Abstract
Given the functional importance of the endoplasmic reticulum (ER), an organelle that performs folding, modification, and trafficking of secretory and membrane proteins to the Golgi compartment, the maintenance of ER homeostasis in insulin-secreting β-cells is very important. When ER homeostasis is disrupted, the ER generates adaptive signaling pathways, called the unfolded protein response (UPR), to maintain homeostasis of this organelle. However, if homeostasis fails to be restored, the ER initiates death signaling pathways. New observations suggest that both chronic hyperglycemia and hyperlipidemia, known as important causative factors of type 2 diabetes (T2D), disrupt ER homeostasis to induce unresolvable UPR activation and β-cell death. This review examines how the UPR pathways, induced by high glucose and free fatty acids (FFAs), interact to disrupt ER function and cause β-cell dysfunction and death.
Keywords: unfolded protein response, ER stress, free fatty acid, glucose, pancreatic β-cell
INTRODUCTION
Modern lifestyles, characterized by the overconsumption of energy-rich foods and reduced physical activity, have dramatically increased the frequency of type 2 diabetes (T2D). T2D is a major cause of morbidity and mortality, decreasing both the quality of life and life expectancy. In 2010, diabetes affected about 285 million people worldwide and is expected to increase by 1.5 fold (439 million people) by 2030 (1). Between 2010 and 2030, there will be a 69% increase in the number of adults with diabetes in developing countries and a 20% increase in developed countries. T2D is a complex heterogeneous group of metabolic conditions characterized by increased levels of blood glucose owing to insulin resistance in adipose tissue, muscle, and liver and/or to impaired insulin secretion from pancreatic β-cells (2). Obesity is mechanistically linked to insulin resistance and T2D. Although both obesity and insulin resistance are associated with T2D, the disease only develops in insulin-resistant subjects with the onset of β-cell dysfunction (3). To adapt to the increased metabolic load caused by obesity and insulin resistance, normal pancreatic islets usually respond by increasing β-cell mass through an increase in β-cell proliferation and neogenesis (4) as well as by enhancing β-cell function (5). However, β-cell adaptation eventually fails as a consequence of genetic and environmental factors that cause a progressive decline in β-cell function and survival. As a consequence, individuals progress from normal glucose tolerance to impaired glucose tolerance and then to established T2D with reduced β-cell mass (3). Although the molecular mechanisms underlying β-cell failure/death remain to be clarified, recent studies suggest that β-cell loss in T2D results from endoplasmic reticulum (ER) stress responses induced by gluco/lipotoxicity (6) and amyloid accumulation (7). In this review, we examine the mechanisms behind UPR-mediated pancreatic cell death with specific attention to the connection between ER stress and T2D.
PANCREATIC β-CELL AND THE ENDOPLASMIC RETICULUM
The islets of Langerhans constitute ~2% of the total pancreatic mass, and each islet is composed of ~2,000 cells of which insulin-secreting β-cells constitute ~60% of the total (8). Insulin, a blood glucose-lowering hormone, is only secreted by the pancreatic β-cells of the islets of Langerhans. After proinsulin is synthesized in the ER, it is processed into its biologically active form and stored in secretory granules. When blood glucose levels increase, insulin is released from storage granules to maintain a normal blood glucose level (9). The β-cell contains a large pool of cytoplasmic proinsulin mRNA (~20% of the total mRNA), one of the most abundant mRNA species (10). One profound example of physiological fluctuation in the protein-folding load in the ER is the unique translational response of pancreatic β-cells to variations in blood glucose (11). Blood glucose level changes lead to a maximum 25-fold increase in proinsulin synthesis, and ~1 × 106 proinsulin molecules are synthesized per minute upon glucose stimulation (12). Therefore, proinsulin synthesis imposes a heavy biosynthetic burden upon the β-cell.
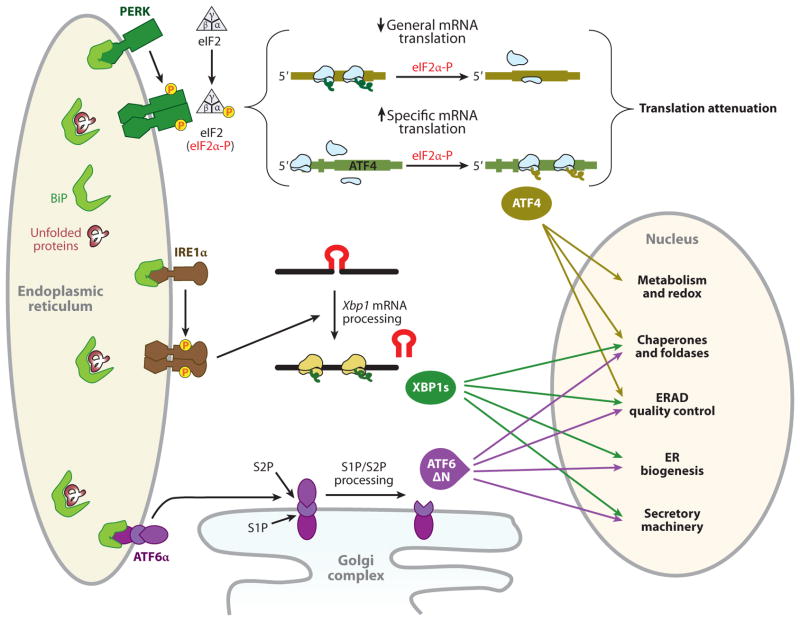
The ER is the first station of the secretory pathway and the site of synthesis for proteins resident in the ER or destined for the Golgi compartment, endosomes, lysosomes, the plasma membrane, or the extracellular milieu (13). It also serves as a site of biosynthesis for steroids, cholesterol, other lipids, and Ca2+ storage, and also as a signaling platform between the nucleus and cytosol (14). Efficient ER function relies on numerous resident quality control factors, such as molecular chaperones and folding enzymes, a high level (several hundred micromolars) of Ca2+, and an oxidative environment. Nascent polypeptides that are translocated into the ER lumen undergo posttranslational modifications and rounds of folding interactions required for optimal function. Properly folded proteins exit the ER and progress through the secretory pathway, whereas irreparably unfolded or misfolded proteins are retro-translocated from the ER and degraded by cytoplasmic proteasomes (15). The flux of nascent polypeptides into the ER is variable because it changes rapidly in response to the physiological state and environmental conditions of the cell. To handle this dynamic situation, cells must adjust their ER protein-folding capacity according to environmental and physiological contexts, thereby ensuring that the quality of membranous and secreted proteins is maintained with high fidelity. Given the importance of ER function for normal cellular function, it is not surprising that altered ER homeostasis affects a diverse number of cellular processes, including transcription, translation, cell cycle control, intracellular signaling, and programmed cell death. When ER homeostasis is altered, signaling pathways are activated, eliciting an adaptive response that is collectively called the unfolded protein response (UPR). The UPR includes expansion of ER size, enhanced folding capacity, reduced protein synthesis through transcriptional and translational controls, and increased clearance of unfolded or misfolded proteins (16). When these mechanisms fail to restore ER homeostasis, cell death signaling pathways are activated (17). There are three transmembrane ER-proximal sensors of unfolded proteins that initiate UPR signaling in mammals: inositol-requiring protein 1 (IRE1), activating transcription factor 6 (ATF6), and PKR-like ER kinase (PERK); all three are present in all cell types (Figure 1). These three main UPR sensors are integral membrane proteins that sense the unfolded protein loaded in the ER lumen and transmit this information across the ER membrane to the cytosol. Considerable progress has been made in our understanding of the signaling pathways and the pathophysiological significance of the UPR. The goal of this review is to summarize our current insight by considering the role of the UPR in T2D as a disease in which there is a structural and pathological change in β-cells.
Figure 1.
The adaptive unfolded protein response (UPR). Activation of three UPR pathways initiates the adaptive endoplasmic reticulum (ER) stress response. During activation of the UPR in mammals, BiP (immunoglobulin heavy chain binding protein, also known as GRP78) is sequestered through binding to unfolded or misfolded polypeptide chains, thereby leading to BiP release from the ER stress sensors for their activation. Unconventional cytoplasmic splicing, mediated by IRE1α, removes a 26-nucleotide intron from unspliced X-box-binding protein 1 (Xbp1) mRNA (encoding 267 amino acids) to produce a translational frameshift, yielding a fusion protein encoded from two evolutionarily conserved open reading frames (16). The fusion protein, XBP1s, acts as a potent transcription factor for expression of UPR target genes involved in protein folding and export from the ER, export and degradation of misfolded proteins, and lipid biosynthesis, to resolve ER stress (16). Upon accumulation of unfolded protein in the ER lumen, oligomerization of the PKR-like ER kinase (PERK) in ER membranes induces its autophosphorylation and kinase domain activation (141, 142). Activated PERK phosphorylates serine 51 on the α-subunit of heterotrimeric eIF2 (143). When eukaryotic translation initiation factor 2α (eIF2α) is phosphorylated, the eIF2 complex shows increased affinity for its guanine nucleotide exchange factor eIF2B and sequesters all available eIF2B. Because the cellular level of eIF2B is 10- to 20-fold lower than the level of eIF2, very small changes in eIF2α phosphorylation can dramatically change the rate of translation initiation (144). Inhibition of general mRNA translation by the phosphorylation of eIF2α reduces accumulation of misfolded protein in the ER lumen (22), thereby protecting the cell from diverse stimuli that perturb the ER homeostasis. In contrast to inhibition of general mRNA translation, the PERK/eIF2α pathway stimulates the translation of several specific mRNAs containing multiple 53-upstream open reading frames, such as Atf4 and Atf5, Chop, Gadd34, and the cationic amino acid transporter 1 (Cat-1, an Na+-independent transporter of L-arginine and L-lysine) (28, 145). Among them, ATF4 activates transcription of the adaptive genes that encode functions in ER protein folding, endoplasmic reticulum–associated degradation (ERAD), amino acid biosynthesis and transportation, and the antioxidative stress response (24). Under ER stress, ATF6α and ATF6βare released from BiP and translocate to the Golgi complex, where they are cleaved by Golgi-resident proteases, first by S1P (site 1 protease) and then in the intramembrane region by S2P (site 2 protease), to release the N-terminal basic leucine zipper protein (bZIP) transcription factor domain (16). The bZIP domain of ATF6α then translocates into the nucleus, where it activates the transcription of genes encoding ER-localized molecular chaperones and folding enzymes, ERAD, protein secretion machineries, and ER biogenesis (146), in some cases in cooperation with XBP1s (42).
SIGNALING FROM TRANSMEMBRANE SENSORS OF THE UNFOLDED PROTEIN RESPONSE
Inositol-Requiring Protein 1–Mediated Signaling Pathways
There are two mammalian homologs of yeast Ire1p: IRE1α, expressed in most cells and tissues with high levels of mRNA expression in the pancreas (18), and IRE1β, mainly expressed in the epithelium of the gastrointestinal tract (19). IRE1α, the most fundamental ER stress sensor, is highly conserved in all eukaryotic cells and is well studied (16). The luminal domain of the IRE1α protein responds to the accumulation of unfolded proteins in the ER and undergoes kinase activation, which triggers a specific endoribonuclease activity in its cytoplasmic domain, where initiation of splicing of the mRNA encoding X-box binding protein 1 (XBP1) occurs (20). β-Cell-specific deletion of Xbp1 causes β-cell failure in a mouse model, suggesting that IRE1α-XBP1 signaling is essential for β-cell function (21). Figure 1 provides a schematic view of the IRE1α-mediated signaling pathway, including the other UPR pathways.
PKR-Like Endoplasmic Reticulum Kinase-Mediated Signaling Pathways
PERK, a type I transmembrane protein located in the ER, has serine/threonine kinase activity in its cytoplasmic domain (Figure 1) (22, 23). The catalytic domain of PERK shares substantial homology with other eIF2α family kinases (GCN2, HRI, and PKR) (24). Approximately half of Perk-null mice, a mouse model of the human genetic disease Wolcott-Rallison syndrome (25), die pre- or postnatally. The surviving half of Perk-null mice gradually develop a multitude of metabolic and growth abnormalities, including hyperglycemia, growth retardation, skeletal defects, and atrophy of the exocrine and endocrine pancreas (26, 27). Different from Perk-deficient mice, all eIF2α A/A homozygous neonates die within 24 h after birth and suffer from hypoglycemia, possibly owing to defective gluconeogenesis [low phosphoenolpyruvate carboxykinase (PEPCK) activity], reduced glycogen storage in the liver, and a deficiency of pancreatic β-cells (28). For β-cell function, PERK activity is required to prevent an abnormal increase in insulin translation in islet cells responding to a high-glucose load (26), but eIF2α phosphorylation is required to control insulin translation in response to both low- and high-glucose conditions (29). Furthermore, it seems that PERK through eIF2α phosphorylation is a positive regulator of ER chaperone and ER-associated degradation (ERAD) function and thereby contributes to ER and Golgi anterograde trafficking, retrotranslocation from the ER to the cytoplasm, and proteasomal degradation (29–31). Therefore, Perk and/or eIF2α phosphorylation-deficient β-cells show retention of misfolded proinsulin in the ER lumen and defective trafficking of proinsulin, and thereby a reduced number of insulin granules in β-cells, indicating that the mutant β-cells experience ER stress, accompanied by increased cell death, leading to progressive diabetes.
In pancreatic β-cells, the extracellular glucose level modulates the activity of the UPR sensors. PERK phosphorylation is differentially regulated by glucose in the β-cell. In β-cell, eIF2α phosphorylation is gradually decreased with the increase of glucose levels. Its phosphorylation inversely correlates with the rate of proinsulin synthesis (32). However, both low blood glucose and chronic high blood glucose activate eIF2α phosphorylation. Chronically high-glucose concentrations stimulate proinsulin transcription and translation. As a consequence, it is believed that proinsulin synthesis overcomes the ER folding machinery, leading to PERK activation to reduce protein influx into the ER (33). However, there remains some controversy whether chronically high-glucose exposure (more than 18 h) actually causes severe ER stress, activating PERK (33, 34). Yet glucose stimulation of β-cells growing in acute high glucose causes eIF2α dephosphorylation, likely through a protein phosphatase 1 (PP1)-like phosphatase (32) that dephosphorylates eIF2α. Although this kinase/phosphatase model can easily explain the changes in eIF2α phosphorylation in response to glucose, it is not known how PP1 is regulated under these conditions. The kinase responsible for low-glucose eIF2α phosphorylation has not been identified (31). It is most likely that PERK is the kinase that phosphorylates eIF2α in low glucose. This is supported by studies from several groups, including Gomez and colleagues (35). Moreover, Gomez et al. (35) propose that PERK may sense levels of cellular ATP/energy in pancreatic β-cells. It has been shown that PERK, but not IRE1α, is activated by a decrease in glucose concentration or intracellular energy level induced by mitochondrial inhibitors (35). Therefore, it is possible that PERK in pancreatic β-cells is also activated by a mechanism independent of IRE1α activation or by the unfolded protein accumulation. It was also reported that a decrease in glucose concentration leads to a concentration-dependent reduction in ER Ca2+ that parallels the activation of PERK and the phosphorylation of eIF2α. It was proposed that an ER Ca2+ decrease is caused by a decrease in SERCA activity, mediated by a reduction in its cell energy status (154). However, this study did not suggest a precise mechanism that described why IRE1α is not activated by an ER Ca2+ decrease, which is induced by low glucose, although it is possible that PERK and IRE1α may have different thresholds for activation in response to a decrease in ER Ca2+. Clearly, further studies are required to elucidate the precise molecular mechanisms involved in energy/glucose-dependent regulation of eIF2α phosphorylation and its biological meaning.
It has been suggested that the cytosolic function of PERK is also controlled by P58IPK, first identified as a PKR inhibitor (36, 37). A more recent study (38), however, suggested that P58IPK localizes mainly to the ER lumen and functions as a molecular cochaperone for BiP in the ER lumen. Therefore, if P58IPK is a major regulator of PERK function, it is likely through some chaperone function (38). Thus, the precise inactivation mechanism of PERK remains to be clarified.
Activating Transcription Factor 6–Mediated Signaling Pathways
ATF6 encodes a basic leucine zipper protein (bZIP)-containing transcription factor localized to the ER membrane (39). Upon accumulation of unfolded protein in the ER, ATF6 traffics to the Golgi complex, where it is cleaved by site 1 and site 2 proteases, S1P and S2P, in a process called regulated intramembrane proteolysis. In mammals, there are two Atf6 genes, Atf6α and Atf6β/creb-rp/g13 (40). Figure 1 provides a schematic view of the ATF6-mediated signaling pathway. The deletion of Atf6α resulted in increased sensitivity to chronic stress in mice challenged with chemical ER stressers, and it was suggested that ATF6α is required to optimize protein folding, secretion, and degradation during ER stress and thus facilitates recovery from acute stress and confers tolerance to chronic stress (41, 42). Unlike ATF6α, the role(s) for ATF6β is still unknown. Moreover, ATF6β (like ATF6α) is dispensable in embryonic and postnatal development, and a deficiency of ATF6β does not alter UPR gene induction (42). However, the double knockout of Atf6α and Atf6β causes early embryonic lethality, suggesting that ATF6α and ATF6β possess at least overlapping functions that are essential for mouse development. ATF6α and ATF6β are ubiquitously expressed (40). However, in recent years, several tissue-specific bZIP transcription factors located in the ER membrane and regulated by regulated intramembrane proteolysis have been identified: CREBH in hepatocytes, the pyloric stomach, and small intestine (43); OASIS in astrocytes (44); BBF2H7/CREB3L2 in most tissues (45); Tisp40 (transcript induced in spermatogenesis) in testis (46); Luman/LZIP/CREB3 in most tissues (47, 48); and CREB4 possibly in most tissues (49). The discovery of these numerous regulated intramembrane proteolysis-activated transcription factors raises a question: Why have cells evolved multiple ATF6-like molecules in the ER? These ATF6-like molecules might be evolutionally chosen to respond to specific conditions of ER stress that can occur in different tissues to activate tissue-specific expression of genes to resolve ER stress. Further studies are required to answer these issues.
Wolfram syndrome (which is caused by mutations in the Wfs1 gene, encoding wolframin) is a disorder of progressive neurodegeneration and β-cell failure leading to diabetes. Wolframin is a transmembrane ER protein involved in preventing ER stress signaling. Recently, Fonseca et al. (50) reported that WFS1 negatively regulates ATF6α through the ubiquitin-proteasome pathway during ER stress. In the absence of ER stress or in late stages of ER stress, WFS1 may limit ER stress by recruiting ATF6α to the E3 ubiquitin ligase, HRD1, for ubiquitination-mediated degradation of ATF6α. Therefore, Wfs1-null murine pancreata display higher levels of ATF6α protein and lower levels of Hrd1 protein compared with those in the control littermates. Additionally, another study suggested that the ectopic overexpression of an active form of ATF6α in β-cells causes apoptosis (51). These results imply that dysregulated ER stress signaling through hyperactivation of ATF6α is one pathogenic pathway for diseases involving chronic, unresolvable ER stress, such as pancreatic β-cell death in diabetes. Therefore, understanding the role of ATF6α in diabetic mouse models is an important step toward understanding the physiological roles of ATF6α in pancreatic β-cells.
UNFOLDED PROTEIN RESPONSE-MEDIATED CELL DEATH
Activation of each arm of the UPR initiates adaptive mechanisms to relieve the stresses accumulating in the ER. The adaptive UPR responses, signaled through activating transcription factor 4 (ATF4), cleaved ATF6α, and spliced XBP1, include inhibition of general mRNA translation by rapid PERK-mediated phosphorylation of eIF2α as well as induction of genes to increase the ER protein folding capacity and remove misfolded proteins from the ER. In 2006, it was recognized that preemptive quality control may also reduce the burden of misfolded substrates entering the ER by inhibiting translocation of many, but not all, polypeptides into the ER lumen (52). Thus, the adaptive pathways maintain cellular function and avoid apoptosis during ER stress. However, if ER stress is severe and chronic, the UPR-mediated efforts to correct the protein-folding defect fail, and then several apoptotic pathways are activated (53). Although both mitochondrial-dependent and -independent cell death pathways (17) execute apoptosis in response to ER stress, it is proposed that the ER serves as an important compartment where apoptotic signals are generated and integrated to elicit cell death in response to the unresolvable accumulation of unfolded proteins. Below, we briefly describe two UPR sensor-mediated cell death pathways.
PERK-Mediated Cell Death Pathways
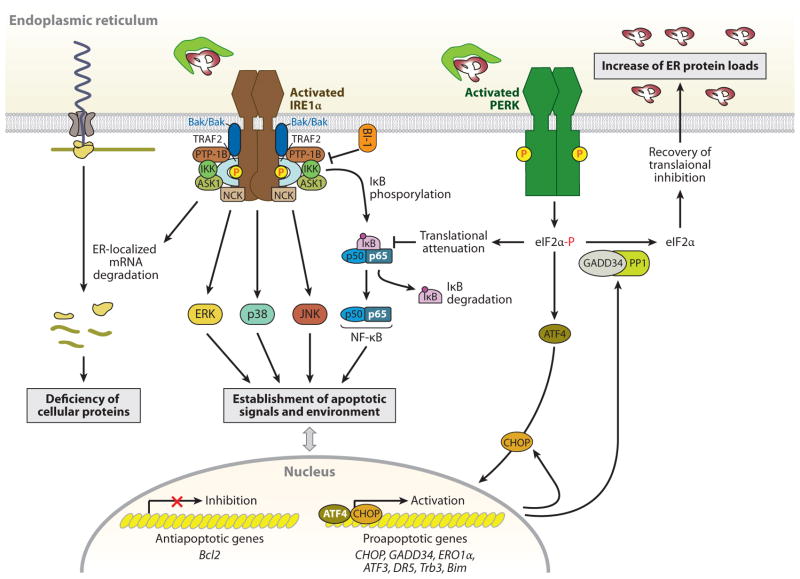
During unresolvable ER stress, sustained activation of the PERK pathway also induces the proapoptotic pathway, similar to IRE1α (Figure 2). There are no reports that show PERK association with adaptors or modulators involves apoptosis. The persistent phosphorylation of eIF2α by PERK increases proapoptotic CHOP/GADD153 (C/EBP homology protein/growth arress and DNA damage 153) expression through the transcription factor ATF4. CHOP is a member of the C/EBP family of bZIP transcription factors (54). The major inducers of the UPR (ATF4, ATF6, and XBP1) regulate Chop through both an ER stress response element and a C/EBP-ATF composite site (55–57). Perk- and Atf4-null cells, as well as eIF2α (S51A) knock-in cells, fail to induce CHOP (28, 58, 59), whereas IRE1α-, Xbp1-, and Atf6α-null cells or mouse liver tissues show increased and/or persistent Chop expression during ER stress (41, 42, 60, 61), suggesting that the PERK-eIF2α-ATF4 pathway is the main contributor toward ER stress-dependent CHOP expression. The deletion of the murine Chop gene attenuates ER stress-induced cell death in cultured fibroblasts and partially protects mice from renal toxicity owing to pharmacological induction of ER stress by tunicamycin (62). Furthermore, Chop deletion (a) prevents neuronal apoptosis induced by ischemia (63) and neuronal oxidative injury in a model of Parkinson’s disease (64) and (b) protects the murine liver from ER stress and oxidative damage induced from clotting factor VIII expression (65), which is prone to mis-folding and pancreatic β-cell death resulting from either accumulation of misfolded mutant proinsulin or exposure to nitric oxide (66, 67). Chop deletion even promotes β-cell survival in multiple diabetic mouse models induced by a leptin receptor deficiency or a high-fat diet treatment with haplo-insufficiency of eIF2α phosphorylation (68). The mechanism by which CHOP-mediated apoptosis occurs in response to ER stress is not well established. It was suggested that overexpression of CHOP decreases expression of the antiapoptotic Bcl-2 protein, depletes cellular glutathione, and exaggerates production of reactive oxygen species (ROS) (69, 70). However, a study of Chop-deleted cells revealed a different view of CHOP-mediated cell death. CHOP directly activates Gadd34, which promotes ER client protein biosynthesis by dephosphorylating eIF2α in stressed cells, and causes further accumulation of high-molecular-weight detergent-resistant stress-associated ER complexes in the ER (Figure 2) (70). Therefore, impaired GADD34 expression reduces client protein load and ER stress in Chop-null cells exposed to ER stress. The Chop- and Gadd34-null mutant cells accumulate fewer high-molecular-weight protein complexes in their stressed ER than wild-type cells. Furthermore, ERO1α (ER oxidoreductase a), α direct CHOP target gene, causes hyperoxidation of the ER to increase abnormal high-molecular-weight protein complexes. Thus, CHOP may disrupt ER function by promoting protein synthesis and oxidation.
Figure 2.
IRE1α- and PERK-mediated cell death pathways. During endoplasmic reticulum (ER) stress, inositol-requiring protein 1α (IRE1α) forms a hetero-oligomeric complex with TNF receptor-associated factor 2 (TRAF2) (76) and apoptosis signal-regulating kinase 1 (ASK1) (77) and then recruits the protein kinase JNK, leading to the activation of JNK (76). The IRE1α-TRAF2 complex recruits IκB kinase (IKK), which phosphorylates inhibitor of κB (IκB), leading to the degradation of IκB and the nuclear translocation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) (147). IRE1α also modulates the activation of other “alarm genes,” such as p38 and ERK (80), possibly by the binding of the Src homology (SH) 2/3 -containing adaptor proteins Nck and TRAF2, respectively. Furthermore, several proapoptotic (i.e., BAX/BAK, AIP1, and maybe PTP-1B) or antiapoptotic proteins (i.e., BI-1) interact with IRE1α, regulating its activation state (81–84). Thus, the formation of a macromolecular signaling complex of IRE1α with several proapoptotic proteins can generate apoptotic signals and establish an apoptotic environment. In addition, the endoribonuclease activity of IRE1α, aside from specific cleavage of Xbp1 mRNA, degrades ER-targeted mRNAs that can decrease cellular functions, such as proinsulin synthesis in β-cells. In contrast to the adaptive response by the PERK-phosphorylated eIF2α-ATF4 pathway, this pathway also contributes to stress-induced cell death by ATF4-mediated induction of proapoptotic genes, including CHOP, ATF3, and GADD34 (16). The induced transcription factor CHOP contributes to increased expression of the proapoptotic factors, such as death receptor 5 (DR5) (148), tribbles-related protein 3 (Trb3) (149), and binding to microtubule (Bim) (150), and it can suppress B cell lymphoma 2 (Bcl2) expression. Bim is also activated through protein phosphatase 2A-mediated dephosphorylation, which prevents its ubiquitination and proteasomal degradation (150). PERK-mediated translational attenuation upregulates NF-κB-dependent transcription because IκB has a shorter half-life than NF-κB, so NF-κB is released to translocate to the nucleus (151). Recovery from translational repression is mediated by eIF2α dephosphorylation by the two regulatory subunits of protein phosphatase 1 (PP1), GADD34 and CReP (constitutive repressor of eIF2α phosphorylation) (16). GADD34 is induced transcriptionally during ER stress by ATF4, whereas CreP is a constitutive activator of PP1. The premature dephosphorylation of eIF2α by the GADD34-PP1 complex restores translation of general mRNAs, which may be detrimental if the ER protein-folding defect is not resolved.
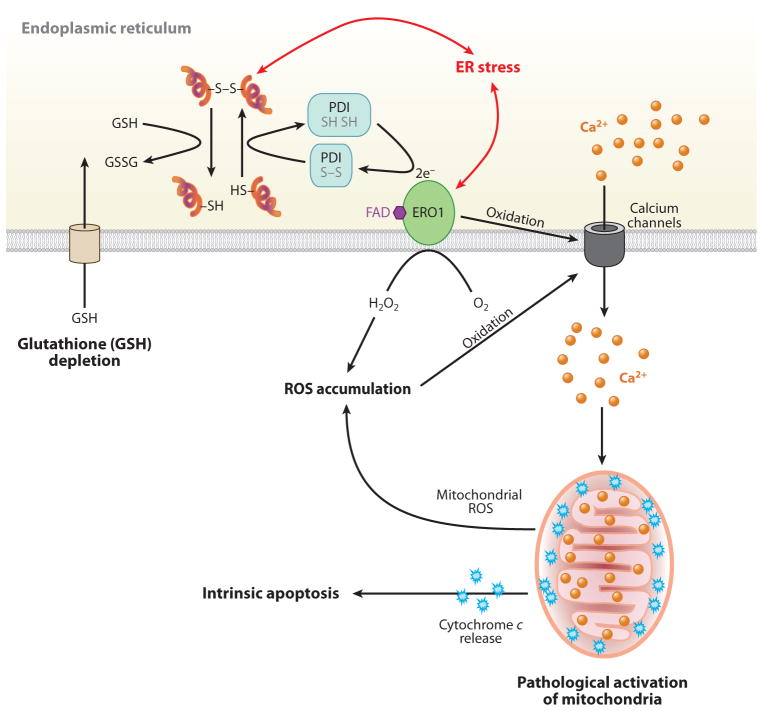
Increasing evidence suggests that an accumulation of misfolded proteins in the ER lumen generates ROS (65, 71, 72). Formation of incorrect intermolecular and/or intramolecular disulfide bonds depletes glutathione that is required for their reduction. Disulfide bond formation in the ER is ushered in by the oxidative folding pathway, catalyzed by protein disulfide isomerase (PDI) and ERO1-mediated oxidation of substrate polypeptides, that produces ROS (Figure 3). For example, overexpression of a misfolded protein CPY (yeast vacuolar protein carboxypeptidase Y) activates the UPR, causes oxidative stress, and induces apoptosis. However, removal of all cysteine residues in CPY reduced the UPR, oxidative stress, and cell death (73). Furthermore, in macrophages, cholesterol and ER stress inducers oxidize inositol-1,4,5-trisphosphate (IP3) receptors in the ER membrane to release Ca2+ and activate Ca2+/calmodulin-dependent protein kinase IIγ (CaMKIIγ) (74). This study suggested also that there are three CaMKIIγ-mediated apoptotic pathways: (a) CaMKIIγ-mediated c-Jun N-terminal kinase ( JNK) activation; (b) CaMKIIγ-increased mi-tochondrial Ca2+; and (c) CaMKIIγ activation of STAT1, a proapoptotic signal transducer (74). These hypotheses are supported by the finding that either Chop or Ero1α deletion in yeast and mice reduces ROS accumulation and protects from ER stress-mediated cell death.
Figure 3.
The role of calcium and reactive oxygen species (ROS) in endoplasmic reticulum (ER) stress-mediated cell death. In the ER lumen, oxidative protein folding is catalyzed by protein disulfide isomerase (PDI) and ER oxidoreductase (ERO1). In this reaction, an oxidant flavin adenine dinucleotide (FAD)-bound ERO1 oxidizes PDI, which subsequently oxidizes folding proteins directly. FAD-bound ERO1 then passes two electrons to molecular oxygen, resulting in the production of hydrogen peroxide (73). During unfolded protein response activation, CHOP-mediated induction of ERO1α hyperoxidizes the ER lumen and causes oxidation-induced activation of the ER Ca2+ release channel inositol 1,4,5-trisphosphate receptor (152), causing a large and transient release of Ca2+ from the ER. Increased cytosolic Ca2+ is taken up into the mitochondrial matrix, and this stimulates mitochondrial ROS production through disruption of mitochondrial electron transport (153). High levels of ROS generated from mitochondria, in turn, further increase Ca2+ release from the ER. The increase in mitochondrial Ca2+ eventually dissociates cytochrome c from the inner membrane cardiolipin, which triggers permeability transition pore opening and cytochrome c release across the outer membrane. Now the vicious cycle of ER calcium release and mitochondrial ROS production activates cytochrome c-mediated apoptosis. In addition, ER stress may cause consumption of excessive cellular glutathione (GSH) because reduced GSH may also assist in reducing nonnative disulfide bonds in misfolded proteins, resulting in the production of oxidized glutathione (GSSG).
Recent studies demonstrated significant increases in ER stress marker proteins P58IPK, BiP, and CHOP in islets in tissue sections from obese diabetic individuals (T2D patients) (75). Additionally, analysis of leptin receptor-deficient (Leprdb/db) mice, as well as other murine models of T2D, revealed that insulin resistance increases proinsulin synthesis in β-cells beyond the capacity for folding of nascent polypeptides within the ER lumen, thereby disrupting ER homeostasis and triggering the UPR (68). The pancreatic β-cells in the Leprdb/db mice displayed slightly increased expression of several UPR genes encoding adaptive functions to improve ER folding capacity, such as BiP, Grp94, Fkbp11, and P58IPK , as well as accumulated oxidative damage. By contrast, the deletion of Chop gene increased expression of UPR and oxidative stress response genes and reduced levels of oxidative damage, such as products of protein oxidation (carbonyls) and lipid peroxidation (hydroxyoctadecadienoic acid). These findings suggest that CHOP can be a fundamental factor linking protein mis-folding in the ER to oxidative stress and apop-tosis in β-cells under conditions of increased insulin demand, such as in T2D. Therefore, small molecules that modulate the expression or transcriptional activity of CHOP may be useful to alleviate ER stress-mediated cell death.
IRE1α-Mediated Cell Death Pathways
Studies have suggested that the IRE1α/ TRAF2/ASK1 complex promotes apoptosis (Figure 2) through JNK phosphorylation (76). Although polyglutamine aggregates are typically cytoplasmic, it was suggested that polyglutamine inhibits the proteasome and induces ER stress. It was demonstrated that Ask1-null primary neurons are resistant to ER stress-induced cell death (77). Activated ASK1 leads to JNK-mediated phosphorylation and activation of the proapoptotic protein Bim (78), but inhibits the antiapoptotic protein Bcl-2 (79). Furthermore, a cell-based chemical library screen identified compounds that enhance phosphorylation of serine 967 of ASK1, promoting 14-3-3 protein binding and thereby suppressing ASK1 function (80). The deficiency of ER-localized proapoptotic Bcl-2 family members BAX and BAK (81), ASK1-interacting protein 1 (AIP1) (82), or protein tyrosine phosphatase-1B (PTP-1B) (83) in cells and mice impairs IRE1α activation, thereby attenuating Xbp1 splicing, JNK phosphorylation, expression of XBP1 target genes, and ER stress-induced apoptosis. Conversely, BAX inhibitor-1 -deficient cells exhibit hyperactivation of IRE1α associated with increased Xbp1 mRNA splicing and upregulation of XBP1s-dependent genes, activation of JNK, and increased cell death (84). On the basis of these compelling data, we speculate that the IRE1α UPRosome initiates multiple signaling responses through interaction with adaptors and modulators in a highly regulated manner. However, more studies are needed to define the physiological significance of these findings.
Recent reports suggest that during unresolvable ER stress, hyperactivation of IRE1α’s RNase causes endonucleolytic decay of many ER-localized mRNAs, including those encoding chaperones, thereby culminating in cellular dysfunction and the death of β-cells as well as other cell types (85, 86). This process was termed regulated IRE1-dependent RNA degradation. It was proposed that the RNase of IRE1α can yield different outputs in RNA cleavage depending on the conditions and/or the intensity of ER stress (85). Under conditions of low-level ER stress or artificial dimerization of IRE1α without ER stress, IRE1α mainly cleaves unspliced Xbp1 mRNA to promote an adaptive response, whereas persistent and/or strong activation causes IRE1α to cleave both unspliced Xbp1 mRNA and ER-targeted mRNAs, including insulin. These findings suggest (a) that chronic exposure of β-cells to high glucose causes ER stress and hyperactivation of IRE1α, leading to degradation of insulin mRNA; and (b) that IRE1α kinase/endoribonuclease can function as an apoptotic switch in response to persistent ER stress.
ENDOPLASMIC RETICULUM STRESS STIMULI AND β-CELL DEATH IN TYPE 2 DIABETES
Obesity is nearly invariably associated with insulin resistance, but T2D only develops in genetically predisposed and insulin-resistant subjects with the onset of β-cell dysfunction (3). Pancreatic β-cell failure and loss of islet mass are the primary determinants in the pathogenesis of T2D. Many mechanisms for β-cell dysfunction and death in T2D have been proposed, including lipotoxicity, glucotoxicity, oxidative stress, amyloid deposition, and others. There is growing evidence that β-cell failure and death are caused by unresolvable ER stress, leading to chronic and/or strong activation of IRE1α and/or PERK. Indeed, ER stress alone can initiate and propagate all the characteristics of β-cell failure and death observed in T2D (28). Here, we review how insulin resistance and obesity may cause ER stress in the β-cell leading to T2D.
Endoplasmic Reticulum Stress by Lipotoxicity
Many studies suggest that high-fat diets and obesity are associated with elevated levels of plasma free fatty acids (FFAs) (2). FFAs are now considered as important mediators of β-cell dysfunction and apoptosis in T2D (3). Long saturated FFAs, such as palmitate, mediate apoptotic β-cell death in vivo and in vitro (87, 88), although both unsaturated and saturated FFAs eventually inhibit proinsulin synthesis and glucose-stimulated insulin secretion in β-cells (89). However, the precise mechanisms causing β-cell dysfunction and apoptosis by saturated FFAs (called lipotoxicity) are not fully understood. Accumulating evidence suggests that saturated long-chain FFAs induce ER stress and thereby cause β-cell failure and cell death, whereas unsaturated long-chain FFAs induce it to a lesser extent (6) and may even protect against these processes in some instances. The mechanisms by which saturated FFAs activate ER stress signaling pathways are also unclear. Several reports demonstrated that palmitate treatment of β-cells and/or islets activates the PERK pathway, including expression of ATF4 and CHOP by eIF2α phosphorylation (Figure 4) (6). However, detection of IRE1α activation and spliced Xbp1 mRNAs’ or XBP1s’ protein was dependent on the palmitate preparation and/or the β-cell lines used in different laboratories (75, 90, 91). Further studies are required under physiologically relevant conditions. There is controversy about whether ATF6α is activated by palmitate. Although some reports suggest that the ATF6α pathway is activated by palmitate treatment (75, 92), other studies did not observe an increase in BiP expression induced by ATF6α activation in palmitate-treated β-cells (90, 93). It is also unknown whether palmitate is a specific inducer for the ATF6α branch because both palmitate and oleate treatment increased expression of known ATF6α-target genes, total Xbp1, and BiP/Grp78 (91). The protective effect of BiP overexpression is also controversial in palmitate-treated β-cells (90). These inconsistencies need reconciliation through direct evidence of ATF6α cleavage and BiP expression in β-cells of palmitate-fed animals. The saturated fatty acid palmitate has multiple deleterious effects on pancreatic β-cells: (a) activation of PKC-δ; (b) accumulation of long-chain acyl-coenzyme As (CoAs) or lipid derivatives, such as diacylglycerol, lysophosphatic acid, and sphingolipids (ceramide and others); and (c) perturbation of ER Ca2+ to increase cytosolic Ca2+. Below, we discuss to what extent each of these mechanisms contributes to ER stress and β-cell apoptosis.
Figure 4.
Apoptotic unfolded protein response (UPR) pathways induced by free fatty acids (FFAs) and chronically high glucose in β-cells. In contrast to unsaturated FFAs, saturated FFAs serve as poor substrates for mitochondrial fatty acid oxidation and de novo triglyceride synthesis. However, saturated FFAs serve as intermediates in ceramide biosynthesis. The saturated FFAs activate UPR pathways (primarily PKR-like ER kinase, PERK) by perturbation of ER Ca2+ mobilization through inhibition of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), or activation of inositol-1,4,5-trisphosphate (IP3) receptors, and/or direct impairment of endoplasmic reticulum (ER) homeostasis. In addition, chronically high glucose increases biosynthesis of proinsulin and islet amyloid polypeptide in β-cells, which increases accumulation of misfolded proteins [insulin and islet amyloid polypeptide (IAPP)] and oxidative protein folding-mediated reactive oxygen species (ROS) production. The oxidative stress created by ROS and toxic IAPP oligomers perturb ER Ca2+ mobilization through activation of IP3 receptors to release ER Ca2+. Perturbation of ER Ca2+ causes protein misfolding in the ER and activates the UPR pathways (primarily inositol-requiring protein 1a, IRE1α) that induce proapoptotic signals, including proinsulin mRNA degradation as described in Figure 2. Abbreviations: ATF4: activating transcription factor 4, ATF6α: activating transcription factor 6α, CHOP: CCAAT-enhancer-binding protein (C/EBP) homology protein, eIF2α-P: phosphorylated form of eukaryotic translation initiation factor 2α.
First, blocking of PKCδ translocation by the phospholipase C inhibitor, U-73122 (94), or direct inhibition of PKCδ by rotterlin (95) substantially reduced palmitate-induced apoptosis. Moreover, overexpression of dominant-negative PKCδ in pancreatic β-cells protected against high-fat diet-induced glucose intolerance and β-cell dysfunction in mice (96). Therefore, it is possible that FFA-induced activation of PKCδ may contribute to β-cell loss in T2D (97). It was recently shown that overexpression of dominant-negative PKCδ inhibited palmitate-induced nuclear accumulation of forkhead box protein O1 (FoxO1) in cultured β-cells and islets (96), although it remains to be confirmed whether apoptosis by activated PKCδ is caused by changes in FoxO1 nuclear localization. In pancreatic β-cells, the FoxO1 transcription factor is implicated in regulating differentiation, proliferation, and apoptosis (98). Furthermore, inhibition of FoxO1, which requires JNK inhibition, protects pancreatic β-cells against FFAs (99). In addition, FoxO1 activity was increased by an ER stress inducer, thapsigargin, and dominant-negative FoxO1 expression protected β-cells from thapsigargin-induced cell death (99). Recently, Qi & Mochly-Rosen (100) reported that during ER stress, PKCδ complexes with c-Abl (a protein tyrosine kinase involved in genotoxic and oxidative stresss) to cause JNK activation (101). Moreover, PKCδ inhibition reduced JNK activation and inhibited ER stress-mediated apoptosis. It is possible that FFAs or ER stress may activate the PKCδ-JNK pathway to activate FoxO1 directly or through inhibition of AKT-mediated insulin signaling. Therefore, the implication of the PKCδ-JNK-FoxO1 pathway in FFA-induced β-cell apoptosis warrants further investigation. It is also important to address how PKCδ is activated in FFA-treated or ER stress-induced β-cells. It is interesting that hepatic XBP1s, or even DNA-binding-defective mutant XBP1s, bind FoxO1 and promote its degradation, and thereby improve glucose homeostasis in leptin-deficient ob/ob T2D model mice (102). This suggests that increased expression of XBP1s in FFA- or ER stress-exposed β-cells may provide a new therapeutic approach to modulate the activity of FoxO1 for the treatment of T2D. However, prolonged XBP1s overexpression interferes with β-cell function via inhibition of insulin, pancreatic duodonal homeobox 1 (PDX1), and v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MafA) expression, eventually leading to β-cell apoptosis (103). Therefore, this issue requires further investigation.
Second, several studies suggest that the differential toxicity between saturated FFAs and unsaturated FFAs is directly related to their ability to promote triglyceride accumulation and fatty acid oxidation (Figure 4) (104, 105). In β-cells, oleate treatment leads to triglyceride accumulation and is well tolerated, whereas palmitate is poorly incorporated into triglyceride and causes ER stress and apoptosis (105). The lipotoxicity caused by excess oleate in acyl-CoA:diacylglycerol transferase 1 (DGAT1)-deficient fibroblasts (DGAT catalyzes the formation of triglycerides from diacylglycerol and acyl-CoA) (104) emphasizes the importance of a harmonious match between cellular lipid influx and lipid utilization. Moreover, other studies have revealed that overexpression of stearoyl-CoA desaturase 1 (SCD1) to desaturate excess saturated FFAs is sufficient to prevent lipotoxicity of palmitate (104, 106). By contrast, knockdown of SCD in INS-1 β-cells decreased desaturation of palmitate to monounsaturated fatty acid, lowered FFA partitioning into complex neutral lipids, and augmented palmitate-induced ER stress and apoptosis (107). The importance of lipid content in ER function was further suggested in diabetic murine models. Loss of SCD1 worsened diabetes in leptin-deficient obese mice (108). In addition, Scd1 and Scd2 mRNA expression was induced in islets from prediabetic hyperinsulinemic Zucker diabetic fatty (ZDF) rats, whereas several fatty acid desaturases, including Scd1 mRNA levels, were markedly reduced in diabetic ZDF rat islets (107).
Studies using inhibitors (e.g., etomoxir or bromopalmitate) or activators (e.g., T090217) of fatty acid mitochondrial β-oxidation suggest that increased fatty acid oxidation is important to prevent lipotoxicity and ER stress (92, 109). For example, overexpression of the mitochondrial fatty acid transporter carnitine palmitoyltransferase 1 alleviated palmitate-induced apoptosis and decreased expression of ER stress markers, eIF2α-P and CHOP, in β-cells (110). Fatty acid oxidation and triglyceride formation were most pronounced in oleate-treated β-cells when compared to palmitate (92, 105), although the responsible genes are not known.
Several saturated lipid intermediates (e.g., lysophosphatidic acid, phosphatidic acid, and diacylglycerols) of the saturated FFA esterification pathway (3) and sphingolipids (such as ceramide) de novo synthesized from saturated FFAs (111, 112) were proposed as toxic molecules that can induce β-cell dysfunction and apoptosis. Among these toxic lipid intermediates, studies using proximal inhibitors (such as myriocin or fumonisin B1) of de novo ceramide synthesis suggested that ceramide induces ER stress and cell death in several cell lines, including β-cells (92, 111, 112). Recently, Boslem et al. (113) employed mass spectrometry in a comprehensive lipidomic screen of MIN6 β-cells treated with palmitate. They observed that the major alterations following palmitate exposure were in the sphingolipid class [glucosylceramide (GlcCer), lactosylceramide, trihexosylceramide] without any significant alteration in the amounts of either ceramide or sphingomyelin. Among the sphingolipid class, the amount of GlcCer changed most significantly, and increased conversion of ceramide to GlcCer by overexpression of GlcCer synthase reduced ER stress and apoptosis and also ameliorated the palmitate-mediated ER-to-Golgi protein trafficking defect. Although these results support ceramide as a causative factor in palmitate-induced β-cell death and possibly in β-cell dysfunction in T2D, it remains unknown how ceramide causes ER stress and cell death without an increase in the steady-state level of ceramide, as other accumulated sphingolipids are not toxic to β-cells.
Third, studies have revealed that ER Ca2+ homeostasis is important for β-cell function. ER Ca2+ measurement using an ER-targeted cameleon showed that the MIN6 β-cell has about 250 μM resting ER Ca2+ (56). The high intraluminal Ca2+ concentration in the ER is important to maintain Ca2+-dependent ER chaperone functions, and Ca2+ released into the cytosol is an important signaling molecule (114, 115). In response to a variety of external stimuli, Ca2+ is released from the lumen of the ER via two Ca2+ channels; the IP3 receptor and the ryanodine receptor. The concentration of Ca2+ in the cytosol is maintained at a low level by active Ca2+ transport into the ER via SERCA, creating a large [Ca2+] gradient between the cytosol and the ER lumen (0.1 μM versus 400 μM). Ca2+ is buffered in the ER by proteins (e.g., calnexin, calreticulin, ORP 150, ERp57, and others) that bear multiple low-affinity Ca2+-binding sites. More importantly, Ca2+ binding to molecular chaperones regulates their activity, and therefore ER Ca2+ directly affects posttranslational protein folding, modification, and trafficking. For example, the interaction between calreticulin and other ER chaperones (PDI and ERp57) depends on the ER Ca2+ concentration. Therefore, blocking the SERCA pump by thapsigargin, a selective inhibitor of SERCA, depletes ER Ca2+, inhibits ER functions, and thereby causes cell death through hyperactivation of the IRE1α and PERK pathways (116). ER stress and cell death could occur if saturated FFAs perturb ER Ca2+ homeostasis through reduction of SERCA activity or by activation of IP3 or ryanodine receptors. A previous study demonstrated that islets from db/db mice, an animal model of typical T2D, lack the initial reduction of intracellular Ca2+ and subsequent intracellular Ca2+ oscillations following stimulation with high glucose, possibly caused by a defect in cytosolic Ca2+ sequestration secondary to a reduction in SERCA activity (117). Several recent studies have indicated that palmitate triggers ER stress in pancreatic β-cells through perturbation of ER Ca2+ levels (Figure 4) (6). Long-term palmitate treatment depleted ER Ca2+ (by 40%) to a greater extent than oleate (by 24%) (91). The reduced ER Ca2+ content is explained by impaired ER Ca2+ uptake, which may reflect inhibition of the SERCA pump in palmitate-treated β-cells. The inhibition of ER Ca2+ uptake was observed at an early time (3 h) after palmitate treatment and was followed by UPR activation. Furthermore, CHOP depletion by siRNA partially protected against palmitate-induced β-cell apoptosis, suggesting that ER stress is involved in palmitate-induced apoptosis through ER Ca2+ depletion. However, this model is challenged by evidence described below. In the absence of extracellular Ca2+, the palmitate-induced Ca2+ signal was mostly inhibited, except for the initiating Ca2+ signal from the ER (118). Several plasma membrane L-type Ca2+ channel blockers (nifedipine, nimodipine, and verapamil) and the KATP channel opener (diazoxide) efficiently inhibited the palmitate-induced Ca2+ signals and significantly reduced CHOP expression and cell death in pancreatic β-cells and islets, whereas the IP3 receptor Ca2+ channel blocker (xestospongin c) and ryanodine receptor Ca2+ channel blocker (dantrolin) did not significantly alter the palmitate-induced Ca2+ signal and did not protect from palmitate-induced apoptosis (95). This suggests that a Ca2+ influx through the voltage-sensitive Ca2+ channel of the L-type coupled to membrane depolarization through closure of the KATP channel is crucial for cytosolic Ca2+ increase in response to palmitate. Moreover, several mono-/polyunsaturated FFAs (oleate, linoleic acid, and α-linolenic acid) in addition to palmitate also elicited intracel-lular Ca2+ signals (119) and reduced ER Ca2+ content (118). This suggests that the increase in cytosolic Ca2+ alone is not sufficient to induce cell death in palmitate-treated pancreatic β-cells. However, the unsaturated FFAs were less toxic than palmitate to β-cells. Futhermore, palmitate immediately induced PERK activation in 5 min and subsequently caused a significant increase in both XBP1s and CHOP in β-cells. Therefore, it is possible that reduced ER Ca2+ content is a prerequisite for activation of palmitate-mediated UPR signaling or apoptosis in pancreatic β-cells. Clearly, further studies are required to unravel the relative contribution of reduced ER Ca2+ content in palmitate-induced ER stress and apoptosis in β-cells.
Endoplasmic Reticulum Stress by Glucotoxicity
In T2D, absolute or relative insulin deficiency associated with insulin resistance causes blood glucose levels to remain high, called hyper-glycemia (120). In chronic hyperglycemia, consistently overstimulated β-cells show a gradual decrease of glucose-induced insulin secretion and insulin gene expression and eventually impaired β-cell function and survival, a process called glucotoxicity. As diabetic hyperglycemia becomes chronic, the glucose that normally serves as fuel or substrate is used to generate detrimental metabolites for β-cells. Glucotoxicity is mediated at least in part by accumulation of excess ROS generated by several metabolic pathways, including mitochondrial oxidative phosphorylation and other alternative metabolic pathways, such as glucose autoxidation, hexosamine metabolism, sorbitol metabolism, and increased protein glycation (120, 121). The pancreatic β-cell is vulnerable to ROS because it has a low antioxidative stress response (122). β-Cells do not express catalase and only low levels of glutathione peroxidase. Furthermore, oxidative stress by elevated ROS reduced proinsulin synthesis by decreasing mRNA expression through inactivation of the β-cell-specific transcription factors, PDX1 and MafA, that regulate expression of proinsulin genes and multiple downstream genes required for β-cell differentiation, proliferation, and survival (123). Therefore, it is thought that oxidative stress is an important factor in β-cell failure.
Another possible mechanism of ROS generation from hyperglycemia-exposed pancreatic β-cells has emerged in recent years. Protein folding pathways in the ER and ROS production are closely linked events (Figure 3) (65, 71, 73). Prolonged UPR activation leads to the accumulation of ROS via two sources: the UPR-regulated oxidative protein folding machinery in the ER and oxidative phosphorylation in mitochondria. First, in hyperglycemia-exposed β-cells, the increased demand for insulin requires increased disulfide bond formation, which generates ROS during the process (73). Moreover, the increased amount of proinsulin synthesis further depletes glutathione, used for reducing nonnative disulfide bonds in misfolded proinsulin molecules. It has been estimated that approximately 25% of the ROS generated in a cell may result from formation of disulfide bonds in the ER during oxidative protein folding (124). Second, ER Ca2+ uptake to mitochondria mediates mitochondrial ROS production through disruption of mitochon-drial electron transport and eventually induces mitochondrial apoptotic pathways (Figure 3). Specifically, more than 50% of the protein synthesized in the ER during glucose stimulation is proinsulin that requires delicate intermolecular disulfide bond formation and exchange (9). If chronic hyperglycemia leads to an increased rate of proinsulin synthesis, it would overcrowd the ER and increase the rate of oxidative protein folding. This would place an enormous burden on the ER and would enhance the probability of protein misfolding. Thus, during hyperglycemia, the accumulation of misfolded proinsulin in the ER lumen can generate ROS by the UPR-regulated oxidative protein folding machinery and by functional perturbation of mitochondria by a Ca2+ leak from the ER. In this manner, glucotoxicity may cause ER stress-regulated ROS accumulation, leading to diminished insulin gene expression, β-cell failure, and apoptosis. However, additional studies need to test the notion whether the hyperglycemia-increased proinsulin synthesis-ER stress-ROS pathway is a main cause for the onset and/or the progression of T2D.
Under conditions of chronic hyperglycemia, increased proinsulin biosynthesis may overwhelm the ER protein folding capacity, leading to UPR activation. Chronic high-glucose exposure (24 h) causes hyper-activation of IRE1α to splice Xbp1 mRNA, whereas acute exposure (1–3 h) to high glucose activates IRE1α without Xbp1 mRNA splicing (Figure 4) (86). During chronically high glucose exposure over several days, hyperactivated IRE1α, which may have a different activation states (85), degrades ER proinsulin mRNA, possibly including ER-localized mRNAs, contributing to the reduction of proinsulin biosynthesis and further β-cell demise (85, 125). Further studies under physiological conditions are necessary to elucidate the validity of these in vitro systems that use prolonged states of high glucose exposure, which are rarely observed in vivo.
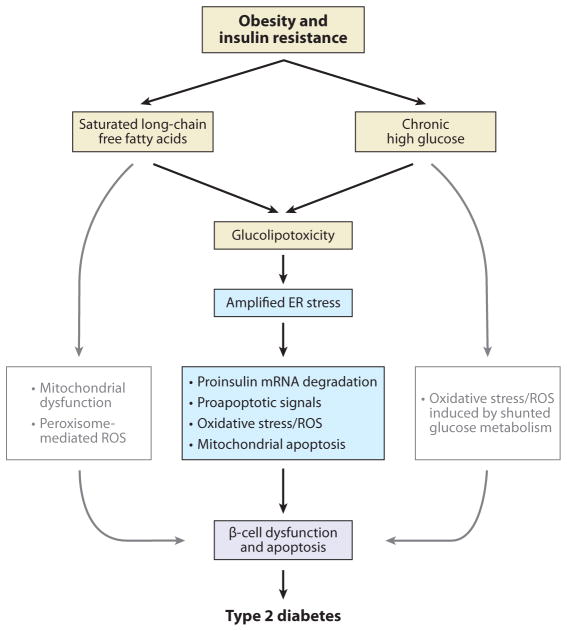
It appears that β-cell death in T2D is not associated with hyperglycemia or hyperlipidemia alone but is a combination of hyperglycemia and hyperlipidemia, which is called glucolipotoxicity (3). FFA-mediated UPR signaling pathways are potentiated by high-glucose cosupplementation of β-cells as high glucose exacerbates β-cell lipotoxicity (Figure 5) (126, 127). Although high glucose activates PERK and IRE1α phosphorylation in palmitate-treated β-cells, it remains to be determined how high glucose synergizes with palmitate to alter ER stress signaling, especially the PERK pathway. In general, acute high glucose, leading to increased proinsulin synthesis and folding, represses PERK activation, which limits excessive proinsulin synthesis and causes expression of the integrated stress response genes (such as Atf4, Chop, Gadd34, and others) (33, 128). The potential role of high glucose as an enhancer of FFA-mediated ER stress now warrants further investigation to fully understand glucolipotoxicity in T2D.
Figure 5.
Amplified endoplasmic reticulum (ER) stress and β-cell death in type 2 diabetes (T2D). Conditions of insulin resistance and obesity cause hyperglycemia and hyperlipidemia, which result in glucolipotoxicity for the β-cell. Studies suggest that free fatty acid-mediated unfolded protein response signaling pathways are potentiated by high-glucose cosupplementation to β-cells as high glucose exacerbates β-cell lipotoxicity (126, 127). The amplified ER stress response leads to β-cell dysfunction and apoptosis through proinsulin mRNA degradation, oxidative stress, proapoptotic signals, and mitochondrial apoptosis, eventually culminating in T2D. Abbreviation: ROS, reactive oxygen species.
Endoplasmic Reticulum Stress by Islet Amyloid
In addition to β-cell failure and insulin resistance, islet hyalinosis (hyaline deposits in β-cells), reported for the first time by Opie in 1901 (129), also plays a role in T2D. The hyaline deposits in pancreatic β-cells occur in approximately 90% of individuals with T2D and are associated with reduced β-cell volume (130). The hyaline deposits are known as amyloids (131), insoluble fibrous protein aggregates sharing specific structural traits. A number of human neurodegenerative diseases (such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease) are now thought to be associated with the formation of amyloids or amyloid-like fibrils (132). In 1987, the islet amyloid in T2D was shown to be an islet amyloid polypeptide (IAPP) (133). It is coex-pressed with insulin in pancreatic β-cells, traffics through the insulin secretory pathway and is secreted with insulin after food ingestion (134). The mature IAPP is a 37-amino acid polypeptide derived from an 89-amino acid precursor by proteolytic processing (135). The primary sequence of processed IAPP in humans, non-human primates, and cats is highly conserved, but rodent IAPPs show sequence differences between the twentieth and twenty-ninth amino acids. In vitro IAPP in humans, nonhuman primates, and cats, but not rodents, forms amyloid fibrils and conveys cellular toxicity to pancreatic β-cells (136). It is believed that the sequence differences in IAPP conveys its propensity to form a fibrillar amyloid in aqueous environments and confers toxicity to β-cells (7). This amyloid hypothesis for T2D was further tested by β-cell-specific overexpression of human IAPP (hIAPP) in transgenic mice. The hIAPP-transgenic mice recapitulated the metabolic characteristics of T2D, i.e., hyperglycemia, impaired insulin secretion, and insulin resistance (7). Recent examination of amyloid toxicity found that the toxic form of amyloidogenic proteins appears not to be the extracellular or intracellular large amyloid deposits detected by Congo red dye under light microscopy but rather smaller intracellular nonfibrillar oligomers that can be detected by a specific antibody against amyloid β protein toxic oligomers (7). Through use of the toxic oligomer-specific antibody, Lin et al. (137) were able to show that toxic oligomers of hIAPP in hIAPP-expressing transgenic mice accumulate in β-cells, although there are no data from the human pancreata from patients with T2D to confirm the existence of these toxic hIAPP oligomers.
How and why IAPP amyloids are formed are important questions to be answered in the future. Although the exact mechanism linking hIAPP oligomer formation and apoptotic β-cell death is unknown, several in vitro studies suggest the notion that the toxicity of IAPP oligomers is related to the formation of a membrane channel inducing an unregulated Ca2+ influx or membrane disruption (Figure 5) (138, 139). Therefore, ER accumulation of the toxic IAPP oligomers might reasonably be expected to induce Ca2+ leakage into the cytoplasm, which can induce ER stress. As predicted, Huang and coworkers (7) observed ER stress in β-cell lines as well as in islets of transgenic mice and rats expressing human IAPP. Several ER stress markers, including CHOP and XBP1s, were observed in both the islets and β-cells expressing hIAPP, whereas overexpression of rat IAPP did not provoke ER stress. Furthermore, siRNA-mediated inhibition of CHOP reduced apoptotic cell death in hIAPP-expressing β-cells. The β-cells of hIAPP transgenic mice have increased polyu-biquitinated proteins, which are not present in transgenic mice that express rat IAPP, implying that β-cells in hIAPP transgenic mice may experience ER stress induced by the accumulation of misfolded proteins. However, there are several challenges to the hIAPP oligomer-mediated ER stress model. Recently, Hull et al. (140) did not detect ER stress in hIAPP-expressing islets, although amyloid formation in these murine islets was associated with reduced β-cell mass. TUNEL-positive β-cells in hIAPP transgenic mice were not always positive for CHOP expression. Moreover, it was curious why the induced CHOP was localized in the perinuclear region instead of in the nucleus. Therefore, experimental designs using chemical chaperones or Chop knockout in hIAPP transgenic mice should provide additional insight into the involvement of other apoptotic pathways. Nevertheless, Huang and coworkers’ data suggest that, in patients with obesity and T2D, hIAPP oligomer-mediated ER stress is an important mechanism, leading to increased β-cell apoptosis.
CONCLUSIONS
T2D develops only when β-cell dysfunction appears. It is thought that β-cell failure is mainly caused by increases in blood glucose and FFAs that cannot be properly disposed or stored. Excess glucose and fatty acids can overload the cell and disrupt ER and mitochondrial functions. Indeed, both chronically high glucose and saturated fatty acids cause β-cell failure and loss in islet mass. It was suggested that chronically high glucose may cause oxidative stress through its metabolism, and saturated FFAs produce toxic lipid metabolites. However, recent studies suggest that high glucose and saturated FFAs interfere with ER function, which subsequently disrupts proinsulin synthesis, folding, and processing in β-cells. The chronically stressed ER generates several proapoptotic signals, induces oxidative stress, and initiates mitochondrial apoptosis. Consequently, β-cell function deteriorates, and cells eventually die.
SUMMARY POINTS.
When ER homeostasis is altered, signaling pathways mediated by three ER-proximal sensors: IRE1, PERK and ATF6 are activated, eliciting an adaptive response that is collectively called the unfolded protein response (UPR). However, when the adaptive mechanisms fail to restore ER homeostasis, several death signaling pathways are activated.
During the death response, the PERK-mediated cell death pathway is mainly initiated by CHOP expression, which causes an increase in misfolded protein accumulation and oxidative stress, activates the mitochondrial death signal through ER Ca2+ release, and regulates expression of pro- and antiapoptotic genes.
During the death response, IRE1α may propagate death by signaling activation of JNK and by regulating IRE1-dependent RNA degradation.
The UPR pathway is activated by chronic exposure to high glucose or saturated long-chain FFAs, and activation of the UPR pathways is amplified by cosupplementation of both high glucose and saturated long-chain FFAs.
The activation of the UPR in T2D β-cells is connected with saturated long-chain fatty acid-mediated proapoptotic mechanisms that include PKCδ activation, accumulation of lipid derivatives, and ER Ca2+ release.
Under chronic hyperglycemia in T2D, increased proinsulin synthesis induces oxidative stress by ROS production and ER Ca2+ release, leading to mitochondria-dependent cell death.
Under chronic hyperglycemia conditions, hyperactivated IRE1α contributes to β-cell failure by degrading proinsulin mRNA, possibly including ER-localized mRNAs. 8. Activation of the UPR induced by ER accumulation of the toxic hIAPP oligomer may be an important mechanism in β-cell death in humans.
FUTURE ISSUES.
Is hyperglycemia and/or hyperlipidemia essential for β-cell failure in T2D?
What is the molecular mechanism of glucose level-dependent differential activation of PERK and IREa in pancreatic β-cells?
How does ceramide activate the UPR? What other lipid intermediates activate or inhibit the UPR?
How does the UPR activate PKCδ in palmitate-treated β-cells?
How does palmitate induce ER calcium release?
How does high glucose activate the UPR in β-cells? Is there any direct evidence of ER stress?
What are the molecular mechanisms involved in the formation of the toxic hIAPP oligomers and UPR induction by hIAPP oligomers?
Further investigation is needed to identify effective chemical chaperones that can improve ER function to prevent hyperactivation of the UPR.
Acknowledgments
We apologize to our colleagues whose work was not cited owing to space limitations. We especially thank Hyun Ju Yoo at the Asan Insitutute of Life Science for helpful comments. This work was funded by the Basic Science Research Program through the National Research Foundation of Korea (2011-0011433) to S.H.B. This work was partially supported by National Institute of Health grants DK042394, DK088227, DK093074, HL052173, and HL057346 to R.J.K.
Glossary
- UPR
unfolded protein response
- IRE1
inositol-requiring protein 1
- ATF6
activating transcription factor 6
- PERK
PKR (double-stranded RNA-dependent kinase)-like endoplasmic reticulum kinase
- Eukaryotic translation initiation factor 2α(eIF2α)
a subunit of heterotrimeric eIF2 complex, which mediates the binding of methionyl-tRNA to ribosome
- Wolcott-Rallison syndrome
a rare autosomal recessive disease, characterized by neonatal/early-onset nonautoimmune insulin-requiring diabetes associated with skeletal dysplasia and growth retardation
- eIF2αA/A
a homozygous mutant mouse model harboring an alanine mutation at the phosphorylation site (serine 51 amino acid) in eIF2α
- SERCA
sarco/ endoplasmic reticulum Ca2+-ATPase
- Preemptive quality control
a cotranslational rerouting pathway reducing the burden of misfolded substrates entering the ER by cytosolic degradation
- ROS
reactive oxygen species
- Leprdb/dbmouse
a mouse model of obesity, diabetes, and dyslipdemia wherein leptin receptor activity is deficient
- UPRosome
a complex of IRE1 α and multiple signaling molecules, which can modulate the amplitude and duration of IRE1α signaling
- FFAs
free fatty acids
- Integrated stress response
eIF2α phosphorylation-dependent, stress-inducible signaling pathways that can be activated by eIF2 kinases (PERK, PKR, HRI, GCN2)
Footnotes
DISCLOSURE STATEMENT
Dr. Kaufman is an Scientific Advisory Board member of Alnylam Pharmaceuticals, Inc., Cambridge, MA; and Proteostasis Therapeutics, Inc., Cambridge, MA.
Contributor Information
Sung Hoon Back, Email: shback@ulsan.ac.kr.
Randal J. Kaufman, Email: rkaufman@sanfordburnham.org.
LITERATURE CITED
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–46. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Investig. 2006;116:1802–12. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, et al. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes. 2005;54:2294–304. doi: 10.2337/diabetes.54.8.2294. [DOI] [PubMed] [Google Scholar]
- 5.Liu YQ, Jetton TL, Leahy JL. β-cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem. 2002;277:39163–68. doi: 10.1074/jbc.M207157200. [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Ladriere L, Igoillo-Esteve M, Moura RF, Cunha DA. Causes and cures for endoplasmic reticulum stress in lipotoxic beta-cell dysfunction. Diabetes Obes Metab. 2010;12(Suppl 2):76–82. doi: 10.1111/j.1463-1326.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 7.Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–16. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter GA. Nutrient-secretion coupling in the pancreatic islet beta-cell: recent advances. Mol Aspects Med. 2001;22:247–84. doi: 10.1016/s0098-2997(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 9.Dodson G, Steiner D. The role of assembly in insulin’s biosynthesis. Curr Opin Struct Biol. 1998;8:189–94. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- 10.Van Lommel L, Janssens K, Quintens R, Tsukamoto K, Vander Mierde D, et al. Probe-independent and direct quantification of insulin mRNA and growth hormone mRNA in enriched cell preparations. Diabetes. 2006;55:3214–20. doi: 10.2337/db06-0774. [DOI] [PubMed] [Google Scholar]
- 11.Goodge KA, Hutton JC. Translational regulation of proinsulin biosynthesis and proinsulin conversion in the pancreatic beta-cell. Semin Cell Dev Biol. 2000;11:235–42. doi: 10.1006/scdb.2000.0172. [DOI] [PubMed] [Google Scholar]
- 12.Schuit FC, In’t Veld PA, Pipeleers DG. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci USA. 1988;85:3865–69. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–88. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 14.McMaster CR. Lipid metabolism and vesicle trafficking: more than just greasing the transport machinery. Biochem Cell Biol. 2001;79:681–92. doi: 10.1139/o01-139. [DOI] [PubMed] [Google Scholar]
- 15.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–72. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 16.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 17.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–30. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 18.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–24. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1β-deficient mice. J Clin Investig. 2001;107:585–93. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirasophon W, Lee K, Callaghan B, Welihinda A, Kaufman RJ. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 2000;14:2725–36. doi: 10.1101/gad.839400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci USA. 2011;108:8885–90. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–74. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Vattem KM, Sood R, An J, Liang J, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal. 2007;9:2357–71. doi: 10.1089/ars.2007.1764. [DOI] [PubMed] [Google Scholar]
- 25.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–9. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 26.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–63. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, McGrath B, Li S, Frank A, Zambito F, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–74. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 29.Back SH, Scheuner D, Han J, Song B, Ribick M, et al. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes. 2010;59:1937–47. doi: 10.2337/db09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–97. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Vander Mierde D, Scheuner D, Quintens R, Patel R, Song B, et al. Glucose activates a protein phosphatase-1-mediated signaling pathway to enhance overall translation in pancreatic beta-cells. Endocrinology. 2007;148:609–17. doi: 10.1210/en.2006-1012. [DOI] [PubMed] [Google Scholar]
- 33.Hou ZQ, Li HL, Gao L, Pan L, Zhao JJ, Li GW. Involvement of chronic stresses in rat islet and INS-1 cell glucotoxicity induced by intermittent high glucose. Mol Cell Endocrinol. 2008;291:71–78. doi: 10.1016/j.mce.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SM, et al. Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia. 2007;50:1442–52. doi: 10.1007/s00125-007-0674-4. [DOI] [PubMed] [Google Scholar]
- 35.Gomez E, Powell ML, Bevington A, Herbert TP. A decrease in cellular energy status stimulates PERK-dependent eIF2alpha phosphorylation and regulates protein synthesis in pancreatic beta-cells. Biochem J. 2008;410:485–93. doi: 10.1042/BJ20071367. [DOI] [PubMed] [Google Scholar]
- 36.Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, et al. Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci USA. 2002;99:15920–25. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee TG, Tang N, Thompson S, Miller J, Katze MG. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratri-copeptide repeat family of proteins. Mol Cell Biol. 1994;14:2331–42. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutkowski DT, Kang SW, Goodman AG, Garrison JL, Taunton J, et al. The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell. 2007;18:3681–91. doi: 10.1091/mbc.E07-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–99. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haze K, Okada T, Yoshida H, Yanagi H, Yura T, et al. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J. 2001;355:19–28. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–64. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–76. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Luebke-Wheeler J, Zhang K, Battle M, Si-Tayeb K, Garrison W, et al. Hepatocyte nuclear factor 4alpha is implicated in endoplasmic reticulum stress-induced acute phase response by regulating expression of cyclic adenosine monophosphate responsive element binding protein H. Hepatology. 2008;48:1242–50. doi: 10.1002/hep.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, et al. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol. 2005;7:186–94. doi: 10.1038/ncb1213. [DOI] [PubMed] [Google Scholar]
- 45.Kondo S, Saito A, Hino S, Murakami T, Ogata M, et al. BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of endoplasmic reticulum stress transducer. Mol Cell Biol. 2007;27:1716–29. doi: 10.1128/MCB.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagamori I, Yabuta N, Fujii T, Tanaka H, Yomogida K, et al. Tisp40, a spermatid specific bZip transcription factor, functions by binding to the unfolded protein response element via the Rip pathway. Genes Cells. 2005;10:575–94. doi: 10.1111/j.1365-2443.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 47.Liang G, Audas TE, Li Y, Cockram GP, Dean JD, et al. Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol. 2006;26:7999–8010. doi: 10.1128/MCB.01046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raggo C, Rapin N, Stirling J, Gobeil P, Smith-Windsor E, et al. Luman, the cellular counterpart of herpes simplex virus VP16, is processed by regulated intramembrane proteolysis. Mol Cell Biol. 2002;22:5639–49. doi: 10.1128/MCB.22.16.5639-5649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stirling J, O’Hare P. CREB4, a transmembrane bZip transcription factor and potential new substrate for regulation and cleavage by S1P. Mol Biol Cell. 2006;17:413–26. doi: 10.1091/mbc.E05-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Investig. 2010;120:744–55. doi: 10.1172/JCI39678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seo HY, Kim YD, Lee KM, Min AK, Kim MK, et al. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases insulin gene expression via up-regulation of orphan nuclear receptor small heterodimer partner. Endocrinology. 2008;149:3832–41. doi: 10.1210/en.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang SW, Rane NS, Kim SJ, Garrison JL, Taunton J, Hegde RS. Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell. 2006;127:999–1013. doi: 10.1016/j.cell.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–76. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–89. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 55.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–65. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida H, Okada T, Haze K, Yanagi H, Yura T, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–67. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida H, Okada T, Haze K, Yanagi H, Yura T, et al. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol Cell Biol. 2001;21:1239–48. doi: 10.1128/MCB.21.4.1239-1248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–79. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y, Hendershot LM. Herp is dually regulated by both the endoplasmic reticulum stress-specific branch of the unfolded protein response and a branch that is shared with other cellular stress pathways. J Biol Chem. 2004;279:13792–99. doi: 10.1074/jbc.M313724200. [DOI] [PubMed] [Google Scholar]
- 60.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–40. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, et al. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11:403–15. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 64.Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95:974–86. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA. 2008;105:18525–30. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Investig. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA. 2001;98:10845–50. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Investig. 2008;118:3378–89. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–77. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15:767–76. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 72.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 73.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–46. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Investig. 2009;119:2925–41. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–63. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 76.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–66. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 77.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–55. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432–37. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol Cell Biol. 1999;19:8469–78. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim I, Shu CW, Xu W, Shiau CW, Grant D, et al. Chemical biology investigation of cell death pathways activated by endoplasmic reticulum stress reveals cytoprotective modulators of ASK1. J Biol Chem. 2009;284:1593–603. doi: 10.1074/jbc.M807308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–76. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 82.Luo D, He Y, Zhang H, Yu L, Chen H, et al. AIP1 is critical in transducing IRE1-mediated endoplasmic reticulum stress response. J Biol Chem. 2008;283:11905–12. doi: 10.1074/jbc.M710557200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu F, Nguyen DT, Stuible M, Dube N, Tremblay ML, Chevet E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem. 2004;279:49689–93. doi: 10.1074/jbc.C400261200. [DOI] [PubMed] [Google Scholar]
- 84.Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–91. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–75. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, et al. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–54. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 87.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–38. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 88.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–96. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 89.Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Investig. 1998;101:1094–101. doi: 10.1172/JCI420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A. Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2008;294:E540–50. doi: 10.1152/ajpendo.00478.2007. [DOI] [PubMed] [Google Scholar]
- 91.Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008;121:2308–18. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choi SE, Jung IR, Lee YJ, Lee SJ, Lee JH, et al. Stimulation of lipogenesis as well as fatty acid oxidation protects against palmitate-induced INS-1 beta-cell death. Endocrinology. 2011;152:816–27. doi: 10.1210/en.2010-0924. [DOI] [PubMed] [Google Scholar]
- 93.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 94.Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, et al. Protein kinase C delta activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes. 2003;52:991–97. doi: 10.2337/diabetes.52.4.991. [DOI] [PubMed] [Google Scholar]
- 95.Choi SE, Kim HE, Shin HC, Jang HJ, Lee KW, et al. Involvement of Ca2+-mediated apoptotic signals in palmitate-induced MIN6N8a beta cell death. Mol Cell Endocrinol. 2007;272:50–62. doi: 10.1016/j.mce.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Hennige AM, Ranta F, Heinzelmann I, Dufer M, Michael D, et al. Overexpression of kinase-negative protein kinase Cdelta in pancreatic beta-cells protects mice from diet-induced glucose intolerance and beta-cell dysfunction. Diabetes. 2010;59:119–27. doi: 10.2337/db09-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Welters HJ, Smith SA, Tadayyon M, Scarpello JH, Morgan NG. Evidence that protein kinase Cdelta is not required for palmitate-induced cytotoxicity in BRIN-BD11 beta-cells. J Mol Endocrinol. 2004;32:227–35. doi: 10.1677/jme.0.0320227. [DOI] [PubMed] [Google Scholar]
- 98.Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal. 2011;14:649–61. doi: 10.1089/ars.2010.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–59. doi: 10.2337/db07-0595. [DOI] [PubMed] [Google Scholar]
- 100.Qi X, Mochly-Rosen D. The PKCdelta -Abl complex communicates ER stress to the mitochondria—an essential step in subsequent apoptosis. J Cell Sci. 2008;121:804–13. doi: 10.1242/jcs.024653. [DOI] [PubMed] [Google Scholar]
- 101.Sun X, Majumder P, Shioya H, Wu F, Kumar S, et al. Activation of the cytoplasmic c-Abl tyrosine kinase by reactive oxygen species. J Biol Chem. 2000;275:17237–40. doi: 10.1074/jbc.C000099200. [DOI] [PubMed] [Google Scholar]
- 102.Zhou Y, Lee J, Reno CM, Sun C, Park SW, et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17:356–65. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allagnat F, Christulia F, Ortis F, Pirot P, Lortz S, et al. Sustained production of spliced X-box binding protein 1 (XBP1) induces pancreatic beta cell dysfunction and apoptosis. Diabetologia. 2010;53:1120–30. doi: 10.1007/s00125-010-1699-7. [DOI] [PubMed] [Google Scholar]
- 104.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–82. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thorn K, Bergsten P. Fatty acid-induced oxidation and triglyceride formation is higher in insulin-producing MIN6 cells exposed to oleate compared to palmitate. J Cell Biochem. 2010;111:497–507. doi: 10.1002/jcb.22734. [DOI] [PubMed] [Google Scholar]
- 106.Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, et al. Increased fatty acid desatu-ration and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta-cells from lipoapoptosis. Diabetes. 2005;54:2917–24. doi: 10.2337/diabetes.54.10.2917. [DOI] [PubMed] [Google Scholar]
- 107.Green CD, Olson LK. Modulation of palmitate-induced endoplasmic reticulum stress and apo-ptosis in pancreatic beta-cells by stearoyl-CoA desaturase and Elovl6. Am J Physiol Endocrinol Metab. 2011;300:E640–49. doi: 10.1152/ajpendo.00544.2010. [DOI] [PubMed] [Google Scholar]
- 108.Flowers JB, Rabaglia ME, Schueler KL, Flowers MT, Lan H, et al. Loss of stearoyl-CoA desaturase-1 improves insulin sensitivity in lean mice but worsens diabetes in leptin-deficient obese mice. Diabetes. 2007;56:1228–39. doi: 10.2337/db06-1142. [DOI] [PubMed] [Google Scholar]
- 109.Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001;50:315–21. doi: 10.2337/diabetes.50.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sol EM, Sargsyan E, Akusjarvi G, Bergsten P. Glucolipotoxicity in INS-1E cells is counteracted by carnitine palmitoyltransferase 1 over-expression. Biochem Biophys Res Commun. 2008;375:517–21. doi: 10.1016/j.bbrc.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 111.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 112.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–42. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 113.Boslem E, MacIntosh G, Preston AM, Bartley C, Busch AK, et al. A lipidomic screen of palmitate-treated MIN6 beta-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem J. 2011;435:267–76. doi: 10.1042/BJ20101867. [DOI] [PubMed] [Google Scholar]
- 114.Bygrave FL, Benedetti A. What is the concentration of calcium ions in the endoplasmic reticulum? Cell Calcium. 1996;19:547–51. doi: 10.1016/s0143-4160(96)90064-0. [DOI] [PubMed] [Google Scholar]
- 115.Corbett EF, Michalak M. Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem Sci. 2000;25:307–11. doi: 10.1016/s0968-0004(00)01588-7. [DOI] [PubMed] [Google Scholar]
- 116.Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, et al. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes. 2009;58:422–32. doi: 10.2337/db07-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roe MW, Philipson LH, Frangakis CJ, Kuznetsov A, Mertz RJ, et al. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J Biol Chem. 1994;269:18279–82. [PubMed] [Google Scholar]
- 118.Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ ho-meostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 119.Schnell S, Schaefer M, Schofl C. Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40. Mol Cell Endocrinol. 2007;263:173–80. doi: 10.1016/j.mce.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 120.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–54. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 122.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–42. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 123.Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006;6:615–19. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 124.Kaufman RJ, Back SH, Song B, Han J, Hassler J. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in beta-cells. Diabetes Obes Metab. 2010;12(Suppl 2):99–107. doi: 10.1111/j.1463-1326.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lipson KL, Ghosh R, Urano F. The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells. PLoS ONE. 2008;3:e1648. doi: 10.1371/journal.pone.0001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bachar E, Ariav Y, Ketzinel-Gilad M, Cerasi E, Kaiser N, Leibowitz G. Glucose amplifies fatty acid–induced endoplasmic reticulum stress in pancreatic beta-cells via activation of mTORC1. PLoS ONE. 2009;4:e4954. doi: 10.1371/journal.pone.0004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanabe K, Liu Y, Hasan SD, Martinez SC, Cras-Méneur C, et al. Glucose and fatty acids synergize to promote B-cell apoptosis through activation of glycogen synthase kinase 3beta independent of JNK activation. PLoS ONE. 2011;6:e18146. doi: 10.1371/journal.pone.0018146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jonas JC, Bensellam M, Duprez J, Elouil H, Guiot Y, Pascal SM. Glucose regulation of islet stress responses and beta-cell failure in type 2 diabetes. Diabetes Obes Metab. 2009;11(Suppl 4):65–81. doi: 10.1111/j.1463-1326.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- 129.Opie EL. The relation of diabetes mellitus to lesions of the pancreas: hyaline degeneration of the islands of Langerhans. J Exp Med. 1901;5:527–40. doi: 10.1084/jem.5.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 131.Ehrlich JC, Ratner IM. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals. Am J Pathol. 1961;38:49–59. [PMC free article] [PubMed] [Google Scholar]
- 132.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 133.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA. 1987;84:8628–32. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Butler PC, Chou J, Carter WB, Wang YN, Bu BH, et al. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–56. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- 135.Sanke T, Bell GI, Sample C, Rubenstein AH, Steiner DF. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988;263:17243–46. [PubMed] [Google Scholar]
- 136.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–60. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 137.Lin CY, Gurlo T, Kayed R, Butler AE, Haataja L, et al. Toxic human islet amyloid polypeptide (h-IAPP) oligomers are intracellular, and vaccination to induce anti-toxic oligomer antibodies does not prevent h-IAPP-induced beta-cell apoptosis in h-IAPP transgenic mice. Diabetes. 2007;56:1324–32. doi: 10.2337/db06-1579. [DOI] [PubMed] [Google Scholar]
- 138.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypep-tide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–98. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 139.Kawahara M, Kuroda Y, Arispe N, Rojas E. Alzheimer’s beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J Biol Chem. 2000;275:14077–83. doi: 10.1074/jbc.275.19.14077. [DOI] [PubMed] [Google Scholar]
- 140.Hull RL, Zraika S, Udayasankar J, Aston-Mourney K, Subramanian SL, Kahn SE. Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress. Diabetologia. 2009;52:1102–11. doi: 10.1007/s00125-009-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 142.Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J Biol Chem. 2000;275:24881–85. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 143.Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16:3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 144.Hershey JWB, Merrick WC. The pathway and mechanism of initiation of protein synthesis. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harb. Lab. Press; 2000. pp. 33–88. [Google Scholar]
- 145.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 146.Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci. 2009;122:1626–36. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–84. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 149.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–55. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 151.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–68. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li G, Mongillo M, Chin KT, Harding H, Ron D, et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–92. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 154.Moore CE, Omikorede O, Gomez E, Willars GB, Herbert TP. PERK activation at low glucose concentration is mediated by SERCA pump inhibition and confers preemptive cytoprotection to pancreatic beta-cells. Mol Endocrinol. 2011;25:315–26. doi: 10.1210/me.2010-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]