Abstract
Background
The previous works about safety evaluation for constructed bladder tissue specific adenovirus are poorly documented. Thus, we investigated the biodistribution and body toxicity of bladder specific oncolytic adenovirus Ad-PSCAE-UPII-E1A (APU-E1A) and Ad-PSCAE-UPII-E1A-AR (APU-E1A-AR), providing meaningful information prior to embarking on human clinical trials.
Materials and Method
Conditionally replicate recombinant adenovirus (CRADs) APU-E1A, APU-EIA-AR were constructed with bladder tissue specific Uroplakin II (UP II) promoter to induce the expression of Ad5E1A gene and E1A-AR fusing gene, and PSCAE was inserted at upstream of promoter to enhance the function of promoter. Based on the cytopathic and anti-tumor effect of bladder cancer, these CRADs were intratumorally injected into subcutaneous xenografts tumor in nude mice. We then determined the toxicity through general health and behavioral assessment, hepatic and hematological toxicity evaluation, macroscopic and microscopic postmortem analyses. The spread of the transgene E1A of adenovirus was detected with RT-PCR and Western blot. Virus replication and distribution were examined with APU-LUC administration and Luciferase Assay.
Results
General assessment and body weight of the animals did not reveal any alteration in general behavior. The hematological alterations of groups which were injected with 5×108 pfu or higher dose (5×109 pfu) of APU-E1A and APU-E1A-AR showed no difference in comparison with PBS group, and only slight increased transaminases in contrast to PBS group at 5×109 pfu of APU-E1A and APU-E1A-AR were observed. E1A transgene did not disseminate to organs outside of xenograft tumor. Virus replication was not detected in other organs beside tumor according to Luciferase Assay.
Conclusions
Our study showed that recombinant adenovirus APU-E1A-AR and APU-E1A appear safe with 5×107 pfu and 5×108 pfu intratumorally injection in mice, without any discernable effects on general health and behavior.
Keywords: Bladder cancer, gene therapy, oncolytic adenovirus, biodistribution, safety assessment
Introduction
Bladder cancer (BC) ranks as the eighth highest incidence in all of the malignant tumors and is the second-leading common genitourinary malignancy [1]. About 30% of bladder cancer patients represent with or progress to disease invading bladder muscle. Standard therapies conducted include radical surgery, radiotherapy and chemotherapy. Although radical cystectomy is performed in patients with invasive BC, half of the patients progress to an advanced stage with metastasis, and most of them die from this aggressive disease within 5 years[2]. Cisplatin or other drugs based systemic chemotherapy with or without radical cystectomy has been empirically adapted with the aim of improving the prognosis of invasive or metastatic BC. However, these therapies remain far from being satisfactory [3, 4].
With the development of genetics and molecular biology, gene therapy has become one of the novel models for the treatment of cancer[5]. The underlying hypothesis is that viral replication leads to intratumoral amplification of the therapeutic virus, leading to the ultimate destruction of infected cancer cells with minimal damage to nonneoplastic tissue. Several clever strategies have been employed to mutagenize and/or select for tumor-specific replicating viruses, or to target the tumor microenvironment to maximize their therapeutic efficacy[6]. A large number of gene therapy applications encompass the use of adenoviral vectors. Adenoviral vectors have a high transduction efficiency, are capable of containing DNA inserts up to 8 kilo bases, have extremely high viral titers (in the range of 1010-1013), and infect both replicating and differentiated cells. Also, since they lack integration, they can not bring about mutagenic effects caused by random integration into the host genome[7]. Bladder-specific conditionally replication competent adenoviruses are attractive therapeutics for bladder cancer because they selectively replicate in tumors then kill tumor cells.
UP II gene expression is bladder specific and differentiation dependent, which provides important information for target gene therapy of bladder tumor [8, 9]. Meanwhile, it is indicated that Prostate Stem Cell Antigen Enhancer (PSCAE, 327bp) not only express in prostate cells [10, 11], but also in bladder cancer epithelial cells. Furthermore, some analyses revealed that the early adenoviral genes (E1A) interact with the androgen receptor (AR) in some AR positive bladder cancer cells, limiting the activity of both E1A and AR [12]. This mutual inhibition led to decreased potency of our early adenoviral vectors. However, fusing gene E1A with AR at downstream of promoter UP II could promote replication of adenovirus. The chimeric fusion allowed augmentation of activity, rather than inhibition [12]. So we constructed selective replicate recombinant adenovirus Ad-PSCAE-UP II -E1A-AR (APU-E1A-AR), Ad-PSCAE-UP II-E1A (APU-E1A) and Ad-PSCAE-UP II-Luc (APU-LUC), which had efficient cytopathic and antitumor effects for bladder cancer cells in vivo and vitro[13].
The results of clinical trials on oncolytic viruses for head and neck, ovarian, brain, and prostate cancers have been encouraging in terms of efficacy with minimal, if any, toxicity [14]. To date there have only been a handful studies that have brought their vectors to clinical trials, and even fewer that have subsequently published the results [15-21]. But, for bladder cancer, uptill now even a single kind of tissue specific oncolytic adevovirus have not went into Phase I Clinical Trial. Collection of data from preclinical studies to access the spread of E1A transgene expression is of the utmost importance in assessing the risks associated with gene therapy. Thus, in this present study, we obtained data describing a comprehensive safety assessment and biodistribution of E1A gene throughout the major organs of mice, which might serve as a prelude to Phase I Clinical Trial for the bladder cancer specific recombinant oncolytic adenovirus APU-E1A-AR and APU-E1A.
Materials and Methods
Construction of Bladder Cancer Specific Recombinant Oncolytic Adenovirus
The plasmids Rp-PSCAE-UP II-E1A and Rp-PSCAE-UP II-LUC had been described previously [9, 11]. A BamHI/EcoRV fragment from RpS-TOAD-PSE-PBN-E1A-ARC685Y (presented by Professor Rodriguez in John Hopkins University, USA) containing E1A-ARC85Y was sub-cloned into Rp-PSCAE-UP II -E1A to get Rp-PSCAE-UP II -E1A-AR (C685Y). These plasmids were recombined with pAdEasy-1 by homologous recombination in colibacillus (BJ51832) and by electroporating competent cells and cultivated in Kana LB culture media to get corresponding recombinant adenovirus vectors APU-E1A, APU-LUC and APU-E1A-AR see Fig. (1). These recombinant vectors were then packaged by calcium phosphate transfection into HEK293 cells. Following transfection, cells were harvested. Cell lysates were purified by caesium chloride (Genmed, USA) gradient, and then the vector was concentrated and dialysed against 2 × phospate-buffered saline (PBS)/2 mM MgCl2 in cellulose filter bags (D1-7spectra, spectrum, USA). The genomic integrity of the final virus preparation was verified by evaluating the expression of transgenes E1 A, UP II, PSCAE and E1A-AR with quantitative polymerase chain reaction (qPCR) using a ROTOR-GEN 3000 (Cobette, Australia) following a standard operating procedure. The genomic titers of the stocks were determined by Tissue Culture Infectious Dose50 Assay (TCID50), and the titers of APU-E1A, APU-LUC and APU-E1A-AR were 1.2×1012, 5.7×1011 and 3.8×1011 vector genomes (vg)/ml separately. Virus aliquots were stored at −80 °C.
Fig. (1).
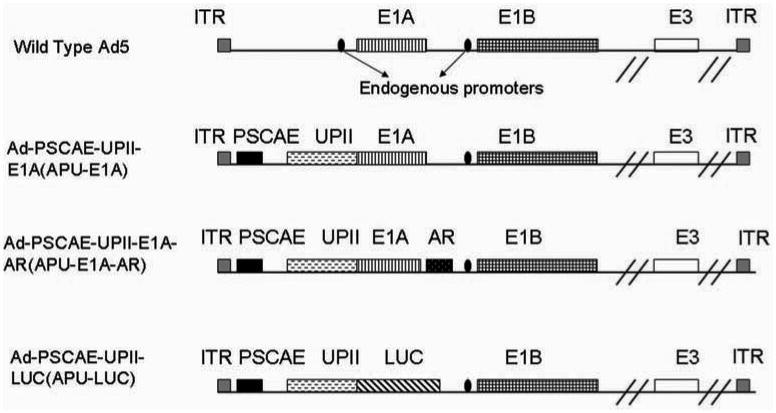
Schematic diagrams of constructed oncolytic adenovirus of APU-E1A, APU-E1A-AR and APU-LUC. APU-E1A is based on Ad5 wherein the Prostate Stem Cell Antigen Enhancer (PSCAE, 327bp) is inserted and endogenous E1a promoter has been replaced by the human UP II promoter. In APU-E1A-AR, the androgen receptor (AR) gene was inserted following E1A. APU-LUC, with LUC gene inserted after UPII, is used to detect the biodistribution of adenovirus.
Animal Experiment
5-week-old (18-24 g) male athymic BALB/c nu-nu mice (SLAC animal laboratories, Shanghai, China) were housed in Animal Laboratory of SPF Housing Facility in Gansu College of Traditional Chinese Medicine, and treated in accordance with approved protocols which were gained from the Laboratory Animal Care and Use Committee of Gansu Province. Animals were housed in groups of two at constant temperature (23 °C) and relative humidity (60%) with free access to food and water and a 12: 12 h light/dark cycle. Mice were injected subcutaneously on each posterior flank with 5×106 EJ cells in 0.2 ml PBS using microliter syringe fitted with a 28-gauge needle. Tumor areas (length × width) were measured on alternate days using Vernier calipers (VWR International, Cat# 62379-531) and tumor volume was calculated using the following formula: volume (a rotational ellipsoid)=M1×M22 ×0.5236, where M1 indicates the long axis, and M2 is the short axis[22]. When the tumors reached about 150mm3 in volume, the mice were divided into eight groups (10 mice per group): (a) control group, phosphate-buffered saline (vehicle) injected intratumorally, 60 μl per day for five consecutive days; (b) APU-LUC, intratumorally 1×108 plaque forming units injected in 60 μl per day for five consecutive days (total 5×108 plaque forming units). (c) APU-E1 A, intratumorally 1×107 plaque forming units injected in a volume of 60 μl per day for five consecutive days (total 5×107 plaque forming units); (d) APU-E1A, intratumorally 1×108 plaque forming units injected in 60 μl per day for five consecutive days (total 5×108 plaque forming units); (e) APU-E1A, intratumorally 1×109 plaque forming units injected in 60 μl per day for five consecutive days (total 5×109 plaque forming units);(f) APU-E1A-AR, intratumorally 1×107 plaque forming units injected in 60 μl per day for five consecutive days (total 5×107 plaque forming units); (g) APU-E1A-AR, intratumorally 1×108 plaque forming units injected in 60 μl per day for five consecutive days (total 5×108 plaque forming units); (h) APU-E1A-AR, intratumorally 1×109 plaque forming units injected in 60 μl per day for five consecutive days (total 5×109 plaque forming units); All mice were euthanized at 8 weeks after surgery.
General Health and Behavioral Assessment
The observation of body weight, morbidity, and mori-bundity of mice were performed twice a week for 8 weeks after injection of increasing viral doses. Meanwhile, the mice were observed in its home cage and scored by a blinded observer for the behaviors: body position, respiration, clonic involuntary movement, tonic involuntary movement, vocalization and palpebral closure. The mice were then gently removed from its cage and scored for the following observations while holding in hand: reactivity to being picked up, ease of handling, palpebral closure, lacrimation, salivation, piloerection, plus a description of any other behaviors observed such as bite marks, missing nails, gauntness or death.
Hepatic and Hematological Toxicity Assessment
After 8 weeks, blood of all of these animals injected with adenovirus, were collected by picking out the eyebull with nipper, centrifuge at 1000 Revolutions Per Minute with a centrifugal apparatus (Biofuge 15R, Heraes, Germany). Liver enzymes (AST, aspartate aminotransferase and ALT, alanine aminotransferase) were analyzed by Auto Biochemical Analyzer (Hitachi 7060, Japan) with IFCC medthod (reagent from Merit Choice Bioengineering, Beijing, China); Hematological profiles were determined with Animal blood analyzer (HEMAVET 950, USA).
Organ Processing
After animals were deeply anesthetized with isoflurane, blood was collected from eye, and the organs were carefully removed from the body, weighted and examined by a macroscopic examination. Each organ was considered to have unremarkable gross pathology if it met specific criteria such as normal coloring, shape texture and no evidence of lesions or tumors. Organs were dissected in the order that the tissues least likely to contain E1A genomes were dissected first and those most likely (i.e. tumor) were dissected last. Separating dissection tools and storage containers that were used for each tissue to minimize cross contamination. The following organs/tissues were harvested in the approximate order as testis, seminal vesicle, kidney, spleen, stomach, heart, lung, brain, bladder, liver, and tumor. These organs were dissected into two part and placed in separate containers, half of the tissues were perfused with 4% paraformaldehyde to processing for Hematoxylin and Eosin (H&E) staining and immunohistochemistry. The other half was fresh frozen in liquid nitrogen for transgene biodistribution by quantitative reverse transcriptase (qRT)-PCR, Western blot and Luciferase reporter analysis.
H&E Staining
After organ tissues were post-fixed in 4% paraformaldehyde/0. 1M phosphate buffer for 24 hours at room temperature, perfused sections were mounted onto poly L-lysine coated slides and left to dry overnight prior to staining. All sections were then processed together for H&E staining using standard methods. Sections were scored for damage by a blinded observer using an Olympus microscope (Olympus AX80, Japan) at ×100 and ×400 magnification. The following markers of cell death/damage were assessed: cellular necrosis, cytoplasmic vacuolation, anachromasis and pyknotic nuclei. Any other abnormalities observed were also noted. The abnormal areas percentage was calculated with software Image-Pro Plus (IPP) 5.1. The scoring system used was: 0 = normal, 1 = very slight (focal damage), 2 = slight (<10% of tissue), 3 = moderate (10–40% of tissue), 4 = severe (40– 100% of damage).
Anti-E1A Immunohistochemistry
The organs were embedded with paraffin and tissue sections were rinsed in PBS-Triton and treated with 3% H2O2 to remove endogenous peroxidase. Following three rinses in PBS-Triton, sections were blocked with goat blood serum for 15 min at room temperature. Then sections were incubated overnight at 4°C with primary polyclonal antibody anti-adenovirus-5 E1A (santa Cruz Bioctechnology). After three rinses with PBS-Triton, sections were incubated at 37 for 15 min with biotin-labeled goat anti-mouse antibody (Sigma, USA). Slides were then treated with ExtrAvidin peroxidase (1: 500 dilutions; Sigma) for 2 h before a final wash in PBS and staining with 0.5 mg/ml 3, 3-diaminobenzidine (Sigma). Finally slides were visualized under an Olympus microscope (Olympus AX80, Japan).
Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction Assay for Transgene Expression
Analysis of transgene expression was performed according to a standard operating procedure. Major organs (testis, seminal vesicle, kidney, heart, lung, spleen, stomach, brain, bladder, liver, and tumor) of adenovirus administrated mice were quickly cut into small pieces with a sterile razor blade, and grounded in a mortar and pestle under liquid nitrogen. Total RNA was isolated from these tissues using the SV Total RNA Isolation System (Promega). cDNA samples were synthesized from 1 μg of RNA, with the PrimeScript® RT reagent Kit (Takara, Dalian, China). Quantitative real-time polymerase chain reaction (qT-PCR) using the SYBER GREEN fluorogenic detection system was performed according to SYBR® Premix Ex Taq™ Perfect Real Time Kit(Takara, Dalian, China).Two microliters of cDNA and 0.2 μM of each primer and 12.5 μl of SYBR® Premix Ex Taq™ mix were used per PCR reaction in a total volume of 25 μl. The primers for E1A gene of oncolytic adenovirus were designed using the primer design software Primer Express (Perkin-Elmer Applied Biosystems, Foster City, Calif). The forward and reverse primers for E1A were: E1A forward, 5′-CCCGAGTCTGTAATGTTGG-3′; E1A reverse, 5′-GTCGTCACTGGGTGGAAA-3′. PCR of an internal control gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was also performed with the following primers: GAPDH for, GGATTTGGTGGTCGTATTGGG; GAPDH rev, GGAAGATGGTGATGGGATT. The primers were evaluated for amplification efficiency and the consistency of replicate wells using the standard thermocycling conditions for the ROTOR-GEN 3000 (Cobette, Australia) system over 45 cycles. Cycling conditions included denaturation at 95°c for 5 sec, annealing and elongation at 60°c for 30 sec. The presence of only one product was confirmed by performing a melt cycle. To validate the assay, tissues from a PBS control animal were subjected to qRT-PCR to determine the sensitivity and specificity of the assay. The number Results were analyzed using the comparative CT method as described by the manufacturer (Rotor-Gene Real-Time Analysis Software 6.1).
Western Blot Analysis of E1A Protein Expression
Major organs (lung, brain, liver, and tumor) of adenovirus administrated mice were grounded in a mortar with pestle under liquid nitrogen. Cell lysate was made by adding RIPA Lysis Buffer (Beyotime, Jiangsu, China) containing 1 mM PMSF (phenylmethyl sulfonylfluoride). The lysate was sonicated for 30 seconds on ice and then centrifuged at 10000 rpm for 5 minutes at 4°C. Protein was quantitated by BCA (bicinchoninic acid) method. Total protein (20 μg) per sample was subjected to electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gel and electrotransferred to nitro cellulose membrane. After blocking with phosphate buffered saline (PBS) containing 5% powdered milk for 2 hours, the membrane was incubated with polyclonal antibody against adenovirus type 5 E1A (Abcam, USA), diluted 1:1000, for 2 hours at 37 °C, washed the membrane with PBS for 3 times and 5 minutes per time, followed by incubation with fluorescent labelled goat anti-mouse IgG (Beyotime, Jiangsu, China), diluted 1:2000. After extensive washing the protein was visualized at Fast Chemiluminescence Image System (ImageQuant 350, GE Healthe care, China). The protein of GAPDH was used as an internal control.
Luciferase Assay for Oncolytic Adenovirus
Tumor tissues and other major organs (liver, lung, and brain) of APU-LUC injected mice were quick-frozen in liquid nitrogen and then grounded to a powder and resuspend at room temperature in 1× Luciferase Cell Culture Lysis Reagent (Promega, USA) with further homogenization. The debris was removed after cell lysis by a brief centrifugation. The supernatant was incubated in a 96-well plate, and LUC were detected with ONE-Glo™ Luciferase Assay System (Promega, USA) on a Plate-Reading Luminometers (Thermo Scientific Varioskan Flash Instrument) at 560 nm.
Statistical Analysis
The results are presented as the Mean±Standard deviation. Statistical analyses were performed using SPSS 15.0 software (USA); maps were made by Sigmaplot 11.0 (USA) running on a Sony-PC compatible computer on the Windows XP operating system. Statistical comparison was determined using One-way analysis of variance. Statistical significance was defined as a P-value <0.05 and was denoted in each of the figures by an asterisk.
Results
General Health and Behavioral Assessment of Oncolytic Adenovirus Injected Mice
To provide bladder specific oncolytic adenovirus APU-E1A-AR and APU-E1A mediated toxicity data, a comprehensive health assessment was performed twice weekly on mice injected intratumorally with oncolytic adenovirus APU-E1A, APU-E1A-AR and APU-LUC. This examination involved assessing the health of the nose, eye, mouth, chin, face, ears, chest, tail and coat, recording whether they appeared normal, as well as recording any abnormalities such as wounds, lumps, scabs, bald patches, hunched posture or noisy breathing. There were no serious adverse events and no changes that could be attributed to injection of oncolytic adenovirus.
To assess the general health and behavior of the mice in their normal environment, functional observations were made in the home cage and in the open field according to a standard operating procedure. These included assessment of body position, respiration, clonic or tonic involuntary movements, vocalization, palpebral closure, reactivity when held in hand, piloerection, salivation and lacrimation. All animals displayed normal health and behavior when assessed for 8 weeks after surgery.
Body Weight, Hepatic and Hematological Toxicity Assessment
Animals that were injected with increasing dose of APU-E1A and APU-E1A-AR gained weight at a steady pace, however, animals that were injected with PBS and APU-Luc gained weight for first 3 weeks and then they showed weight loss after 21st day Fig. (2a). Liver enzymes (aspartate aminotransferase and alanine aminotransferase) were determined at 56th day after injection of different virus doses. At 5×109 pfu of APU-E1A and APU-E1A-AR, only slightly increased transaminases in contrast with PBS group were observed Fig. (2b). The hematological alterations of groups which were injected with 5×108 pfu or higher dose (5×109 pfu) of APU-E1A and APU-E1A-AR had no difference comparing with PBS group Figs. (2c, 2d).
Fig. (2).
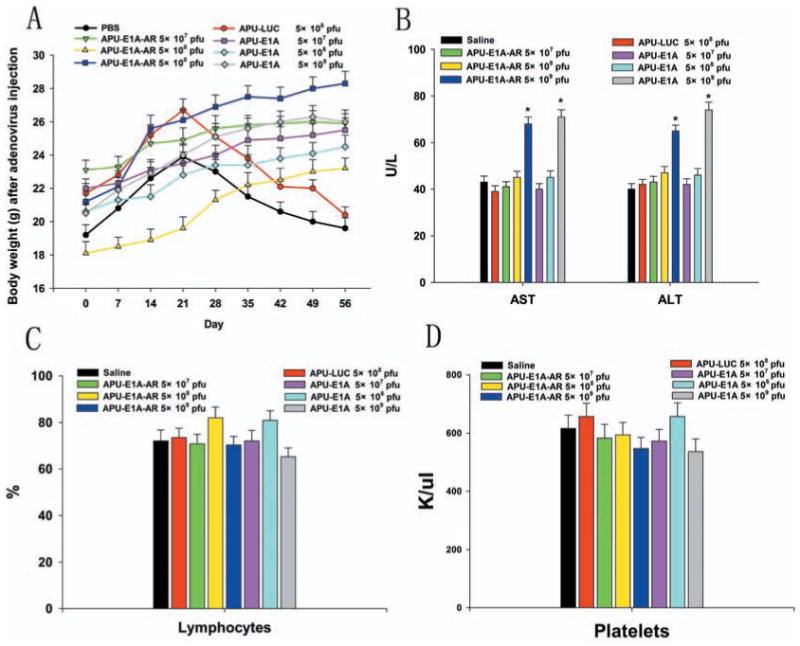
Body weight, hepatic and hematological toxicity profile after increasing single doses of oncolytic adenovirus administration in immunocompetent mice. (A) Average weights of mice from injection to 56 day, immediately prior to euthanasia. (B) The average value for alanine aminotransferase (AST) and aspartate aminotransferase (ALT). (C) The lymphocyte percentage in white blood cell and (D) average value for platelet. Phosphate-buffered saline (PBS) administration was used in the control group. Mean values ± SD of 5–10 mice/group were depicted. * significant (P < 0.05) by One-way analysis of variance compared with the PBS group.
Macroscopic and Microscopic Postmortem Analyses
Following euthanasia, major organs of animals were examined macroscopically and weighed. All organs examined appeared normal, with no evidence of lesions or tumors. The organs from different doses of APU-LUC, APU-E1A and APU-E1A-AR animals were of similar size as PBS-treated animals and there were no significant differences in relative organ weight between these groups Fig. (3a).
Fig. (3).
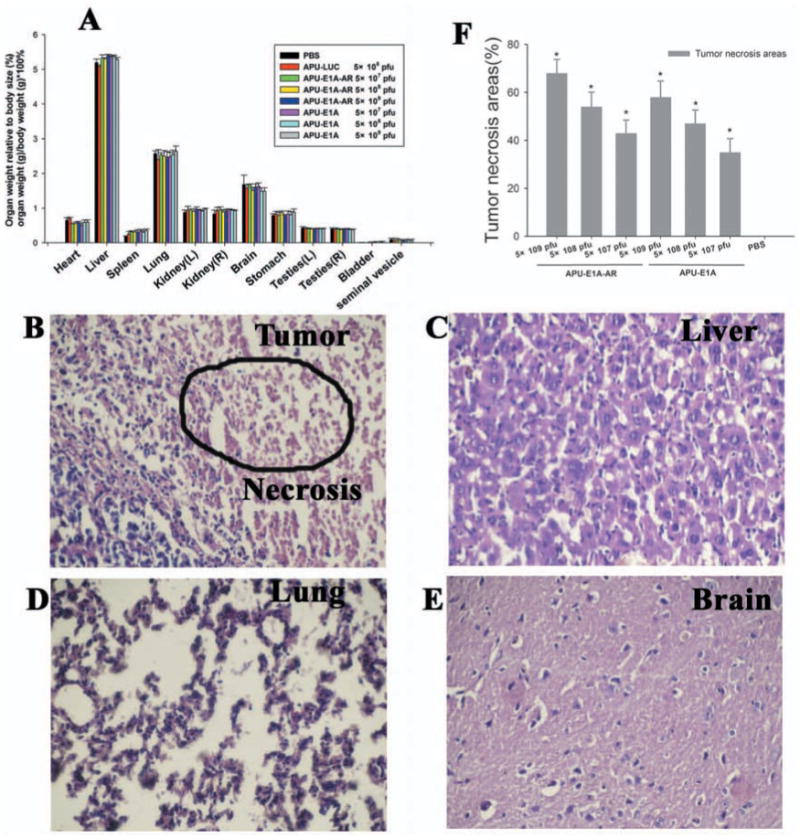
Macroscopic and microscopic postmortem analysis (×400 magnification). (A) Weights of organs at sacrifice (n = 5-10 for both groups). Error bars indicate the mean ± SEM. (B–E) H&E staining of the (B) tumor, (C) liver, (D) lung and (E) brain, from animals that were injected with 5×109 pfu APU-E1A-AR. The cellular necrosis of tumor tissue are shown (circle), according to IPP analysis, the tumor necrosis was severe (68% of damage, score of 4). (F) The tumor necrosis area percentage (%) were calculated with software Image-Pro Plus (IPP) 5.1 (n = 5-10 for both groups). Error bars indicate the mean ± SEM. * P <0.05 comparing with PBS control.
Tumor, liver, lung and brain tissue sections from multiple positions across the dorsal–ventral axis were stained with H&E, calculated with software Image-Pro Plus (IPP) 5.1 and scored for damage on a scale of 1–4 by an observer who was blinded to the presence of cellular necrosis, cytoplasmic vacuolation, anachromasis and pyknotic nuclei. Normal tissues was observed in all major organs of 5×109 pfu APU-E1A and APU-E1A-AR and PBS control animals, except the presence of cellular necrosis in the tumors, which was severe (68% of damage, score of 4) Figs. (3b-e). With the increase dose of adenovirus, the tumor necrosis area percentage was accordingly increased, and had significant meaning comparing with PBS group Fig. (3f). This phenomenon can be attributed to the oncolytical function of adenoviruses.
E1A immunostaining was performed on the perfused mice major organs (tumor, liver, lung, brain, heart, spleen and kidney) to confirm the expression of E1A in APU-E1A and APU-E1A-AR treated mice. By comparing with the control mice, expression levels of E1A showed an increase in tumor tissues staining in the APU-E1A-AR injected mice following increased doses. However, the anti-E1A immunostaining were negative in other organs, such as liver, lung, heart, brain, spleen and kidney in APU-E1A-AR injected mice Fig. (4). The result is same with mice injected with oncolytic adenovirus APU-E1A (data not shown).
Fig. (4).
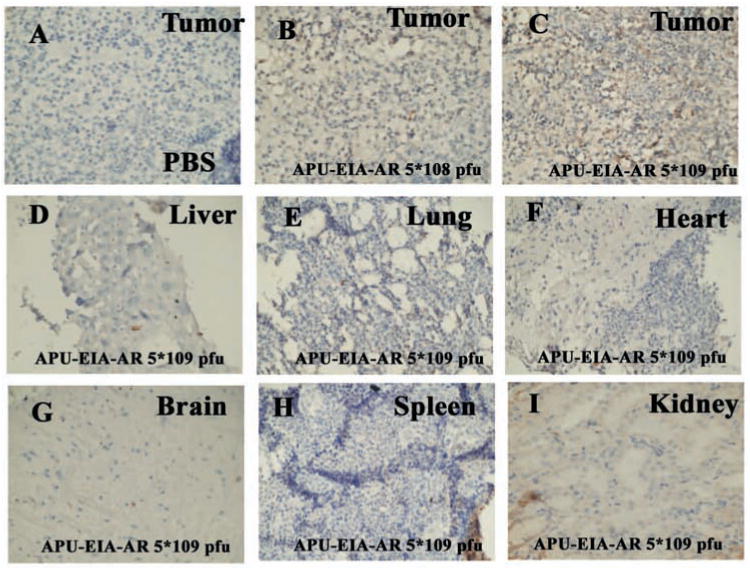
Anti-E1A immunostaining (×400 magnification). Immunohischemistry for anti-E1A is shown on tumor (A-C), liver (D), lung (E), heart (F), brain (G), spleen (H) and kidney (I) from PBS-treated mice (A), APU-E1A-AR 5×108 pfu treated mice (B) and APU-E1A-AR 5×109 pfu treated mice (C-I).
Biodistribution of E1A Transgene
To assess the diffusion of E1A transgene, total RNA was extracted from major organs (tumor, liver, lung, brain, heart, spleen and kidney) from animals at 56th day after injection with increasing dose of APU-E1A and APU-E1A-AR, and analyzed using RT-PCR to access the level of E1A expression. Tumor tissues showed extremely high expression of E1A transgene (P<0.05) in comparison with PBS group, confirming the expression of the E1A in the xenograft tumor because of the tissue specific UP II promoter and PSCAE enhancer. Moreover, the level of E1A was increasing corresponding with the increasing doses of oncolytic adenovirus. In the liver, lung and spleen tissues, very low E1A transgene were detected in comparison with PBS control, which had no significance meaning (p>0.05). Meanwhile, in other tissues, such as brain, heart and kidney, the expression of E1A were negative Fig. (5).
Fig. (5).
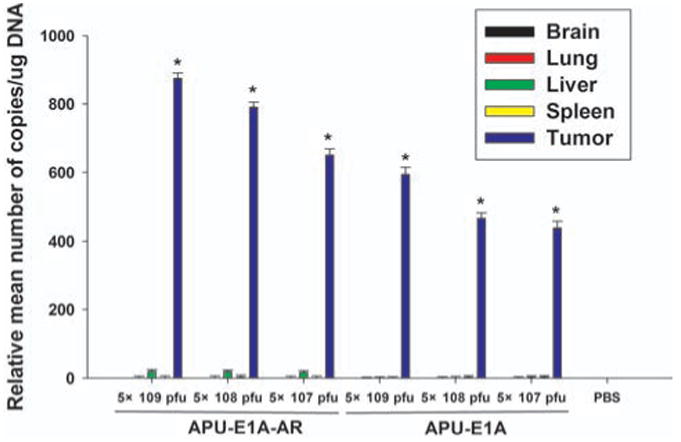
Biodistribution of E1A transgene with qRT-PCR. Relative mean number of copies of E1A transgene (standardized to GAPDH) was qualified in triplicates using Rotor-Gene Real-Time Analysis Software 6.1. * P <0.05 comparing with PBS control.
Western Blot Analysis of E1A Protein Biodistribution
In order to evaluate the virus replication and distribution in different organs, the E1A protein expression in major organs of mice injected with increasing doses of APU-E1A-AR and APU-E1A were detected with western blot analysis. It showed that after injection with oncolytic adenovirus APU-E1A-AR and APU-E1A, E1A protein was detected only in tumor tissues; it did not express in other tissues such as liver, lung and brain Fig. (6a).
Fig. (6).
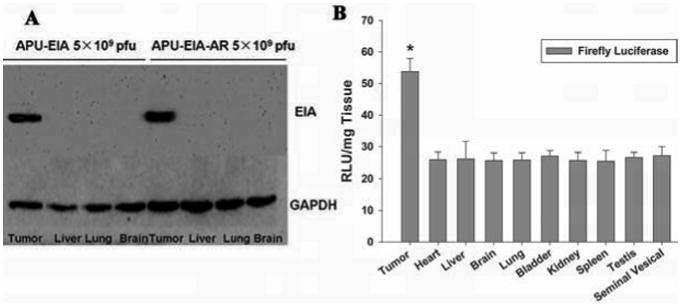
(A) Western blot analysis of E1A protein. Tumor and other major organs (Liver, Lung, Brain) of high dose APU-E1A and APU-E1A-AR injected mice were detected in triplicates, and GAPDH was control. (B) Luciferase reporter assay for APU-LUC. Tumor and other major organs of 5×108 pfu APU-LUC injected mice were detected in triplicates. * p<0.05 comparing with other organs.
Luciferase Reporter Assay for Oncolytic Adenovirus
To examine adenovirus replication and distribution in mice, we use tumor tissues and major organs from mice treated with constructed adenovirus APU-LUC, LUC were detected with Luciferase Assay System. It showed that a higher RLU was detected in tumor tissues, which has significant difference comparing with other organs (p<0.05) Fig. (6b).
Discussion
Several studies have been conducted on the application of gene therapies using CRADs for the treatment of bladder cancer [22, 23]. The CRADs are able to lyse the infected tumor cells and deliver cytotoxic proteins directly into tumor cells. Moreover, because of the unique mode of tumor destruction, oncolytic virotherapy has the potential to augment the antineoplastic activity of chemotherapy and radiation therapy [24, 25]. Meanwhile, intravesicular therapy of refractory superficial bladder cancer employing an oncolytic adenovirus would allow for local administration and efficient delivery of virus to bladder tumor [26], so we can conveniently use oncolytic adenovirus bladder instillation to reduce the toxicity [27]. It is postulated that oncolytic adenovirus intravesical therapies with better tumor penetration is a promising new approach for bladder cancer resistant to conventional treatments.
Based on the anti-tumor efficiency of oncolytic adenovirus, the most important field of gene therapy is the analysis of toxicity and transgene biodistribution throughout the body, which is critical for assessing the relative risk to benefit ratio of new preclinical therapies. Several years ago, most clinical trails about safety and toxicity information on systemic oncolytic adenoviruses were obtained with Onyx-015 in different tumor types [28, 29]. To promote the target effect of adenovirus and antitumor effect, more and more gene modified oncolytic adenoviruses were used for various cancers. Recent clinical studies with these more potent oncolytic adenoviruses had indicated that toxicity needs to be monitored carefully when systemic administration is recommended [20, 30, 31]. Though multiple groups have been involved in developing gene therapy strategies for bladder cancer, to date, there are very few published studies of biodistribution following intratumorally injection of bladder specific oncolytic adenovirus.
APU-E1A-AR and APU-E1A are both demonstrated to be anti-tumor efficient for bladder cancer, and APU-E1A-AR is more efficient for AR positive bladder cancer. APU-E1A-AR combines four different genetic modifications to achieve a selective and potent anti-tumor effect. E1A is responsible for adenovirus replication and oncolytic function. The UP II promoter is designed to provide efficient replication and infectivity only in bladder tumor cells and avoid E1A expression and subsequent virus replication in normal cells. The PSCAE insertions are designed to enhance the function of UP II promoter. Fusing gene AR with E1A at downstream of promoter UP II could promote replication of adenovirus in AR positive bladder cancer. The cytopathic effect of APU-E1A, APU-E1A-AR were evaluated in bladder transitional cell carcinoma (TCC) cell lines T24, 5637, BIU87, and EJ cells. We observed apoptosis of bladder cancer cells which were interfered with oncolytic adenovirus by Flow Cytometry and TUNEL, indicating the probable mechanism of cytopathic effect. We also investigated the antitumor effect of recombinant adenovirus for bladder cancer in BALB/c nu/nu mice xenograft model. We had demonstrated that these oncolytic recombinant adenovirus with 5×107 pfu could cause significant inhibition of tumor growth and increased survival of mice in several models of human cancer [13].
In our study, based on the efficacy of bladder specific oncolytic adenovirus APU-E1A-AR and APU-E1A, we determined the toxicity through general health and behavioral assessment, hepatic and hematological toxicity evaluation, macroscopic and microscopic postmortem analyses, as well as the spread of the transgene E1A and adenovirus replication in major organs. Toxicity and efficacy are the main parameters of a pre-clinical study. Although presented separately, these are parts of a unity. So we use mice xerograph tumor model to do safety assessment, and the dose were 5×108 pfu as well as 5×109 pfu to evaluate the toxicicy. Intravesical injection is the most possible way to use in clinical for bladder cancer, so we use oncolytic adenovirus intra-tumorally injection to make the safety assessment.
It is reported that intratumorally administration of oncolytic adenovirus in mice leads to a toxicity characterized by increased serum levels of transaminases, degeneration of liver tissue, and severe weight loss [32, 33]. In this study, we did not get a differential toxicity between high doses of APU-E1A-AR, APU-E1A and PBS at a systemic level, from general health, macroscopic and microscopic postmortem analyses. APU-E1A-AR, APU-E1A did not lead to animal weight loss, however, PBS and APU-Luc gained weight for first 3 weeks, and then had certain weight loss. It is postulated that this change may be due to the consumption function of tumor after 21st day. Behavioral assessment of animals did not reveal any alternation in general behavior, exploration, or locomotion. The lymphocyte percentage in white blood cells and the average level of platelets did not change significantly at different doses of adenovirus. The first dose at which increased serum levels of transaminases could be observed was 5×109 pfu. This dose caused a nearly twofold increase in ALT and AST than was observed with PBS. These results are similar to other results with selectively replicating adenovirus [34, 35]. Hence we could conclude that APU-E1A-AR, APU-E1A at 5 × 107 pfu and 5 × 108 pfu does not lead to the toxicity associated with virus replication.
Real time quantitative PCR is widely used for the detection of viral transgene biodistribution [36]. The adenovirus life cycle is separated by the DNA replication process into two phases: an early and a late phase. E1A is one of the most important early transcriptional genes of adenovirus, it is necessary for adenovirus replication. Hence, detecting E1A mRNA expression with RT-PCR, rather than DNA expression, can indicate not only the existence of adenovirus but also the early transcription and replication state of adenovirus. Accordingly, E1A transgene expression in major perfused organs of mice injected with oncolytic adenovirus APU-E1A-AR and APU-E1A was detected by qRT-PCR. In tumor tisssues, the E1A expression was significantly higher, while outside of tumor tissues, E1A transgene were detected in liver, brain, lung and spleen with very low levels. In other organs, E1A were not disseminated. In western blot assays, E1A protein was only detected in tumor tissue. While in LUC assay for APU-LUC injected mice, although slight LUC expression is also detected in other tissues (background signal of the analytical method), it demonstrated that LUC was much more intensive in tumor and had significant difference comparing with other organs. This was in accord with the previous results. This was expected, as the addition of the UP II and PSCAE sequence should improve the translation of E1A only when the promoter is active in bladder tumor cells. So we can conclude that the adenovirus we constructed has replication specificity only in bladder tumor, and they did not replicate or express in other places, which showed the desired toxicity can be induced and at the same time ensuring the whole safety.
In summary, intratumorally injection of APU-E1A-AR and APU-E1A at dose 5×107 pfu and 5×108 pfu appears safe, with no discernable effects on general health and behavior. This present study provided strong supporting data that complement the ongoing clinical program for APU-E1A-AR and APU-E1A gene therapy for patients with bladder cancer. Future improvements may be pursued by doing long-term toxicity assessment with other kinds of normal animals, such as rat and dogs, complete preclinical toxicology studies including hematologic profiles, metabolism regulation, Ames and in vitro chromosomal mutagenicity studies, viral persistence studies, viral integration studies, teratogenicity studies and clinical trails.
Acknowledgments
This work was supported by grants from NSFC “Surface Project” (81172437) and was supported by the Fundamental Research Funds for the Central Universities (lzujbky-2012-148). The authors thank Yongjie Wu (Institute of Pharmacology, School of Basic Medical Science, Lanzhou University) for technical assistance, Wenhui Zhang (Laboratory Center for Medical Science, Lanzhou University) for assistance with clinical hepatic chemistry and hematological analysis, Baoguang Shi (pathology department in Second Hospital of Lanzhou University) for assistance with histopathological and immunohistochemistry analysis and Jing-Cheng (Laboratory Center for Medical Science, Lanzhou University) for assistance with qRT-PCR experiments.
Footnotes
Conflict of Interest Statement: None declared.
References
- 1.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 2.Ghoneim MA, Abol-Enein H. Management of muscle-invasive bladder cancer: an update. Nat Clin Pract Urol. 2008;5:501–8. doi: 10.1038/ncpuro1202. [DOI] [PubMed] [Google Scholar]
- 3.ABCM-a C. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet. 2003;361:1927–34. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 4.Hussain SA, James ND. The systemic treatment of advanced and metastatic bladder cancer. Lancet Oncol. 2003;4:489–97. doi: 10.1016/s1470-2045(03)01168-9. [DOI] [PubMed] [Google Scholar]
- 5.Karamouzis MV, Argiris A, Grandis JR. Clinical applications of gene therapy in head and neck cancer. Curr Gene Ther. 2007;7:446–57. doi: 10.2174/156652307782793487. [DOI] [PubMed] [Google Scholar]
- 6.Kaur B, Cripe TP, Chiocca EA. “Buy one get one free”: armed viruses for the treatment of cancer cells and their microenvironment”. Curr Gene Ther. 2009;9:341–55. doi: 10.2174/156652309789753329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody SL, Crystal RG. Adenovirus-mediated in vivo gene transfer. Ann N Y Acad Sci. 1994;716:90–101. doi: 10.1111/j.1749-6632.1994.tb21705.x. discussion 101-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Ramesh N, Chen Y, et al. Identification of human uroplakin II promoter and its use in the construction of CG8840, a urothelium-specific adenovirus variant that eliminates established bladder tumors in combination with docetaxel. Cancer Res. 2002;62:3743–50. [PubMed] [Google Scholar]
- 9.He XD, Wang ZP, Wei HY, et al. Construction of urothelium-specific recombinant adenovirus and its inhibition in bladder cancer cell. Urol Int. 2009;82:209–13. doi: 10.1159/000200802. [DOI] [PubMed] [Google Scholar]
- 10.Latham JP, Searle PF, Mautner V, et al. Prostate-specific antigen promoter/enhancer driven gene therapy for prostate cancer: construction and testing of a tissue-specific adenovirus vector. Cancer Res. 2000;60:334–41. [PubMed] [Google Scholar]
- 11.Wang D, Wang Z, Tian J, et al. Prostate stem cell antigen enhancer and uroplakin II promoter based bladder cancer targeted tissue-specific vector. Urol Oncol. 2008;28:164–9. doi: 10.1016/j.urolonc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Hoti N, Li Y, Chen CL, et al. Androgen receptor attenuation of Ad5 replication: implications for the development of conditionally replication competent adenoviruses. Mol Ther. 2007;15:1495–503. doi: 10.1038/sj.mt.6300223. [DOI] [PubMed] [Google Scholar]
- 13.Zhai Z, Wang Z, Fu S, et al. Antitumor effects of bladder cancer-specific adenovirus carrying E1A-androgen receptor in bladder cancer. Gene Ther. 2012 doi: 10.1038/gt.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemminki A. From molecular changes to customised therapy. Eur J Cancer. 2002;38:333–8. doi: 10.1016/s0959-8049(01)00368-9. [DOI] [PubMed] [Google Scholar]
- 15.Burke J. Virus therapy for bladder cancer. Cytokine Growth Factor Rev. 2010;21:99–102. doi: 10.1016/j.cytogfr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Ganly I, Kirn D, Eckhardt G, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 17.Jiang H, Gomez-Manzano C, Lang FF, et al. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther. 2009;9:422–7. doi: 10.2174/156652309789753356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freytag SO, Stricker H, Peabody J, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007;15:636–42. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- 19.Freytag SO, Khil M, Stricker H, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–76. [PubMed] [Google Scholar]
- 20.Small EJ, Carducci MA, Burke JM, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–17. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.DeWeese TL, van der Poel H, Li S, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–72. [PubMed] [Google Scholar]
- 22.Terao S, Shirakawa T, Kubo S, et al. Midkine promoter-based conditionally replicative adenovirus for targeting midkine-expressing human bladder cancer model. Urology. 2007;70:1009–13. doi: 10.1016/j.urology.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Ramesh N, Ge Y, Ennist DL, et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor--armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. 2006;12:305–13. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda S, Fujiwara T, Shirakawa Y, et al. Telomerase-dependent oncolytic adenovirus sensitizes human cancer cells to ionizing radiation via inhibition of DNA repair machinery. Cancer Res. 2010;70:9339–48. doi: 10.1158/0008-5472.CAN-10-2333. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Gao L, Yeagy B, et al. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–9. [PubMed] [Google Scholar]
- 26.Ramesh N, Memarzadeh B, Ge Y, et al. Identification of pretreatment agents to enhance adenovirus infection of bladder epithelium. Mol Ther. 2004;10:697–705. doi: 10.1016/j.ymthe.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Satoh M, Abe H, et al. Oncolytic viral therapy by bladder instillation using an E1A, E1B double-restricted adenovirus in an orthotopic bladder cancer model. Urology. 2006;68:674–81. doi: 10.1016/j.urology.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Hamid O, Varterasian ML, Wadler S, et al. Phase II trial of intravenous CI-1042 in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:1498–504. doi: 10.1200/JCO.2003.09.114. [DOI] [PubMed] [Google Scholar]
- 29.Nemunaitis J, Cunningham C, Buchanan A, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–59. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 30.Toth K, Djeha H, Ying B, et al. An oncolytic adenovirus vector combining enhanced cell-to-cell spreading, mediated by the ADP cytolytic protein, with selective replication in cancer cells with deregulated wnt signaling. Cancer Res. 2004;64:3638–44. doi: 10.1158/0008-5472.CAN-03-3882. [DOI] [PubMed] [Google Scholar]
- 31.Working PK, Lin A, Borellini F. Meeting product development challenges in manufacturing clinical grade oncolytic adenoviruses. Oncogene. 2005;24:7792–801. doi: 10.1038/sj.onc.1209045. [DOI] [PubMed] [Google Scholar]
- 32.Engler H, Machemer T, Philopena J, et al. Acute hepatotoxicity of oncolytic adenoviruses in mouse models is associated with expression of wild-type E1a and induction of TNF-alpha. Virology. 2004;328:52–61. doi: 10.1016/j.virol.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 33.Shayakhmetov DM, Gaggar A, Ni S, et al. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–91. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cascallo M, Alonso MM, Rojas JJ, et al. Systemic toxicity-efficacy profile of ICOVIR-5, a potent and selective oncolytic adenovirus based on the pRB pathway. Mol Ther. 2007;15:1607–15. doi: 10.1038/sj.mt.6300239. [DOI] [PubMed] [Google Scholar]
- 35.Tsukuda K, Wiewrodt R, Molnar-Kimber K, et al. An E2F-responsive replication-selective adenovirus targeted to the defective cell cycle in cancer cells: potent antitumoral efficacy but no toxicity to normal cell. Cancer Res. 2002;62:3438–47. [PubMed] [Google Scholar]
- 36.Lovatt A. Applications of quantitative PCR in the biosafety and genetic stability assessment of biotechnology products. J Biotechnol. 2002;82:279–300. doi: 10.1016/S1389-0352(01)00043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


