Abstract
Respiratory brainstem neurons fulfill critical roles in controlling breathing: they generate the activity patterns for breathing and contribute to various sensory responses including changes in O2 and CO2. These complex sensorimotor tasks depend on the dynamic interplay between numerous cellular building blocks that consist of voltage-, calcium-, and ATP-dependent ionic conductances, various ionotropic and metabotropic synaptic mechanisms, as well as neuromodulators acting on G-protein coupled receptors and second messenger systems. As described in this review, the sensorimotor responses of the respiratory network emerge through the state-dependent integration of all these building blocks. There is no known respiratory function that involves only a small number of intrinsic, synaptic, or modulatory properties. Because of the complex integration of numerous intrinsic, synaptic, and modulatory mechanisms, the respiratory network is capable of continuously adapting to changes in the external and internal environment, which makes breathing one of the most integrated behaviors. Not surprisingly, inspiration is critical not only in the control of ventilation, but also in the context of “inspiring behaviors” such as arousal of the mind and even creativity. Far-reaching implications apply also to the underlying network mechanisms, as lessons learned from the respiratory network apply to network functions in general.
Introduction
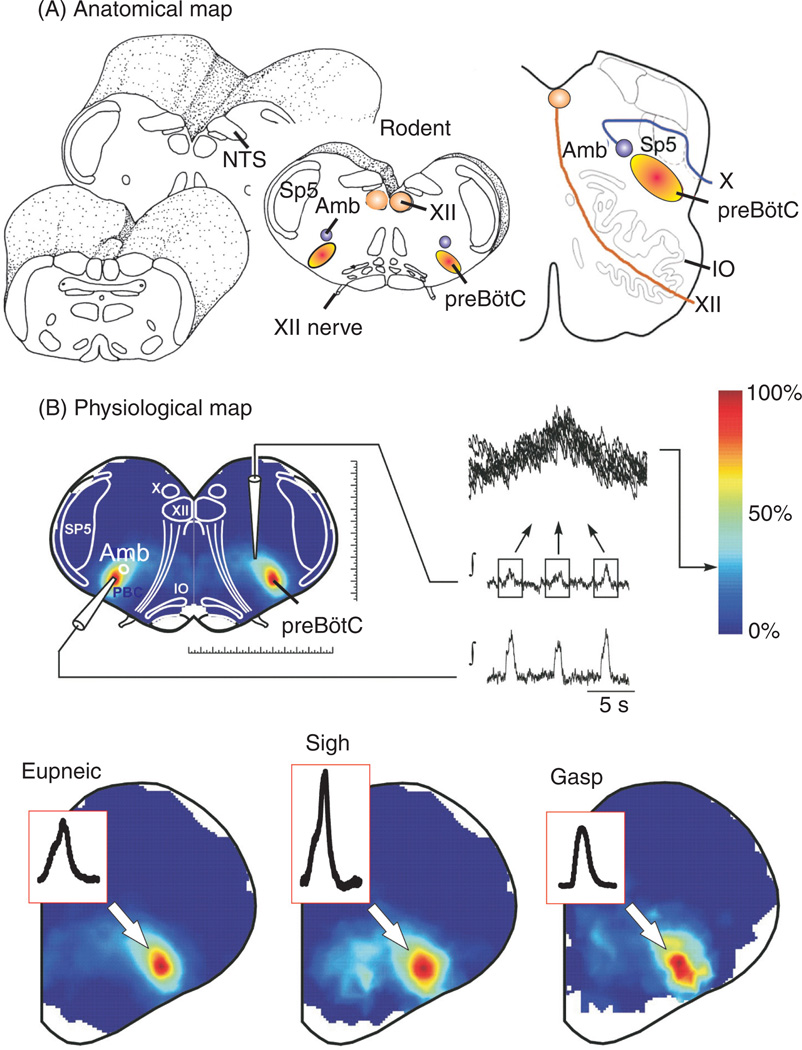
The enormous metabolic costs associated with endothermy and the need to maintain the brain and most other organs active continuously made mammals entirely dependent on a continuous supply of oxygen, a highly energetic element which is acquired through breathing (433). Without breathing, mammals typically survive for only a few minutes. Moreover, breathing needs to be continuously adapted to the metabolic needs of the behaving organism, making breathing one of the most integrated behaviors. Various metabolic and behavioral conditions modulate breathing including vocalization, sleep, arousal, fear, exercise, hypoxia, and hypercapnia. Breathing also modulates behaviors such as fear, arousal, and cognitive states. “Being inspired” or “having an inspiration” are commonly used expressions that reflect the close interaction between breathing and higher brain functions. The abilities to breathe, regulate gas exchange, and adapt to various metabolic and behavioral challenges critically depend on the cellular properties of the respiratory neurons within the brainstem. As elegantly researched by the French Professor Francois Clarac (78), the concept that the brainstem plays a crucial role in controlling breathing was first proposed by the English Professor Thomas Willis (599). In 1812, the French physiologist Julien-Jean-Cesar Legallois performed transections at various brainstem levels and was the first to conclude that only a very small circumscribed area in the medulla is critical for breathing (286). This observation was subsequently confirmed by Mary Jean-Pierre Flourens, who named this small circumscribed area the noeud vital, to reflect the fact that this area is of vital importance (153, 432). In 1991, Smith et al. identified a very circumscribed area in the ventrolateral medulla, which when lesioned in the isolated brainstem-spinal cord of neonatal rats abolished respiratory activity. This area continued to generate respiratory rhythmic activity even when isolated in thin medullary slices (Fig. 1). Smith et al. (516) termed the area responsible for respiratory rhythm generation the pre-Bötzinger complex (preBötC, Fig. 1A). More recently, the preBötC was identified in humans and it was demonstrated that anatomical alterations in the preBötC were associated with distinct breathing abnormalities (488).
Figure 1.
Anatomical and physiological characterization of the pre-Bötzinger complex (preBötC) in the ventrolateral medulla. (A) Anatomical maps of brainstem regions from rodent [left (436)] and human (right, modified, with permission, from reference (489) containing the preBötC. The location of the preBötC is anatomically characterized by the same transverse section as the nucleus ambiguus (Amb), inferior olive (IO), nucleus tractus solitarius (NTS), and hypoglossal nucleus (XII). (B) Isolating the preBötC in a single medullary brainstem slice from rodents preserves rhythmic neuronal activity implicated in the generation of inspiratory activity. Heat maps of activity show both an anatomical and physiological overlap of neuronal activities representing fictive eupnea (left), sighs (center), and gasps (right). Modified, with permission, from reference 299.
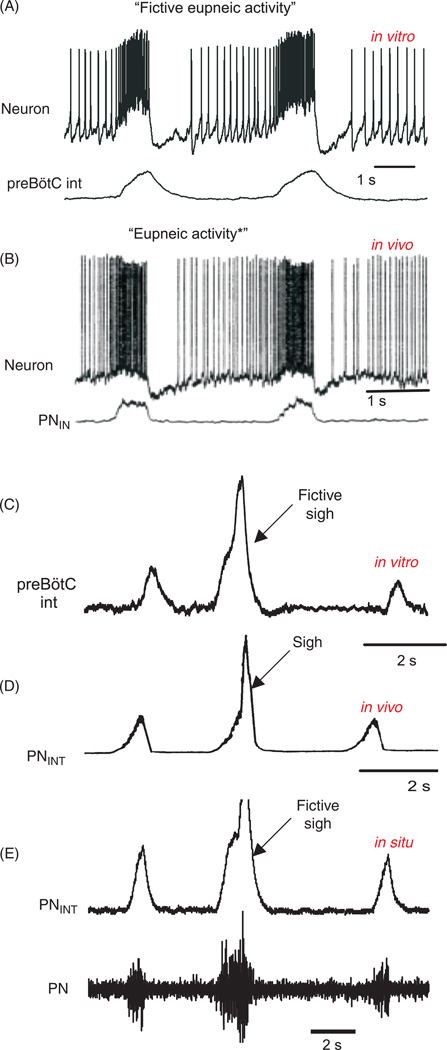
The isolated preBötC generates three physiologically distinct respiratory activity patterns. These activity patterns are similarly seen in vivo and in the so-called working heart brain-stem preparation, and are referred to as normal respiratory activity pattern (or eupneic activity, Figs. 1B, 2A,B), sigh-activity pattern (Figs. 1B, 2C-2E), and gasping pattern (299). Mapping the pattern of these three activities reveals a complete anatomical overlap within the preBötC (Fig. 1B). The ability to isolate the preBötC in a functional manner provided rigorous insights not only into the cellular mechanisms that are critical for rhythm generation but also the mechanisms that govern the reconfiguration of the respiratory network to generate the three different activity patterns under different metabolic conditions. These in vitro studies were paralleled by studies performed in a variety of in vivo preparations. The first demonstration that lesioning of the preBötC indeed abolishes breathing in vivo (440), was followed by numerous studies that continue to confirm its importance for various aspects of breathing in health and disease (36, 184, 194, 334, 335, 337, 432, 489, 520, 537, 544, 545, 555, 594). However, it must be emphasized that the preBötC is embedded in a larger neuronal network that is distributed throughout the nervous system. Thus, brainstem respiratory neurons are not only present within the preBötC but different types of cellular properties found in different brainstem areas contribute to different aspects of the breathing rhythm, as well as chemosensation (67, 98, 145, 168, 195, 197, 217, 236, 237, 277, 336, 360, 463, 515, 528). Selective lesion experiments and the ability to preserve specific respiratory functions following experimental isolation have helped not only to better define the role of the preBötC, but there are also various other areas that have benefited from in vitro approaches. For example, the experimental isolation of areas such as the retrotrapezoid nucleus (RTN) (193, 196, 284), the locus ceruleus (LC), (13, 133, 370, 620, 621), raphe nucleus (87, 586), and the nucleus tractus solitarius (NTS) (85, 96, 370) have helped us to gain insights into the cellular mechanisms contributing to chemosensation. One important next challenge will be to understand how these cellular properties are integrated into a larger network, to understand how breathing behavior with all its complexity and adaptability is produced. In this review, we will discuss the cellular properties that are known to be critical for various aspects of breathing, based on studies in various in vitro and in vivo preparations. These studies have not only provided important insights into the neuronal control of breathing, but they have also led to the formulation of principles that govern network interactions and behavioral control in general. It is these general implications that make the respiratory network a great model for understanding how the nervous system generates behavior. Thus, we hope that this review can provide insights that are not only of interest for those working in the field of breathing, but also for anyone interested in neuronal network functions and behavior in general.
Figure 2.
Simultaneous intracellular whole cell recordings and integrated extracellular population recordings from pre-Bötzinger (preBötC) respiratory neurons (A) in vitro from a mouse brain slice exhibiting “fictive” eupneic activity and (B) in vivo from an anesthetized rat, during “eupneic activity” (modified, wih permission, from reference 490). (C) Integrated population recordings in vitro of a fictive sigh recorded with a surface electrode from the preBötC in a mouse brain slice and (E) from a working heart-brainstem preparation (WHBP). (D) A sigh recorded in vivo from the phrenic nerve (PN) from an anesthetized cat (modified, with permission, from reference 76).
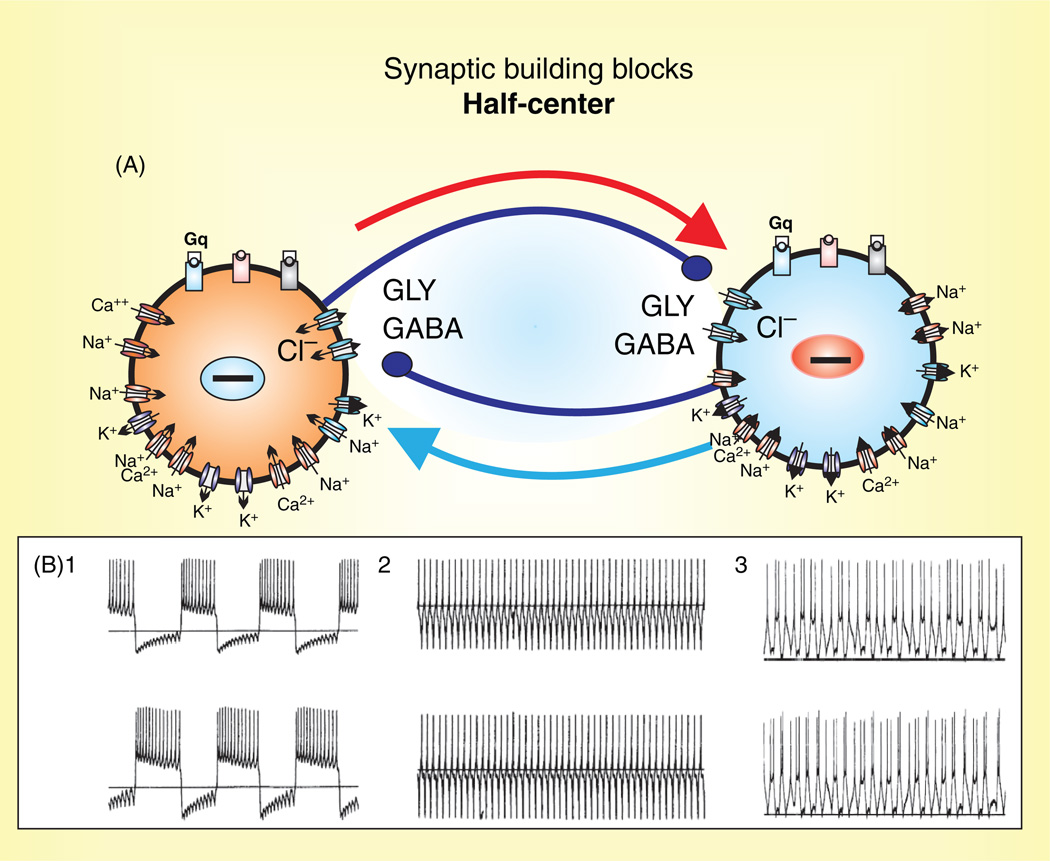
The Building Block Hypothesis for Generating Respiratory Network Activity
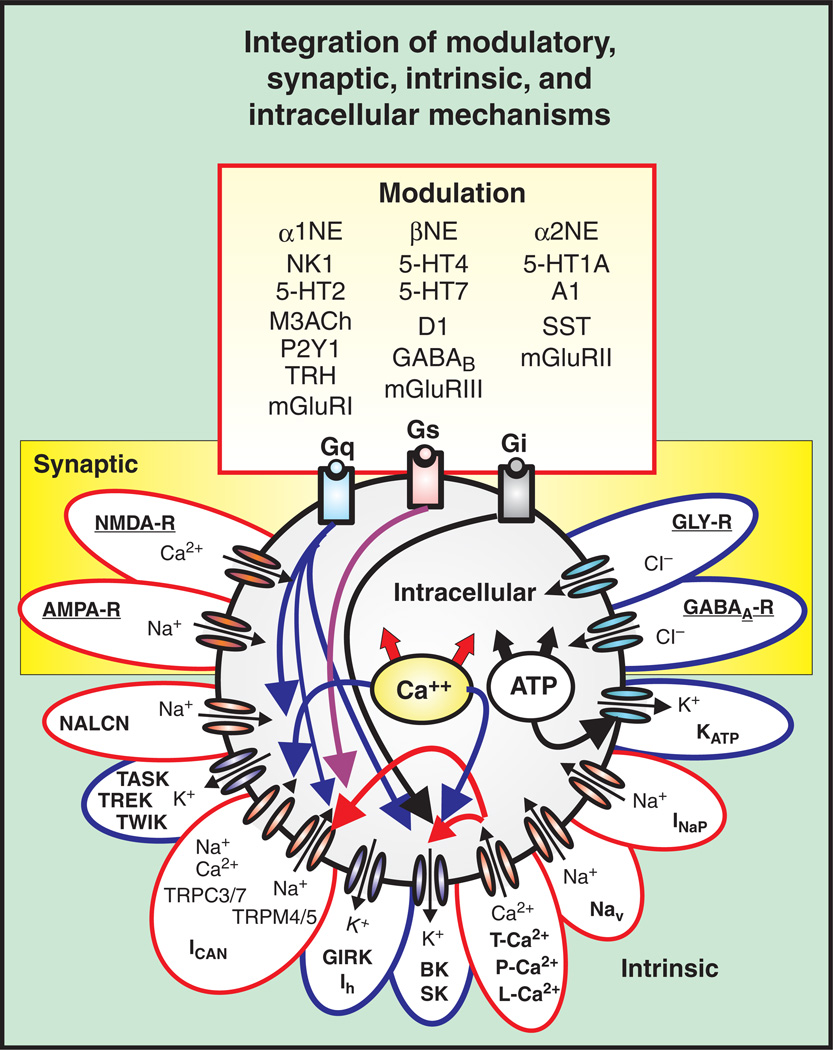
The search for common principles that underlie the generation of activity patterns, of complex behaviors and processes such as sensori-motor integration, and of learning and memory has led to the important realization that neuronal networks are not hard-wired. The respiratory network is no exception. Synaptic, cellular, and modulatory processes that define network functions are continuously changing and homeostatically regulated. The availability of modern tools and initiatives that may allow the identification of all synaptic connections in a “connectome” of the brain will provide important insights into the architecture of neuronal networks (294, 295, 526, 527). But understanding the wiring of neuronal networks is just one aspect that defines a functioning network. A network connection is not just excitatory or inhibitory; it is also defined by its iono- and metabotropic mechanisms, its many pre- and post-synaptic ionic currents, its surface receptors, and its complex intracellular mechanisms that are dynamically regulated in an activity-, state-, time-, and context-dependent manner. In this scenario, reciprocally organized inhibitory connections, recurrent excitation, persistent sodium currents, calcium activated nonspecific cation currents, neuromodulators acting on G-protein coupled receptors, and second messenger pathways interacting intracellularly are all building blocks that are dynamically arranged in many different ways to produce a network output. Similar network outputs can be assembled by many different combinations of these building blocks. Removing any of these building blocks may alter the overall output. The network will continue to operate in a manner that is difficult to predict, since these building blocks are not simple “Lego” blocks that have different colors and forms. These building blocks follow many nonlinear rules with regard to the effects that they exert and the conditions during which they are activated. These ideas are by no means novel. More than 20 years ago, Peter Getting wrote a very influential review on the emerging principles governing network operation, and he spelled out the concept that synaptic and intrinsic properties need to be considered as building blocks of a neuronal network (173). The review by Peter Getting is still as relevant today as it was 20 years ago. What has changed is the increased understanding of the molecular and cellular determinants of these building blocks. The respiratory network is a good example for which there has been an explosion in our understanding of the cellular properties of respiratory brainstem neurons. Yet, our understanding of how these properties interact to form a breath is still limited. While it is relatively easy to synthesize the available information into a framework that could explain how the respiratory network generates the respiratory rhythm, the emerging picture will always be incomplete and may even be misleading. But, it seems obvious that the respiratory network does not depend on only one building block, but rather on multiple intrinsic, synaptic, intracellular, and modulatory processes that interact with each other (Fig. 3). Figure 3 can only provide an overview of some of the cellular mechanisms involved in the neuronal control of breathing. A more complete account of the mechanisms involved will be given in the context of this review. But, it is important to emphasize that the combination of these building blocks is not always the same, and different configurations define different states of the respiratory network. Moreover, even the simplest behavioral state is not determined by only one network state. For example, the apparently simple “gasping” state is the result of different network states, as is suggested by the different types of known gasping behaviors (180). Similarly, what is defined as “eupneic breathing” is not generated by a fixed network state, but may differ from species to species and preparation to preparation, causing much confusion in the field of respiration (435). The “eupneic” breaths of a cat, rat, or human, of a neonate or adult, of a mammal living at low or high altitude will all differ. Even within the same species there are individual differences in eupneic breathing and the same individual will not generate every eupneic breath in the same manner.
Figure 3.
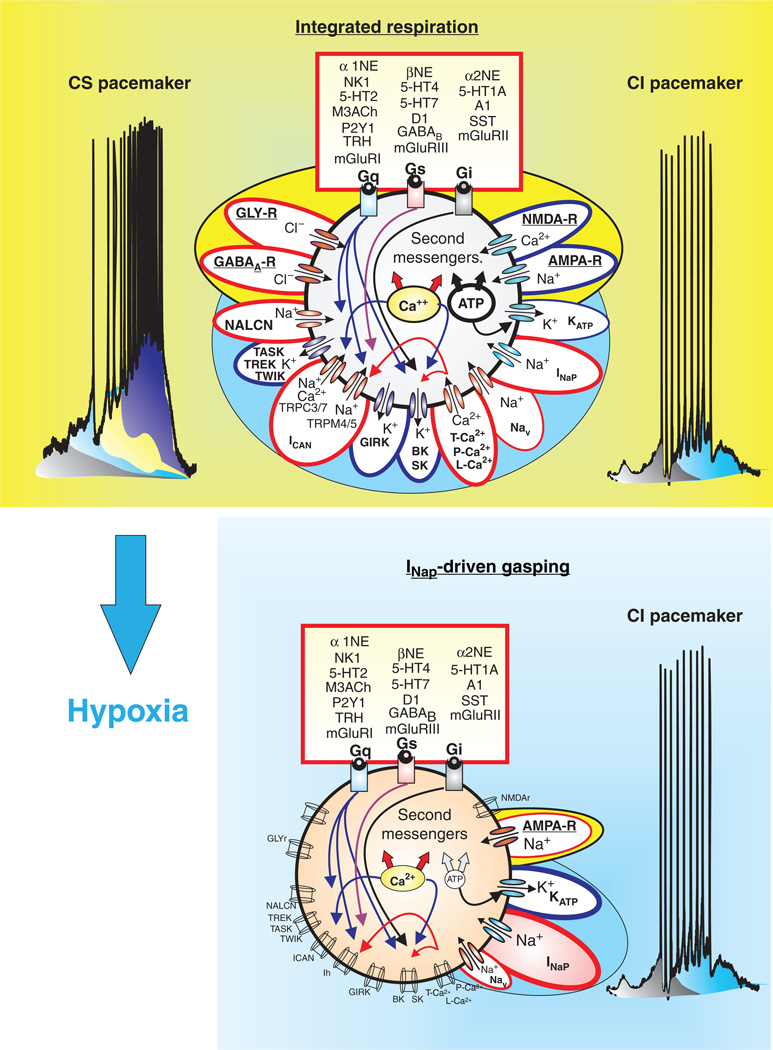
Putative inspiratory neurons of the preBötC integrate modulatory, synaptic, intrinsic, and intracellular mechanisms that give rise to bursting. Modulation commonly involves a cascade of mechanisms mediated through metabotropic glutamate receptors (mGLUR-I, II, III), noradrenergic receptors (α1, α2, and β-NE), serotonergic receptors (5HT-1A, 2, 4, and 7), peptidergic (NK1-R), and purinergic receptors (P2Y1) that represent G-protein-coupled protein receptors (Gs, Gi, and Gq) and act on various intracellular signal transductions. Synaptic mechanisms involve ionotropic glutamatergic (AMPA-R, NMDA-R), GABAergic (GABAA-R), and glycinergic receptors (GLY-R) that rapidly change membrane potential and hence, neuronal excitability when activated. Intrinsic mechanisms refer to other membrane conductances that are not strictly synaptic, but also regulate neuronal excitability. These include, but are not limited to: (1) leak conductances, (TREK, TWIK, TASK, and NALCN); (2) calcium conductances (T, P, and L-Ca2+); (3) K+ conductances (KATP, BK, SK); (4) sodium conductances (Nav, INaP); and nonspecific cation conductances, ICAN (TRPC-3, 7, and TRPM-4,5). Intracellular mechanisms refer to the molecules and ions regulating intracellular signaling cascades and ultimately lead to changes in excitability. For example, Ca2+ and ATP influence neuronal excitability through indirect mechanisms affecting the conductances generated through various channels such as the KATP, and ICAN. Blue outlines represent hyperpolarizing conductances, while red outlines represent depolarizing conductances.
From a historical perspective, our understanding of neuronal network function has been driven by numerous debates over the most important principles or building blocks that determine network functions and behaviors. Whether the field of respiration received more than its fair share of divisive debates compared to other fields of network functions is irrelevant, but there have been many debates beginning with the discussion of central pattern generators (CPGs) versus chains of reflexes (182, 183, 506, 507), the debates over the importance of reciprocal synaptic inhibition versus recurrent synaptic excitation, pacemaker versus network properties, in vitro versus in vivo (467) persistent sodium current versus calcium-activated nonspecific current (CAN) (390), and individual pacemakers versus group pacemakers (106). Perhaps it is time to accept the conclusion that networks are not functioning based on single mechanisms, and that a behavior, even if it looks the same, is not the result of a single network configuration (422), that networks depend not only on CPGs, but also on numerous interacting reflexes, and that they involve persistent sodium and CAN currents, and single and group pacemakers. If we can lay these debates aside, we can begin to better appreciate the dynamic principles that govern not only the respiratory network and breathing, but also all networks and behaviors in general. In this review, we will describe many of the cellular properties that constitute some of the building blocks that govern the respiratory network. We will also discuss some of the interactions that make this network so dynamic and adaptable.
Determinants of Autonomous Activity and Excitability
Overview
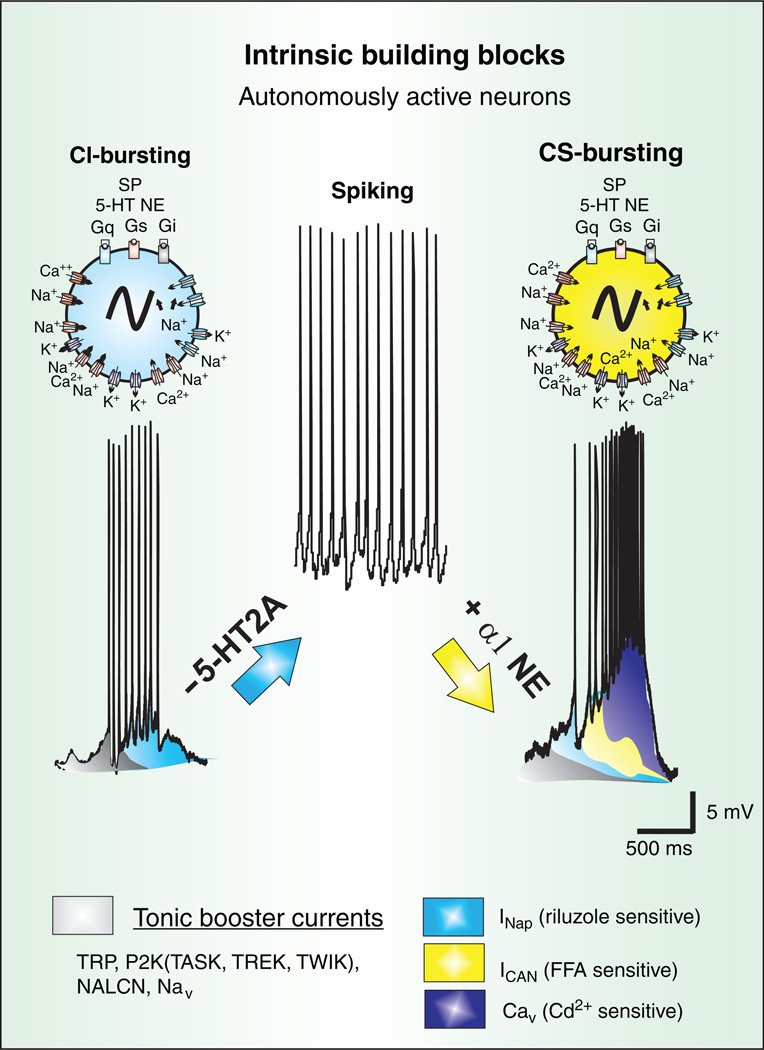
The ability of respiratory brainstem neurons to autonomously (=intrinsically) generate action potentials (=spiking) and bursting has received considerable attention in the field of respiration (243, 434, 442, 516) (Fig. 4). Yet, autonomous discharge properties are not a unique feature of the respiratory network. Autonomous neuronal activity is found virtually everywhere in the nervous system, including the neocortex, subthalamic nucleus, amydgala, nucleus basalis, globus pallidum, LC, raphe nuclei, hippocampus, inferior olive, thalamus, suprachiasmatic nucleus, substantia nigra (SNc), ventral tegmental area, and the cerebellum (57, 68, 95, 115, 147, 181, 249, 251, 304, 394, 414, 430, 431, 458, 472, 543, 574). Indeed, autonomously generated activity provides an important intrinsic drive for many behavioral functions, a concept that was first proposed more than a century ago (182, 183). Intrinsic activity is now considered as an important driving principle, not only for all rhythmic motor patterns including breathing, but is also critical for the generation of higher brain functions (31, 206, 279, 429, 617). Indeed, accepting that the brain is an intrinsic activity generator has provided novel ways to characterize the neuronal substrate of complex behaviors and to explain and diagnose the neuronal basis of many neurological disorders (188, 355, 524, 596, 617). Thus, understanding the cellular properties that underlie the intrinsic activity that gives rise to respiratory activity has implications that go far beyond its immediate relevance for breathing. Conversely, the intrinsic ionic currents involved in the generation of the respiratory rhythm play critical roles, not only within the respiratory network, but also in driving other networks throughout the nervous system.
Figure 4.
Autonomously active neurons are part of the spectrum of intrinsic building blocks responsible for rhythmic population bursts in the preBötC. At the cellular level, a diversity of mechanisms exist to produce several forms of bursting that have different pharmacological properties. Some neurons express cadmium-insensitive bursting (CI) that is riluzole sensitive, and therefore, appears to be mediated by INaP (blue). Other neurons express cadmium-sensitive bursting (CS), predominantly mediated by both voltage-dependent Ca2+ currents (Cav) and a FFA-sensitive ICAN. Moreover, pharmacological blockade of 5HT2A receptors can turn CI-bursting into tonic spiking while activation of a1-noradrenergic receptors in tonic spiking neurons into CS-bursting. This type of conditional bursting demonstrates that the role of autonomously active neurons in the preBötC may not be fixed. Hence, while both forms of bursting, CI and CS, depend on dominant current(s) to drive spontaneous bursting, all putative inspiratory neurons of the preBötC appear to possess tonic boosting currents (grey), the INaP (blue), ICAN (yellow), and Cav (purple).
Neuronal activities are commonly defined as “autonomous” if these activities do not require synaptic input to be generated (434, 539). According to this nomenclature, autonomously active neurons are also referred to as pacemakers. Among the autonomously active neurons there are two general types: (a) neurons that are generating intrinsically spiking activity (i.e., are not silent) (434, 539). These neurons will be referred to as autonomous spiking pacemakers (539). (b) Neurons that are capable of bursting in the absence of synaptic input, which will be defined as autonomous bursting pacemakers (213). In this review, we will make no distinction between the term “intrinsic” or “autonomous.” Both terms indicate that this activity pattern emerges as a cellular property within an individual neuron without the need of extrinsic drive. But, there are several considerations that are important to emphasize.
Autonomously bursting and spiking neurons may not constitute different neuron types
Autonomously bursting and spiking may characterize different activity states of the same neuron (Fig. 4). The biophysical mechanisms underlying the dynamic transitions between spiking and bursting have been extensively studied in other model networks using a variety of computational approaches (91, 509, 522). Within the respiratory system as well as other known rhythm generating networks, neuromodulators play critical roles in transforming spiking into bursting neurons and vice versa (442, 578). Specific examples within the respiratory network are neurons that transition from a bursting into an autonomously spiking state following the blockade of endogenous 5-HT2A receptor activation (405) or that transition from autonomously spiking to bursting in the presence of norepinephrine (NE) (578) or substance P (405). The role of thyrotropin-releasing hormone (TRH) in inducing bursting has been elegantly demonstrated in the NTS (99).
Autonomously active and silent neurons may not constitute different neuron types
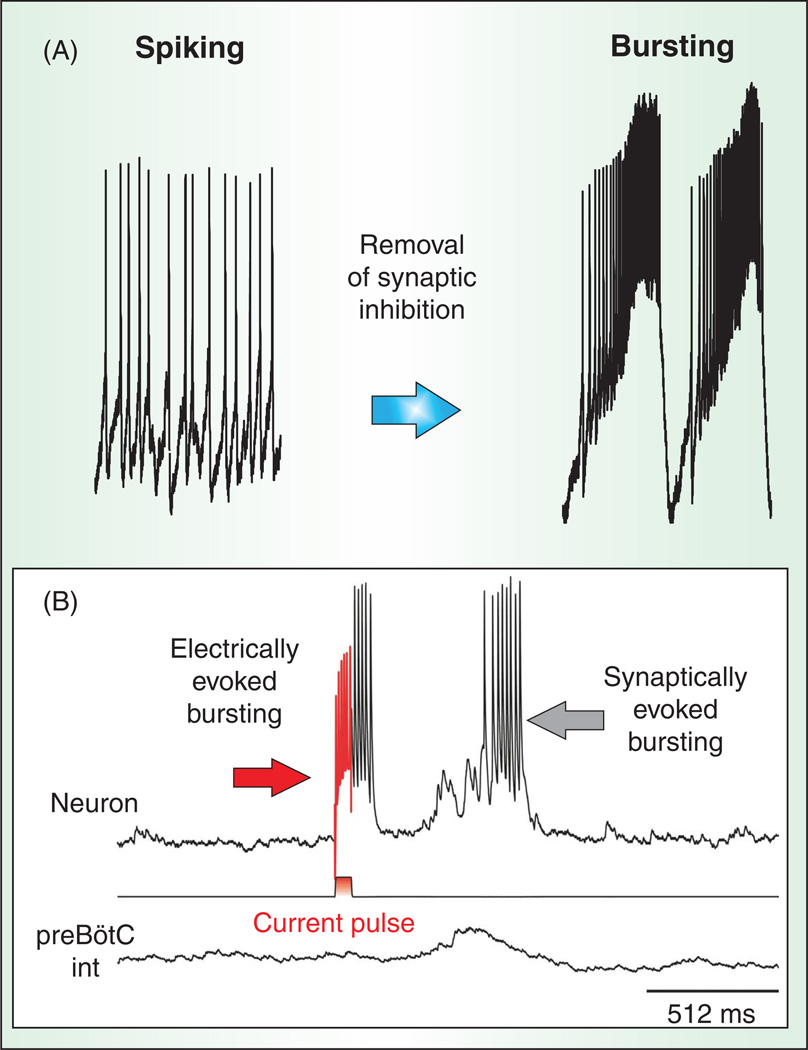
Again, computational models and various experimental approaches can demonstrate how neurons transit into the different states of activity (522). Whether a neuron is autonomously active or silent depends on various factors, including the types of modulatory inputs, but to a large degree also on the details of the synaptic inputs. Depending on the amount and type of inward current or the balance between inward and outward currents, a neuron may not be sufficiently depolarized in its resting state to be autonomously active in the absence of synaptic input. For such a neuron, synaptic excitatory inputs are required to drive the membrane potential into the voltage range that will activate a burst or spiking activity. But this is also the case when bursting neurons are embedded into the functional network. In this case, synaptic inhibition can suppress autonomous bursting, and synaptic excitatory input is required to activate bursts [Fig. 5; (434, 564)].
Figure 5.
Synaptic modulation of bursting properties. (A) Pharmacological removal of synaptic inhibition can alter the propensity of a respiratory neuron to switch from a tonic spiking into a bursting mode. (B) In a simultaneous intracellular and population recording, a burst of action potentials can be triggered in the cell by a brief positive current injection (rd) or by synaptic input occurring during the population burst.
Synaptic isolation is an experimental tool to characterize activity state
It is important to emphasize that synaptic isolation is only an experimental tool to better define the mechanisms that underlie bursting and spike generation (554). The fact that pacemaker neurons were more frequently identified within the in vitro network than in the in vivo network has no functional implications. Irrespective of the in vitro or in vivo condition, in the intact network these pacemaker mechanisms will be integrated in the synaptically active rhythmic network (Fig. 5). As will be discussed in more detail in later sections, the cellular mechanisms that underlie pacemaker bursting and autonomous spiking will regulate synaptic activity and in turn are regulated by synaptic activity.
The same types of ionic currents can lead to autonomous bursting, spiking, or silence
A neuron’s activity is determined by the balance of its inward and outward currents and different ratios of the same currents can generate different neuronal activities. Any modulatory change in the ratio of inward and outward currents and any synaptically evoked change in a neuron’s membrane potential will alter the propensity to burst and transform an autonomously bursting neuron into a silent neuron or vice versa (306). Thus, the same neuron can autonomously burst under certain conditions, but it may require synaptic activation in other conditions. Moreover, conditions that alter the degree of synaptic inhibition will affect the ability of the neuron to autonomously burst (Fig. 5). Changes in the ratio of inward and outward currents can not only be caused acutely via neuromodulators or synaptic inputs, but it is likely that it could also be changed in a long-term manner through various forms of plasticity that includes homeostatic plasticity and long-term facilitation. However, such long-term effects on the propensity to burst have not been studied in the respiratory network, but they are well known in other systems (261).
Autonomously bursting neurons in the functional network
First described in in vitro preparations, autonomously spiking and bursting respiratory neurons (103, 268, 338, 404, 554) have also been observed in the so-called in situ preparation (531). However, it remains unknown whether autonomously bursting neurons also burst in the intact alert animal. Bursting may be suppressed and/or enhanced by synaptic and/or neuromodulatory inputs. The fact that this is still unknown should not imply that bursting does not exist in intact animals. Unfortunately, demonstrating bursting in intact animals will continue to be challenging, since synaptic isolation of a neuron from an area as critical as the preBötC will inevitably lead to the death of the animal (440, 544). Yet, irrespective of the uncertainty as to whether respiratory neurons burst more strongly or more weakly in the intact animal, the types of inward currents that are acutely identified in pacemaker neurons under in vitro conditions are likely also present in the intact animal. These inward currents will interact with synaptic and modulatory inputs, following principles that can in part be studied more rigorously in reduced preparations.
Two types of bursting mechanisms in the preBötC
In neurons of the respiratory network, two types of inward currents have received considerable attention (554): the persistent sodium current (INaP) and the CAN cation current (ICAN). Both currents give rise to autonomous spiking and bursting activity and both currents interact with synaptic and modulatory inputs, which in the functional network contribute to respiratory rhythm generation. The two inward conductances significantly differ, not only in their activation and inactivation properties, but most likely also in their contributions to the formation of the respiratory rhythm (578). Pharmacological approaches help to better define how these conductances contribute to generation of autonomous and synaptically evoked bursting. Neurons in which bursting depends on ICAN stop bursting when exposed to flufenamic acid (FFA), lanthanum (105, 404) or cadmium, a blocker of calcium currents (554). Because of this pharmacological approach, these neurons are referred to as “cadmium-sensitive” (CS) pacemaker neurons (554). Neurons that rely on INaP to generate bursts continue to do so even in the presence of cadmium and are, therefore, called “cadmium-insensitive” (CI) pacemaker neurons (554).
It is important, however, to emphasize that this pharmacological approach identifies only the inward current that is critical to promote bursting in a given neuron. This does not imply that this inward current is the only inward current that is important in a particular neuron. In fact, the majority of respiratory neurons most likely possess both conductances (103, 105). Thus, CS pacemakers cease to burst following the blockade of ICAN because the unblocked inward currents are not sufficient to promote intrinsic bursting, not because these neurons possess no other inward currents. Figure 4 proposes a cascade of different ionic currents contributing to the generation of bursting in these neurons. Indeed, following the blockade of ICAN 22% of CS pacemakers continue to autonomously generate action potentials (404), most likely because of the presence of INaP. Moreover, both inward currents very likely interact closely with each other: within the same neuron, as well as within the population of neurons that are involved in the generation of the respiratory rhythm as proposed by modeling studies (558). Mounting evidence from in vitro and in vivo preparations indicates that respiratory activity persists following the blockade of INaP and ICAN alone, and ceases only when both mechanisms are blocked (401, 404). Yet, the concept that both inward currents interact and together are critical for respiratory rhythm generation, is not shared by all laboratories in the field. Some laboratories emphasize INaP-dependent mechanisms (265), while others emphasize ICAN-dependent mechanisms as the driving principle of rhythm generation within the preBötC (105, 392). Based primarily on computational modeling, some believe that INaP is essential only in the in vitro, but not in the in vivo network and they dismiss the ICAN altogether (464), while others question the necessity of the INaP in the in vitro network, attributing rhythm generation primarily to ICAN-dependent mechanisms (105, 392). Early characterizations of inward currents in the functional in vivo respiratory network provided convincing evidence that INaP plays a role in amplifying synaptic drive potentials in medullary respiratory neurons of the intact network (341). Moreover, chelating intracellular calcium as well as elegant voltage- and current-clamp studies showed that calcium-dependent mechanisms that include low- and high-voltage-activated calcium currents and calcium-dependent potassium currents contribute significantly to the shape of ongoing drive potentials and are, therefore, likely to be critical for rhythm and pattern generation (419, 455). Thus, there are many different inward conductances that serve as building blocks of the respiratory network (Fig. 3).
Concluding remarks
The intrinsic firing properties of respiratory neurons are determined by a combination of various distinct inward and outward currents, leading to the phenotypes of silent, autonomous spiking, or autonomous bursting neurons. However, these firing properties are not fixed, but rather are constantly modulated by multiple mechanisms. In the functional network synaptic, intrinsic, and neuromodulatory mechanisms act in concert to promote the generation of respiratory activity. Understanding these intricate interactions requires a thorough understanding of the underlying cellular and molecular properties, as will be discussed in the following sections.
The Inward Conductances Within the Respiratory Network
This section will describe in more detail the physiology, pharmacology, and molecular biology of the inward currents that have been extensively studied within the respiratory network: the voltage-dependent sodium currents, nonselective cation “leak” currents, calcium currents, and calcium-dependent cation currents. Although these currents are described separately, we would like to reemphasize that this should not imply that they also function in separation. There is ample evidence indicating that all three types of inward currents are important building blocks in the generation of the respiratory rhythm.
The persistent sodium current (INaP) and its role in regulating neuronal excitability
Overview
Voltage-dependent sodium currents (Nav) play critical roles in the generation of intrinsic excitability and information processing in general. These currents are important contributors to the autonomous generation of action potentials and bursts of activity. Thus, not surprisingly, these currents have received considerable attention, not only in the field of respiratory control, but also in most areas that deal with intrinsically active neuronal networks.
Molecular biology
Nav channels are composed of a pore-forming α-subunit and one or two associated β-subunits (63, 64). Although nine functional types of α-subunit mRNAs have been identified, only five are expressed within the central nervous system (NaV1.1, NaV1.2, NaV1.3, NaV1.5, and NaV1.6) (113, 175, 176). Single cell RT-PCR reveals the expression of multiple NaV transcripts in acutely dissociated preBötC neurons from P0-P15 neonatal rats (NaV1.1, NaV1.2, and NaV1.6) (424). Because INaP likely results from modal gating produced by conventional Nav channels, any of these transcripts may potentially underlie INaP in the preBötC. Four different types of β-subunits are expressed in various combinations in different neurons (611, 613). They modify the biophysical and pharmacological properties of the α-subunit and thus seem to play important functional roles (38, 232). Yet, little is known about the role of β-subunits within the respiratory network. In the neocortex, mutations in the β1-gene (SCNA1B) have been associated with epilepsy (580) and the β-subunits seem to confer insensitivity to antiepilepsy drug therapy (567). The cytoplasmic tail of NaVβ4 acts as an endogenous blocking protein that delays Nav channels from entering persistent fast-inactivated states by rapid, unstable binding upon activation and unbinding at negative voltages, resulting in a “resurgent” sodium current upon repolarizations. This resurgent sodium current may mediate rapid repetitive firing in some neurons (8, 16).
Physiology
Nav currents exhibit two distinct inactivation properties. The fast transiently activated Nav current provides the initial depolarization of action potentials, while the noninactivating low-voltage-activated persistent Nav current (INaP) gives rise to autonomous spiking and bursting. The INaP contributes to the generation of intrinsic activity in neurons distributed through-out the nervous system (44, 109, 542), and is typically activated at around −60 mV, reaching its peak current amplitude at −40 to −20 mV (7, 60, 103, 250, 321, 328, 424, 574). However, the voltage-operating range within which INaP is active may be extended to even more negative membrane potentials (opening at −80 mV) in some preparations, such as the presynaptic terminals at the Calyx of Held (221), offering the possibility of a larger influence of INaP at subthreshold potentials in more situations than were previously appreciated.
Due to its role in generating autonomous bursting, INaP has been extensively studied in the respiratory network (51, 103, 265, 465). Ptak and colleagues (424) found that the peak persistent sodium conductance, current density, and input resistance of preBötC neurons were greater than in neurons isolated from the neighboring region of the rostral VRG. The properties of INaP can explain many of the discharge characteristics of autonomously active respiratory neurons. The voltage dependency of the INaP can, for example, explain why the same neuron can assume different autonomous activity states. As demonstrated experimentally and computationally, increasing INaP density transitions a neuron from a silent to a bursting state and from a bursting into an autonomously active spiking state (51, 103, 554). Moreover, INaP density is greater in autonomously bursting neurons when compared to non-bursting neurons (103). However, it must be emphasized that the activity state does not necessarily depend on the absolute persistent sodium current density, but rather on the balance between INaP and outward leak currents (103, 265, 425).
Pharmacology
INaP can be blocked with riluzole (568) and low concentrations of TTX (160), which abolishes bursting in the majority of CI bursting pacemaker neurons. However, even with a bath concentration of 20 to 50 µmol/L riluzole plus 200 µmol/L cadmium, 29% of CI pacemaker neurons continue to burst (404). This means that there is currently no pharmacological tool available that blocks all autonomously bursting pacemaker neurons in slice preparations. This has the important, yet often overlooked, implication that no pharmacological approach in slices can prove that pacemaker neurons are not essential. Moreover, even though riluzole blocks 71% of CI pacemakers more than half of them (59%) continue to spike autonomously in the presence of riluzole. Thus, while riluzole reduces the number of bursting pacemakers, this substance can neither block bursting altogether, nor can it eliminate the impact of autonomously generated activity on driving network activity (404). Thus, it is not surprising that focal bilateral microinjection of riluzole into the preBötC fails to block respiratory rhythmic activity (390).
Another reason why it is difficult to test whether INaP is obligatory for respiratory rhythm generation is the challenge to experimentally separate the transient and persistent sodium currents. Riluzole modulates both of these components to approximately the same degree (424), but preferably binds to and stabilizes NaV channels in late closed-state conformations, and thus can be used as a pharmacological tool to only preferentially block INaP, while minimizing the block of transient sodium currents. Yet, while riluzole leads to a relatively greater reduction of the persistent (INaP) versus transient sodium current component, there cannot be a selective blockade of INaP alone. The ratio between the blocked persistent versus blocked transient sodium current will be concentration dependent. This is particularly complicated in a slice preparation in which riluzole needs to reach its neuronal target via diffusion. Thus, pharmacologically applied riluzole (as well as TTX) results in a spatially and temporally nonuniform pharmacological attenuation of INaP (265). This could explain why in slices respiratory rhythmic activity persists when riluzole is applied alone. Interestingly, one advantage of the respiratory network isolated in the in situ preparation is that substances can reach their neuronal targets not just by diffusion, but also via the artificial cerebrospinal fluid (CSF) that is infused into the nervous tissue through the still working heart and blood supply. Thus, limited diffusion is not an issue in this preparation. Importantly, this in situ preparation is exquisitely and consistently sensitive to riluzole applications (578). However, this finding seems to not be consistent with a study by St-John et al. (532). These authors found that riluzole does not block eupneic activity.
NALCN may be a significant component of the leak inward current within the preBötC
Another likely source of persistent inward cation current in the preBötC was recently revealed through a series of elegant studies, primarily from the laboratory of Dejian Ren at the University of Pennsylvania, combining molecular cloning and mouse molecular genetics. Lu and colleagues (315) reported the cloning and functional expression of a sodium-leak-channel (NALCN) channel, an unconventional member of the extended 4-domain NaV/CaV gene family, which encodes a TTX-insensitive, nonvoltage-activated, nonselective cation channel. NALCN is evolutionarily conserved, with clear orthologs in Drosophila (α-1U) (302) and Caenorhabditis elegans (nca-1, nca-2) (518). Mutations of these orthologs in Drosophila and C. elegans result in behavioral phenotypes consistent with altered neuronal excitability and susceptibility to volatile anesthetics (367, 609). Following heterologous expression in HEK293 cells under bi-ionic recording conditions, NALCN produced constitutive currents with unusual ionic selectivity, conducting Na+, K+, and Cs+ relatively indiscriminately, and to a lesser extent Ca2+ [PNa(1.3) > PK(1.2) > PCs(1.0) > PCa(0.5)] (315). This current exhibited unusual pharmacological properties, atypical of voltage-dependent NaV or CaV channels. TTX (10 µmol/L) and several conventional organic CaV blockers (Nifedipine 100 µmol/L; dialtizem 1 mmol/L) failed to block NALCN currents. In addition, neither Ni2+ (1 mmol/L) nor La3+ (100 µmol/L) blocked NALCN. However, significant block (80%) was observed with Gd3+ (10 µmol/L), verapamil (1 mmol/L), Cd2+ (1 mmol/L), and Co2+ (1 mmol/L) (315). The sensitivity of NALCN to riluzole has not been reported. Significantly, targeted null mutations of NALCN in mice resulted in animals that died within 24 h after birth, due to an elevated rate of prolonged apneas (> 5–10 s), and, ultimately, respiratory failure (315). En bloc recordings from C4 phrenic nerves of isolated brainstem-spinal cord preparations revealed a 6-fold reduction of expiratory burst frequency in homozygous NALCN KO pups compared to wild type (WT), consistent with a central component to this respiratory defect. A central neuronal defect was further supported by recordings from WT and NALCN KO hippocampal neurons, which showed that NALCN underlies a native persistent inward current, which contributes a approximately 10 to 20 mV depolarization to WT resting membrane potentials (315).
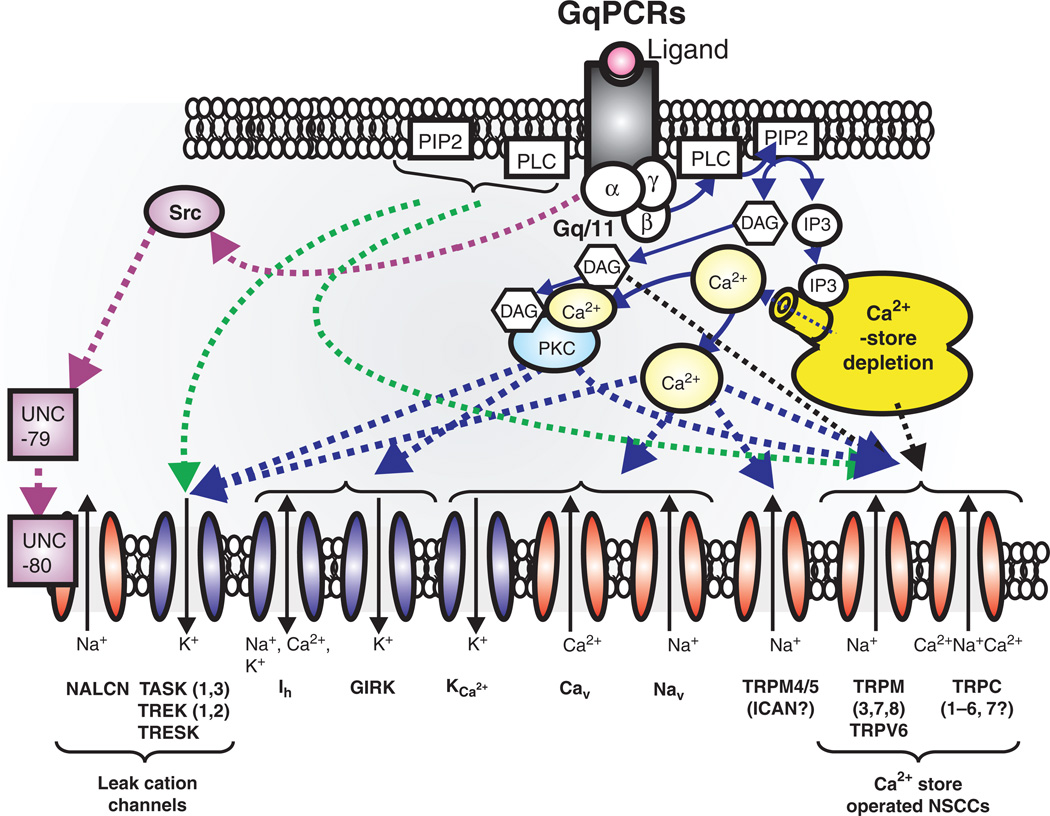
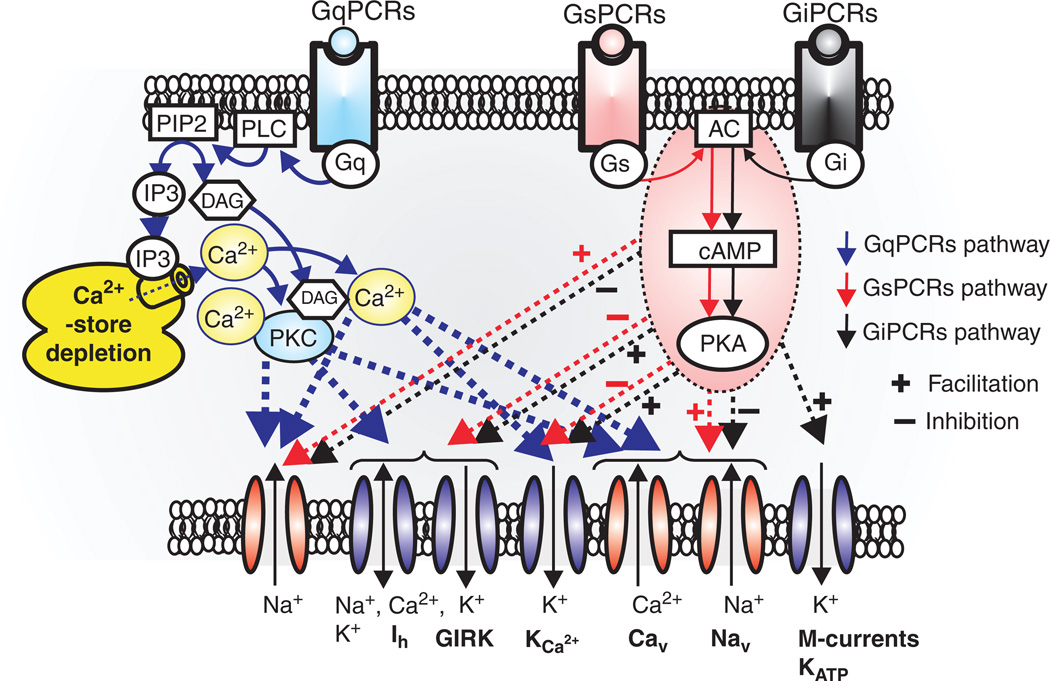
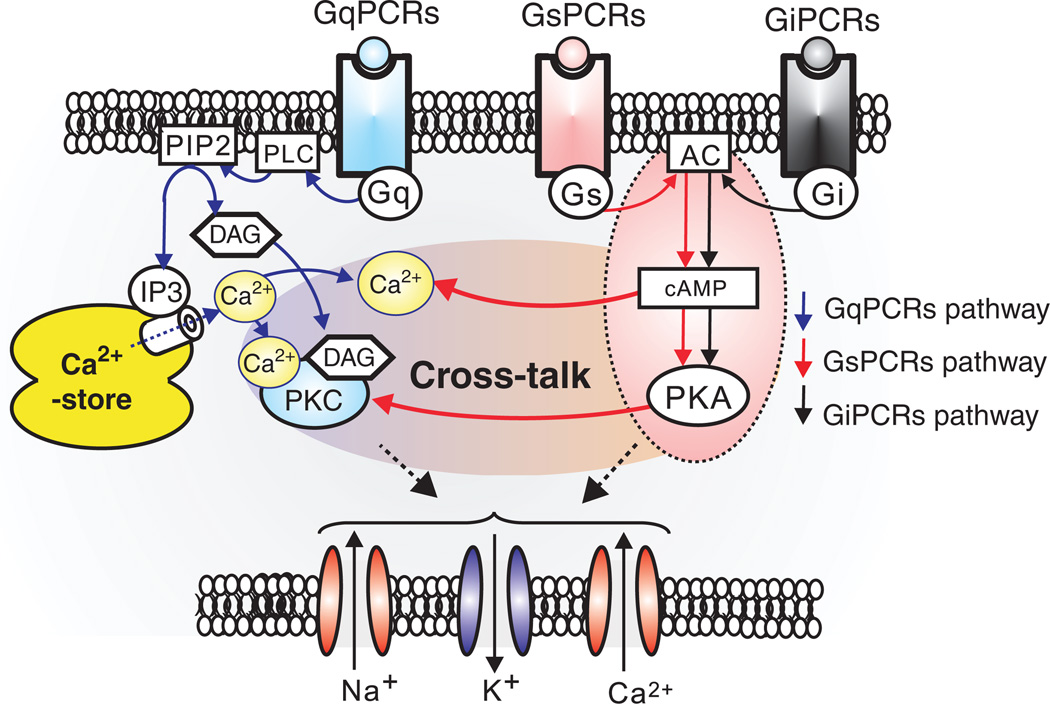
As will be discussed in more detail below, neuromodulation is critical for sustaining respiratory rhythms in the preBötC (186) and regulating the state dependence of the respiratory neural network. Interestingly, NALCN activity is profoundly regulated by several G-protein coupled receptors (GPCRs), including the Neurokinin-1 receptor (NK1) that binds Substance P, the M3 muscarinic receptor, and the CaSR receptor that senses extracellular Ca2+ (315, 316) (267, 540). Activation of NK1 or M3 receptor greatly augments NALCN currents through an unconventional signaling pathway that utilizes a Src family kinase (SFK), instead of heterotrimeric G-proteins. This signaling pathway is absolutely dependent upon the assembly of the NALCN channel protein with UNC-80, a conserved intracellular scaffolding protein first discovered in C. elegans (491), into a functional signaling complex (316, 540) (Fig. 15). By contrast, activation of CaSR by normal levels of extracellular Ca2+ (2.0 mmol/L), signals to inhibit NALCN through a conventional G-protein pathway, but again requires the assembly of NALCN with UNC-80 and a second related scaffolding protein, UNC-79 (317) (Fig. 15). Significantly, UNC-79 KO mouse lines also die perinatally, within 48 h of birth, and exhibit the same symptoms of respiratory failure as NALCN KO mice, although less severe. Independently, a forward genetic screen in mice also identified a dominant mutant allele of unc-79 (Lightweight) that dies perinatally as homozygous mutants. When assayed as heterozygous adults, these mutants exhibit altered sensitivity to acute isoflurane anesthesia and ethanol intoxication (525).
Figure 15.
GqPCRs regulates not only voltage-dependent cation channels, but also transient receptor potential (TRP) and leak cation channels. GqPCRs-induced depletion of Ca2+-store facilitates activation of Ca2+-store operated NSCCs, such as TRPC (1–6, 7), TRPM (3, 7, 8) and TRPV6. On the other hand, TRPM4/5 may be directly activated by elevation of internal Ca2+ concentration (300, 576). GqPCRs seem to modulate activity of K2P (TASK, TREK, and TRESK) and sodium-leak-channel-nonspecific (NALCN) channels. In particular, GqPCRs act through Src protein controls NALCN channels through both UNC-79 and UNC-80 (316, 317, 491, 540). Abbreviations; P2K (“two-pore” potassium channels, TASK, TREK, and TRESK channels), TRPM (transient receptor potential melastatin), TRPV(transient receptor potential vanilloid), TRPC (transient receptor potential canonical), Ca2+ store operated NSCCs (nonselective cation currents), and Src (sarcoma) is a proto-oncogenic and nonreceptor tyrosine kinase.
Taken together, these studies suggest that NALCN may contribute to a critical persistent inward cation current in respiratory neurons in the preBötC that is highly susceptible to regulation by neuromodulators are essential for sustaining respiratory rhythms. This channel may have gone unrecognized by earlier studies, due to the limitations of existing pharmacological tools. Further electrophysiological studies with preBötC slice preparations from NALCN and UNC-79 mutant mouse lines may prove highly revealing.
Voltage-dependent calcium currents and their roles in regulating respiratory network activities
Overview
Until early 2000 most studies using respiratory rhythmic brainstem slice preparations assumed that the persistent sodium current (INaP) was the major inward current responsible for generating autonomous pacemaker activity in respiratory neurons (103,331). Moreover, modeling studies provided convincing arguments that a rhythm could emerge through the activation and inactivation properties of INaP in these neurons (51, 102). The focus on INaP in in vitro studies created the impression that a “simple” respiratory rhythm is generated under in vitro conditions which emerges entirely through the persistent sodium current, a conclusion that is still implied by some studies (464). Yet, also for the in vitro network, it should be obvious that calcium currents play critical and heterogeneous roles. Calcium-sensitive dyes reveal that during periods of spontaneous bursting, Ca2+ concentrations within pacemaker neurons rise as Ca2+ ions enter through voltage-sensitive ion channels (265), and intrinsic calcium oscillations have been found in respiratory neurons (347). Not only in vitro, but also many in vivo studies indicate that voltage-dependent calcium currents and calcium-dependent mechanisms regulate various important aspects of respiratory rhythm generation (419, 450, 451). Some of the roles for calcium currents will be reviewed in this section. Molecularly, all voltage-dependent Ca2+ channels (Cav) contain a pore-forming α1-subunit that determines their main biophysical and pharmacologic properties. There are three major families of α1-subunits that contribute to the L, P/Q, N, R, and T-type calcium currents (22, 121, 156, 445). The R-type channels are known to contribute to exocytosis at many synapses (6, 246), including the mossy fiber-CA3 synapse in the hippocampus (169). But, to the best of our knowledge, the role of the R-type calcium current in the neuronal control of breathing remains unknown. Because it is very likely that this current also plays an important role in regulating respiratory activity, this issue clearly-deserves more in-depth studies.
The L-type channels
The L-type channels belong to the Cav1 subfamily. These channels have slow activation and inactivation kinetics (156, 301). For Cav1.3, it has been shown that it is involved in generating pacemaker activity in the substantia nigra (427), but it is unlikely that this subunit plays a critical role in generating autonomous pacemaker activity in the respiratory network. However, L-type calcium currents may still contribute to the generation of autonomous pacemaker activity. In the functional respiratory network, L-type calcium channels amplify drive potentials and increase spike frequency in some but not all respiratory neurons (297, 384). Calcium influx though L-type calcium channels is known to increase during hypoxia in respiratory neurons (349, 351). This effect could potentially contribute to the augmentation seen during the initial phase of hypoxia (407).
Three respective members from the Cav2 subfamily contribute to the P/Q-, N-, and R-type calcium currents. Although the activation and inactivation kinetics of these channels are very similar, these channels are characterized by their differential sensitivity to blockade by a variety of biological toxins (307, 376, 383, 505). All of these antagonists act on the channels from the outside of the cell membrane.
The α1A (Cav2.1)-containing (P/Q-type) calcium channels
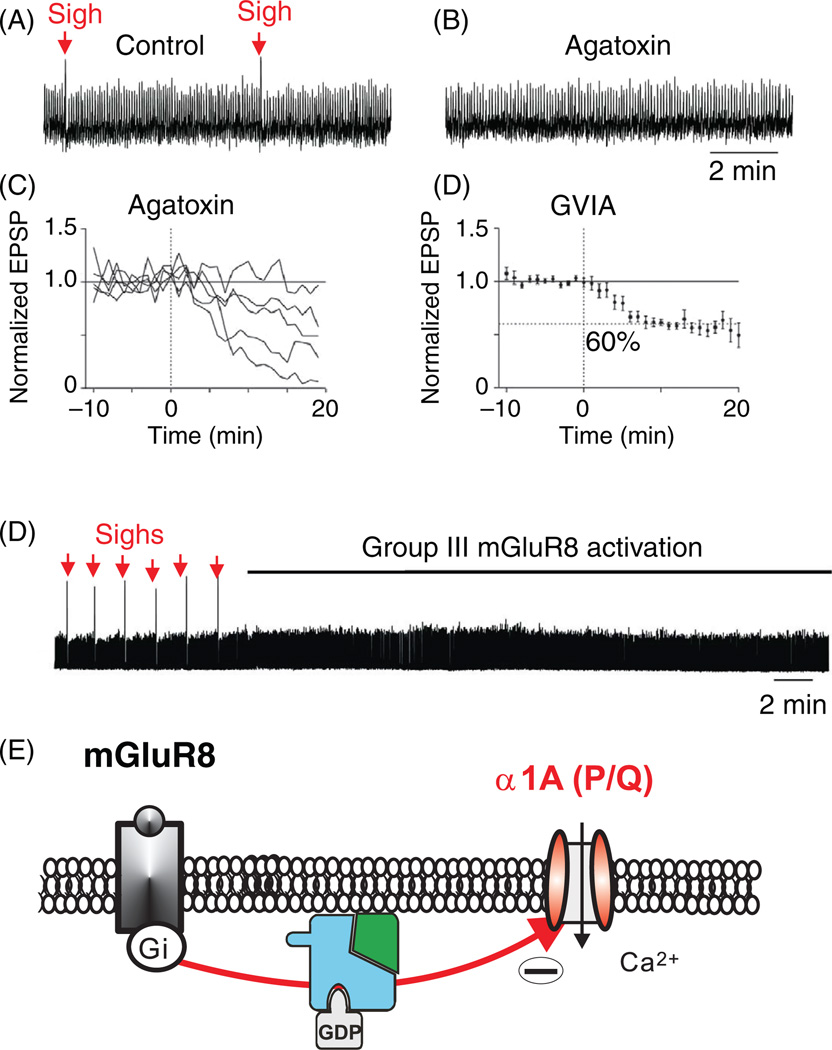
P/Q-type channels are sensitive to specific spider toxins (ω-Agatoxin IVA or TK) (346). These channels were first identified in Purkinje cells (307). They are expressed in the dendrites (215) and soma (346), where they play an important role in controlling the action potential firing rate (275, 570). As shown, for example, in other systems, P/Q-type channels can contribute to bursting in Purkinje cells (155) and other neurons. Within the respiratory network, P/Q-type calcium channels trigger neurotransmitter release at central excitatory synapses (297). Interestingly, the effect of P/Q-type calcium channel blockade was variable, as excitatory post-synaptic potential (EPSP) amplitudes were reduced in some respiratory neurons by only 8% and in others by more than 90%, suggesting that only a subset of excitatory synaptic connections within the respiratory network depends primarily upon P/Q-type channels (Fig. 6C). This finding may have important systems-level implications, because the P/Q-type channel blocker ω-agatoxin TK completely abolishes sigh generation at concentrations that do not eliminate normal respiratory activity in the preBötC (297). This raises the possibility that within the respiratory network, a subnetwork consisting of P/Q-type calcium-channel-dependent excitatory synapses may be critical for the generation of sighs (296, 297). Moreover, this subnetwork possesses metabotropic glutamate (mGluR8) receptors coupled to these P/Q-type Ca2+ channels (Fig. 6D,E). But, P/Q-type channels are not only involved in the generation of sighs. These α1A (CaV2.1)-containing channels also augment the amplitude and duration of drive potentials of preinspiratory and inspiratory neurons, which in turn increases spike frequency during the respiratory burst (297, 384). P/Q-type calcium channels also contribute to an increased regularity of normal respiratory activity. Thus, P/Q-type channels serve a modulatory role for normal respiratory activity, and apparently are essential for the generation of sighs (297).
Figure 6.
Generation of fictive sighs depends on the activation of P/Q-type calcium channels. Pharmacological blockade of the P/Q type channels with ω-agatoxin TK specifically abolishes sighs. (A) Sighs recorded under control conditions in population recordings from the preBötC (sighs indicated by red arrows) and (B) after bath application of ω-agatoxin TK, at low concentrations (modified, with permission, from reference 297). (C) Reduction of the amplitude of intracellularly recorded evoked EPSPs (by electrical stimulation of the contralateral preBötC) in respiratory neurons by pharmacological blockade of P/Q-type calcium channels. Individual responses of five neurons to ω-agatoxin TK [120 nmol/L] show a variable response, with a minimum of 8% reduction and a maximum of 90% reduction. (D) Individual responses to the N-type specific calcium channel blocker GVIA [0.5 µmol/L], showing a homogeneous 40% reduction of evoked EPSPs (modified, with permission, from reference 297). (E) Activation of the metabotropic glutamate receptors (mGluR8) leads to specific inhibition of “fictive sighs” recorded from the preBötC (modified, with permission, from reference 296). (F) Hypothesized mechanism of action for the inhibition of P/Q-type calcium channels by activated mGluR8 receptors, through a direct inhibitory interaction with the β-subunit of the heterotrimeric G-protein.
The α1B (N-type) calcium channels
N-type channels are not just modulatory, but essential for generating normal respiration (eupnea) in vivo. Acute blockade of the N-type calcium channel within the preBötC abolishes eupneic activity in vivo (440). By contrast, in in vitro systems, rhythmic respiratory activity persists following the blockade of N-type calcium currents with bath-applied ω–conotoxin GVIA (297). In the in vitro network, N-type calcium currents contribute to only 40% of the amplitude of glutamatergic EP-SCs generated between respiratory preBötC neurons (297). It is possible that the in vivo network depends more on N-type calcium-dependent synaptic mechanisms than the in vitro respiratory network. Although unproven, this hypothesis would be consistent with the idea that synaptic mechanisms are more important in the in vivo network, as proposed in the computational study by Rubin et al. (463). But many other possible reasons could explain the differences. These experiments were performed at significantly different ages (neonatal in vitro vs. adult in vivo) and species (mouse in vitro vs. cat in vivo).
N-type calcium currents activated during each action potential significantly influences their width and shape. The shape of action potentials in turn directly correlates to the amount of neurotransmitter release in presynaptic terminals. Calcium influx through N-type calcium currents also activates large (BK) and small (SK) conductance calcium-dependent K+ channels (KCa) (26, 27, 326) or CAN cation channels. The coupling between N-type calcium current and KCa currents could explain why respiratory frequency increases in vitro upon blockade of N-type calcium currents (297, 384). Block-ade of N-type calcium channels also increases the frequency of sigh activity, an effect associated with the elimination of the post-sigh apnea (297). Blockade of N-type calcium channels also augments respiratory drive potentials, which suggests that KCa currents play critical roles in shaping drive potentials in vitro (297, 384, 616). This conclusion is consistent with insights gained from the in vivo respiratory network. Under in vivo conditions, buffering intracellular calcium with 1, 2-bis(o-aminophenoxy)ethane-N,N,N′,N′- tetraacetic acid (BAPTA) resulted in blocking potassium currents. This revealed that intracellular calcium is important for activating calcium-dependent potassium currents that play critical roles in shaping the drive potentials of expiratory neurons (66, 451). These calcium-dependent mechanisms also seem to constitute an important off-switch mechanism involved in phase termination (416, 439, 451), but the molecular or pharmacological identity of these calcium channels remains unknown under in vivo conditions.
The Cav3 family encodes T-type channels
The T-type channels (Cav3) are located both on and near the soma and at more distal dendritic sites and can be detected in all brain regions including the neocortex, hippocampus, thalamus, cerebellum, and inferior olivary nucleus (244, 333, 485, 579). T-type channels have several unique properties that allow them to mediate pacemaker activity and rhythmic burst firing, as has been demonstrated in thalamic relay neurons (86, 226, 329, 536). However, so far no one has demonstrated this type of autonomous bursting activity within the respiratory network. But this does not mean that T-type calcium currents play no role in respiratory rhythm generation. Respiratory neurons within the preBötC possess T-type calcium currents (127, 385). Indeed, within the same anatomical location, the preBötC, rhythmic respiratory neurons express a larger T-type conductance compared to neurons not rhythmically active with respiration (126). When the resting membrane potential is below −70 mV, neurons expressing T-type currents can generate a high-frequency burst of action potentials (86, 234, 305). For medullary respiratory neurons, it was demonstrated that synaptic mechanisms are critical for removing inactivation from low-threshold calcium currents, which enables rebound excitation in inspiratory, as well as expiratory, neurons in vivo (419, 439, 451). This rebound depolarization persists after intracellular blockade of sodium currents, which demonstrates that they are mediated by calcium currents (451).
A new and exciting twist with regard to T-type calcium channels is their functionally significant window current. Although not shown for respiratory neurons, the window component of the T-type current can significantly contribute to the resting membrane potential of thalamic neurons, as well as to the up state of intrinsically generated slow oscillations (114). Moreover, in this study it was possible to evoke bursts of action potentials at depolarized potentials, where the majority of T-type calcium channels are known to be inactivated (114). This may also have important functional implications for the respiratory network, as rhythmically active preBötC neurons are characterized by a large T-type conductance with a large window current (126).
Calcium-activated nonselective cationic currents (ICAN) and their roles in rhythm generation and neuromodulation
Overview
Until a decade ago, no model of respiratory rhythm generation considered ICAN as a major cellular mechanism, and it was assumed that bursting in autonomous pacemaker neurons is primarily driven by the voltage-dependent characteristics of the persistent sodium current (INaP) (51, 102). While voltage-dependent calcium currents (Cav)were known to play critical roles in synaptic transmission and amplifying synaptic inputs, calcium-dependent mechanisms were thought to be modulatory in nature in pacemaker neurons. The first indication that calcium-dependent mechanisms could also be critical for autonomous bursting came with the discovery that pacemaker neurons cease to burst in the presence of cadmium, a blocker of Cav (554). However, it was not possible to unambiguously link this bursting property to a specific voltage-dependent calcium channel, as this autonomous bursting was not reliably blocked by specific blockers of the L-, N-, P-, or Q-type calcium currents. It was subsequently demonstrated that bursting ceases in most (82%) CS pace-maker neurons in the presence of FFA and lanthanum (404). Because these substances block ICAN, it was concluded that ICAN gives rise to bursting in this type of pacemaker neuron (404). But it must be emphasized that FFA does not abolish bursting in 100%ofCS pacemakers, an important caveatwhen assessing the obligatory role of ICAN (105, 554). Subsequent studies confirmed that ICAN is critical for bursting in CS pace-maker neurons (105, 404), and attributed ICAN as a major burst mechanism involved in amplifying synaptic drive potentials (392, 462).
ICAN-type currents have been described in a variety of nonneuronal cells (81, 327) as well as neurons (82, 108, 397). Because ICAN does not inactivate, it is ideal for promoting bursts and long-lasting plateau potentials (107, 359). ICAN is calcium dependent, and may not be directly depended on membrane voltage to be activated. However, ICAN is indirectly affected by voltage, because various Cav currents contribute to intracellular calcium currents and thus to the activation of ICAN (282, 397). In addition, ICAN can be activated by calcium released from internal stores (347). A recently published computational model by Toporikova and Butera (558) suggests that intracellular calcium oscillations may give rise to the autonomous bursting of CS pacemaker neurons, an interesting hypothesis that will need to be tested experimentally. Experimental evidence for intracellular dendritic calcium waves was demonstrated in isolated neurons from the preBötC. These calcium waves activated TRPM4/5 channels within the somatic compartment (347). These intracellularly generated calcium waves were facilitated by depolarization and activation of mGluR1/5 (347).
Molecular biology
ICAN was identified pharmacologically in respiratory neurons and its molecular identity is still somewhat uncertain. But it is very likely this channel belongs molecularly to the transient receptor potential (TRP) family (21, 89). TRP channels consist of six transmembrane spanning segments (S1-S6) and a cation permeable pore (S5-S6) (345). The family of TRP channels is diverse, with seven subfamilies, six of which are functional in various mammalian tissues including nervous tissue (372). Within the brain, various forms of TRP channels are involved in temperature sensing (62), taste (622), hearing, and mechanosensation (88). They have also been implicated in neuronal outgrowth (TRPM7) (1) and cell survival (TRPC5) (189). In addition, a number of studies show that postsynaptic currents evoked by metabotropic glutamate receptors (mGluR1) are carried by TRPC1 channels, suggesting a role in synaptic plasticity and neuromodulation (23, 256, 560). The role of TRP and metabotropic receptors in neuromodulation will be discussed in a later section that describes in more detail interactions associated with G-protein coupled receptors.
It is possible that different isoforms of the TRP family contribute to different aspects of ICAN within the respiratory network. It has been demonstrated that TRPM4 is regulated by phosphatidylinositol 4,5 (PIP2), and that intracellularly applied PIP2-analogues augment the inspiratory drive potential in respiratory neurons. Together with the observation that drive potentials are sensitive to FFA, this set of studies suggested that ICAN, in the form of TRPM4, plays a critical role in boosting excitatory drive in respiratory neurons (89, 248, 371). Consistent with this hypothesis, some studies indicate that TRPM4 as well as TRPM5 is expressed in the preBötC. How-ever, Ben-Mabrouk and Tryba (21) were unable to reproduce expression of TRPM4 in Western Blots taken from the area that generates respiratory rhythmic activity. This negative result could have various methodological reasons, as discussed by Ben-Mabrouk and Tryba (21), thus, single-cell RT-PCR approaches taken specifically from respiratory neurons may shed more light on this unresolved issue. In a characterization that focused on the modulation of respiratory activity by substance P, Ben-Mabrouk and Tryba (21) proposed that ICAN depends on TRPC3 or TRPC7 channels. According to this study, the antagonist (SKF-96365) blocks NK-1 receptor activation by substance P, presumably via a blockade of TRPC channels. SKF-96365 specifically blocked the effects of substance P on CS pacemakers, further supporting the notion that TRPC channels may play a critical role in the NK1-mediated modulation of ICAN-dependent bursting in pacemaker neurons.
Contribution of different inward currents to the onset of a respiratory cycle
While in the previous sections the different inward currents were separately discussed, they will likely all contribute to the onset of a respiratory cycle. This section will consider these currents in a more functional context.
Contribution of INaP-dependent bursting mechanisms to the onset of a respiratory cycle
INaP-dependent bursting neurons (CI pacemakers) were extensively studied in transverse slice preparations and their bursting characteristics simulated in computational models (51, 103–105, 268). According to these combined studies, INaP inactivation contributes to the termination of the burst. The slow kinetics of the recovery from this inactivation seem to determine the timing of burst onset. In the functional network, excitatory and inhibitory synaptic inputs and other inward and outward currents will influence this voltage-dependent recovery process. Synaptically evoked inputs will be able to slow or protract the recovery from inactivation, and an INaP-dependent intrinsic burst will be triggered either earlier or later in the respiratory cycle or not at all. Thus, the amount of synaptic excitation needed to trigger a protracted burst will depend on the inactivation state of the INaP current, but also on the presence and strength of other inward and outward currents that also will be described in this review. It will also depend on the amount of concurrent inhibition that these neurons receive. Immediately following a network burst, the threshold for activating the intrinsic burst will be highest and thus the probability for intrinsic bursting to contribute to the initiation of an inspiratory burst will be very small. But as time following the network burst increases, the likelihood that an INaP-dependent burst will be activated will also increase. This may explain why in autonomously bursting neurons ectopic bursts are often generated prior to the subsequent population burst (143, 404, 563). Assuming that bursting neurons are embedded in the respiratory network, these ectopic bursts may contribute to the initiation of the network burst (442). But to tease apart to what extent an ectopic burst is generated intrinsically or synaptically is difficult and it is most likely that the threshold for synaptic or intrinsic activation of a burst will vary among individual neurons and even individual respiratory cycles. The amount of available INaP, the inactivation status, the amount of synaptic inputs, and the location of ion channels and synaptic receptors on the dendrite and soma of the respiratory neurons will not be the same for any given neuron at any given time. This also explains why ectopic bursts are generated in some but not all neurons during some but not all respiratory cycles. This leads to another important conclusion. Bursting could potentially act as a nonlinear amplifier in some cycles and an initiating mechanism in other cycles, and the onset of each population burst will depend on the overall integration of all these intrinsic and synaptic factors at the cellular and network level. Thus, it will be impossible to know which neuron and what mechanism will contribute to what degree to the onset of the inspiratory activity at any given respiratory cycle. We conclude that there will unlikely be a situation in which a cycle onset will be purely determined synaptically or intrinsically, or only by one particular mechanism.
Contribution of ICAN-dependent bursting to the onset of a respiratory cycle
While voltage-dependent properties are largely responsible for determining INaP-dependent bursting as described earlier, there are different parameters that determine the activation of ICAN-dependent bursting. Indeed, much is already known about the various synaptic and metabotropic determinants (89, 100, 347, 392, 442). In principle, the onset of an ICAN burst will be determined by an increase in intracellular calcium that will lead to the opening of TRP channels, which then results in the depolarizing influx of Na+ (101). In addition to the spontaneous activation of intracellular dendritic calcium waves, as demonstrated in the dendrites of respiratory neurons (347), many processes are known to raise intracellular calcium and will, therefore, contribute to the opening of TRP channels. mGluR 1/5 receptors seem to be important for the activation of these calcium waves that in turn activate ICAN (347). In one of the later sections, we will discuss in more detail various neuromodulators that have been implicated in the activation of channels in the TRP superfamily. Moreover, every action potential will open voltage-gated calcium currents. It follows that CS pacemaker neurons and ICAN activation in general will have some voltage-dependent properties even though the TRP channels. However, the voltage dependency of the CS bursting may be different from that of CI bursting or the functionally activated INaP inward current (434). Action potentials generated during the population burst will create sufficient calcium influx to contribute to the activation of ICAN current. Indeed, calcium-imaging studies have demonstrated that substantial and widespread somatic Ca2+ influx is generated by TTX-sensitive action potentials (358), which seem to complement the intrinsically generated dendritic calcium waves (347). Thus, the excitatory synaptic input contributes to the triggering of the ICAN burst, in part through voltage-gated calcium currents that are activated by action potentials.
Contribution of a background current to the onset of a respiratory cycle
Bursting pacemaker neurons are continuously depolarized by a sodium-dependent background current that renders them spontaneously active (77, 566). This background current will contribute to the generation of action potentials that will intrinsically ramp up prior to the ICAN-dependent burst. Assuming that approximately 50% of bursting neurons are excitatory (357), this intrinsic background current will contribute to an intrinsically generated ramp-up of EPSPs preceding the population burst (101). This ramp occurs not only from bursting pacemakers, but also from the numerous autonomously spiking neurons within the respiratory network. Clearly, in the functional network, spike generation will never be purely intrinsic, as autonomous spiking will evoke synaptic events in postsynaptic neurons. Thus, the onset of the respiratory cycle constitutes a synergy between intrinsic voltage- and calcium-dependent and synaptic mechanisms.
Contribution of calcium and other inward currents to the onset of respiratory cycle
Once initiated, the respiratory burst will bring the membrane potential to the voltage range that will also activate other voltage-dependent ionic conductances and channels, including the transient INa currents and the various voltage-gated calcium currents (P/Q-, L-, and N-type calcium channels) that were discussed in prior sections. The depolarization by these voltage-gated channels may suffice to remove Mg2+ block leading to the voltage-dependent opening of the NMDA receptor (NMDA-R), thus further contributing to the amplitude of the burst (439). The generation of the burst will become regenerative, as any of these currents will elevate the membrane potential to a level that will open more voltage-gated ion channels, which in turn will raise the calcium levels, which then will increase the activation of ICAN. The earlier detailed cellular considerations focus on the generation of the inspiratory burst within the preBötC. But the reader should be reminded that this will be only one aspect in the generation of the respiratory rhythm. A very elegant study by Paarmann described in detail how different types of NMDA-R splice variants have different deactivation kinetics in different regions of the medulla, which may have interesting functional implications (388, 389). Moreover, the exact type of NMDA-R subunit activated within the preBötC may change during development, which could also have functional consequences (303). In addition, it is known that NMDA-R play widespread differential roles in the generation of the respiratory rhythm (61, 84, 146, 154, 356, 417, 418) and the hypoxic response (382). Although, the ongoing quest for the obligatory mechanisms has demonstrated that NMDA-R are not essential for functional respiratory rhythm generation (164), it should be clear that these receptors are important building blocks in the generation of the respiratory rhythm, chemosensation, and motor patterning.
Concluding remarks
Several inward currents are important for the function of respiratory activity. These include not only voltage gated sodium and calcium currents (i.e., INaP), but also voltage-insensitive conductances (i.e., background currents, and leak channels). Inward currents have distinct activation and inactivation properties that have different functional consequences on the firing pattern of respiratory neurons. Although these properties shape the firing properties at the cellular level, they will ultimately affect the network output, which in turn will contribute to the generation of the different patterns of respiratory activity (i.e., eupnea, gasping, and sighs). Yet, our understanding of the functional integration of these cellular properties is still incomplete. While some currents, such as the INaP current, were extensively studied, we have much to learn about the integration of the different TRP channels and the NALCN current, and as yet nothing is known about the R-type calcium currents.
Potassium Currents and Their Contribution to Respiratory Rhythm Generation
The balance between inward currents as described in the Section “The Inward Conductance Within the Respiratory Network” and a large catalog of outward currents determines how a neuron drives synaptic and modulatory activity, and how it responds to its synaptic and modulatory inputs. As will be discussed in this section, potassium channels play critical roles in this complex and dynamic interplay. Potassium currents are known to determine key aspects of neuronal excitability, including basal subthreshold membrane potential, the shape of the repolarizing phase of action potentials, and the envelope of action potential bursts. Thus, potassium channels are expected to play equally important roles in respiratory rhythm generation. However, we are just beginning to appreciate the full extent of their numerous contributions. Initial studies of respiratory brainstem neurons relied nearly exclusively upon the application of various potassium channel blockers, many of which, however, offer relatively poor molecular specificity. More recently, molecular genetic approaches have been applied to identify and manipulate specific potassium channel genes, especially by employing genetically engineered mouse strains. Nonetheless, significant challenges remain in linking the role of individual potassium channels to the macroscopic electrical behavior of a circuit as complex as the respiratory network, even in its reduced form in the isolated brain slice. These challenges include the neuronal heterogeneity of circuit elements involved in rhythm generation, their potential redundancies, and the relative paucity of information about the detailed microcircuitry between these circuit elements. The development of further experimental tools, including additional molecular markers for specific key circuit elements, will undoubtedly help to overcome these challenges in the future.
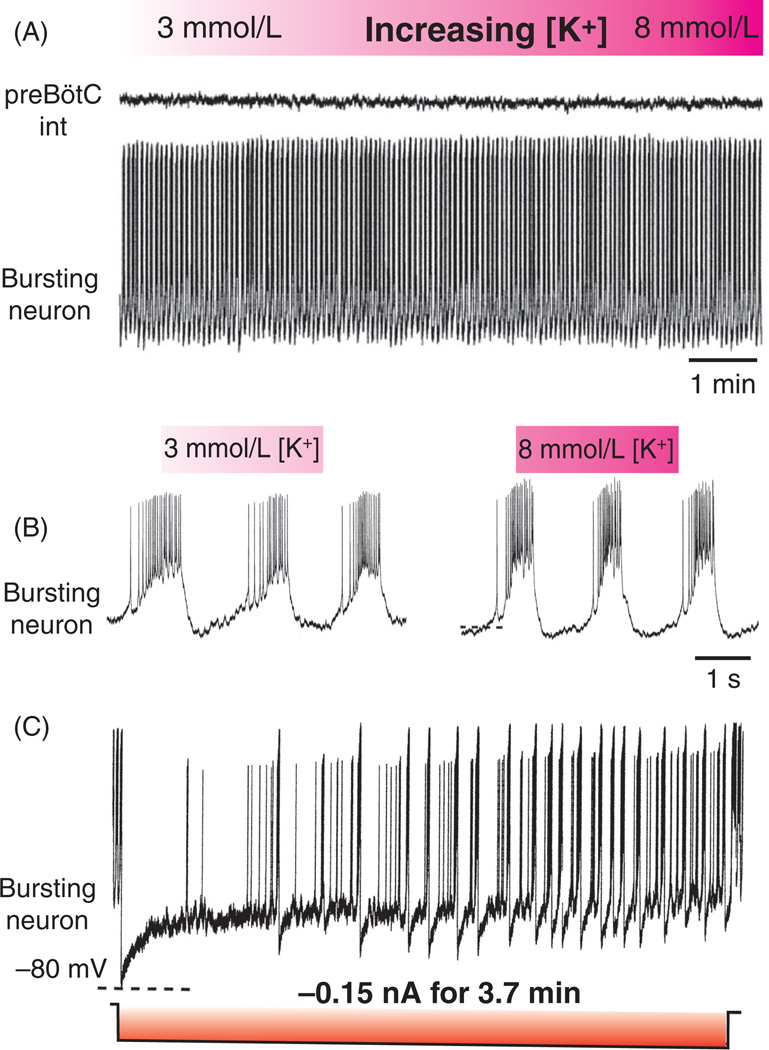
Although, it is well established that the isolated preBötC can generate respiratory rhythmic activity at physiological potassium concentrations (165, 459, 460, 516, 564), isolated slices are usually investigated at elevated external [K+]o, typically between 5 and 9 mmol/L. A number of studies have suggested that raising external [K+]o alters the balance between potassium leak channels and sodium leak channels constitutively open at subthreshold membrane potentials (265, 390, 468). Elevated external [K+]o would be expected to depolarize the potassium reversal potential, thus reducing the normal driving force for a hyperpolarizing outward potassium conductance through an open potassium leak channel. In combination with a countervailing depolarizing drive from an inward sodium leak current, this reduction of potassium conductance may provide sufficient basal excitation to trigger additional cellular and/or circuit mechanisms to generate sustained patterned neuronal output. Support for this hypothesis is provided by computational and pharmacological studies of the preBötC circuit (468). A network model of 50 interconnected excitatory neurons (no contributions from inhibitory neurons was considered in this model) was simulated with varying ratios of potassium and sodium leak conductance, and subjected to a simulated step elevation in external [K+]o. Computationally, synchronized network bursting was found to be triggered within a narrow window of elevated [K+]o (5–9 mmol/L), primarily determined by the ratio of potassium (gK) to sodium leak (gNa) conductance, and the size of excitatory synaptic conductance (gEdr). This computational model was supported by experimental recordings from preBötC slices. Experimentally, rhythmic respiratory bursts were extracellularly recorded from slices in 7 mmol/L [K+]o, then silenced in 3 mmol/L [K+]o. Extracellular recorded rhythms could be subsequently reestablished by blocking potassium channels with [4-aminopyridine (4-AP), 100 µmol/L, or tetraethylammonium (TEA), 4 mmol/L]. Conversely, application of riluzole (25–50 µmol/L), a blocker of INaP, was sufficient to abolish rhythmicity. These experimental results were incorporated into a computational model that suggested that neuronal potassium and sodium leak conductances are critical determinants of preBötC rhythmicity. While this generalized conclusion is certainly correct, it must be emphasized that pharmacological blockade of all potassium channels can provide only a very coarse insight into the complex dynamics of potassium currents interacting with other membrane and network properties, in particular, because this study was based only on extracellular recordings. Unfortunately, this and many subsequent computational studies often overlooked important experimental evidence that could have provided a more refined view of network and pacemaker activity. As shown in Figure 7, pacemaker neurons possess a background Na+ current which stabilizes their bursting activity against changes in extracellular K+ currents. As shown in this figure, the pacemaker neuron can burst at 3 mmol/L extracellular K+ (Fig. 7A,B) and its bursting does not change much when the extracellular K+ concentration is raised to 8 mmol/L. This current can be revealed by long-lasting hyperpolarizing current injections (Fig. 7C). In response to this imposed hyperpolarization, the neuron ceases to burst, but as it depolarizes intrinsically bursting is resumed. Thus, unlike the neurons proposed in various computational models, biological pacemaker neurons do not require artificial elevation of extracellular potassium to autonomously burst (77, 564, 566). In the following section, we aim at providing a detailed overview of the role of potassium outward currents and their dynamic and complex interplay with inward currents.
Figure 7.
Pacemaker neurons are able to burst throughout a range of extracellular potassium concentrations with the contribution of the persistent sodium current. (A) An intracellular recording from an individual pacemaker neuron illustrating autonomous bursting throughout a range of extracellular potassium concentrations (3–8 mmol/L) without significant changes to membrane potential. (B) Traces expanded from A of the autonomously bursting pacemaker at 3 mmol/L (left) and 8 mmol/L (right) extracellular K+. (C) Autonomous bursting involves INaP as revealed by long-lasting hyperpolarizing current injections that cause the neuron to cease bursting, but as it intrinsically depolarizes bursting is resumed. Hence, pacemaker neurons do not require artificial elevation of extracellular potassium to autonomously burst (77, 564, 566).
Molecularly, potassium channels from the “two-pore” potassium channel gene family (177, 290) may encode, at least in part, the potassium leak conductance observed in neurons of the respiratory network. Voltage-clamp recordings from inspiratory preBötC neurons identify an outwardly rectifying, halothane-sensitive potassium leak current, and single cell RT-PCR reveal the presence of TASK1 and TASK3 potassium channel transcripts, consistent with this possibility (264). However, these results do not preclude the possibility that other “two-pore” subunits, or other classes of subthreshold potassium channels such as inward rectifiers (KIR) may also contribute to the potassium leak current. Their potential roles in central chemosensitivity will be discussed later.
Voltage-dependent potassium currents can be broadly classified into noninactivating “delayed rectifier”-type (KDR) and transient “A-type” (KA) currents (192, 214, 612). 4-AP preferentially blocks KA currents, although a subset of noninactivating K+ potassium channels encoded by Kv3 genes are equally sensitive to blockade by 4-AP (447, 591). KA currents were observed in inspiratory preBötC neurons, at a higher frequency than expiratory neurons (205). Space-clamp limitations precluded detailed biophysical analysis of this current in all whole-cell recordings, but steady-state activation and inactivation profiles of this current in well-clamped excised inside-out patches are consistent with potassium channels encoded by Kv4.2 or Kv4.3. In slice preparations, 4-AP reversibly broadens the width of individual action potentials and shortens the latency to first spike, in response to step injections of depolarizing current. Dramatically, in slices generating fictive eupneic rhythms, 4-AP disrupts the regularity of population bursts by inspiratory preBötC neurons (both amplitude and frequency are affected), as well as the efficacy of transmission of respiratory drive to downstream hypoglossal motor pools. The authors suggest that KA currents in inspiratory neurons serve to buffer excitability against sporadic excitatory input. This allows neurons to respond only to highly synchronous and sustained excitatory synaptic input. Knock-out mouse strains for Kv4.2 and Kv4.3 are available (374, 375), but to our knowledge no obvious respiratory defects have yet been described.
DR-type K+ channels
A large number of potassium channel species may underlie DR-type potassium currents (KDR) in the preBötC, including members of the voltage-dependent Kv1, Kv2, and Kv3 gene families among others (214, 470, 592), but few detailed molecular examinations specific for the preBötC exist yet in the literature. Among KDR currents that may be found in preBötC, two non-Kv channels deserve particular notice. Because of the large and dynamic influxes of Na+ and Ca2+ in respiratory neurons with underlying regular bursting potentials, repolarizing potassium channels sensitive to changes in the internal concentrations of these cations may play a particularly important role in shaping and terminating burst potentials. Two such potassium channels are large-conductance calcium-dependent potassium channels (BK-KCa) [composed of KCNMA1 (Slo1) α-subunits and KCNMB1-4 (BK-β) β- subunits], and sodium/chloride-dependent potassium channels (KNa) [encoded by KCNT1 (Slo2.2 or SLACK) and KCNT2 (Slo2.1 or SLICK) α-subunits, in homo- or heteromeric combinations] (29, 52, 471, 615). Both BK-KCa and KNa channels are widely distributed in the brain, and found in high abundance in the brainstem (29, 30, 116, 125, 260). The role for BK-KCa channels in preBötC activity was investigated with the highly selective BK-KCa blocker, paxiline (616, 619) and the less well-characterized BK-KCa activator NS-1619 (616). Blocking BK channels with paxiline significantly pro-longs the length and frequency of burst potentials in both autonomous pacemaker and nonbursting neurons recorded from the preBötC in synaptic isolation by a cocktail of postsynaptic blockers (blocking glutamatergic, GABAergic, and glycinergic receptors). Paradoxically, the BK-KCa activator, NS-1619, under similar conditions, also increases both duration and frequency of bursts in both neuronal types, for still unknown reasons. Interestingly, in preBötC slices neither pharmacological manipulation of BK-KCa channels dramatically altered measured parameters of fictive eupneic or hypoxic respiratory rhythms. This suggests that despite the ability of BK-KCa channels to shape the electrical properties of individual isolated neurons, in the context of intact preBötC circuits these perturbations are buffered by other compensatory cellular or circuit-wide mechanisms. More dramatic effects on fictive eupneic rhythms were reported with the application of quinidine (100 µmol/L), a nonselective blocker of KNa channels (269, 606). Bath application of quinidine completely silenced eupneic bursts in a reversible fashion, concomitant with an approximately 14 mV depolarization of resting potentials in preBötC neurons. In synaptically isolated neurons, quinidine augments the size and slows the subsequent repolarization to a baseline potential of bursts, in response to step injections of depolarizing current. These results should be interpreted with caution because quinidine has been shown to be a broad spectrum K+-channel blocker (150, 209). In another study, voltage-clamp recordings, in combination with gene-specific siRNA knockdown transfections, from a variety of cultured neuronal cell types (olfactory mitral cells, striatal medium spiny neurons, and cortical pyramidal cells) indicate that a large component of noninactivating KNa current is widely present in most neurons, spatially coupled to INaP provided by TTX-sensitive NaV channels (47). Therefore, it would not be unreasonable to suspect that KNa channels may play a major role in shaping respiratory rhythms. However, the findings of Krey et al. (269), as the authors note, need to be interpreted with caution because quinidine is a highly nonselective pharmacological agent, which can effectively block many species of potassium channels even at the relatively low concentrations used. Mouse knockout strains for Slo1 (339, 477) and Slo2.2 (Salkoff, unpublished) have been created that may be used to more directly address their roles in preBötC circuitry, but to the best of our knowledge, no specific respiratory defects have yet been described.
One other KDR channel gene deserves note. This is the KCNQ2 gene, which is a member of the five gene family in vertebrates (KCNQ1-5) (71, 271, 289, 487, 512, 581, 582, 607). This gene family encodes subunits that combine in homo- and heteromeric fashion to assemble “M-type” potassium channels (KM) (42, 43). These potassium channels, by virtue of their unusually slow kinetics of activation and deactivation and relatively hyperpolarized conductance-voltage profiles, are thought to be major stabilizers of basal neuronal excitability. In addition, KCNQ potassium channels are exquisitely sensitive to modulation by various intracellular signaling pathways, including most prominently those coupled to muscarinic receptors. Consistent with their general importance, human mutations of four KCNQ genes (KCNQ1-4) are associated with a variety of hereditary diseases including epilepsy (Benign Familial Neonatal Convulsions or BFNC; KCNQ2, KCNQ3), long-QT syndrome (LQT1; KCNQ1), and congenital progressive deafness (nonsyndromic sensorineural deafness type 2 or DFNA2; KCNQ4) (238, 288, 534). Watanabe et al. (589) described a mouse knockout strain of KCNQ2 that exhibits hypersensitivity to epileptogenic drugs, when assayed as heterozygous individuals carrying only one functional copy of the gene. Interestingly, all homozygous KO neonates die within 24 h of birth due to pulmonary atelectasis and apparent respiratory failure. Whether respiratory failure can be attributed to a central defect in brainstem patterning mechanisms remains unexamined, to our knowledge. However, there is an increasing awareness that human epileptic patients suffer from a collateral risk of sudden unexpected death (SUDEP) at rates 24 to 40 times higher than the general healthy population (148). The underlying causes of SUDEP remain unknown. Conceivably, KCNQ2 may play a critical role in both cortical circuits controlling epilepsy and brain-stem respiratory neurons, and thus provide a mechanistic link between these two neural dysfunctions.
Small-conductance calcium-dependent K+ Channels (SK-KCa)
Respiratory phenotypes have been explicitly examined in several other mutant mouse strains engineered to delete or alter the expression of specific classes of potassium channels. These include apamin-sensitive SK3 potassium channels (34), neuronal ATP-sensitive potassium (KATP) channels formed by Kir6.2 (343, 353) and SUR1 subunits (4, 230), and pH-sensitive inward rectifier (Kir2.2; 387) and “two-pore” potassium channels [TASK1, TASK3; (363); TASK2, (172)]. In general, none of these genetic manipulations of single potassium channel genes yielded overtly catastrophic respiratory phenotypes, as all strains were viable, even if mutant phenotypes were observed in the context of isolated preBötC slices or single neuronal recordings. This may reflect the intrinsic subtlety or redundancy of control exerted by individual potassium channels within the intact preBötC respiratory circuit.
SK3 encodes one of four mammalian subunits that form nonvoltage-dependent, calcium-dependent SK potassium channels (SK1-4) (33, 263). Bond et al. (34) engineered a strain of mice with a tetracycline-based genetic switch inserted into the 5’ untranslated regulatory region of the SK3 gene. This permitted conditional overexpression of SK3 under the control of its native promoter, triggered by the withdrawal of doxycycline (dox) chronically administered by feeding. Animals thus induced to overexpress SK3 were found to have a compromised response to moderate hypoxia (8% O2), assessed by whole animal plethysmography. WT animals respond to this moderate hypoxic challenge with an elevated rate of breathing (~150 inspirations/min), but SK3 overexpression severely blunted this response (~55 inspirations/min) and produced prolonged periods of apnea. This effect was entirely reversible by reestablishing chronic dox treatment and shutting off SK3 overexpression. Although SK3 is abundantly expressed in brainstem structures including the preBötC, expression is also found in many non-neural tissues. Thus, these respiratory effects could result from either peripheral or central mechanisms mediated by SK3. Evidence supporting a central role for SK-type potassium channels in the generation of respiratory rhythms came from pharmacological studies with in vitro preBötC slices (616). Bath application of 1-ethyl-2-benzimidazolinone (1-EBIO), an SK-KCa channel activator, reduced the amplitude and frequency of inspiratory preBötC bursts in a dose-dependent manner, whereas apamin, an SK-KCa specific blocker, significantly increased burst rate and irregularity. Significantly, at low concentrations that did not decrease burst rate, augmenting SK-KCa channel activity with 1-EBIO selectively abolished fictive “sighs,” one of several modes of bursting which can be observed from preBötC slices. Correspondingly, inhibiting SK-KCa activity with apamin increased the frequency of sighs, along with increasing the general excitability of the slice. Taken together, these studies suggest an important and perhaps uniquely selective role for SK-KCa-type channels in regulating the overall excitability of the preBötC respiratory network.
ATP-dependent K+ channels
KATP potassium channels are regulated by intracellular concentrations of ATP and phosphoinositol signaling lipids (3, 4 phosphoinositol-bis-phosphate, PIP2), and thus they serve as sensitive effectors linking the metabolic status of cells with membrane potential. Decreases in cytosolic ATP activate KATP channels, and thus these channels may serve to produce a rapid protective hyperpolarization in response to acute hypoxia. In addition, these channels may also subserve continuous homeostatic regulation of excitability in cells designed to sense or experience high metabolic demand. Most notably, KATP channels have been studied in pancreatic β-islet cells in the context of compromised insulin release, but these channels are also widely distributed in the various brain regions, including glucose-responsive neurons of the ventromedial hypothalamus (342) and midbrain dopaminergic neurons (533). In preBötC slices, KATP channels were found in relatively high abundance, through a combination of patch-clamp recordings and single-cell RT-PCR, particularly in inspiratory respiratory neurons (200, 350). The high abundance of these channels allowed their activity to be simultaneously monitored in on-cell patches under voltage clamp, along with unclamped transients corresponding to action potentials generated by these neurons. Under normoxic conditions, the activity of KATP channels appeared to regularly fluctuate in phase with inspiratory bursts of action potentials. This discovery leads to the important concept that these channels dynamically modulate the firing patterns of these neurons in a cycle-by-cycle manner even under baseline conditions.
Concluding remarks
Like inward currents, K+ currents act as diverse determinants of excitability. They provide stability to subthreshold Vm oscillations and these currents dynamically shape neuronal firing behavior. Both pharmacological and genetic manipulations of K+ currents further demonstrate their roles in maintaining proper control over breathing and respiratory network activity. But our understanding of the differential physiological and pathophysiological roles of the highly diverse classes of potassium channels is still very limited. Understanding, for example, how potassium currents contribute to the sudden unexplained death of epilepsy is one area of important clinical relevance.
The interactions between Intrinsic and Synaptic Properties
Overview
Synaptic mechanisms are cellular building blocks that are as important as the various inward and outward currents discussed in the previous sections. The search for the synaptic components that are critical for generating rhythmic activity patterns has been shaped by history. Following Graham Brown’s (182) proposed concept of a CPG network came an equally influential publication in which he proposed a mechanism that could explain the emerging rhythmicity (182, 183). According to his proposal, two groups of functionally antagonistic groups of neurons are connected via reciprocal inhibition and Brown suggested that this so called “half-center” will give rise to the alternating rhythmic activity that characterizes many rhythmic activity patterns, including locomotion (182). This powerful hypothesis inspired many laboratories around the world to investigate the synaptic mechanisms in a variety of small and larger invertebrate and vertebrate neuronal networks. This quest led to the demonstration that indeed the majority of the networks reveal some degree of reciprocal organization involving synaptic inhibitory mechanisms (173, 203, 324, 400, 503). Moreover, early theoretical models confirmed the hypothesis that a network based purely on synaptic interactions could generate rhythmicity without the need to incorporate autonomously bursting neurons (411). However, as more experimental insights were gained, it became clear that certain intrinsic membrane properties were critical to control phase transitions in half center networks (588). Ionic mechanisms such as the hyperpolarization-activated cation current (Ih current) were discovered. This channel is activated during the inhibitory phase which is exerted by the functionally antagonist group of neurons (503, 514, 588). The depolarization caused by the Ih current could then activate low-voltage activated T-type calcium currents that would trigger the onset of the next rhythmic phase [“escape mechanism”; (503, 588)]. Alternatively, an active neuron could fall below the threshold to release the inhibitory transmitter that would release the silenced neurons from their inhibition [“release mechanism”; (514, 588)]. This half-center model also inspired many computational and experimental studies within the field of mammalian respiration (3, 14, 381, 466).
However, the idea that a reciprocally organized inhibitory network will generate “automatically” rhythmic activity alternating between two groups of neurons or between two or three phases of the rhythmical activity may be an oversimplification. Even if various intrinsic currents are incorporated in a network, it cannot be automatically assumed that a reciprocally organized inhibitory network will generate rhythmicity. Principle insights were gained into the constraints and requirements associated with the half-center network using the dynamic clamp or related approaches (503, 523). With these approaches, isolated neurons can be connected to a half-center network in which intrinsic and synaptic properties can be precisely regulated by a computer interface to explore the conditions that allow the generation of different activity patterns. This approach reveals that, depending on the time constants of the inhibitory potentials, the current density of the various inward and outward currents and various other parameters, the same half-center connectivity is capable of generating not only alternating stable rhythmic activity but also various other forms of activity patterns including synchrony, antiphase spiking, silence, or activity that was stuck in one particular phase [Figure 8B; (503, 523)]. Moreover, modeling studies suggest that reciprocal inhibition can play a critical role not only in generating antiphase activity, but also stable synchrony between bursting neurons (235). Thus, even if one were certain that the respiratory network is strictly organized in a reciprocal manner, it is impossible to predict whether this network structure will promote the generation of alternating rhythmicity. However, irrespective of these uncertainties, it should be clear that massive synaptic inhibitory interactions are present within the respiratory network and it is very likely that they do play critical roles in various aspects of the respiratory rhythm. In this section, we want to highlight some of the hypothesized roles.
Figure 8.
Synaptic inhibition and its potential role in the generation of rhythmic network activity. (A) Schematic cartoon of a half center model with neurons connected by reciprocal inhibition. (B) Neurons connected to a dynamic clamp establish an artificial half center and can produce a variety of patterned outputs, as shown. Examples of a half-center model exhibiting (B1) alternating slow oscillations, (B2) antiphasic spiking, or (B3) high-frequency half-center oscillation. Output of the half-center model is highly dependent upon the synaptic and ionic conductance parameters defined by the dynamic clamp (modified, with permission, from reference 503).
Inhibitory synaptic connections within the respiratory network
Synaptic inhibition in the in vivo network
There is much evidence for the role of synaptic inhibition within the respiratory network and many elegant in vivo studies have pioneered this research area. By employing different types of in vivo models, widespread inhibitory connections have been demonstrated between all types of respiratory neurons (484). Reciprocal inhibitory connections exist, for example, among expiratory neurons, as demonstrated by cross-correlation analyses (141, 504, 556). Inhibitory connectivity was also characterized by antidromic mapping, which showed that expiratory Bötzinger complex neurons with an augmenting firing pattern are extensively connected via inhibitory synapses to different classes of neurons (239). Elegant intracellular studies of respiratory rhythmic depolarization patterns revealed extensive inhibitory connections between all known types of respiratory neurons (14, 449, 484). By manipulating the drive potentials of intracellularly recorded neurons, it was demonstrated that synaptic inhibition plays a critical role in shaping expiratory activity in vivo (259). Moreover, glycinergic and GABAergic inhibition plays a critical role in generating the inspiratory ramp in vivo (199, 482, 483), and inhibition is critical for regulating the duration of inspiratory activity (146). Synaptic inhibition also has an important role in controlling the gain of respiratory drive potentials, as shown for various respiratory neurons (270, 624). Interestingly, there are various forms of inhibitory control mechanisms, which include phasic and tonic inhibition occurring during the silent and active respiratory phases that differentially regulate ongoing respiratory activity, and behaviorally associated changes in respiratory activity (110, 624). Over the past couple of years, there has been renewed interest in understanding how synaptic inhibition shapes respiratory activity and how different areas of the respiratory network communicate via synaptic inhibition. Most of these studies confirm the importance of synaptic inhibition in the generation of various characteristics of the respiratory rhythm (3, 12, 462, 515), and they also demonstrate that inhibition is an important determinant for the interaction between different areas of the respiratory network. One of these studies shows, for example, inhibitory interactions between the parafacial respiratory group (pFRG) and the preBötC (166). The pFRG seems to be critical for the generation of active expiration, as shown in a series of elegant studies using modern in vivo approaches (142, 144).
Inhibition in the in vitro network
While it is sometimes implied that synaptic inhibition is only important in the generation of the in vivo intact rhythm, it should be emphasized that the importance of synaptic inhibition has been extensively demonstrated within the isolated network. In the rhythmically active network, bursting neurons are bombarded by barrages of synaptic inhibition (441). Fifty percent of inspiratory neurons are thought to be glycinergic (600), and GABAergic inhibition by inspiratory neurons is also critical (278). When exposed to blockers of synaptic inhibition, the preBötC loses the ability to generate different phases of activity, and neurons that are active in phase with postinspiration begin to discharge in phase with inspiration (41, 299, 441, 496). The population activity generated by the isolated respiratory network changes from a bell-shaped, augmenting-like inspiratory activity to a decrementing short duration inspiratory activity upon blockade of synaptic inhibition (299). As also shown in vivo, synaptic inhibition occurs not only during the expiratory phase, but also concurrently during inspiration in the in vitro network (441). This inhibitory connectivity seems to contribute to the suppression of bursting in some but not all bursting neurons (564). Thus, inhibition controls the degree of bursting within the respiratory network. In a modeling study, it has been demonstrated that weak inhibition concurrently arriving at bursting neurons cannot only suppress bursting, but also induce bursts and complete synchronization in networks that possess strong desynchronizing connections (20). This modeling study is an important reminder that synaptic interactions can result in network dynamics and activity patterns that are not necessarily expected by intuition. The role of concurrent inhibition and the regulation of pacemaker properties are presumably also important in the intact network. While it is known that concurrent inhibition does exist in the intact network (118), it remains difficult to unambiguously demonstrate pacemaker neurons in an intact animal.
Blockade of synaptic inhibition
Although reciprocally organized network structures were discovered in numerous neuronal networks and proposed to be the driving principle for many neuronal networks (11, 53, 161, 190, 330, 412, 413, 466, 493, 535, 546), it became increasingly clear that the majority of networks continue to generate rhythmicity even in the absence of synaptic inhibition (202, 252, 293). Thus, in retrospect it is not surprising that respiratory rhythmic activity persisted following the blockade of synaptic inhibition in vitro (441, 496) and in situ (443). Although these findings were confusing to many researchers who believed in the half-center organization as the only driving principle of rhythm generation, this discovery does not imply that all neuronal networks are pacemaker driven or that synaptic inhibition is not important in any neuronal network. The finding that respiratory rhythmic activity can be generated in the absence of synaptic inhibition should never be interpreted as proof that synaptic inhibition is not important. Instead, it is another example for the building block hypothesis, indicating that synaptic inhibition is one of several building blocks that establish rhythmicity in the respiratory network. Thus, it is important to emphasize that although the persistence of rhythmicity in the absence of synaptic inhibition was first discovered in the isolated respiratory network, it does not imply that the rhythm generated in vitro does not involve synaptic inhibition.
Consistent with the building block hypothesis, one can arrive at the following general statement: if the respiratory network or any network continues to generate rhythmicity in the absence of any given building block, it does not mean that this particular cellular property is unimportant for the generation of this particular rhythm. Nor does it imply that the rhythm functions in the network without this particular building block. This conclusion applies not only to the various glycinergic or GABAergic mechanisms but also to the INaP current, the ICAN current, or any other current. This lesson has been learned not only from the respiratory network, but also from studies performed in neuronal networks across the animal kingdom over the past century. Therefore, it should be questioned whether the search for the obligatory rhythm generating mechanism or the building block on which the entire rhythm depends is really meaningful. Is this search merely a theoretical exercise that potentially causes more harm than benefit to the understanding of the mechanisms underlying rhythm generation? In analogy: will we better understand an airplane if we identify the part that is most critical? Can there be just one part that is critical? Is it not more important to understand how the different parts work together to make a functional airplane rather than trying to determine which part is the most critical one?
Synaptic inhibition and the control of the motor output
Synaptic inhibition plays not only a critical role in generating the respiratory rhythm, the respiratory patterns, and the different respiratory phases, but also in regulating the respiratory motor output. In fact, the search for the inhibitory mechanisms that shape motor output has an equally long history as the studies that characterized the inhibitory mechanisms of the respiratory network (e.g., reference 25). Although it is not within the scope of this review to cover this exciting research area in sufficient depth, it is important to emphasize that synaptic inhibition plays a critical role in actively shaping the respiratory motor output within the XII and phrenic nucleus (35, 378, 395, 396, 511, 571, 572). One important source for the synaptic inhibition observed in the hypoglossal motoneurons arrives from GABAergic neurons located within the nucleus of roller (571, 572), which illustrates that the respiratory motor nuclei are not “follower nuclei” that are simple targets of the centrally generated respiratory rhythm and that relay this centrally generated activity simply to the muscles, but that instead these nuclei play a critical role in actively shaping the activity motor pattern, involving different sets of premotor nuclei.
Excitatory synaptic connections within the respiratory network
Excitatory synaptic transmission and the generation of normal respiratory activity (eupneic activity)
While the role of synaptic inhibition in respiratory rhythm generation has been a matter of ongoing debate, it was never questioned that synaptic excitation is critical for respiratory rhythm generation. AMPA receptors (AMPA-R) were identified early on as critical contributors to the generation of drive potentials in respiratory neurons (170, 501). Consistent with this conclusion, respiratory rhythmic activity at the level of the preBötC ceases upon the blockade of AMPA-R (165, 187, 296, 554). However, it is well established that multiple excitatory synaptic mechanisms are active within the respiratory rhythmic network. This multiplicity is critical for the generation of the large repertoire of behavioral functions that are served by this rhythm-generating network. A study by Ireland et al. (231) has elegantly demonstrated that the type of non-NMDA-R (probably AMPA) that mediate glutamatergic synaptic transmission within the preBötC are different from the non-NMDA-R mediating the drive to motoneurons (231).
For the generation of normal respiratory activity, there has been an increased understanding of how glutamatergic mechanisms and intrinsic membrane properties interact with each other to generate a burst of inspiratory activity. The activation of postsynaptic glutamatergic receptors leads to a transient Ca2+ influx that in turn activates a signaling cascade cumulating in the activation of ICAN that then causes a dramatic amplification of the inspiratory drive potential (392). An elegant study by Mironov illustrates how metabotropic glutamate receptors (mGluR 1,5) interact with a dendritic, intracellularly generated calcium transient which propagates to the soma. There it causes the activation of ICAN that then leads to a burst of activity, which is synchronized by non-NMDA-R, CNQX-sensitive synapses (347). The emerging calcium wave will lead to the activation of ICAN that is synchronized by synaptic mechanisms.
Subsequent computational studies that were aimed at finding the obligatory mechanism suggested that respiratory rhythmicity emerges from an interconnected network of glutamatergic neurons without the ability for autonomous bursting (464). These theoretical considerations are consistent with the so-called group pacemaker hypothesis as first proposed by Rekling and others (143, 446). While there is no question that glutamatergic synapses play a critical role in triggering ICAN, and thereby amplifying synaptic drive potentials, this hypothesis has been linked to a debate that questions the role of autonomous voltage-dependent bursting properties in respiratory rhythm generation. As discussed earlier, the usefulness of searching for the obligatory rhythm generation mechanism in a network that has a large catalogue of other synaptic, ionic, and modulatory mechanisms is questionable and potentially confusing to those outside the field. It is particularly confusing because nobody can deny the existence of bursting pacemaker neurons within the respiratory network. Moreover, there is currently no tool to unambiguously test the obligatory role of bursting pacemakers. Unfortunately, it is often overlooked that no pharmacological approach can specifically block all bursting neurons (404). As also discussed in this review, networks are highly dynamic and any manipulation of synaptic or intrinsic membrane properties will inevitably also alter the configuration of the network, making it impossible to know to what extent a network follows the same rules as before the manipulation. Although the search for the obligatory mechanism may create the impression of wild disagreement between the different groups of researchers, there is really more agreement than disagreement. There is little disagreement that glutamatergic synaptic transmission is highly diverse and complex, that these mechanisms are essential for the formation of the eupneic network activity, and that the intrinsic membrane property reflected in the activation of the ICAN is a critical mechanism in respiratory rhythm generation (143, 406). There is also general agreement that mGluR receptors are important contributors to respiratory rhythm generation (89, 296, 347, 392). Moreover, nobody disagrees that various forms of autonomous pacemaker activity do exist within the preBötC (243, 268, 404, 554, 563, 564, 566). Although there is little direct experimental evidence for direct recurrent excitation within the respiratory network, recent studies have demonstrated that isolated preBötC neurons can form small synaptically coupled micronetworks that can give rise to rhythmic bursts (347). Although the respiratory rhythm ceases in the presence of FFA and riluzole (404, 566), we do not know exactly why this is happening, in particular, since there is no drug available that specifically blocks all types of autonomously bursting neurons (as discussed earlier). Thus, any pharmacological manipulation will leave some bursting neurons functionally active. Aside from theoretical considerations of extreme conditions, there is not much evidence that the respiratory network will ever generate the rhythm based on only synaptic or only intrinsic membrane properties.
Differential synaptic and intrinsic mechanisms for the generation of normal and sigh respiratory activity
Within the preBötC, glutamatergic transmission differentially contributes to different forms of respiratory rhythms: in particular, in the simultaneous generation of eupneic and sigh activity (296, 297, 299). Both of these activities are generated in the isolated preBötC, and they are characterized by two distinct integrated waveform patterns and distinct population burst frequencies (299). Although distinctive at the activity level, there is not much evidence for two distinct populations of neurons, except for a small number of neurons that are active only during the sigh, but not eupneic activity (563). Indeed, as shown in Figure 1B, there is complete anatomical overlap between the areas involved in the generation of these activities. This poses the interesting issue of how the same population of preBötC neurons is capable of generating two distinct rhythms with very different timing and amplitude parameters: a fast, small amplitude eupneic and a slow, high-amplitude sigh activity (Fig. 2A,D). Lieske and Ramirez (296) proposed that the production of these two activities involves the differential activation of glutamatergic synapses with distinct properties. Fictive sigh burst generation seems to involve a glutamatergic synapse that is coupled to the P/Q-type Ca2+ channel and that is modulated by the activation of the group III metabotropic glutamate receptor (mGluR), mGluR8 (Fig. 6E). Moreover, sighs are more sensitive to the blockade of AMPA receptors (296). Thus, incomplete blockade of AMPA-R abolishes only fictive sighs. Based on these findings, it was suggested that sighs are emerging through excitatory connections with these specific properties that are different from those responsible for the generation of eupneic activity. The excitatory synaptic mechanisms contributing to the generation of fictive eupneic activity and, in particular, inspiratory drive potentials, are more dependent on the NMDA-R and also involve mGluR1 and mGluR5 (296, 297, 392), that is, metabotropic glutamate receptors that are distinct from those responsible for the generation of the sighs.
But, the differential generation of sigh and normal respiratory activities will not only depend on synaptic mechanisms. Blockade of the INaP current seems to abolish sighs (404), and Tryba et al. (563) identified a type of CI pacemaker neuron that depends on INaP and that concurrently exhibits two types of autonomous bursting with distinct characteristics (Fig. 9B): large amplitude bursts that are autonomously generated at a slow frequency, and small amplitude bursts that are generated at a faster frequency. As will be discussed in more detail in the section on neuromodulation, oxotremorine selectively inhibits the faster, small amplitude bursts, while it selectively activates the generation of the large amplitude bursts (Fig. 9B). As shown in the slower time scale, the two types of bursts are concurrently generated as oxotremorine is being washed out (Fig. 9B, lower trace). This finding is very intriguing because at the network level oxotremorine inhibits the fast and small amplitude eupneic activity and dramatically increases the frequency of the large-amplitude sigh activity (Fig. 9A). This raises the question of whether these two burst types are transmitted via different types of glutamatergic synapses that differentially amplify these signals to generate two rhythmic activities. Although many questions remain unresolved, the differential generation of sighs and normal respiratory activity is clearly another example indicating that rhythm generation emerges through the differential integration of synaptic, intrinsic, and modulatory properties.
Figure 9.
Network and cellular responses of respiratory neurons to muscarinic receptor activation. (A) Bath application of the muscarinic agonist oxotremorine stimulates fictive sigh activity (red arrows), while inhibiting eupneic activity (blue arrows). (B) Bath application of oxotremorine induces the generation of two distinct burst patterns recorded from a CI pacemaker neuron, isolated from fast synaptic transmission (modified, with permission, from reference 563).
Concluding remarks
While synaptic inhibition, synaptic excitation, and intrinsic neuronal properties are known contributors to the functional output from respiratory networks, it must be recognized that these circuit and intrinsic properties do not function in isolation from one another. They are integrated to generate stability while at the same time retain the dynamic responsiveness of the network to changing intrinsic and external environments. Understanding the details of synaptic integration in the larger respiratory network that includes the various distributed brainstem components will be one of the important challenges for the future.
Cellular Properties and the Integration of Central Chemosensation to O2 and CO2
Overview
To guarantee adequate ventilation at any given time, the respiratory network needs to quickly respond to changes in O2, CO2, and pH. The discovery of peripheral chemoreceptors that are exquisitely sensitive to O2, together with lesion experiments, led to the concept that peripheral chemosensors sense O2, while the central nervous system (CNS), in particular, the ventral surface of the medulla, senses CO2 (210, 212, 309, 310, 380, 478, 479, 480, 481). Although it has always been acknowledged that these different sites interact to form the respiratory responses (76, 171, 211, 285, 310, 325, 380), an anatomical separation between CO2 sensitivity originating within the CNS and O2 sensitivity originating in peripheral sensory organs dominated the field of respiration for many decades. Peripheral sensory neurons in the carotid body are thought to provide the primary neuronal input mediating acute respiratory responses, with central chemosensitive neurons contributing a secondary component and underlying more long-term adaptive responses. However, while it is widely considered as only a “secondary component,” there is an increased appreciation of the importance of the CNS in sensing both O2 and CO2.
Lessons learned from the central respiratory network can provide important insights that go beyond the immediate relevance for the neuronal control of breathing. As will be discussed in this section, the nervous system needs to maintain a very fine balance between hypoxia and hyperoxia to avoid the detrimental effects associated with any deviation from this balance. Any change in a network’s activity state will alter this balance if not concurrently coupled with an adequate network response. Depending on the particular behavioral, environmental, or intrinsic metabolic conditions, different neurons and different networks across the brain will be affected in a very differential manner. A “master peripheral or central sensor” for O2 and CO2 unlikely provides the adequate local sensitivity required for maintaining this fine homeostasis at the level of different neuronal networks distributed throughout the nervous system. Neuronal networks across the brain need to be capable of adjusting and maintaining this balance locally and must, therefore, also be able to sense O2 and CO2 locally. This is particularly important because the majority of neuronal networks across the nervous system are continuously active (206, 279). These networks are, therefore, in continuous demand of an adequate supply of O2 and CO2. Slight shifts in this ongoing intrinsic activity will be associated with changes in oxygen consumption that varies from network to network and from moment to moment. Each network needs to adequately respond to slight changes in oxygen consumption andCO2 production. Because 95% of the brain’s metabolic energy is devoted to maintaining ongoing intrinsic activity that characterizes our resting state (429), the process of chemosensation cannot be turned on or off by a master regulator. More likely, chemosensation must be considered as a continuous process that forms an integral part of a network’s ability to intrinsically generate activity.
Oxygen sensing and the integration within the preBötC network
Overview
The concept that chemosensation is an integral part of a network’s ability to intrinsically generate activity is best exemplified by the preBötC. Known for its role in respiratory rhythm generation, the preBötC also integrates incoming sensory information and has an exquisite intrinsic sensitivity to changes in its biochemical environment. Moreover, neurons in the preBötC and ventral respiratory group are located in proximity to arterioles in newborn mice (139). The responsiveness and reconfiguration of the preBötC has been extensively studied in reduced slice preparations, which is perhaps the most direct demonstration that this rhythm generating network is capable of intrinsically sensing oxygen, as other peripheral mechanisms can be excluded (213, 437, 548). But, the conclusions gained from this in vitro network are also supported by in vivo studies in which experimentally induced central hypoxia mediates biphasic changes in ventilation and motor output (90, 521). Solomon et al. (521), in particular, demonstrated that focal hypoxia in preBötC leads to an initial excitation of respiratory motor output, further supporting the notion that this important rhythm-generating network is also involved in the chemosensory response.
What makes oxygen sensitivity a truly integral part of this network is that different aspects of the underlying rhythm generating mechanisms express different sensitivities to changes in the oxygen environment. Thus, we propose that the rhythm generating mechanisms themselves constitute important sensors of O2. These sensitive mechanisms are the same cellular properties that are also discussed in this review in context of respiratory rhythm generation. Important for these considerations is the discovery that the isolated preBötC is capable of generating multiple patterns of rhythmic activity during changes in its experimental oxygen environment: eupneic activity, sigh activity, and gasping activity (299). Alterations in O2 evoke a sequence of changes in various synaptic (Fig. 10B) and cellular mechanisms (Fig. 10C). These alterations result in a dramatic change in the firing patterns of the neurons that are embedded within the functional network (Fig. 11A) and the firing patterns of the autonomously active neurons as characterized independently from the network (Fig. 11B). These changes transform the network from a fully integrated, very complex network that depends on multiple network and autonomous mechanisms to one that is dominated by pacemaker neurons that are dependent on the persistent sodium current as first postulated by Pena et al. (404) (Figure 12).
Figure 10.
Network reconfiguration of the preBötC during hypoxia, at the cellular and network level. (A) Typical biphasic network activity response of the preBötC during hypoxia, assessed by integrated population recording from the preBötC. After an early augmentation phase, activity enters a late depression phase. During this response, activity changes from a “fictive eupneic” to a “fictive gasping” mode of activity. Note the generation of multiple “fictive sighs” during the augmentation phase. (B1) Plot of spontaneous excitatory and inhibitory postsynaptic currents (ESPCs and IPSCs) recorded from a preBötC respiratory neuron in response to hypoxia. Note the dramatic reduction of IPSCs during hypoxia. (B2) IPSCs recorded in vivo from a respiratory neuron of an anesthetized cat, before and during hypoxia (modified, with permission, from reference 486). (C) Average activity of three subsets of autonomous active neurons, in response to hypoxia. Tonically firing autonomous spiking and cadmium-sensitive (CS) pacemaker neurons hyperpolarize and cease firing during hypoxia (State I and State II). (D) By contrast, cadmium-insensitive (CI) pacemaker neurons continue to fire under hypoxia, even with persistent exposure to hypoxic conditions (State III) (modified, with permission, from references 404 and 553).
Figure 11.
Examples of activity pattern changes in respiratory neurons during hypoxia. (A1) Current-clamp recording from an inspiratory neuron showing an augmenting burst pattern under control normoxic conditions, switching to a sharply rising and decrementing burst under hypoxia. (A2) This change in network-initiated burst patterns coincides with a decrease in spontaneous inhibitory postsynaptic currents (IPSCs), measured in voltage clamp. (A3) Example of an expiratory neuron that receives inhibitory synaptic input under normoxic conditions, switching to excitatory synaptic input under hypoxia. (B) Examples of autonomous activity in three subsets of respiratory neurons isolated from fast synaptic transmission, before and during hypoxia. Tonic firing neurons and CS-pacemaker neurons become silent, while CI-pacemaker neurons continue to generate bursting activity under hypoxia.
Figure 12.
The diverse interaction and relative contribution of multiple mechanisms (see Figure 3) in a single neuron gives rise to a neuronal population that possesses heterogeneous properties used to generate bursting and ultimately contribute to the eupneic rhythm. This integrated respiration during eupnea provides both stability and dynamic responsiveness. Hypoxia reconfigures the network to a state dominated by the INaP, KATP, and the AMPA receptor (AMPA-R) and various forms of neuromodulation. At the cellular level, INaP appears to be the basis for bursting involved with the gasping rhythm (i.e., INaP-driven gasping).
The time courses of the hypoxic response
The hypoxic response begins with a biphasic response (48, 130, 198, 369). An initial frequency and amplitude augmentation and increased number of sighs is followed by a frequency depression (Fig. 10A). In severe hypoxia, this marks the beginning of a process that leads to a gasping state (548). The transition from eupnea into gasping can be gradual, both in vitro (213, 299) and in vivo (587). During the transition into gasping, respiratory bursts can vary in rise time and burst duration. This transitory phase has been referred to as “pregasping” in vivo (587). Thus, one cannot assume that the respiratory network is fully reconfigured and in a gasping state when exposing the network to only very brief hypoxic or ischemic conditions or when exposing the network to mild hypoxia. In this pregasping state, some of characteristics of the network and modulatory response may still resemble that of the eupneic state, such as the duration of the inspiratory burst, while others like the rise time of the inspiratory burst may already look like gasping (530, 559). Figure 10 illustrates the matched time courses of the response of the network (Fig. 10A), of the synaptic excitatory (Fig. 10B1, excitatory post-synaptic currents (EPSCs)) and inhibitory activity (Fig. 10B1, inhibitory post-synaptic currents (IPSCs)), as well as the neurons that are autonomously active (Fig. 10C): the autonomously spiking neurons, CS pacemakers, and CI pacemakers (404, 554). The average responses reveal that autonomously spiking neurons cease to discharge first, followed by the cessation of CS pacemaker neurons, while CI pacemakers continue to be active throughout hypoxia. Thus, the pregasping state is characterized in part by the cessation of the autonomously active neurons and the continuously decreasing contribution from CS pacemaker neurons. But the gasping state is only reached when CI pacemaker neurons become the sole drivers (“State III”, Fig. 10B, blue). This network configuration becomes sensitive to riluzole, which blocks CI pacemaker neurons as well as the respiratory network during the gasping state [Figure 12; (401, 404)].
The hypoxic response and postnatal development
The time course of the hypoxic response changes during postnatal development. The augmentation phase of neonatal mammals, also including pre- and full-term human infants, is typically relatively brief, and it is followed by a sustained depression that can be maintained for a relatively long time (48, 191, 198, 283, 433, 473). In neonatal mammals, there is also an increased time to the first gasp, and autorescucitations are successful even after prolonged exposure to severe hypoxia (402). A sustained frequency depression with the generation of fictive gasps can also be seen in the neonatal in vitro preparations, suggesting that these effects are centrally generated and may originate within the medullary network. By contrast, adult mammals typically generate a sustained frequency augmentation followed by a depression that quickly leads to apnea (167, 227, 433, 452, 562). The time to the first gasp is shorter in mature compared to neonatal animals and the ability to resuscitate decreases with maturation (402). The shorter respiratory response leading to apnea, coupled with an enhanced augmentation in severe hypoxia is also similar to the response seen in vitro (437). As the mice mature, there seems to also be a decreased coupling between the rhythm that is generated within the preBötC and the XII motor output as demonstrated in vitro (402, 438).
The response to graded hypoxia
The respiratory network also shows a differential response when exposed to graded levels of sustained hypoxia. A dependency on the persistent sodium current (Fig. 12) is found only in severe hypoxia, and sustained severe hypoxia is also required to significantly alter the rise time of the inspiratory burst from an augmenting or bell-shaped bursting to a decrementing bursting (213). Both of these characteristics are the hallmarks of gasping. By contrast, the frequency of respiratory activity is altered even in moderate hypoxic conditions and is gradually affected as the hypoxic conditions become more severe. Thus, it is conceivable that the mechanisms governing the respiratory frequency have different oxygen sensitivity than those determining the rise time and riluzole dependency. The respiratory network begins to show a dramatic frequency increase as synaptic inhibition gets depressed and autonomously active neurons are beginning to shut down (Fig. 10A-C), but at a time when CS pacemakers are still active (Fig. 10C). This leads to the conclusion that the hypoxic response of the respiratory network is the consequence of multiple oxygen sensitivities inherent in the multiple synaptic, cellular, and network mechanisms that govern the different aspects of neuronal network activity (213).
Differential network sensitivities to pharmacological blockade
It is hypothesized that under well oxygenated and moderately hypoxic conditions, the respiratory network is not dependent on riluzole-sensitive mechanisms (213, 404) because additional mechanisms exist, such as FFA-sensitive bursting mechanisms (including CS pacemakers), calcium-dependent mechanisms, GABAergic, glycinergic, and glutamatergic synaptic mechanisms, plus a host of metabotropic mechanisms that also contribute to respiratory rhythm generation (Fig. 12, integrated respiration). This network state is already complex under in vitro conditions, and it is even more complex in the in vivo state (112, 464, 515). As the network transitions into the gasping mode, it becomes sensitive to riluzole [(401, 404), Figure 12"INaP-driven gasping”]. Despite the caveats associated with the use of riluzole as discussed in this review, it is striking that experiments performed in all the available preparations ranging from slices to whole animals arrive at similar conclusions, that is, the respiratory network under well- to moderately oxygenated conditions is not sensitive to the blockade of riluzole either in vitro (390, 404), in situ (532), or in vivo (401). In all these studies, the authors conclude that in this oxygenated state the respiratory network depends on multiple network mechanisms (404). In this state, the respiratory network is able to generate frequency and amplitude changes that may involve partial changes in network configuration.
Role of ATP and KATP channels in the hypoxic response
The KATP channels seem to play a critical role in the biphasic response to hypoxia (200, 348). As ATP levels drop in moderate and severe hypoxia, the activation of KATP channels could protect the respiratory network against unmitigated Ca2+ influx that can lead to Ca2+ overload and neurotoxicity. This protective response has been studied not only within the respiratory network, but also in networks throughout the nervous system (15). Demonstrated by Mironov et al. (350), the opening of a K+ conductance facilitates the closing of L-type Ca2+ channels during the frequency depression. The identity of this K+ conductance was proposed to be KATP, as it was inhibited by tolbutamide and glibanclamide, and opened with diazoxide. Hypoxic activation of KATP will concurrently occur with the phasic activation of the KATP that was already present in well oxygenation conditions. This phasic activation is thought to be linked to the mitochondrial membrane potential (ΔΨ) that oscillates in phase with the population rhythm as a potential mechanism to prevent Ca2+ overload, via mitochondrial uptake of intracellular Ca2+ (352). Hence, the hypoxia-mediated increased Ca2+ influx is mitigated by the activation of KATP that is accompanied with mitochondrial Ca2+ uptake. This process will preserve and contribute to rhythmogenesis during the initial phase of hypoxia. The rundown of the ICa in inspiratory neurons of the preBötC was delayed with hypoxia. Moreover, pharmacological blockade of L-type Ca2+ channels eliminates the initial frequency augmentation, while accelerating the occurrence of frequency depression (351).
While a drop in intracellular ATP may activate KATP channels, it is also known that during hypoxia extracellular ATP is actually released throughout the ventrolateral medulla from glia sources, which may contribute to the hypoxic ventilatory response via purinergic receptors (178, 179). Putative inspiratory neurons of the preBötC express the metabotropic purinergic receptor (P2Y1R) which when activated by ATP, opens an inward current that increases excitability, similar to that observed during the hypoxic frequency augmentation of the preBötC (312).
The role for KATP channels was also examined at the behavioral level using a mouse strain in which Kir6.2 was genetically deleted (353, 386). Deletion of Kir6.2 was found to clearly abolish the gasping response induced by decapitation. In response to moderate hypoxia (5.5% O2), Kir6.2 KO mice exhibited a significant intensification of the initial “augmentation” phase, manifested as an increase in both inspiratory frequency and length of the augmentation phase. In addition, the strength of “sighs” was diminished, although “sigh” frequency appeared unaffected in the Kir6.2 KO line. Oyamada et al. (386) suggested that the role for Kir6.2 in these hypoxic responses may be age dependent, as the respiratory effects of Kir6.2 deletion appear to be more prominent in older animals (4 weeks compared to 2 weeks postnatal). Future studies utilizing in vitro preBötC slices from Kir6.2 KO mice under controlled normoxic and hypoxic conditions may help unravel central versus peripheral contributions of KATP to respiratory control. It may also help to better integrate the findings at the behavioral level with those obtained at the cellular level.
The cessation of ICAN-dependent burst mechanisms
It is conceivable that the transition into gasping involves a severe drop in ATP levels that may result in KATP channels potentially inhibiting ICAN-dependent bursting mechanisms. The suppression of ICAN dependent mechanisms during hypoxia is a hallmark of the network reconfiguration (404). Given the importance of ICAN-dependent mechanisms in regulating the amplitude and shape of inspiratory activity (122, 392, 578), the loss of these mechanisms may contribute to the characteristic change in the shape of inspiratory activity from an augmenting, eupneic burst (Fig. 11, upper left panel) to a rapidly rising and decrementing gasping shape in severe hypoxia (Fig. 11, upper right panel).
The depression of synaptic inhibition
KATP may also mediate the inhibition of inhibitory neurons within the preBötC respiratory network. There is much evidence for the role of inhibitory neurons in the preBötC (69, 357), and it is known that many autonomously active respiratory neurons shut down at the onset of the hypoxic response (404). As shown in Figure 10C, the cessation of autonomously active neurons coincides with the dramatic decrease in the number of inhibitory postsynaptic potentials with hypoxia (299). As shown in the in vivo network, endogenously generated adenosine will accumulate and could also play a critical role in the generation of the depression of synaptic transmission (486). The decrease in synaptic inhibition and the cessation of autonomously active neurons also overlaps in part with the beginning of the frequency augmentation at the network level (Fig. 10A). However, based on in vitro experiments, the depression of synaptic inhibition seems not to be responsible for the frequency change, because pharmacological blockade of synaptic inhibition does not mimic the biphasic frequency changes (Lieske et al., 2000). By contrast, the hypoxia-induced synaptic depression seems to contribute to the alteration of the shape of the inspiratory burst from augmenting into decrementing (Fig. 11A, upper panel). This shape change can also be mimicked pharmacologically by blocking glycinergic mechanisms (299). Moreover, this decrease in synaptic inhibition will also contribute to the loss of expiratory activity and the phase switch of postinspiratory/ expiratory activity into inspiration, as can be seen in the isolated preBötC during exposure to severe hypoxia (438). Figure 11A exemplifies an expiratory neuron (11A, third panel on the left) as it loses the phasic inhibition and becomes rhythmically active in phase with inspiration [Figure 11A, third panel on the right (298, 299)]. This figure also illustrates the loss of IPSCs as the respiratory neurons are transiting into severe hypoxia (Fig. 11A, voltage clamp recording). Many of the in vitro findings resemble those obtained under in vivo conditions. A detailed characterization of changes as they occur within the in vivo respiratory network has been described by Richter et al. (452). At the behavioral level, the transition into severe hypoxia is also accompanied by a reconfiguration at the level of motoneurons. For example, the activity of the thyro-arythenoid muscle changes from expiratory to inspiratory, and a cessation of expiratory activity occurs at the level of the intercostal muscles (529).
For the preBötC, it was first proposed that in this reduced network state when ICAN-dependent mechanisms and synaptic inhibition are significantly reduced, CI pacemaker mechanisms become the main driver of rhythmic activity and exposure to riluzole causes the cessation of respiratory activity (404). This network state characterizes gasping with all its properties including a shortened inspiratory rise time and duration, a reduction in inhibitory synaptic mechanisms, and a silencing of ICAN-dependent bursting mechanisms. The aforementioned discussion also illustrates that the fact that this network state becomes riluzole sensitive is just one of many consequences associated with this network reconfiguration. In addition, INaP could become critical because hypoxia directly activates INaP as shown in other systems (245, 407). Clearly, the aforementioned changes in cellular properties are only some of the many changes that will contribute to the hypoxic response and network reconfiguration. There are many additional alterations that likely contribute but are not yet fully understood. Hypoxia is also associated with an increase in extracellular glutamate which will affect both ionotropic and metabotropic glutamate receptors. Moreover, hypoxia also activates Ca2+ channels (538) and as will be discussed in the Section “Cellular basis for chemosensing in preBotC neurons with a particular emphasis on the role of potassium currents” various K+ currents also play critical roles in chemosensation (240, 291). These currents will likely contribute equally not only to respiratory rhythm generation, but also to the hypoxic response of this rhythm-generating network.
Conclusion: Hypoxia and the generation of three distinct network states
In this section, we describe how the cellular building blocks involved in respiratory rhythm generation are also involved in governing the hypoxic response of the respiratory network level, not only in vitro, but also in vivo. Many concurrent processes contribute to the orchestrated response seen at the network and behavioral level. Many of these changes are interactive and occur gradually, as the network is increasingly exposed to hypoxia. Yet, there are clearly marked changes that characterize distinct network states (Fig. 10D): the first distinct state is the control state, in which the network operates on multiple cellular and network mechanisms (Fig. 10D, State I). This state is not sensitive to the blockade of riluzole or FFA when applied alone (401, 404). State II is characterized by the cessation of the autonomously active spiking neurons and the cessation of synaptic inhibition (Fig. 10D, State II). During this distinct state, the network configuration is characterized by the loss of expiratory activity and the transition from a frequency augmentation to a frequency depression. State III occurs as the CS pacemakers cease to discharge, which renders the network dependent on the activity of the CI pacemaker neurons, making it sensitive to the blockade of riluzole (Fig. 10D, State III). During this third distinct state, the network is generating gasping in severe hypoxia that is an activity that is in many aspects significantly different from the activity of the well-oxygenated state I.
Cellular basis for chemosensing in preBötC neurons with a particular emphasis on the role of potassium currents
At the cellular level, changes in pH and O2 are likely mediated by molecular mechanisms sensitive to protons (for hypercapnia), and immediate metabolic correlates of mitochondrial function, such as ATP, NAD+/NADH redox potential, or reactive oxygen species (ROS) (for hypoxia and hyperoxia associated with reperfusion). Within the brainstem, several structures and cell types have been studied for intrinsic chemosensitivity, including the serotonergic neurons of the raphe (133, 494), noradrenergic neurons of the LC (133, 149, 456) and neurons (362, 363), and glia (135, 136, 178, 593) in the retrotrapeziod nucleus (RTN). Although chemosensitivity can clearly be demonstrated in many cases for cells in these individual brainstem nuclei, a unique role of any particular structure for in vivo respiratory regulation remains uncertain. It also remains unclear whether one particular region plays a dominant role, or whether chemosensitivity emerges through a distributed network response involving all these and other nuclei (97, 197, 426, 448). In this context, several potassium channels have been examined as candidate chemosensors, particularly for acidosis within the normal physiological range produced under nonpathological hypercapnic conditions (pH 7.0–7.4). TASK1 (KCNK3), and TASK3 (KCNK9) are acidsensitive, outwardly rectifying “two-pore” potassium channels which contribute to constitutive K+ leak (24). Potassium conduction through these channels is blocked by external protons (IC50 of pH 7.3 for TASK1 and pH 6.5 for TASK3), by protonation of a titratable histidine residue conserved in both channels near the outer mouth of the channel pore (361). Mulkey et al. (363) examined the role for TASK1 and TASK3 as central respiratory sensors for hypercapnia by generating mouse strains deleted for TASK1 and TASK3, as single and double homozygous KO strains. Despite the widespread expression of both channel transcripts in brainstem, homozygous TASK1/TASK3 KO mice were viable and displayed no gross behavioral phenotypes. Recordings from chemosensitive neurons from the raphe revealed a blunted pH response (between pH 6.9–7.5) in mutant neurons deleted for TASK1 and TASK3 (24). However, similar recordings in chemosensitive RTN neurons revealed no difference in the same mutant background. In addition, whole animal plethysmography failed to demonstrate respiratory differences between WT and mutant animals in response to progressive hypercapnic challenges (3%, 5%, and 10% CO2). Thus, neither of these potassium channels appears to play an obligatory role for regulating normal respiratory responses to hypercapnia. More recently, a mouse KO strain was reported for TASK2 (KCNK5),which is actually a more structurally distant member of the two-pore gene family, despite its name (172). Although TASK2 is primarily expressed in non-neuronal tissue, lower expression was observed in restricted brain regions by driving the expression of lacZ (β-galactosidase) under the control of the native TASK2 promoter. A small bilateral cluster of neurons in the RTN were found to express TASK2, and these neurons were missing in the Phox2b27Ala/+ mutant background which mimics human congenital central hypoventilation syndrome (CCHS). In the Phox2b mouse model of CCHS there is a developmental lost of pH-sensitive glutamergic neurons in the RTN. Several studies suggest that these neurons likely mediate, at least in part, reflexive increases in respiration in response to hypercapnia (2, 117). Functionally, the loss of TASK2 was found to both augment acute respiratory response to hypercapnia (< 15 min) and abolish long-term “acclimated” suppression of respiratory frequency (> 30 min). Recordings of phrenic motoneuron bursts from isolated “en bloc” preparations provided support for the hypothesis that these effects could be attributed, at least in part, to central mechanisms. TASK2 KO preparations failed to exhibit a normal 50% decrease in expiratory burst frequency with anoxia, although responses to hypercapnia, normocapnic acidosis, or normocapnic alkalinization were not significantly different from WT. The mechanism for how TASK2 channels may sense O2, as suggested by these studies, as well as the details of how central mechanisms may integrate with peripheral mechanisms, remain to be explored more fully. Taken together, these studies suggest that the functional contributions of individual two-pore potassium channels to central respiratory circuits are probably redundant.
Although TASK channels are sensitive to external pH, chemosensitive raphe neurons, and perhaps other neurons, sense hypercapnia by changes in internal pH. Therefore, potassium channels that display demonstrated modulation by internal pH within the normal physiological range are attractive candidates for pH sensors in chemosensitive neurons. Among the class of inward rectifier potassium channels encoded by the large Kir gene family, heteromeric channels formed by Kir4.1 and Kir5.1 have emerged as a potential candidate for a pH-sensor active in respiratory neurons (240, 604). Heteromeric Kir4.1/Kir5.1 potassium channels are specifically inhibited by internal protons, with an IC50 of approximately 7.4 and an IC80 of approximately 7.1. This mirrors the normal nonpathological working range of internal pH experienced by neurons subjected to hypercapnia. Both Kir4.1 and Kir5.1 transcripts are found abundantly in brainstem, although expression is widespread and does not appear restricted to respiratory-related regions (602). Specific blockers for Kir4.1/Kir5.1 are not available, so pharmacological manipulation to study the role for this channel in preBötC rhythmogenesis cannot be performed. In addition to its role as a neuronal pH sensor, this channel has also been proposed to serve as the pH sensor in astrocytes in the RTN, where it may contribute to chemosensitivity by modulating pH-dependent purinergic transmission between glia and respiratory neurons (178, 179, 593). Mouse lines null for either Kir4.1 or Kir5.1 have not been described, but respiration has been examined in a mouse KO line for Kir2.2 (387). Respiratory responses to hypercapnia were examined by whole animal plethysmography, but only modest differences were observed between WT and Kir2.2 mutants, and only transitory between postnatal days 14 and 15. This result is perhaps not unexpected since Kir2.2 does not exhibit overt pH sensitivity, although it likely can form heteromeric channels with Kir2.3 that does exhibit inhibition by external pH (569). Clearly, future experimentation with Kir4.1 or Kir5.1 mouse KO lines could be very revealing.
Concluding remarks
Central chemosensation emerges through the integration of the same cellular processes that are also involved in the generation of respiratory activity patterns. Ionic conductances such as K+-channels possess properties sensitive to molecular cues for changes in local oxygen and carbon dioxide microenvironments. The different synaptic and intrinsic membrane properties have different sensitivities to changes in their metabolic environment. In the case of hypoxia, these differential sensitivities give rise to a complex reconfiguration of the respiratory network, resulting in the transition from eupneic into gasping activity. The integration of chemosensitive properties within the very mechanisms that are also responsible for the generation of respiratory activity preserves respiratory functionality during changes in the metabolic environment that ultimately promotes survival during common yet sometimes unexpected environmental challenges.
G-protein Coupled Receptors and the Modulation of Cellular Properties
Overview
The cellular properties that govern excitability, connectivity, and chemosensitivity within the respiratory network are under continuous influence from numerous endogenously released neuromodulators. The neuromodulators best studied within the respiratory network include NE, serotonin (5-HT), acetylcholine (ACh), substance P (SP), ATP, TRH, somatostatin (SST), dopamine (DA), endorphins, and adenosine. Yet, there are many potential modulators that have not been examined so far. The intracellular mechanisms that underlie a neuromodulator’s action are diverse and interactive, and we are just beginning to appreciate this complexity. Indeed, for most neuromodulators very little is known about their specific actions within the respiratory network; thus, we can only infer from studies conducted in other systems. Almost all known neuromodulators act on G-protein coupled metabotropic receptors (Fig. 13, GPCRs). These metabotropic receptors are activated not only by neuromodulators, but also by the classical neurotransmitters glutamate and GABA, which creates a fascinating, highly complex interaction between metabotropic and ionotropic receptors (Fig. 3). Moreover, release of modulators and transmitters can be tonic and/or phasic, which adds a dynamic component to neuromodulation that is little understood. Neuromodulation targets tonically active and rhythmically bursting neurons and their synaptic interactions within the respiratory network, which results in a complex web of modulatory interactions inseparable from the rhythm generating and chemosensory functions of the rhythmically active respiratory network.
Figure 13.
Downstream pathways and effector channels for Gq/11, Gs, and Gi protein coupled receptors. Gs protein coupled receptors (GsPCRs) and Gq/11 protein coupled receptors (GqPCRs) are in general excitatory systems, and Gi protein coupled receptors (GiPCRs) are inhibitory systems. Both GsPCRs and GiPCRs regulate AC, cAMP, and PKA, and GqPCRs facilitate Ca2+ release from Ca2+ store and protein kinase C (PKC). These second messenger proteins up- and downregulate open probability of several voltage-dependent cation channels. PIP2 (phosphatidylinositol 4,5-biphosphate), PLC (phospholipase C), IP3 (inositol-1,4,5-trisphosphate), DAG (diacylglycerol), PKC, AC (adenylyl cyclase), cAMP (cyclic AMP), PKA (protein kinase A), ICAN (calcium-activated nonspecific cation current), Ih (hyperpolarization activated non-selective cation channels), GIRK (G protein coupled inward rectifier K+ channels), KCa2+ (Ca2+-activated K+ channels), Cav (voltage-dependent Ca2+ channels), Nav (voltage-dependent Na+ channels), M-current (muscarinic receptor activated K+ channels), and KATP (ATP-sensitive K+ channels).
The isolated respiratory network containing the preBötC has contributed significantly to our current understanding of this dynamic interplay. Although portrayed by some as a “simple network,” the preBötC when isolated in a slice preparation is continuously and endogenously modulated by numerous neuromodulators, which include NE, 5-HT, Ach, and SP (405, 406, 497, 498, 499, 500, 563, 565, 578). These neuromodulators regulate different cellular mechanisms that are critical for rhythm and pattern generation, as well as chemoreception. As similarly discussed for chemosensation, neuromodulation is also an integral part of the respiratory rhythm generating process. Neuromodulators exert their dynamic influence via a multitude of receptors, second messenger systems, and regulatory processes that also alter intracellular calcium. The diversity of surface receptors coupled to a large number of diverse intracellular mechanisms allows neuromodulators to tightly regulate neuronal discharge patterns and to control many different aspects of network activity. Our understanding of these interactions within the respiratory network has benefited from lessons learned from the multineuromodulatory network located within the crustacean stomatogastric (STG) ganglion. Although composed of only 25 to 30 cells, more than 25 neuromodulators and neuropeptides were identified within this network (323, 492, 513). Many neuropeptides in the STG bind to GPCRs (CCAP, CabTrp, mACh, Proc, FLRF, and PRCH) and partly converge to the same cation channels (541). It is very likely that many of the rules identified in this small but not simple invertebrate network, also apply to the relatively small but not simple mammalian respiratory network.
To achieve a better appreciation of the complexity and diversity of neuromodulation, we will begin with a brief general overview of the molecular biology that underlies neuromodulation. Neuromodulators that bind to GPCRs are generally classified into Gq/11-protein coupling GPCRs (GqPCRs), Gs-protein coupling GPCRs (GsPCRs), and Gi/o-protein coupling GPCRs (GiPCRs) (Fig. 13). The so-called GqPCRs activate phospholipase C (PLC) that induces the hydrolysis of phosphatidylinositol 4, 5-biphosphate (PIP2). The product of PIP2 hydrolysis is diacylglycerol (DAG) and inositol- 1,4,5-trisphosphate (IP3), which is important for the activation of the IP3 receptor on intracellular Ca2+ stores. Production of DAG and elevated intracellular Ca2+ may typically lead to the activation of protein kinase C (PKC) (Fig. 13). As demonstrated in a variety of systems, Ca2+ store depletion activates several ion channels (403). By contrast, the GsPCRs and GiPCRs are coupled to adenylyl cyclase (AC), cAMP, and protein kinase A (PKA) activity, which can then directly regulate ion channels (373), among other effectors (Fig. 13).
All three GPCRs and their downstream pathways engage in extensive cross-talk (Fig. 14). This cross-talk significantly affects physiological functions, and must, therefore, be considered when trying to understand how neuromodulation and plasticity affect respiratory functions. Initial computational approaches described the existence of cross-talk between PKA and PKC and different GPCRs in the invertebrate Aplysia (17). Subsequent computational models also incorporated cross-talk between GqPCRs and GiPCRs (151). In addition, there is prominent experimental evidence for cross-talk between separate GPCR signaling pathways. For example, PKA-PKC cross-talk exists in the presynaptic terminals attached to the nucleus basalis of Maynert (272) and in heart cells (608). cAMP leads not only to the activation of PKA, but also to the elevation of intracellular Ca2+, suggesting that this interaction results from cross-talk between PKA and PKC (457). These findings are very relevant for the respiratory system. Activation of both 5-HT1A (GiPCRs) and group mGluR (GqPCRs) results not only in PKA, but also PKC activation (157, 454). Cross-talk involving downstream cAMP has been reported for 5-HT1A and β-NE (GsPCRs) receptors (584). The cross-talk between these two receptors may also affect the level of voltage-gated Ca2+ influx (585). Additional examples of cross-talk exist between M3 ACh (GqPCRs) and β-NE, M3 ACh and M2 ACh (GsPCRs), kappa-opioid (GiPCRs), and β-NE receptors (220, 274, 408, 495). Within the respiratory network, cross-talk has also been identified in the neuronal control of long-term facilitation (218). Although cross-talk is highly relevant for understanding all aspects of neuromodulation, for simplicity, the next sections discuss these pathways separately.
Figure 14.
Cross-talk between Gq/11 and Gs system. It has been thought that each of the GqPCRs and Gs(i)PCRs systems are separated. However, recent studies suggest GsPCRs-related cAMP production or activation of PKA stimulates elevation of intracellular Ca2+ and activation of PKC (17, 151, 272, 457, 608).
The role of neuromodulators and G-protein coupled metabotropic receptors in regulating cation channels within the respiratory network
The activation of GPCRs modulates the gating properties of numerous ion channels (Fig. 3, Fig. 13). Several of these channels were discussed in previous sections, in the context of respiratory rhythm generation, chemoreception, and synaptic integration. Here, we will revisit some of these channels in the context of neuromodulation.
TRP channels and their modulation by GPCRs
TRP channels play critical roles in integrating not only intrinsic and synaptic properties, but also the modulatory inputs mediated by GPCRs. Our understanding of the diverse roles of TRP channels is still very limited for the respiratory system, However based on studies in a variety of other systems it can be expected that TRP channels will play equally important and diverse roles within the respiratory network. Activation of GqPCR can induce Ca2+ store depletion, which in turn facilitates the opening of store-operated nonselective cation currents (NSCCs) encoded by members of the TRP channel superfamily (152, 258, 469, 519, 601, 614), including TRPV6, and some TRPM and TRPC genes. The activation of TRPC channels by substance P could be mediated in part by this calcium-dependent mechanism (Fig. 15). Ben-Mabrouk and Tryba reported that the effect of NK1 receptor activation is inhibited by TRP channel blockers, and because the preBötC region contains TRPC3/7 immunostaining, it was suggested that TRPC3/7 receptors are a target NK1 receptor of modulation (21). Besides activating TRPC channels, GqPCRs also activate TRPV6 channels via their coupling to other second messenger cascades such as PIP2, DAG, and PKC (5, 561, 618). GqPCRs also activate TRPM4/5 through their coupling to DAG or IP3 induced Ca2+ release from internal stores (300, 576). This may be relevant for the neuronal control of breathing, since TRPM4/5 may be the ICAN, as postulated by Crowder et al. (89) and Mironov (347). ICAN was first described as a critical determinant of the respiratory network (404) at a time when the field was entirely focused on the role of INaP (51, 103, 104, 332, 446). Even though the exact molecular identity of ICAN remains controversial, it seems to be modulated by mGluRs 1/5 (89, 347, 392). In the functional network, ICAN is modulated by various modulators acting through GqPCRs. GqPCR receptors are known to activate TRPC (1, 2, 3, 5 and 6), TRPV6, and TRPM (3, 4, 5, 7, and 8) via a variety of second messenger systems (Fig. 15). It seems very likely that the effect of norepinephrine acting on the α-1 receptor is mediated through these GqPCR-activated pathways (Fig. 15). Norepinephrine specifically induces ICAN-dependent bursting via α-1 adrenergic receptors in autonomously spiking neurons, which facilitates and amplifies inspiratory activity (578). Following the blockade of ICAN with FFA, α-1-mediated amplification of inspiratory drive potentials was abolished (578). Interestingly, TRPM4/5 is activated by elevated internal Ca2+ that may result from receptor-mediated release of Ca2+ from internal stores (Liman, 2007; Vennekens and Nilius, 2007). This would be consistent with the hypothesized role of TRPM4 being activated in the soma of respiratory neurons following the activation of dendritic calcium waves (347). Because of their effects on cytosolic calcium, GqPCRs can activate TRPM4/5 in various ways, for example, by coupling to PIP2 or by IP3-induced Ca2+ release from intracellular stores (300, 576). Thus, in addition to phasic mGluR receptor-mediated activation of ICAN (89, 347, 392), it seems likely that many of the known endogenously active neuromodulators will contribute to “tonic” activation of ICAN within the respiratory network (578).
Modulation of channels of the K2P superfamily
GqPCRs are not only coupled to TRP channels, but they also modulate channels of the K2P superfamily which includes TREK, TASK, and TRESK (Fig. 15). Various forms of modulation have been described for these channels in a variety of systems. For example, both TREKs (1 and 2) are reported to be inhibited by the activation of PKC (131, 247, 364). But full suppression of TREK channels may also depend upon direct phosphorylation by PKA, acting through a reduction in PIP2 sensitivity (311). A channel that has been implicated in the neuronal control of breathing is the TASK channel (18, 19, 264, 363). TASK channels are targeted by substance P and other neuromodulators, and they are directly inhibited by the activated Gq α-subunit itself, or via the Gq-induced PLC-PIP2 pathway which may directly suppress this channel without phosphorylation by PKC (72, 92). The GqPCR-dependent regulation of the TRESK channels seems to differ from the modulatory regulation of TREK and TASK (131) in that Ca2+/calmodulin-dependent calcineurin directly affects TRESK, while TREK and TASK channels are unaffected by Ca2+ signaling (93). Although we are still at the beginning of our understanding of how respiratory neurons are modulated by different modulators, these examples illustrate that GqPCRs activation can potentially target many types of TRP and K2P channels which may be either activated or inhibited via various different intracellular mechanisms. These neurmodulators likely act by binding to GPCRs which then modulate TRP and/or K2P channels. Neutomodulators known to be critical for respiratory control include NE, 5-HT, ACh, SP, ATP, adenosine, TRH, dopamine, SST, GABA, and glutamate (112).
Norepinephrine and the modulation of GPCRs
Moreover, most of these modulators bind not only to GqPCRs, but also, to a variety of other GsPCRs or GiPCRs (Fig. 2; Fig. 14). NE receptors, for example, can be divided into three types of GPCRs, α-1, α-2, and β-NE receptors, which couple to Gq/11, Gi/o, and Gs-protein, respectively. From studies in a variety of systems we know that each of GPCRs affects a different complement of target ion channels. For example, NE binding to α-1 NE receptors (GqPCRs) suppress Cs+- sensitive K+ channels in human osteoblast, Ba2+- and TEA-sensitive heat-activated K+ channels in rat DRG neuron, and cardiac delayed rectifier K+ currents (262, 583, 605). α-2 NE receptors (GiPCRs) modulate, for example, N-type voltage-gated Ca2+ channels in cultured cerebellar neurons, delayed-rectifier K+ current, and Na+ current in rat sympathetic neurons (73, 292). β-NE receptors (GsPCRs) inhibit Ba2+- and TEA-sensitive heat-activated K+ channels in rat DRG neurons (605). Within the respiratory network, we know that NE produces differential modulation on the two main types of respiratory pacemakers, resulting in an amplitude modulation of CS bursting pacemakers and frequency modulation for CI bursting pacemakers (578), but how exactly modulation of these bursting mechanisms is achieved remains unknown.
Serotonin, GPCRs, and implications for SIDS
A similar complexity of modulatory effects underlies modulation by serotonin. With the exception of the ionotropic 5-HT3 receptor, the various subgroups of 5-HT receptors can be classified according to their pharmacological profiles of binding to various synthetic agonists and antagonists (Fig. 3). Serotonin binding to 5-HT1A receptors regulates hyperpolarization activated non-selective cation channels (Ih) in rat DRG and N- and L-type Ca2+ channels in NTS (55, 233). Activation of 5-HT2 receptors decreases K+ conductance and Ca2+- dependent K+ currents in subthalamic neurons (603). 5-HT4 receptors facilitate L-type Ca2+ channels in rat ventricular cardiomyocytes and Ih channels in CA1 pyramidal cells (32, 70). 5-HT7 receptors modulate Ih in rat DRG neurons (56). Within the respiratory network, 5-HT2, 5-HT4, and 5-HT7 receptors have been recognized as important modulators (201). In the preBötC, activation of 5-HT2 alters the bursting properties in CI and CS pacemaker neurons, effects that seem to be regulated through PKC (405). Bursting in CI pacemaker neurons seems to depend on activation by basally released serotonin, as blockade of 5-HT receptors abolishes bursting and reduces the persistent sodium current in CI bursting pacemaker neurons (405). As shown in vivo, in vitro, and in situ, the respiratory network becomes dependent on the persistent sodium current during hypoxia-induced gasping (399, 401, 404) and blockade of continuously activated 5-HT2 receptors abolishes gasping and abnormal respiratory activity (565). This is an interesting finding in the context of sudden infant death syndrome (SIDS), as there is corroborative evidence that gasping is an important arousal and autoresuscitation mechanism, and that serotonergic mechanisms are significantly disturbed in SIDS patients (40, 46, 119, 120, 398, 444, 565, 590). Thus, a disturbance in serotonergic mechanisms could lead to a failure to autoresuscitate via a diminished drive for gasping (404, 565). Disturbances in serotonergic mechanisms could become particularly significant during hypoxic conditions when 5-HT levels are normally elevated. 5-HT levels rise coincident with the onset of respiratory depression (455), an observation that is consistent with c-FOS staining which indicates that raphe neurons are activated during hypoxia (132). However, it would be wrong to suggest that 5-HT2 activation is the only mechanism that determines respiratory activity during hypoxia. Activation of 5-HT1A receptors in the preBötC abolishes respiratory activity, which is consistent with in vivo data that suggest that 5-HT1A receptors are expressed on respiratory neurons and that receptor’s activation suppresses excitability by activating on potassium currents (281, 453). Indeed, the existing data suggest a push-pull mechanism that relies on the differential activation of different serotonin receptors to determine the transitions from hypoxic augmentation to depression and, ultimately, to gasping. One possible scenario is that 5-HT1A receptor activation leads to the shutdown of the majority of respiratory neurons resulting in respiratory depression, thus protecting these neurons from excitotoxicity, as postulated by Richter et al. (455). By contrast, subsequent 5-HT2A activation may mediate the activation of bursting in persistent sodium-dependent pacemaker neurons which continue to be active to generate gasping, thus contributing to a critical role in autorescucitation, as postulated by Tryba et al. (565) and Pena et al. (404). But serotonin may not only play a critical role in hypoxic depression and autoresuscitation. A role of serotonin has also been implicated in recovery from opioid-mediated respiratory depression, since activation of 5- HT4 and 5-HT7 receptors can restore the respiratory rhythm and inhibition by opioids (322, 454).
Substance P and integration with other neuromodulators
Serotonin is coreleased with substance P (SP), and like 5-HT, SP also plays a critical role in generating hypoxic response (28, 273, 502, 547, 577, 597). SP activates the respiratory frequency at the network and behavioral level (105, 112, 185, 186, 405, 510, 555). In the in vitro and in vivo respiratory network SP stabilizes the respiratory rhythm. Like serotonin, SP slowly depolarizes spiking neurons and activates bursting in CS pacemakers. In CI pacemakers, it causes an increase in burst amplitude, frequency, and duration (406). SP activates neurokinin-1 (NK1) receptors (186) coupled to Gq/11-proteins that in turn activate phospholipase C (PLC). This intracellular cascade induces ICAN which contributes to the SP effects on burst amplitude. As described above, these modulatory effects involve the activation of TRP channels. How serotonin, substance P, as well as norepinephrine interact in a state-dependent manner has recently been studied in both intact and in vitro preparations (112). Based on these studies, it was concluded that the activation of NK1 receptors may work only within a narrow range of the awake-sleep state, as the activity of NK1 receptors is completely masked by the concurrent activation of α-1 NE or 5-HT2 receptors (112). This study focused on the interaction and convergence of these neuromodulators at the level of the GqPCRs. More detailed information will be needed as it relates to the potential cross-talk and intracellular interactions that govern this convergence (111).
Acetylcholine and the modulatory control of breathing
Cholinergic inputs provide equally important modulatory drive to the respiratory system, where they modulate nicotinic and muscarinic receptors (50, 80, 174, 204, 368, 497, 498, 500). Muscarinic ACh (mACh) receptors can be classified into two types: (1) M2 and M4 ACh receptors acting as GiPCRs, while (2) M1, M3, and M5 Ach receptors act as GqPCRs. All of these ACh receptor subtypes are localized within the CNS. As shown in a variety of different systems, M2 AChRs generally increase K+ and decrease Ca2+ conductances, while M3 AChR inhibit TREK-2 channels (247), activate the NALCN channel (540), and inhibit the TASK-1 channel (37). Within the preBötC, M3 ACh receptor antagonists increase the input resistance of preBötC inspiratory neurons, suggesting that cation channels (K+ channels) are closing under this condition (500). Muscarinic modulation has also been linked to SIDS, as prenatal nicotinic exposure seems to significantly suppress muscarinic receptor signaling and activates nicotinic modulation (80). This is interesting, as muscarinic receptor activation seems to be involved in the generation of sighs and concurrent depression of eupneic respiratory activity, thus representing another form of a push-pull mechanism for respiratory control (563). At the cellular level, cholinergic modulation differentially altered two types of bursting in a subset of CI pacemakers (563). As observed by recordings from a single neuron, cholinergic activation concurrently enhanced the frequency of large-amplitude bursting, while inhibiting the frequency of the relatively small-amplitude bursts. As demonstrated in a study by Tryba et al. (563), muscarinic activation altered not only the ratio of autonomously generated low and large amplitude bursts at the cellular level, but it also altered the ratio between small and large amplitude population bursts at the network level. The frequency of low-amplitude bursts representing fictive eupneic activity was inhibited, while the frequency of large amplitude bursts representing fictive sigh activity was increased. Thus, the shift in the autonomous activity generated within single neurons mirrored the activity of the network. The frequency of large amplitude sighs was significantly increased and small amplitude eupneic bursts were significantly decreased both in slices and the working heart brainstem preparation, which suggests that this is not just an “in vitro artifact” (563). This opens the intriguing hypothetical possibility that this modulatory push-pull mechanism may originate at the level of single neurons. These effects are likely mediated by M3 muscarinic receptors. But again, it must be emphasized that this most certainly is not the complete pictire, as ACh modulates respiratory activity not only via muscarinic, but also nicotinic receptors (497, 498, 499, 500, 563).
The generation of sighs has also been implicated in the arousal and autoresuscitation response, a failure of which may lead to SIDS (158, 404, 550, 551). Relevant for SIDS is also the fact that cholinergic mechanisms play a critical role at the interface between breathing and sleep (287). Intrinsic cholinergic drive is in part mediated by the pedunculo-pontine tegmental area, an area which is known to cause breathing instabilities and an increased generation of postsigh apneas (476). Thus, cholinergic modulation is not only relevant in the context of SIDS, but also sleep apnea (59, 253, 557). Carbachol injections into the peduncolo-pontine tegmental area significantly increase the apnea index in intact rats (59). Moreover, glutamate injection into this region causes erratic breathing and apneas (474, 475). These respiratory effects are possibly mediated by cholinergic action on the respiratory neurons within the preBötC, involving mechanisms studied by Tryba et al. (563), especially since the respiratory effects seem to be closely related to cholinergic neurons (557). Much like the situation already discussed for the preBötC, a complex integration with serotonergic modulation also plays a critical role in these pontine modulatory effects (476). Although it goes beyond the scope of this review, to fully understand the neuronal control of breathing and its state-dependent relationship to sleep and wakefulness, it will be important to integrate all modulatory processes not only within the medulla as emphasized in this review, but also within the pons, hypothalamus, and many other supraspinal regions (65). These modulatory processes potentially include cholinergic (94), serotonergic (575), cannabinoids (58), orexinergic (124, 228, 254, 276, 377, 549, 552, 598, 610), adenosine (573), and histaminergic mechanisms (123, 354).
Prenatal nicotine exposure and long-term plasticity
Given that sleep and breathing are closely linked by their common cholinergic mechanisms, and that nicotine is a major risk factor in SIDS, there is increased interest in understanding the consequences of prenatal nicotine exposure on the developing respiratory network (54, 80, 159, 223, 225, 420, 421). Indeed, it has been elegantly demonstrated that prenatal nicotine exposure decreases excitatory synaptic transmission within the XII nucleus and increases intrinsic excitability of XII motoneurons (421). Moreover, prenatal nicotine exposure increases the frequency of spontaneous apneas and, most dramatically it blunts the hypercapnic response and reduces the hypoxic response (137, 224). Prenatal nicotine exposure also alters long-term plasticity, an effect that becomes particularly relevant in the context of repeated hypoxic exposures (163). Direct effects on the respiratory network alter the respiratory frequency due to an effect on the α4/β2 nicotinic ACh receptors located presynaptically on glutamatergic neurons (319, 420). However, at the same time, prenatal nicotine exposure strengthens GABAergic synaptic transmission (318). An interesting opportunity to study the developmental impact of prenatal nicotine exposure is offered by the amphibian model system which exhibits different distinct developmental stages (45).
ATP and purinergic modulation
Neuromodulatory processes associated with ATP have also received considerable attention. In particular, this is the case, since ATP is the major energy source for the maintenance of cells, and also serves as an important extracellular agonist for ATP-specific receptors referred to as purinergic P2 receptors (P2Rs) (49). P2Rs can be divided into P2X and P2Y receptors. The former receptor type form non-GPCR ionotropic receptors, while the latter are metabotropic GPCRs. As discussed earlier for the other modulators, many different ionic mechanisms are associated with purinergic modulation. ATP binding to P2Y1 receptors (GqPCRs) affects Ih channels in trigeminal neurons (222), Ca2+ signaling in cultured rat hippocampal astrocytes (266), and inwardly rectifying cation permeability in Xenopus oocytes (379). The respiratory rhythm generated within the preBötC is sensitive to the activation of both P2X2 and P2Y1 receptors, which excite the respiratory rhythm during hypoxia conditions (179, 313). The P2Y1R-mediated increase in respiratory frequency appears to involve the activation of a mixed cationic conductance within the preBötC (312). The role of adenosine in mediating the hypoxic response has also been studied in vivo (453, 455, 486).
ATP is cleared from the extracellular space by being sequentially metabolized to ADP, AMP (595), and adenosine (257). Adenosine has long been known to reduce neurotransmitter release (207). Thus, metabolized adenosine that derives from ATP performs a negative feedback on both pre- and postsynaptic sites. Adenosine binds to adenosine (A) receptors classified as A1 (GiPCRs), A2A (GsPCRs), A2B (GsPCRs and GqPCRs), and A3 receptors (GiPCRs). Activation of A1 receptors inhibits neuronal excitability mediated by the modulation of N-type Ca2+ channels, both within the peripheral nerve system (PNS) and the CNS (365, 623), and facilitates G-protein-coupled inwardly rectifying K+ (GIRK) channels and small conductance Ca2+-dependent K+ (SK-KCa) channels (79). A3 adenosine receptors attenuate the Ca2+ rise accompanying NMDA-R activation in retinal ganglion cells (619), and activate a PKC-sensitive Cl− current in ciliary epithelial cells (508). Within the respiratory network, adenosine acting on A1 receptors hyperpolarizes respiratory neurons, whereas blockade of A1 receptors increases respiratory activity, suggesting that A1 receptors are tonically activated in preBötC respiratory neurons (486).
TRH, dopamine, and somatostatin
Early on, thyrotropin-releasing hormone (TRH) receptors have been implicated in respiratory control (99). Two types of TRH receptors (TRH1 and TRH2) have recently been recognized as GqPCRs (129). Within the respiratory network, TRH receptors are localized in NTS and the hypoglossal nucleus (XII nucleus). Further, it was recently published that TRH is coreleased with serotonin within the preBötC from the raphe obscurus (423). TRH induces rhythmic bursting in neurons within respiratory-related regions of the guinea pig NTS (99) and it stimulates central respiratory rhythmic activity (219). However, it is still unknown what molecular type(s) of TRH receptors (TRH1 or TRH2) mediate(s) the effects on respiratory rhythms (111, 216).
Dopamine has also been implicated in respiratory control (280). The dopamine receptors are divided into two types: (1) D1-like (D1 and D5) (GsPCRs) and (2) D2-like (D2, 3 and 4) (GiPCRs). Much is known about the role of D1- like receptors in a variety of systems. These receptors modulate voltage-dependent Ca2+ channels in rat striatal aspiny neurons, Ih conductance in rat retinal ganglion cells, resting K+ conductance in mouse subthalamic nucleus, and nonselective cation channel in primary cultured striatal neurons (10, 74, 314, 320). Within the respiratory network, D1-like receptor agonists prevent opioid receptor induced tonic discharges in expiratory neurons (280). D2-like (including D4) receptors activate nonselective cationic conductances in dorsal raphe serotonin neurons (9), and it has been demonstrated that D4 receptors disperse synchronization of respiratory neurons within the pFRG (162).
Somatostatin (SST) receptors have been used to define the anatomical limits of the preBötC (488). All SST receptor subtypes belong to GiPCRs. SST1 receptors activate inwardly rectifying K+ conductance (75, 241, 255). In insulin-producing INS-1 cells SST2 and SST3 receptors suppress R-type voltage-gated Ca2+ channels (340). SST4 receptors inhibit spiking and L-type voltage-gated Ca2+ channels in rat retinal ganglion cells (140), and they couple to Kv7 (KCNQ) channels (M-currents) in hippocampal CA1 pyramidal neurons (428). SST5 receptors activate both KATP channels and GIRK channels in MIN-6 cells (517). Thus, in most systems Gi/o protein coupling of SST receptors has an inhibitory action. Not surprisingly, within the respiratory network SST receptors suppress the respiratory rhythm (308). But the specific subtypes of SST receptors are still not identified in the preBötC (308, 544).
Metabotropic GABA and glutamate receptors
GABA and glutamate receptors should also be considered in the context of neuromodulation. GABAB receptors are metabotropic receptors (GiPCRs), and within the respiratory network GABAB receptors modulate GIRK channels (415), Ba2+-sensitive K+ conductances (242), and voltage-dependent calcium currents (393). The mGluRs are generally divided into group I, II, and III receptors (296). Group I mGluRs are predominantly localized postsynaptically, while group II and III mGluRs are expressed at both pre- and postsynaptic sites (373). In a variety of systems it has been demonstrated that group I mGluRs modulate ICAN currents, Ih currents, TRP-like channels, and K2P channels (39, 72, 82, 128, 138, 256, 391, 392). For inspiratory neurons, it has been demonstrated that glutamate binds to group I mGluRs, which supports ICAN-dependent mechanisms (347, 392). Moreover, glutamate seems to bind also to group III mGluRs to modulate the generation of sighs [Figure 6 (296)]. Interestingly, group III mGluRs are known to exert their effects via the cAMP second messenger pathway involving the activation of Gi-proteins [Figure 13 (83, 366)]. However, pharmacological manipulations suggest that the effects on the sighs involve a direct inhibition of P/Q type calcium channels via the G-protein βγ-subunits because manipulating the downstream second messenger pathways had no effects on the sigh (208, 229, 296, 344).
The endogenous release of neuromodulators within the respiratory network
There is a large body of literature on the endogenous release of neuromodulators within the respiratory network. Nore-pinephrine is, for example, released from A5 and A6 nuclei, which provides the preBötC with local medullary input from noradrenergic neurons (112). Endogenous NE release continuously facilitates the respiratory rhythm through an integrated activation of α-1, α-2, and β-noradrenergic receptors (112). Under synaptically isolated conditions, NE differently affects different types of inspiratory neurons within the preBötC in vitro (578). NE induces ICAN-dependent bursting properties in spiking neurons (Fig. 4), and depolarizes CI bursting pacemakers and increases their burst frequency. In CS pacemakers, NE increases the burst amplitude of the depolarizing drive potential, as well as the number of action potentials during the burst. But NE does not affect the burst frequency of CS pacemakers (578). This differential effect is preserved at the network level, since only the modulation of population burst amplitude, but not frequency, depends on the activation of ICAN (578). Therefore, by inducing bursting in pacemaker neurons, NE cannot only change the balance between bursting and spiking pacemakers, but also the differential modulation of frequency, amplitude, and burst shape among CI and CS pacemakers, which may have very specific consequences at the network level.
The preBötC receives, in addition, innervation from 5-HT and SP containing terminals derived from raphe nuclei, and it has been demonstrated that 5-HT and SP are indeed functionally co-released within the preBötC (423). Ruangkittisakul et al. reported that the preBötC generates eupneic rhythmic activity under the influence of caudal structures and tonically released TRH-like transmitters, while eupnea-sigh activity is predominantly controlled by the influence of rostral structures and SP-like transmitters (461). This raises the interesting concept that different rostro-caudal structures of the respiratory network may be associated with distinct modulatory influences that differentially regulate different modes of respiratory activities. Electrostimulation of the raphe magnus facilitates 5-HT release and leads to the activation of 5-HT2 receptors within the preBötC, which resulted in an increase in respiratory frequency (112). The respiratory rhythm within the preBötC is also continuously modulated by direct cholinergic projections. Physostigmine, an acetylcholinesterase inhibitor, microinjected into the preBötC, increases the respiratory rhythm, which suggests that the respiratory rhythm is continuously activated by basal endogenous ACh release (500). Although it is sometimes automatically assumed that modulatory effects within the preBötC are neuronal in nature, it must be emphasized that glia cells likely play an equally critical role in neuromodulation. ATP, for example, is stored and released from glia cells located at the ventral surface of medulla. These glia cells likely play an important role in respiratory rhythm generation, in response to changes in pH and hypoxic conditions (178, 528).
Concluding remarks
Complex modulation of cellular activity in respiratory neurons is achieved through a variety of neuromodulators. Various intracellular second messenger pathways are activated by G-protein coupled receptors, with the potential for interplay and cross-talk between these pathways. An understanding of these complex interactions will provide important insights into the modulation of respiratory activity and its ability to adapt to environmental and behavioral cues. Pathophysiological consequences may arise from disorders of these neuromodulatory mechanisms. While most existing studies focus on neuronal modulation, much needs to be learned about the modulatory role of glia cells.
Conclusion
This review provided a broad overview of the various cellular properties of brainstem respiratory neurons. We described the various inward and outward currents, G-protein coupled receptors, and intracellular mechanisms that modulate these ionic currents and the functional context in which these currents operate. The emerging picture is exceedingly complex and dynamic. This dynamic property applies not only for the processes that govern the generation of the respiratory rhythm, but also for the processes that adapt the respiratory network to changes in the behavioral and metabolic states of the organism. Indeed, all cellular properties are integrated to varying degrees into every aspect of respiratory function, and it is impossible to functionally separate roles such as rhythm and pattern formation, chemosensation, and neuromodulation. The various cellular properties described in this review function as building blocks of the respiratory network and it is safe to conclude that there is no function that depends on just a single cellular property or principle. These building blocks are dynamically activated and deactivated, and they converge and diverge in the behaving animal. Even an apparently similar type of breathing behavior, such as eupnea, may involve different configurations of the respiratory network. The artificial removal of any of these building blocks will evoke transitions into different network states that may or may not replicate certain aspects of a given behavior. Although much has been learned about cellular integration within the preBötC, it is clear this network is integrated in a wider network that extends throughout the neuronal axis. Together, all these interactions are responsible for the generation of breathing, which is clearly one of the most integrated and important behaviors of any organism.
Acknowledgements
This study was supported by NIH grants to JMR.
References
- 1.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. Akey role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 2.Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:19–25. doi: 10.1016/j.resp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, th, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 5.Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- 6.Albillos A, Neher E, Moser T. R-type Ca2+channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci. 2000;20:8323–8330. doi: 10.1523/JNEUROSCI.20-22-08323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alzheimer C, Schwindt PC, Crill WE. Postnatal development of a persistent Na+ current in pyramidal neurons from rat sensorimotor cortex. J Neurophysiol. 1993;69:290–292. doi: 10.1152/jn.1993.69.1.290. [DOI] [PubMed] [Google Scholar]
- 8.Aman TK, Raman IM. Inwardly permeating Na ions generate the voltage dependence of resurgent Na current in cerebellar Purkinje neurons. J Neurosci. 2010;30:5629–5634. doi: 10.1523/JNEUROSCI.0376-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aman TK, Shen RY, Haj-Dahmane S. D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J Pharmacol Exp Ther. 2007;320:376–385. doi: 10.1124/jpet.106.111690. [DOI] [PubMed] [Google Scholar]
- 10.Aosaki T, Kiuchi K, Kawaguchi Y. Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. J Neurosci. 1998;18:5180–5190. doi: 10.1523/JNEUROSCI.18-14-05180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arshavsky YI, Deliagina TG, Orlovsky GN, Panchin YV, Popova LB, Sadreyev RI. Analysis of the central pattern generator for swimming in the mollusk Clione. Ann N Y Acad Sci U S A. 1998;860:51–69. doi: 10.1111/j.1749-6632.1998.tb09038.x. [DOI] [PubMed] [Google Scholar]
- 12.Baekey DM, Molkov YI, Paton JF, Rybak IA, Dick TE. Effect of baroreceptor stimulation on the respiratory pattern: Insights into respiratory-sympathetic interactions. Respir Physiol Neurobiol. 2010;174:135–145. doi: 10.1016/j.resp.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballantyne D, Andrzejewski M, Muckenhoff K, Scheid P. Rhythms, synchrony and electrical coupling in the Locus coeruleus. Respir Physiol Neurobiol. 2004;143:199–214. doi: 10.1016/j.resp.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Ballantyne D, Richter DW. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballanyi K. Protective role of neuronalKATP channels in brain hypoxia. J Exp Biol. 2004;207:3201–3212. doi: 10.1242/jeb.01106. [DOI] [PubMed] [Google Scholar]
- 16.Bant JS, Raman IM. Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons. Proc Natl Acad Sci U S A. 2010;107:12357–12362. doi: 10.1073/pnas.1005633107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxter DA, Canavier CC, Clark JW, Jr, Byrne JH. Computational model of the serotonergic modulation of sensory neurons in Aplysia. J Neurophysiol. 1999;82:2914–2935. doi: 10.1152/jn.1999.82.6.2914. [DOI] [PubMed] [Google Scholar]
- 18.Bayliss DA, Sirois JE, Talley EM. The TASK family: Two-pore domain background K+ channels. Mol Interv. 2003;3:205–219. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- 19.Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K(+) channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- 20.Belykh I, Shilnikov A. When weak inhibition synchronizes strongly desynchronizing networks of bursting neurons. Phys Rev Lett. 2008;101:078102. doi: 10.1103/PhysRevLett.101.078102. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Mabrouk F, Tryba AK. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur J Neurosci. 2010;31:1219–1232. doi: 10.1111/j.1460-9568.2010.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benarroch EE. Neuronal voltage-gated calcium channels: Brief overview of their function and clinical implications in neurology. Neurology. 2010;74:1310–1315. doi: 10.1212/WNL.0b013e3181da364b. [DOI] [PubMed] [Google Scholar]
- 23.Bengtson CP, Tozzi A, Bernardi G, Mercuri NB. Transient receptor potential-like channels mediate metabotropic glutamate receptor EPSCs in rat dopamine neurones. J Physiol. 2004;555:323–330. doi: 10.1113/jphysiol.2003.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger AJ. Respiratory gating of phrenic motoneuron responses to superior laryngeal nerve stimulation. Brain Res. 1978;157:381–384. doi: 10.1016/0006-8993(78)90046-x. [DOI] [PubMed] [Google Scholar]
- 26.Berkefeld H, Fakler B. Repolarizing responses of BKCa-Cav complexes are distinctly shaped by their Cav subunits. J Neurosci. 2008;28:8238–8245. doi: 10.1523/JNEUROSCI.2274-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 28.Berner J, Shvarev Y, Lagercrantz H, Bilkei-Gorzo A, Hokfelt T, Wickstrom R. Altered respiratory pattern and hypoxic response in transgenic newborn mice lacking the tachykinin-1 gene. J Appl Physiol. 2007;103:552–559. doi: 10.1152/japplphysiol.01389.2006. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharjee A, von Hehn CA, Mei X, Kaczmarek LK. Localization of the Na+-activated K+ channel Slick in the rat central nervous system. J Comp Neurol. 2005;484:80–92. doi: 10.1002/cne.20462. [DOI] [PubMed] [Google Scholar]
- 31.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 32.Birkeland JA, Swift F, Tovsrud N, Enger U, Lunde PK, Qvigstad E, Levy FO, Sejersted OM, Sjaastad I. Serotonin increases L-type Ca2 current and SRCa2 + +content through 5-HT4 receptors in failing rat ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H2367–H2376. doi: 10.1152/ajpheart.01375.2006. [DOI] [PubMed] [Google Scholar]
- 33.Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–311. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, Storck T, Baetscher M, Jerecic J, Maylie J, Knaus HG, Seeburg PH, Adelman JP. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000;289:1942–1946. doi: 10.1126/science.289.5486.1942. [DOI] [PubMed] [Google Scholar]
- 35.Bou-Flores C, Berger AJ. Gap junctions and inhibitory synapses modulate inspiratory motoneuron synchronization. J Neurophysiol. 2001;85:1543–1551. doi: 10.1152/jn.2001.85.4.1543. [DOI] [PubMed] [Google Scholar]
- 36.Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- 37.Boyd DF, Millar JA, Watkins CS, Mathie A. The role of Ca2+ stores in the muscarinic inhibition of the K+ current IK(SO) in neonatal rat cerebellar granule cells. J Physiol. 2000;529(Pt 2):321–331. doi: 10.1111/j.1469-7793.2000.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brackenbury WJ, Isom LL. Voltage-gated Na+ channels: Potential for beta subunits as therapeutic targets. Expert Opin Ther Targets. 2008;12:1191–1203. doi: 10.1517/14728222.12.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brager DH, Johnston D. Plasticity of intrinsic excitability during longterm depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Broadbelt KG, Barger MA, Paterson DS, Holm IA, Haas EA, Krous HF, Kinney HC, Markianos K, Beggs AH. Serotonin-related FEV gene variant in the sudden infant death syndrome is a common polymorphism in the African-American population. Pediatr Res. 2009;66:631–635. doi: 10.1203/PDR.0b013e3181bd5a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 42.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- 43.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci. 2000;20:4829–4843. doi: 10.1523/JNEUROSCI.20-13-04829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brundage CM, Taylor BE. Neuroplasticity of the central hypercapnic ventilatory response: Teratogen-induced impairment and subsequent recovery during development. Dev Neurobiol. 2010;70:726–735. doi: 10.1002/dneu.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Budelli G, Hage TA, Wei A, Rojas P, Jong YJ, O’Malley K, Salkoff L. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat Neurosci. 2009;12:745–750. doi: 10.1038/nn.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bureau MA, Zinman R, Foulon P, Begin R. Diphasic ventilatory response to hypoxia in newborn lambs. J Appl Physiol. 1984;56:84–90. doi: 10.1152/jappl.1984.56.1.84. [DOI] [PubMed] [Google Scholar]
- 49.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 50.Burton MD, Nouri M, Kazemi H. Acetylcholine and central respiratory control: Perturbations of acetylcholine synthesis in the isolated brainstem of the neonatal rat. Brain Res. 1995;670:39–47. doi: 10.1016/0006-8993(94)01249-h. [DOI] [PubMed] [Google Scholar]
- 51.Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999;82:382–397. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- 52.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 53.Calabrese RL, Nadim F, Olsen OH. Heartbeat control in the medicinal leech: A model system for understanding the origin, coordination, and modulation of rhythmic motor patterns. JNeurobiol. 1995;27:390–402. doi: 10.1002/neu.480270311. [DOI] [PubMed] [Google Scholar]
- 54.Campos M, Bravo E, Eugenin J. Respiratory dysfunctions induced by prenatal nicotine exposure. Clin Exp Pharmacol Physiol. 2009;36:1205–1217. doi: 10.1111/j.1440-1681.2009.05214.x. [DOI] [PubMed] [Google Scholar]
- 55.Cardenas CG, Del Mar LP, Scroggs RS. Two parallel signaling pathways couple 5HT1A receptors to N- and L-type calcium channels in C-like rat dorsal root ganglion cells. J Neurophysiol. 1997;77:3284–3296. doi: 10.1152/jn.1997.77.6.3284. [DOI] [PubMed] [Google Scholar]
- 56.Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J Physiol. 1999;518(Pt 2):507–23. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cardin JA, Palmer LA, Contreras D. Stimulus-dependent gamma (30–50 Hz) oscillations in simple and complex fast rhythmic bursting cells in primary visual cortex. J Neurosci. 2005;25:5339–5350. doi: 10.1523/JNEUROSCI.0374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carley DW, Paviovic S, Janelidze M, Radulovacki M. Functional role for cannabinoids in respiratory stability during sleep. Sleep. 2002;25:391–398. [PubMed] [Google Scholar]
- 59.Carley DW, Radulovacki M. Role of peripheral adenosine A(1) receptors in the regulation of sleep apneas in rats. Exp Neurol. 1999;159:545–550. doi: 10.1006/exnr.1999.7167. [DOI] [PubMed] [Google Scholar]
- 60.Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cassus-Soulanis S, Foutz AS, Denavit-Saubie M. Involvement of NMDA receptors in inspiratory termination in rodents: Effects of wakefulness. Brain Res. 1995;679:25–33. doi: 10.1016/0006-8993(95)00205-5. [DOI] [PubMed] [Google Scholar]
- 62.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen- Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 63.Catterall WA. Molecular properties of brain sodium channels: An important target for anticonvulsant drugs. Adv Neurol. 1999;79:441–456. [PubMed] [Google Scholar]
- 64.Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 65.Chamberlin NL, Saper CB. A brainstem network mediating apneic reflexes in the rat. J Neurosci. 1998;18:6048–6056. doi: 10.1523/JNEUROSCI.18-15-06048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Champagnat J, Richter DW. The roles of K+conductance in expiratory pattern generation in anaesthetized cats. J Physiol. 1994;479(Pt 1):127–138. doi: 10.1113/jphysiol.1994.sp020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Champagnat J, Morin-Surun MP, Fortin G, Thoby-Brisson M. Developmental basis of the rostro-caudal organization of the brainstem respiratory rhythm generator. Philos Trans R Soc Lond B Biol Sci. 2009;364:2469–2476. doi: 10.1098/rstb.2009.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan V, Starr PA, Turner RS. Bursts and oscillations as independent properties of neural activity in the parkinsonian globus pallidus internus. Neurobiol Dis. 2011;41:2–10. doi: 10.1016/j.nbd.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chao D, Xia Y. Ionic storm in hypoxic/ischemic stress: Can opioid receptors subside it? Prog Neurobiol. 2010;90:439–470. doi: 10.1016/j.pneurobio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chapin EM, Haj-Dahmane S, Torres G, Andrade R. The 5-HT(4) receptor-induced depolarization in rat hippocampal neurons is mediated by cAMP but is independent of I(h) Neurosci Lett. 2002;324:1–4. doi: 10.1016/s0304-3940(02)00113-1. [DOI] [PubMed] [Google Scholar]
- 71.Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, Leppert M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet. 1998;18:53–55. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- 72.Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003;22:5403–5411. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen BS, Peng H, Wu SN. Dexmedetomidine, an alpha2-adrenergic agonist, inhibits neuronal delayed-rectifier potassium current and sodium current. Br J Anaesth. 2009;103:244–254. doi: 10.1093/bja/aep107. [DOI] [PubMed] [Google Scholar]
- 74.Chen L, Yang XL. Hyperpolarization-activated cation current is involved in modulation of the excitability of rat retinal ganglion cells by dopamine. Neuroscience. 2007;150:299–308. doi: 10.1016/j.neuroscience.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 75.Chen SK, Ko GY, Dryer SE. Somatostatin peptides produce multiple effects on gating properties of native cone photoreceptor cGMP-gated channels that depend on circadian phase and previous illumination. J Neurosci. 2007;27:12168–12175. doi: 10.1523/JNEUROSCI.3541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cherniack NS, Edelman NH, Lahiri S. Hypoxia and hypercapnia as respiratory stimulants and depressants. Respir Physiol. 1970–1971;11:113–126. doi: 10.1016/0034-5687(70)90107-6. [DOI] [PubMed] [Google Scholar]
- 77.Chevalier M, Ben-Mabrouk F, Tryba AK. Background sodium current underlying respiratory rhythm regularity. Eur J Neurosci. 2008;28:2423–2433. doi: 10.1111/j.1460-9568.2008.06537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clarac F. Some historical reflections on the neural control of locomotion. Brain Res Rev. 2008;57:13–21. doi: 10.1016/j.brainresrev.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 79.Clark BD, Kurth-Nelson ZL, Newman EA. Adenosine-evoked hyperpolarization of retinal ganglion cells is mediated by G-protein-coupled inwardly rectifying K+ and small conductance Ca2+-activated K channel activation. J Neurosci. 2009;29:11237–11245. doi: 10.1523/JNEUROSCI.2836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coddou C, Bravo E, Eugenin J. Alterations in cholinergic sensitivity of respiratory neurons induced by pre-natal nicotine: A mechanism for respiratory dysfunction in neonatal mice. Philos Trans R Soc Lond B Biol Sci. 2009;364:2527–2535. doi: 10.1098/rstb.2009.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colquhoun D, Neher E, Reuter H, Stevens CF. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981;294:752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- 82.Congar P, Leinekugel X, Ben-Ari Y, Crepel V. A long-lasting calciumactivated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 84.Connelly CA, Otto-Smith MR, Feldman JL. Blockade of NMDA receptor-channels by MK-801 alters breathing in adult rats. Brain Res. 1992;596:99–110. doi: 10.1016/0006-8993(92)91537-o. [DOI] [PubMed] [Google Scholar]
- 85.Conrad SC, Nichols NL, Ritucci NA, Dean JB, Putnam RW. Development of chemosensitivity in neurons from the nucleus tractus solitarii (NTS) of neonatal rats. Respir Physiol Neurobiol. 2009;166:4–12. doi: 10.1016/j.resp.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Contreras D. The role of T-channels in the generation of thalamocortical rhythms. CNS Neurol Disord Drug Targets. 2006;5:571–585. doi: 10.2174/187152706779025526. [DOI] [PubMed] [Google Scholar]
- 87.Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol. 2009;168:49–58. doi: 10.1016/j.resp.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003;39:585–588. doi: 10.1016/s0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- 89.Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBotzinger complex. J Physiol. 2007;582:1047–1058. doi: 10.1113/jphysiol.2007.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curran AK, Rodman JR, Eastwood PR, Henderson KS, Dempsey JA, Smith CA. Ventilatory responses to specific CNS hypoxia in sleeping dogs. J Appl Physiol. 2000;88:1840–1852. doi: 10.1152/jappl.2000.88.5.1840. [DOI] [PubMed] [Google Scholar]
- 91.Cymbalyuk G, Shilnikov A. Coexistence of tonic spiking oscillations in a leech neuron model. J Comput Neurosci. 2005;18:255–263. doi: 10.1007/s10827-005-0354-7. [DOI] [PubMed] [Google Scholar]
- 92.Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIKrelated acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 93.Czirjak G, Toth ZE, Enyedi P. The two-pore domain K+ channel, TRESK, is activated by the cytoplasmic calcium signal through calcineurin. J Biol Chem. 2004;279:18550–18558. doi: 10.1074/jbc.M312229200. [DOI] [PubMed] [Google Scholar]
- 94.Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- 95.de Oliveira RB, Howlett MC, Gravina FS, Imtiaz MS, Callister RJ, Brichta AM, van Helden DF. Pacemaker currents in mouse locus coeruleus neurons. Neuroscience. 2010;170:166–177. doi: 10.1016/j.neuroscience.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 96.Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- 97.Dean JB, Nattie EE. Central CO2 chemoreception in cardiorespiratory control. J Appl Physiol. 2010;108:976–978. doi: 10.1152/japplphysiol.00133.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dean JB, Putnam RW. The caudal solitary complex is a site of central CO(2) chemoreception and integration of multiple systems that regulate expired CO(2) Respir Physiol Neurobiol. 2010;173:274–287. doi: 10.1016/j.resp.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985;229:67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- 100.Del Negro CA, Hayes JA, Pace RW, Brush BR, Teruyama R, Feldman JL. Synaptically activated burst-generating conductances may underlie a group-pacemaker mechanism for respiratory rhythm generation in mammals. Prog Brain Res. 2010;187:111–136. doi: 10.1016/B978-0-444-53613-6.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Del Negro CA, Hayes JA, Rekling JC. Dendritic calcium activity precedes inspiratory bursts in preBotzinger complex neurons. J Neurosci. 2011;31:1017–1022. doi: 10.1523/JNEUROSCI.4731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DelNegro CA, Johnson SM, Butera RJ, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. III. Experimental tests of model predictions. J Neurophysiol. 2001;86:59–74. doi: 10.1152/jn.2001.86.1.59. [DOI] [PubMed] [Google Scholar]
- 103.Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-botzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- 104.Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: An emergent network property? Neuron. 2002;34:821–830. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- 105.Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Del Negro CA, Pace RW, Hayes JA. What role do pacemakers play in the generation of respiratory rhythm? Adv Exp Med Biol. 2008;605:88–93. doi: 10.1007/978-0-387-73693-8_15. [DOI] [PubMed] [Google Scholar]
- 107.Derjean D, Bertrand S, Nagy F, Shefchyk SJ. Plateau potentials and membrane oscillations in parasympathetic preganglionic neurones and intermediolateral neurones in the rat lumbosacral spinal cord. J Physiol. 2005;563:583–596. doi: 10.1113/jphysiol.2004.076802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Di Prisco GV, Pearlstein E, Le Ray D, Robitaille R, Dubuc R. A cellular mechanism for the transformation of a sensory input into a motor command. J Neurosci. 2000;20:8169–8176. doi: 10.1523/JNEUROSCI.20-21-08169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: Modulation by slow inactivation. Neuron. 2003;39:109–120. doi: 10.1016/s0896-6273(03)00360-x. [DOI] [PubMed] [Google Scholar]
- 110.Dogas Z, Krolo M, Stuth EA, Tonkovic-Capin M, Hopp FA, McCrimmon DR, Zuperku EJ. Differential effects of GABAA receptor antagonists in the control of respiratory neuronal discharge patterns. J Neurophysiol. 1998;80:2368–2377. doi: 10.1152/jn.1998.80.5.2368. [DOI] [PubMed] [Google Scholar]
- 111.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci. 2010;30:8251–8262. doi: 10.1523/JNEUROSCI.5361-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Donahue LM, Coates PW, Lee VH, Ippensen DC, Arze SE, Poduslo SE. The cardiac sodium channel mRNA is expressed in the developing and adult rat and human brain. Brain Res. 2000;887:335–343. doi: 10.1016/s0006-8993(00)03033-x. [DOI] [PubMed] [Google Scholar]
- 114.Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci. 2010;30:99–109. doi: 10.1523/JNEUROSCI.4305-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drion G, Bonjean M, Waroux O, Scuvee-Moreau J, Liegeois JF, Sejnowski TJ, Sepulchre R, Seutin V. M-type channels selectively control bursting in rat dopaminergic neurons. Eur J Neurosci. 2010;31:827–835. doi: 10.1111/j.1460-9568.2010.07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dryer SE, Dourado MM, Wisgirda ME. Characteristics of multiple Ca(2+)-activated K +channels in acutely dissociated chick ciliary-ganglion neurones. J Physiol. 1991;443:601–627. doi: 10.1113/jphysiol.1991.sp018854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duffin J, Tian GF, Peever JH. Functional synaptic connections among respiratory neurons. Respir Physiol. 2000;122:237–246. doi: 10.1016/s0034-5687(00)00162-6. [DOI] [PubMed] [Google Scholar]
- 119.Duncan JR, Garland M, Myers MM, Fifer WP, Yang M, Kinney HC, Stark RI. Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: Implications for sudden infant death syndrome. J Appl Physiol. 2009;107:1579–1590. doi: 10.1152/japplphysiol.91629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 122.Dunmyre JR, Del Negro CA, Rubin JE. Interactions of persistent sodium and calcium-activated nonspecific cationic currents yield dynamically distinct bursting regimes in a model of respiratory neurons. J Comput Neurosci. 2011;31:305–328. doi: 10.1007/s10827-010-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dutschmann M, Bischoff AM, Busselberg D, Richter DW. Histaminergic modulation of the intact respiratory network of adult mice. Pflugers Arch. 2003;445:570–576. doi: 10.1007/s00424-002-0904-z. [DOI] [PubMed] [Google Scholar]
- 124.Dutschmann M, Kron M, Morschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kolliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol. 2007;159:232–235. doi: 10.1016/j.resp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 125.Egan TM, Dagan D, Kupper J, Levitan IB. Na(+)-activated K+ channels are widely distributed in rat CNS and in Xenopus oocytes. Brain Res. 1992;584:319–321. doi: 10.1016/0006-8993(92)90913-t. [DOI] [PubMed] [Google Scholar]
- 126.Elsen FP, Ramirez JM. Calcium currents of rhythmic neurons recorded in the isolated respiratory network of neonatal mice. J Neurosci. 1998;18:10652–10662. doi: 10.1523/JNEUROSCI.18-24-10652.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elsen FP, Ramirez JM. Postnatal development differentially affects voltage-activated calcium currents in respiratory rhythmic versus nonrhythmic neurons of the pre-Botzinger complex. J Neurophysiol. 2005;94:1423–1431. doi: 10.1152/jn.00237.2005. [DOI] [PubMed] [Google Scholar]
- 128.Ene FA, Kalmbach A, Kandler K. Metabotropic glutamate receptors in the lateral superior olive activate TRP-like channels: Age- and experience-dependent regulation. J Neurophysiol. 2007;97:3365–3375. doi: 10.1152/jn.00686.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Engel S, Gershengorn MC. Thyrotropin-releasing hormone and its receptors–a hypothesis for binding and receptor activation. Pharmacol Ther. 2007;113:410–419. doi: 10.1016/j.pharmthera.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 130.England SJ, Melton JE, Douse MA, Duffin J. Activity of respiratory neurons during hypoxia in the chemodenervated cat. J Appl Physiol. 1995;78:856–861. doi: 10.1152/jappl.1995.78.3.856. [DOI] [PubMed] [Google Scholar]
- 131.Enyedi P, Czirjak G. Molecular background of leak K+ currents: Twopore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 132.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 133.Erlichman JS, Boyer AC, Reagan P, Putnam RW, Ritucci NA, Leiter JC. Chemosensory responses to CO2 in multiple brain stem nuclei determined using a voltage-sensitive dye in brain slices from rats. J Neurophysiol. 2009;102:1577–1590. doi: 10.1152/jn.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Erlichman JS, Cook A, Schwab MC, Budd TW, Leiter JC. Heterogeneous patterns of pH regulation in glial cells in the dorsal and ventral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;286:R289–R302. doi: 10.1152/ajpregu.00245.2003. [DOI] [PubMed] [Google Scholar]
- 135.Erlichman JS, Hewitt A, Damon TL, Hart M, Kurascz J, Li A, Leiter JC. Inhibition of monocarboxylate transporter 2 in the retrotrapezoid nucleus in rats:Atest of the astrocyte-neuron lactate-shuttle hypothesis. J Neurosci. 2008;28:4888–4896. doi: 10.1523/JNEUROSCI.5430-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol. 1998;85:1599–1604. doi: 10.1152/jappl.1998.85.5.1599. [DOI] [PubMed] [Google Scholar]
- 137.Eugenin J, Otarola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von Bernhardi R. Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: A probable link to sudden infant death syndrome. J Neurosci. 2008;28:13907–13917. doi: 10.1523/JNEUROSCI.4441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Faber ES, Sedlak P, Vidovic M, Sah P. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience. 2006;137:781–794. doi: 10.1016/j.neuroscience.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 139.Falk S, Rekling JC. Neurons in the preBotzinger complex and VRG are located in proximity to arterioles in newborn mice. Neurosci Lett. 2009;450:229–234. doi: 10.1016/j.neulet.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 140.Farrell SR, Raymond ID, Foote M, Brecha NC, Barnes S. Modulation of voltage-gated ion channels in rat retinal ganglion cells mediated by somatostatin receptor subtype 4. J Neurophysiol. 2010;104:1347–1354. doi: 10.1152/jn.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fedorko L, Duffin J, England S. Inhibition of inspiratory neurons of the nucleus retroambigualis by expiratory neurons of the Botzinger complex in the cat. Exp Neurol. 1989;106:74–77. doi: 10.1016/0014-4886(89)90146-5. [DOI] [PubMed] [Google Scholar]
- 142.Feldman JL. Looking forward to breathing. Prog Brain Res. 2011;188:213–218. doi: 10.1016/B978-0-444-53825-3.00019-X. [DOI] [PubMed] [Google Scholar]
- 143.Feldman JL, Del Negro CA. Looking for inspiration: New perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feldman JL, Janczewski WA. Point:Counterpoint: The parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. Counterpoint: The preBotC is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100:2096–2097. doi: 10.1152/japplphysiol.00119.2006. discussion 2097–2098, 2103–2108. [DOI] [PubMed] [Google Scholar]
- 145.Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Feldman JL, Windhorst U, Anders K, Richter DW. Synaptic interaction between medullary respiratory neurones during apneusis induced by NMDA-receptor blockade in cat. J Physiol. 1992;450:303–323. doi: 10.1113/jphysiol.1992.sp019128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Feng SS, Jaeger D. The role of SK calcium-dependent potassium currents in regulating the activity of deep cerebellar nucleus neurons: A dynamic clamp study. Cerebellum. 2008;7:542–546. doi: 10.1007/s12311-008-0077-1. [DOI] [PubMed] [Google Scholar]
- 148.Ficker DM. Sudden unexplained death and injury in epilepsy. Epilepsia. 2000;41(Suppl 2):S7–S12. doi: 10.1111/j.1528-1157.2000.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 149.Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol. 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fishman MC, Spector I. Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc Natl Acad Sci U S A. 1981;78:5245–5249. doi: 10.1073/pnas.78.8.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Flaherty P, Radhakrishnan ML, Dinh T, Rebres RA, Roach TI, Jordan MI, Arkin AP. A dual receptor crosstalk model of G-protein-coupled signal transduction. PLoS Comput Biol. 2008;4:e1000185. doi: 10.1371/journal.pcbi.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fleig A, Penner R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 153.Flourens MJ-P. Recherches Expérimentales sur les Propriétés et les Fonctions du Système Nerveux dans les Animaux Vertébrés [Experimental research on the properties and functions of the nervous system in vertebrate animals] Paris: Crevot; 1824. [Google Scholar]
- 154.Foutz AS, Champagnat J, Denavit-Saubie M. Involvement of N-methyl- D-aspartate (NMDA) receptors in respiratory rhythmogenesis. Brain Res. 1989;500:199–208. doi: 10.1016/0006-8993(89)90314-4. [DOI] [PubMed] [Google Scholar]
- 155.Fox AP, Cahill AL, Currie KP, Grabner C, Harkins AB, Herring B, Hurley JH, Xie Z. N- and P/Q-type Ca2+ channels in adrenal chromaffin cells. Acta Physiol (Oxf) 2008;192:247–261. doi: 10.1111/j.1748-1716.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- 156.Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Francesconi A, Duvoisin RM. Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: Selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc Natl Acad Sci U S A. 2000;97:6185–6190. doi: 10.1073/pnas.97.11.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Franco P, Szliwowski H, Dramaix M, Kahn A. Polysomnographic study of the autonomic nervous system in potential victims of sudden infant death syndrome. Clin Auton Res. 1998;8:243–249. doi: 10.1007/BF02277969. [DOI] [PubMed] [Google Scholar]
- 159.Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir Physiol Neurobiol. 2008;164:80–86. doi: 10.1016/j.resp.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.French CR, Sah P, Buckett KJ, Gage PW. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. JGenPhysiol. 1990;95:1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Friesen WO, Poon M, Stent GS. Neuronal control of swimming in the medicinal leech. IV. Identification of a network of oscillatory interneurones. J Exp Biol. 1978;75:25–43. doi: 10.1242/jeb.75.1.25. [DOI] [PubMed] [Google Scholar]
- 162.Fujii M, Umezawa K, Arata A. Dopaminergic modulation on respiratory rhythm in rat brainstem-spinal cord preparation. Neurosci Res. 2004;50:355–359. doi: 10.1016/j.neures.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 163.Fuller DD, Dougherty BJ, Sandhu MS, Doperalski NJ, Reynolds CR, Hayward LF. Prenatal nicotine exposure alters respiratory long-term facilitation in neonatal rats. Respir Physiol Neurobiol. 2009;169:333–337. doi: 10.1016/j.resp.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Funk GD, Johnson SM, Smith JC, Dong XW, Lai J, Feldman JL. Functional respiratory rhythm generating networks in neonatal mice lacking NMDAR1 gene. J Neurophysiol. 1997;78:1414–1420. doi: 10.1152/jn.1997.78.3.1414. [DOI] [PubMed] [Google Scholar]
- 165.Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: Role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- 166.Funke F, Muller M, Dutschmann M. Reconfiguration of respiratoryrelated population activity in a rostrally tilted transversal slice preparation following blockade of inhibitory neurotransmission in neonatal rats. Pflugers Arch. 2008;457:185–195. doi: 10.1007/s00424-008-0509-2. [DOI] [PubMed] [Google Scholar]
- 167.Gabel RA, Weiskopf RB. Ventilatory interaction between hypoxia and [H+] at chemoreceptors of man. J Appl Physiol. 1975;39:292–296. doi: 10.1152/jappl.1975.39.2.292. [DOI] [PubMed] [Google Scholar]
- 168.Garcia AJ, III, Zanella S, Koch H, Doi A, Ramirez JM. Networks within networks The neuronal control of breathing. Prog Brain Res. 2011;188:31–50. doi: 10.1016/B978-0-444-53825-3.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gasparini S, Kasyanov AM, Pietrobon D, Voronin LL, Cherubini E. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J Neurosci. 2001;21:8715–8721. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ge Q, Feldman JL. AMPA receptor activation and phosphatase inhibition affect neonatal rat respiratory rhythm generation. J Physiol. 1998;509(Pt 1):255–266. doi: 10.1111/j.1469-7793.1998.255bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gesell R, Lapides J, Levin M. Interaction of central and peripheral chemical control of breathing. Am. J. Pkytiol. 1940;130:155–170. [Google Scholar]
- 172.Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- 174.Gillis RA, Walton DP, Quest JA, Namath IJ, Hamosh P, Dretchen KL. Cardiorespiratory effects produced by activation of cholinergic muscarinic receptors on the ventral surface of the medulla. J Pharmacol Exp Ther. 1988;247:765–773. [PubMed] [Google Scholar]
- 175.Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 176.Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 177.Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- 178.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: Role in hypoxic ventilatory response. J Neurosci. 2005;25:1211–1218. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gozal D, Torres JE, Gozal YM, Nuckton TJ. Characterization and developmental aspects of anoxia-induced gasping in the rat. Biol Neonate. 1996;70:280–288. doi: 10.1159/000244377. [DOI] [PubMed] [Google Scholar]
- 181.Graef JD, Huitt TW, Nordskog BK, Hammarback JH, Godwin DW. Disrupted thalamic T-type Ca2+ channel expression and function during ethanol exposure and withdrawal. J Neurophysiol. 2011;105:528–540. doi: 10.1152/jn.00424.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Graham Brown T. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond. 1911;84B:308–319. [Google Scholar]
- 183.Graham Brown T. On the nature of the fundamental activity of the nervous centres: Together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol. 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J, Del Negro CA. Developmental origin of preBotzinger complex respiratory neurons. J Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Greka A, Navarro B, Oancea E, Duggan A, Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 190.Grillner S, Deliagina T, Ekeberg O, el Manira A, Hill RH, Lansner A, Orlovsky GN, Wallen P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends Neurosci. 1995;18:270–279. [PubMed] [Google Scholar]
- 191.Grunstein MM, Hazinski TA, Schlueter MA. Respiratory control during hypoxia in newborn rabbits: Implied action of endorphins. J Appl Physiol. 1981;51:122–130. doi: 10.1152/jappl.1981.51.1.122. [DOI] [PubMed] [Google Scholar]
- 192.Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B, Garcia ML, Grissmer S, Jan LY, Karschin A, Kim D, Kuperschmidt S, Kurachi Y, Lazdunski M, Lesage F, Lester HA, McKinnon D, Nichols CG, O’Kelly I, Robbins J, Robertson GA, Rudy B, Sanguinetti M, Seino S, Stuehmer W, Tamkun MM, Vandenberg CA, Wei A, Wulff H, Wymore RS. International Union of Pharmacology. XLI. Compendium of voltagegated ion channels: Potassium channels. Pharmacol Rev. 2003;55:583–586. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- 193.Guyenet PG. The 2008 Carl Ludwig Lecture: Retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guyenet PG, Wang H. Pre-Botzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J Neurophysiol. 2001;86:438–446. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- 195.Guyenet PG, Mulkey DK. Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol. 2010;173:244–255. doi: 10.1016/j.resp.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Haddad GG, Mellins RB. Hypoxia and respiratory control in early life. Annu Rev Physiol. 1984;46:629–643. doi: 10.1146/annurev.ph.46.030184.003213. [DOI] [PubMed] [Google Scholar]
- 199.Haji A, Remmers JE, Connelly C, Takeda R. Effects of glycine and GABA on bulbar respiratory neurons of cat. J Neurophysiol. 1990;63:955–965. doi: 10.1152/jn.1990.63.5.955. [DOI] [PubMed] [Google Scholar]
- 200.Haller M, Mironov SL, Karschin A. RichterDW.Dynamic activation of K(ATP) channels in rhythmically active neurons. J Physiol. 2001;537:69–81. doi: 10.1111/j.1469-7793.2001.0069k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 202.Harris-Warrick RM. General principles of rhythmogenesis in central pattern generator networks. Prog Brain Res. 2010;187:213–222. doi: 10.1016/B978-0-444-53613-6.00014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annu Rev Neurosci. 1991;14:39–57. doi: 10.1146/annurev.ne.14.030191.000351. [DOI] [PubMed] [Google Scholar]
- 204.Hatori E, Sakuraba S, Kashiwagi M, Kuribayashi J, Tsujita M, Hosokawa Y, Takeda J, Kuwana S. Association of nicotinic acetylcholine receptors with central respiratory control in isolated brainstemspinal cord preparation of neonatal rats. Biol Res. 2006;39:321–330. doi: 10.4067/s0716-97602006000200014. [DOI] [PubMed] [Google Scholar]
- 205.Hayes JA, Mendenhall JL, Brush BR, DelNegro CA. 4-Aminopyridinesensitive outward currents in preBotzinger complex neurons influence respiratory rhythm generation in neonatal mice. J Physiol. 2008;586:1921–1936. doi: 10.1113/jphysiol.2008.150946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y, Evans AC. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One. 2009;4:e5226. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hedqvist P, Fredholm BB. Effects of adenosine on adrenergic neurotransmission; prejunctional inhibition and postjunctional enhancement. Naunyn Schmiedebergs Arch Pharmacol. 1976;293:217–223. doi: 10.1007/BF00507344. [DOI] [PubMed] [Google Scholar]
- 208.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 209.Hermann A, Gorman AL. Action of quinidine on ionic currents of molluscan pacemaker neurons. J Gen Physiol. 1984;83:919–940. doi: 10.1085/jgp.83.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Heymans C, Bouckaert JJ. Sinus caroticus and respiratory reflexes: I. Cerebral blood flow and respiration. Adrenaline apnoea. J Physiol. 1930;69:254–266. doi: 10.1113/jphysiol.1930.sp002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Heymans C, Neil E. Rcflexogenic areas of the cardiovatcular tyttem. London: Churchill. 1958 [Google Scholar]
- 212.Heymans JF, Heymans C. Sur les modifications directes et sur la regulation reflexe de l’activite du centre respiratoire de la tfite isolee du chien. Arch Int Pharmacodyn Ther. 1927;33:273–372. [Google Scholar]
- 213.Hill AA, Garcia AJ, III, Zanella S, Upadhyaya R, Ramirez JM. Graded reductions in oxygenation evoke graded reconfiguration of the isolated respiratory network. J Neurophysiol. 2011;105:625–639. doi: 10.1152/jn.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hille B. Ion Channels of ExcitableMembranes. Sunderland, MA, USA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- 215.Hillman D, Chen S, Aung TT, Cherksey B, Sugimori M, Llinas RR. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A. 1991;88:7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hodges MR, Richerson GB. The role of medullary serotonin (5- HT) neurons in respiratory control: Contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2(A) receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol. 2010;588:255–266. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Holtman JR, Jr, Anastasi NC, Norman WP, Dretchen KL. Effect of electrical and chemical stimulation of the raphe obscurus on phrenic nerve activity in the cat. Brain Res. 1986;362:214–220. doi: 10.1016/0006-8993(86)90446-4. [DOI] [PubMed] [Google Scholar]
- 220.Hornigold DC, Mistry R, Raymond PD, Blank JL, Challiss RA. Evidence for cross-talk between M2 and M3 muscarinic acetylcholine receptors in the regulation of second messenger and extracellular signalregulated kinase signalling pathways in Chinese hamster ovary cells. Br J Pharmacol. 2003;138:1340–1350. doi: 10.1038/sj.bjp.0705178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huang H, Trussell LO. Control of presynaptic function by a persistent Na(+) current. Neuron. 2008;60:975–979. doi: 10.1016/j.neuron.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang W, Xiu Y, Yan JA, He WJ, Zhao YD, Hu ZA, Ruan HZ. Facilitation of Ih channels by P2Y1 receptors activation in Mesencephalic trigeminal neurons. Neurosci Lett. 2010;482:156–159. doi: 10.1016/j.neulet.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 223.Huang YH, Brown AR, Costy-Bennett S, Luo Z, Fregosi RF. Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir Physiol Neurobiol. 2004;143:1–8. doi: 10.1016/j.resp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 224.Huang YH, Brown AR, Cross SJ, Cruz J, Rice A, Jaiswal S, Fregosi RF. Influence of prenatal nicotine exposure on development of the ventilatory response to hypoxia and hypercapnia in neonatal rats. J Appl Physiol. 2010;109:149–158. doi: 10.1152/japplphysiol.01036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Huang ZG, Griffioen KJ, Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. Nicotinic receptor activation occludes purinergic control of central cardiorespiratory network responses to hypoxia/ hypercapnia. J Neurophysiol. 2007;98:2429–2438. doi: 10.1152/jn.00448.2007. [DOI] [PubMed] [Google Scholar]
- 226.Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992;12:3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hwang JC, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol. 1983;55:793–798. doi: 10.1152/jappl.1983.55.3.793. [DOI] [PubMed] [Google Scholar]
- 228.Igarashi N, Tatsumi K, Nakamura A, Sakao S, Takiguchi Y, Nishikawa T, Kuriyama T. Plasma orexin-A levels in obstructive sleep apneahypopnea syndrome. Chest. 2003;124:1381–1385. doi: 10.1378/chest.124.4.1381. [DOI] [PubMed] [Google Scholar]
- 229.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 230.Inagaki N, Gonoi T, Clement JPth, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 231.Ireland MF, Lenal FC, Lorier AR, Loomes DE, Adachi T, Alvares TS, Greer JJ, Funk GD. Distinct receptors underlie glutamatergic signalling in inspiratory rhythm-generating networks and motor output pathways in neonatal rat. J Physiol. 2008;586:2357–2370. doi: 10.1113/jphysiol.2007.150532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Isom LL. Sodium channel beta subunits: Anything but auxiliary. Neuroscientist. 2001;7:42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- 233.Iwahori Y, Ikegaya Y, Matsuki N. Hyperpolarization-activated current I(h) in nucleus of solitary tract neurons: Regional difference in serotonergic modulation. Jpn J Pharmacol. 2002;88:459–462. doi: 10.1254/jjp.88.459. [DOI] [PubMed] [Google Scholar]
- 234.Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. JPhysiol. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jalil S, Belykh I, Shilnikov A. Fast reciprocal inhibition can synchronize bursting neurons. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:045201. doi: 10.1103/PhysRevE.81.045201. [DOI] [PubMed] [Google Scholar]
- 236.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006a;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Janczewski WA, Feldman JL. Novel data supporting the two respiratory rhythm oscillator hypothesis. Focus on “respiration-related rhythmic activity in the rostral medulla of newborn rats”. J Neurophysiol. 2006b;96:1–2. doi: 10.1152/jn.00246.2006. [DOI] [PubMed] [Google Scholar]
- 238.Jentsch TJ. Neuronal KCNQ potassium channels: Physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 239.Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Botzinger complex in the cat. Exp Brain Res. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- 240.Jiang C, Xu H, Cui N, Wu J. An alternative approach to the identification of respiratory central chemoreceptors in the brainstem. Respir Physiol. 2001;129:141–157. doi: 10.1016/s0034-5687(01)00301-2. [DOI] [PubMed] [Google Scholar]
- 241.Jiang N, Furue H, Katafuchi T, Yoshimura M. Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neurosci Res. 2003;47:97–107. doi: 10.1016/s0168-0102(03)00183-4. [DOI] [PubMed] [Google Scholar]
- 242.Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: Role of Gi/o protein-mediated mechanisms. J Appl Physiol. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- 243.Johnson SM, Smith JC, Funk GD, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J Neurophysiol. 1994;72:2598–2608. doi: 10.1152/jn.1994.72.6.2598. [DOI] [PubMed] [Google Scholar]
- 244.Joksovic PM, Doctor A, Gaston B, Todorovic SM. Functional regulation of T-type calcium channels by s-nitrosothiols in the rat thalamus. J Neurophysiol. 2007;97:2712–2721. doi: 10.1152/jn.00926.2006. [DOI] [PubMed] [Google Scholar]
- 245.Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497(Pt 2):337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kamp MA, Krieger A, Henry M, Hescheler J, Weiergraber M, Schneider T. Presynaptic ‘Ca2.3-containing’ E-type Ca channels share dual roles during neurotransmitter release. Eur J Neurosci. 2005;21:1617–1625. doi: 10.1111/j.1460-9568.2005.03984.x. [DOI] [PubMed] [Google Scholar]
- 247.Kang D, Han J, Kim D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am J Physiol Cell Physiol. 2006;291:C649–C656. doi: 10.1152/ajpcell.00047.2006. [DOI] [PubMed] [Google Scholar]
- 248.Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch. 2008;457:77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]
- 249.Kass JI, Mintz IM. Silent plateau potentials, rhythmic bursts, and pacemaker firing: Three patterns of activity that coexist in quadristable subthalamic neurons. Proc Natl Acad Sci U S A. 2006;103:183–188. doi: 10.1073/pnas.0506781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kay AR, Sugimori M, Llinas R. Kinetic and stochastic properties of a persistent sodium current in mature guinea pig cerebellar Purkinje cells. J Neurophysiol. 1998;80:1167–1179. doi: 10.1152/jn.1998.80.3.1167. [DOI] [PubMed] [Google Scholar]
- 251.Khateb A, Fort P, Serafin M, Jones BE, Muhlethaler M. Rhythmical bursts induced by NMDA in guinea-pig cholinergic nucleus basalis neurones in vitro. J Physiol. 1995;487(Pt 3):623–638. doi: 10.1113/jphysiol.1995.sp020905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol. 2011;21:100–109. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 253.Kim J, Bhattacharjee R, Snow AB, Capdevila OS, Kheirandish-Gozal L, Gozal D. Myeloid-related protein 8/14 levels in children with obstructive sleep apnoea. Eur Respir J. 2010;35:843–850. doi: 10.1183/09031936.00075409. [DOI] [PubMed] [Google Scholar]
- 254.Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Orexin-A and ghrelin depolarize the same pedunculopontine tegmental neurons in rats: An in vitro study. Peptides. 2009;30:1328–1335. doi: 10.1016/j.peptides.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 255.Kim SJ, Chung WH, Rhim H, Eun SY, Jung SJ, Kim J. Postsynaptic action mechanism of somatostatin on the membrane excitability in spinal substantia gelatinosa neurons of juvenile rats. Neuroscience. 2002;114:1139–1148. doi: 10.1016/s0306-4522(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 256.Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- 257.Kirkpatrick KA, Burnstock G. Evidence that the inhibition of ATP release from sympathetic nerves by adenosine is a physiological mechanism. Gen Pharmacol. 1992;23:1045–1050. doi: 10.1016/0306-3623(92)90284-q. [DOI] [PubMed] [Google Scholar]
- 258.Kiselyov K, Shin DM, Kim JY, Yuan JP, Muallem S. TRPC channels: Interacting proteins. Handb Exp Pharmacol. 2007;179:559–574. doi: 10.1007/978-3-540-34891-7_33. [DOI] [PubMed] [Google Scholar]
- 259.Klages S, Bellingham MC, Richter DW. Late expiratory inhibition of stage 2 expiratory neurons in the cat–a correlate of expiratory termination. J Neurophysiol. 1993;70:1307–1315. doi: 10.1152/jn.1993.70.4.1307. [DOI] [PubMed] [Google Scholar]
- 260.Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca(2+)-activatedK+channels in rat brain: Targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Koch H, Huh SE, Elsen FP, Carroll MS, Hodge RD, Bedogni F, Turner MS, Hevner RF, Ramirez JM. Prostaglandin E2-induced synaptic plasticity in neocortical networks of organotypic slice cultures. J Neurosci. 2010;30:11678–11687. doi: 10.1523/JNEUROSCI.4665-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kodama D, Togari A. Modulation of potassium channels via the alpha(1B)-adrenergic receptor in human osteoblasts. Neurosci Lett. 2010;485:102–106. doi: 10.1016/j.neulet.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 263.Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 264.Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. JNeurosci. 2010;30:4273–4284. doi: 10.1523/JNEUROSCI.4017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Koizumi H, Smith JC. Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Botzinger complex in vitro. J Neurosci. 2008;28:1773–1785. doi: 10.1523/JNEUROSCI.3916-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Koizumi S, Saito Y, Nakazawa K, Nakajima K, Sawada JI, Kohsaka S, Illes P, Inoue K. Spatial and temporal aspects of Ca2+ signaling mediated by P2Y receptors in cultured rat hippocampal astrocytes. Life Sci. 2002;72:431–442. doi: 10.1016/s0024-3205(02)02273-7. [DOI] [PubMed] [Google Scholar]
- 267.Koon HW, Shih D, Karagiannides I, Zhao D, Fazelbhoy Z, Hing T, Xu H, Lu B, Gerard N, Pothoulakis C. Substance P modulates colitisassociated fibrosis. Am J Pathol. 2010;177:2300–2309. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- 269.Krey RA, Goodreau AM, Arnold TB, Del Negro CA. Outward currents contributing to inspiratory burst termination in preBotzinger complex neurons of neonatal mice studied in vitro. Front Neural Circuits. 2010;4:124. doi: 10.3389/fncir.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Krolo M, Stuth EA, Tonkovic-Capin M, Dogas Z, Hopp FA, McCrimmon DR, Zuperku EJ. Differential roles of ionotropic glutamate receptors in canine medullary inspiratory neurons of the ventral respiratory group. J Neurophysiol. 1999;82:60–68. doi: 10.1152/jn.1999.82.1.60. [DOI] [PubMed] [Google Scholar]
- 271.Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- 272.Kubota H, Katsurabayashi S, Moorhouse AJ, Murakami N, Koga H, Akaike N. GABAB receptor transduction mechanisms, and cross-talk between protein kinases A and C, in GABAergic terminals synapsing onto neurons of the rat nucleus basalis of Meynert. J Physiol. 2003;551:263–276. doi: 10.1113/jphysiol.2003.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kumar GK, Prabhakar NR. Tachykinins in the control of breathing by hypoxia: Pre- and post-genomic era. Respir Physiol Neurobiol. 2003;135:145–154. doi: 10.1016/s1569-9048(03)00033-8. [DOI] [PubMed] [Google Scholar]
- 274.Kurian N, Hall CJ, Wilkinson GF, Sullivan M, Tobin AB, Willars GB. Full and partial agonists of muscarinic M3 receptors reveal single and oscillatory Ca2+ responses by beta 2-adrenoceptors. J Pharmacol Exp Ther. 2009;330:502–512. doi: 10.1124/jpet.109.153619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kuruma A, Inoue T, Mikoshiba K. Dynamics of Ca(2+) and Na(+) in the dendrites of mouse cerebellar Purkinje cells evoked by parallel fibre stimulation. Eur J Neurosci. 2003;18:2677–2689. doi: 10.1111/j.1460-9568.2003.02977.x. [DOI] [PubMed] [Google Scholar]
- 276.Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol. 2008;164:204–212. doi: 10.1016/j.resp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 277.Kuwaki T, Li A, Nattie E. State-dependent central chemoreception: A role of orexin. Respir Physiol Neurobiol. 2010;173:223–229. doi: 10.1016/j.resp.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kuwana S, Tsunekawa N, Yanagawa Y, Okada Y, Kuribayashi J, Obata K. Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Botzinger complex. Eur J Neurosci. 2006;23:667–674. doi: 10.1111/j.1460-9568.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- 279.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanismof attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 280.Lalley PM. D1-dopamine receptor agonists prevent and reverse opiate depression of breathing but not antinociception in the cat. Am J Physiol Regul Integr Comp Physiol. 2005;289:R45–R51. doi: 10.1152/ajpregu.00868.2004. [DOI] [PubMed] [Google Scholar]
- 281.Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol. 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- 282.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 283.Lawson EE, Long WA. Central origin of biphasic breathing pattern during hypoxia in newborns. J Appl Physiol. 1983;55:483–488. doi: 10.1152/jappl.1983.55.2.483. [DOI] [PubMed] [Google Scholar]
- 284.Lazarenko RM, Fortuna MG, Shi Y, Mulkey DK, Takakura AC, Moreira TS, Guyenet PG, Bayliss DA. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K(+) current. J Neurosci. 2010;30:9324–9334. doi: 10.1523/JNEUROSCI.1956-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lee LY, Millhorn HTJ. Central ventilatory responses to O, and CO, at three levels of carotid chemoreceptor stimulation. Respir Physiol. 1975;35:310–333. doi: 10.1016/0034-5687(75)90007-9. [DOI] [PubMed] [Google Scholar]
- 286.Legallois J-J-C. Expériences sur le Principe de la Vie, Notamment sur Celui des Mouvements du Coeur, et sur le Siège de ce Principe [Experiments on the vital principle in particular on that of heart movements and the site of this principle] Hautel, Paris. 1812 [Google Scholar]
- 287.Leonard TO, Lydic R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J Neurosci. 1997;17:774–785. doi: 10.1523/JNEUROSCI.17-02-00774.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Leppert MF, Singh N. Susceptibility genes in human epilepsy. Semin Neurol. 1999;19:397–405. doi: 10.1055/s-2008-1040854. [DOI] [PubMed] [Google Scholar]
- 289.Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, Steinmeyer K. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem. 2000;275:22395–22400. doi: 10.1074/jbc.M002378200. [DOI] [PubMed] [Google Scholar]
- 290.Lesage F, Lazdunski M. Molecular and functional properties of twopore- domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- 291.Leyssens A, Nowicky AV, Patterson L, Crompton M, Duchen MR. The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i and [Ca2+]i studied in isolated rat cardiomyocytes. J Physiol. 1996;496(Pt 1):111–128. doi: 10.1113/jphysiol.1996.sp021669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Li C, Horn JP. Differential Inhibition of Ca2+ channels by alpha2- adrenoceptors in three functional subclasses of rat sympathetic neurons. J Neurophysiol. 2008;100:3055–3063. doi: 10.1152/jn.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li WC, Roberts A, Soffe SR. Specific brainstem neurons switch each other into pacemaker mode to drive movement by activating NMDA receptors. J Neurosci. 2010;30:16609–16620. doi: 10.1523/JNEUROSCI.3695-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lichtman JW, Sanes JR. Ome sweet ome: What can the genome tell us about the connectome? Curr Opin Neurobiol. 2008;18:346–353. doi: 10.1016/j.conb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lichtman JW, Livet J, Sanes JR. A technicolour approach to the connectome. Nat Rev Neurosci. 2008;9:417–422. doi: 10.1038/nrn2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. II. Intrinsic modulation by metabotropic glutamate receptors. J Neurophysiol. 2006a;95:1334–1344. doi: 10.1152/jn.00506.2004. [DOI] [PubMed] [Google Scholar]
- 297.Lieske SP, Ramirez JM. Pattern-specific synaptic mechanisms in a multifunctional network. I. Effects of alterations in synapse strength. J Neurophysiol. 2006b;95:1323–1333. doi: 10.1152/jn.00505.2004. [DOI] [PubMed] [Google Scholar]
- 298.Lieske SP, Thoby-Brisson M, Ramirez JM. Reconfiguration of the central respiratory network under normoxic and hypoxic conditions. Adv Exp Med Biol. 2001;499:171–178. doi: 10.1007/978-1-4615-1375-9_27. [DOI] [PubMed] [Google Scholar]
- 299.Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: Eupnea, sighs and gasps [see comment] Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- 300.Liman ER. TRPM5 and taste transduction. Handb Exp Pharmacol. 2007;179:287–298. doi: 10.1007/978-3-540-34891-7_17. [DOI] [PubMed] [Google Scholar]
- 301.Lipscombe D, Helton TD, Xu W. L-type calcium channels: The low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- 302.Littleton JT, Ganetzky B. Ion channels and synaptic organization: Analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 303.Liu Q, Wong-Riley MT. Postnatal development of N-methyl-D-aspartate receptor subunits 2A, 2B, 2C, 2D, and 3B immunoreactivity in brain stem respiratory nuclei of the rat. Neuroscience. 2010;171:637–654. doi: 10.1016/j.neuroscience.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Llano DA, Sherman SM. Differences in intrinsic properties and local network connectivity of identified layer 5 and layer 6 adult mouse auditory corticothalamic neurons support a dual corticothalamic projection hypothesis. Cereb Cortex. 2009;19:2810–2826. doi: 10.1093/cercor/bhp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- 306.Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 307.Llinas RR, Sugimori M, Cherksey B. Voltage-dependent calcium conductances in mammalian neurons. The P channel. Ann N Y Acad Sci U S A. 1989;560:103–111. doi: 10.1111/j.1749-6632.1989.tb24084.x. [DOI] [PubMed] [Google Scholar]
- 308.Llona I, Eugenin J. Central actions of somatostatin in the generation and control of breathing. Biol Res. 2005;38:347–352. doi: 10.4067/s0716-97602005000400006. [DOI] [PubMed] [Google Scholar]
- 309.Loeschcke HH. A concept of the role of intracranial chemosensitivity in respiratory control. In cerebrospinal fluid and the regulation of respiration. Oxford: Blackwell. 1965;183:210. [Google Scholar]
- 310.Loeschcke HH, Mitchell RA, Katsaros B, Perkins JF, Konig A. Interaction of intracranial chemosensitivity with peripheral afferents to the respiratory centers. Ann N Y Acad Sci U S A. 1963;109:651–660. doi: 10.1111/j.1749-6632.1963.tb13495.x. [DOI] [PubMed] [Google Scholar]
- 311.Lopes CM, Remon JI, Matavel A, Sui JL, Keselman I, Medei E, Shen Y, Rosenhouse-Dantsker A, Rohacs T, Logothetis DE. Protein kinase A modulates PLC-dependent regulation and PIP2-sensitivity of K channels. Channels (Austin) 2007;1:124–134. doi: 10.4161/chan.4322. [DOI] [PubMed] [Google Scholar]
- 312.Lorier AR, Lipski J, Housley GD, Greer JJ, Funk GD. ATP sensitivity of preBotzinger complex neurones in neonatal rat in vitro: Mechanism underlying a P2 receptor-mediated increase in inspiratory frequency. J Physiol. 2008;586:1429–1446. doi: 10.1113/jphysiol.2007.143024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lorier AR, Peebles K, Brosenitsch T, Robinson DM, Housley GD, Funk GD. P2 receptors modulate respiratory rhythm but do not contribute to central CO2 sensitivity in vitro. Respir Physiol Neurobiol. 2004;142:27–42. doi: 10.1016/j.resp.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 314.Loucif AJ, Woodhall GL, Sehirli US, Stanford IM. Depolarisation and suppression of burst firing activity in the mouse subthalamic nucleus by dopamine D1/D5 receptor activation of a cyclic-nucleotide gated nonspecific cation conductance. Neuropharmacology. 2008;55:94–105. doi: 10.1016/j.neuropharm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 315.Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell. 2007;129:371–383. doi: 10.1016/j.cell.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 316.Lu B, Su Y, Das S, Wang H, Wang Y, Liu J, Ren D. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature. 2009;457:741–744. doi: 10.1038/nature07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lu B, Zhang Q, Wang H, Wang Y, Nakayama M, Ren D. Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex. Neuron. 2010;68:488–499. doi: 10.1016/j.neuron.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Luo Z, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure increases the strength of GABA(A) receptor-mediated inhibition of respiratory rhythm in neonatal rats. J Physiol. 2004;561:387–393. doi: 10.1113/jphysiol.2004.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Luo Z, McMullen NT, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure alters glycinergic and GABAergic control of respiratory frequency in the neonatal rat brainstem-spinal cord preparation. Respir Physiol Neurobiol. 2007;157:226–234. doi: 10.1016/j.resp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 320.Ma LQ, Liu C, Wang F, Xie N, Gu J, Fu H, Wang JH, Cai F, Liu J, Chen JG. Activation of phosphatidylinositol-linked novel D1 dopamine receptors inhibits high-voltage-activated Ca2+ currents in primary cultured striatal neurons. J Neurophysiol. 2009;101:2230–2238. doi: 10.1152/jn.90345.2008. [DOI] [PubMed] [Google Scholar]
- 321.Magistretti J, Alonso A. Biophysical properties and slow voltagedependent inactivation of a sustained sodium current in entorhinal cortex layer-II principal neurons: A whole-cell and single-channel study. J Gen Physiol. 1999;114:491–509. doi: 10.1085/jgp.114.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- 323.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 324.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 325.Marek W, Prabhakar NR, Loeschcke HH. Effects of electrical stimulation of chemosensory afferents in different phases of the respiratory cycle. Fedn Proc. 1978;37:903–908. [Google Scholar]
- 326.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 327.Maruyama Y, Peterson OH. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini. Nature. 1982;299:159–161. doi: 10.1038/299159a0. [DOI] [PubMed] [Google Scholar]
- 328.Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 330.McCrea DA, Rybak IA. Modeling the mammalian locomotor CPG: Insights frommistakes and perturbations. Prog Brain Res. 2007;165:235–253. doi: 10.1016/S0079-6123(06)65015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.McCrimmon DR, Monnier A, Ptak K, Zummo G, Zhang Z, Alheid GF. Respiratory rhythm generation: PreBotzinger neuron discharge patterns and persistent sodium current. Adv Exp Med Biol. 2001;499:147–152. doi: 10.1007/978-1-4615-1375-9_23. [DOI] [PubMed] [Google Scholar]
- 332.McCrimmon DR, Ramirez JM, Alford S, Zuperku EJ. Unraveling the mechanism for respiratory rhythm generation. Bioessays. 2000;22:6–9. doi: 10.1002/(SICI)1521-1878(200001)22:1<6::AID-BIES3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 333.McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW. Ca(V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci. 2006;24:2581–2594. doi: 10.1111/j.1460-9568.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- 334.McKay LC, Feldman JL. Unilateral ablation of pre-Botzinger complex disrupts breathing during sleep but not wakefulness. Am J Respir Crit Care Med. 2008;178:89–95. doi: 10.1164/rccm.200712-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Mellen NM. Degeneracy as a substrate for respiratory regulation. Respir Physiol Neurobiol. 2010;172:1–7. doi: 10.1016/j.resp.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Mellen NM, Mishra D. Functional anatomical evidence for respiratory rhythmogenic function of endogenous bursters in rat medulla. J Neurosci. 2010;30:8383–8392. doi: 10.1523/JNEUROSCI.5510-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BKlarge conductance Ca2+-activated K+channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 340.Mergler S, Singh V, Grotzinger C, Kaczmarek P, Wiedenmann B, Strowski MZ. Characterization of voltage operated R-type Ca2+ channels in modulating somatostatin receptor subtype 2- and 3-dependent inhibition of insulin secretion from INS-1 cells. Cell Signal. 2008;20:2286–2295. doi: 10.1016/j.cellsig.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 341.Mifflin S, Richter DW. The effects of QX-314 on medullary respiratory neurones. Brain Res. 1987;420:22–31. doi: 10.1016/0006-8993(87)90235-6. [DOI] [PubMed] [Google Scholar]
- 342.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 343.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- 345.Minke B. Drosophila mutant with a transducer defect. Biophys Struct Mech. 1977;3:59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- 346.Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 347.Mironov SL. Metabotropic glutamate receptors activate dendritic calcium waves and TRPM channels which drive rhythmic respiratory patterns in mice. J Physiol. 2008;586:2277–2291. doi: 10.1113/jphysiol.2007.149021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mironov SL, Hartelt N, Ivannikov MV. Mitochondrial K(ATP) channels in respiratory neurons and their role in the hypoxic facilitation of rhythmic activity. Brain Res. 2005;1033:20–27. doi: 10.1016/j.brainres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 349.Mironov SL, Langohr K. Mechanisms of Na+andCa2+influx into respiratory neurons during hypoxia. Neuropharmacology. 2005;48:1056–1065. doi: 10.1016/j.neuropharm.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 350.Mironov SL, Langohr K, Haller M, Richter DW. Hypoxia activates ATP-dependent potassium channels in inspiratory neurones of neonatal mice. J Physiol. 1998;509(Pt 3):755–66. doi: 10.1111/j.1469-7793.1998.755bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Mironov SL, Richter DW. L-type Ca2+ channels in inspiratory neurones of mice and their modulation by hypoxia. J Physiol. 1998;512(Pt 1):75–87. doi: 10.1111/j.1469-7793.1998.075bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Mironov SL, Richter DW. Oscillations and hypoxic changes of mitochondrial variables in neurons of the brainstem respiratory centre of mice. J Physiol. 2001;533:227–236. doi: 10.1111/j.1469-7793.2001.0227b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Miyake A, Yamada K, Kosaka T, Miki T, Seino S, Inagaki N. Disruption of Kir6.2-containing ATP-sensitive potassium channels impairs maintenance of hypoxic gasping in mice. Eur J Neurosci. 2007;25:2349–2363. doi: 10.1111/j.1460-9568.2007.05499.x. [DOI] [PubMed] [Google Scholar]
- 354.Miyamoto K, Iwase M, Kimura H, Homma I. Central histamine contributes to the inspiratory off-switch mechanism via H1 receptors in mice. Respir Physiol Neurobiol. 2004;144:25–33. doi: 10.1016/j.resp.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 355.Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Monteau R, Gauthier P, Rega P, Hilaire G. Effects of N-methyl-D-aspartate (NMDA) antagonist MK-801 on breathing pattern in rats. Neurosci Lett. 1990;109:134–139. doi: 10.1016/0304-3940(90)90551-j. [DOI] [PubMed] [Google Scholar]
- 357.Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBotzinger complex of neonatal mouse. J Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Morgado-Valle C, Beltran-Parrazal L, DiFranco M, Vergara JL, Feldman JL. Somatic Ca2+ transients do not contribute to inspiratory drive in preBotzinger Complex neurons. J Physiol. 2008;586:4531–4540. doi: 10.1113/jphysiol.2008.154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Morschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci. 2009;364:2517–2526. doi: 10.1098/rstb.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Morton MJ, O’Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K(+) channels TASK-1 and -2. Pflugers Arch. 2003;445:577–583. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- 362.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 363.Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Murbartian J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- 365.Mynlieff M, Beam KG. Adenosine acting at anA1receptor decreases N-type calcium current in mouse motoneurons. J Neurosci. 1994;14:3628–3634. doi: 10.1523/JNEUROSCI.14-06-03628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Nakanishi S. The molecular diversity of glutamate receptors. Prog Clin Biol Res. 1994;390:85–98. [PubMed] [Google Scholar]
- 367.Nash HA, Scott RL, Lear BC, Allada R. An unusual cation channel mediates photic control of locomotion in Drosophila. Curr Biol. 2002;12:2152–2158. doi: 10.1016/s0960-9822(02)01358-1. [DOI] [PubMed] [Google Scholar]
- 368.Nattie EE, Li AH. Ventral medulla sites of muscarinic receptor subtypes involved in cardiorespiratory control. J Appl Physiol. 1990;69:33–41. doi: 10.1152/jappl.1990.69.1.33. [DOI] [PubMed] [Google Scholar]
- 369.Neubauer JA, Melton JE, Edelman NH. Modulation of respiration during brain hypoxia. J Appl Physiol. 1990;68:441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- 370.Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol. 2008;605:348–352. doi: 10.1007/978-0-387-73693-8_61. [DOI] [PubMed] [Google Scholar]
- 371.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 373.Niswender CM, Conn PJ. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Niwa N, Wang W, Sha Q, Marionneau C, Nerbonne JM. Kv4.3 is not required for the generation of functional Ito,f channels in adult mouse ventricles. J Mol Cell Cardiol. 2008;44:95–104. doi: 10.1016/j.yjmcc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Norris AJ, Nerbonne JM. Molecular dissection of I(A) in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv4.3, and Kv1.4 alpha-subunits. J Neurosci. 2010;30:5092–5101. doi: 10.1523/JNEUROSCI.5890-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 377.Nunez A, Rodrigo-Angulo ML, Andres ID, Garzon M. Hypocretin/ Orexin neuropeptides: Participation in the control of sleepwakefulness cycle and energy homeostasis. Curr Neuropharmacol. 2009;7:50–59. doi: 10.2174/157015909787602797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.O’Brien JA, Sebe JY, Berger AJ. GABA(B) modulation of GABA(A) and glycine receptor-mediated synaptic currents in hypoglossal motoneurons. Respir Physiol Neurobiol. 2004;141:35–45. doi: 10.1016/j.resp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 379.O’Grady SM, Elmquist E, Filtz TM, Nicholas RA, Harden TK. A guanine nucleotide-independent inwardly rectifying cation permeability is associated with P2Y1 receptor expression in Xenopus oocytes. J Biol Chem. 1996;271:29080–29087. doi: 10.1074/jbc.271.46.29080. [DOI] [PubMed] [Google Scholar]
- 380.O’Regan RG, Majcherczyk S. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol. 1982;100:23–40. doi: 10.1242/jeb.100.1.23. [DOI] [PubMed] [Google Scholar]
- 381.Ogilvie MD, Gottschalk A, Anders K, Richter DW, Pack AI. A network model of respiratory rhythmogenesis. Am J Physiol. 1992;263:R962–R975. doi: 10.1152/ajpregu.1992.263.4.R962. [DOI] [PubMed] [Google Scholar]
- 382.Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D. NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol. 1998;84:853–861. doi: 10.1152/jappl.1998.84.3.853. [DOI] [PubMed] [Google Scholar]
- 383.Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: The omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- 384.Onimaru H, Ballanyi K, Homma I. Contribution of Ca2+-dependent conductances to membrane potential fluctuations of medullary respiratory neurons of newborn rats in vitro. J Physiol. 2003;552:727–741. doi: 10.1113/jphysiol.2003.049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Oyamada Y, Murai M, Harada N, Ishizaka A, Okada Y. Age-dependent involvement of ATP-sensitive potassium channel Kir6.2 in hypoxic ventilatory depression of mouse. Respir Physiol Neurobiol. 2008;162:80–84. doi: 10.1016/j.resp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 387.Oyamada Y, Yamaguchi K, Murai M, Hakuno H, Ishizaka A. Role of Kir2.2 in hypercapnic ventilatory response during postnatal development of mouse. Respir Physiol Neurobiol. 2005;145:143–151. doi: 10.1016/j.resp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 388.Paarmann I, Frermann D, Keller BU, Hollmann M. Expression of 15 glutamate receptor subunits and various splice variants in tissue slices and single neurons of brainstem nuclei and potential functional implications. J Neurochem. 2000;74:1335–1345. doi: 10.1046/j.1471-4159.2000.0741335.x. [DOI] [PubMed] [Google Scholar]
- 389.Paarmann I, Frermann D, Keller BU, Villmann C, Breitinger HG, Hollmann M. Kinetics and subunit composition of NMDA receptors in respiratory-related neurons. J Neurochem. 2005;93:812–824. doi: 10.1111/j.1471-4159.2005.03027.x. [DOI] [PubMed] [Google Scholar]
- 390.Pace R, Mackay D, Feldman J, Del Negro C. Role of persistent sodium current in mouse preBötzinger complex neurons and respiratory rhythm generation. J Physiol. 2007;580:485–496. doi: 10.1113/jphysiol.2006.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Pace RW, Del Negro CA. AMPA and metabotropic glutamate receptors cooperatively generate inspiratory-like depolarization in mouse respiratory neurons in vitro. Eur J Neurosci. 2008;28:2434–2442. doi: 10.1111/j.1460-9568.2008.06540.x. [DOI] [PubMed] [Google Scholar]
- 392.Pace RW, Mackay DD, Feldman JL, DelNegro CA. Inspiratory bursts in the preBotzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol. 2010;58:123–147. doi: 10.1016/S1054-3589(10)58006-2. [DOI] [PubMed] [Google Scholar]
- 394.Pare D, Pape HC, Dong J. Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: Electrophysiological properties and morphological features. J Neurophysiol. 1995;74:1179–1191. doi: 10.1152/jn.1995.74.3.1179. [DOI] [PubMed] [Google Scholar]
- 395.Parkis MA, Dong X, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: Phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Parkis MA, Feldman JL, Robinson DM, Funk GD. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J Neurosci. 2003;23:8152–8158. doi: 10.1523/JNEUROSCI.23-22-08152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Partridge LD, Valenzuela CF. Ca2+ store-dependent potentiation of Ca2+-activated non-selective cation channels in rat hippocampal neurones in vitro. J Physiol. 1999;521(Pt 3):617–27. doi: 10.1111/j.1469-7793.1999.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Paterson DS, Rivera KD, Broadbelt KG, Trachtenberg FL, Belliveau RA, Holm IA, Haas EA, Stanley C, Krous HF, Kinney HC, Markianos K. Lack of association of the serotonin transporter polymorphism with the sudden infant death syndrome in the San Diego Dataset. Pediatr Res. 2010;68:409–413. doi: 10.1203/PDR.0b013e3181f2edf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Paton JF, St-John WM. Counterpoint: Medullary pacemaker neurons are essential for gasping, but not eupnea, in mammals. J Appl Physiol. 2007;103:718–720. doi: 10.1152/japplphysiol.00003.2007a. discussion 721-2. [DOI] [PubMed] [Google Scholar]
- 400.Pearson KG, Ramirez JM. Neuron, Networks and Motor Behavior. Cambridge, Massachusetts, London, England: MIT Press: 1997. Sensory modulation of pattern-generating circuits; pp. 225–237. [Google Scholar]
- 401.Pena F, Aguileta MA. Effects of riluzole and flufenamic acid on eupnea and gasping of neonatal mice in vivo. Neurosci Lett. 2007;415:288–293. doi: 10.1016/j.neulet.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 402.Pena F, Meza-Andrade R, Paez-Zayas V, Gonzalez-Marin MC. Gasping generation in developing Swiss-Webster mice in vitro and in vivo. Neurochem Res. 2008;33:1492–1500. doi: 10.1007/s11064-008-9616-x. [DOI] [PubMed] [Google Scholar]
- 403.Pena F, Ordaz B. Non-selective cation channel blockers: Potential use in nervous system basic research and therapeutics. Mini RevMed Chem. 2008;8:812–819. doi: 10.2174/138955708784912166. [DOI] [PubMed] [Google Scholar]
- 404.Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 405.Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Pena F, Ramirez JM. Hypoxia-induced changes in neuronal network properties. Mol Neurobiol. 2005;32:251–283. doi: 10.1385/MN:32:3:251. [DOI] [PubMed] [Google Scholar]
- 408.Pepe S, Xiao RP, Hohl C, Altschuld R, Lakatta EG. ‘Cross talk’ between opioid peptide and adrenergic receptor signaling in isolated rat heart. Circulation. 1997;95:2122–2129. doi: 10.1161/01.cir.95.8.2122. [DOI] [PubMed] [Google Scholar]
- 409.Perez-Reyes E. Molecular characterization of T-type calcium channels. Cell Calcium. 2006;40:89–96. doi: 10.1016/j.ceca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 410.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 411.Perkel DH, Mulloney B. Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science. 1974;185:181–183. doi: 10.1126/science.185.4146.181. [DOI] [PubMed] [Google Scholar]
- 412.Perrins R, Soffe SR. Local effects of glycinergic inhibition in the spinal cord motor systems for swimming in amphibian embryos. JNeurophysiol. 1996;76:1025–1035. doi: 10.1152/jn.1996.76.2.1025. [DOI] [PubMed] [Google Scholar]
- 413.Perrins R, Soffe SR. Influence of glycinergic inhibition on spinal neuron excitability during amphibian tadpole locomotion. Ann N Y Acad Sci U S A. 1998;860:472–474. doi: 10.1111/j.1749-6632.1998.tb09080.x. [DOI] [PubMed] [Google Scholar]
- 414.Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms. 2010;25:432–441. doi: 10.1177/0748730410385204. [DOI] [PubMed] [Google Scholar]
- 415.Pfeiffer A, Zhang W. Postnatal development of GABAB-receptor-mediated modulation of potassium currents in brainstem respiratory network of mouse. Respir Physiol Neurobiol. 2007;158:22–29. doi: 10.1016/j.resp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 416.Pierrefiche O, Champagnat J, Richter DW. Calcium-dependent conductances control neurones involved in termination of inspiration in cats. Neurosci Lett. 1995;184:101–104. doi: 10.1016/0304-3940(94)11179-m. [DOI] [PubMed] [Google Scholar]
- 417.Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubie M. The bulbar network of respiratory neurons during apneusis induced by a blockade of NMDA receptors. Exp Brain Res. 1992;89:623–639. doi: 10.1007/BF00229887. [DOI] [PubMed] [Google Scholar]
- 418.Pierrefiche O, Foutz AS, Champagnat J, Denavit-Saubie M. NMDAand non-NMDAreceptorsmay play distinct roles in timing mechanisms and transmission in the feline respiratory network. J Physiol. 1994;474:509–523. doi: 10.1113/jphysiol.1994.sp020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Pierrefiche O, Haji A, Bischoff A, Richter DW. Calcium currents in respiratory neurons of the cat in vivo. Pflugers Arch. 1999;438:817–826. doi: 10.1007/s004249900090. [DOI] [PubMed] [Google Scholar]
- 420.Pilarski JQ, Fregosi RF. Prenatal nicotine exposure alters medullary nicotinic and AMPA-mediated control of respiratory frequency in vitro. Respir Physiol Neurobiol. 2009;169:1–10. doi: 10.1016/j.resp.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Pilarski JQ, Wakefield HE, Fuglevand AJ, Levine RB, Fregosi RF. Developmental nicotine exposure alters neurotransmission and excitability in hypoglossal motoneurons. J Neurophysiol. 2011;105:423–433. doi: 10.1152/jn.00876.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- 423.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Ptak K, Zummo GG, Alheid GF, Tkatch T, Surmeier DJ, McCrimmon DR. Sodium currents in medullary neurons isolated from the pre- Botzinger complex region. J Neurosci. 2005;25:5159–5170. doi: 10.1523/JNEUROSCI.4238-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Purvis LK, Smith JC, Koizumi H, Butera RJ. Intrinsic bursters increase the robustness of rhythm generation in an excitatory network. J Neurophysiol. 2007;97:1515–1526. doi: 10.1152/jn.00908.2006. [DOI] [PubMed] [Google Scholar]
- 426.Putnam RW. CO2 chemoreception in cardiorespiratory control. J Appl Physiol. 2010;108:1796–1802. doi: 10.1152/japplphysiol.01169.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Putzier I, Kullmann PH, Horn JP, Levitan ES. Cav1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J Neurosci. 2009;29:15414–15419. doi: 10.1523/JNEUROSCI.4742-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Qiu C, Zeyda T, Johnson B, Hochgeschwender U, de Lecea L, Tallent MK. Somatostatin receptor subtype 4 couples to the M-current to regulate seizures. J Neurosci. 2008;28:3567–3576. doi: 10.1523/JNEUROSCI.4679-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 430.Ramcharan EJ, Gnadt JW, Sherman SM. Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Vis Neurosci. 2000;17:55–62. doi: 10.1017/s0952523800171056. [DOI] [PubMed] [Google Scholar]
- 431.Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci U S A. 2005;102:12236–12241. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Ramirez JM. The human pre-Botzinger complex identified. Brain. 2011;134:8–10. doi: 10.1093/brain/awq357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Ramirez JM, Folkow LP, Blix AS. Hypoxia tolerance in mammals and birds: From the wilderness to the clinic. Annu Rev Physiol. 2007;69:113–143. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- 434.Ramirez JM, Koch H, Garcia IA, Doi A, Zanella S. The role of spiking and bursting pacemakers in the neuronal control of breathing. J Biol Physics. 2011;37:241–261. doi: 10.1007/s10867-011-9214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Ramirez JM, Lieske SP. Commentary on the definition of eupnea and gasping. Respir Physiol Neurobiol. 2003;139:113–119. doi: 10.1016/s1569-9048(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 436.Ramirez JM, Quellmalz UJ, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol. 1996;491(Pt 3):799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Ramirez JM, Quellmalz UJ, Wilken B, Richter DW. The hypoxic response of neurones within the in vitro mammalian respiratory network. J Physiol. 1998;507(Pt 2):571–582. doi: 10.1111/j.1469-7793.1998.571bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Ramirez JM, Quellmalz UJ, Wilken B. Developmental changes in the hypoxic response of the hypoglossus respiratory motor output in vitro. J Neurophysiol. 1997;78:383–392. doi: 10.1152/jn.1997.78.1.383. [DOI] [PubMed] [Google Scholar]
- 439.Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1996;6:817–825. doi: 10.1016/s0959-4388(96)80033-x. [DOI] [PubMed] [Google Scholar]
- 440.Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Botzinger complex in vivo eliminates breathing but not gasping. J Physiol. 1998;507(Pt 3):895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Ramirez JM, Telgkamp P, Elsen FP, Quellmalz UJ, Richter DW. Respiratory rhythm generation in mammals: Synaptic and membrane properties. Respir Physiol. 1997;110:71–85. doi: 10.1016/s0034-5687(97)00074-1. [DOI] [PubMed] [Google Scholar]
- 442.Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: An integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 443.Ramirez JM, Viemari JC. Determinants of inspiratory activity. Respir Physiol Neurobiol. 2005;147:145–157. doi: 10.1016/j.resp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 444.Rand CM, Berry-Kravis EM, Zhou L, Fan W, Weese-Mayer DE. Sudden infant death syndrome: Rare mutation in the serotonin system FEV gene. Pediatr Res. 2007;62:180–182. doi: 10.1203/PDR.0b013e3180a725a0. [DOI] [PubMed] [Google Scholar]
- 445.Randall A, Benham CD. Recent advances in the molecular understanding of voltage-gated Ca2 +channels. Mol Cell Neurosci. 1999;14:255–272. doi: 10.1006/mcne.1999.0795. [DOI] [PubMed] [Google Scholar]
- 446.Rekling JC, Feldman JL. PreBotzinger complex and pacemaker neurons: Hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- 447.Rettig J, Wunder F, Stocker M, Lichtinghagen R, Mastiaux F, Beckh S, Kues W, Pedarzani P, Schroter KH, Ruppersberg JP, Rudiger V, Olaf P. Characterization of a Shaw-related potassium channel family in rat brain. EMBO J. 1992;11:2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 449.Richter DW, Ballantyne D, Remmers JE. The differential organization of medullary post-inspiratory activities. Pflugers Arch. 1987;410:420–427. doi: 10.1007/BF00586520. [DOI] [PubMed] [Google Scholar]
- 450.Richter DW, Ballanyi K, Schwarzacher S. Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1992;2:788–793. doi: 10.1016/0959-4388(92)90135-8. [DOI] [PubMed] [Google Scholar]
- 451.Richter DW, Champagnat J, Jacquin T, Benacka R. Calcium currents and calcium-dependent potassium currents in mammalian medullary respiratory neurones. J Physiol. 1993;470:23–33. doi: 10.1113/jphysiol.1993.sp019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U. Response of the medullary respiratory network of the cat to hypoxia. J Physiol. 1991;443:231–256. doi: 10.1113/jphysiol.1991.sp018832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Richter DW, Lalley PM, Pierrefiche O, Haji A, Bischoff AM, Wilken B, Hanefeld F. Intracellular signal pathways controlling respiratory neurons. Respir Physiol. 1997;110:113–123. doi: 10.1016/s0034-5687(97)00077-7. [DOI] [PubMed] [Google Scholar]
- 454.Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: Guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 455.Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514(Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Ritucci NA, Dean JB, Putnam RW. Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Am J Physiol Cell Physiol. 2005;289:C1094–C1104. doi: 10.1152/ajpcell.00329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Roberts CD, Dvoryanchikov G, Roper SD, Chaudhari N. Interaction between the second messengers cAMP and Ca2+in mouse presynaptic taste cells. J Physiol. 2009;587:1657–1668. doi: 10.1113/jphysiol.2009.170555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Rouchet N, Waroux O, Lamy C, Massotte L, Scuvee-Moreau J, Liegeois JF, Seutin V. SK channel blockade promotes burst firing in dorsal raphe serotonergic neurons. Eur J Neurosci. 2008;28:1108–1115. doi: 10.1111/j.1460-9568.2008.06430.x. [DOI] [PubMed] [Google Scholar]
- 459.Ruangkittisakul A, Panaitescu B, Ballanyi K. K(+) and Ca(2)(+) dependence of inspiratory-related rhythm in novel “calibrated” mouse brainstem slices. Respir Physiol Neurobiol. 2010;175:37–48. doi: 10.1016/j.resp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 460.Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, Funk GD, Ballanyi K. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci. 2008;28:2447–2458. doi: 10.1523/JNEUROSCI.1926-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote networkdependent burst oscillations. Proc Natl Acad SciUS A. 2009;106:2939–2944. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Rubin JE, Bacak BJ, Molkov YI, Shevtsova NA, Smith JC, Rybak IA. Interacting oscillations in neural control of breathing: Modeling and qualitative analysis. J Comput Neurosci. 2010;30:607–632. doi: 10.1007/s10827-010-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Rubin JE, Shevtsova NA, Ermentrout GB, Smith JC, Rybak IA. Multiple rhythmic states in a model of the respiratory central pattern generator. J Neurophysiol. 2009;101:2146–2165. doi: 10.1152/jn.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Rybak IA, Shevtsova NA, Ptak K, McCrimmon DR. Intrinsic bursting activity in the pre-Botzinger complex: Role of persistent sodium and potassium currents. Biol Cybern. 2004;90:59–74. doi: 10.1007/s00422-003-0447-1. [DOI] [PubMed] [Google Scholar]
- 466.Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: Insights from deletions during fictive locomotion. JPhysiol. 2006;577:617–639. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Rybak IA, St John WM, Paton JF. Models of neuronal bursting behavior: Implications for in-vivo versus in-vitro respiratory rhythmogenesis. Adv Exp Med Biol. 2001;499:159–164. doi: 10.1007/978-1-4615-1375-9_25. [DOI] [PubMed] [Google Scholar]
- 468.Rybak IA, Shevtsova NA, St-John WM, Paton JF, Pierrefiche O. Endogenous rhythm generation in the pre-Botzinger complex and ionic currents: Modelling and in vitro studies. Eur J Neurosci. 2003;18:239–257. doi: 10.1046/j.1460-9568.2003.02739.x. [DOI] [PubMed] [Google Scholar]
- 469.Salido GM, Sage SO, Rosado JA. TRPC channels and store-operated Ca(2+) entry. Biochim Biophys Acta. 2009;1793:223–230. doi: 10.1016/j.bbamcr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 470.Salkoff L, Baker K, Butler A, Covarrubias M, Pak MD, Wei A. An essential ‘set’ of K+ channels conserved in flies, mice and humans. Trends Neurosci. 1992;15:161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- 471.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 472.Sanchez-Alonso JL, Munoz-Cuevas J, Vicente-Torres MA, Colino A. Role of low-voltage-activated calcium current on the firing pattern alterations induced by hypothyroidism in the rat hippocampus. Neuroscience. 2010;171:993–1005. doi: 10.1016/j.neuroscience.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 473.Sankaran K, Wiebe H, Seshia MM, Boychuk RB, Cates D, Rigatto H. Immediate and late ventillatory response to high and low O2 in preterm infants and adult subjects. Pediatr Res. 1979;13:875–878. doi: 10.1203/00006450-197908000-00001. [DOI] [PubMed] [Google Scholar]
- 474.Saponjic J, Radulovacki M, Carley DW. Respiratory pattern modulation by the pedunculopontine tegmental nucleus. Respir Physiol Neurobiol. 2003;138:223–237. doi: 10.1016/j.resp.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 475.Saponjic J, Radulovacki M, Carley DW. Modulation of respiratory pattern and upper airway muscle activity by the pedunculopontine tegmentum: Role of NMDA receptors. Sleep Breath. 2006;10:195–202. doi: 10.1007/s11325-006-0075-9. [DOI] [PubMed] [Google Scholar]
- 476.Saponjic J, Radulovacki M, Carley DW. Monoaminergic system lesions increase post-sigh respiratory pattern disturbance during sleep in rats. Physiol Behav. 2007;90:1–10. doi: 10.1016/j.physbeh.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 477.Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Schlaefke ME, Loeschcke HH. Lokalisation an der Regulation von Atmung und Kreislauf beteiligten Gebietes an der ventralen OberflSche de Medulla oblongata durch Kalteblockade. Pfliigers Arch. 1967;297:201–220. [Google Scholar]
- 479.Schlaefke ME, See WR, Herker-See A, Loeschcke HH. Respiratory response to hypoxia and hypercapnia after elimination of central chemosensitivity. Pflugers Arch. 1979;381:241–248. doi: 10.1007/BF00583255. [DOI] [PubMed] [Google Scholar]
- 480.Schlaefke ME, See WR, Massion WH, Loeschcke HH. Die Rolle ‘spezifischer’ und ‘ unspezifischer’ Afferenzen fur den Antrieb de Atmung, untersucht durch Reizung und Blockade von Afferenzen an der decerebrierten Katze. Pfliigers Arch. 1969;31a:198–212. doi: 10.1007/BF00586928. [DOI] [PubMed] [Google Scholar]
- 481.Schlaefke ME, Pokorski M, See WR, Kille JF, Loeschcke HH. Chemosensitive neurons on the ventral medullary surface. Bull. Physio- Pathol. Respir. 1975;11:277–284. [PubMed] [Google Scholar]
- 482.Schmid K, Bohmer G, Gebauer K. GABAA receptor mediated fast synaptic inhibition in the rabbit brain-stem respiratory system. Acta Physiol Scand. 1991a;142:411–420. doi: 10.1111/j.1748-1716.1991.tb09175.x. [DOI] [PubMed] [Google Scholar]
- 483.Schmid K, Bohmer G, Gebauer K. Glycine receptor-mediated fast synaptic inhibition in the brainstem respiratory system. Respir Physiol. 1991b;84:351–361. doi: 10.1016/0034-5687(91)90129-7. [DOI] [PubMed] [Google Scholar]
- 484.Schmid K, Foutz AS, Denavit-Saubie M. Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res. 1996;710:150–160. doi: 10.1016/0006-8993(95)01380-6. [DOI] [PubMed] [Google Scholar]
- 485.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 486.Schmidt C, Bellingham MC, Richter DW. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J Physiol. 1995;483(Pt 3):769–781. doi: 10.1113/jphysiol.1995.sp020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- 488.Schwarzacher SW, Rub U, Deller T. Neuroanatomical characteristics of the human pre-Botzinger complex and its involvement in neurode-generative brainstem diseases. Brain. 2010;134:24–35. doi: 10.1093/brain/awq327. [DOI] [PubMed] [Google Scholar]
- 489.Schwarzacher SW, Pestean A, Günther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- 490.Schwarzacher SW, Wilhelm Z, Anders K, Richter DW. The medullary respiratory network in the rat. J Physiol. 1991;435:631–644. doi: 10.1113/jphysiol.1991.sp018529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Sedensky MM, Meneely PM. Genetic analysis of halothane sensitivity in Caenorhabditis elegans. Science. 1987;236:952–954. doi: 10.1126/science.3576211. [DOI] [PubMed] [Google Scholar]
- 492.Selverston AI. A neural infrastructure for rhythmic motor patterns. Cell Mol Neurobiol. 2005;25:223–244. doi: 10.1007/s10571-005-3154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Selverston AI, Moulins M. Oscillatory neural networks. Annu Rev Physiol. 1985;47:29–48. doi: 10.1146/annurev.ph.47.030185.000333. [DOI] [PubMed] [Google Scholar]
- 494.Severson CA, Wang W, Pieribone VA, Dohle CI, Richerson GB. Midbrain serotonergic neurons are centralpHchemoreceptors. NatNeurosci. 2003;6:1139–1140. doi: 10.1038/nn1130. [DOI] [PubMed] [Google Scholar]
- 495.Shan J, Yu XC, Fung ML, Wong TM. Attenuated “cross talk” between kappa-opioid receptors and beta-adrenoceptors in the heart of chronically hypoxic rats. Pflugers Arch. 2002;444:126–132. doi: 10.1007/s00424-002-0814-0. [DOI] [PubMed] [Google Scholar]
- 496.Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: Differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- 497.Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: Effects mediated by M3-like receptors in pre-Botzinger complex inspiratory neurons. J Neurophysiol. 2000;83:1243–1252. doi: 10.1152/jn.2000.83.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Shao XM, Feldman JL. Mechanisms underlying regulation of respiratory pattern by nicotine in pre-Botzinger complex. J Neurophysiol. 2001;85:2461–2467. doi: 10.1152/jn.2001.85.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Shao XM, Feldman JL. Pharmacology of nicotinic receptors in pre-Botzinger complex that mediate modulation of respiratory pattern. J Neurophysiol. 2002;88:1851–1858. doi: 10.1152/jn.2002.88.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Shao XM, Feldman JL. Cholinergic neurotransmission in the pre- Botzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience. 2005;130:1069–1081. doi: 10.1016/j.neuroscience.2004.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Shao XM, Ge Q, Feldman JL. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBotzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J Physiol. 2003;547:543–553. doi: 10.1113/jphysiol.2002.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Sharma SD, Raghuraman G, Lee MS, Prabhakar NR, Kumar GK. Intermittent hypoxia activates peptidylglycine alpha-amidating monooxygenase in rat brain stem via reactive oxygen species-mediated proteolytic processing. J Appl Physiol. 2009;106:12–19. doi: 10.1152/japplphysiol.90702.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Sharp AA, Skinner FK, Marder E. Mechanisms of oscillation in dynamic clamp constructed two-cell half-center circuits. J Neurophysiol. 1996;76:867–883. doi: 10.1152/jn.1996.76.2.867. [DOI] [PubMed] [Google Scholar]
- 504.Shen L, Li YM, Duffin J. Inhibitory connections among rostral medullary expiratory neurones detected with cross-correlation in the decerebrate rat. Pflugers Arch. 2003;446:365–372. doi: 10.1007/s00424-003-1024-0. [DOI] [PubMed] [Google Scholar]
- 505.Sher E, Clementi F. Omega-conotoxin-sensitive voltage-operated calcium channels in vertebrate cells. Neuroscience. 1991;42:301–307. doi: 10.1016/0306-4522(91)90376-y. [DOI] [PubMed] [Google Scholar]
- 506.Sherrington CS. On the proprioceptive system especially in its reflex aspect. Brain. 1906;29:467–482. [Google Scholar]
- 507.Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Shi C, Szczesniak A, Mao L, Jollimore C, Coca-Prados M, Hung O, Kelly ME. A3 adenosine and CB1 receptors activate a PKC-sensitive Cl- current in human nonpigmented ciliary epithelial cells via a G beta gamma-coupled MAPK signaling pathway. Br J Pharmacol. 2003;139:475–486. doi: 10.1038/sj.bjp.0705266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Shilnikov A, Calabrese RL, Cymbalyuk G. Mechanism of bistability: Tonic spiking and bursting in a neuron model. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:056214. doi: 10.1103/PhysRevE.71.056214. [DOI] [PubMed] [Google Scholar]
- 510.Shvarev YN, Lagercrantz H, Yamamoto Y. Biphasic effects of substance P on respiratory activity and respiration-related neurones in ventrolateral medulla in the neonatal rat brainstem in vitro. Acta Physiol Scand. 2002;174:67–84. doi: 10.1046/j.1365-201x.2002.00926.x. [DOI] [PubMed] [Google Scholar]
- 511.Singer JH, Berger AJ. Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons. J Neurophysiol. 1999;81:1608–1616. doi: 10.1152/jn.1999.81.4.1608. [DOI] [PubMed] [Google Scholar]
- 512.Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, McHarg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- 513.Skiebe P. Neuropeptides are ubiquitous chemical mediators: Using the stomatogastric nervous system as a model system. J Exp Biol. 2001;204:2035–2048. doi: 10.1242/jeb.204.12.2035. [DOI] [PubMed] [Google Scholar]
- 514.Skinner FK, Kopell N, Marder E. Mechanisms for oscillation and frequency control in reciprocally inhibitory model neural networks. J Comput Neurosci. 1994;1:69–87. doi: 10.1007/BF00962719. [DOI] [PubMed] [Google Scholar]
- 515.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Smith PA, Sellers LA, Humphrey PP. Somatostatin activates two types of inwardly rectifying K +channels in MIN-6 cells. J Physiol. 2001;532:127–142. doi: 10.1111/j.1469-7793.2001.0127g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Snutch TP, Monteil A. The sodium “leak” has finally been plugged. Neuron. 2007;54:505–507. doi: 10.1016/j.neuron.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 519.Soboloff J, Spassova M, Hewavitharana T, He LP, Luncsford P, Xu W, Venkatachalam K, van Rossum D, Patterson RL, Gill DL. TRPC channels: Integrators of multiple cellular signals. Handb Exp Pharmacol. 2007:575–591. doi: 10.1007/978-3-540-34891-7_34. [DOI] [PubMed] [Google Scholar]
- 520.Solomon IC. Modulation of gasp frequency by activation of pre- Botzinger complex in vivo. J Neurophysiol. 2002;87:1664–1668. doi: 10.1152/jn.00742.2001. [DOI] [PubMed] [Google Scholar]
- 521.Solomon IC, Edelman NH, Neubauer JA. Pre-Botzinger complex functions as a central hypoxia chemosensor for respiration in vivo. J Neurophysiol. 2000;83:2854–2868. doi: 10.1152/jn.2000.83.5.2854. [DOI] [PubMed] [Google Scholar]
- 522.Sorensen JB. Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Pflugers Arch. 2004;448:347–362. doi: 10.1007/s00424-004-1247-8. [DOI] [PubMed] [Google Scholar]
- 523.Sorensen ME, DeWeerth SP. Functional consequences of model complexity in rhythmic systems. II. Systems performance of model and hybrid oscillators. J Neural Eng. 2007;4:189–196. doi: 10.1088/1741-2560/4/3/003. [DOI] [PubMed] [Google Scholar]
- 524.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Speca DJ, Chihara D, Ashique AM, Bowers MS, Pierce-Shimomura JT, Lee J, Rabbee N, Speed TP, Gularte RJ, Chitwood J, Medrano JF, Liao M, Sonner JM, Eger EI, 2nd, Peterson AS, McIntire SL. Conserved role of unc-79 in ethanol responses in lightweight mutant mice. PLoS Genet. 2010;12(6(8)):e1001057. doi: 10.1371/journal.pgen.1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Sporns O. The human connectome: A complex network. Ann N Y Acad Sci USA. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 527.Sporns O, Tononi G, Kotter R. The human connectome: A structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Spyer KM, Gourine AV. Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc Lond B Biol Sci. 2009;364:2603–2610. doi: 10.1098/rstb.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.St-John WM. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol. 1997;82:375–376. doi: 10.1152/jappl.1997.82.2.375. [DOI] [PubMed] [Google Scholar]
- 530.St-John WM, Leiter JC. Discharge of the hypoglossal nerve cannot distinguish eupnea from gasping, as defined by phrenic discharge, in the in situ mouse. J Appl Physiol. 2009;107:686–695. doi: 10.1152/japplphysiol.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.St-John WM, Stornetta RL, Guyenet PG, Paton JF. Location and properties of respiratory neurones with putative intrinsic bursting properties in the rat in situ. J Physiol. 2009;587:3175–3188. doi: 10.1113/jphysiol.2009.170308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.St-John WM, Waki H, Dutschmann M, Paton JF. Maintenance of eupnea of in situ and in vivo rats following riluzole: A blocker of persistent sodium channels. Respir Physiol Neurobiol. 2007;155:97–100. doi: 10.1016/j.resp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 533.Stanford IM, Lacey MG. Regulation of a potassium conductance in rat midbrain dopamine neurons by intracellular adenosine triphosphate (ATP) and the sulfonylureas tolbutamide and glibenclamide. J Neurosci. 1995;15:4651–4657. doi: 10.1523/JNEUROSCI.15-06-04651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci. 2004;5:400–408. doi: 10.1038/nrn1388. [DOI] [PubMed] [Google Scholar]
- 535.Stent GS, Kristan WB, Jr, Friesen WO, Ort CA, Poon M, Calabrese RL. Neuronal generation of the leech swimming movement. Science. 1978;200:1348–1357. doi: 10.1126/science.663615. [DOI] [PubMed] [Google Scholar]
- 536.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 537.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol. 2002;444:207–220. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- 538.Sun MK, Reis DJ. Hypoxia-activated Ca2+ currents in pacemaker neurones of rat rostral ventrolateral medulla in vitro. J Physiol. 1994;476:101–116. [PMC free article] [PubMed] [Google Scholar]
- 539.Surmeier DJ, Mercer JN, Chan CS. Autonomous pacemakers in the basal ganglia: Who needs excitatory synapses anyway? Curr Opin Neurobiol. 2005;15:312–318. doi: 10.1016/j.conb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 540.Swayne LA, Mezghrani A, Varrault A, Chemin J, Bertrand G, Dalle S, Bourinet E, Lory P, Miller RJ, Nargeot J, Monteil A. The NALCN ion channel is activated by M3 muscarinic receptors in a pancreatic beta-cell line. EMBO Rep. 2009;10:873–880. doi: 10.1038/embor.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci. 2000;20:6752–6759. doi: 10.1523/JNEUROSCI.20-18-06752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- 543.Tai C, Hines DJ, Choi HB, Macvicar BA. Plasma membrane insertion of TRPC5 channels contributes to the cholinergic plateau potential in hippocampal CA1 pyramidal neurons. Hippocampus. 2010;21:958–967. doi: 10.1002/hipo.20807. [DOI] [PubMed] [Google Scholar]
- 544.Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of preBotzinger complex neurons in adult rats. J Comp Neurol. 2010;518:1862–1878. doi: 10.1002/cne.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Taylor AL, Cottrell GW, Kristan WB., Jr Analysis of oscillations in a reciprocally inhibitory network with synaptic depression. Neural Comput. 2002;14:561–581. doi: 10.1162/089976602317250906. [DOI] [PubMed] [Google Scholar]
- 547.Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol. 2002;88:206–213. doi: 10.1152/jn.2002.88.1.206. [DOI] [PubMed] [Google Scholar]
- 548.Telgkamp P, Ramirez JM. Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J Neurophysiol. 1999;82:2163–2170. doi: 10.1152/jn.1999.82.5.2163. [DOI] [PubMed] [Google Scholar]
- 549.Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- 550.Thach BT. The role of respiratory control disorders in SIDS. Respir Physiol Neurobiol. 2005;149:343–353. doi: 10.1016/j.resp.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 551.Thach BT. Some aspects of clinical relevance in the maturation of respiratory control in infants. J Appl Physiol. 2008;104:1828–1834. doi: 10.1152/japplphysiol.01288.2007. [DOI] [PubMed] [Google Scholar]
- 552.Thankachan S, Kaur S, Shiromani PJ. Activity of pontine neurons during sleep and cataplexy in hypocretin knock-out mice. J Neurosci. 2009;29:1580–1585. doi: 10.1523/JNEUROSCI.5151-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Thoby-Brisson M, Ramirez JM. Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. J Neurosci. 2000;20:5858–5866. doi: 10.1523/JNEUROSCI.20-15-05858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86:104–112. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- 555.Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G. Emergence of the pre-Botzinger respiratory rhythm generator in the mouse embryo. J Neurosci. 2005;25:4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Tian GF, Peever JH, Duffin J. Mutual inhibition between Botzinger-complex bulbospinal expiratory neurons detected with cross-correlation in the decerebrate rat. Exp Brain Res. 1999;125:440–446. doi: 10.1007/s002210050701. [DOI] [PubMed] [Google Scholar]
- 557.Topchiy I, Waxman J, Radulovacki M, Carley DW. Functional topography of respiratory, cardiovascular and pontine-wave responses to glutamate microstimulation of the pedunculopontine tegmentum of the rat. Respir Physiol Neurobiol. 2010;173:64–70. doi: 10.1016/j.resp.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Toporikova N, Butera RJ. Two types of independent bursting mechanisms in inspiratory neurons: An integrative model. J ComputNeurosci. 2010;30:515–528. doi: 10.1007/s10827-010-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–227. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- 560.Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neurosci. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- 561.Trebak M, Hempel N, Wedel BJ, Smyth JT, Bird GS, Putney JW., Jr Negative regulation of TRPC3 channels by protein kinase Cmediated phosphorylation of serine 712. Mol Pharmacol. 2005;67:558–563. doi: 10.1124/mol.104.007252. [DOI] [PubMed] [Google Scholar]
- 562.Trippenbach T, Richter DW. AckerH.Hypoxia and ion activitieswithin the brain stem of newborn rabbits. J Appl Physiol. 1990;68:2494–2503. doi: 10.1152/jappl.1990.68.6.2494. [DOI] [PubMed] [Google Scholar]
- 563.Tryba AK, Pena F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol. 2008;99:2114–2125. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Tryba AK, Pena F, Ramirez JM. Stabilization of bursting in respiratory pacemaker neurons. J Neurosci. 2003;23:3538–3546. doi: 10.1523/JNEUROSCI.23-08-03538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: A rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Tryba AK, Ramirez JM. Background sodium current stabilizes bursting in respiratory pacemaker neurons. J Neurobiol. 2004;60:481–489. doi: 10.1002/neu.20050. [DOI] [PubMed] [Google Scholar]
- 567.Uebachs M, Opitz T, Royeck M, Dickhof G, Horstmann MT, Isom LL, Beck HO. Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel beta subunits via paradoxical effects on persistent sodium currents. J Neurosci. 2010;30:8489–8501. doi: 10.1523/JNEUROSCI.1534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- 569.Ureche ON, Baltaev R, Ureche L, Strutz-Seebohm N, Lang F, Seebohm G. Novel insights into the structural basis of pH-sensitivity in inward rectifier K+channels Kir2.3. Cell Physiol Biochem. 2008;21:347–356. doi: 10.1159/000129629. [DOI] [PubMed] [Google Scholar]
- 570.Usowicz MM, Sugimori M, Cherksey B, Llinas R. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 1992;9:1185–1199. doi: 10.1016/0896-6273(92)90076-p. [DOI] [PubMed] [Google Scholar]
- 571.van Brederode JF, Berger AJ. GAD67-GFP+ neurons in the nucleus of roller. II. Subthreshold and firing resonance properties. J Neurophysiol. 2011;105:249–278. doi: 10.1152/jn.00492.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.van Brederode JF, Yanagawa Y, Berger AJ. GAD67-GFP+ neurons in the Nucleus ofRoller:Apossible source of inhibitory input to hypoglossal motoneurons. I. Morphology and firing properties. J Neurophysiol. 2011;105:235–248. doi: 10.1152/jn.00493.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci. 2009;29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.van Drongelen W, Koch H, Elsen FP, Lee HC, Mrejeru A, Doren E, Marcuccilli CJ, Hereld M, Stevens RL, Ramirez JM. Role of persistent sodium current in bursting activity of mouse neocortical networks in vitro. J Neurophysiol. 2006;96:2564–2577. doi: 10.1152/jn.00446.2006. [DOI] [PubMed] [Google Scholar]
- 575.Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep-disordered breathing. Am J Respir Crit Care Med. 1996;153:776–786. doi: 10.1164/ajrccm.153.2.8564132. [DOI] [PubMed] [Google Scholar]
- 576.Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb Exp Pharmacol. 2007;179:269–285. doi: 10.1007/978-3-540-34891-7_16. [DOI] [PubMed] [Google Scholar]
- 577.Viemari JC, Burnet H, Bevengut M, Hilaire G. Perinatal maturation of the mouse respiratory rhythm-generator: In vivo and in vitro studies. Eur J Neurosci. 2003;17:1233–1244. doi: 10.1046/j.1460-9568.2003.02561.x. [DOI] [PubMed] [Google Scholar]
- 578.Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- 579.Vinet R, Vargas FF. L- and T-type voltage-gated Ca2+ currents in adrenal medulla endothelial cells. Am J Physiol. 276:H1313–H1322. doi: 10.1152/ajpheart.1999.276.4.H1313. [DOI] [PubMed] [Google Scholar]
- 580.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. Febrile seizures and generalized epilepsy associatedwith a mutation in the Na+-channel beta1 subunit gene SCN1B. NatGenet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 581.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 582.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassiumchannel gene:KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 583.Wang S, Xu DJ, Cai JB, Huang YZ, Zou JG, Cao KJ. Rapid component I(Kr) of cardiac delayed rectifier potassium currents in guinea-pig is inhibited by alpha(1)-adrenoreceptor activation via protein kinase A and protein kinase C-dependent pathways. Eur J Pharmacol. 2009;608:1–6. doi: 10.1016/j.ejphar.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 584.Wang SJ, Cheng LL, Gean PW. Cross-modulation of synaptic plasticity by beta-adrenergic and 5-HT1A receptors in the rat basolateral amygdala. J Neurosci. 1999;19:570–577. doi: 10.1523/JNEUROSCI.19-02-00570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Wang SJ, Coutinho V, Sihra TS. Presynaptic cross-talk of betaadrenoreceptor and 5-hydroxytryptamine receptor signalling in the modulation of glutamate release from cerebrocortical nerve terminals. Br J Pharmacol. 2002;137:1371–1379. doi: 10.1038/sj.bjp.0705045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pH(o) and P(CO2) J Physiol. 2002;540:951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Wang W, Fung ML, St John WM. Pontile regulation of ventilatory activity in the adult rat. J Appl Physiol. 1993;74:2801–2811. doi: 10.1152/jappl.1993.74.6.2801. [DOI] [PubMed] [Google Scholar]
- 588.Wang XJ, Rinzel J. Spindle rhythmicity in the reticularis thalami nucleus: Synchronization among mutually inhibitory neurons. Neuroscience. 1993;53:899–904. doi: 10.1016/0306-4522(93)90474-t. [DOI] [PubMed] [Google Scholar]
- 589.Watanabe H, Nagata E, Kosakai A, Nakamura M, Yokoyama M, Tanaka K, Sasai H. Disruption of the epilepsy KCNQ2 gene results in neural hyperexcitability. J Neurochem. 2000;75:28–33. doi: 10.1046/j.1471-4159.2000.0750028.x. [DOI] [PubMed] [Google Scholar]
- 590.Weese-Mayer DE, Ackerman MJ, Marazita ML, Berry-Kravis EM. Sudden Infant Death Syndrome: Review of implicated genetic factors. Am J Med Genet A. 2007;143A:771–788. doi: 10.1002/ajmg.a.31722. [DOI] [PubMed] [Google Scholar]
- 591.Wei A, Covarrubias M, Butler A, Baker K, Pak M, Salkoff L. K current diversity is produced by an extended gene family conserved +in Drosophila and mouse. Science. 1990;248:599–603. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]
- 592.Wei A, Jegla T, Salkoff L. Eight potassium channel families revealed by the C. elegans genome project. Neuropharmacology. 1996;35:805–829. doi: 10.1016/0028-3908(96)00126-8. [DOI] [PubMed] [Google Scholar]
- 593.Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Botzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol. 2004;97:1629–1636. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- 595.Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther. 2002;303:439–444. doi: 10.1124/jpet.102.035113. [DOI] [PubMed] [Google Scholar]
- 596.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in firstdegree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Wickstrom HR, Berner J, Holgert H, Hokfelt T, Lagercrantz H. Hypoxic response in newborn rat is attenuated by neurokinin-1 receptor blockade. Respir Physiol Neurobiol. 2004;140:19–31. doi: 10.1016/j.resp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 598.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Willis T. Cerebri Anatome: Cui Accessit Nervorum Descriptio et Usus [The anatomy of the brain and nerves] Flesher, London. 1664 [Google Scholar]
- 600.Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J, Hulsmann S. Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflugers Arch. 2009;458:459–469. doi: 10.1007/s00424-009-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Wissenbach U, Niemeyer BA. Trpv6. Handb Exp Pharmacol. 2007;179:221–234. doi: 10.1007/978-3-540-34891-7_13. [DOI] [PubMed] [Google Scholar]
- 602.Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol. 2004;197:179–191. doi: 10.1007/s00232-004-0652-4. [DOI] [PubMed] [Google Scholar]
- 603.Xiang Z, Wang L, Kitai ST. Modulation of spontaneous firing in rat subthalamic neurons by 5-HT receptor subtypes. J Neurophysiol. 2005;93:1145–1157. doi: 10.1152/jn.00561.2004. [DOI] [PubMed] [Google Scholar]
- 604.Xu H, Yang Z, Cui N, Chanchevalap S, Valesky WW, Jiang C. A single residue contributes to the difference between Kir4.1 and Kir1.1 channels in pH sensitivity, rectification and single channel conductance. J Physiol. 2000;528(Pt 2):267–277. doi: 10.1111/j.1469-7793.2000.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Yamamoto S, Kanno T, Yamada K, Yasuda Y, Nishizaki T. Dual regulation of heat-activated K+ channel in rat DRG neurons via alpha(1) and beta adrenergic receptors. Life Sci. 2009;85:167–171. doi: 10.1016/j.lfs.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 606.Yang B, Gribkoff VK, Pan J, Damagnez V, Dworetzky SI, Boissard CG, Bhattacharjee A, Yan Y, Sigworth FJ, Kaczmarek LK. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology. 2006;51:896–906. doi: 10.1016/j.neuropharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 607.Yang WP, Levesque PC, Little WA, Conder ML, Ramakrishnan P, Neubauer MG, Blanar MA. Functional expression of two KvLQT1- related potassium channels responsible for an inherited idiopathic epilepsy. J Biol Chem. 1998;273:19419–19423. doi: 10.1074/jbc.273.31.19419. [DOI] [PubMed] [Google Scholar]
- 608.Yao L, Fan P, Jiang Z, Gordon A, Mochly-Rosen D, Diamond I. Dopamine and ethanol cause translocation of epsilonPKC associated with epsilonRACK: Cross-talk between cAMP-dependent protein kinase A and protein kinase C signaling pathways. Mol Pharmacol. 2008;73:1105–1112. doi: 10.1124/mol.107.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Yeh E, Ng S, Zhang M, Bouhours M, Wang Y, Wang M, Hung W, Aoyagi K, Melnik-Martinez K, Li M, Liu F, Schafer WR, Zhen M. A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans. PLoS Biol. 2008;6:e55. doi: 10.1371/journal.pbio.0060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- 611.Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Yu FH, Catterall WA. The VGL-chanome: A protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- 613.Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S. TRPC channels as STIM1-regulated SOCs. Channels (Austin) 2009;3:221–225. doi: 10.4161/chan.3.4.9198. [DOI] [PubMed] [Google Scholar]
- 615.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 616.Zavala-Tecuapetla C, Aguileta MA, Lopez-Guerrero JJ, Gonzalez- Marin MC, Pena F. Calcium-activated potassium currents differentially modulate respiratory rhythm generation. Eur J Neurosci. 2008;27:2871–2884. doi: 10.1111/j.1460-9568.2008.06214.x. [DOI] [PubMed] [Google Scholar]
- 617.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 618.Zhang L, Saffen D. Muscarinic acetylcholine receptor regulation of TRP6 Ca2 +channel isoforms. Molecular structures and functional characterization. J Biol Chem. 2001;276:13331–13339. doi: 10.1074/jbc.M008914200. [DOI] [PubMed] [Google Scholar]
- 619.Zhang M, Hu H, Zhang X, Lu W, Lim J, Eysteinsson T, Jacobson KA, Laties AM, Mitchell CH. The A3 adenosine receptor attenuates the calcium rise triggered by NMDA receptors in retinal ganglion cells. Neurochem Int. 2010;56:35–41. doi: 10.1016/j.neuint.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Zhang X, Cui N, Wu Z, Su J, Tadepalli JS, Sekizar S, Jiang C. Intrinsic membrane properties of locus coeruleus neurons in Mecp2-null mice. Am J Physiol Cell Physiol. 2010;298:C635–C646. doi: 10.1152/ajpcell.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Zhang X, Su J, Cui N, Gai H, Wu Z, Jiang C. The disruption of central CO2 chemosensitivity in amousemodel ofRett syndrome. AmJ Physiol Cell Physiol. 2011;301:C729–C738. doi: 10.1152/ajpcell.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 623.Zhu Y, Ikeda SR. Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]
- 624.Zuperku EJ, McCrimmon DR. Gain modulation of respiratory neurons. Respir Physiol Neurobiol. 2002;131:121–133. doi: 10.1016/s1569-9048(02)00042-3. [DOI] [PubMed] [Google Scholar]