Abstract
Arginine metabolism by oral bacteria via the arginine deiminase system (ADS) increases the local pH, which can neutralize the effects of acidification from sugar metabolism and reduce the cariogenicity of oral biofilms. To explore the relationship between oral arginine metabolism and dental caries experience in children, we measured ADS activity in oral samples from 100 children and correlated it with their caries status and type of dentition. Supragingival dental plaque was collected from tooth surfaces that were caries-lesion-free (PF) and from dentinal (PD) and enamel (PE) caries lesions. Regardless of children’s caries status or type of dentition, PF (378.6) had significantly higher ADS activity compared with PD (208.4; p < .001) and PE (194.8; p = .005). There was no significant difference in the salivary arginolytic activity among children with different caries status. Mixed-model analysis showed that plaque caries status is significantly associated with ADS activity despite children’s age, caries status, and dentition (p < .001), with healthy plaque predicting higher ADS activity compared with diseased plaque. Plaque arginine metabolism varies greatly among children and tooth sites, which may affect their susceptibility to caries.
Keywords: oral biolfim, dental plaque, dental caries, bacteria, arginine, risk factor
Introduction
Dental caries is one of the most common infectious and chronic oral diseases of children (Petersen, 2003) and is associated with costly treatment. In recent years, there has been an alarming rise in childhood caries, especially among underserved populations (Dye et al., 2010). Despite significant efforts to prevent and treat caries, including the use of fluoride, caries still remains a major public health problem. Importantly, caries can have life-long consequences on the health of children by affecting nutrition, growth, and development (Skeie et al., 2006). Early identification of at-risk children and early intervention can significantly reduce the risk for caries development. Thus, there is an urgent need to identify novel and more effective strategies for caries risk assessment and interventions.
As has long been recognized, a caries lesion occurs when acids produced by bacterial glycolysis of dietary carbohydrates cause tooth demineralization. Current evidence suggests that alkali production from the metabolism of salivary substrates, such as arginine or urea, inhibits tooth demineralization by neutralizing glycolytic acids, while positively affecting the ecology of oral biofilms (Shu et al., 2007; Nascimento et al., 2009; Morou-Bermudez et al., 2011). Our previous studies revealed that an increased risk for caries is associated with reduced alkali-producing capacity of bacteria colonizing the adult oral cavity (Shu et al., 2007; Nascimento et al., 2009). A technology containing arginine was demonstrated to have significant effects in inhibiting caries in children (Acevedo et al., 2008). Although the cost-effective use and potential of this technology to reduce caries appears high, the underlying basis for the impact of alkali production in caries pathogenesis has yet to be fully disclosed. Likewise, the capacity of oral bacteria from children with different caries experience to produce alkali from arginine has not been explored.
Oral alkali production via arginine is a highly promising but under-explored approach for caries risk assessment and for caries prevention. Arginine is found free in saliva in micromolar concentrations (Van Wuyckhuyse et al., 1995) and is also abundant in salivary peptides and proteins. In oral biofilms, arginine is mainly metabolized by the arginine deiminase system (ADS) of oral bacteria, which yields citrulline, ornithine, CO2, ATP, and ammonia (Burne and Marquis, 2000). A substantial knowledge base about the physiology and genetics of the ADS in oral bacteria has been established (Marquis et al., 1987; Casiano-Colon and Marquis, 1988; Burne et al., 1991; Curran et al., 1998; Dong et al., 2004; Griswold et al., 2004; Zeng et al., 2006; Liu and Burne, 2009), but the role of arginine metabolism in oral ecology and oral health has not been thoroughly studied in humans. The main goal of this study was to explore the relationship between ADS activity and caries experience in children. In addition, we examined whether the arginolytic capacity of oral bacterial populations could be correlated with the bacterial colonization site and type of dentition.
Materials & Methods
One hundred children ages 2 to 14 yrs were recruited. The wide age range used in this study was selected so that we could examine a spectrum of different dentitions, which may influence oral biofilm ecology and therefore its arginolytic capacity. The selection process excluded children who: (i) had been treated with antibiotics within the preceding 3 mos, (ii) were taking any medication, or (iii) had orthodontic appliances. Parent-administered questionnaires were used to collect information about: (a) demographics, (b) oral health practices, (c) dietary habits, and (d) medical and (e) dental histories. Informed consent was obtained from parents or legal guardians of each child under a protocol approved by the Institutional Review Board of the University of Florida Health Science Center.
Children were grouped by caries status: caries-lesion-free (CF) had no reported or clinical evidence of caries experience [decayed, missing, and filled teeth (DMFT) = 0]; caries-active (CA) had at least 2 active, dentinal, cavitated, and unrestored caries lesions (DT ≥ 2, MFT ≥ 0); and caries experienced (CE) had previous experience of caries but absence of caries activity (DT = 0; MFT ≥ 0). For all CE children, the recorded restorations (FT) had been placed at least 6 mos prior to this study. Children were also grouped by type of dentition, as primary, mixed, or permanent dentitions.
A single examiner (M.M.N.) conducted all clinical examinations and determined the children’s caries status and type of dentition. Caries lesions were recorded according to the International Caries Detection and Assessment System (ICDAS) criteria, which range from 0 to 6 (Ismail et al., 2007). Teeth were examined before and after removal of plaque, as well as before and after being dried with compressed air for 5 sec. The activity of caries lesions was determined by clinical appearance, plaque stagnation, and tactile sensation. The range of ICDAS scores as a function of caries-status group was CF and CE (no activity, ICDAS = 0) and CA (active lesions, ICDAS = 0-6). The threshold for defining the CA group was the presence of at least 2 ICDAS scores of 5 and/or 6 (dentinal, cavitated lesions); however, CA children could also present other types of caries lesions with lower ICDAS scores (enamel and dentin, non-cavitated lesions).
Children were required to refrain from oral hygiene procedures for at least 8 hrs prior to the collection of saliva and plaque, which was performed between 8 and 11 a.m. We collected whole unstimulated saliva by asking the child to expectorate into a sterile plastic tube. We were unable to collect saliva from 11 young children. After saliva collection, supragingival plaque was collected separately from: (i) tooth surfaces that were caries-lesion-free (PF; ICDAS = 0); (ii) active, enamel caries lesions (PE; ICDAS = 1-3); and (iii) active, dentinal caries lesions (PD; ICDAS ≥ 4). PF were collected from all participating children, whereas PD and PE were collected only from CA children. Each plaque sample was obtained by pooling material from at least 2 different tooth sites of similar health condition by means of sterile periodontal curettes, and more than one type of sample could have been collected from the same child. PD was recovered from the internal surfaces of dentinal caries lesions without removing the infected dentin as well as from the surrounding margins. No plaque was collected from superficial root surfaces. Plaque samples contaminated by blood were rejected and not analyzed in this study. The plaque samples were transferred to sterile micro-centrifuge tubes containing 250 μL of 10 mM sodium phosphate buffer (pH 7.0). The oral samples were immediately transported on ice to the laboratory to be analyzed or, if necessary, snap-frozen and stored at -80oC, which does not adversely affect ADS activity.
Before enzymatic assays were conducted, saliva and plaque samples were dispersed by external sonication for 2 cycles of 15 sec, with cooling on ice during the interval. Plaque samples were then washed once with 10 mm Tris-maleate (pH 7.0) and re-suspended in 500 μL of the same buffer. We measured the arginolytic capacity of saliva and plaque bacteria by monitoring citrulline production from arginine (Liu et al., 2008). For the accommodation of small samples of site-specific plaque, the assays used a nano-drop scale on the Biotek Synergy H4 with microspot quantification. ADS activity was normalized to protein content (Nascimento et al., 2009) and defined as nmol of citrulline generated [minute x (mg protein)] -1.
For descriptive analysis, distributions of percentages and means were calculated when appropriate. We used a t test or analysis of variance (ANOVA) to evaluate continuous variables, with the chi-square test used for categorical variables. A linear mixed model was also used for data analysis, with the SAS procedure of PROC MIXED. ADS activity was examined as a function of both level 1 (teeth level) predictors and level 2 (individual level) predictors. The Akaike’s Information Criterion (AIC) was used to select a better-fitted model, which indicated a smaller AIC value. All data management and statistical analyses were performed with SAS procedures (SAS 9.1.3, SAS Institute Inc., Cary, NC, USA).
Results
The demographic characteristics and DMFT scores of the children who participated in the study are presented in Table 1. The distribution of the children’s caries status by type of dentition was: primary (19 CF, 10 CA, and 2 CE), mixed (24 CF, 20 CA, and 7 CE), and permanent (9 CF, 8 CA, and 1 CE). The mean ages for children at the primary, mixed, and permanent dentitions were 3.5 (± 1.2; range, 2-6 yrs), 8.7 (± 2.0; range, 5-13 yrs), and 12.6 (± 0.9; range, 11-14 yrs) yrs, respectively.
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Dentition | Primary | Mixed | Permanent | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Caries Status | Caries-lesion-free | Caries-active | Caries Experienced | Caries-lesion-free | Caries-active | Caries Experienced | Caries-lesion-free | Caries-active | Caries Experienced | Total |
| Number of Decayed Missing and Filled Teeth (DMFT) | 0 | 4.9 (±2.7) | 2 (±0) | 0 | 2.8 (±1.6) | 1.6 (±0.8) | 0 | 4.9 (±1.8) | 1 (±0) | 1.6 (±2.3) |
| Age | 2.9 (± 0.9) | 4.2 (± 1.1) | 5 (± 0) | 8.5 (± 2.0) | 9.1 (± 1.8) | 8.4 (± 2.1) | 12.5 (± 1.1) | 12.6 (± 0.8) | 13 (± 0) | 7.8 (± 3.6) |
| Gender | ||||||||||
| Male | 11 (21) | 3 (6) | 1 (2) | 14 (27) | 11 (21) | 4 (8) | 2 (4) | 5 (9) | 1 (2) | 52 (100) |
| Female | 8 (17) | 7 (15) | 1 (2) | 10 (21) | 9 (19) | 3 (6) | 7 (14) | 3 (6) | 0 | 48 (100) |
| Ethnicity | ||||||||||
| Hispanic | 1 (17) | 2 (32) | 1 (17) | 1 (17) | 1 (17) | 0 | 0 | 0 | 0 | 6 (100) |
| Not Hisp. | 18 (19) | 8 (9) | 1 (1) | 23 (24) | 19 (20) | 7 (7) | 9 (10) | 8 (9) | 1 (1) | 94 (100) |
| Race | ||||||||||
| White | 12 (21) | 3 (5) | 2 (3) | 17 (30) | 9 (16) | 6 (11) | 7 (12) | 1 (2) | 0 | 57 (100) |
| Black | 6 (18) | 4 (12) | 0 | 6 (18) | 8 (24) | 1 (3) | 2 (6) | 7 (21) | 0 | 34 (100) |
| Asian | 1 (20) | 1 (20) | 0 | 0 | 2 (40) | 0 | 0 | 0 | 1 (20) | 5 (100) |
| Other | 0 | 2 (50) | 0 | 1 (25) | 1 (25) | 0 | 0 | 0 | 0 | 4 (100) |
Age is shown in years. DMFT and age are shown as [Mean (±SD)]; SD, standard deviation. Gender, ethnicity, and race are shown as [N (%)]. Percentages are within rows for each demographic characteristic. Other race: Pacific Islander, Native American, Indian, Alaska Native, Native Hawaiian, and others.
The ADS activity levels of oral samples collected from children of different caries status and dentitions are presented in Table 2. ADS activity of saliva samples ranged from 1.1 to 372.1 units (mg protein)-1, and no differences were observed in saliva ADS activity among the groups. In general, ADS activity of plaque samples ranging from 0.1 to 968.5 units (mg protein)-1 was higher than that of saliva samples. Specifically, the range of ADS activities for the PF samples was 0.1 to 968.5, PD was 34.5 to 753.8, and PE was 34.9 to 747.4 units (mg protein)-1 of samples. The mean ADS activity of plaque collected from CF children (377.1 ± 252.6) of all types of dentition was higher than, but not statistically different from, that of CE (299.3 ± 230.6) and CA (291.7 ± 233.3) children. Among the children with mixed dentition, the mean ADS activity of all plaque from CF (403.1 ± 257.5) was higher than that of CA (294.4 ± 224.2) children, with a marginally significant difference (p = .059) between these two groups (Appendix Fig.).
Table 2.
Activity Levels of the Arginine Deiminase System of Saliva and Site-specific Dental Plaque Collected from the Children
| Dentition | Primary | Mixed | Permanent | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Caries Status | Caries-lesion-free | Caries-active | Caries Experienced | Caries-lesion-free | Caries-active | Caries Experienced | Caries-lesion-free | Caries-active | Caries Experienced |
| Plaque Caries-lesion-free | 329.8 ± 234.6 (25) | 301.7 ± 234.5 (11) | 368.1 ± 362.9 (3) | 403.1 ± 257.5 (42) | 397.7 ± 251.9 (20) | 258.2 ± 187.0 (11) | 381.9 ± 256.1 (17) | 540.2 ± 215.7 (10) | 421.6 ± 0.0 (2) |
| Plaque Enamel Caries | - | 61.1 (1) | - | - | 232.8 ± 208.0 (12) | - | - | 141.2 ± 52.9 (6) | - |
| Plaque Dentinal Caries | - | 247.1 ± 220.6 (8) | - | - | 220.7 ± 143.0 (18) | - | - | 113.9 ± 63.3 (7) | - |
| Saliva | 56.0 ± 39.6 (12) | 66.2 ± 65.6 (8) | 191.8 ± 122.1 (2) | 67.2 ± 30.1 (22) | 49.7 ± 64.5 (20) | 91.9 ± 102.0 (7) | 77.7 ± 106.3 (9) | 46.0 ± 27.9 (8) | 22.4 (1) |
The arginine deiminase system (ADS) activity was expressed as nmol of citrulline generated [minute x (mg of protein)] -1. The ADS activity levels of plaque and saliva samples are shown as [Mean ± SD (N)]; SD, standard deviation; N, number of samples.
Regardless of caries status or type of dentition, PF (377.1 ± 253.3) samples had significantly higher levels of ADS activity compared with PD (208.4 ± 160.1; p < .001) and PE (194.8 ± 176.0; p < .001) samples. Although not statistically significant, the distribution of ADS activity by plaque site and type of dentition showed increased ADS activity in PF samples with age, or from primary to permanent dentitions (Fig.). The mean ADS activity of PF (primary = 324.9 ± 247.5; mixed = 379.8 ± 251.9) was significantly higher than that of PD (primary = 247.1 ± 220.6; mixed = 220.7 ± 143.0) and PE (primary = 61.1 ± 0; mixed = 232.8 ± 208.0) for the primary (PF vs. PD, p = .0001; PF vs. PE, p = .0005) and mixed (PF vs. PD, p = .003; PF vs. PE, p = .034) dentitions. There were comparable levels of ADS activity between PD and PE samples in the mixed and permanent dentitions.
Figure.
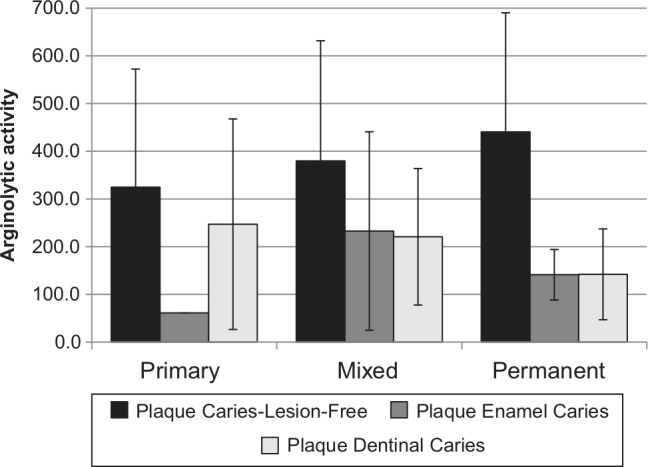
Mean activity levels of the arginine deiminase system of site-specific plaque from children with different types of dentition. Arginolytic activity is represented by the activity of the arginine deiminase system (ADS) and expressed as nmol of citrulline generated [minute x (mg of protein)]–1.
Mixed-model analysis by using plaque caries status and children’s age, with dentition and caries status as predictors of ADS activity, demonstrated that plaque caries status is significantly correlated with ADS activity, despite the other factors (p < .001), with healthy plaque (PF) predicting higher levels of ADS activity compared with diseased plaque (PD and PE).
Discussion
The most significant finding of this study is that the capacity of plaque bacteria to metabolize arginine varies greatly among children and tooth sites of different caries status, which may profoundly affect the resistance or susceptibility of the hosts to dental caries. Specifically, plaque bacteria from tooth surfaces that were caries-lesion-free had higher ADS activity than those from caries lesions. Importantly, our findings highlight that arginolysis may be a novel and fundamental risk assessment criterion and support the concept that increasing the arginolytic potential of children’s dental plaque may be an effective caries intervention strategy. In view of growing efforts to understand the relationship of the composition of the oral microbiome to health and disease, and previous observations that bacterial profiles change with the stages of childhood caries and also differ between the primary and permanent dentitions (Aas et al., 2008; Crielaard et al., 2011), our findings provide compelling support for the continued investigation of oral alkali production as a novel approach to the control of caries.
This study also provided the first analysis of the arginolytic potential of bacteria collected from site-specific supragingival plaque. We observed an extremely high degree of variability in ADS activity among plaque samples, in some cases greater than 10,000-fold. In in vitro studies, a five-fold decrease in alkali-generating potential was shown to markedly diminish the pH-moderating potential of oral bacteria (Clancy and Burne, 1997; Clancy et al., 2000). Plaque bacteria from tooth surfaces without caries lesions showed the widest range of ADS activities. Even though the bacteria from these “healthy” plaque samples showed an overall greater capacity to metabolize arginine compared with plaque bacteria of caries lesions, high levels of ADS activity were also detected in some samples of carious plaque. Notably, human supragingival plaque harbors a highly diverse bacterial community, and the microbial composition of healthy plaque differs substantially from that of carious plaque (Aas et al., 2008). Recent work by our group provides evidence that bacterial heterogeneity in arginolytic capacity is related to intra- and inter-species variation in the regulation of the ADS, likely associated with evolution of adaptive strategies for acid tolerance and nutrient limitation (manuscript submitted). Our research findings to date are consistent with the “ecological plaque hypothesis” (Marsh, 2006), supporting that cariogenesis by oral biofilms is a complex polymicrobial process and disclosing that cariogenic biofilms have both acidogenic and alkalinogenic potentials, as do “healthy” biofilms. Therefore, bacterial metabolism resulting in caries lesions is closely intertwined with the balance between acid and alkali production, and multiple bacterial species with acidogenic or alkalinogenic traits can influence the disease process.
In agreement with our previous study in adults (Nascimento et al., 2009), the ADS activity of plaque samples was generally higher than that produced by salivary samples in children. Higher ureolytic activity in plaque compared with saliva was also observed in a longitudinal study with children (Morou-Bermudez et al., 2011). In fact, the oral microbiota of saliva has been shown to be different from that of plaque in children independent of the presence or absence of caries (Ling et al., 2010). In the present study, no differences in salivary ADS activity were detected among the children’s caries groups. This observation is also in agreement with the findings of our previous study, in which the use of qPCR did not reveal a statistically significant association between the salivary proportions of 2 recognized arginolytic species, S. sanguinis and S. gordonii, and the caries status of adults (Nascimento et al., 2009). It is possible that salivary ADS levels cannot be used as a predictor of caries risk for children.
In spite of the abundance of in vitro data showing an important role for alkali production in inhibiting caries—and in spite of microbiological and microbiome studies showing associations of base-producing organisms with dental health (Becker et al., 2002; Aas et al., 2008; Gross et al., 2010; Crielaard et al., 2011)—this study directly addressed the question whether the change in the microbial flora that is seen when caries develops is also associated with a change in the alkali-producing capacity of oral biofilms. It also provides indirect evidence that enhancing arginolytic potential could have the effect of arresting and possibly reversing the caries process. It is even more critical to evaluate these processes in children because of the significant void in our knowledge of the ontogeny and interactions of the oral microbiome in these populations, the higher risk of children for caries, and the potential for rapid translation to caries risk assessment and interventions. Future studies will focus on the physiological and genetic characterization of the oral microbiome of caries-active and caries-lesion-free children, which will greatly assist in defining the microbiological and molecular basis for the heterogeneity in arginolytic capacity of oral biofilms. Similarly, additional clinical studies may be useful to: (i) confirm that the supplementation of arginine to plaque bacteria is effective against caries; (ii) ensure that arginine does not diminish the impact of fluoride in biofilms; and (iii) optimize formulations for caries control. Other areas worthy of investigation include exploring probiotic applications to enhance oral arginolysis and prevent caries. Collectively, this information will facilitate the rationale design of strategies that rely on alkali production for caries risk assessment and interventions.
Supplementary Material
Footnotes
This work was supported by the University of Florida College of Dentistry and by the National Institute of Dental and Craniofacial Research (Grant DE10362).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, et al. (2008). Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol 46:1407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo AM, Montero M, Rojas-Sanchez F, Machado C, Rivera LE, Wolff M, et al. (2008). Clinical evaluation of the ability of CaviStat in a mint confection to inhibit the development of dental caries in children. J Clin Dent 19:1-8. [PubMed] [Google Scholar]
- Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, et al. (2002). Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA, Marquis RE. (2000). Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193:1-6. [DOI] [PubMed] [Google Scholar]
- Burne RA, Parsons DT, Marquis RE. (1991). Environmental variables affecting arginine deiminase expression in oral streptococci. In: Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, DC: American Society for Microbiology. [Google Scholar]
- Casiano-Colon A, Marquis RE. (1988). Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol 54:1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy A, Burne RA. (1997). Construction and characterization of a recombinant ureolytic Streptococcus mutans and its use to demonstrate the relationship of urease activity to pH modulating capacity. FEMS Microbiol Lett 151:205-211. [DOI] [PubMed] [Google Scholar]
- Clancy KA, Pearson S, Bowen WH, Burne RA. (2000). Characterization of recombinant, ureolytic Streptococcus mutans demonstrates an inverse relationship between dental plaque ureolytic capacity and cariogenicity. Infect Immun 68:2621-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, Keijser BJ. (2011). Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran TM, Ma Y, Rutherford GC, Marquis RE. (1998). Turning on and turning off the arginine deiminase system in oral streptococci. Can J Microbiol 44:1078-1085. [DOI] [PubMed] [Google Scholar]
- Dong Y, Chen YY, Burne RA. (2004). Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J Bacteriol 186:2511-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BA, Arevalo O, Vargas CM. (2010). Trends in paediatric dental caries by poverty status in the United States, 1988-1994 and 1999-2004. Int J Paediatr Dent 20:132-143. [DOI] [PubMed] [Google Scholar]
- Griswold A, Chen YY, Snyder JA, Burne RA. (2004). Characterization of the arginine deiminase operon of Streptococcus rattus FA-1. Appl Environ Microbiol 70:1321-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, et al. (2010). Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol 48:4121-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, et al. (2007). The International Caries Detection and Assessment System (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol 35:170-178. [DOI] [PubMed] [Google Scholar]
- Ling Z, Kong J, Jia P, Wei C, Wang Y, Pan Z, Huang W, Li L, Chen H, Xiang C. (2010). Analysis of oral microbiota in children with dental caries by pcr-dgge and barcoded pyrosequencing. Microbial ecology 60:677-690. [DOI] [PubMed] [Google Scholar]
- Liu Y, Burne RA. (2008). Environmental and growth phase regulation of the Streptococcus gordonii arginine deiminase genes. Appl Environ Microbiol 74:5023-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dong Y, Chen YY, Burne RA (2008). Environmental and growth phase regulation of the Streptococcus gordonii arginine deiminase genes. Appl Environ Microbiol 74:5023-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis RE, Bender GR, Murray DR, Wong A. (1987). Arginine deiminase system and bacterial adaptation to acid environments. Appl Environ Microbiol 53:198-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD. (2006). Dental plaque as a biofilm and a microbial community – implications for health and disease. BMC Oral Health 6(Suppl 1):14S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morou-Bermudez E, Elias-Boneta A, Billings RJ, Burne RA, Garcia-Rivas V, Brignoni-Nazario V, et al. (2011). Urease activity in dental plaque and saliva of children during a three-year study period and its relationship with other caries risk factors. Arch Oral Biol. 56:1282-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. (2009). Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol 24:89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen P. (2003). The World Oral Health Report 2003: continuous improvement of oral health in the 21st century – the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol 31(Suppl 1):3-23. [DOI] [PubMed] [Google Scholar]
- Shu M, Morou-Bermudez E, Suarez-Perez E, Rivera-Miranda C, Browngardt CM, Chen YY, et al. (2007). The relationship between dental caries status and dental plaque urease activity. Oral Microbiol Immunol 22:61-66. [DOI] [PubMed] [Google Scholar]
- Skeie MS, Raadal M, Strand GV, Espelid I. (2006). The relationship between caries in the primary dentition at 5 years of age and permanent dentition at 10 years of age – a longitudinal study. Int J Paediatr Dent 16:152-160. [DOI] [PubMed] [Google Scholar]
- Van Wuyckhuyse BC, Perinpanayagam HE, Bevacqua D, Raubertas RF, Billings RJ, Bowen WH, et al. (1995). Association of free arginine and lysine concentrations in human parotid saliva with caries experience. J Dent Res 74:686-690. [DOI] [PubMed] [Google Scholar]
- Zeng L, Dong Y, Burne RA. (2006). Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J Bacteriol 188:941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


