Abstract
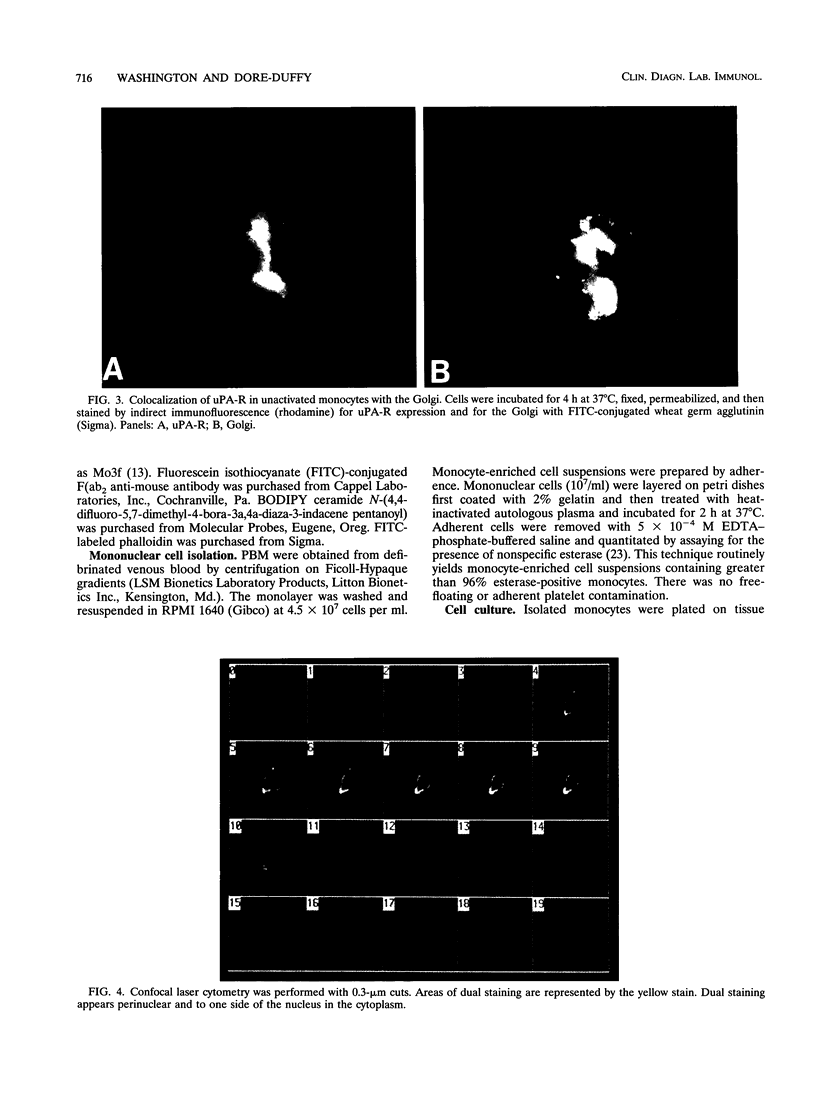
Peripheral blood monocytes exposed to bacterial products, phorbol esters, cyclic AMP, and cyclic AMP analogs express cell surface activation protein Mo3, which is the human urokinase plasminogen activator receptor (uPA-R). uPA-R is expressed by circulating monocytes from patients with multiple sclerosis (MS). We examined the role of cytoskeletal elements in the surface expression and subcellular distribution of uPA-R in nonactivated and lipopolysaccharide-activated monocytes and in monocytes from patients with MS. By using immunofluorescence techniques and confocal laser microscopy, we found that in unactivated monocytes, cytoplasmic uPA-R is found to one side of the nucleus, colocalizing with the Golgi. Upon activation with lipopolysaccharide, cytoplasmic Mo3-uPA-R becomes dispersed throughout the cytoplasm and projections concomitant with an increase in the monocyte perimeter (spreading). Cytoplasmic dispersion, as well as cell surface deposition, is dependent on microtubule integrity. Cell surface deposition of uPA-R upon activation is reduced by colchicine, which disrupts microtubules; however, once associated at the cell surface, uPA-R becomes associated with microfilaments via vinculin. Disruption of microfilaments with cytochalasin also alters surface expression of immunologically reactive uPA-R, as well as the distribution pattern. Monocytes from patients with MS display the uPA-R distribution pattern characteristic of an activated monocyte.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 1993 Feb;15(2):105–111. doi: 10.1002/bies.950150206. [DOI] [PubMed] [Google Scholar]
- Cohen R. L., Xi X. P., Crowley C. W., Lucas B. K., Levinson A. D., Shuman M. A. Effects of urokinase receptor occupancy on plasmin generation and proteolysis of basement membrane by human tumor cells. Blood. 1991 Jul 15;78(2):479–487. [PubMed] [Google Scholar]
- Dore-Duffy P., Donovan C., Todd R. F., 3rd Expression of monocyte activation antigen Mo3 on the surface of peripheral blood monocytes from patients with multiple sclerosis. Neurology. 1992 Aug;42(8):1609–1614. doi: 10.1212/wnl.42.8.1609. [DOI] [PubMed] [Google Scholar]
- Dumler I., Petri T., Schleuning W. D. Interaction of urokinase-type plasminogenactivator (u-PA) with its cellular receptor (u-PAR) induces phosphorylation on tyrosine of a 38 kDa protein. FEBS Lett. 1993 May 3;322(1):37–40. doi: 10.1016/0014-5793(93)81106-a. [DOI] [PubMed] [Google Scholar]
- Estreicher A., Mühlhauser J., Carpentier J. L., Orci L., Vassalli J. D. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990 Aug;111(2):783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Wallner B. P., Mattaliano R. J., Tizard R., Burne C., Frey A., Hession C., McGray P., Sinclair L. K., Chow E. P. Two human 35 kd inhibitors of phospholipase A2 are related to substrates of pp60v-src and of the epidermal growth factor receptor/kinase. Cell. 1986 Jul 18;46(2):191–199. doi: 10.1016/0092-8674(86)90736-1. [DOI] [PubMed] [Google Scholar]
- Hébert C. A., Baker J. B. Linkage of extracellular plasminogen activator to the fibroblast cytoskeleton: colocalization of cell surface urokinase with vinculin. J Cell Biol. 1988 Apr;106(4):1241–1247. doi: 10.1083/jcb.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Liu D. Y., Todd R. F., 3rd A monoclonal antibody specific for a monocyte-macrophage membrane component blocks the human monocyte response to migration inhibitory factor. J Immunol. 1986 Jul 15;137(2):448–455. [PubMed] [Google Scholar]
- Masucci M. T., Blasi F. The receptor for human urokinase: a potential target for anti-invasive and anti-metastatic therapy. Thromb Res Suppl. 1990;11:49–60. doi: 10.1016/0049-3848(90)90391-o. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Taguchi M., Kovacs E. J., Young H. A., Oppenheim J. J. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986 Apr 15;136(8):2883–2891. [PubMed] [Google Scholar]
- Min H. Y., Semnani R., Mizukami I. F., Watt K., Todd R. F., 3rd, Liu D. Y. cDNA for Mo3, a monocyte activation antigen, encodes the human receptor for urokinase plasminogen activator. J Immunol. 1992 Jun 1;148(11):3636–3642. [PubMed] [Google Scholar]
- Niedbala M. J., Sartorelli A. C. Plasminogen activator mediated degradation of subendothelial extracellular matrix by human squamous carcinoma cell lines. Cancer Commun. 1990;2(5):189–199. [PubMed] [Google Scholar]
- Saris C. J., Tack B. F., Kristensen T., Glenney J. R., Jr, Hunter T. The cDNA sequence for the protein-tyrosine kinase substrate p36 (calpactin I heavy chain) reveals a multidomain protein with internal repeats. Cell. 1986 Jul 18;46(2):201–212. doi: 10.1016/0092-8674(86)90737-3. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Hall G. L., Limjuco G., Chin J., Schmidt J. A. Interleukin 1 beta is localized in the cytoplasmic ground substance but is largely absent from the Golgi apparatus and plasma membranes of stimulated human monocytes. J Exp Med. 1988 Feb 1;167(2):389–407. doi: 10.1084/jem.167.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Adams M. D., Rogers C. E., Tripp L. R., Liu D. Y. Evidence for an intracellular pool of a migration inhibitory factor-associated, activation antigen of human mononuclear phagocytes, Mo3e. J Immunol. 1988 Feb 15;140(4):1095–1100. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Alvarez P. A., Brott D. A., Liu D. Y. Bacterial lipopolysaccharide, phorbol myristate acetate, and muramyl dipeptide stimulate the expression of a human monocyte surface antigen, Mo3e. J Immunol. 1985 Dec;135(6):3869–3877. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Mizukami I. F., Vinjamuri S. D., Trochelman R. D., Hancock W. W., Liu D. Y. Human mononuclear phagocyte activation antigens. Blood Cells. 1990;16(1):167–182. [PubMed] [Google Scholar]
- Vassalli J. D., Wohlwend A., Belin D. Urokinase-catalyzed plasminogen activation at the monocyte/macrophage cell surface: a localized and regulated proteolytic system. Curr Top Microbiol Immunol. 1992;181:65–86. doi: 10.1007/978-3-642-77377-8_3. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]