 68% similarity to stcQ in the ST gene cluster in A. nidulans (17). No intron has been identified in the coding region. We tentatively named it ordB due to its possible function as an oxidoreductase in aflatoxin synthesis. However, no pathway-specific involvement of this gene has yet been defined.
aflY (hypA).
Adjacent to the aflX gene, another new gene, aflY (hypA), which encodes a polypeptide with homology to a hypothetical protein, was also identified. The aflY gene encodes a polypeptide of 495 amino acids with unknown function. No aflY gene homolog was identified in the A. nidulans gene cluster.
CONCLUDING REMARKS
Genes involved in most of the bioconversion steps in the aflatoxin/ST biosynthetic pathway have been confirmed through either gene disruption or enzymatic studies. However, details of several biological conversion steps and of genes responsible for the reactions have not yet been deciphered. Among the 25 genes identified in the aflatoxin biosynthetic pathway gene cluster, the functions of 19 in aflatoxin biosynthesis have been assigned and the functions of 6 are unassigned. Among the genes assigned to the pathway steps, the placements of aflE (norA), aflF (norB), and aflN (verA) were based on their homologies to aflatoxin or ST genes and their functions have not been experimentally confirmed (Table 1). It has been demonstrated (109, 110, 117) that additional enzymes are required for G-group toxin formation in A. parasiticus. However, an enzyme(s) or corresponding gene(s) for such reactions has yet to be identified. The possibility remains that one or more of these unassigned genes, such as aflU, aflX, and/or aflY, on the basis of their predicted enzymatic functions, might be involved in G-group toxin formation.
Aflatoxins and ST share almost identical biochemical pathways. The majority of the homologous genes and their enzymes involved in the two pathways, except for the last two steps, are identified (Table 1). However, no gene homologs are identified for aflatoxin cluster genes (aflD, aflF, aflT, aflU, and aflY) in the ST gene cluster and for ST cluster genes (stcC, stcD, stcH, stcM, stcR, stcT, and stcX) in the aflatoxin gene cluster. The aflS gene homolog was located in the ST gene cluster. However, its function is unclear and no name has yet been given (Daren Brown, personal communication). Note that aflP and aflQ are not essential to ST biosynthesis since ST is the final product in A. nidulans. These two genes are either nonfunctional or have been lost in the evolutionary process since no homologous genes have been identified either within or outside the ST gene cluster (Table 1). The possibility exists that some of the genes involved in aflatoxin and ST biosynthesis are located somewhere outside the gene clusters. The genetic control of aflatoxin biosynthesis in relation to primary metabolism and environmental stimuli is apparently beyond this defined gene cluster (18, 38, 39, 46, 49, 78, 80, 122). Identification of all of the genes and global regulators involved in and related to aflatoxin biosynthesis in the fungal system is a daunting challenge. A. flavus genomics and microarray technologies (75, 121, 122) will provide a new avenue for deciphering such mechanisms and unraveling these regulatory elements governing aflatoxin biosynthesis.
REFERENCES
- 1.Bennett, J. W. 1981. Loss of norsolorinic acid and aflatoxin production by a mutant of Aspergillus parasiticus. J. Gen. Microbiol. 124:429-432. [Google Scholar]
- 2.Bennett, J. W. 1987. Mycotoxins, mycotoxicoses, mycotoxicology and Mycopathologia. Mycopathologia 100:3-5. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, J. W., and S. B. Christensen. 1983. New perspectives on aflatoxin biosynthesis. Adv. Appl. Microbiol. 29:53-92. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, J. W., and L. L. Lasure. 1985. Conventions for gene symbols, p. 538-544. In J. W. Bennett and L. L. Lasure (ed.), Gene manipulations in fungi. Academic Press, New York, N.Y.
- 5.Bennett, J. W., L. S. Lee, and A. F. Cucullu. 1976. Effect of dichlorvos on aflatoxin and versicolorin A production in Aspergillus parasiticus. Bot. Gaz. 137:318-324. [Google Scholar]
- 6.Bennett, J. W., L. S. Lee, S. M. Shoss, and G. H. Boudreaux. 1980. Identification of averantin as an aflatoxin B1 precursor: placement in the biosynthetic pathway. Appl. Environ. Microbiol. 39:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett, J. W., P.-K. Chang, and D. Bhatnagar. 1997. One gene to whole pathway: the role of norsolorinic acid in aflatoxin research. Adv. Appl. Microbiol. 45:1-15. [DOI] [PubMed] [Google Scholar]
- 8.Bennett, J. W., and M. Klich. 2003. Mycotoxins. Clin. Microbiol. Rev. 16:497-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger-Deguee, M., and Y. Bergar. 1982. Structure of versicolorone isolated from Aspergillus versicolor. Phytochemistry 21:1449-1451. [Google Scholar]
- 10.Bhatnagar, D., A. H. J. Ullah, and T. E. Cleveland. 1988. Purification and characterization of a methyltransferase from Aspergillus parasiticus SRRC 163 involved in aflatoxin biosynthetic pathway. Prep. Biochem. 18:321-349. [DOI] [PubMed] [Google Scholar]
- 11.Bhatnagar, D., T. E. Cleveland, and D. G. I. Kingston. 1991. Enzymological evidence for separate pathways for aflatoxin B1 and B2 biosynthesis. Biochemistry 30:4343-4350. [DOI] [PubMed] [Google Scholar]
- 12.Bhatnagar, D., K. C. Ehrlich, and T. E. Cleveland. 1992. Oxidation-reduction reactions in biosynthesis of secondary metabolites, p. 255-286. In D. Bhatnagar, E. B. Lillehoj, and D. K. Arora (ed.), Handbook of applied mycology: mycotoxins in ecological systems. Marcel Dekker, New York, N.Y.
- 13.Bhatnagar, D., K. C. Ehrlich, and T. E. Cleveland. 1993. Biochemical characterization of an aflatoxin B2 producing mutant of Aspergillus flavus. FASEB J. 7:A1234. [Google Scholar]
- 14.Bhatnagar, D., J. Yu, and K. C. Ehrlich. 2002. Toxins of filamentous fungi. Chem. Immunol. 81:167-206. [DOI] [PubMed] [Google Scholar]
- 15.Bhatnagar, D., K. C. Ehrlich, and T. E. Cleveland. 2003. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 61:83-93. [DOI] [PubMed] [Google Scholar]
- 16.Brown, D. W., T. H. Adams, and N. P. Keller. 1996. Aspergillus has distinct fatty acid synthases for primary and secondary metabolism. Proc. Natl. Acad. Sci. USA 19:14873-14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown, D. W., J.-H. Yu, H. S. Kelkar, M. Fernandes, T. C. Nesbitt, N. P. Keller, T. H. Adams, and T. J. Leonard. 1996. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 93:1418-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvo, A. M., R. A. Wilson, J. W. Bok, and N. P. Keller. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cary, J. W., M. Wright, D. Bhatnagar, R. Lee, and F. S. Chu. 1996. Molecular characterization of an Aspergillus parasiticus dehydrogenase gene, norA, located on the aflatoxin biosynthesis gene cluster. Appl. Environ. Microbiol. 62:360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cary, J. W., N. Barnaby, K. C. Ehrlich, and D. Bhatnagar. 1999. Isolation and characterization of experimentally induced, aflatoxin biosynthetic pathway deletion mutants of Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 51:808-812. [DOI] [PubMed] [Google Scholar]
- 21.Cary, J. W., J. M. Dyer, K. C. Ehrlich, M. S. Wright, S. H. Liang, and J. E. Linz. 2002. Molecular and functional characterization of a second copy of the aflatoxin regulatory gene, aflR-2, from Aspergillus parasiticus. Biochim. Biophys. Acta 1576:316-323. [DOI] [PubMed] [Google Scholar]
- 22.Chang, P.-K. 2003. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol. Genet. Genomics 268:711-719. [DOI] [PubMed] [Google Scholar]
- 23.Chang, P.-K., C. D. Skory, and J. E. Linz. 1992. Cloning of a gene associated with aflatoxin B1 biosynthesis in Aspergillus parasiticus. Curr. Genet. 21:231-233. [DOI] [PubMed] [Google Scholar]
- 24.Chang, P.-K., J. W. Cary, D. Bhatnagar, T. E. Cleveland, J. W. Bennett, J. E. Linz, C. P. Woloshuk, and G. A. Payne. 1993. Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Appl. Environ. Microbiol. 59:3273-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang, P.-K., J. W. Cary, J. Yu, D. Bhatnagar, and T. E. Cleveland. 1995. Aspergillus parasiticus polyketide synthase gene, pksA, a homolog of Aspergillus nidulans wA, is required for aflatoxin B1. Mol. Gen. Genet. 248:270-277. [DOI] [PubMed] [Google Scholar]
- 26.Chang, P.-K., K. C. Ehrlich, J. Yu, D. Bhatnagar, and T. E. Cleveland. 1995. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl. Environ. Microbiol. 61:2372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang, P.-K., J. Yu, D. Bhatnagar, and T. E. Cleveland. 1999. Repressor-AFLR interaction modulates aflatoxin biosynthesis in Aspergillus parasiticus. Mycopathologia 147:105-112. [DOI] [PubMed] [Google Scholar]
- 28.Chang, P.-K., J. Yu, D. Bhatnagar, and T. E. Cleveland. 1999. The carboxy-terminal portion of the aflatoxin pathway regulatory protein AFLR of Aspergillus parasiticus activates GAL1::lacZ gene expression in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 65:2508-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang, P.-K., J. Yu, K. C. Ehrlich, S. M. Boue, B. G. Montalbano, D. Bhatnagar, and T. E. Cleveland. 2000. The aflatoxin biosynthesis gene adhA in Aspergillus parasiticus is involved in conversion of 5′-hydroxyaverantin to averufin. Appl. Environ. Microbiol. 66:4715-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang, P.-K., and J. Yu. 2002. Characterization of a partial duplication of the aflatoxin gene cluster in Aspergillus parasiticus ATCC 56775. Appl. Microbiol. Biotechnol. 58:632-636. [DOI] [PubMed] [Google Scholar]
- 31.Chiou, C. H., M. Miller, D. L. Wilson, F. Trail, and J. E. Linz. 2002. Chromosomal location plays a role in regulation of aflatoxin gene expression in Aspergillus parasiticus. Appl. Environ. Microbiol. 68:306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleveland, T. E. 1989. Conversion of dihydro-O-methylsterigmatocystin to aflatoxin B2 by Aspergillus parasiticus. Arch. Environ. Contam. Toxicol. 18:429-433. [DOI] [PubMed] [Google Scholar]
- 33.Cleveland, T. E., and D. Bhatnagar. 1991. Molecular regulation of aflatoxin biosynthesis, p. 270-287. In G. A. Bray and D. H. Ryan (ed.), Mycotoxins, cancer and health. Pennington Center Nutrition Series, vol. 1. LSU Press, Baton Rouge, La.
- 34.Cleveland, T. E., and D. Bhatnagar. 1992. Molecular strategies for reducing aflatoxin levels in crops before harvest, p. 205-228. In D. Bhatnagar and T. E. Cleveland (ed.), Molecular approaches to improving food quality and safety. Van Nostrand Reinhold, New York, N.Y.
- 35.Cleveland, T. E., J. W. Cary, R. L. Brown, D. Bhatnagar, J. Yu, P.-K. Chang, C. A. Chlan, and K. Rajasekaran. 1997. Use of biotechnology to eliminate aflatoxin in preharvest crops. Bull. Inst. Compr. Agric. Sci. Kinki Univ. 5:75-90. [Google Scholar]
- 36.Clutterbuck, A. J. 1973. Gene symbols in Aspergillus nidulans. Genet. Res. 21:201-296. [DOI] [PubMed] [Google Scholar]
- 37.Cole, R. J., and E. H. Cox. 1987. Handbook of toxic fungal metabolites. Academic Press, New York, N.Y.
- 38.Cotty, P. J. 1988. Aflatoxin and sclerotial production by Aspergillus flavus: influence of pH. Phytopathology 78:1250-1253. [Google Scholar]
- 39.Demain, A. L. 1972. Cellular and environmental factors affecting the synthesis and excretion of metabolites. J. Appl. Chem. Biotechnol. 22:345-372. [Google Scholar]
- 40.Detroy, R. W., S. Freer, and A. Ciegler. 1973. Aflatoxin and anthraquinone biosynthesis by nitrosoquanidine-derived mutants of Aspergillus parasiticus. Can. J. Microbiol. 19:1373-1378. [DOI] [PubMed] [Google Scholar]
- 41.Dutton, M. F. 1988. Enzymes and aflatoxin biosynthesis. Microbiol. Rev. 52:274-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehrlich, K. C., B. G. Montalbano, D. Bhatnagar, and T. E. Cleveland. 1998. Alteration of different domains in AFLR affects aflatoxin pathway metabolism in Aspergillus parasiticus transformants. Fungal Genet. Biol. 23:279-287. [DOI] [PubMed] [Google Scholar]
- 43.Ehrlich, K. C., J. W. Cary, and B. G. Montalbano. 1999. Characterization of the promoter for the gene encoding the aflatoxin biosynthetic pathway regulatory protein AFLR. Biochim. Biophys. Acta 1444:412-417. [DOI] [PubMed] [Google Scholar]
- 44.Ehrlich, K. C., B. G. Montalbano, and J. W. Cary. 1999. Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticus. Gene 230:249-257. [DOI] [PubMed] [Google Scholar]
- 45.Feng, G. H., and T. J. Leonard. 1995. Characterization of the polyketide synthase gene (pksL1) required for aflatoxin biosynthesis in Aspergillus parasiticus. J. Bacteriol. 177:6246-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng, G. H., and T. J. Leonard. 1998. Culture conditions control expression of the genes for aflatoxin and sterigmatocystin biosynthesis in Aspergillus parasiticus and A. nidulans. Appl. Environ. Microbiol. 64:2275-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandes, M., N. P. Keller, and T. H. Adams. 1998. Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol. Microbiol. 28:1355-1365. [DOI] [PubMed] [Google Scholar]
- 48.Fitzell, D. L., R. Singh, D. P. H. Hsieh, and E. L. Motell. 1977. Nuclear magnetic resonance identification of versicolor hemiacetal acetate as an intermediate in aflatoxin biosynthesis. Agric. Food Chem. 25:1193-1197. [DOI] [PubMed] [Google Scholar]
- 49.Flaherty, J. E., and G. A. Payne. 1997. Overexpression of afIR leads to upregulation of pathway gene expression and increased aflatoxin production in Aspergillus flavus. Appl. Environ. Microbiol. 63:3995-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsieh, D. P., C. C. Wan, and J. A. Billington. 1989. A versiconal hemiacetal acetate converting enzyme in aflatoxin biosynthesis. Mycopathologia 107:121-126. [DOI] [PubMed] [Google Scholar]
- 51.Ito, Y., S. W. Peterson, D. T. Wicklow, and T. Goto. 2001. Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section Flavi. Mycol. Res. 105:233-239. [Google Scholar]
- 52.Jelinek, C. F., A. E. Pohland, and G. E. Wood. 1989. Worldwide occurrence of mycotoxins in foods and feeds—an update. J. Assoc. Off. Anal. Chem. 72:223-230. [PubMed] [Google Scholar]
- 53.Kelkar, H. S., N. P. Keller, and T. H. Adams. 1996. Aspergillus nidulans stcP encodes an O-methyltransferase that is required for sterigmatocystin biosynthesis. Appl. Environ. Microbiol. 62:4296-4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelkar, H. S., S. Hernant, T. W. Skloss, J. F. Haw, N. P. Keller, and T. H. Adams. 1997. Aspergillus nidulans stcL encodes a putative cytochrome P-450 monooxygenase required for bisfuran desaturation during aflatoxin/sterigmatocystin biosynthesis. J. Biol. Chem. 272:1589-1594. [DOI] [PubMed] [Google Scholar]
- 55.Keller, N. P., J. H. C. Dischinger, D. Bhatnagar, T. E. Cleveland, and A. H. J. Ullah. 1993. Purification of a 40-kilodalton methyltransferase active in the aflatoxin biosynthetic pathway. Appl. Environ. Microbiol. 59:479-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keller, N. P., N. J. Kantz, and T. H. Adams. 1994. Aspergillus nidulans verA is required for production of the mycotoxin sterigmatocystin. Appl. Environ. Microbiol. 60:1444-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller, N. P., S. Segner, D. Bhatnagar, and T. H. Adams. 1995. stcS, a putative P-450 monooxygenase, is required for the conversion of versicolorin A to sterigmatocystin in Aspergillus nidulans. Appl. Environ. Microbiol. 61:3628-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller, N. P., D. Brown, R. A. E. Butchko, M. Fernandes, H. Kelkar, C. Nesbitt, S. Segner, D. Bhatnagar, T. E. Cleveland, and T. H. Adams. 1995. A conserved polyketide mycotoxin gene cluster in Aspergillus nidulans, p. 263-277. In J. L. Richard (ed.), Molecular approaches to food safety issues involving toxic microorganisms. Alaken, Fort Collins, Colo.
- 59.Keller, N. P., C. M. H. Watanabe, H. S. Kelkar, T. H. Adams, and C. A. Townsend. 2000. Requirement of monooxygenase-mediated steps for sterigmatocystin biosynthesis by Aspergillus nidulans. Appl. Environ. Microbiol. 66:359-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klich, M. A., E. J. Mullaney, C. B. Daly, and J. W. Cary. 2000. Molecular and physiological aspects of aflatoxin and sterigmatocystin biosynthesis by Aspergillus tamarii and A. ochraceoroseus. Appl. Microbiol. Biotechnol. 53:605-609. [DOI] [PubMed] [Google Scholar]
- 61.Kusumoto, K., and D. P. Hsieh. 1996. Purification and characterization of the esterases involved in aflatoxin biosynthesis in Aspergillus parasiticus. Can. J. Microbiol. 42:804-810. [DOI] [PubMed] [Google Scholar]
- 62.Lancaster, M. D., F. P. Jenkins, and J. M. Phillip. 1961. Toxicity associated with certain samples of groundnuts. Nature 192:1095-1096. [Google Scholar]
- 63.Lee, L. W., C. H. Chiou, and J. E. Linz. 2002. Function of native OmtA in vivo and expression and distribution of this protein in colonies of Aspergillus parasiticus. Appl. Environ. Microbiol. 68:5718-5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang, S.-H., C. D. Skory, and J. E. Linz. 1996. Characterization of the function of the ver-1A and ver-1B genes, involved in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 62:4568-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang, S.-H., T. S. Wu, R. Lee, F. S. Chu, and J. E. Linz. 1997. Analysis of mechanisms regulating expression of the ver-1 gene, involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 63:1058-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin, B. K., and J. A. Anderson. 1992. Purification and properties of versiconal cyclase from Aspergillus parasiticus. Arch. Biochem. Biophys. 293:67-70. [DOI] [PubMed] [Google Scholar]
- 67.Mahanti, N., D. Bhatnagar, J. W. Cary, J. Joubran, and J. E. Linz. 1996. Structure and function of fas-1A, a gene encoding a putative fatty acid synthetase directly involved in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 62:191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsushima, K., P.-K. Chang, J. Yu, K. Abe, D. Bhatnagar, and T. E. Cleveland. 2001. Pre-termination in aflR of Aspergillus sojae inhibits aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 55:585-589. [DOI] [PubMed] [Google Scholar]
- 69.Matsushima, K., K. Yashiro, Y. Hanya, K. Abe, K. Yabe, and T. Hamasaki. 2001. Absence of aflatoxin biosynthesis in koji mold (Aspergillus sojae). Appl. Microbiol. Biotechnol. 55:771-776. [DOI] [PubMed] [Google Scholar]
- 70.Mayorga, M. E., and W. E. Timberlake. 1992. The developmentally regulated Aspergillus nidulans wA gene encodes a polypeptide homologous to polyketide and fatty acid syntheses. Mol. Gen. Genet. 235:205-212. [DOI] [PubMed] [Google Scholar]
- 71.McGuire, S. M., J. C. Silva, E. G. Casillas, and C. A. Townsend. 1996. Purification and characterization of versicolorin B synthase from Aspergillus parasiticus. Catalysis of the stereodifferentiating cyclization in aflatoxin biosynthesis essential to DNA interaction. Biochemistry 35:11470-11486. [DOI] [PubMed] [Google Scholar]
- 72.Meyers, D. M., G. O'Brian, W. L. Du, D. Bhatnagar, and G. A. Payne. 1998. Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl. Environ. Microbiol. 64:3713-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minto, R. E., and C. A. Townsend. 1997. Enzymology and molecular biology of aflatoxin biosynthesis. Chem. Rev. 97:2537-2556. [DOI] [PubMed] [Google Scholar]
- 74.Motomura, M., N. Chihaya, T. Shinozawa, T. Hamasaki, and K. Yabe. 1999. Cloning and characterization of the O-methyltransferase I gene (dmtA) from Aspergillus parasiticus associated with the conversions of demethylsterigmatocystin to sterigmatocystin and dihydrodemethylsterigmatocystin to dihydrosterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 65:4987-4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O'Brian, G. R., A. M. Fakhoury, and G. A. Payne. 2003. Identification of genes differentially expressed during aflatoxin biosynthesis in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet. Biol. 39:118-127. [DOI] [PubMed] [Google Scholar]
- 76.Papa, K. E. 1979. Genetics of Aspergillus flavus: complementation and mapping of aflatoxin mutants. Genet. Res. 34:1-9. [DOI] [PubMed] [Google Scholar]
- 77.Papa, K. E. 1982. Norsolorinic acid mutant of Aspergillus flavus. J. Gen. Microbiol. 128:1345-1348. [Google Scholar]
- 78.Payne, G. A., and W. M. Hagler, Jr. 1983. Effect of specific amino acids on growth and aflatoxin production by Aspergillus parasiticus and Aspergillus flavus in defined media. Appl. Environ. Microbiol. 46:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Payne, G. A., G. J. Nystrom, D. Bhatnagar, T. E. Cleveland, and C. P. Woloshuk. 1993. Cloning of the afl-2 gene involved in aflatoxin biosynthesis from Aspergillus flavus. Appl. Environ. Microbiol. 59:156-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Payne, G. A., and M. P. Brown. 1998. Genetics and physiology of aflatoxin biosynthesis. Annu. Rev. Phytopathol. 36:329-362. [DOI] [PubMed] [Google Scholar]
- 81.Payne, G. A. 1998. Process of contamination by aflatoxin-producing fungi and their impacts on crops, p. 279-306. In K. K. Sinha and D. Bhatnagar (ed.), Mycotoxins in agriculture and food safety, vol. 9. Marcel Dekker, New York, N.Y.
- 82.Peterson, S. W., Y. Ito, B. W. Horn, and T. Goto. 2001. Aspergillus bombycis, a new aflatoxigenic species and genetic variation in its sibling species, A. nomius. Mycologia 93:689-703. [Google Scholar]
- 83.Prieto, R., and C. P. Woloshuk. 1997. ord1, an oxidoreductase gene responsible for conversion of O-methylsterigmatocystin to aflatoxin in Aspergillus flavus. Appl. Environ. Microbiol. 63:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prieto, R., G. L. Yousibova, and C. P. Woloshuk. 1996. Identification of aflatoxin biosynthesis genes by genetic complementation in an Aspergillus flavus mutant lacking the aflatoxin gene cluster. Appl. Environ. Microbiol. 62:3567-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakuno, E., K. Yabe, and H. Nakajima. 2003. Involvement of two cytosolic enzymes and a novel intermediate, 5′-oxoaverantin, in the pathway from 5′-hydroxyaverantin to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 69:6418-6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schroeder, H. W., R. J. Cole, R. D. Grigsby, and H. Hein, Jr. 1974. Inhibition of aflatoxin production and tentative identification of an aflatoxin intermediate “versiconal acetate” from treatment with dichlorvos. Appl. Microbiol. 27:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silva, J. C., and C. A. Townsend. 1996. Heterologous expression, isolation, and characterization of versicolorin B synthase from Aspergillus parasiticus. J. Biol. Chem. 272:804-813. [DOI] [PubMed] [Google Scholar]
- 88.Silva, J. C., R. E. Minto, C. E. Barry, K. A. Holland, and C. A. Townsend. 1996. Isolation and characterization of the versicolorin B synthase gene from Aspergillus parasiticus: expansion of the aflatoxin B1 biosynthetic cluster. J. Biol. Chem. 271:13600-13608. [DOI] [PubMed] [Google Scholar]
- 89.Skory, C. D., P.-K. Chang, J. Cary, and J. E. Linz. 1992. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 58:3527-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skory, C. D., P.-K. Chang, and J. E. Linz. 1993. Regulated expression of the nor-1 and ver-1 genes associated with aflatoxin biosynthesis. Appl. Environ. Microbiol. 59:1642-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takahashi, T., P.-K. Chang, K. Matsushima, J. Yu, Y. Koyama, K. Abe, D. Bhatnagar, and T. E. Cleveland. 2002. Nonfunctionality of Aspergillus sojae aflR in a strain of Aspergillus parasiticus with a disrupted aflR gene. Appl. Environ. Microbiol. 68:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Townsend, C. A., S. B. Christensen, and K. Trautwein. 1984. Hexanoate as a starter unit in polyketide synthesis. J. Am. Chem. Soc. 106:3868-3869. [Google Scholar]
- 93.Townsend, C. A., K. A. Plavcan, K. Pal, S. W. Brobst, M. S. Irish, E. W. Ely, and J. W. Bennett. 1988. Hydroxyversicolorone: isolation and characterization of a potential intermediate in aflatoxin biosynthesis. J. Org. Chem. 53:2472-2477. [Google Scholar]
- 94.Townsend, C. A. 1997. Progress towards a biosynthetic rationale of the aflatoxin pathway. Pure Appl. Chem. 58:227-238. [Google Scholar]
- 95.Trail, F., P.-K. Chang, J. Cary, and J. E. Linz. 1994. Structural and functional analysis of the nor-1 gene involved in the biosynthesis of aflatoxins by Aspergillus parasiticus. Appl. Environ. Microbiol. 60:4078-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trail, F., N. Manhanti, and J. Linz. 1995. Molecular biology of aflatoxin biosynthesis. Microbiology 141:755-765. [DOI] [PubMed] [Google Scholar]
- 97.Trail, F., N. Mahanti, M. Rarick, R. Mehigh, S. H. Liang, R. Zhou, and J. E. Linz. 1995. Physical and transcriptional map of an aflatoxin gene cluster in Aspergillus parasiticus and functional disruption of a gene involved early in the aflatoxin pathway. Appl. Environ. Microbiol. 61:2665-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vederas, J. C., and T. T. Nakashima. 1980. Biosynthesis of averufin by Aspergillus parasiticus: detection of 180-label by 13C NMR isotope shifts. J. Chem. Soc. Chem. Commun. 4:183-185. [Google Scholar]
- 99.Watanabe, C. M. H., D. Wilson, J. E. Linz, and C. A. Townsend. 1996. Demonstration of the catalytic roles and evidence for the physical association of type I fatty acid syntheses and a polyketide synthase in the biosynthesis of aflatoxin B1. Chem. Biol. 3:463-469. [DOI] [PubMed] [Google Scholar]
- 100.Watanabe, C. M., and C. A. Townsend. 2002. Initial characterization of a type I fatty acid synthase and polyketide synthase multienzyme complex NorS in the biosynthesis of aflatoxin B1. Chem. Biol. 9:981-988. [DOI] [PubMed] [Google Scholar]
- 101.Woloshuk, C. P., K. R. Foutz, J. F. Brewer, D. Bhatnagar, T. E. Cleveland, and G. A. Payne. 1994. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl. Environ. Microbiol. 60:2408-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yabe, K. 2003. Pathway and genes of aflatoxin biosynthesis, p. 227-251. In F. Fierro and J. Francisco (ed.), Microbial secondary metabolites: biosynthesis, genetics and regulation. Research Signpost, Trivandrum, India.
- 103.Yabe, K., Y. Ando, and T. Hamasaki. 1988. Biosynthetic relationship among aflatoxins B1, B2, G1, and G2. Appl. Environ. Microbiol. 54:2101-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yabe, K., Y. Ando, J. Hashimoto, and T. Hamasaki. 1989. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl. Environ. Microbiol. 55:2172-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yabe, K., Y. Ando, and T. Hamasaki. 1991. A metabolic grid among versiconal hemiacetal acetate, versiconol acetate, versiconol and versiconal during aflatoxin biosynthesis. J. Gen. Microbiol. 137:2469-2475. [DOI] [PubMed] [Google Scholar]
- 106.Yabe, K., Y. Nakamura, H. Nakajima, Y. Ando, and T. Hamasaki. 1991. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 57:1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yabe, K., and T. Hamasaki. 1993. Stereochemistry during aflatoxin biosynthesis: cyclase reaction in the conversion of versiconal to versicolorin B and racemization of versiconal hemiacetal acetate. Appl. Environ. Microbiol. 59:2493-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yabe, K., Y. Matsuyama, Y. Ando, H. Nakajima, and T. Hamasaki. 1993. Stereochemistry during aflatoxin biosynthesis: conversion of norsolorinic acid to averufin. Appl. Environ. Microbiol. 59:2486-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yabe, K., K. Matsushima, T. Koyama, and T. Hamasaki. 1998. Purification and characterization of O-methyltransferase I involved in conversion of demethylsterigmatocystin to sterigmatocystin and of dihydrodemethylsterigmatocystin to dihydrosterigmatocystin during aflatoxin biosynthesis. Appl. Environ. Microbiol. 64:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yabe, K., M. Nakamura, and T. Hamasaki. 1999. Enzymatic formation of G-group aflatoxins and biosynthetic relationship between G- and B-group aflatoxins. Appl. Environ. Microbiol. 65:3867-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yabe, K., N. Chihaya, S. Hamamatsu, E. Sakuno, T. Hamasaki, H. Nakajima, and J. W. Bennett. 2003. Enzymatic conversion of averufin to hydroxyversicolorone and elucidation of a novel metabolic grid involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 69:66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yao, R. C., and D. P. H. Hsieh. 1974. Step of dichlorvos inhibition in the pathway of aflatoxin biosynthesis. Appl. Microbiol. 28:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu, J., J. W. Cary, D. Bhatnagar, T. E. Cleveland, N. P. Keller, and F. S. Chu. 1993. Cloning and characterization of a cDNA from Aspergillus parasiticus encoding an O-methyltransferase involved in aflatoxin biosynthesis. Appl. Environ. Microbiol. 59:3564-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu, J., P.-K. Chang, J. W. Cary, M. Wright, D. Bhatnagar, T. E. Cleveland, G. A. Payne, and J. E. Linz. 1995. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus. Appl. Environ. Microbiol. 61:2365-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu, J., P.-K. Chang, G. A. Payne, J. W. Cary, D. Bhatnagar, and T. E. Cleveland. 1995. Comparison of the omtA genes encoding O-methyltransferases involved in aflatoxin biosynthesis from Aspergillus parasiticus and A. flavus. Gene 163:121-125. [DOI] [PubMed] [Google Scholar]
- 116.Yu, J., P.-K. Chang, J. W. Cary, D. Bhatnagar, and T. E. Cleveland. 1997. avnA, a gene encoding a cytochrome P-450 monooxygenase is involved in the conversion of averantin to averufin in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 63:1349-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu, J., P.-K. Chang, J. W. Cary, K. C. Ehrlich, B. Montalbano, J. M. Dyer, D. Bhatnagar, and T. E. Cleveland. 1998. Characterization of the critical amino acids of an Aspergillus parasiticus cytochrome P450 monooxygenase encoded by ordA involved in aflatoxin B1, G1, B2, and G2 biosynthesis. Appl. Environ. Microbiol. 64:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu, J., P.-K. Chang, D. Bhatnagar, and T. E. Cleveland. 2000. Genes encoding cytochrome P450 and monooxygenase enzymes define one end of the aflatoxin pathway gene cluster in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 53:583-590. [DOI] [PubMed] [Google Scholar]
- 119.Yu, J., C. P. Woloshuk, D. Bhatnagar, and T. E. Cleveland. 2000. Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 248:157-167. [DOI] [PubMed] [Google Scholar]
- 120.Yu, J., D. Bhatnagar, and K. C. Ehrlich. 2002. Aflatoxin biosynthesis. Rev. Iberoam. Micol. 19:191-200. [PubMed] [Google Scholar]
- 121.Yu, J., D. Bhatnagar, and T. E. Cleveland. 2002. Aspergillus flavus genomics for elimination of aflatoxin contamination. Mycopathologia 155:10. [Google Scholar]
- 122.Yu, J., D. Bhatnagar, T. E. Cleveland, and W. C. Nierman. 2002. Aspergillus flavus EST technology and its applications for eliminating aflatoxin contamination. Mycopathologia 155:6. [Google Scholar]
- 123.Yu, J. Genetics and biochemistry of mycotoxin synthesis. In D. K. Arora (ed.), Handbook of fungal biotechnology, 2nd ed., in press. Marcel Dekker, New York, N.Y.
- 124.Yu, J., P.-K. Chang, D. Bhatnagar, and T. E. Cleveland. 2003. Cloning and functional expression of an esterase gene in Aspergillus parasiticus. Mycopathologia 156:227-234. [DOI] [PubMed] [Google Scholar]
- 125.Yu, J.-H., and T. J. Leonard. 1995. Sterigmatocystin biosynthesis in Aspergillus nidulans requires a novel type I polyketide synthase. J. Bacteriol. 177:4792-4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu, J.-H., R. A. Butchko, M. Fernandes, N. P. Keller, T. J. Leonard, and T. H. Adams. 1996. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 29:549-555. [DOI] [PubMed] [Google Scholar]