Abstract
Rationale
Recent findings have shown a complexly regulated 5-HT system as it is linked to different kinds of aggression.
Objective
We focus on (1) phasic and tonic changes of 5-HT and (2) state and trait of aggression, and emphasize the different receptor subtypes, their role in specific brain regions, feed-back regulation and modulation by other amines, acids and peptides.
Results
New pharmacological tools differentiate the first three 5-HT receptor families and their modulation by GABA, glutamate and CRF. Activation of 5-HT1A, 5-HT1B and 5-HT2A/2C receptors in mesocorticolimbic areas, reduce species-typical and other aggressive behaviors. In contrast, agonists at 5-HT1A and 5-HT1B receptors in the medial prefrontal cortex or septal area can increase aggressive behavior under specific conditions. Activation of serotonin transporters reduce mainly pathological aggression. Genetic analyses of aggressive individuals have identified several molecules that affect the 5-HT system directly (e.g., Tph2, 5-HT1B, 5-HT transporter, Pet1, MAOA) or indirectly (e.g., Neuropeptide Y, αCaMKII, NOS, BDNF). Dysfunction in genes for MAOA escalates pathological aggression in rodents and humans, particularly in interaction with specific experiences.
Conclusions
Feedback to autoreceptors of the 5-HT1 family and modulation via heteroreceptors are important in the expression of aggressive behavior. Tonic increase of the 5-HT2 family expression may cause escalated aggression, whereas the phasic increase of 5-HT2 receptors inhibits aggressive behaviors. Polymorphisms in the genes of 5-HT transporters or rate-limiting synthetic and metabolic enzymes of 5-HT modulate aggression, often requiring interaction with the rearing environment.
Keywords: aggression, serotonin, GABA, glutamate, CRF, raphe, prefrontal cortex, hypothalamus, septum, gene regulation
1. Preamble
Novel findings with tools from molecular genetics and receptor pharmacology in conjunction with more differentiating behavioral and clinical analysis begin to focus on the prominent role of brain serotonin in the predisposition to initiate impulsive aggressive behavior and the termination of bursts of aggressive behavior (Lesch and Merschdorf 2000; Miczek et al. 2002; Miczek et al. 2007b; Nelson and Chiavegatto 2001; Quadros et al. 2009b). The venerable serotonin deficiency hypothesis as simplifying principle, linking low serotonin activity to the propensity to engage in aggressive behavior, is yielding to a more differentiating interpretation of the accruing data (de Boer and Koolhaas 2005; Miczek et al. 2007b). The current review highlights several emerging themes of the past decade.
First, the dimension of impulsive aggressive behavior is a heritable trait that runs in families and appears amplified by salient experiences during a critical postnatal developmental period. The genetic architecture for synthetic and metabolic enzymes, receptor and particularly transporter molecules in serotonergic neurons provides ample targets for important life events to ultimately promote increased impulsive aggressive behavior (Caspi et al. 2002).
Second, superimposed on the serotonergic tone, plastic changes in the impulse flow in serotonin pathways to the forebrain are evident while an individual anticipates an aggressive or defensive act (Ferrari et al. 2003). As aggressive experiences accumulate, neuroadaptive changes in serotonergic dorsal raphé cells projecting to terminals in the forebrain are manifested in serotonin impulse flow and particularly in receptor regulation. These forms of neuroplasticity in serotonergic projections to the prefrontal cortex appear important for anticipating and preparing for imminent aggressive or defensive acts.
Third, one site of regulatory influences on cellular activity in the dorsal raphé nuclei (DRN) is the population of somatodendritic autoreceptors, and stimulation of these receptors inhibits impulse flow in serotonergic cells which, in turn, decreases escalated aggressive behavior in rodent models. Repeated somatodendritic autoreceptor stimulation or antagonism have been explored in order to enhance clinical management of affective disorders, including dysfunctions in social intercourse.
Fourth, excitatory and inhibitory transmitters such as glutamate and GABA synapse with serotonergic cells in the dorsal raphé nuclei, and the modulation of serotonergic activity by these inputs profoundly impacts aggressive behavior. Several members of the GABA and glutamate receptor subtypes, located on serotonergic cells, have already been identified as key targets for several drugs such as alcohol and benzodiazepines in their escalating and inhibiting effects on aggression.
Fifth, among the many peptides that modulate serotonin at the somata and terminals, CRF, vasopressin and opioid peptides are noteworthy for their profound effect on social and aggressive behavior. In rodent models, CRF1 receptor antagonists effectively and selectively reduce alcohol-heightened aggression by action on serotonergic neurons in the DRN, although these findings await translation into the clinic.
The last decade has seen also a concerted effort to translate more readily preclinical and clinical findings as is evident by two developments (1) an increasing focus on escalated types of aggressive behavior in animal models and (2) by operationally and functionally defined aggressive behaviors in clinical assessments.
2. Definition of Aggression
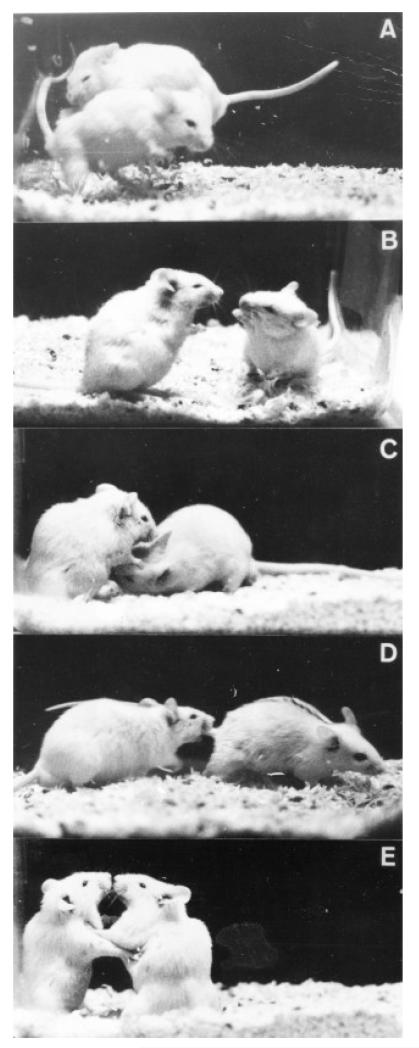
Most psychopharmacological research on serotonin and aggression is motivated by gaining insights into pathological aggression, both in human and veterinary medicine (Volavka et al. 2005). When working with laboratory models of animal aggression, it is useful to consider the ethological foundation of aggressive behavior such as its phylogenetic and ontogenetic origins and its functional significance for the individual and the species. Aggressive behavior comprises communicative signals, acts and postures for the purpose of obtaining a specific goal or in defense against threatening stimuli (Miczek et al. 2002). These behaviors occur in the context of competing for food and other resources that are important to an individual’s survival and reproduction (resident-intruder aggression), defense of a territory or offspring (territorial and maternal aggression), or in response to fear or frustration (Miczek et al. 2001). In this sense, the occurrence of aggressive behavior raises the fitness of the individual and enhances the survival of the species. For example, resident-intruder confrontations may represent male-male rivalries, establishing and maintaining a dominance hierarchy (Figure 1). So-called isolation-induced aggression captures many elements of a resident who excludes other breeding males from the territory (Brain and Benton 1979; Miczek et al. 2001; Table 1).
Figure 1. Mouse agonistic behavior.
Behaviors of resident and intruder mice engaged in an aggressive confrontation: (a) the resident leaps and bites the intruder as the intruder attempts to escape; (b) the resident (right) threatens as the intruder (left) holds a defensive upright posture; (c) the resident investigates the intruder’s anogenital region; (d) the resident pursues the fleeing intruder; (e) both resident and intruder engage in a mutual upright defensive posture. Reprinted with permission from Miczek and O’Donnell (1978).
Table 1. Types of aggressive behavior in preclinical models.
| A. Species-typical aggressive behavior | |||
|---|---|---|---|
| Situational or Experimental variable |
Agonistic behavioral measurements |
References | |
|
Dominant resident, mainly in primates and rats |
In a stable colony where dominance hierarchy is to be established and maintained. |
Frequency and duration of agonistic acts, postures, and displays including supplants, threat, pursue, and fight. |
Mehlman et al. 1994
Higley et al. 1996 Fairbanks et al. 1999 Bennett et al. 2002 Bernstein et al. 1974 Steiniger 1950 Vandenbergh 1967 |
|
Territorial resident (resident- intruder test), mainly in mice and hamsters |
Requires an established territory. In the laboratory, home-cage of experimental male (resident) where it is pair-housed with a female. A male stimulus animal (intruder) that is group housed with other males is introduced into resident’s cage. |
Frequency of attack bite, sideways threat, tail-rattle, pursue, upright posture. Latency to the first bite. |
Van Oortmerssen and Bakker 1981
Eibl-Eibesfeldt 1950 Miczek and O’Donnell 1978 Crawley et al. 1975 |
| BMaternal aggression, mainly in rats, mice, and hamsters |
Home-cage of lactating females from postpartum day 1 to 7. Either male or female of intruder is introduced into dam’s cage. |
Frequency of attack bite (especially directed at the snout and the face), sideways threat, tail-rattle, pursue, upright posture. Latency to the first bite |
Hurst 1987 Sgoifo et al. 1992 Lonstein and Gammie 2000 Noirot et al. 1975 Haney et al. 1989 |
|
Female aggression, mainly in primates and rodents. |
Dominant hierarchy among female monkeys. In the laboratory settings, female rodent pair-housed with a breeding male. Sexually matured female is introduced as an intruder. |
Harrassing attacks by dominant female. Frequency of attack bite, sideways threat, tail-rattle, pursue, upright posture. Latency to the first bite |
Smuts 1986
Palanza et al. 2005 DeBold and Miczek 1981 Zitzman et al. 2005 |
|
Isolation-induced aggression (similar to territorial aggression) |
Male isolated for same time, ranging from 24 h to 8 weeks prior to resident-intruder encounter. |
Frequency of attack bite, sideways threat, tail-rattle, pursue, upright posture. Latency to the first bite. |
Malick 1979
Valzelli and Bernasconi 1979 Cairns and Nakelski 1971 Yen et al. 1959 |
| B. Escalated aggressive behavior | |||
|
Situational or Experimental variable |
Agonistic behavioral measurements |
References | |
|
Alcohol- heightened aggression, mainly in rats and mice |
Animals receive ethanol (1.0g/kg) intraperitoneally or orally before the resident-intruder encounter. |
(These 3 methods measure aggressive behavior in same residentintruder method) Frequency of attack bite, sideways threat, tail-rattle, pursue, upright posture. Latency to the first bite Targets of attack bites (head, dorsal areas, ventral areas, appendages) |
Peeke and Figler 1981 Blanchard et al. 1987 Miczek et al 1992, 1998a Miczek and de Almeida 2001 |
|
Social provocations (instigations), mainly in hamsters, mice, and rats |
A resident male pre-exposed to another breeding male in his home- cage without direct agonistic interaction (stimulus animals are behind protective screen), followed by resident-intruder encounter. |
Heiligenberg 1974
Potegal and Tenbrink 1984 Potegal 1991 Fish et al. 1999 |
|
|
Frustration- heightened aggression |
A resident male trained to obtain rewards. Before the resident-intruder encounter, reward is omitted. |
Berkowitz 1993
De Almeida and Miczek 2002 |
|
|
Aggression induced by low glucocorticoids |
Animals are adrenalectomized and implanted with a low corticosterone pellet |
Haller et al. 2001 | |
|
Affective defense (“rage”), mainly in cats |
Electrical stimulation (0.2–0.8 mA, 63 Hz, 1 ms per half cycle duration) delivered in medial hypothalamus or midbrain periaqueductal gray. |
Hissing, arching of the back, retraction of the ears, piloerection, unsheathing of the claws, papillary dilatation and paw striking |
Leyhausen 1979
Siegel et al. 1999 Hess 1954 |
Based on distal and proximal antecedent conditions, the behavioral topography, and the consequences, aggressive behavior can be differentiated as offensive or defensive (Blanchard and Blanchard 1977; Brain 1979). In rats, a set of specific defensive behaviors occurs in response to either predator or conspecific attack, and comprises escape, freezing, defensive postures and threats (Blanchard et al. 2003; Pellis and Pellis 1988; Rasia-Filho et al. 2008). Defensive behaviors can be a response to threatening or fear-provoking stimuli and, usually, result in escapes (Brain 1979). For example, maternal aggressive behavior is seen in postpartum female rodents in order to protect offspring against male intruders, and this type of aggression includes both defensive and offensive elements (Lucion and de Almeida 1996; Parmigiani et al. 1998).
Violence in animals is a controversial term in animal ethology. This term has been hypothesized to be related to escalated, pathological and abnormal forms of aggression characterized by rapid attack latencies, prolonged and frequent aggressive behavior and attack bites (Miczek et al. 2002; Miczek et al. 2003). These parameters are quantitative, in that violence is expected to show shorter attack latencies and higher frequencies and longer durations of consummatory behavior than adaptive aggression. Measures of a qualitative nature have been proposed independently, where violence is considered qualitatively different from adaptive aggression. For instance, attach bites aimed at vulnerable parts of the opponent’s body are considered characteristic of abnormal aggression (Haller et al. 2005). A few additional qualitative facets have been studied, namely lack of ritualistic behaviors as measured by Attack/Threat (A/T) ratios (Haller et al. 2005) and context independent attacks (Koolhaas 1978) aimed at the opponent regardless of its sex or state (free-living/anaesthetized/dead) or the environment (home/neutral cage). In that sense, violence can therefore in principle refer to an escalated (hyper-) aggression (quantitative) or to an abnormal form of aggression (qualitative), or even to aggression that is both escalated and abnormal (both), which is unsurprisingly rare (for review see Natarajan and Caramashi 2010).
Aggressive behaviors in humans share commonalities with those in non-human animals, but they differ from most of them in their complexity. While social norms set the boundaries of appropriate aggressive behavior, inappropriate aggressive behavior in the form of interpersonal violence represents serious mental and social problems (Ferris et al. 2008). Aggressive behavior is a symptom in several psychiatric diseases, as detailed in the DSM-IV R and the updated DSM-V which is scheduled for publication in 2012, such as schizophrenia, brief reactive psychosis, anxiety disorder, adjustment disorder, impulse control disorder, antisocial personality disorder, attention deficit disorder, mania/depression, PTSD, autism, and substance abuse (Boles and Miotto 2003; Raine 2002; Rydén et al. 2009; Volavka et al. 2005).
A useful scheme considers human aggression as defensive, premeditated (e.g., predatory and instrumental) or impulsive-hostile in nature (Stoff and Vitiello 1996; Vitiello and Stoff 1997). Especially the premeditated and impulsive types of aggressive behaviors are diagnosed as pathological (i.e. in need of treatment). A converging pattern of empirical data links impulsive, but not premeditated, aggression to biological, environmental, and also to pharmacological or psychological treatment response factors (Coccaro et al. 2010).
Methodologically (see Table 2), human aggressive behavior as a state is assessed using protocols according to which individuals are provoked in competitive situations with fictitious opponents and that provide opportunities to engage in measurable aggressive responses (for review see Miczek et al. 2002). The assessment of aggressive traits in human subjects are accomplished by psychometric measures like inventories, questionnaires and scales. These laboratory measures of aggressive behavior have been used successfully in research on the role of 5-HT (Table 2). A critical challenge for this and similar experimental approaches is to relate the laboratory measures of aggression to aggressive and violent acts outside of the laboratory. It also remains difficult to discern subtypes of human aggression with laboratory measurement techniques. Table 2 summarizes the psychometric instruments which identify individuals with contrasting aggressive traits such as the impulsive-reactive-hostile-affective subtype versus the controlled-proactive-instrumental-predatory subtype (Stoff and Vitiello 1996).
Table 2. Experimental protocols for assessing 5-HT effects on human aggressive behavior.
| A. Experimental manipulations | |||
|---|---|---|---|
| Experimental Manipulation | Measurement | Trait/State | References |
| Aggressive responses toward a competitor are measured in the form of electric shock settings |
Activate buttons at 5–10 settings, each corresponding to a different intensity or duration of electric shock |
State |
Buss 1961
Godlaski and Giancola 2009 |
| A fictitious instigator or competitor is the target of aggressive responses that are measured in the form of electric shock deliveries |
Setting of electric shock level on a scale from 1–10 |
State |
Taylor 1967
Chermack and Giancola 1997 |
| The subjects are provoked by having points subtracted that are earned in a competitive task. The point losses are attributed to a fictitious opponent, but are actually determined by a computer program according to a random schedule. Subjects responded by retaliation of point subtractions (= aggressive responses |
Number of point subtractions from a fictitious competition |
State |
Cherek and Heistad 1971
Cherek and Lane 1999 Gowin et al. 2010 |
| Aggression was defined as delivery of electric shocks to a fictitious opponent |
Use of a modified version of the Buss aggression machine. Setting of shock level on a scale from 1–5 |
State |
Zeichner and Pihl 1979
Giancola et al. 2009 |
| B. Psychometric inventories | |||
| Psychometric Assessment | Instrument | Trait/State | References |
| Aggression, impulsive and hostility are measured by Minnesota Multiphasic Personality Inventory MMPI |
Inventory | Trait |
McKinley et al. 1948
Nagtegaal and Rassin 2004 |
| Buss-Durkee Hostility Inventory (BDHI), a self-rating scale of anger and hostility. 66 items with false/true answers; also contains 7 scales: assault, indirect aggression, irritability, negativism, resentment, suspicion and verbal aggression |
Inventory | Trait | Buss and Durkee 1957 |
| Anger and anxiety are measured by State-Trait Anger Expression Inventory (STAXI) |
Inventory | State/Trait |
Spielberg et al. 1973
Kim et al. 2009b |
| Aggression is measured by Beck Anxiety Inventory and Beck Depression Inventory |
Inventory | Trait |
Beck et al. 1961
Lamar et al. 2009 |
3. Aggressive “trait” vs. “state”
Based on early clinical and preclinical studies, the most frequently reiterated hypothesis links a serotonin deficiency to individuals presenting impulsive, hostile, and violent behavior (Brown and Goodwin 1986; Goldman et al. 1992; Lesch and Merschdorf 2000; Linnoila and Virkkunen 1992; Mann 1999; Valzelli 1977). These individuals may benefit particularly from pharmacological treatments aimed at inhibiting 5-HT transporters (using SSRIs such as fluoxetine, citalopram), or activating 5-HT1A (buspirone) or blocking 5-HT2A receptors (risperidone). Acutely, these drugs induce phasic changes in 5-HT function that are associated with their transient anti-aggressive effects. Using in vivo microdialysis techniques, transient changes in 5-HT extracellular levels can be monitored before, during, after and in anticipation of an aggressive encounter in rats. In one study, reduced 5-HT levels in the prefrontal cortex were revealed during and after the aggressive confrontation, while no changes in 5-HT were detected in another terminal region, the nucleus accumbens (Van Erp and Miczek 2000; Figure 2). By contrast, chronic treatment with these anti-aggressive compounds may promote yet to be defined neuroadaptive changes in 5-HT function that are associated with the emergence of therapeutic effects (e.g., autoreceptor desensitization).
Figure 2. Dopamine and serotonin during aggression.
Measurements of extracellular dopamine and serotonin via in vivo microdialysis in resident male rats before, during, and after a confrontation with an intruder. (a) In the nucleus accumbens (top panel), dopamine levels (gray circles) rise and remain elevated after the confrontation, while serotonin levels (black diamonds) do not significantly change. (b) In the prefrontal cortex (bottom panel), dopamine levels rise after the confrontation, while serotonin decline and remain lower after the confrontation. Samples were collected every 10 min and levels are expressed as mean percent of baseline ± SEM. Baseline was measured for 50 min before the fight. The vertical light gray bar indicates the occurrence of the 10-min fight. * and ** represent significant differences from baseline (dashed line) at the p < 0.05 and p < 0.01 levels, respectively. Reprinted with permission from Van Erp and Miczek (2000).
On the other hand, genetic studies focus on aggression as a “trait”. While it is clear that these aggressive traits are polygenic, it is remarkable that in several cases a gene-environment interaction is required for the increased propensity to engage in violent outbursts, as observed with TPH2, MAO-A and 5-HTT polymorphisms (see below). For example, a SNP in TPH2 gene (A2051C) has been shown to have a link to aggressive behavior in rhesus monkeys. Individuals that have an AA/AC genotype show increased aggressive acts compared to those with a CC genotype when they were reared without their mother (peer-reared). This difference disappeared when individuals of both genotypes were reared by their mothers (Chen et al. 2010). In this review, we will discuss the effects of genes on aggressive behaviors with a focus on the interaction with salient environmental events.
In addition, gene-gene interactions are also of interest and need to be examined in the future. For example, Passamonti et al. (2008) showed interactions between 5-HTT and MAOA polymorphisms, and those interactions exerted stronger effects on the activity of the anterior cingulate cortex, one of the brain areas implicated in impulsivity, including impulsive aggression. Many other genes may have subtle effects on aggressive phenotypes and it is possible that those genes have complex epistatic interactions from which stronger effects emerge (Miczek et al. 2001). In rodents, most genetic studies on aggression in the past 15 years make use of conventional knockout techniques in which the expression of a gene is generally deleted in the whole body, affecting all developmental stages and inducing compensatory changes in other genes (trait-like change; see Table 3). Novel tools, including conditional knockout, viral vector microinfusion, or drug-induceable knockout technique, can produce transient and local changes in gene expression, enabling the examination of more “phasic” changes in gene expression and how they affect aggressive behavior. The use of these techniques may reduce some discrepancies in the results from genetic and pharmacological studies of 5-HT function in aggression.
Table 3. Genes and aggressive behavior in mice.
| Gene | Abb | Chr | Background strain (Generations of backcross) |
Type of aggression | Effects | Serotonin function |
References |
|---|---|---|---|---|---|---|---|
| Plasmacytoma expressed transcript 1 |
Pet1 (Fev) |
1 | Mix C57BL/6 and 129Sv |
Isolation-induced resident aggression |
Increased | 90% reduction of brain 5-HT |
Hendricks et al. 2003 |
| Arginine vasopressin receptor 1B |
V1bR | 1 | Mix C57BL/6 and 129/SvJ |
Isloation-induced aggression |
Suppressed | Wersinger et al. 2002 | |
| Neutral cage aggression |
Suppressed | ||||||
| Aadenosine A1 receptor |
A1AR | 1 | Mix C57BL and 129/OlaHsd |
Isolation-induced resident aggression |
Increased | Gimenez-Llort et al. 2002 | |
| Regulators of G protein signaling 2 |
Rgs2 | 1 | C57BL/6J (N5) | Social dominance test |
Suppressed | Oliveira-dos-Santos et al. 2000 | |
| v-abl Abelson murine leukemia viral oncogene homolog 2 (Abelson-related gene) |
Arg | 1 | Mix C57BL/6 and 129/SvJ |
Resident aggression | Suppressed | Koleske et al. 1998 | |
| Glutamic acid decarboxylase 2 |
GAD65 | 2 | Mix C57BL/6 and CBA2 |
Isolation-induced resident aggression |
Suppressed | Stork et al. 2000 | |
| Calcium channel, voltage-dependent, N type, α1B subunit |
Cav2.2 | 2 | Mix C57BL/6J and 129S4/SvJae |
Isolation-induced resident aggression |
Increased | Increased hypothalamus 5- HT |
Kim et al. 2009a |
| dopamine β- hydroxylase |
Dbh | 2 | Mix C57BL/6J and 129/SvEv |
Isolation-induced resident aggression |
Suppressed | Marino et al. 2005 | |
| Brain-derived neurotrophic factor [+/−] |
BDNF | 2 | C57BL/6J (>N10) | Isolation-induced resident aggression |
Increased | Decreased brain 5- HT |
Lyons et al. 1999 |
| β2-microglobulin | β2m | 2 | 129S2/SvPas (Mix C57BL/6J?) |
Resident aggression | Suppressed | Loconto et al. 2003 | |
| Oxytocin | Oxt | 2 | Mix C57BL/6J and 129SvEv |
Isolation-induced resident aggression |
Increased | Winslow et al. 2000 | |
| Resident aggression | Increased | ||||||
| Suppressed | DeVries et al. 1997 | ||||||
| membrane metallo endopeptidase (enkephalinase) |
NEP | 3 | Mix C57BL/6J and 129Sv |
Resident aggression | Increased | Fischer et al. 2000 | |
| Cannabinoid receptor 1 | CB1 | 4 | CD1 (N15) | Isolation-induced resident aggression |
Increased[1] | Martin et al. 2002 | |
| Preproenkephalin | Enk | 4 | Mix CD1 and 129 |
Isolation-induced resident aggression |
Increased[1] | Konig et al. 1996 | |
| endothelial nitric oxide synthase |
eNOS | 5 | Mix C57BL/6 and 129Sv |
Resident aggression | Suppressed | Increased 5-HT turnover |
Demas et al. 1999 |
| Neutral cage aggression |
Suppressed | ||||||
| Interleukin-6 | IL-6 | 5 | Mix C57BL/6, 129/SvEv |
Isolation-induced resident aggression |
Increased[2] | No difference in brain 5-HT concentration |
Alleva et al. 1998 |
| Adrenergic receptor, α2c |
Naα2C | 5 | C57BL/6J (N7) | Isolation-induced resident aggression |
Increased | Sallinen et al. 1998 | |
| Neuronal nitric oxide synthase |
nNOS | 5 | Mix C57BL/6J, 129Sv, DBA2 |
Resident aggression | Increased | Reduced brain 5- HT turnover |
Nelson et al. 1995 |
| Maternal aggression |
Suppressed | Gammie and Nelson 1999 | |||||
| Acetylcholinesterase | AChE | 5 | 129S6/SvEvTac | Home-cage hierarchy |
Suppressed | Duysen et al. 2002 | |
| Adenylate cyclase activating polypeptide 1 receptor 1 |
PAC1 | 6 | Mix C57BL/6J and 129Sv |
Resident aggression | Suppressed | Nicot et al. 2004 | |
| Tachykinin receptor 1 | NK-1r | 6 | Mix C57BL/6 and 129Sv |
Isolation-induced resident aggression |
Suppressed | De Felipe et al. 1998 | |
| A cluster of vomeronasal receptor genes |
V1Ra/b | 6 | 129/SvEv | Maternal aggression |
Suppressed | Del Punta et al. 2002 | |
| Oxytocin receptor | Oxtr | 6 | Mix C57BL/6 and 129Sv |
Isloation-induced aggression |
Increased | Takayanagi et al. 2005 | |
| Cage-mate injury | Increased | ||||||
| Histamine receptor H1 | H1 | 6 | Mix C57BL/6J and 129 |
Isolation-induced resident aggression |
Suppressed[2] | Increased 5-HT turnover |
Yanai et al. 1998 |
| Amyloid β(A4) precursor proteinbinding, family A, member 2 |
X11L | 7 | C57BL/6 | Competitive feeding |
Subordinate | Increased Hypothalamus 5- HT |
Sano et al. 2009 |
| Transient receptor potential cation channel, subfamily C, member 2 |
TRP2 | 7 | Mix C57BL/6J and 129Sv |
Resident aggression | Suppressed | Stowers et al. 2002 | |
| Neuropeptide Y receptor Y1 |
Y1 | 8 | Mix C57BL/6 and 129SvJ |
Resident aggression | Increased | Reduced TPH mRNA expression in the raphe nuclei |
Karl et al. 2004 |
| Prostaglandin E receptor 1 (Subtype EP1) |
EP1 | 8 | C57BL/6 (N5) | Neutral cage aggression |
Increased | No difference in 5- HT turnover |
Matsuoka et al. 2005 |
| Norepinephrine transporter |
NET | 8 | Mix C57BL/6J and 129SvJ |
Isolation-induced resident aggression |
Increased[1] | Haller et al. 2002 | |
| Neural cell adhesion molecule 1 |
NCAM1 | 9 | C57BL/6J (>N5) | Isolation-induced resident aggression |
Increased | Stork et al. 1997 | |
| Aromatase (Cyp19a1) | Ar | 9 | Mix C57BL/6 and 129S/SvEv |
Resident aggression | Suppressed | Matsumoto et al. 2003 | |
| Aggression toward female |
Increased | ||||||
| 5-hydroxytryptamine (serotonin) receptor 1B |
5-HT1B | 9 | 129S2/SvPas | Resident aggression | Increased | Deleted 5-HT1B receptor expression |
Saudou et al. 1994 |
| Maternal aggression |
Increased | Brunner and Hen 1997 | |||||
| Estrogen Receptor-α | ERα | 10 | Mix C57BL/6J and 129 |
Resident aggression | Suppressed | Ogawa et al. 1998b | |
| Female aggression | Increased | Ogawa et al. 1998a | |||||
| Fyn tryrosine kinase | Fyn | 10 | Mix C57BL/6 and CBA |
Isolation-induced resident aggression |
Suppressed | Miyakawa et al. 2001 | |
| nuclear receptor subfamily 2, group E, member 1 |
Nr2e1 | 10 | C57BL/6J (>N6) | Resident aggression | Increased | Young et al. 2002 | |
| Adenosine A2a receptor |
A2AR | 10 | CD1 (N4) | Isolation-induced resident aggression |
Increased | Ledent et al. 1997 | |
| Tryptophan hydroxylase 2 |
Tph2 | 10 | FVB/N (N7) | Home-cage injury | Increased | Reduced brain 5- HT |
Alenina et al. 2009 |
| Glutamate receptor, ionotropic, AMPA1 (α1) |
GluR-A | 11 | C57BL/6J (N5) | Isolation-induced aggression |
Suppressed | No difference in brain 5-HT level |
Vekovischeva et al. 2004 |
| Neutral cage aggression |
Suppressed | ||||||
| 5-hydroxytryptamine (serotonin) transporter |
SERT | 11 | C57BL/6J (N8) | Isolation-induced resident aggression |
Suppressed | Increased extracelluler 5-HT |
Holmes et al. 2002 |
| Estrogen Receptor-β | ERβ | 12 | Mix C57BL/6J and 129 |
Resident aggression | Increased[1] | Ogawa et al. 1999 | |
| Dopamine transporter | DAT | 13 | Mix C57BL/6J and 129SvJ |
Dyadic encounter aggression |
Increased | Rodriguiz et al. 2004 | |
| 5-hydroxytryptamine (serotonin) receptor 1A |
5-HT1A | 13 | 129S1/Sv | Resident aggression | Suppressed | Deleted 5-HT1A receptor expression |
Zhuang et al. 1999 |
| Catechol-O- methyltransferase 1 [+/−] |
COMT | 16 | Mix C57BL/6J and 129SvJ |
Neutral cage aggression |
Increased | No difference in brain 5-HT level |
Gogos et al. 1998 |
| Calcium/calmodulin- dependent protein kinase II alpha [+/−] |
αCaMK II |
18 | Mix C57BL/6, 129/OU, BALB/c |
Defensive aggression |
Increased | Reduced 5-HT release in the dorsal raphe nucleus |
Chen et al. 1994 |
| Melanocortin-5 receptor |
MC5R | 18 | C57BL/6 (>N7) | Neutral cage aggression |
Suppressed | Morgan et al. 2004 | |
| Monoamine oxidase A | MAOA | X | C3H/HeJ | Resident aggression | Increased | Increased brain 5- HT up to 9 fold |
Cases et al. 1995 |
| Cage-mate injury | Increased | ||||||
| Cyclic nucleotide– gated channel a2 |
Cnga2 | X | Mix C57BL/6J and 129SvEv |
Resident aggression | Suppressed | Mandiyan et al. 2005 | |
| Guanosine diphosphate (GDP) dissociation inhibitor 1 |
Gdi1 | X | C57BL/6J (N5) | Isolation-induced resident aggression |
Suppressed | D’Adamo et al. 2002 | |
| Androgen receptor[3] | AR | X | C57BL/6 and 129SvEv |
Isolation-induced resident aggression |
Suppressed[2] | Raskin et al. 2009 |
[+/−]: Data obtained from heterozygote of knockout mouse
Behavioral change only at the first encounter
Behavior change after repeated exposure
Nestine-Cre-LoxP conditional knockout mice that selectively lack AR expression in the nervous system.
4. 5-HT receptors
4.1. Pharmacology of 5-HT receptors and aggression
So far, most evidence implicates the 5-HT1 and 5-HT2 families of receptors in aggressive behaviors (Miczek et al. 2002; Olivier 2004), with some initial evidence for the involvement of 5-HT3 receptors as well (McKenzie-Quirk et al. 2005; Ricci et al. 2004; Rudissaar et al. 1999).
Clinically, the 5-HT1A receptor partial agonist buspirone can reduce aggressive behavior in mentally retarded patients (Kavoussi et al. 1997; Ratey et al. 1991). This compound has been used for the management of aggressive outbursts associated with neuropsychiatric disorders in adults and children (Connor and Steingard 1996; Pabis and Stanislav 1996). However, clinical studies have mostly focused on patients with multiple diagnoses and clinical symptoms, undergoing treatment with various drugs simultaneously (Brahm et al. 2008; Levy et al. 2005; Pabis and Stanislav 1996; Ratey et al. 1989; Ratey and O’Driscoll 1989). Thus, more controlled studies for different clinical populations are necessary to assess the efficacy – and side effect profile – of buspirone and other 5-HT1A agents as selective anti-aggressive medications. In preclinical investigations, systemic administration of 5-HT1A receptor agonists promotes anti-aggressive effects in several species, including fish, amphibian, birds, rodents, guinea pigs and non-human primates (Bell and Hobson 1994; Blanchard et al. 1988; Clotfelter et al. 2007; de Boer et al. 1999; de Boer et al. 2000; de Boer and Koolhaas 2005; Dompert et al. 1985; Haug et al. 1990; Joppa et al. 1997; Lindgren and Kantak 1987; McMillen et al. 1988; Miczek et al. 1998b; Muehlenkamp et al. 1995; Nikulina et al. 1992; Olivier et al. 1992; Sanchez et al. 1993; Sperry et al. 2003; Ten Eyck 2008; Tompkins et al. 1980). Only one exception was observed in fruit flies (Drosophila melanogaster), in which treatment with the 5-HT1A agonist, 8-OH-DPAT, escalated aggressive behavior (Johnson et al. 2009). Selective antagonists of 5-HT1A receptors, such as WAY-100635, block the anti-aggressive effect of 5-HT1A agonists, while having no reliable effects on aggression per se (de Boer and Koolhaas 2005; Mendoza et al. 1999; Miczek et al. 1998b).
Laboratory studies have found that the anti-aggressive effects of 5-HT1A agonists in vertebrates are consistently accompanied by non-specific effects including sedation, slow motor routines, stereotypic behavior or reduced social interest (de Boer and Koolhaas 2005; Miczek et al. 1998b; Olivier et al. 1995). However, some 5-HT1A agonists, at least in a ferally derived rat strain, can selectively reduce aggressive behavior without affecting other non-aggressive behaviors, (i.e., alnespirone and S-15535; (de Boer et al. 1999; de Boer et al. 2000; de Boer and Koolhaas 2005). It is possible that those compounds act on a subpopulation of 5-HT1A receptors to exert this anti-aggressive effect, and thereby are more behaviorally specific.
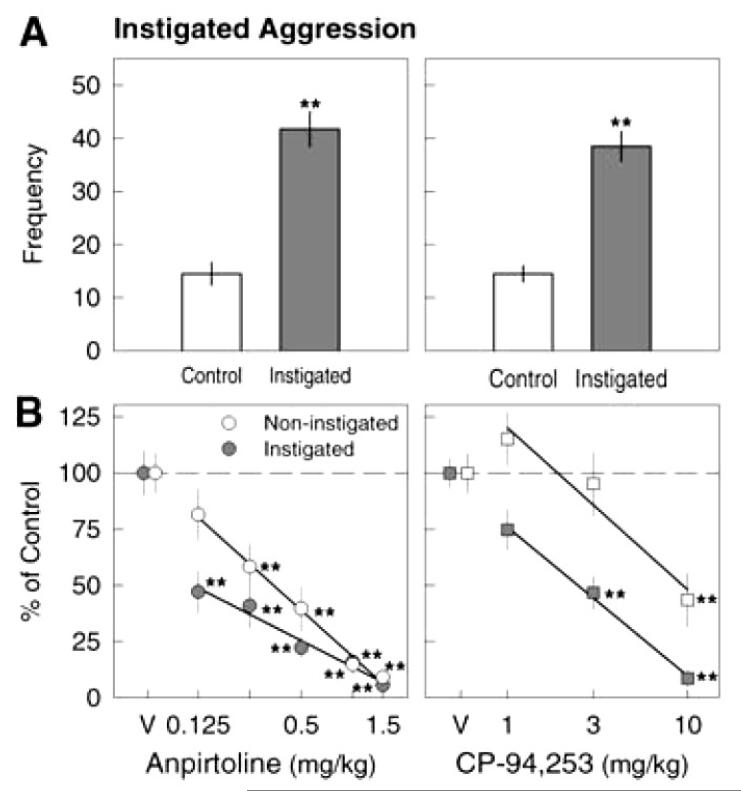
Despite the absence of clinically approved drugs, preclinical work suggests that targeting 5-HT1B receptors may have more specific anti-aggressive effects than 5-HT1A manipulations. In mice and rats, the systemic administration of 5-HT1B agonists reduces aggressive behavior without sedation, or motor or sensory impairment (de Almeida et al. 2001; de Almeida and Miczek 2002; de Boer and Koolhaas 2005; Fish et al. 1999; Miczek et al. 2002; Miczek et al. 2004; Olivier et al. 1990; Olivier 2004; Figure 3). These effects were antagonized by the 5-HT1B/1D antagonist GR-127935, further confirming the involvement of 5-HT1B in mediating the anti-aggressive effects (de Boer and Koolhaas 2005). However, differences in the binding domain of 5-HT1B receptors of humans and rodents may yield different pharmacological selectivity and specificity of 5-HT1B agonists (Olivier 2004).
Figure 3.
A. Effects of social instigation on aggressive behavior by a resident mouse toward a male intruder. Bars represent the mean frequency ±SEM (vertical lines) of attack bites under control (light gray) and instigated (dark gray) conditions. Asterisks denote statistical significance from control (**P<0.01). B. Preferential reduction of instigated aggressive behavior by the 5-HT1B agonist anpirtoline (left panel, filled circles) and CP-94,253 (right panel, filled squares). Symbols represent the mean frequency of attack bites, expressed as a percentage of vehicle (V) baseline, ±SEM. Light gray symbols represent non-instigated fighting and dark gray symbols represent instigated levels of fighting. Asterisks denote significance from vehicle baseline (P<0.05). Adapted from Fish et al. (1999) and de Almeida and Miczek (2002).
Determination of the critical brain regions and specific mechanisms underlying the anti-aggressive effects of 5-HT1A and 5-HT1B receptor agonists still remains to be resolved (Table 4). Neurobiological studies have associated the effects of 5-HT1A and 5-HT1B agonists with reduced 5-HT neuronal firing and release in projection sites (Adell et al. 2001; Bonvento et al. 1992; Sprouse and Aghajanian 1987), suggestive of presynaptic mechanisms mediating the anti-aggressive effects of these drugs. Activation of 5-HT1A and 5-HT1B inhibitory autoreceptors in the dorsal raphé nucleus (DRN) with microinfusion of selective receptor agonists consistently reduced aggressive behavior in rats and mice, but with concomitant reduction of motor activity and social interactions (Bannai et al. 2007; Faccidomo et al. 2008; Mos et al. 1993; Van Der Vegt et al. 2003). Infusion of a 5-HT1A agonist into the median raphé nucleus (MRN) also reduced aggressive behavior of lactating female rats (de Almeida and Lucion 1997).
Table 4. Modulation of aggressive behaviors after local infusion of drugs targeting 5-HT receptors in selected brain regions.
| Brain Region | Type of Aggression, Species |
Target; Drugs and Doses | Pharmacological Effects | References |
|---|---|---|---|---|
| DRN | Resident aggression, male rats |
5-HT1A: 8-OH-DPAT, 1-10 μg. 5-HT1A/5-HT1B: Eltopronazine, 1-30 μg (agonists) |
↓ aggressive behavior, with inactivity and decreased social interaction |
Mos et al. 1993 |
| Resident aggression, male rats |
5-HT1A: Alnespirone, 25 μg (agonist) | ↓ aggression; no side effects | Van der Vegt et al. 2003 | |
| Alcohol-escalated aggression, male mice |
5-HT1A: 8-OH-DPAT, 1.0 μg 5-HT1B: CP-94253, 1.0 μg (agonists) |
8-OH-DPAT and CP-94253: ↓ baseline aggression, with reduced motor activity; no effects on alcoho- lrelated aggression |
Faccidomo et al. 2008 | |
| Schedule-heightened aggression, male mice |
5-HT1B: CP-93129, 0.1-1.0 μg (agonist) |
↓ escalated aggression; reduced walking behavior |
Bannai et al. 2007 | |
| Alcohol-escalated aggression, male mice |
5-HT1B: CP-93129, 0.1-1.0 μg (agonist) |
↓ baseline and alcohol-related aggression (0.5-1.0 μg); concomitant reduction in motor activity |
Faccidomo et al. submitted | |
| Maternal aggression, rats |
5-HT1A: 8-OH-DPAT, 0.56 μg (agonist) |
↑ maternal aggression (0.56 μg); no motor effects DPAT-escalated aggression prevented by infusion of CP-93129 (1.0 μg) into the orbitofrontal cortex |
Veiga et al. 2010 | |
| MRN | Maternal aggression, rats |
5-HT1A: 8-OH-DPAT, 0.2-2.0 μg (agonist) |
↓ maternal aggression; no side effects |
De Almeida and Lucion, 1997 |
| PAG | Maternal aggression, rats |
Dorsal PAG 5-HT1A: 8-OH-DPAT, 0.2-2.0 μg (agonist) |
↓ maternal aggression (0.2-2.0 μg); no side effects |
de Almeida and Lucion, 1997 |
| Maternal aggression, rats |
Dorsal PAG 5-HT2A/2C: α-methyl-5-HT maleate, 0.2-1.0 μg (agonist) 5-HT2A/2C: ketanserin, 1.0 μg (antagonist) |
α-methyl-5-HT maleate: ↓ maternal aggression; no motor effects Ketanserin: no effects on aggression, decreased motor activity |
de Almeida et al. 2005 | |
| Hypothalamic- stimulated defensive aggression, cats |
PAG 5-HT1A: 8-OH-DPAT*, 0.016 ng -1.0 μg 5-HT2C: DOI*, 3.57 ng–0.54 μg (agonists) |
8-OH-DPAT: ↓ defensive hissing (0.66 – 1.0 μg), effect prevented by antagonist p-MPPI. No motor effect DOI: facilitation of defensive hissing (0.54 μg) |
Shaikh et al. 1997 | |
| Septal nuclei | Maternal aggression, rats |
Medial septal nucleus 5-HT1A: 8-OH-DPAT, 0.2-2.0 μg (agonist) |
↑ maternal aggression (0.2-0.5 μg); reduced activity only with highest dose (2.0 μg) |
de Almeida and Lucion, 1997 |
| Maternal aggression, rats |
Medial septal nucleus 5-HT2A/2C: alpha-methyl-5-HT maleate, 0.2-1.0 μg (agonist) 5-HT2A/2C: ketanserin, 1.0 μg (antagonist) |
No effects on aggressive or non- aggressive behaviors (agonist) Ketanserin: no effects on aggression, but decreased motor activity |
de Almeida et al. 2005 |
DRN = dorsal raphé nucleus; MRN = median raphé nucleus; PAG = periaqueductal gray area;
doses calculated based on the following molecular weights (MW) of the compounds (as available at Sigma-Aldrich): 8-OH-DPAT hydrobromide, MW=328.29; CGS-12066 maleate salt, MW=450.41; DOI hydrochloride, MW=357.62.
The overall relevance of presynaptic mechanisms for the anti-aggressive effects of these manipulations is challenged by several reports that lesions or depletion of 5-HT neurons (e.g., using tryptophan hydroxylase inhibitor PCPA, or the neurotoxin 5,7-dihydroxytryptamine (5,7-DHT)) do not affect the anti-aggressive effects of 5-HT1A and 5-HT1B agonists (de Almeida et al. 2001; Miczek et al. 1998b; Sanchez and Hyttel 1994; Sijbesma et al. 1991). While these data suggest postsynaptic 5-HT1 receptors as critical sites of action, these pharmacological depletions spared a subpopulation of receptors.
In projection sites of 5-HT neurons, 5-HT1B receptors likely modulate 5-HT release from synaptic terminals as autoreceptors, whereas both 5-HT1A and 5-HT1B receptors modulate postsynaptic neurons (Olivier et al. 1992). In most studies, local activation of 5-HT1A and 5-HT1B in projection regions (e.g., medial preoptic area, lateral septum, orbitofrontal cortex, anterior hypothalamus, medial hypothalamus, periacqueductal gray) promote reduction of aggressive behavior under different procedures and species (see Table 4). Interestingly, under conditions that may promote escalated aggression, such as consumption of moderate doses of alcohol or maternal aggression, a 5-HT1B or 5-HT1A agonist further increased levels of aggressive behavior when infused into the medial prefrontal cortex (Faccidomo et al. 2008) or the medial septal area (de Almeida and Lucion 1997), respectively. Further studies are required to delineate the mechanisms for such pro-aggressive effects.
Atypical antipsychotic agents (e.g., risperidone) with significant antagonist action at 5-HT2A receptors have been successfully used to reduce aggressive outbursts in patients diagnosed with various neuropsychiatric disorders (Buckley et al. 1997; Buitelaar et al. 2001; Czobor et al. 1995; De Deyn et al. 1999; Fava 1997; Keck, Jr. et al. 2000; Zarcone et al. 2001). On the contrary, some reports cast doubt on these routine uses of antipsychotics (Swanson et al. 2008; Tyrer et al. 2008). The placebo treatment group showed the greatest recovery from aggressive challenges compared to antipsychotic drug groups in people with intellectual disability (Tyrer et al. 2008). In animal models, risperidone and other drugs with more selective action as 5-HT2A antagonists (e.g., ketanserin, ritanserin and MDL 100907) reduce aggressive behaviors in a behaviorally non-specific manner (Rodriguez-Arias et al. 1998; Sakaue et al. 2002; Shih et al. 1999; White et al. 1991).
Activation of 5-HT2A and 5-HT2C receptors by DOI and other substituted phenylisopropylamines also reduce aggressive behavior in several species including flies, amphibians, mice and rats (Bonson et al. 1994; de Almeida and Lucion 1994; Johnson et al. 2009; Muehlenkamp et al. 1995; Olivier et al. 1995; Sanchez et al. 1993; Ten Eyck 2008). However, the effects of 5-HT2 ligands are accompanied by sedative effects in the same dose range as the anti-aggressive effects. Local infusion of 5-HT2A/2C agonist into the PAG reduces maternal aggression in rats (de Almeida et al. 2005), whereas microinjections into the medial hypothalamus and into the PAG increased defensive aggression in cats (Hassanain et al. 2003; Shaikh et al. 1997; see Table 4). This latter effect is likely linked to the role of 5-HT2A/2C receptors in anxiety-like behavior (Lucki and Wieland 1990; Nogueira and Graeff 1995). The development of more selectively acting pharmacological tools will allow a more adequate differentiation of 5-HT2 receptor subtypes, and promises to dissociate the anti-aggressive and sedative effects.
4.2. Genetics of 5-HT receptors and aggression
The 5-HT1B receptor is the first molecule that has been linked to aggression by using the genetic knockout technique. Male mice with disrupted 5-HT1B receptor expression (Htr1b−/−) increased aggressive behavior in the resident-intruder test relative to wild-type residents after a month of isolation (Saudou et al. 1994; Table 3; but see Bouwknecht et al. 2001). However, due to very low, close to zero, aggressive behavior in the wild-type mice (129/Sv-ter), the number of attacks in Htr1b−/− was very low and the latency to initiate was very long compared to other strains of mice. These mice displayed behavioral disinhibition in other behavioral tests including hyperlocomotor activity (Brunner et al. 1999; Ramboz et al. 1995), drug intake (Crabbe et al. 1996; Rocha et al. 1998), measures of anxiety-like behavior (Brunner et al. 1999; Malleret et al. 1999), and autonomic hyperreactivity to novelty (Bouwknecht et al. 2001). Females of Htr1b−/− also show increased aggressive behavior during the postpartum period (Brunner and Hen 1997). These results have been interpreted to suggest a role for 5-HT1B receptors in the inhibition of aggressive and impulsive behaviors.
Linkage analysis on a SNP in the 5′UTR region of the 5-HT1B gene, A161T, found a significant correlation between this SNP and the history of aggression in subjects who completed violent suicides (Zouk et al. 2007). Individuals with the T161 locus had higher lifetime aggressive behaviors. T161 polymorphism had reduced transcriptional activity of 5-HT1B receptor (Sun et al. 2002), and thus lower 5-HT1B receptor expression may be related to lifetime aggression in suicidal victims. By contrast, other SNPs in the 5-HT1B showed an opposite pattern. A linkage study of 5-HT1B with alcoholism and antisocial personality disorder showed that the G861C polymorphism had significant linkage with antisocial alcoholism in two groups (Lappalainen et al. 1998). Specifically, C861, the SNP with higher 5-HT1B receptor expression (Huang et al. 1999), was related to antisocial behavior in alcoholics. However, these associations between the 5-HT1B polymorphisms (G861C, G261T, or C129T) and aggression/antisocial behavior are not seen in other studies (Huang et al. 1999; Kranzler et al. 2002; Sinha et al. 2003; Van den Berg et al. 2008). Therefore, findings from pharmacological manipulation, genetic deletion and polymorphism studies of the 5-HT1B receptor do not follow a simple and consistent scheme. This suggests that the role of 5-HT1B receptors may depend on the types of aggressive behaviors or tonic and phasic level of aggression.
In contrast to the important role of 5-HT1A receptors in the neural control of aggressive behavior based on pharmacological evidence, no prominent linkage has been reported with polymorphisms in the 5-HT1A gene and aggression so far. However, there is evidence for a correlation between 5-HT1A receptor expression and aggression. A human PET study found a higher 5-HT1A receptor distribution in prefrontal cortex of subjects with higher aggression scores based on a self-report questionnaire (Witte et al. 2009). Also, rats selected for higher defensive reactions showed reduced 5-HT1A receptor expression in several brain areas (Popova et al. 1998). It is possible that the polymorphisms which directly or indirectly affect 5-HT1A receptor transcription may be associated with either aggressive or defensive responses. 5-HT1A receptor knockout mice engaged in less aggressive behavior relative to wild-type controls, which also implicates the possible involvement of the 5-HT1A gene in aggressive behavior (Zhuang et al. 1999; Table 3). Given the consistent pharmacological data on anti-aggressive effects of 5-HT1A agonists in clinical and preclinical studies, lack of complementary genetic data remains disconcerting.
Platelet 5-HT2A receptor binding is increased in patients with personality disorders and in a psychiatric population with greater lifetime aggression scores (Coccaro et al. 1997; McBride et al. 1994). In postmortem brains, lifetime aggression was positively correlated with prefrontal 5-HT2A receptor binding in suicide victims (Oquendo et al. 2006). Therefore, it is possible that polymorphisms that affect the level of expression of 5-HT2A receptors can be associated with self-directed aggression. In some samples, significant linkage was found between polymorphisms in the 5-HT2A receptor, T102C, A1438G and His452Tyr, and aggressive-impulsive trait or adolescent-onset antisocial behavior in humans (Assal et al. 2004; Bjork et al. 2002; Burt and Mikolaiewski 2008; Nomura et al. 2006). But others have reported no such link between aggression and 5-HT2A polymorphisms (Khait et al. 2005; Van den Berg et al. 2008). Again, the success with pharmacotherapeutic management of aggressive patients using compounds with affinity for 5-HT2A receptors would suggest that violence-prone individuals may be characterized by distinctive 5-HT polymorphisms.
5. 5-HTT
5.1. Pharmacology of 5-HTT and aggressive behavior
Blocking serotonin transporter molecules is effective in reducing and preventing aggressive behavior in humans and non-human animals, presumably due to increased brain 5-HT levels. Clinically, blocking 5-HT transporters with the administration of selective serotonin reuptake inhibitors (SSRIs), reduces aggressive outbursts and violent behavior in psychiatric patients (Barkan et al. 2006; Blader 2006; Bond 2005; Coccaro and Kavoussi 1997; New et al. 2004; Reist et al. 2003; Walsh and Dinan 2001), with therapeutic effects being usually observed after chronic treatment (>3 weeks). However, there are occasional reports that SSRIs may facilitate aggressivity and suicidal behavior, and the causes for these unusual outcomes remain to be determined (Spigset 1999; Troisi et al. 1995).
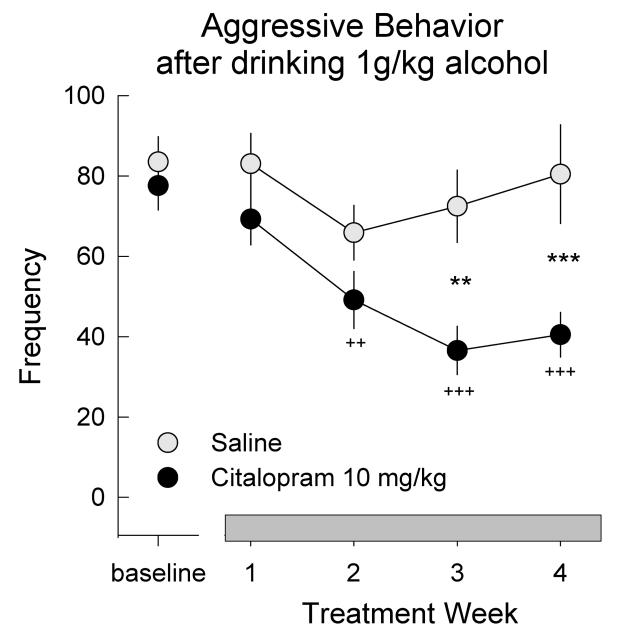
In animal models, both acute and chronic treatment with SSRIs can dose-dependently reduce aggressive behavior (Carrillo et al. 2009; Delville et al. 1996; Olivier et al. 1989; Pinna et al. 2003). Acute administration of several SSRIs (e.g., fluoxetine, fluvoxamine, sertraline) reduced aggression in different contexts and species, including rodents and non-human primates (Carrillo et al. 2009; Cutler et al. 1997; Delville et al. 1996; Fairbanks et al. 2001; Ferris et al. 1997; Fuller 1996; Ho et al. 2001; Sanchez and Meier 1997). Chronically, daily treatment with the SSRI citalopram abolished the escalated levels of aggression induced by a moderate dose of alcohol over the course of three weeks, with modest reductions in baseline levels of aggression in mice (Caldwell and Miczek 2008; Figure 4). On the other hand, chronic SSRI treatment may restore competent agonistic behavior in placid, non-aggressive laboratory rats (Mitchell et al. 1991; Mitchell 2005; Mitchell and Redfern 1992; Mitchell and Redfern 1997). Thus, the anti-aggressive effects of SSRIs are more prominent in conditions of escalated aggressive behavior such as those promoted by alcohol (Caldwell and Miczek 2008).
Figure 4.
Effects of repeated, twice daily administration of the SSRI citalopram (10 mg/kg, i.p.) on aggressive behavior in mice after drinking 1.0 g/kg alcohol in operant self-administration panels. Frequency of aggressive acts (± SEM) is defined as sum of attack bites, threats, pursuits and tail rattles, and was analyzed in 5 min confrontations against a male intruder, during the course of fours weeks of citalopram (or saline control) treatment. + symbols represent differences from baseline (++ p<0.01; +++ p<0.001); * symbols represent group (citalopram vs. saline-controls) differences (** p<0.01; *** p<0.001). Adapted from Caldwell & Miczek (2008).
Mechanistically, acute or chronic administration of citalopram (or the more potent isomer escitalopram) both elevate extracellular levels of 5-HT in the prefrontal cortex of rats, suggesting increased cortical 5-HT as a putative therapeutic mechanism for SSRIs’ effects on aggression and other mood disorders (Ceglia et al. 2004). However, the anti-aggressive effects of another SSRI, fluoxetine, might be primarily mediated by actions on neurosteroids and GABA transmission, and only secondarily via 5-HT (Pinna et al. 2003; Pinna et al. 2006). Furthermore, long-term effects of SSRIs likely recruit pre- and post-synaptic mechanisms and neuroplastic events that contribute to their therapeutic effects (Benmansour et al. 1999; Blier and de Montigny 1998; Ceglia et al. 2004; Pineyro et al. 1994).
5.2. Genetics of 5-HTT and aggressive behavior
A variation in the length of 5′-flanking transcriptional control region (promoter) of the 5-HTT gene (the serotonin-transporter-gene-linked polymorphic region; 5-HTTLPR) has been identified in humans (Heils et al. 1996), great apes and rhesus monkeys (Lesch et al. 1997). This variation affects the transcriptional activity of the 5-HTT gene, and the short length (s) allele reduces 5-HTT expression in vitro and lowers the prolactin response to clomipramine in human, which reflects reduced 5-HT function, compared to the homozygote of long length allele (l/l) (Heils et al. 1995; Lesch et al. 1996; Whale et al. 2000). An association study in humans showed that individuals with one or two copies of the s allele (s/s, s/l) were characterized by higher anxiety, depression, hostility and aggression, and lower agreeableness than individuals of the l/l homozygote in both sexes (Lesch and Merschdorf 2000). Higher frequency of the s allele was observed in alcoholics accompanied with high impulsivity and antisocial behaviors (type 2 alcoholism) compared to alcoholics without antisocial behavior (type 1) or healthy controls (Hallikainen et al. 1999). Consistent with the human polymorphism, rhesus monkeys that possess the s allele engaged in higher rates of aggressive behaviors compared to l/l individuals (Jarrell et al. 2008; Lesch and Merschdorf 2000).
A genotype-environment interaction can be found in 5-HTT polymorphism. Rhesus monkeys with the s allele had lower 5-hydroxyindoleacetic acid (5HIAA) in CSF than l/l individuals when they were reared without their mother (peer-reared). This difference disappeared when both were reared by their mothers (Bennett et al. 2002). Peer-reared monkeys showed increased aggression-related behavior as well as altered CSF 5HIAA levels (Higley et al. 1991; Kraemer et al. 1989). In humans, higher suicide ideations or attempts were observed in individuals carrying the s allele than in l/l homozygotes when they encountered a number of stressful life events, but not in less stressful situations (Caspi et al. 2003). Therefore, it is possible that animals with the s allele are more vulnerable to the stressful challenges, and subsequently escalate their aggressive behaviors towards others and themselves. However, the effect of the s allele on aggression differed among sexes (Cadoret et al. 2003) and even cultures (Baca-Garcia et al. 2004).
Seemingly contrary results were observed in mice with a deletion of the 5-HTT gene (Slc6a4). Homozygote and heterozygote 5-HTT knockout mice on a C57BL/6J background showed fewer attack bites and longer latencies to start fighting relative to wild-type mice in the resident-intruder test (Holmes et al. 2002; Table 3). 5-HTT knockout mice have lower 5-HT uptake and higher extracellular 5-HT concentrations in the forebrain compared to wild-type (Mathews 2004). 5-HTT knockout mice underwent changes in more than 50 phenotypes including morphological, physiological, sensory, and behavioral functions (Murphy and Lesch 2008), and thus those pleiotropic changes of other phenotypes may contribute to the reduction of aggressive behaviors in these mice. Comparable findings on 5-HTT and aggression were reported in the rat. 5-HTT knockout rats on a Wistar/Crl background also showed reduced offensive behaviors and longer attack latencies compared to wild-type rats (Homberg et al. 2007). Therefore, genetic ablation of 5-HTT consistently reduced aggressive behaviors in rodents.
6. Monoamine oxidase A (MAOA)
6.1. Pharmacology of MAOA and aggression
Inhibition of MAOA reduces the oxidative metabolism of monoamines, thus presumably increasing the availability of 5-HT and other monoamines in the brain. Despite the early recognized importance of MAO inhibitors as antidepressants, there are only a few preclinical studies that systematically evaluated the effects of MAO inhibitors on aggression (Miczek 1987). For the most part, non-selective inhibitors of both MAOA and MAOB (e.g. phenelzine, isocarboxazid, tranylcypromine) show acute anti-aggressive effects in doses that also alter motor and other non-aggressive behaviors (DaVanzo et al. 1966; Sofia 1969; Valzelli et al. 1967; Welch and Welch 1968). Clinically, non-selective MAO inhibitors or selective MAOB inhibitors can be useful in the pharmacological management of personality disorders that include impulsive aggression and suicidal tendencies as important symptoms, but are accompanied by an unfavorable profile of side effects (Hollander 1999; Raj 2004).
6.2. Genetics of MAOA and aggression
The gene for MAOA was the first candidate identified as a determinant in the susceptibility for aggression in humans, and it has remained the focus of most genetic and epigenetic studies. Brunner and colleagues (Brunner et al. 1993b) identified a large Dutch kindred with a syndrome of borderline mental retardation and dysregulated impulsive aggression. All affected males showed aggressive outbursts, and some exhibited sexually aberrant behavior, attempted murder and arson. Linkage and sequence analyses showed that all affected males in this family possessed one missense mutation in the MAOA gene on the X chromosome, so that MAOA function was completely disturbed (Brunner et al. 1993a). The affected males had higher serotonin and lower metabolites of NE, DA, and 5-HT in the urine (Brunner et al. 1993a). MAOA also is of significance in the probability of fighting in animals. Male mice with disrupted MAOA gene on either C3H/He or 129Sv background showed escalated aggressive behaviors compared to wild-types, as is evident by skin wounds among the cage-mates and a short latency to initiate attacks in the resident-intruder test (Cases et al. 1995; Scott et al. 2008). MAOA-deficient mice also showed a large increase in 5-HT and NE, and a subtle DA elevation, in the brain and liver (Cases et al. 1995; Kim et al. 1997; Table 3). It is likely that the change of 5-HT function is the cause of the behavioral changes in the MAOA-deficient mice. Ketanserin and MDL100907, antagonists that preferentially bind to 5-HT2A receptors, blocked the escalated aggression in the MAOA mutant mice (Shih et al. 1999). Depletion of 5-HT by PCPA during the early developmental stage improved some behavioral and brain structural abnormality in the MAOA-deficient mice (Cases et al. 1995; Cases et al. 1996).
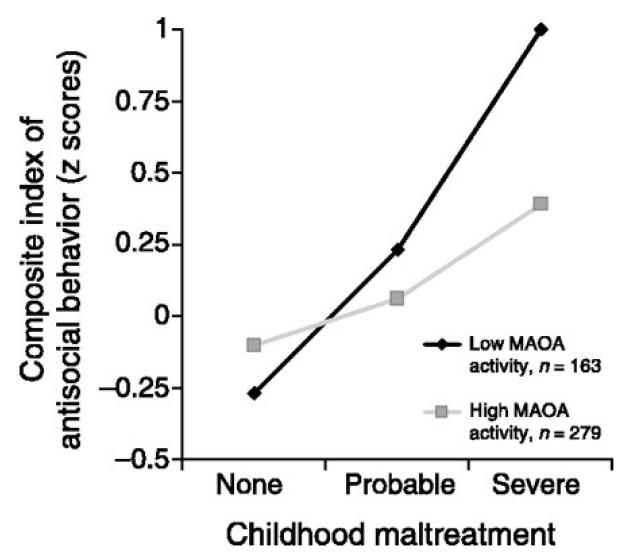
Variable-number tandem repeat (VNTR) polymorphism, which exists on the upstream region of the MAOA gene, regulates MAOA expression depending on the number of repeats: Alleles with 3.5 or 4 repeats have 2-10 times higher transcription than 3 or 5 repeat alleles in vitro (Denney et al. 1999; Sabol et al. 1998). A prominent interaction between MAOA genotype and environment on aggressive behavior has been reported (Caspi et al. 2002; Figure 5). Under stressful rearing conditions, such as abuse or neglect, or exposure to traumatic life events in the first 15 years of their lives, individuals with low MAOA expression (MAOA-L) polymorphisms showed higher propensity to have criminal arrests and a violent history, adolescent conduct disorder, and also higher aggressive disposition in self-report questionnaire compared to individuals with higher MAOA expression (MAOA-H) allele or MAOA-L individuals without abuse (Caspi et al. 2002; Foley et al. 2004; Frazzetto et al. 2007; Kim-Cohen et al. 2006; Weder et al. 2009; Widom and Brzustowicz 2006). If rearing environments were lumped together, the effect of MAOA genotype disappeared (Fresan et al. 2007) or sometimes MAOA-H individuals reported higher aggression using data from interviews and questionnaires (Manuck et al. 2000; Manuck et al. 2002). Therefore, individuals with MAOA-L allele are vulnerable to environmental factors and show a high propensity to engage in aggressive behaviors only when they are in a stressful environment. These findings are consistent in males, but not in females (Sjoberg et al. 2007). Similarly, rhesus monkeys have a repeat length variation polymorphism (rhMAOA-LPR) in the MAOA gene, and this polymorphism is also linked to aggression. Monkeys with a low-activity allele exhibited higher aggressive behavior and tend to attain higher dominance rank when they were reared by their mother. In contrast, when they were reared separately from their parents (peer-reared), monkeys with the low-activity allele engaged in less aggressive behavior (Newman et al. 2005). This inhibition of aggression has been attributed to increased fear and anxiety in peer-reared monkeys (Higley and Suomi 1986).
Figure 5.
Means on the composite index of antisocial behavior as a function of MAOA activity and a childhood history of maltreatment. MAOA activity is the gene expression level associated with allelic variants of the functional promoter polymorphism, grouped into low and high activity; childhood maltreatment is grouped into 3 categories of increasing severity. The antisocial behavior composite is standardized (z score) to a M = 0 and SD = 1; group differences are interpretable in SD unit differences (d). Reprinted with permission from Caspi et al. (2002).
Neuroimaging studies have indicated pronounced differences in volume and activity of limbic system and neocortical areas between individuals with MAOA-L and MAOA-H (see Buckholtz and Meyer-Lindenberg 2008 for a review). fMRI analysis in healthy human volunteers showed that MAOA-L males had smaller limbic and orbitofrontal volumes, and higher activity in amygdala and hippocampus during aversive recall (Meyer-Lindenberg et al. 2006), that may be related to violent behavior in MAOA-L individuals. Alia-Klein et al. (2008) reported that lower MAOA activity in cortical and subcortical brain areas is associated with high aggression measured by self-report questionnaire, independent from MAOA polymorphism. These data show that the MAOA activity is one of the determinants for the vulnerability to aggression, especially the interaction between MAOA polymorphism and salient social experiences can escalate the aggression and also change relevant brain structures.
7. Modulation of serotonergic activity by other systems
The 5-HT neurons in the raphé nuclei are modulated by other amines, acids, peptides and steroids (Adell et al. 2002). Recently, several efforts were undertaken to uncover the nature of the neural systems that modulate 5-HT neurons to promote escalated aggressive behaviors. Here we will focus briefly on inhibitory and excitatory neurotransmitters and some neuropeptides in terms of their interaction with 5-HT system. The more general role of those molecules on aggressive behavior was reviewed recently (Miczek et al. 2007b).
7.1 GABA
The inhibitory neurotransmitter γ-aminobutyric acid (GABA) plays a crucial role in the modulation of the dorsal raphé nuclei (DRN). Large number of GABA interneurons and distal GABAergic afferents can be found in the DRN (Belin et al. 1983; Gervasoni et al. 2000; Nanopoulos et al. 1982; Wang et al. 1992), and both GABAA and GABAB receptors are expressed in the DRN (Bowery et al. 1987). In vitro electrophysiology studies have shown that the activation of the GABAA and GABAB receptors on the 5-HT neurons both inhibit 5-HT cell firings (Colmers and Williams 1988; Gallager and Aghajanian 1976; Innis and Aghajanian 1987; Judge et al. 2004). On the other hand, in vivo microdialysis studies have shown that the GABAA and GABAB receptors in the DRN differentially modulate 5-HT release depending on the projection sites (Tao et al. 1996). We recently found that the pharmacological activation of GABAB receptors in the DRN escalated aggressive behaviors in mice (Takahashi et al. submitted). Interestingly, only under the influence of alcohol, local administration of GABAA receptor agonist muscimol also heightened aggressive behaviors (Takahashi et al. 2010). By contrast, intra-DRN muscimol inhibited aggressive behaviors in rats (Van Der Vegt et al. 2003) or was without effect on aggression in the absence of alcohol (Takahashi et al. 2010). Therefore, both subtypes of GABA receptors are involved in escalated forms of aggressive behavior via different mechanisms. In vivo microdialysis showed that GABAB activation in the DRN increased extracellular 5-HT level in the medial prefrontal cortex (Takahashi et al., submitted; Figure 6). This result suggests that the phasic activation of 5-HT system may be able to promote certain types of escalated aggressive behaviors in mice.
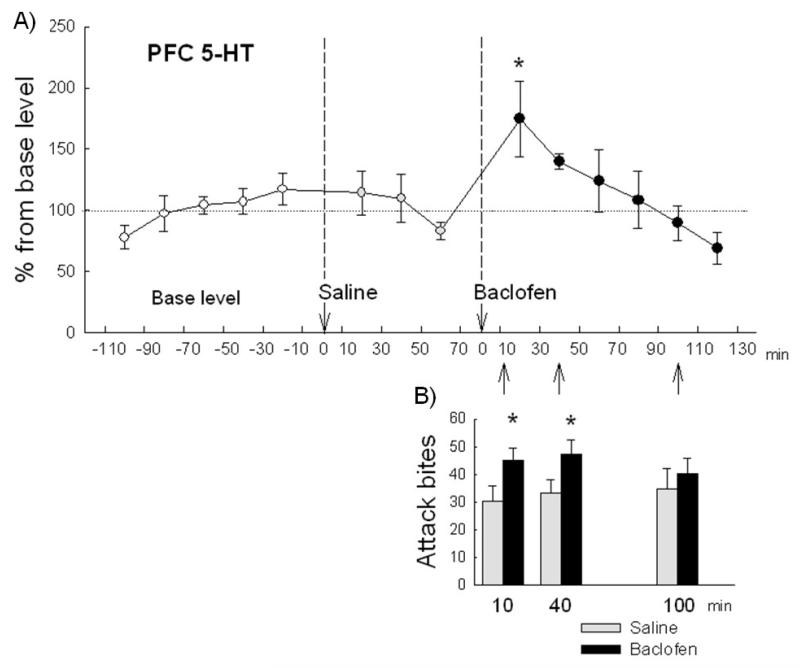
Figure 6. Extracellular 5-HT concentration in the medial prefrontal cortex (mPFC) of mice after GABAB receptor activation in the dorsal raphe nucleus (DRN).
(A) Baclofen microinjected into the DRN increased the 5-HT level in the mPFC whereas saline injection did not change the 5-HT level. Twenty minutes samples were collected 5 samples for baseline, 3 samples after saline injection, and 6 samples after baclofen (0.06 nmol) injection. Data are expressed as percentage of baseline (n=7). * p<.05 compared to the baseline. (B) The effect of 0.06 nmol baclofen on attack bites after the different interval (10, 40 and 100 min, corresponding to the time period of fraction 9, 11, and 14 in the microdialysis, respectively). Escalated attack bites were observed both 10 and 40 minutes after the intra-DRN baclofen injection. * p<.05 compared to corresponding vehicle control.
7.2 Glutamate
The DRN receives prominent glutamate input by the descending projections from the lateral habenula, periaqueductal gray, lateral hypothalamus, interpeduncular nucleus and medial prefrontal cortex (Aghajanian and Wang 1977; Behzadi et al. 1990; Kalen et al. 1986; Maciewicz et al. 1981). Both the N-metyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolaproprionate/kainate (AMPA/kainate) are localized on serotonergic neurons and increase the 5-HT release in the DRN and its projection areas (Celada et al. 2001; Pallotta et al. 1998; Tao et al. 1996; Tao and Auerbach 2000; Vandermaelen et al. 1986). Systemic administrations of classic antagonists of NMDA receptors, including phencyclidine (PCP) and dizocilpine (MK-801), can increase aggressive behavior (Burkhalter and Balster 1979; Krsiak 1974; McAllister 1990; Musty and Consroe 1982; Rewerski et al. 1971; Wilmot et al. 1987), while other studies find that these compounds are suppressive and sedative due to their strong side-effects (Belozertseva and Bespalov 1999; Lang et al. 1995; Miczek and Haney 1994; Tyler and Miczek 1982). Anatomically discrete analysis is required to identify the sites of action for NMDA receptors that produce enhanced aggressive behavior. The 5-HT system is one of the candidates, especially the descending glutamatergic projection from the medial prefrontal cortex (mPFC) to the DRN will be interesting to investigate. The prefrontal cortex (PFC) has been implicated in the emotion regulation including aggression (Davidson et al. 2000; Miczek et al. 2007a). This PFC-DRN glutamatergic projections are involved in the controllability or emotion regulation (Amat et al. 2005) and further study will be required to address the role of this PFC-DRN glutamatergic neurons on aggressive behaviors.
7.3 CRF
The DRN is innervated by Corticotropin-Releasing Factor (CRF) immunoreactive fibers, and presents both subtypes of CRF receptors, CRF1 and CRF2 (Chalmers et al. 1995; Potter et al. 1994; Swanson et al. 1983). Evidence suggests that CRF, CRF receptors and other peptides of the CRF family (Urocortins), play key modulatory roles on DRN serotonin neurons (see Valentino and Commons 2005). Electrophysiological and microdialysis studies consistently report that i.c.v. or intra-DRN microinjections of CRF, or drugs targeting CRF receptors, exert potent modulatory control over 5-HT neural firing (Kirby et al. 2000; Lowry et al. 2000), and 5-HT output to limbic, striatal and prefrontal cortical regions (Amat et al. 2004; Amat et al. 2005; Forster et al. 2008; Lukkes et al. 2008; Meloni et al. 2008; Price et al. 1998; Price and Lucki 2001).
A role for CRF, CRF1 and CRF2 receptors in aggressive behavior has been indicated by studies on maternal- and inter-male aggression in mice and hamsters (D’Anna et al. 2005; Farrokhi et al. 2004; Gammie et al. 2004; Gammie et al. 2005; Gammie et al. 2007; Gammie and Stevenson 2006). In rats, there is evidence that low doses of CRF themselves may facilitate or induce pro-aggressive effects after i.c.v. or intra-amygdala infusions (Elkabir et al. 1990; Tazi et al. 1987). Under conditions of escalated aggression promoted by moderate doses of alcohol in male mice, CRF1 receptors are a promising target for pharmacological intervention. Systemically, antagonists of CRF1 receptors reduce alcohol-heightened aggression, but also reduce baseline levels of aggressive behavior (Quadros et al. 2009a). When locally administered into the DRN, CRF1 antagonists (e.g., CP-154526 or MTIP) prevent the escalated levels of aggression observed after consumption of alcohol, with no side effects on other behaviors. Remarkably, such anti-aggressive effects of CRF1 antagonists can be abolished with the infusion of 8-OH-DPAT into the DRN, which transiently slows 5-HT impulse flow. On the other hand, microinfusion of a CRF2 antagonist (Astressin-2B) into the DRN escalates aggressive behavior (Quadros et al. 2009a).
Thus, the modulation of aggressive behaviors by CRF systems depends on the species (mice, rats) and type of aggression (species-typical, maternal or escalated aggression). Initial evidence suggests the 5-HT cells in the DRN as one of the critical sites for such modulation in the escalated aggression promoted by alcohol, with presumably opposing roles for CRF1 and CRF2 receptors.
7.4. Vasopressin
Arginine vasopressin (AVP) is a neuropeptide that modulates a variety of social behaviors including pair-bonding, social recognition, maternal behavior, and aggression (Albers and Bamshad 1998; Coccaro et al. 1998; Ferris 1992; Goodson 2008; Koolhaas et al. 1990; Neumann et al. 2010; Winslow et al 1993). Selective antagonist of vasopressin V1a receptors (SRX251, [d(CH2)5Tyr(Me)AVP]) inhibited inter-male aggression (Ferris et al. 2006; Ferris and Potegal 1988), indicating the involvement of V1a receptors in aggressive behaviors. The interaction between AVP and 5-HT has been shown to be critical for certain types of aggressive behaviors. In humans, a positive correlation has been observed between AVP concentrations in the CSF and the life history of aggression, a composite measure of trait aggression. Also, there was a positive correlation between AVP concentrations and prolactin responses to a challenge with d-fenfluramine (Coccaro et al 1998). This result indicates that individuals that have higher aggression ratings tend to have a high AVP concentration in the CSF and a hyporesponsive 5-HT system. Neuronal interactions between AVP containing neurons and 5-HT neurons are found in the anterior hypothalamus (Ferris et al. 1997; Ferris et al. 1999), and this AVP-5-HT link is implicated in aggression. Microinjection of AVP into the anterior hypothalamus increased aggressive behavior in hamsters, and systemic fluoxetine (5-HTT inhibitor) treatment blocked the pro-aggressive effect of AVP (Delville et al. 1996; Ferris et al. 1997). Therefore, 5-HT may have an inhibitory function on the AVP induced heightened aggression. In contrast, mice with disrupted Ca2+ channel expression (Cav2−/−) showed escalated level of aggressive behavior and also higher AVP concentration in the CSF. However, these animals showed overactivation of the dorsal raphe 5-HT neurons and increased 5-HT concentration in the hypothalamus (Kim et al. 2009). Further investigation is anticipated to uncover how AVP and 5-HT interact and whether there are specific types of aggression that require the activity of either 5-HT or AVP systems independently.
8. Other molecules that directly or indirectly affect 5-HT pathways and aggression
Here we will briefly discuss selected molecules that directly or indirectly affect serotonergic pathways and modulate aggressive behaviors based mainly on findings from gene knockout mouse studies, as summarized in Table 3. For more reviews on genes and aggressive behavior, see Maxson and Canastar (2007), Miczek et al. (2001) and Nelson and Chiavegatto (2001).
8.1. Pet-1 (also known as Fev)
Pet-1, one of the transcription factors, is specifically expressed in the serotonergic raphé neurons, and has a critical role in 5-HT neural development. Deletion of Pet-1 expression reduced 5-HT level in the forebrain, and also depleted expression of TPH, 5-HTT, and the vesicular monoamine transporter 2 (Vmat2). In the resident-intruder test, Pet-1 knockout mice (Pet-1−/−) engaged in higher frequency and intensity of attacks toward a conspecific male (Hendricks et al. 2003). These increases in aggressive behavior in Pet-1−/− mice are embedded in broad behavioral disruptions that extended to maternal and anxiety-like behaviors (Hendricks et al. 2003; Lerch-Haner et al. 2008), and it is possible that those other behavioral changes promote indirectly aggressive behaviors.
8.2. Brain-derived neurotrophic factor (BDNF)
BDNF has several important roles in the neuron including neuronal survival, development, differentiation and plasticity. Mice with decreased BDNF expression including knockout (BDNF+/−) and conditional knockout (BDNF2L/1LNes-cre and BDNF2L/2LCk-Cre) all showed increased inter-male aggression (Chan et al. 2006; Lyons et al. 1999). Higher hippocampal extracellular levels of 5-HT were observed in BDNF+/− mice compared to wild-type (Deltheil et al. 2008), but fluoxetine reduced their heightened aggressive behavior (Lyons et al. 1999). All mutants changed 5-HT2A receptor expression, however BDNF+/− showed increased 5-HT2A expression in the lateral frontal cortex and hypothalamus (Lyons et al. 1999) whereas BDNF2L/1LNes-Cre and BDNF2L/2LCk-Cre exhibit reduced 5-HT2A receptor expression in the prefrontal cortex (Chan et al. 2006; Rios et al. 2006). A SNP in the BDNF gene, Val66Met, has attracted strong interest because of its association with mood disorders and hippocampal function in humans (Egan et al. 2003; Neves-Pereira et al. 2002). Knock-in mice with this human Met allele also showed an increased aggressive behavior and changed the response to SSRI treatment (Chen et al. 2006). In contrast to the consistent results on aggressive behavior among BDNF mutant mice, studies on polymorphisms of the BDNF gene in aggressive behavior in humans remain to be resolved. No association was observed between Val66Met polymorphism and proneness to violence in a Chinese male sample (Tsai et al. 2005). Other SNPs in the BDNF gene may be associated with high impulsivity in children with ADHD (Oades et al. 2008).
8.3. Neuronal nitric oxide (nNOS)
Nitric oxide, a free radical gas which diffuses across membranes, is involved in several cellular functions (for review see Calabrese et al. 2007). Mice lacking neuronal nitric oxide synthase (nNOS−/−) show various deficits in their physiological development and also behavior (Huang et al. 1993). nNOS−/− males, but not females, showed higher duration of aggressive behavior and also displayed much fewer submissive postures compared to wild-types (Nelson et al. 1995). Serotonergic dysfunction was observed in the nNOS−/− mice, specifically reduced 5-HT turnover in the brain and deficient 5-HT1A and 5-HT1B receptor function (Chiavegatto et al. 2001). Escalated aggression in the nNOS−/− was rescued by 5-HTP treatment which increased 5-HT level and turnover. These findings point to an important role of nitric oxide for the normal 5-HT function, and thus increased aggression nNOS−/− may be induced by changing 5-HT activity.
8.4. Neuropeptide Y (NPY)
NPY controls primarily food intake, energy balance, and metabolic regulation (Herzog 2003). This molecule which is critical for energy homeostasis is also implicated in aggressive behavior (Emeson and Morabito, 2005). Male mice with deleted expression of the Y1-receptor (Y1−/−) showed obesity and reduced energy homeostasis (Kushi et al. 1998), and also exhibited increased aggressive behaviors in the resident-intruder test (Karl et al. 2004). However, this escalated aggression in Y1−/− was observed only in the home cage, not in the novel environment. This result suggests a specific increase in territorial aggression which may be related to altered 5-HT function of Y1−/− (Karl et al. 2004). TPH mRNA expression in the raphé nuclei was reduced in the Y1−/− mice. In addition, 5-HT1A agonist treatment reduced escalated aggression in Y1−/− mice.
8.5. α-Calcium-Calmodulin Kinase II (α-CaMKII)
α-CaMKII is a neural specific enzyme and has been shown to be involved in long-term potentiation (LTP; Silva et al. 1992). Heterozygotes of α-CaMKII knockout mice showed escalated defensive aggression but not offensive aggression (Chen et al. 1994). In the resident-intruder test, resident α-CaMKII heterozygotes showed aggressive behavior similar to wild-type mice. In contrast, when the mutant was tested as an intruder, they exhibited high defensive reactions toward the resident. Reduced 5-HT release was observed in the dorsal raphé of α-CaMKII mutants in vitro, and thus the changed 5-HT function may be associated with defensive aggression in this mouse.
Gene targeting techniques in mice have identified a number of genes involved in aggressive behaviors (Table 3) and these animals offer insights into the simple serotonin-aggression link. The direction of the change in the 5-HT system is not always the same (Table 3); some knockout mice that showed escalated aggressive behaviors also showed a reduction in 5-HT tone (e.g. Pet1, nNOS, NPY, α-CaMKII) but others showed a clear increase of 5-HT release in the brain (e.g. MAOA, BDNF, Cav2.2). Further examination of 5-HT changes in dissected brain regions and also of changes in specific 5-HT receptor subtypes in these knockout mice will clarify more details of the serotonin-aggression link.
9. Concluding Remarks
In summary, understanding the complex role of 5-HT in aggression requires consideration of multiple factors, including (1) the type of aggressive behavior, its topography and function, (2) the genetic background of the individual and the typical phenotype for this background, (3) the trait characteristics of the human subject or the mouse (i.e., Mus musculus are a pugnacious species and many inbred strains are quite placid), and (4) the situational conditions under which aggressive behaviors has been engendered. From a clinical perspective, the impulsive, hostile, explosive type of aggressive behavior is part of a more broadly defined trait that has been linked to profound changes in the expression of MAO-A, 5-HTT, and 5-HT receptors. A growing number of studies that suggest important gene-environment interactions involving genes that regulate 5-HT transmission (e.g., monoamine oxidase and serotonin transporters) and stressful life events are of particular interest. When associated, these conditions are critical in determining an increased vulnerability to initiate violent acts and aggressive outbursts (e.g., Bennett et al. 2002; Caspi et al. 2002; Caspi et al. 2003). By associating the specific characteristics of the individual, the environment and the type of social interaction, the serotonin-aggression link can be further explored, providing new pharmacological and molecular targets for interventions.
From a therapeutic perspective, it seems clear that manipulations of 5-HT transmission are effective for the management of aggressive behavior, despite the common observation of side effects. Traditionally, pharmacological manipulations that increase 5-HT function are effective in reducing aggression in clinical and preclinical settings. However, whether such increases in 5-HT transmission are the main relevant mechanism for the anti-aggressive effects remains in dispute. For example, agonists of the 5-HT1 family of receptors and citalopram (SSRI) promote opposite changes in extracellular concentrations of 5-HT in terminal regions, despite both having anti-aggressive effects. Such observations suggest that drugs acting at different 5-HT molecular targets may recruit different neuropharmacological mechanisms in order to promote their anti-aggressive effects, likely involving actions on specific receptor subpopulations and downstream signaling pathways. Of particular clinical interest, the development of neuroplastic changes seems to accompany the emergence of anti-aggressive effects after chronic treatment with SSRIs. More recently, the identification of pharmacological targets that directly or indirectly modulate 5-HT function, such as receptors for glutamate, GABA and neuropeptides, show promising results for more selective anti- and pro-aggressive effects.
References
- Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Artigas F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J Neurochem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- Albers HE, Bamshad M. Role of vasopressin and oxytocin in the control of social behavior in Syrian hamsters (Mesocricetus auratus) Prog Brain Res. 1998;119:395–408. doi: 10.1016/s0079-6123(08)61583-6. [DOI] [PubMed] [Google Scholar]
- Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, Telang F, Shumay E, Biegon A, Craig IW, Henn F, Wang GJ, Volkow ND, Fowler JS. Brain monoamine oxidase a activity predicts trait aggression. J Neurosci. 2008;28:5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva E, Cirulli F, Bianchi M, Bondiolotti GP, Chiarotti F, De Acetis L, Panerai AE. Behavioural characterization of interleukin-6 overexpressing or deficient mice during agonistic encounters. Eur J Neurosci. 1998;10:3664–3672. doi: 10.1046/j.1460-9568.1998.00377.x. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, Cummings JL. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Arch Neurol. 2004;61:1249–1253. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Vaquero C, Diaz-Sastre C, Garcia-Resa E, Saiz-Ruiz J, Fernandez-Piqueras J, de Leon J. Lack of association between the serotonin transporter promoter gene polymorphism and impulsivity or aggressive behavior among suicide attempters and healthy volunteers. Psychiatry Res. 2004;126:99–106. doi: 10.1016/j.psychres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphé nucleus of mice. Psychopharmacology. 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- Barkan T, Peled A, Modai I, Weizman A, Rehavi M. Characterization of the serotonin transporter in lymphocytes and platelets of schizophrenia patients treated with atypical or typical antipsychotics compared to healthy individuals. Eur Neuropsychopharmacol. 2006;16:429–436. doi: 10.1016/j.euroneuro.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CA, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Behzadi G, Kalen P, Parvopassu F, Wiklund L. Afferents to the median raphe nucleus of the rat: retrograde cholera toxin and wheat germ conjugated horseradish peroxidase tracing, and selective d-[3H]aspartate labelling of possible excitatory amino acid inputs. Neuroscience. 1990;37:77–100. doi: 10.1016/0306-4522(90)90194-9. [DOI] [PubMed] [Google Scholar]
- Belin MF, Nanopoulos D, Didier M, Aguera M, Steinbusch H, Verhofstad A, Maitre M, Pujol JF. Immunohistochemical evidence for the presence of γ-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983;275:329–339. doi: 10.1016/0006-8993(83)90994-0. [DOI] [PubMed] [Google Scholar]
- Bell R, Hobson H. 5-HT1A receptor influences on rodent social and agonistic behavior: A review and empirical study. Neurosci Biobehav Rev. 1994;18:325–338. doi: 10.1016/0149-7634(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Bespalov AY. Effects of NMDA receptor channel blockade on aggression in isolated male mice. Aggress Behav. 1999;25:381–396. [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19:10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. Its causes, consequences and control. McGraw-Hill; New York: 1993. Aggression. [Google Scholar]
- Bernstein IS, Rose RM, Gordon TP. Behavioral and environmental events influencing primate testosterone levels. J Hum Evol. 1974;3:517–525. [Google Scholar]
- Bjork JM, Moeller FG, Dougherty DM, Swann AC, Machado MA, Hanis CL. Serotonin 2a receptor T102C polymorphism and impaired impulse control. Am J Med Genet. 2002;114:336–339. doi: 10.1002/ajmg.10206. [DOI] [PubMed] [Google Scholar]
- Blader JC. Pharmacotherapy and postdischarge outcomes of child inpatients admitted for aggressive behavior. J Clin Psychopharmacol. 2006;26:419–425. doi: 10.1097/01.jcp.0000227356.31203.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Rodgers RJ, Hendrie CA, Hori K. ‘Taming’ of wild rats (Rattus rattus) by 5HT1A agonists buspirone and gepirone. Pharmacol Biochem Behav. 1988;31:269–278. doi: 10.1016/0091-3057(88)90345-0. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard CD. Aggressive behavior in the rat. Behav Biol. 1977;21:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Blanchard DC, Hall J. Ethanol effects on aggression of rats selected for different levels of aggressiveness. Pharmacol Biochem Behav. 1987;27:641–644. doi: 10.1016/0091-3057(87)90187-0. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. Biol Psychiatry. 1998;44:313–323. doi: 10.1016/s0006-3223(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Boles SM, Miotto K. Substance abuse and violence—A review of the literature. Aggress Violent Behav. 2003;8:155–174. [Google Scholar]
- Bond AJ. Antidepressant treatments and human aggression. Eur J Pharmacol. 2005;526:218–225. doi: 10.1016/j.ejphar.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Johnson RG, Fiorella D, Rabin RA, Winter JC. Serotonergic control of androgen-induced dominance. Pharmacol Biochem Behavior. 1994;49:313–322. doi: 10.1016/0091-3057(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Bonvento G, Scatton B, Claustre Y, Rouquier L. Effect of local injection of 8-OH-DPAT into the dorsal or median raphe nuclei on extracellular levels of serotonin in serotonergic projection areas in the rat brain. Neurosci Lett. 1992;137:101–104. doi: 10.1016/0304-3940(92)90308-t. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der GJ, Maes RA, Hen R, Olivier B. Absence of 5-HT1B receptors is associated with impaired impulse control in male 5-HT1B knockout mice. Biol Psychiatry. 2001;49:557–568. doi: 10.1016/s0006-3223(00)01018-0. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Brahm NC, Fast GA, Brown RC. Buspirone for autistic disorder in a woman with an intellectual disability. Ann Pharmacother. 2008;42:131–137. doi: 10.1345/aph.1K427. [DOI] [PubMed] [Google Scholar]
- Brain P, Benton D. The interpretation of physiological correlates of differential housing in laboratory rats. Life Sci. 1979;24:99–115. doi: 10.1016/0024-3205(79)90119-x. [DOI] [PubMed] [Google Scholar]
- Brain PF. Hormones, Drugs and Aggression. Eden Press; Montreal, Canada: 1979. [Google Scholar]
- Brown GL, Goodwin FK. Cerebrospinal fluid correlates of suicide attempts and aggression. In: Mann JJ, editor. Psychobiology of Suicidal Behavior. Vol. 487. New York Academy of Sciences; New York: 1986. pp. 175–220. Annals of the New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- Brunner D, Hen R. Insights into the neurology of impulsive behavior from serotonin receptor knockout mice. Ann NY Acad Sci. 1997;836:81–105. doi: 10.1111/j.1749-6632.1997.tb52356.x. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, Vanoost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase-A. Science. 1993a;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen MR, Vanzandvoort P, Abeling NGGM, Vangennip AH, Wolters EC, Kuiper MA, Ropers HH, Vanoost BA. X-Linked borderline mental-retardation with prominent behavioral disturbance—phenotype, genetic localization, and evidence for disturbed monoamine metabolism. Am J Hum Genet. 1993b;52:1032–1039. [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Ibrahim ZY, Singer B, Orr B, Donenwirth K, Brar PS. Aggression and schizophrenia: Efficacy of risperidone. J Am Acad Psychiatry Law. 1997;25:173–181. [PubMed] [Google Scholar]
- Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman TM. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry. 2001;62:239–248. doi: 10.4088/jcp.v62n0405. [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, Balster RL. The effects of phencyclidine on isolation-induced aggression in mice. Psychol Rep. 1979;45:571–576. doi: 10.2466/pr0.1979.45.2.571. [DOI] [PubMed] [Google Scholar]
- Burt SA, Mikolaiewski AJ. Preliminary evidence that specific candidate genes are associated with adolescent-onset antisocial behavior. Aggress Behav. 2008;34:437–445. doi: 10.1002/ab.20251. [DOI] [PubMed] [Google Scholar]
- Buss AH. The Psychology of Aggression. Wiley and Sons, New York Buss AH, Durkee A (1957) An inventory for assessing different kinds of hostility. J Consult Psychol. 1961;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Langbehn D, Caspers K, Troughton EP, Yucuis R, Sandhu HK, Philibert R. Associations of the serotonin transporter promoter polymorphism with aggressivity, attention deficit, and conduct disorder in an adoptee population. Compr Psychiatry. 2003;44:88–101. doi: 10.1053/comp.2003.50018. [DOI] [PubMed] [Google Scholar]
- Cairns RB, Nakelski JS. On fighting in mice: Ontogenetic and experiential determinants. J Comp Physiol Psychol. 1971;74:354–364. doi: 10.1037/h0030584. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Caldwell EE, Miczek KA. Long-term citalopram maintenance in mice: selective reduction of alcohol-heightened aggression. Psychopharmacology. 2008;196:407–416. doi: 10.1007/s00213-007-0972-z. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Coppersmith GA, Melloni RH., Jr. The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology. 2009;205:349–368. doi: 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, DeMaeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: Role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Acconcia S, Fracasso C, Colovic M, Caccia S, Invernizzi RW. Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. Br J Pharmacol. 2004;142:469–478. doi: 10.1038/sj.bjp.0705800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centenaro LA, Vieira K, Zimmermann N, Miczek KA, Lucion AB, de Almeida RM. Social instigation and aggressive behavior in mice: role of 5-HT1A and 5-HT1B receptors in the prefrontal cortex. Psychopharmacology. 2008;201:237–248. doi: 10.1007/s00213-008-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JP, Unger TJ, Byrnesa J, Rios M. Examination of behavioral deficits triggered by targeting BDNF in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Chen C, Rainnie DG, Greene RW, Tonegawa S. Abnormal fear response and aggressive behavior in mutant mice deficient for α-calcium-calmodulin kinase II. Science. 1994;265:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- Chen GL, Novak MA, Meyer JS, Kelly BJ, Vallender EJ, Miller GM. The effect of rearing experience and TPH2 genotype on HPA axis function and aggression in Rhesus monkeys: a retrospective analysis. Horm Behav. 2010;57:184–91. doi: 10.1016/j.yhbeh.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherek DR, Heistad GT. Fixed-interval induced aggression. Psychon Sci. 1971;25:7–8. [Google Scholar]
- Cherek DR, Lane SD. Laboratory and psychometric measurements of impulsivity among violent and nonviolent female parolees. Biol Psychiatry. 1999;46:273–280. doi: 10.1016/s0006-3223(98)00309-6. [DOI] [PubMed] [Google Scholar]
- Chermack ST, Giancola PR. The relation between alcohol and aggression: An integrated biopsychosocial conceptualization. Clin Psychol Rev. 1997;17:621–649. doi: 10.1016/s0272-7358(97)00038-x. [DOI] [PubMed] [Google Scholar]
- Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci U S A. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotfelter ED, O’Hare EP, McNitt MM, Carpenter RE, Summers CH. Serotonin decreases aggression via 5-HT1A receptors in the fighting fish Betta splendens. Pharmacol Biochem Behav. 2007;87:222–231. doi: 10.1016/j.pbb.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Sheline YI, Berman ME, Csernansky JG. Impulsive aggression in personality disorder correlates with platelet 5-HT2A receptor binding. Neuropsychopharmacology. 1997;16:211–216. doi: 10.1016/S0893-133X(96)00194-7. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Kavoussi RJ. Aggression, suicidality, and intermittent explosive disorder: serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharmacology. 2010;35:435–444. doi: 10.1038/npp.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Williams JT. Pertussis toxin pretreatment discriminates between pre- and postsynaptic actions of baclofen in rat dorsal raphe nucleus in vitro. Neurosci Lett. 1988;93:300–306. doi: 10.1016/0304-3940(88)90099-7. [DOI] [PubMed] [Google Scholar]
- Cologer-Clifford A, Simon NG, Lu SF, Smoluk SA. Serotonin agonist-induced decreases in intermale aggression are dependent on brain region and receptor subtype. Pharmacol Biochem Behav. 1997;58:425–430. doi: 10.1016/s0091-3057(97)00295-5. [DOI] [PubMed] [Google Scholar]
- Connor DF, Steingard RJ. A clinical approach to the pharmacotherapy of aggression in children and adolescents. In: Ferris CF, Grisso T, editors. Understanding Aggressive Behavior in Children. Ann NY Acad Sci. Vol. 794. 1996. pp. 290–307. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Schleidt WM, Contrera JF. Does social environment decrease propensity to fight in male mice? Behav Biol. 1975;15:73–83. doi: 10.1016/s0091-6773(75)92105-7. [DOI] [PubMed] [Google Scholar]
- Cutler MG, Rodgers RJ, Jackson JE. Behavioural effects in mice of subchronic buspirone, ondansetron, and tianeptine. I. Social interactions. Pharmacol Biochem Behav. 1997;56:287–293. doi: 10.1016/s0091-3057(96)00241-9. [DOI] [PubMed] [Google Scholar]
- Czobor P, Volavka J, Meibach RC. Effect of risperidone on hostility in schizophrenia. J Clin Psychopharmacol. 1995;15:243–249. doi: 10.1097/00004714-199508000-00002. [DOI] [PubMed] [Google Scholar]
- D’Adamo P, Welzl H, Papadimitriou S, Raffaele dB, Tiveron C, Tatangelo L, Pozzi L, Chapman PF, Knevett SG, Ramsay MF, Valtorta F, Leoni C, Menegon A, Wolfer DP, Lipp HP, Toniolo D. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum Mol Genet. 2002;11:2567–2580. doi: 10.1093/hmg/11.21.2567. [DOI] [PubMed] [Google Scholar]
- D’Anna KL, Stevenson SA, Gammie SC. Urocortin 1 and 3 impair maternal defense behavior in mice. Behav Neurosci. 2005;119:1061–1071. doi: 10.1037/0735-7044.119.4.1061. [DOI] [PubMed] [Google Scholar]
- DaVanzo JP, Daugherty M, Ruckart R, Kang L. Pharmacological and biochemical studies in isolation-induced fighting mice. Psychopharmacologia. 1966;9:210–219. doi: 10.1007/BF02198481. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA. 5-HT1B receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology. 2006;185:441–450. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ. Maternal aggression in Wistar rats: effect of 5-HT2A/2C receptor agonist and antagonist microinjected into the dorsal periaqueductal gray matter and medial septum. Braz J Med Biol Res. 2005;38:597–602. doi: 10.1590/s0100-879x2005000400014. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Lucion A. Effects of intracerebroventricular administration of 5-HT receptor agonists on the maternal aggression of rats. Eur J Neurosci. 1994;264:445–448. doi: 10.1016/0014-2999(94)00548-6. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Lucion AB. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology. 1997;134:392–400. doi: 10.1007/s002130050476. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Miczek KA. Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) in mice: inhibition by anpirtoline - a 5-HT1B receptor agonist. Neuropsychopharmacology. 2002;27:171–181. doi: 10.1016/S0893-133X(02)00291-9. [DOI] [PubMed] [Google Scholar]
- de Almeida RMM, Nikulina EM, Faccidomo S, Fish EW, Miczek KA. Zolmitriptan--a 5-HT1B/D agonist, alcohol, and aggression in mice. Psychopharmacology. 2001;157:131–141. doi: 10.1007/s002130100778. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: A pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Lesourd M, Mocaer E, Koolhaas JM. Selective antiaggressive effects of alnespirone in resident-intruder test are mediated via 5-hydroxytryptamine1A receptors: A comparative pharmacological study with 8-hydroxy-2-dipropylaminotetralin, ipsapirone, buspirone, eltoprazine, and WAY-100635. J Pharmacol Exp Ther. 1999;288:1125–1133. [PubMed] [Google Scholar]
- de Boer SF, Lesourd M, Mocaer E, Koolhaas JM. Somatodendritic 5-HT1A autoreceptors mediate the anti-aggressive actions of 5-HT1A receptor agonists in rats: An ethopharmacological study with S-15535, alnespirone, and WAY-100635. Neuropsychopharmacology. 2000;23:20–33. doi: 10.1016/S0893-133X(00)00092-0. [DOI] [PubMed] [Google Scholar]
- De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PL, Eriksson S, Lawlor BA. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999;53:946–955. doi: 10.1212/wnl.53.5.946. [DOI] [PubMed] [Google Scholar]
- De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJH, Laird JMA, Belmonte C, Cervero F, Hunt SP. Altered nocieption, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- DeBold JF, Miczek KA. Sexual dimorphism in the hormonal control of aggressive behavior of rats. Pharmacol Biochem Behav. 1981;14:89–93. doi: 10.1016/s0091-3057(81)80015-9. [DOI] [PubMed] [Google Scholar]
- Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Guilloux JP, Nicolas L, Delomenie C, Reperant C, Le Maitre E, Leroux-Nicollet I, Benmansour S, Coudore F, David DJ, Gardier AM. Consequences of changes in BDNF levels on serotonin neurotransmission, 5-HT transporter expression and function: studies in adult mice hippocampus. Pharmacol Biochem Behav. 2008;90:174–183. doi: 10.1016/j.pbb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: Interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav. 1996;59:813–816. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Demas GE, Kriegsfeld LJ, Blackshaw S, Huang P, Gammie SC, Nelson RJ, Snyder SH. Elimination of aggressive behavior in male mice lacking endothelial nitric oxide synthase. J Neurosci. 1999;19:1–5. doi: 10.1523/JNEUROSCI.19-19-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male shin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet. 1999;105:542–551. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Young WS, Nelson RJ. Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. J Neuroendocrinol. 1997;9:363–368. doi: 10.1046/j.1365-2826.1997.t01-1-00589.x. [DOI] [PubMed] [Google Scholar]
- Dompert WU, Glaser T, Traber J. 3H-TVX Q 7821: Identification of 5-HT1 binding sites as target for a novel putative anxiolytic. Naunyn Schmiedebergs Arch Pharmacol. 1985;328:467–470. doi: 10.1007/BF00692918. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Stribley JA, Fry DL, Hinrichs SH, Lockridge O. Rescue of the acetylcholinesterase knockout mouse by feeding a liquid diet; phenotype of the adult acetylcholinesterase deficient mouse. Brain Res Dev Brain Res. 2002;137:43–54. doi: 10.1016/s0165-3806(02)00367-x. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eibl-Eibesfeldt I. Beiträge zur Biologie der Haus- und der Ährenmaus nebst einigen Beobachtungen an anderen Nagern. Z Tierpsychol. 1950;7:558–587. [Google Scholar]
- Elkabir DR, Wyatt ME, Vellucci SV, Herbert J. The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept. 1990;28:199–214. doi: 10.1016/0167-0115(90)90018-r. [DOI] [PubMed] [Google Scholar]
- Emeson RB, Morabito MV. Food fight: the NPY-serotonin link between aggression and feeding behavior. Sci STKE. 2005;2005:e12. doi: 10.1126/stke.2772005pe12. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Bannai M, Miczek KA. Escalated aggression after alcohol drinking in male mice: dorsal raphe and prefrontal cortex serotonin and 5-HT1B Receptors. Neuropsychopharmacology. 2008;33:2888–2899. doi: 10.1038/npp.2008.7. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Fontenot MB, Phillips-Conroy JE, Jolly CJ, Kaplan JR, Mann JJ. CSF monoamines, age and impulsivity in wild grivet monkeys (Cercopithecus aethiops aethiops) Brain Behav Evol. 1999;53:305–312. doi: 10.1159/000006601. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology. 2001;24:370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- Farrokhi C, Blanchard DC, Griebel G, Yang M, Gonzales C, Markham C, Blanchard RJ. Effects of the CRF1 antagonist SSR125543A on aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2004;77:465–469. doi: 10.1016/j.pbb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Fava M. Psychopharmacologic treatment of pathologic aggression. Psychiatr Clin North Am. 1997;20:427–451. doi: 10.1016/s0193-953x(05)70321-x. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Van Erp AMM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Ferris C. Role of vasopressin in aggressive and dominant/subordinate behaviors. Ann N Y Acad Sci. 1992;652:212–226. doi: 10.1111/j.1749-6632.1992.tb34357.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Lu SF, Messenger T, Guillon CD, Heindel N, Miller M, Koppel G, Robert BF, Simon NG. Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharmacol Biochem Behav. 2006;83:169–174. doi: 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Delville Y. Serotonin regulation of aggressive behavior in male golden hamsters (Mesocricetus auratus) Behav Neurosci. 1999;113:804–815. doi: 10.1037//0735-7044.113.4.804. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008;9:111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer HS, Zernig G, Schuligoi R, Miczek KA, Hauser KF, Gerard C, Saria A. Alterations within the endogenous opioid system in mice with targeted deletion of the neutral endopeptidase (“enkephalinase”) gene. Regul Pept. 2000;96:53–58. doi: 10.1016/s0167-0115(00)00200-7. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A. Early trauma and increased risk for physical aggression during adulthood: the moderating role of MAOA genotype. PLoS One. 2007;2:e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresan A, Camarena B, Apiquian R, Aguilar A, Urraca N, Nicolini H. Association study of MAO-A and DRD4 genes in schizophrenic patients with aggressive behavior. Neuropsychobiology. 2007;55:171–175. doi: 10.1159/000106477. [DOI] [PubMed] [Google Scholar]
- Fuller RW. Fluoxetine effects on serotonin function and aggressive behavior. In: Ferris CF, editor. Understanding aggressive behavior in children. The New York Academy of Sciences. New York, New York: 1996. pp. 90–97. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Aghajanian GK. Effect of antipsychotic drugs on the firing of dorsal raphe cells. II. Reversal by picrotoxin. Eur J Pharmacol. 1976;39:357–364. doi: 10.1016/0014-2999(76)90145-x. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Bethea ED, Stevenson SA. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC Neurosci. 2007;8:17. doi: 10.1186/1471-2202-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Stevenson SA, Bale TL, D’Anna KL. Elevated stress sensitivity in corticotropin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behav Brain Res. 2005;160:169–177. doi: 10.1016/j.bbr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci. 2004;118:805–814. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci. 1999;19:8027–8035. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Stevenson SA. Intermale aggression in corticotropin-releasing factor receptor 1 deficient mice. Behav Brain Res. 2006;171:63–69. doi: 10.1016/j.bbr.2006.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Levinson CA, Corman MD, Godlaski AJ, Morris DH, Phillips JP, Holt JC. Men and women, alcohol and aggression. Exp Clin Psychopharmacol. 2009;17:154–164. doi: 10.1037/a0016385. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Fernandez-Teruel A, Escorihuela RM, Fredholm BB, Tobena A, Pekny M, Johansson B. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- Godlaski AJ, Giancola PR. Executive functioning, irritability, and alcohol-related aggression. Psychol Addict Behav. 2009;23:391–403. doi: 10.1037/a0016582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Dean M, Brown GL, Bolos AM, Tokola R, Virkkunen M, Linnoila M. D2 dopamine receptor genotype and cerebrospinal fluid homovanillic acid, 5-hydroxyindoleacetic acid and 3-methoxy-4-hydroxyphenylglycol in alcoholics in Finland and the United States. Acta Psychiatrica Scandinavica. 1992;86:351–357. doi: 10.1111/j.1600-0447.1992.tb03279.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Swann AC, Moeller FG, Lane SD. Zolmitriptan and human aggression: interaction with alcohol. Psychopharmacology. 2010;210:521–31. doi: 10.1007/s00213-010-1851-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Bakos N, Rodriguiz RM, Caron MG, Wetsel WC, Liposits Z. Behavioral responses to social stress in noradrenaline transporter knockout mice: effects on social behavior and depression. Brain Res Bull. 2002;58:279–284. doi: 10.1016/s0361-9230(02)00789-x. [DOI] [PubMed] [Google Scholar]
- Haller J, Mikics E, Halasz J, Toth M. Mechanisms differentiating normal from abnormal aggression: glucocorticoids and serotonin. Eur J Pharmacol. 2005;526:89–100. doi: 10.1016/j.ejphar.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Haller J, van de Schraaf J, Kruk MR. Deviant forms of aggression in glucocorticoid hyporeactive rats: a model for ‘pathological’ aggression? J Neuroendocrinol. 2001;13:102–107. doi: 10.1046/j.1365-2826.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- Hallikainen T, Saito T, Lachman HM, Volavka J, Pohjalainen T, Ryynanen OP, Kauhanen J, Syvalahti E, Hietala J, Tiihonen J. Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Mol Psychiatry. 1999;4:385–388. doi: 10.1038/sj.mp.4000526. [DOI] [PubMed] [Google Scholar]
- Haney M, DeBold JF, Miczek KA. Maternal aggression in mice and rats towards male and female conspecifics. Aggress Behav. 1989;15:443–453. [Google Scholar]
- Hassanain M, Bhatt S, Siegel A. Differential modulation of feline defensive rage behavior in the medial hypothalamus by 5-HT1A and 5-HT2 receptors. Brain Res. 2003;981:201–209. doi: 10.1016/s0006-8993(03)03036-1. [DOI] [PubMed] [Google Scholar]
- Haug M, Wallian L, Brain PF. Effects of 8-OH-DPAT and fluoxetine on activity and attack by female mice towards lactating intruders. Gen Pharmacol. 1990;21:845–849. doi: 10.1016/0306-3623(90)90443-p. [DOI] [PubMed] [Google Scholar]
- Heiligenberg W. Processes governing behavioral states of readiness. Adv Study Behav. 1974;5:173–200. [Google Scholar]
- Heils A, Teufel A, Petri S, Seemann M, Bengel D, Balling U, Riederer P, Lesch KP. Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. J Neural Transm Gen Sect. 1995;102:247–254. doi: 10.1007/BF01281159. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knockout models. Eur J Pharmacol. 2003;480:21–29. doi: 10.1016/j.ejphar.2003.08.089. [DOI] [PubMed] [Google Scholar]
- Hess WR. Das Zwischenhirn. Benno Schwabe & Co.; Basel: 1954. [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ. Parental behavior in primates. In: Sluckin W, editor. Parental Behavior in Animals and Humans. Blackwell Press; Oxford: 1986. pp. 152–207. [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology. 1991;103:551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- Ho HP, Olsson M, Westberg L, Melke J, Eriksson E. The serotonin reuptake inhibitor fluoxetine reduces sex steroid-related aggression in female rats: an animal model of premenstrual irritability? Neuropsychopharmacology. 2001;24:502–510. doi: 10.1016/S0893-133X(00)00219-0. [DOI] [PubMed] [Google Scholar]
- Hollander E. Managing aggressive behavior in patients with obsessive-compulsive disorder and borderline personality disorder. J Clin Psychiatry 60 Suppl. 1999;15:38–44. [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology. 2002;161:160–167. doi: 10.1007/s00213-002-1024-3. [DOI] [PubMed] [Google Scholar]
- Homberg JR. Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. Eur J Neurosci. 2007;26:2066–2073. doi: 10.1111/j.1460-9568.2007.05839.x. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Huang YY, Grailhe R, Arango V, Hen R, Mann JJ. Relationship of psychopathology to the human serotonin1B genotype and receptor binding kinetics in postmortem brain tissue. Neuropsychopharmacology. 1999;21(2):238–246. doi: 10.1016/S0893-133X(99)00030-5. [DOI] [PubMed] [Google Scholar]
- Hurst JL. Behavioral variation in wild house mice (Mus domesticus Rutty): A quantitative assessment of female social organization. Anim Behav. 1987;35:1846–1857. [Google Scholar]
- Innis RB, Aghajanian GK. Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur J Pharmacol. 1987;143:195–204. doi: 10.1016/0014-2999(87)90533-4. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson O, Becnel J, Nichols CD. Serotonin 5-HT2 and 5-HT1A-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience. 2009;158:1292–1300. doi: 10.1016/j.neuroscience.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joppa MA, Rowe RK, Meisel RL. Effects of serotonin 1A or 1B receptor agonists on social aggression in male and female Syrian hamsters. Pharmacol Biochem Behav. 1997;58:349–353. doi: 10.1016/s0091-3057(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Ingram CD, Gartside SE. GABA receptor modulation of 5-HT neuronal firing: characterization and effect of moderate in vivo variations in glucocorticoid levels. Neurochem Int. 2004;45:1057–1065. doi: 10.1016/j.neuint.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Kalen P, Pritzel M, Nieoullon A, Wiklund L. Further evidence for excitatory amino acid transmission in the lateral habenular projection to the rostral raphe nuclei: lesion-induced decrease of high affinity glutamate uptake. Neurosci Lett. 1986;68:35–40. doi: 10.1016/0304-3940(86)90225-9. [DOI] [PubMed] [Google Scholar]
- Karl T, Lin S, Schwarzer C, Sainsbury A, Couzens M, Wittmann W, Boey D, von Horsten S, Herzog H. Y1 receptors regulate aggressive behavior by modulating serotonin pathways. Proc Natl Acad Sci U S A. 2004;101:12742–12747. doi: 10.1073/pnas.0404085101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavoussi R, Armstead P, Coccaro E. The neurobiology of impulsive aggression. Psychiatr Clin North Am. 1997;20:395–403. doi: 10.1016/s0193-953x(05)70319-1. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr., Strakowski SM, McElroy SL. The efficacy of atypical antipsychotics in the treatment of depressive symptoms, hostility, and suicidality in patients with schizophrenia. J Clin Psychiatry. 2000;61(Suppl 3):4–9. [PubMed] [Google Scholar]
- Khait VD, Huang YY, Zalsman G, Oquendo MA, Brent DA, Harkavy-Friedman JM, Mann JJ. Association of serotonin 5-HT2A receptor binding and the T102C polymorphism in depressed and healthy Caucasian subjects. Neuropsychopharmacology. 2005;30:166–172. doi: 10.1038/sj.npp.1300578. [DOI] [PubMed] [Google Scholar]
- Kim C, Jeon D, Kim YH, Lee CJ, Kim H, Shin HS. Deletion of N-type Ca2+ channel Cav2.2 results in hyperaggressive behaviors in mice. J Biol Chem. 2009;284:2738–2745. doi: 10.1074/jbc.M807179200. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Shih JC, Chen K, Chen L, Bao SW, Maren S, Anagnostaras SG, Fanselow MS, DeMaeyer E, Seif I, Thompson RF. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YR, Jahng JW, Min SK. Association between the serotonin transporter gene (5-HTTLPR) and anger-related traits in Korean schizophrenic patients. Neuropsychobiology. 2009b;59:165–171. doi: 10.1159/000218079. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, Baltimore D. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM. Hypothalamically induced intraspecific aggressive behaviour in the rat. Exp Brain Res. 1978;32:365–375. doi: 10.1007/BF00238708. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Van der Brink THC, Roozendaal B, Boorsma F. Medial amygdala and aggressive behavior: Interaction between testosterone and vasopressin. Aggress Behav. 1990;16:223–229. [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, Ebert MH, Schmidt DE, McKinney WT. A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in Rhesus monkeys. Neuropsychopharmacology. 1989;2:175–189. doi: 10.1016/0893-133x(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Hernandez-Avila CA, Gelernter J. Polymorphism of the 5-HT1B receptor gene (HTR1B): Strong within-locus linkage disequilibrium without association to antisocial substance dependence. Neuropsychopharmacology. 2002;26:115–122. doi: 10.1016/S0893-133X(01)00283-4. [DOI] [PubMed] [Google Scholar]
- Krsiak M. Behavioral changes and aggressivity evoked by drugs in mice. Res Commun Chem Pathol Pharmacol. 1974;7:237–257. [PubMed] [Google Scholar]
- Kushi A, Sasai H, Koizumi H, Takeda N, Yokoyama M, Nakamura M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc Natl Acad Sci U S A. 1998;95:15659–15664. doi: 10.1073/pnas.95.26.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Cutter WJ, Rubia K, Brammer M, Daly EM, Craig MC, Cleare AJ, Murphy DG. 5-HT, prefrontal function and aging: fMRI of inhibition and acute tryptophan depletion. Neurobiol Aging. 2009;30:1135–1146. doi: 10.1016/j.neurobiolaging.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Lang A, Harro J, Soosaar A, Koks S, Volke V, Oreland L, Bourin M, Vasar E, Bradwejn J, Mannisto PT. Role of N-methyl-D-aspartic acid and cholecystokinin receptors in apomorphine-induced aggressive behaviour in rats. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:363–370. doi: 10.1007/BF00169076. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Long JC, Eggert M, Ozaki N, Robin RW, Brown GL, Naukkarinen H, Virkkunen M, Linnoila M, Goldman D. Linkage of antisocial alcoholism to the serotonin 5-HT1B receptor gene in 2 populations. Arch Gen Psychiatry. 1998;55:989–994. doi: 10.1001/archpsyc.55.11.989. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Mueller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;264:1537–1551. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in Rhesus monkeys. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Levy M, Berson A, Cook T, Bollegala N, Seto E, Tursanski S, Kim J, Sockalingam S, Rajput A, Krishnadev N, Feng C, Bhalerao S. Treatment of agitation following traumatic brain injury: a review of the literature. NeuroRehabilitation. 2005;20:279–306. [PubMed] [Google Scholar]
- Leyhausen P. Cat behavior: predatory and social behavior of domestic and wild cats. Garland STPM Press; New York: 1979. [Google Scholar]
- Lindgren T, Kantak KM. Effects of serotonin receptor agonists and antagonists on offensive aggression in mice. Aggress Behav. 1987;13:87–96. [Google Scholar]
- Linnoila VMI, Virkkunen M. Aggression, suicidality, and serotonin. J Clin Psychiatry. 1992;53:46–51. [PubMed] [Google Scholar]
- Loconto J, Papes F, Chang E, Stowers L, Jones EP, Takada T, Kumanovics A, Fischer LK, Dulac C. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucion AB, de Almeida RMM. On the dual nature of maternal aggression in rats. Aggress Behav. 1996;22:365–373. [Google Scholar]
- Lucki I, Wieland S. 5-Hydroxytryptamine-1A receptors and behavioral responses. Neuropsychopharmacology. 1990;3:481–493. [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciewicz R, Foote WE. Excitatory projection from the interpeduncular nucleus to central superior raphe neurons. Brain Res. 1981;225:179–183. doi: 10.1016/0006-8993(81)90327-9. [DOI] [PubMed] [Google Scholar]
- Malick JB. The pharmacology of isolation-induced aggressive behavior in mice. In: Essman WB, editor. Current Developments in Psychopharmacology. SP Medical and Scientific Books; New York: 1979. pp. 1–27. [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Muldoon MF, Ferrell RE. Central nervous system serotonergic responsivity and aggressive disposition in men. Physiol Behav. 2002;77:705–709. doi: 10.1016/s0031-9384(02)00922-8. [DOI] [PubMed] [Google Scholar]
- Marino MD, Bourdelat-Parks BN, Liles LC, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161:197–203. doi: 10.1016/j.bbr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Mathews TA. Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods. 2004;140:169–181. doi: 10.1016/j.jneumeth.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology. 2003;77:416–424. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Furuyashiki T, Yamada K, Nagai T, Bito H, Tanaka Y, Kitaoka S, Ushikubi F, Nabeshima T, Narumiya S. Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci U S A. 2005;102:16066–16071. doi: 10.1073/pnas.0504908102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson SC, Canastar A. The genetics of aggression in mice. In: Flannery D, Vazsonyi AT, Waldman I, editors. The Cambridge Handbook of Violent Behavior and Aggression. Cambridge University Press; New York: 2007. pp. 91–110. [Google Scholar]
- McAllister KH. Ethological analysis of the effects of MK-801 upon aggressive male mice: similarity to chlordiazepoxide. Pharmacol Biochem Behav. 1990;37:101–106. doi: 10.1016/0091-3057(90)90048-m. [DOI] [PubMed] [Google Scholar]
- McBride PA, Brown RP, DeMeo M, Keilp J, Mieczkowski T, Mann JJ. The relationship of platelet 5-HT2 receptor indexes to major depressive disorder, personality traits, and suicidal behavior. Biol Psychiatry. 1994;35:295–308. doi: 10.1016/0006-3223(94)90033-7. [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Girasa KA, Allan AM, Miczek KA. 5-HT3 receptors, alcohol and aggressive behavior in mice. Behav Pharmacol. 2005;16:163–170. doi: 10.1097/00008877-200505000-00005. [DOI] [PubMed] [Google Scholar]
- McKinley JC, Hathaway SR, Meehl PE. The Minnesota multiphasic personality inventory: the K-scale. J Consult Psychol. 1948;12:20–31. doi: 10.1037/h0061377. [DOI] [PubMed] [Google Scholar]
- McMillen BA, DaVanzo EA, Scott SM, Song AH. N-alkyl-substituted aryl-piperazine drugs: Relationship between affinity for serotonin receptors and inhibition of aggression. Drug Dev Res. 1988;12:53–62. [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Reedy CL, Cohen BM, Carlezon WA., Jr. Activation of raphe efferents to the medial prefrontal cortex by corticotropin-releasing factor: correlation with anxiety-like behavior. Biol Psychiatry. 2008;63:832–839. doi: 10.1016/j.biopsych.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza DL, Bravo HA, Swanson HH. Antiaggresive and anxiolytic effects of gepirone in mice, and their attenuation by WAY 100635. Pharmacol Biochem Behav. 1999;62:499–509. doi: 10.1016/s0091-3057(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA. The psychopharmacology of aggression. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology, Volume 19: New Directions in Behavioral Pharmacology. Plenum; New York: 1987. pp. 183–328. [Google Scholar]
- Miczek KA, Barros HM, Sakoda L, Weerts EM. Alcohol and heightened aggression in individual mice. Alcohol Clin Exp Res. 1998a;22:1698–1705. [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM. Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology. 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM, Kravitz EA, Rissman EF, de Boer SF, Raine A. Neurobiology of escalated aggression and violence. J Neurosci. 2007a;27:11803–11806. doi: 10.1523/JNEUROSCI.3500-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo S, de Almeida RMM, Bannai M, Fish EW, DeBold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo SP, Fish EW, DeBold JF. Neurochemistry and molecular neurobiology of aggressive behavior. In: Blaustein J, editor. Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology. Springer; New York: 2007b. pp. 285–336. [Google Scholar]
- Miczek KA, Fish EW, DeBold JF, de Almeida RMM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology. 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–257. doi: 10.1016/j.yhbeh.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Haney M. Psychomotor stimulant effects of d-amphetamine, MDMA and PCP: Aggressive and schedule-controlled behavior in mice. Psychopharmacology. 1994;115:358–365. doi: 10.1007/BF02245077. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Hussain S, Faccidomo S. Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology. 1998b;139:160–168. doi: 10.1007/s002130050701. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and l-dopa. Psychopharmacology. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, Tornatzky W, DeBold JF, Vatne TM. Alcohol and “bursts” of aggressive behavior: Ethological analysis of individual differences in rats. Psychopharmacology. 1992;107:551–563. doi: 10.1007/BF02245270. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ. Antidepressant treatment and rodent aggressive behaviour. Eur J Pharmacol. 2005;526:147–162. doi: 10.1016/j.ejphar.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Fletcher A, Redfern PH. Is antidepressant efficacy revealed by drug-induced changes in rat behaviour exhibited during social interaction? Neurosci Biobehav Rev. 1991;15:539–544. doi: 10.1016/s0149-7634(05)80145-9. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Redfern PH. Acute and chronic antidepressant drug treatments induce opposite effects in the social behavior of rats. J Psychopharmacol. 1992;6:241–257. doi: 10.1177/026988119200600218. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Redfern PH. Potentiation of the time-dependent, antidepressant-induced changes in the agonistic behaviour of resident rats by the 5-HT1A receptor antagonist, WAY-100635. Behav Pharmacol. 1997;8:585–606. doi: 10.1097/00008877-199711000-00016. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Takao K, Niki H. Differential effect of Fyn tyrosine kinase deletion on offensive and defensive aggression. Behav Brain Res. 2001;122:51–56. doi: 10.1016/s0166-4328(01)00171-1. [DOI] [PubMed] [Google Scholar]
- Morgan C, Thomas RE, Ma W, Novotny MV, Cone RD. Melanocortin-5 receptor deficiency reduces a pheromonal signal for aggression in male mice. Chem Senses. 2004;29:111–115. doi: 10.1093/chemse/bjh011. [DOI] [PubMed] [Google Scholar]
- Mos J, Olivier B, Poth M, Van Oorschot R, Van Aken H. The effects of dorsal raphe administration of eltoprazine, TFMPP and 8-OH-DPAT on resident intruder aggression in the rat. Eur J Pharmacol. 1993;238:411–415. doi: 10.1016/0014-2999(93)90877-k. [DOI] [PubMed] [Google Scholar]
- Muehlenkamp F, Lucion A, Vogel WH. Effects of selective serotenergic agonists on aggressive behavior in rats. Pharmacol Biochem Behav. 1995;50:671–674. doi: 10.1016/0091-3057(95)00351-7. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Musty RE, Consroe PF. Phencyclidine produces aggressive behavior in rapid eye movement sleep-deprived rats. Life Sci. 1982;30:1733–1738. doi: 10.1016/0024-3205(82)90307-1. [DOI] [PubMed] [Google Scholar]
- Nagtegaal MH, Rassin E. The usefulness of the thought suppression paradigm in explaining impulsivity and aggression. Pers Individ Dif. 2004;37:1233–1244. [Google Scholar]
- Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF. Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res. 1982;232:375–389. doi: 10.1016/0006-8993(82)90281-5. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Caramaschi D. Animal violence demystified. Front Behav Neurosci. 2010;4:9. doi: 10.3389/fnbeh.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends Neurosci. 2001;24:713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Front Behav Neurosci. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: Evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Buchsbaum MS, Hazlett EA, Goodman M, Koenigsberg HW, Lo J, Iskander L, Newmark R, Brand J, Flynn K, Siever LJ. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology. 2004;176:451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nicot A, Otto T, Brabet P, Dicicco-Bloom EM. Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. J Neurosci. 2004;24:8786–8795. doi: 10.1523/JNEUROSCI.1910-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Avgustinovich DF, Popova NK. Role of 5HT1A receptors in a variety of kinds of aggressive behavior in wild rats and counterparts selected for low defensiveness towards Man. Aggress Behav. 1992;18:357–364. [Google Scholar]
- Nogueira RL, Graeff FG. Role of 5HT receptor subtypes in the modulation of dorsal periaqueductal gray generated aversion. Pharmacol Biochem Behav. 1995;52:1–6. doi: 10.1016/0091-3057(94)00402-5. [DOI] [PubMed] [Google Scholar]
- Noirot E, Goyens J, Buhot MC. Aggressive behavior of pregnant mice towards males. Horm Behav. 1975;6:9–17. doi: 10.1016/0018-506x(75)90018-5. [DOI] [PubMed] [Google Scholar]
- Nomura M, Kusumi I, Kaneko M, Masui T, Daiguji M, Ueno T, Koyama T, Nomura Y. Involvement of a polymorphism in the 5-HT2A receptor gene in impulsive behavior. Psychopharmacology. 2006;187:30–35. doi: 10.1007/s00213-006-0398-z. [DOI] [PubMed] [Google Scholar]
- Oades RD, Lasky-Su J, Christiansen H, Faraone SV, Sonuga-Barke EJS, Banaschewski T, Chen W, Anney RJL, Buitelaar JK, Ebstein RP, Franke B, Gill M, Miranda A, Roeyers H, Rothenberger A, Sergeant JA, Steinhausen HC, Taylor EA, Thompson M, Asherson P. The influence of serotonin- and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behav Brain Funct. 2008;4:48. doi: 10.1186/1744-9081-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson J-A, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene-deficient (βERKO) male and female mice. Proc Natl Acad Sci U S A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-β gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998a;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-β gene disruption in male mice. Endocrinology. 1998b;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- Oliveira-dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, Mariathasan S, Sasaki T, Wakeham A, Ohashi PS, Roder JC, Barnes CA, Siderovski DP, Penninger JM. Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc Natl Acad Sci U S A. 2000;97:12272–12277. doi: 10.1073/pnas.220414397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B. Serotonin and aggression. Ann N Y Acad Sci. 2004;1036:382–392. doi: 10.1196/annals.1330.022. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, Rasmussen D. Behavioural pharmacology of the serenic, eltoprazine. Rev Drug Metab Drug Interact. 1990;8:31–83. doi: 10.1515/dmdi.1990.8.1-2.31. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, Tulp MTM, van der Poel AM. Animal models of anxiety and aggression in the study of serotonergic agents. In: Langer SZ, editor. Serotonin receptor subtypes: Pharmacological significance and clinical implications. Karger; Basel: 1992. pp. 67–79. [Google Scholar]
- Olivier B, Mos J, Van der Heyden J, Hartog J. Serotonergic modulation of social interactions in isolated male mice. Psychopharmacology. 1989;97:154–156. doi: 10.1007/BF00442239. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J, Van Oorschot R, Hen R. Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry. 1995;28:80–90. doi: 10.1055/s-2007-979624. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Russo SA, Underwood MD, Kassir SA, Ellis SP, Mann JJ, Arango V. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol Psychiatry. 2006;59:235–243. doi: 10.1016/j.biopsych.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Pabis DJ, Stanislav SW. Pharmacotherapy of aggressive behavior. Ann Pharmacother. 1996;30:278–287. doi: 10.1177/106002809603000312. [DOI] [PubMed] [Google Scholar]
- Palanza P, Della Seta D, Ferrari PF, Parmigiani S. Female competition in wild house mice depends upon timing of female/male settlement and kinship between females. Anim Behav. 2005;69:1259–1271. [Google Scholar]
- Pallotta M, Segieth J, Whitton PS. N-Methyl-d-aspartate receptors regulate 5-HT release in the raphe nuclei and frontal cortex of freely moving rats: differential role of 5-HT1A autoreceptors. Brain Res. 1998;783:173–178. doi: 10.1016/s0006-8993(97)01333-4. [DOI] [PubMed] [Google Scholar]
- Parmigiani S, Ferrari PF, Palanza P. An evolutionary approach to behavioral pharmacology: using drugs to understand proximate and ultimate mechanisms of different forms of aggression in mice. Neurosci Biobehav Rev. 1998;23:143–153. doi: 10.1016/s0149-7634(98)00016-5. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Gioia MC, Magariello A, Muglia M, Quattrone A, Fera F. Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage. 2008;40:1264–1273. doi: 10.1016/j.neuroimage.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Peeke HVS, Figler MH. Modulation of aggressive behavior in fish by alcohol and congeners. Pharmacol Biochem Behav 14 Suppl. 1981;1:79–84. doi: 10.1016/s0091-3057(81)80013-5. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting in the Syrian golden hamster Mesocricetus auratus Waterhouse, and its relationship to serious fighting during postweaning development. Dev Psychobiol. 1988;21:323–337. doi: 10.1002/dev.420210404. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P, Dennis T, de Montigny C. Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci. 1994;14:3036–3047. doi: 10.1523/JNEUROSCI.14-05-03036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology. 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK, Avgustinovich DF, Kolpakov VG, Plyusnina IZ. Specific [3H]8-OH-DPAT binding in brain regions of rats genetically predisposed to various defense behavior strategies. Pharmacol Biochem Behav. 1998;59:793–797. doi: 10.1016/s0091-3057(97)00504-2. [DOI] [PubMed] [Google Scholar]
- Potegal M. Attack priming and satiation in female golden hamsters: Tests of some alternatives to the aggression arousal interpretation. Aggress Behav. 1991;17:327–335. [Google Scholar]
- Potegal M, Tenbrink L. Behavior of attack-primed and attack-satiated female golden hamsters (Mesocricetus auratus) J Comp Psychol. 1984;98:66–75. [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LJ, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IM, Miguel TM, DeBold JF, Miczek KA. 2009 Neuroscience Meeting Planner. Society for Neuroscience; Chicago, IL: 2009a. Opposing action of CRF1 vs. CRF2 receptors in the dorsal raphé: Modulation of alcohol-heightened aggression. Program No.445.5/T8. Online. [Google Scholar]
- Quadros IM, Takahashi A, Miczek KA. Serotonin and aggression. In: Müller CP, Jacobs BL, editors. Handbook of the Behavioral Neurobiology of Serotonin. Academic Press; 2009b. pp. 687–714. [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. J Abnorm Child Psychol. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Ratey JJ, O’Driscoll GA. Buspirone as a habilitative drug for patients with a dual diagnosis. Family Practive Recertification. 1989;11:38–45. [Google Scholar]
- Ratey JJ, Sovner R, Mikkelsen E, Chmielinski HE. Buspirone therapy for maladaptive behavior and anxiety in developmentally disabled persons. J Clin Psychiatry. 1989;50:382–384. [PubMed] [Google Scholar]
- Raj YP. Psychopharmacology of borderline personality disorder. Curr Psychiatry Rep. 2004;6:225–231. doi: 10.1007/s11920-004-0068-y. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Saudou F, Amara DA, Belzung C, Segu L, Misslin R, Buhot MC, Hen R. 5-HT1B receptor knock out - Behavioral consequences. Behav Brain Res. 1995;73:305–312. doi: 10.1016/0166-4328(96)00119-2. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Giovenardi M, de Almeida RM. Drugs and aggression. Recent Pat CNS Drug Discov. 2008;3:40–49. doi: 10.2174/157488908783421456. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratey J, Sovner R, Parks A, Rogentine K. Buspirone treatment of aggression and anxiety in mentally retarded patients: a multiple-baseline, placebo lead-in study. J Clin Psychiatry. 1991;52:159–162. [PubMed] [Google Scholar]
- Reist C, Nakamura K, Sagart E, Sokolski KN, Fujimoto KA. Impulsive aggressive behavior: open-label treatment with citalopram. J Clin Psychiatry. 2003;64:81–85. [PubMed] [Google Scholar]
- Rewerski W, Kostowski W, Piechocki T, Rylski M. The effects of some hallucinogens on aggressiveness of mice and rats. I. Pharmacology. 1971;5:314–320. doi: 10.1159/000136205. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Grimes JM, Melloni RH., Jr. Serotonin type 3 receptors modulate the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2004;118:1097–1110. doi: 10.1037/0735-7044.118.5.1097. [DOI] [PubMed] [Google Scholar]
- Rios M, Lambe EK, Liu RJ, Teillon S, Liu JH, Akbarian S, Roffler-Tarlov S, Jaenisch R, Aghajanian GK. Severe deficits in 5-HT2A-mediated neurotransmission in BDNF conditional mutant mice. J Neurobiol. 2006;66:408–420. doi: 10.1002/neu.20233. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Scearce-Levie K, Lucas JJ, Hiroi N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Minarro J, Aguilar MA, Pinazo J, Simon VM. Effects of risperidone and SCH 23390 on isolation-induced aggression in male mice. Eur Neuropsychopharmacol. 1998;8:95–103. doi: 10.1016/s0924-977x(97)00051-5. [DOI] [PubMed] [Google Scholar]
- Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Rudissaar R, Pruus K, Skrebuhhova T, Allikmets L, Matto V. Modulatory role of 5-HT3 receptors in mediation of apomorphine-induced aggressive behaviour in male rats. Behav Brain Res. 1999;106:91–96. doi: 10.1016/s0166-4328(99)00095-9. [DOI] [PubMed] [Google Scholar]
- Rydén E, Thase ME, Straht D, Aberg-Wistedt A, Bejerot S, Landen M. A history of childhood attention-deficit hyperactivity disorder (ADHD) impacts clinical outcome in adult bipolar patients regardless of current ADHD. Acta Psychiatr Scand. 2009;120:239–246. doi: 10.1111/j.1600-0447.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Sowa C, Sakamoto Y, Nishihara B, Koyama Y, Baba A, Matsuda T. Modulation by 5-HT2A receptors of aggressive behavior in isolated mice. Jpn J Pharmacol. 2002;89:89–92. doi: 10.1254/jjp.89.89. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M. Adrenergic α2c-receptors modulate the acoustic startle reflex, prepulse inhibition, and aggression in mice. J Neurosci. 1998;18:3035–3042. doi: 10.1523/JNEUROSCI.18-08-03035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Hyttel J, Moltzen EK. The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology. 1993;110:53–59. doi: 10.1007/BF02246950. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Hyttel J. Isolation-induced aggression in mice: Effects of 5-hydroxytryptamine uptake inhibitors and involvement of postsynaptic 5-HT1A receptors. Eur J Pharmacol. 1994;264:241–247. doi: 10.1016/0014-2999(94)00470-6. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Psychopharmacology. 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- Sano Y, Ornthanalai VG, Yamada K, Homma C, Suzuki H, Suzuki T, Murphy NP, Itohara S. X11-like protein deficiency is associated with impaired conflict resolution in mice. J Neurosci. 2009;29:5884–5896. doi: 10.1523/JNEUROSCI.5756-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, Lemeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Scott AL, Bortolato M, Chen K, Shih JC. Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. NeuroReport. 2008;19:739–743. doi: 10.1097/WNR.0b013e3282fd6e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgoifo A, Stilli D, Musso E, Mainardi D, Parmigiani S. Offensive and defensive bite-target topographies in attacks by lactating rats. Aggress Behav. 1992;17:47–52. [Google Scholar]
- Shaikh MB, De Lanerolle NC, Siegel A. Serotonin 5-HT1A and 5-HT2/1C receptors in the midbrain periaqueductal gray differentially modulate defensive rage behavior elicited from the medial hypothalamus of the cat. Brain Res. 1997;765:198–207. doi: 10.1016/s0006-8993(97)00433-2. [DOI] [PubMed] [Google Scholar]
- Shih JC, Ridd MJ, Chen K, Meehan WP, Kung MP, Seif I, De Maeyer E. Ketanserin and tetrabenazine aggression in mice lacking monoamine oxidase A. Brain Res. 1999;835:104–112. doi: 10.1016/s0006-8993(99)01478-x. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Sijbesma H, Schipper J, De Kloet ER, Mos J, Van Aken H, Olivier B. Postsynaptic 5-HT1 receptors and offensive aggression in rats: A combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Behav. 1991;38:447–458. doi: 10.1016/0091-3057(91)90305-l. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Sinha R, Cloninger CR, Parsian A. Linkage disequilibrium and haplotype analysis between serotonin receptor 1B gene variations and subtypes of alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2003;121B:83–88. doi: 10.1002/ajmg.b.20064. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Wargelius HL, Leppert J, Lindstrom L, Oreland L. Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosicial factors. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- Smuts BB. Sexual competition and mate choice. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. University of Chicago Press; Chicago: 1986. pp. 385–399. [Google Scholar]
- Sofia RD. Structural relationship and potency of agents which selectively block mouse killing (muricide) behavior in rats. Life Sciences. 1969;8:1201–1210. doi: 10.1016/0024-3205(69)90049-6. [DOI] [PubMed] [Google Scholar]
- Sperry TS, Thompson CK, Wingfield JC. Effects of acute treatment with 8-OH-DPAT and fluoxetine on aggressive behaviour in male song sparrows (Melospiza melodia morphna) J Neuroendocrinol. 2003;15:150–160. doi: 10.1046/j.1365-2826.2003.00968.x. [DOI] [PubMed] [Google Scholar]
- Spielberg CD, Sharma S, Singh M. Development of Hindi edition of state-trait anxiety inventory. Indian J Psychol. 1973;48:11–20. [Google Scholar]
- Spigset O. Adverse reactions of selective serotonin reuptake inhibitors - Reports from a spontaneous reporting system. Drug Saf. 1999;20:277–287. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Steiniger F. Beitrag zur Soziologie und sonstigen Biologie der Wanderratte. Z Tierpsychol. 1950;7:356–379. [Google Scholar]
- Stoff DM, Vitiello B. Role of serotonin in aggression of children and adolesecents: biochemical and phamacological studies. In: Stoff DM, editor. Aggression and Violence: Genetic, Neurobiological and Biosocial Perspectives. Lawrence Erlbaum Associates; Mahwah, NJ: 1996. pp. 101–123. [Google Scholar]
- Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H, Cremer H, Schachner M. Increased intermale aggression and neuroendocrine response in mice deficient for the neural cell adhesion molecule (NCAM) Eur J Neurosci. 1997;9:1117–1125. doi: 10.1111/j.1460-9568.1997.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Sun HF, Chang YT, Fann CS, Chang CJ, Chen YH, Hsu YP, Yu WY, Cheng AT. Association study of novel human serotonin 5-HT1B polymorphisms with alcohol dependence in Taiwanese Han. Biol Psychiatry. 2002;51:896–901. doi: 10.1016/s0006-3223(01)01366-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swanson JW, Swartz MS, Van Dorn RA, Volavka J, Monahan J, Stroup TS, Mcevoy JP, Wagner HR, Elbogen EB, Lieberman JA. Comparison of antipsychotic medication effects on reducing violence in people with schizophrenia. Br J Psychiatry. 2008;193:37–43. doi: 10.1192/bjp.bp.107.042630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kwa C, DeBold JF, Miczek KA. GABAA receptors in the dorsal raphé nucleus of mice: Escalation of aggression after alcohol consumption. Psychopharmacology. 2010;211:467–77. doi: 10.1007/s00213-010-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABAB receptor modulation of serotonin neurons in the dorsal raphé nucleus and escalation of aggression in mice. J Neurosci. doi: 10.1523/JNEUROSCI.1814-10.2010. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Regulation of serotonin release by GABA and excitatory amino acids. J Psychopharmacol. 2000;14:100–113. doi: 10.1177/026988110001400201. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br J Pharmacol. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SP. Aggressive behavior and physiological arousal as a function of provocation and the tendency to inhibit aggression. J Pers. 1967;35:297–310. doi: 10.1111/j.1467-6494.1967.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Tazi A, Dantzer R, Le Moal M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist blocks stress-induced fighting in rats. Regul Pept. 1987;18:37–42. doi: 10.1016/0167-0115(87)90048-6. [DOI] [PubMed] [Google Scholar]
- Ten Eyck GR. Serotonin modulates vocalizations and territorial behavior in an amphibian. Behav Brain Res. 2008;193:144–147. doi: 10.1016/j.bbr.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Tompkins EC, Clemento AJ, Taylor DP, Perhach JL., Jr. Inhibition of aggressive behavior in Rhesus monkeys by buspirone. Res Commun Psychol Psychiatr Behav. 1980;5:337–352. [Google Scholar]
- Troisi A, Vicario E, Nuccetelli F, Ciani N, Pasini A. Effects of fluoxetine on aggressive behavior of adult inpatients with mental retardation and epilepsy. Pharmacopsychiatry. 1995;28:1–4. doi: 10.1055/s-2007-979593. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Liao DL, Yu YW, Chen TJ, Wu HC, Lin CH, Cheng CY, Hong CJ. A study of the association of (Val66Met) polymorphism in the brain-derived neurotrophic factor gene with alcohol dependence and extreme violence in Chinese males. Neurosci Lett. 2005;381:340–343. doi: 10.1016/j.neulet.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Tyler CB, Miczek KA. Effects of phencyclidine on aggressive behavior in mice. Pharmacol Biochem Behav. 1982;17:503–510. doi: 10.1016/0091-3057(82)90311-2. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Oliver-Africano PC, Ahmed Z, Bouras N, Cooray S, Deb S, Murphy D, Hare M, Meade M, Reece B, Kramo K, Bhaumik S, Harley D, Regan A, Thomas D, Rao B, North B, Eliahoo J, Karatela S, Soni A, Crawford M. Risperidone, haloperidol, and placebo in the treatment of aggressive challenging behaviour in patients with intellectual disability: a randomised controlled trial. Lancet. 2008;371:57–63. doi: 10.1016/S0140-6736(08)60072-0. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Valzelli L. About a “specific” neurochemistry of aggressive behavior. In: Delgado JMR, editor. Behavioral Neurochemistry. Spectrum Publications, Inc.; New York: 1977. pp. 113–132. [Google Scholar]
- Valzelli L, Bernasconi S. Aggressiveness by isolation and brain serotonin turnover changes in different strains of mice. Neuropsychobiology. 1979;5:129–135. doi: 10.1159/000117674. [DOI] [PubMed] [Google Scholar]
- Valzelli L, Giacalone E, Garattini S. Pharmacological control of aggressive behavior in mice. Eur J Pharmacol. 1967;2:144–146. doi: 10.1016/0014-2999(67)90040-4. [DOI] [PubMed] [Google Scholar]
- Van den Berg L, Vos-Loohuis M, Schilder MBH, van Oost BA, Hazewinkel HAW, Wade CM, Karlsson EK, Lindblad-Toh K, Liinamo AE, Leegwater PAJ. Evaluation of the serotonergic genes htr1A, htr1B, htr2A, and slc6A4 in aggressive behavior of golden retriever dogs. Behav Genet. 2008;38:55–66. doi: 10.1007/s10519-007-9179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Vegt BJ, Lieuwes N, van de Wall EH, Kato K, Moya-Albiol L, Martinez-Sanchis S, de Boer SF, Koolhaas JM. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci. 2003;117:667–674. doi: 10.1037/0735-7044.117.4.667. [DOI] [PubMed] [Google Scholar]
- Van Erp AMM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oortmerssen GA, Bakker TCM. Artificial selection for short and long attack latencies in wild Mus musculus domesticus. Behav Genet. 1981;11:115–126. doi: 10.1007/BF01065622. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. The development of social structure in free-ranging Rhesus monkeys. Behaviour. 1967;29:179–194. doi: 10.1163/156853967x00109. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Matheson GK, Wilderman RC, Patterson LA. Inhibition of serotonergic dorsal raphe neurons by systemic and iontophoretic administration of buspirone, a non-benzodiazepine anxiolytic drug. Eur J Pharmacol. 1986;129:123–130. doi: 10.1016/0014-2999(86)90343-2. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, de Almeida RMM. Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Braz J Med Biol Res. 2007;40:825–830. doi: 10.1590/s0100-879x2006005000113. [DOI] [PubMed] [Google Scholar]
- Veiga CP, Miczek KA, Lucion AB, de Almeida RMM. Social Instigation and maternal aggression in rats: role of 5-HT1A and 5-HT1B receptors in the dorsal raphé nucleus and prefrontal cortex. Psychopharmacology. 2010 doi: 10.1007/s00213-010-2083-5. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekovischeva OY, Aitta-aho T, Echenko O, Kankaanpaa A, Seppala T, Honkanen A, Sprengel R, Korpi ER. Reduced aggression in AMPA-type glutamate receptor GluR-A subunit-deficient mice. Genes Brain Behav. 2004;3:253–265. doi: 10.1111/j.1601-1848.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Stoff DM. Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36:307–315. doi: 10.1097/00004583-199703000-00008. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Citrome L, McQuade RD, Carson WH, Kostic D, Hardy S, Marcus R. Efficacy of aripiprazole against hostility in schizophrenia and schizoaffective disorder: Data from 5 double-blind studies. J Clin Psychiatry. 2005;66:1362–1366. doi: 10.4088/jcp.v66n1103. [DOI] [PubMed] [Google Scholar]
- Walsh MT, Dinan TG. Selective serotonin reuptake inhibitors and violence: a review of the available evidence. Acta Psychiatr Scand. 2001;104:84–91. doi: 10.1034/j.1600-0447.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29:943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, Kaufman J. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biol Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BL, Welch AS. Rapid modification of isolation-induced aggressive behavior and elevation of brain catecholamines and serotonin by the quick-acting monoamine-oxidase inhibitor pargyline. Commun Behav Biol. 1968;1:347–351. [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Whale R, Quested DJ, Laver D, Harrison PJ, Cowen PJ. Serotonin transporter (5-HTT) promoter genotype may influence the prolactin response to clomipramine. Psychopharmacology. 2000;150:120–122. doi: 10.1007/s002130000432. [DOI] [PubMed] [Google Scholar]
- White SM, Kucharik RF, Moyer JA. Effects of serotonergic agents on isolation-induced aggression. Pharmacol Biochem Behav. 1991;39:729–736. doi: 10.1016/0091-3057(91)90155-u. [DOI] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the “cycle of violence”: childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Wilmot CA, Vander Wende C, Spoerlein MT. The effects of phencyclidine on fighting in differentially housed mice. Pharmacol Biochem Behav. 1987;28:341–346. doi: 10.1016/0091-3057(87)90450-3. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Witte AV, Floel A, Stein P, Savli M, Mien LK, Wadsak W, Spindelegger C, Moser U, Fink M, Hahn A, Mitterhauser M, Kletter K, Kasper S, Lanzenberger R. Aggression is related to frontal serotonin-1A receptor distribution as revealed by PET in healthy subjects. Hum Brain Mapp. 2009;30:2558–2570. doi: 10.1002/hbm.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai K, Son LZ, Endou M, Sakurai E, Nakagawasai O, Tadano T, Kisara K, Inoue I, Watanabe T, Watanabe T. Behavioral characterization and amounts of brain monoamines and their metabolites in mice lacking histamine H1 receptors. Neuroscience. 1998;87:479–487. doi: 10.1016/s0306-4522(98)00167-5. [DOI] [PubMed] [Google Scholar]
- Yen CY, Stanger RL, Millman N. Ataractic suppression of isolation-induced aggressive behavior. Arch Int Pharmacodyn Ther. 1959;123:179–185. [PubMed] [Google Scholar]
- Young KA, Berry ML, Mahaffey CL, Saionz JR, Hawes NL, Chang B, Zheng QY, Smith RS, Bronson RT, Nelson RJ, Simpson EM. Fierce: a new mouse deletion of Nr2e1; violent behaviour and ocular abnormalities are background-dependent. Behav Brain Res. 2002;132:145–158. doi: 10.1016/s0166-4328(01)00413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone JR, Hellings JA, Crandall K, Reese RM, Marquis J, Fleming K, Shores R, Williams D, Schroeder SR. Effects of risperidone on aberrant behavior of persons with developmental disabilities: I. A double-blind crossover study using multiple measures. Am J Ment Retard. 2001;106:525–538. doi: 10.1352/0895-8017(2001)106<0525:EOROAB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zeichner A, Pihl RO. Effects of alcohol and behavior contingencies on human-aggression. J Abnorm Psychol. 1979;88:153–160. doi: 10.1037//0021-843x.88.2.153. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21:S52–S60. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Zitzman D, DeBold JF, Miczek KA. 2005 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2005. Positive modulation of the GABAA receptor heightens aggression in ovariectomized, nulliparous female mice. Program No. 76.15. Online. [Google Scholar]
- Zouk H, McGirr A, Lebel V, Benkelfat C, Rouleau G, Turecki G. The effect of genetic variation of the serotonin 1B receptor gene on impulsive aggressive Behavior and suicide. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:996–1002. doi: 10.1002/ajmg.b.30521. [DOI] [PubMed] [Google Scholar]