Abstract
Objective
To study challenging behavior (destruction, aggression, self-injury, stereotypy) in children with Smith-Lemli-Opitz syndrome (SLOS) using a biobehavioral model that helps distinguish biological from socially mediated variables influencing the behavior.
Background
SLOS is an autosomal-recessive syndrome of multiple malformations and intellectual disability resulting from a genetic error in cholesterol synthesis in all cells and tissues, including brain. The exact cause of the challenging behavior in SLOS is unclear, but defective brain cholesterol synthesis may contribute. Because the precise genetic and biochemical etiology of SLOS is known, this disorder is a good model for studying biological causes of challenging behavior.
Method
In a preliminary application of a biobehavioral model, we studied the association between cholesterol levels (as a biochemical indicator of disease severity) and behavior subtype (“biological” vs “learned”) in 13 children with SLOS. Parents completed a questionnaire that categorized challenging behavior as influenced primarily by social or nonsocial (thus, presumably biological) factors.
Results
The severity of the cholesterol synthesis defect correlated significantly with behavior subtype classification for 1 of 2 challenging behaviors. Greater severity of the cholesterol synthesis defect was associated with behavior being classified as primarily influenced by biological factors.
Conclusion
The interplay between challenging behavior and defective cholesterol synthesis in SLOS may help explain biological influences on the behavior. Our findings have implications for research on the effectiveness of behavioral and medical treatments for behavioral difficulties in SLOS and other neurodevelopmental disorders.
Keywords: Smith-Lemli-Opitz syndrome, challenging behavior, biobehavioral, cholesterol, behavioral assessment
Smith-Lemli-Opitz syndrome (SLOS) is a syndrome of multiple malformations and intellectual disability, caused by a genetic error in cholesterol biosynthesis. Homozygous or compound heterozygous mutations of the gene DHCR7 encoding the enzyme 3β-hydroxysterol-Δ7 reductase1–3 impair cholesterol synthesis, leading to cholesterol insufficiency and a buildup of potentially toxic byproducts of cholesterol synthesis, 7-dehydrocholesterol and 8-dehydrocholesterol, in blood and other tissues. Although there is as yet no direct way to measure brain cholesterol production, it is assumed to be defective in SLOS. The overall defect in cholesterol synthesis can range on a continuum from mild to severe. The more severe the defect, the more severe the physical and developmental manifestations.4–6
The clinical manifestations of SLOS are extremely variable. Severely affected individuals typically have multiple physical abnormalities (eg, toe syndactyly, dysmorphic facies, congenital heart defects), intellectual disability, and behavior problems. Mildly affected individuals exhibit minor physical abnormalities and learning disabilities. Many individuals with SLOS also have microcephaly, hypotonia, feeding problems, and functional gastrointestinal problems.
The behavioral “phenotype” of individuals with SLOS has been reported to include pervasive irritability,7 hyperactivity,8 aggressive behavior,9 self-injury,8,10 and behavioral symptoms of autism such as repetitive and stereotyped flicking or flapping of hands.11,12 Larger, systematic evaluations have shown that at least half of patients display aggressive and/or self-injurious behavior.9,12 In non-SLOS populations, including some people who are in need of voluntary or involuntary psychiatric hospitalization, lower levels of cholesterol have been associated with extreme emotions and with violent and/or suicidal behavior,13,14 although these findings have raised some methodological questions.15 Interestingly, study participants more representative of the general population have shown improvement in their emotions when following diets that lower their plasma cholesterol levels.15 While the association between cholesterol and the emotional and behavioral difficulties of non-SLOS populations requires more study, cholesterol clearly plays an important role in behavior.
Early incidence estimates of SLOS based on clinical features suggested rates between 1:20,000 and 1:60,000 live births9,16; a 2001 estimate based on carrier frequency predicted a higher incidence of 1:1,590 to 1:13,000.17 The actual incidence is likely somewhere in between.
Beyond simply documenting the behavioral characteristics of SLOS, we must study the factors that influence the challenging behavior before we can fully understand the syndrome’s behavioral presentation and, potentially, guide intervention selection. Studying the causes of challenging behavior and learning whether specific single gene-induced biochemical defects contribute to the behavior might also have implications for treatment.
Mace and Mauk18 advocated a biobehavioral approach to studying behavioral difficulties, particularly self-injury, in individuals with neurodevelopmental conditions. This approach involves blending behavioral assessment and biomedical models in investigating challenging behavior. Using the biobehavioral model allows the teasing apart of the potential influences of biological and socially mediated factors on challenging behavior, to determine which are primary. For example, is a boy hitting himself because of the effects of deficient brain cholesterol or because he has learned that doing so results in his getting attention from his parents or an escape from schoolwork? Once we know the primacy of these factors, we can classify targeted behavior as “learned” (ie, primarily influenced by socially mediated variables), “biological” (ie, less influenced by socially mediated variables, and presumably influenced by biological variables, eg, in this boy, deficient brain cholesterol synthesis), or both, and we are better prepared to treat it.19 A biobehavioral approach has been used to understand and treat problem behavior in people with such conditions as Prader-Willi syndrome,20 Cornelia de Lange syndrome,21 Lesch-Nyhan syndrome,22 and Smith-Magenis syndrome.23
Until now, the biobehavioral model had not been applied to studying challenging behavior in individuals with SLOS. The model could be particularly effective in SLOS because the precise genetic and biochemical defects are known, so that it might be possible to correlate defective cholesterol synthesis with behavior.
In a preliminary study, we applied a biobehavioral model to challenging behavior in SLOS by testing the association between a biochemical indicator of disease severity (sterol ratio) and behavior subtype (learned vs biological) in 13 affected children. We hypothesized that the worse the impairment in synthesizing cholesterol, the greater the association with behavior being classified as primarily biologically mediated.
METHODS
Participants
We studied 8 boys and 5 girls aged from approximately 2 to 13 years (M = 4.89 years) with biochemically determined SLOS (Table 1). All participants lived with their parents, and all school-age children attended a private or public school.
TABLE 1.
Participant Characteristics and Behavior Classification
| Type of Challenging Behavior (Category‡) | ||||||
|---|---|---|---|---|---|---|
| Participant | Age (years) | Sex | IQ Ratio | Sterol Ratio+ | 1 | 2 |
| 1 | 4.33 | Male | 35† | 0.004 | Destruction (L) | Self-injury (L) |
| 2 | 6.75 | Male | 23* | 0.147 | Stereotypy (B) | Destruction (B) |
| 3 | 11.33 | Female | 51* | 0.028 | Destruction (L) | Aggression (L) |
| 4 | 2.83 | Male | 81† | 0.002 | Aggression (L) | Destruction (L) |
| 5 | 2.33 | Male | 31* | 0.147 | Self-injury (B) | Destruction (L) |
| 6 | 3.00 | Female | 26† | 0.159 | Aggression (L) | Self-injury (L) |
| 7 | 12.17 | Female | 28* | 0.342 | Stereotypy (B) | Aggression (B) |
| 8 | 3.08 | Male | 30† | 0.009 | Aggression (L) | Destruction (L) |
| 9 | 5.17 | Female | 28† | 0.081 | Self-injury (B) | Stereotypy (L) |
| 10 | 1.92 | Male | 41† | 0.312 | Destruction (B) | -- |
| 11 | 3.92 | Male | 35† | 0.137 | Self-injury (B) | Stereotypy (B) |
| 12 | 2.10 | Female | 73† | 0.152 | Self-injury (L) | Self-injury (L) |
| 13 | 3.67 | Male | 29† | 0.217 | Destruction (L) | -- |
Sterol ratio = (7-dehydrocholesterol + 8-dehydrocholesterol)/cholesterol
IQ ratio derived from Stanford Binet Intelligence Scales, 4th ed.26
IQ ratio derived from Mullen Scales of Early Learning, AGS ed.27
Behavior is categorized as B (biological) or L (learned) according to Motivation Assessment Scale22 scores.
The children were a subset of patients enrolled in a longitudinal study of SLOS that has been in progress since 1995, with evolution of focus and methods. Patients were recruited into the longitudinal study via advertisements to parent support groups and by direct referrals from physician colleagues familiar with the study. We chose the 13 patients for this preliminary study because all had completed our measures of interest.
Every 3 to 12 months, the children in the longitudinal study underwent a range of medical and developmental evaluations during a weeklong inpatient stay at the Oregon Health & Science University General Clinical Research Center (now the Clinical and Translational Research Center). During each admission, we tested the children’s sterol levels and their intelligence and development, and their parents evaluated their problematic behaviors. All 13 of our participants completed at least 1 admission for which they ate an essentially cholesterol-free diet both several weeks before and during the admission.24 Eleven of our 13 patients also completed at least 1 admission before and during which they ate a high-cholesterol diet. A high-cholesterol diet has been evaluated as a potential treatment for SLOS.25 For these 11 children, we analyzed the developmental data from the first high-cholesterol admission during which both child and parents completed the measures of interest. For participants #1 and #12, who never returned for a high-cholesterol admission, we analyzed results from a low-cholesterol diet admission.
The Institutional Review Board at Oregon Health & Science University approved this study. Parents of all participants provided informed consent.
Measures
Identifying Challenging Behaviors
During each admission, we gave parents a nonvalidated standardized questionnaire, termed the Behavior Problem Questionnaire (Freeman, unpublished questionnaire, 2006) that we had developed for the longitudinal study to gather information about the occurrence and frequency of 30 specific challenging behaviors. We grouped the behaviors into 4 general categories—self-injury, stereotypic behavior, aggression, and property destruction. We based the categories and specific behaviors on the literature about common forms of challenging behavior in individuals with intellectual and/or developmental disabilities.26,27 We left space on the questionnaire for parents to write in specific patterns of their child’s behavior that were consistent with these categories. We asked the parents to complete the questionnaire, first reporting their child’s maladaptive behaviors over the preceding month and estimating their frequency, and then noting the 2 specific challenging behaviors that had most concerned them during that month.
Classifying Challenging Behaviors
We used the Motivation Assessment Scale (MAS)28 to classify our sample’s maladaptive behaviors as biological or learned. The MAS is a 16-item questionnaire in which parents or caregivers report on how likely their child’s challenging behavior is to occur in various situations (eg, when involved in a task, when denied access to a preferred object or activity). For this study, we asked the parents to complete a MAS for each of the behaviors that had most concerned them during the past month.
The MAS has 4 subscales. Three of them assess whether a targeted behavior is influenced primarily by socially mediated variables: attention (behavior occurs primarily in situations in which the person has not had much interaction or in which others respond to challenging behavior by providing interaction), escape (behavior occurs primarily in situations that involve demands being placed on the person or in which the person is allowed to escape from or avoid tasks or activities), and tangible (behavior occurs primarily in situations that involve limited or no access to preferred activities or stimuli, or in which the person gains access to the preferred activities or stimuli through negative behavior). The fourth subscale assesses whether the behavior is influenced primarily by nonsocial factors: sensory (behavior occurs in situations that do not seem to be influenced by social surroundings or in which the behavior seems primarily self-stimulatory). Respondents answer each item using a 7-point Likert scale (0 = never to 6 = always). Ratings are tallied to provide a total score for each subscale. Mean subscale score is determined by dividing the total score by 4. Relative rankings of subscales are identified by assigning “1” to the highest mean score, “2” to the next highest, and so on. Adequate reliability and validity of MAS scores have been established.28,29
Biochemical Severity
We used the (7-dehydrocholesterol [DHC] + 8-DHC)/cholesterol ratio as the biochemical indicator of disease severity.30 When the enzyme 3β-hydroxysterol-Δ7 reductase has low activity because of a gene mutation, the precursors (7-DHC and its isomer, 8-DHC) accumulate and the product (cholesterol) decreases. The ratio of these precursors to the product provides an indirect estimate of the enzyme activity. We drew the blood for this test during a low-cholesterol diet admission, to provide a baseline indicator of cholesterol synthesis, unaffected by interventions.31
We measured plasma sterol concentrations by capillary-column gas chromatography on a Perkin Elmer (Waltham, MA) gas chromatograph (Model AutoSystemXL) or Agilent (Santa Clara, CA) gas chromatograph (Model 6890N) with a CP-Wax57 column (25 m, 0.32 mmID, 0.25-µm film; Chrompack Inc, Raritan, NJ) or ZB1701 column (30 m, 0.25 mmID, 0.25-µm film; Phenomenex, Torrance, CA). For calibration, we used internal standards (5α-cholestane or epicoprostanol) and authentic cholesterol standards.
Intelligence and Developmental Tests
If possible during a high-cholesterol diet admission, participants completed either the Stanford-Binet Intelligence Scales, 4th ed (SB4)32 or the Mullen Scales of Early Learning, AGS ed (MSEL).33 These standardized measures assess a child’s current intellectual (SB4) or developmental (MSEL) functioning, yielding a summary standard score (mean = 100, SD = 15). However, many of our participants had such severe cognitive and/or developmental delays that they did not reach base levels on the tests and we could not assign them a standard score. Therefore, we calculated a ratio intelligence quotient (IQ) for each participant. For those children who completed the SB4, we derived the ratio IQ by dividing the age equivalent of the child’s SB4 summary score by chronological age, and then multiplying by 100.4 For the MSEL, we derived the ratio IQ by calculating the mean of the age equivalences of 4 tested subscales (Visual Reception, Fine Motor, Receptive Language, and Expressive Language) used to calculate standard scores, dividing that mean by the child’s chronological age, and multiplying by 100.34,35 The resulting ratio IQ scores suggested that our participants’ function ranged from severely impaired to average (Table 1).
RESULTS
Type and Biobehavioral Categorization of Challenging Behavior
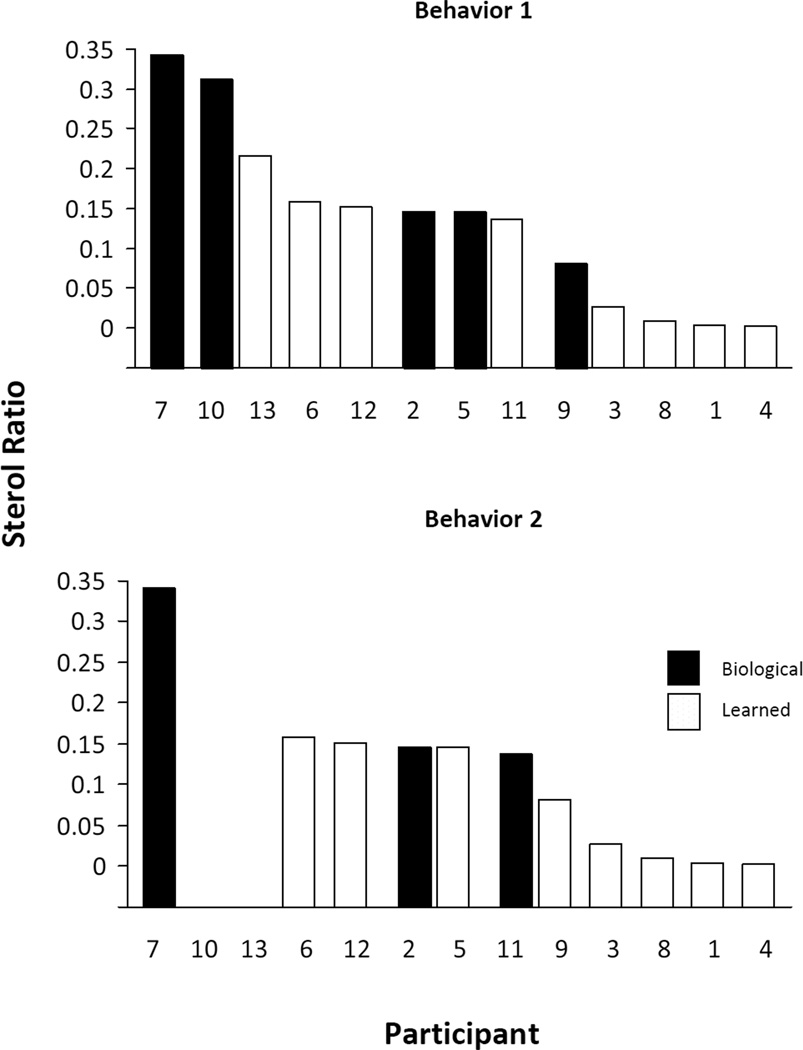
Parents of all 13 participants reported that their child had engaged in at least 1 problematic behavior during the preceding month (Table 1); the parents of 11 of the participants also reported a second problematic behavior. Many parents selected the most frequent challenging behaviors. Based on the MAS scores, we classified the first challenging behavior of 5 children as biological and that of the other 8 children as learned (Figure 1, top graph). For the second challenging behavior, we classified 3 as biological and 8 as learned (Figure 1, bottom graph).
FIGURE 1.
Association between behavior classification (biological vs learned) and sterol ratio, expressed as (7-dehydrocholesterol + 8-dehydrocholesterol)/cholesterol, in 13 children with Smith-Lemli-Opitz syndrome. According to their parents, behaviors 1 and 2 were the child’s most concerning behaviors during the previous month. The children are shown by patient number, from the most severe to the mildest cholesterol defect.
Association Between Biochemical Severity and Behavior Classification
For each of the problematic behaviors, we performed a separate 2-tailed point-biserial correlation to test the strength of the association between the dichotomous biobehavioral categorization variable (biological vs learned) and the continuous factor of biochemical severity. The association for challenging behavior #1 approached significance, and the association was significant for challenging behavior #2 (Table 2). For challenging behavior #2, higher biochemical severity was associated with the biological subtype (Figure 1).
TABLE 2.
Association of Behavior Classification with Sterol Ratio, Ratio IQ, and Age
| Challenging Behavior | ||||
| 1 | 2 | |||
| Variable | r | P | r | P |
| Sterol ratio | 0.537 | 0.058 | 0.629 | 0.038 |
| Ratio IQ | −0.41 | 0.16 | −0.37 | 0.26 |
| Age | 0.23 | 0.46 | 0.46 | 0.15 |
Association of Behavior Classification with Ratio IQ and Age
We performed point biserial correlations to test whether the participants’ challenging behavior classification was better accounted for by association with the other potentially relevant factors of intellectual or developmental level and age.36,37 Our results showed only nonsignificant associations (Table 2).
DISCUSSION
In 13 children with SLOS, we determined the behaviors that parents found most problematic, and whether these behaviors were biologically based or learned. Our results partially confirmed our hypothesis that a more severe cholesterol synthesis defect in children with SLOS would be more significantly associated with challenging behavior categorized as biological. We confirmed this hypothesis with 1 of the 2 problematic behaviors; for the other, the association showed a trend but failed to reach significance. Our analyses showed that classification as biological vs learned correlates with biochemical severity, but not with age or intellectual and/or developmental level, factors that other reports have linked to a higher frequency of challenging behavior.36,37
To our knowledge, this is the first application of the biobehavioral model to SLOS. Our study is part of a growing body of research that combines behavioral and medical sciences to address behavioral difficulties. Research has consistently shown that behavioral assessment procedures, such as those that we used in this study, lead to selection of effective behavioral interventions.38 Further, patients’ responsiveness to behavioral interventions may be impacted by whether the challenging behavior is being maintained by socially mediated vs nonsocial factors.39 Research has also shown that behavioral assessments are useful in evaluating medication effects.40 Collectively, these findings support the importance of investigating challenging behavior in SLOS using a biobehavioral model that involves testing the relative influence of biological and learned factors on behavior, as well as the influence of both medical and behavioral treatments.
The primary limitation of our study was our using the MAS as the sole means of classifying challenging behavior. Although the MAS has been used for studying influences on behavior problems of individuals with neurodevelopmental disorders29 and has been shown to produce results similar to those of more objective observational methods,41,42 researchers have advocated using the MAS in tandem with observational measures.43 Further, some researchers have questioned the accuracy of the MAS’s psychometric properties.44–46 The original proponents of the biobehavioral model18 described combining descriptive behavioral assessment strategies, like those used in this study, with observational strategies to classify behavior. The original proponents also proposed distinguishing between behavior that is maintained by automatic reinforcement (ie, the act of engaging in the behavior in and of itself produces reinforcing outcomes, such as a tactile sensation from hitting oneself or a stimulating sound by banging on a wall) and behavior influenced primarily by biological factors. Our methods did not allow for such a distinction. We chose to use the MAS because it does allow for an initial investigation using the biobehavioral model, with lower response effort required of participants and families, and the results would help us determine whether further study using observational methods would be warranted.
Given that we used only the MAS, we might be thought premature in classifying challenging behavior as primarily biological or learned. We suggest, however, that our findings are a useful preliminary application of the biobehavioral model to the challenging behavior of patients with SLOS. Future research applying the biobehavioral model to behavioral difficulties in SLOS should use both parent reports and more comprehensive and robust behavioral assessment measures. Such measures are particularly important given the multiple complex biological, physical, and developmental factors that affect patients’ behavior.
The other major limitation of our study was the small sample size, which may not be representative of all individuals with SLOS and may have limited our statistical power. However, given the low prevalence of SLOS in the general population, our group of 13 children was adequate for a first investigation of challenging behavior using the biobehavioral framework. Further, our sample included children with a wide range of developmental and intellectual levels and of biochemical severity, consistent with what is known about the SLOS population as a whole. Future studies of larger samples will be important to confirm our findings.
Our focus on children with SLOS may be considered a limitation, as our findings cannot be generalized to older individuals with the syndrome. No longitudinal data exist about whether behavior changes as affected individuals age; however, parent reports suggest improvement, eg, in sleep, that may be accounted for by maturation.9 Thus, future investigations of the biobehavioral model across the age spectrum will be important to determine whether age is an important variable.
A minor limitation of the study is that it is not yet possible to test brain cholesterol synthesis directly, so we had to rely on peripheral plasma sterol levels. Brain cholesterol synthesis likely correlates with peripheral synthesis, as reflected in plasma sterol levels,47 but we have no way to be sure.
A final limitation is that it is not yet known whether the sequelae of SLOS relate to cholesterol deficiency, 7-DHC accumulation, or both. Thus, it remains unclear which specific measure of synthesis deficiency is best for study as it relates to challenging behavior.
Our results suggest future research into the treatment of challenging behavior in SLOS. The few intervention studies to date have focused primarily on the effects of dietary cholesterol supplementation, without any controlled investigation of behavioral treatments.10,48–50 Our findings suggest the importance of studying both biological and environmental influences on challenging behavior in SLOS, and on treatment. Studies of challenging behavior in other disorders may serve as a guide for this research. For example, Sidener and colleagues51 showed that behavioral interventions may be effective in treating phenotypic behavior that is not mediated by social contingencies; however, LeBlanc and colleagues39 suggested that behavior that is not primarily socially mediated (and is thus considered “biologically” driven) may be less responsive to behavioral intervention than is learned behavior. Discrepant results may be explained, eg, by unique features of particular challenging behavior patterns or underlying mechanisms of behavior in specific conditions.
More studies using the biobehavioral model in SLOS and similar genetic disorders may help explain whether challenging behaviors respond differently to behavioral and medical interventions based on biological or learned subtype. Any treatment decisions for addressing problematic behavior in SLOS must consider multiple sources of information due to the complexity of the syndrome. Future research should more fully articulate the role of behavioral assessment in planning treatment.
Another important area of study is the effect of cholesterol supplements and other medical interventions on challenging behavior in SLOS. Dietary cholesterol supplementation does not improve developmental progress in youth with SLOS.4 Case reports have suggested that patients with SLOS have an improved disposition and more acceptable behavior when using cholesterol supplements48–50; however, if supplements improve mood and behavioral at all, the pathway is not clear, given that cholesterol does not cross the blood-brain barrier.52 Further, a small randomized clinical trial by Tierney et al53 suggests that cholesterol supplementation does not alter challenging behavior in SLOS.
Patients with SLOS have been tested with lipophilic statins, which do cross the blood-brain barrier.30 In an in vitro study of fibroblasts from patients with SLOS,54 simvastatin decreased 7-DHC concentrations and increased cholesterol synthesis when there was at least some activity of 3β-hydroxysterol-Δ7 reductase. The increased conversion was shown to be associated with increased expression of the enzyme. Because, unlike cholesterol, simvastatin crosses from blood to brain, simvastatin therapy may help to correct the biochemical defect in the central nervous system.
Adding bile acids to cholesterol supplements has shown some benefit.31,55 Because SLOS is a cholesterol deficiency disorder and cholesterol is the precursor of bile acid, it was initially thought that SLOS was likely to lead to a deficiency of bile acids. The addition of bile acids could correct the deficiency and increase the absorption of dietary cholesterol.31 This approach has lost favor, however, because of side effects,55,56 lack of convincing evidence of benefit,56 and limited availability of the beneficial formulations of bile acids.
Given observed oxidative stress in SLOS,57 patients may benefit from antioxidants.
Future research should evaluate more rigorously how cholesterol supplementation, statins, bile acids, antioxidants, and other medications affect challenging behavior, and whether dietary and medical interventions in individuals whose behavior has been identified as “biological” might have different effects from those in individuals whose behavior is significantly motivated by social or environmental factors. We might hypothesize that the effects of interventions that alter cholesterol synthesis or accumulation would be more pronounced for individuals whose behavior has been identified as biological.
In conclusion, our findings suggest the potential benefits of blending behavioral and medical approaches in studying the behavior of individuals with genetic conditions. Our data suggest future lines of inquiry into challenging behavior in SLOS, and the role of cholesterol in behavior more generally. As noted, lower cholesterol levels have been associated with extreme emotions and violent and/or suicidal behavior in other clinical populations.13,14 Thus, studying SLOS, whose precise biochemical defect in cholesterol metabolism is understood, may have far-reaching implications for our understanding of how cholesterol can affect behavior.
ACKNOWLEDGMENTS
The authors acknowledge Jennifer Penfield and Jessica Adsit for their study coordination, and Karen Grant and Julie Canfield for their assistance with data collection. The authors are grateful to the children and families who participated in the study and to the health care providers who referred children.
Supported by National Institutes of Health grants R01 HL073980 to R.D.S. and M01 RR000334 to Oregon Health & Science University. Publication supported in part by the Oregon Clinical and Translational Research Institute, grant UL1 RR024140 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Wassif CA, Maslen C, Kachilele-Linjewile S, et al. Mutations in the human sterol delta7-reductase gene at 11q12-13 cause Smith-Lemli-Opitz syndrome. Am J Hum Genet. 1998;63:55–62. doi: 10.1086/301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterham HR, Wijburg F, Hennekam RC, et al. Smith-Lemli-Opitz syndrome is caused by mutations in the 7-dehydrocholesterol reductase gene. Am J Hum Genet. 1998;63:329–338. doi: 10.1086/301982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzky BU, Witsch-Baumgartner M, Erdel M, et al. Mutations in the Delta7-sterol reductase gene in patients with the Smith-Lemli-Opitz syndrome. Proc Natl Acad Sci USA. 1998;95:8181–8186. doi: 10.1073/pnas.95.14.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikora DM, Ruggiero M, Petit-Kekel K, et al. Cholesterol supplementation does not improve developmental progress in Smith-Lemli-Opitz syndrome. J Pediatr. 2004;114:783–791. doi: 10.1016/j.jpeds.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Tint GS, Salen G, Batta AK, et al. Correlation of severity and outcome with plasma sterol levels in variants of the Smith-Lemli-Opitz syndrome. J Pediatr. 1995;127:82–87. doi: 10.1016/s0022-3476(95)70261-x. [DOI] [PubMed] [Google Scholar]
- 6.Witsch-Baumgartner M, Fitzky BU, Ogorelkova M, et al. Mutational spectrum in the Delta7-sterol reductase gene and genotype-phenotype correlation in 84 patients with Smith-Lemli-Opitz syndrome. Am J Hum Genet. 2000;66:402–412. doi: 10.1086/302760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley RI. A new face for an old syndrome. Am J Med Genet Part A. 1997;65:251–256. doi: 10.1002/(sici)1096-8628(19970131)68:3<251::aid-ajmg1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Opitz JM. RSH (so-called Smith-Lemli-Opitz) syndrome. Curr Opin Pediatr. 1999;11:353–362. doi: 10.1097/00008480-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Ryan AK, Bartlett K, Clayton P, et al. Smith-Lemli-Opitz syndrome: a variable clinical and biochemical phenotype. J Med Genet. 1998;35:558–565. doi: 10.1136/jmg.35.7.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tint GS, Irons M, Elias ER, et al. Defective cholesterol biosynthesis associated with Smith-Lemli-Opitz syndrome. N Engl J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 11.Sikora DM, Pettit-Kekel K, Penfield J, et al. The near-universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz Syndrome. Am J Med Genet. 2006;104A:1511–1518. doi: 10.1002/ajmg.a.31294. [DOI] [PubMed] [Google Scholar]
- 12.Tierney E, Nwokoro NA, Porter FD, et al. Behavior phenotype in the RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet. 2001;98:191–200. doi: 10.1002/1096-8628(20010115)98:2<191::aid-ajmg1030>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Roaldset JO, Bakken AM, Bjorkly S. A prospective study of lipids and serotonin as risk markers of violence and self-harm in acute psychiatric patients. Psych Res. 2011;186:293–299. doi: 10.1016/j.psychres.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Atmaca M, Kuloglu M, Tezcan E, et al. Serumleptinandcholesterolvaluesinviolentandnon-violentsuicideattempters. Psych Res. 2008;158:87–91. [Google Scholar]
- 15.Weidner G, Connor SL, Gerhard GT, et al. The effects of dietary cholesterol-lowering on psychological symptoms: a randomised controlled study. Psychol Health Med. 2009;14:255–261. doi: 10.1080/13548500902730101. [DOI] [PubMed] [Google Scholar]
- 16.Battaile K, Battaile B, Merkens L, et al. Carrier frequency of the common mutation IVS8-1G.C in DHCR7 and estimate of the expected incidence of Smith-Lemli-Opitz syndrome. Mol Genet Metab. 2001;72:67–71. doi: 10.1006/mgme.2000.3103. [DOI] [PubMed] [Google Scholar]
- 17.Lowry RB, Yong SL. Borderline normal intelligence in the Smith–Lemli–Opitz (RSH) syndrome. Am J Med Genet. 1980;5:137–143. doi: 10.1002/ajmg.1320050205. [DOI] [PubMed] [Google Scholar]
- 18.Mace FC, Mauk JE. Bio-behavioral diagnosis and treatment of self-injury. Ment Retard Dev Disabil Res Rev. 1995;1:104–110. [Google Scholar]
- 19.Iwata BA, Vollmer TR, Zarcone JR, et al. Treatment classification and selection based on behavioral function. In: Van Houten R, Axelrod S, editors. Behavior Analysis and Treatment. New York: Plenum; 1998. pp. 101–125. [Google Scholar]
- 20.Didden R, Korzilius H, Curfs L. Skin-picking in individuals with Prader-Willi syndrome: prevalence, functional assessment, and its co-morbidity with compulsive and self-injurious behaviors. J Appl Res Intellect Disabil. 2007;20:409–419. [Google Scholar]
- 21.Moss J, Oliver C, Hall S, et al. The association between environmental events and self-injurious behavior in Cornelia de Lange syndrome. J Intellect Disabil Res. 2005;49:269–277. doi: 10.1111/j.1365-2788.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- 22.Olson L, Houlihan D. A review of behavioral treatments used for Lesch-Nyhan syndrome. Behav Modif. 2000;24:202–222. doi: 10.1177/0145445500242003. [DOI] [PubMed] [Google Scholar]
- 23.Taylor L, Oliver C. The behavioural phenotype of Smith-Magenis syndrome: evidence for a gene-environment interaction. J Intellect Disabil Res. 2008;52:1–12. doi: 10.1111/j.1365-2788.2008.01066.x. [DOI] [PubMed] [Google Scholar]
- 24.Steiner RD, Linck LM, Flavell DP, Lin DS, Conner WE. Sterol balance in the Smith-Lemli-Opitz syndrome: reduction in whole body cholesterol synthesis and normal bile acid production. J. Lipid Res. 2000;41:1437–1447. [PubMed] [Google Scholar]
- 25.Merkens LS, Connor WE, Linck LM, et al. Effects of dietary cholesterol on plasma lipoproteins in Smith-Lemli-Opitz syndrome. Pediatr Res. 2004;56:726–732. doi: 10.1203/01.PDR.0000141522.14177.4F. (now 26) [DOI] [PubMed] [Google Scholar]
- 26.Thompson T, Gray DB. Sage Focus Editions. Vol. 170. Thousand Oaks, CA: Sage; 1994. Destructive behavior in developmental disabilities: diagnosis and treatment; pp. 24–48. 1994. [Google Scholar]
- 27.Emerson E, Kiernan C, Alborz A, et al. The prevalence of challenging behavior: a total population study. Res Dev Disabil. 2001;22:77–93. doi: 10.1016/s0891-4222(00)00061-5. [DOI] [PubMed] [Google Scholar]
- 28.Durand VM, Crimmins DB. Motivation Assessment Scale Administration Guide. Syracuse, NY: Program Development Associates; 1992. [Google Scholar]
- 29.Durand VM, Crimmins DB. Identifying the variables maintaining self-injurious behaviors. J Autism Dev Disord. 1988;18:99–117. doi: 10.1007/BF02211821. [DOI] [PubMed] [Google Scholar]
- 30.Haas D, Garbade SF, Vohwinkel C, et al. Effects of cholesterol and simvastatin treatment in patients with Smith-Lemli-Opitz syndrome (SLOS) J Inherit Metab Dis. 2007;30:375–387. doi: 10.1007/s10545-007-0537-7. [DOI] [PubMed] [Google Scholar]
- 31.Irons M, Elias ER, Abuelo D, et al. Treatment of Smith-Lemli-Opitz syndrome: results of a multicenter trial. Am J Med Genet. 1997;68:311–314. [PubMed] [Google Scholar]
- 32.Thorndike RL, Hagen EP, Sattler JM. Stanford-Binet Intelligence Scale. 4th ed. Rolling Meadows, IL: Riverside Publishing Co; 1986. [Google Scholar]
- 33.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 34.Richler J, Bishop SL, Kleinke JR, et al. Restricted and repetitive behaviors in young children with autism spectrum disorders. J Autism Dev Disord. 2007;37:73–85. doi: 10.1007/s10803-006-0332-6. [DOI] [PubMed] [Google Scholar]
- 35.Bishop SL, Guthrie W, Coffing M, et al. Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. Am J Intellect Dev Disabil. 2011;116:331–343. doi: 10.1352/1944-7558-116.5.331. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saloviita T. The structure and correlates of self-injurious behavior in an institutional setting. Res Dev Disabil. 2000;21:501–511. doi: 10.1016/s0891-4222(00)00055-x. [DOI] [PubMed] [Google Scholar]
- 37.Baghdadli A, Pascal C, Grisi S, et al. Risk factors for self-injurious behaviours among 222 young children with autistic disorders. J Intellect Disabil Res. 2003;47:622–627. doi: 10.1046/j.1365-2788.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- 38.Iwata BA, Pace GM, Dorsey MF, et al. The functions of self-injurious behavior: an experimental-epidemiological analysis. J Appl Behav Anal. 1994;27:215–240. doi: 10.1901/jaba.1994.27-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeBlanc LA, Patel MR, Carr JE. Recent advances in the assessment of aberrant behavior maintained by automatic reinforcement in individuals with developmental disabilities. J Behav Ther Exp Psychiatry. 2000;31:137–154. doi: 10.1016/s0005-7916(00)00017-3. [DOI] [PubMed] [Google Scholar]
- 40.Zarcone JR, Lindauer SL, Morse PS, et al. Effects of risperidone on destructive behavior of persons with developmental disabilities: III. functional analysis. Am J Ment Retard. 2004;109:310–321. doi: 10.1352/0895-8017(2004)109<310:EORODB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Hall SS. Comparing descriptive, experimental and informant-based assessments of problem behaviors. Res Dev Disabil. 2005;26:514–526. doi: 10.1016/j.ridd.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Wasano LC, Borrano JC, Kohn CS. Brief report: a comparison of indirect versus experimental strategies for the assessment of pica. J Autism Devel Dis. 2009;39:1582–1586. doi: 10.1007/s10803-009-0766-8. [DOI] [PubMed] [Google Scholar]
- 43.Duker PC, Sigafoos J. The motivation assessment scale: reliability and construct validity across three topographies of behavior. Res Dev Disabil. 1998;19:131–141. doi: 10.1016/s0891-4222(97)00047-4. [DOI] [PubMed] [Google Scholar]
- 44.Crawford J, Bockel B, Schauss S, et al. A comparison of methods for the functional assessment of stereotypic behavior. J Assoc Pers Sev Handicaps. 1992;17:77–86. [Google Scholar]
- 45.Zarcone JR, Rodgers TA, Iwata BA, et al. Reliability analysis of the motivation assessment scalerA failure to replicate. Res Dev Disabil. 1991;12:349–360. doi: 10.1016/0891-4222(91)90031-m. [DOI] [PubMed] [Google Scholar]
- 46.Singafoos J, Kerr M, Roberts D. Interrater reliability of the motivation assessment scale: failure to replicate with aggressive behavior. Res Dev Disabil. 1994;15:333–342. doi: 10.1016/0891-4222(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 47.Teunissen CE, Dijkstra CD, Polman CH, et al. Decreased levels of the brain specific 24S-hydroxycholesterol and cholesterol precursors in serum of multiple sclerosis patients. Neurosci Lett. 2003;347:159–162. doi: 10.1016/s0304-3940(03)00667-0. [DOI] [PubMed] [Google Scholar]
- 48.Elias ER, Irons MB, Hurley AD, et al. Clinical effects of cholesterol supplementation in six patients with the Smith-Lemli-Opitz syndrome (SLOS) Am J Med Genet. 1997;68:305–310. doi: 10.1002/(sici)1096-8628(19970131)68:3<305::aid-ajmg11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 49.Martin A, Koenig K, Scahill L, et al. Smith-Lemli-Opitz syndrome (letters to the editor) J Am Acad Child Adolesc Psychiatry. 2001;40:506–507. doi: 10.1097/00004583-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Nowaczyk MJ, Whelan DT, Heshka TW, et al. Smith-Lemli-Opitz syndrome: a treatable inherited error of metabolism causing mental retardation. CMAJ. 1999;161:165–170. [PMC free article] [PubMed] [Google Scholar]
- 51.Sidener TM, Carr JE, Firth AM. Superimposition and withholding of edible consequences as treatment for automatically reinforced stereotypy. J Appl Behav Anal. 2005;38:121–124. doi: 10.1901/jaba.2005.58-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2001;45:1–23. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Tierney E, Conley SK, Goodwin H, et al. Analysis of short-term behavioral effects of dietary cholesterol supplementation in Smith–Lemli–Opitz syndrome. Am J Med Genet. 2010;152A:91–95. doi: 10.1002/ajmg.a.33148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wassif CA, Krakowiak PA, Wright BS, Gewandter JS, Sterner AL, Javitt N, Yergey AL, Porter FD. ResidualcholesterolsynthesisandsimvastatininductionofcholesterolsynthesisinSmith-Lemli-Opitzsyndromefibroblasts. Mol Genet Metab. 2005;85(2):96–107. doi: 10.1016/j.ymgme.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Irons M, Elias RE, Tint GS, et al. Abnormal cholesterol metabolism in the Smith-Lemli-Opitz syndrome: report of the clinical and biochemical findings in four patients and treatment in one patient. Am J Med Genet. 1994;50:347–352. doi: 10.1002/ajmg.1320500409. [DOI] [PubMed] [Google Scholar]
- 56.Nwokoro NA, Mulvihill JJ. Cholesterol and bile acid replacement therapy in children and adults with Smith-Lemli-Opitz (SLO/RSH) syndrome. Am J Med Genet. 1997;68:315–321. doi: 10.1002/(sici)1096-8628(19970131)68:3<315::aid-ajmg13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 57.Korade Z, Xu L, Shelton R, et al. Biological activities of 7-dehydrocholesterol-derived oxysterols: implications for Smith-Lemli-Opitz syndrome. J Lip Res. 2010;51:3259–3269. doi: 10.1194/jlr.M009365. [DOI] [PMC free article] [PubMed] [Google Scholar]