Abstract
Nociceptin/orphanin FQ peptide receptor (NOP) agonists produce antinociceptive effects in animal models after spinal administration and potentiate μ-opioid receptor (MOP)-mediated antinociception. This study determined the antinociceptive effects of spinally administered bifunctional NOP/MOP ligands and the antinociceptive functions of spinal NOP and MOP receptors in mice. Antinociceptive effects of bifunctional NOP/MOP ligands BU08028 [(2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol] and SR16435 [1-(1-(2,3,3α,4,5,6-hexahydro-1H-phenalen-1-yl)piperidin-4-yl)-indolin-2-one] were pharmacologically compared with the putative bifunctional ligand buprenorphine, selective NOP agonist SCH221510 [3-endo-8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol] and selective MOP agonist morphine in neuropathic and inflammatory pain models. Additionally, the degree of tolerance development to the antiallodynic effects of SR16435 and buprenorphine were determined after repeated intrathecal administration. Our data indicated that BU08028 and SR16435 were more potent than morphine and SCH221510 in attenuating nerve injury-induced tactile allodynia and inflammation-induced thermal hyperalgesia. Coadministration of receptor-selective antagonists further revealed that both NOP and MOP in the spinal cord mediated the antiallodynic effects of BU08028 and SR16435, but intrathecal buprenorphine-induced antiallodynic effects were primarily mediated by MOP. Repeated intrathecal administration of SR16435 resulted in reduced and slower development of tolerance to its antiallodynic effects compared with buprenorphine. In conclusion, both NOP and MOP receptors in the spinal cord independently drive antinociception in mice. Spinally administered bifunctional NOP/MOP ligands not only can effectively attenuate neuropathic and inflammatory pain, but also have higher antinociceptive potency with reduced tolerance development to analgesia. Such ligands therefore display a promising profile as spinal analgesics.
Introduction
Nociceptin/orphanin FQ receptor (NOP), the fourth member of the opioid family with similarities in localization and cellular actions to the classic opioid receptors, is implicated in the modulation of pain responses (Lambert, 2008; Largent-Milnes and Vanderah, 2010; Calo and Guerrini, 2013). The NOP system in the brain and spinal cord is upregulated in rodents under neuropathic and inflammatory pain conditions (Jia et al., 1998; Briscini et al., 2002). In rodents, activation of spinal NOP is shown to produce antinociception in acute, neuropathic, and inflammatory pain (Tian et al., 1997; Hao et al., 1998; Obara et al., 2005). In nonhuman primates, however, NOP agonists produce antinociception by systemic as well as spinal administration and without μ-opioid-associated side effects (Ko et al., 2009; Hu et al., 2010). Overall, these studies indicate that one of the main sites of antinociceptive actions for NOP agonists is the spinal cord. This makes NOP a potential target for spinal analgesia.
Spinal NOP and μ-opioid receptor (MOP) independently drive antinociception in preclinical pain models. In rodents, spinal administration of NOP agonists potentiated morphine-induced antinociception in the absence of motor dysfunction (Tian et al., 1997; Courteix et al., 2004; Reiss et al., 2008). In nonhuman primates as well, NOP activation potentiated MOP-mediated antinociception, devoid of side effects such as respiratory depression (Hu et al., 2010; Cremeans et al., 2012). These studies emphasize the importance of bifunctional ligands, which can simultaneously activate both NOP and MOP receptors. Such ligands may have a wider therapeutic window and the ability to treat severe pain conditions. Additionally, coactivation of NOP and MOP may also result in slower tolerance development because both receptor pools are used to a lesser degree to achieve analgesia. Collectively, bifunctional NOP/MOP ligands may have a promising clinical value and need to be investigated in animal models.
Bifunctional ligands with varying degrees of affinity and efficacy at NOP and MOP were tested in rodents for their antinociceptive effects after systemic administration (Spagnolo et al., 2008; Toll et al., 2009; Khroyan et al., 2011b). However, it is not known how NOP and MOP receptors in the spinal cord contribute to antinociception when bifunctional ligands are directly injected in the spinal cord. One of the putative bifunctional ligands is buprenorphine, which has high binding affinity and agonist activity at MOP (Lewis, 1985). Although buprenorphine has extremely low binding affinity for NOP (Toll et al., 1998), systemic buprenorphine-induced antinociception was enhanced in rodents when NOP antagonists were systemically administered (Lutfy et al., 2003; Khroyan et al., 2009), indicating the suppression of buprenorphine’s antinociception by the NOP component. However, it is unclear how spinal NOP directly modulates antinociceptive effects of buprenorphine. It is therefore important to pharmacologically characterize antinociceptive actions of bifunctional NOP/MOP ligands and buprenorphine after acute and repeated spinal administration.
Our study determined the function of NOP versus MOP receptors in the spinal cord to produce antinociception against neuropathic pain, relative to inflammatory pain, by using selective and bifunctional ligands. In particular, we studied bifunctional ligands with high affinity and partial agonist activity at NOP and MOP—BU08028 [2-(N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl)-3,3-dimethylpentan-2-ol] and SR16435 [1-(1-(2,3,3α,4,5,6-hexahydro-1H-phenalen-1-yl)piperidin-4-yl)-indolin-2-one]—but without agonist activity at δ- and κ-opioid receptors (Khroyan et al., 2007, 2011a), and we compared them with the selective NOP and MOP agonists as well as buprenorphine. In addition, we compared potential tolerance development to the antiallodynic effects of SR16435 and buprenorphine, which have similar duration of actions, after repeated intrathecal administration.
Materials and Methods
Animals
Male ICR mice weighing 25–30 g were used (Harlan Industries, Indianapolis, IN). Mice were housed five per cage with food and water ad libitum and a 12-hour light/dark cycle under standard laboratory conditions. All procedures were conducted in accordance with the University Committee on the Use and Care of Animals at the University of Michigan (Ann Arbor, MI) and the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Bethesda, MD).
Chronic Constriction Injury
Nerve injury was induced by ligation of the sciatic nerve using the method developed by Bennett and Xie (1988) modified for mice. Briefly, mice were anesthetized with ketamine (80 mg/kg i.p.)/xylazine (12 mg/kg i.p.). An incision was made between the gluteus superficialis and biceps femoris muscles in the right leg to expose the sciatic nerve. Four chromic gut ligatures were tied loosely around the sciatic nerve at 1-mm intervals in such a way that the epineural blood flow was occluded but not arrested. The wound was closed by suturing the muscles and the skin.
Carrageenan-Induced Paw Inflammation
Mice were lightly anesthetized with 3% isoflurane and received an intraplantar injection of 50 µl λ-carrageenan (2%; Sigma-Aldrich, St. Louis, MO) dissolved in saline in the right hind paw.
Drug Administration
Morphine sulfate, naltrexone, buprenorphine (National Institute on Drug Abuse, Bethesda, MD), and SR16435 (Astraea Therapeutics, Mountain View, CA) were dissolved in sterile water. SCH221510 [3-endo-8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol] (Tocris Bioscience, Bristol, UK), BU08028 (University of Bath, Bath, UK), and J-113397 [1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one] (Tocris Bioscience) were dissolved in 1:1:8 ratio of dimethylsulfoxide (DMSO), Tween 80, and sterile water.
All drugs were administered intrathecally in the volume of 5 μl as previously described elsewhere (Fairbanks, 2003). Briefly, the mouse was secured by a firm grip on the pelvic girdle, and the drugs were injected by lumbar puncture between the L5/L6 vertebrae using a 30-gauge needle attached to a 10-μl Hamilton syringe. Mice in the control group received an intrathecal injection of the appropriate vehicle.
Behavioral Analyses
Tactile Allodynia.
Tactile allodynia was measured in mice with chronic constriction injury as paw withdrawal thresholds in response to probing with calibrated von Frey filaments in a manner described by Chaplan et al. (1994). Mice were habituated for 45 minutes in suspended cages designed with wire-mesh floors. von Frey filaments with buckling weights ranging from 0.04 to 2 g were then applied perpendicularly to the plantar surface of the ipsilateral paw and held for 5 seconds. A positive response was indicated by a sharp withdrawal of the paw. For each mouse, the testing began with a von Frey filament corresponding to the weight of 0.4 g. If the animal made a positive response, the next filament with lower force was applied. If the response was negative, the next filament with higher force was used. Each response was recorded, and the experiment ended once the animal had made five responses after the first positive response.
The 50% paw withdrawal threshold was determined by the Dixon nonparametric method (Dixon, 1980). If the animal made four consecutive positive responses, a score of 0.04 g was assigned. If the animal made four consecutive negative responses, a score of 2 g was assigned. Baseline values for paw withdrawal thresholds were obtained in all mice before the induction of nerve injury. Changes in tactile allodynia in response to drug treatment were determined 2 weeks after the nerve injury was induced.
Thermal Hyperalgesia
Thermal hyperalgesia was measured in mice with paw inflammation induced by the intraplantar carrageenan injection. Thermal hyperalgesia was measured using the hot plate method, with the surface temperature maintained at 52°C. Mice were placed on the hot plate, and the latency for withdrawal of the carrageenan-treated paw from the hot plate was measured. A cutoff latency of 30 seconds (s) was used to avoid tissue damage. Baseline values for paw withdrawal latencies were obtained in all mice before the induction of paw inflammation. Changes in thermal hyperalgesia in response to drug treatment were determined 2 hours after the injection of carrageenan in the paw.
Experimental Design
Mice with nerve injury developed significant tactile allodynia within 2 weeks after the chronic constriction injury of the sciatic nerve and showed reduced withdrawal thresholds of the ipsilateral paw in response to von Frey filaments. No changes were observed in withdrawal thresholds of the contralateral paw. Hence, all tests were conducted by probing only the ipsilateral paw. Mice that received the intraplantar injection of carrageenan developed thermal hyperalgesia 2 hours later and showed significant reduction in the paw withdrawal latency of the ipsilateral paw in response to noxious heat. Once the predrug values for paw withdrawal thresholds and paw withdrawal latencies were obtained, drugs were intrathecally administered. Separate groups of animals (n = 6–8 per group) were used to study each dosing condition. All behavioral experiments were conducted by experimenters blinded to the condition.
The first part of the study was conducted to characterize the potency, duration, and effectiveness of selective NOP agonist SCH221510, selective MOP agonist morphine, and the bifunctional ligands BU08028, SR16435, and buprenorphine against neuropathic and inflammatory pain. Mice with nerve injury-induced tactile allodynia received an intrathecal injection of SCH221510 (0.3–10 µg), morphine (0.3–10 µg), BU08028 (0.03–1 µg), SR16435 (0.1–3 µg), buprenorphine (0.1–3 µg), or vehicle. Similarly, the mice with carrageenan-induced thermal hyperalgesia received an intrathecal injection of SCH221510 (0.1–3.0 µg), morphine (0.1–3.0 µg), BU08028 (0.001–0.1 µg), SR16435 (0.03–1 µg), buprenorphine (0.01–1 µg), or vehicle.
The doses of morphine and SCH221510 were selected based on the previous studies in rodent pain models using NOP or MOP agonists (Hao et al., 1998; Sounvoravong et al., 2004; Obara et al., 2005). We hypothesized that for a compound with the ability to activate NOP and MOP receptors it may be ideal to use doses that were 3 to 10 times lower than those of SCH221510 and morphine. Once the doses that produced approximately 50% antinociception were obtained, the dose-response curves for all compounds were characterized. Because no difference was noted in the nociceptive thresholds of the mice that had received intrathecal saline or the mixture of dimethylsulfoxide (DMSO), Tween 80, and water, the data were pooled for all vehicle groups. After the mice had received the drugs or vehicle, their behavioral analyses were conducted at time points of 0.5, 1, 2, 3, 4, 24, and 48 hours.
The second part of the study was conducted to determine the function of spinal NOP and MOP receptors to produce antiallodynia against neuropathic pain. Doses of SCH221510 (10 µg), morphine (10 µg), BU08028 (1 µg), SR16435 (3 µg), and buprenorphine (3 µg), which produced near maximal antiallodynia, were selected. Antagonists, which included naltrexone (0.3–3.0 µg) and J-113397 (0.3–3.0 µg), or a single solution containing 3 µg of both naltrexone and J-113397, were administered intrathecally as a 10-minute pretreatment. Mice that received intrathecal morphine were administered naltrexone (0.3–3.0 µg) or J-113397 (3 µg). Mice that received intrathecal SCH221510 were administered J-113397 (0.3–3.0 µg) or naltrexone (3 µg). Highest doses of antagonists naltrexone (3 µg), J-113397 (3 µg), or the combination of both antagonists (3 µg), which completely blocked the antiallodynic effects of the selective agonists, were chosen as a 10-minute pretreatment to BU08028, SR16435, or buprenorphine. A group of mice in each agonist treatment also received the intrathecal pretreatment of the respective vehicle.
On the day of testing, the predrug values for paw withdrawal thresholds were measured. The mice then were injected with the assigned antagonist doses. Selective or bifunctional ligands were administered 10 minutes later. Paw withdrawal thresholds were again measured at 30 minutes after the agonist treatment to determine the effects of antagonist pretreatment. A separate group of mice received all antagonist treatments alone followed by intrathecal vehicle administration. No changes in nociceptive thresholds were observed. Hence, the data are not shown.
The third part of the study was conducted to determine the rate and degree of tolerance development to the antiallodynic effects of buprenorphine (3 µg) and SR16435 (3 µg) in mice with neuropathic pain. Doses that produced near maximal antiallodynia with similar duration of action were chosen for the tolerance study. For buprenorphine and SR16435, the area under the curve was calculated for the percentage of the maximum possible effect (%MPE) of their antiallodynic effects up to 48 hours at 3 µg. Based on the area under the curve, the schedule of drug administration was determined. SR16435 was injected three times a day separated by 4 hours, whereas buprenorphine was injected 2 times a day separated by 6 hours. Both compounds were administered for 5 days, and the paw withdrawal thresholds were measured each day at 0.5 hours after their administration.
This schedule of repeated drug administration may be more relevant to a clinical setting in which the drug is readministered once its analgesic effects start to diminish. Previous studies have used a dosing schedule of more than two times a day for intrathecal administration (Davis and Inturrisi, 1999; Choi et al., 2004; Hopkins et al., 2004). No signs of distress or overt pain behaviors were observed in these mice after the repeated intrathecal injections.
Statistical Analysis
Antiallodynic and antihyperalgesic effects were quantified in each animal as %MPE at each time point and drug dose. The following formula was used to quantify %MPE: % MPE = [(Postdrug value for a behavioral response (g or s) − Predrug value for a behavioral response/(Cutoff value − Predrug value for a behavioral response) × 100. If the animal did not respond to any von Frey filament or did not respond before 30 seconds, a score of 100% MPE was assigned.
The mean values (± S.E.M.) were calculated from individual animals for all behavioral end points. Multiple comparisons were made using repeated measures two-way analysis of variance. Post hoc analyses were conducted using the Bonferroni test. Comparisons of data at a single time point were made using one-way analysis of variance followed by the Dunnett test. P < 0.05 was considered statistically significant for all tests. The 50% effective dose (ED50) plus 95% confidence interval (CI) values were determined from the %MPE of each drug at the 0.5-hour time point.
Results
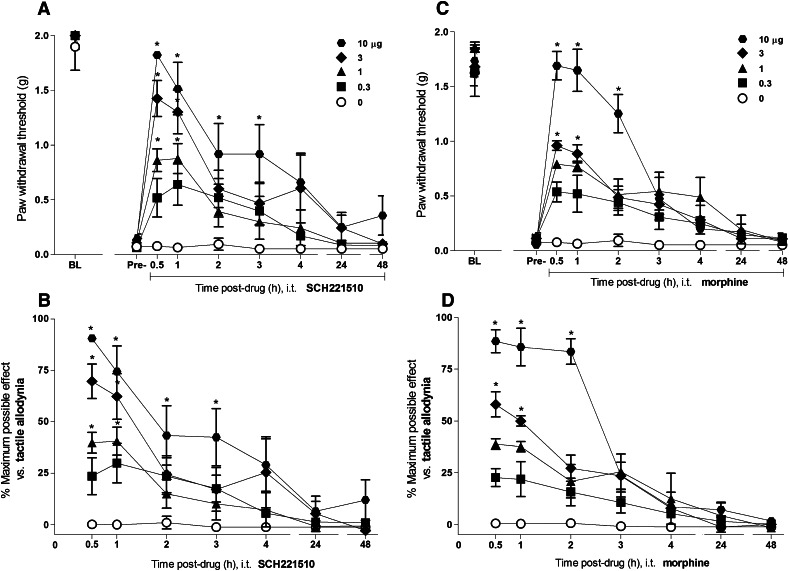
Figure 1 illustrates the time course and antiallodynic effects of intrathecal SCH221510 (0.3–10 µg) and morphine (0.3–10 µg) on tactile allodynia in mice with chronic constriction injury. Before the surgical manipulation, all mice maintained high paw withdrawal thresholds close to the cutoff value (1.8 ± 0.1 g). Statistically significant reduction in the paw withdrawal thresholds was measured at 2 weeks after the nerve injury was induced (0.09 ± 0.01 g). Intrathecal SCH221510 produced an increase in the paw withdrawal thresholds, thereby increasing %MPE for antiallodynia in dose-dependent [(F(4,25) = 4.4, P < 0.05] and time-dependent [(F(6,150) = 43.2, P < 0.05] manners. Similarly, intrathecal morphine produced antiallodynic effects in dose-dependent [F(4,25) = 79.2, P < 0.05] and time-dependent [F(6,150) = 52.2, P < 0.05] manners. Both SCH221510 and morphine at 10 µg showed near maximal %MPE of 91 ± 2 and 89 ± 6, respectively, elevating the paw withdrawal thresholds close to their presurgery values. For both drugs, the peak antiallodynic effect was observed at 0.5 hours after drug administration. These effects of SCH221510 lasted for 3 hours; for morphine, they lasted for 2 hours. Nociceptive thresholds did not change in the mice that had received the intrathecal injection of a vehicle.
Fig. 1.
Antiallodynic effects of intrathecal administration of SCH221510 and morphine in mice with neuropathic pain. Effects of SCH221510 (A) and morphine (C) on paw withdrawal thresholds (g). Percentage of maximum possible effect of SCH221510 (B) and morphine (D) for attenuating tactile allodynia. BL, baseline values before induction of nerve injury. Pre-, predrug values before intrathecal administration of drugs. Behavioral responses were measured at 0.5, 1, 2, 3, 4, 24, and 48 hours after drug administration. Each value represents the mean ± S.E.M. (n = 6). Symbols represent different dosing conditions in different groups of mice. *Statistically significant difference from the vehicle controls (○, 0 µg) (P < 0.05).
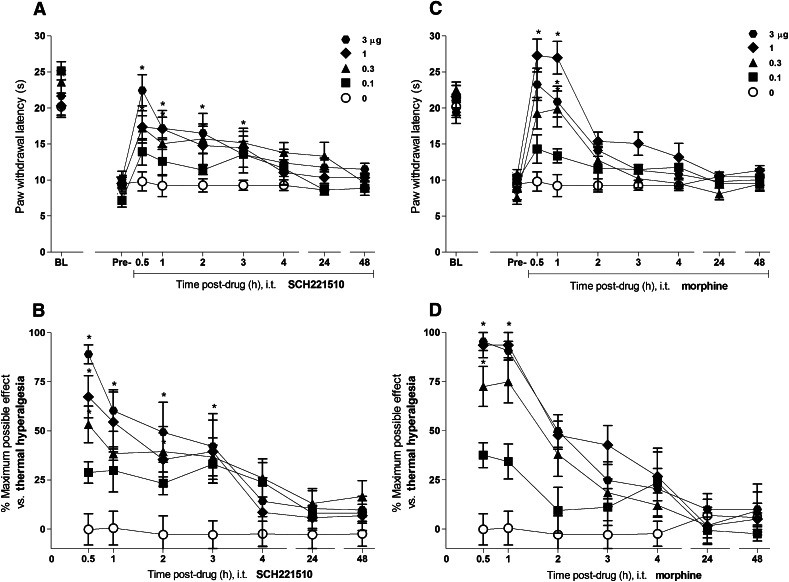
Figure 2 illustrates the time course and antihyperalgesic effects of intrathecal SCH221510 (0.1–3 µg) and morphine (0.1–3 µg) on thermal hyperalgesia in mice with carrageenan-induced paw inflammation. Before the induction of inflammation, all mice showed high paw withdrawal latencies (21.8 ± 0.1 seconds). Significant reduction in the paw withdrawal latencies was measured at 2 hours after the carrageenan injection (9.2 ± 0.1 seconds). Intrathecal SCH221510 produced an increase in the paw withdrawal latencies and %MPE for its antihyperalgesic effects in dose-dependent [F(4,25) = 5.9, P < 0.05] and time-dependent [(F(6,150) = 11.6, P < 0.05] manners. Similarly, intrathecal morphine produced antihyperalgesic effects in dose-dependent [F(4,25) = 14, P < 0.05] and time-dependent [F(6,150) = 42.2, P < 0.05] manners. Both SCH221510 and morphine at 3 µg showed near maximal %MPE of 89 ± 5 and 95 ± 5, respectively, elevating the paw withdrawal latencies close to their precarrageenan values. For both drugs, the peak antihyperalgesic effect was observed at 0.5 hours after drug administration. These effects of SCH221510 lasted for 3 hours whereas for morphine they lasted for 1 hour. Paw withdrawal latencies did not change in the mice that had received the intrathecal injection of a vehicle.
Fig. 2.
Antihyperalgesic effects of intrathecal administration of SCH221510 and morphine in mice with inflammatory pain. Effects of SCH221510 (A) and morphine (C) on paw withdrawal latencies (sec). Percentage of maximum possible effect of SCH221510 (B) and morphine (D) for attenuating thermal hyperalgesia. BL, baseline values before induction of paw inflammation. Pre-, predrug values before intrathecal administration of drugs. Behavioral responses were measured at 0.5, 1, 2, 3, 4, 24, and 48 hours after drug administration. Each value represents the mean ± S.E.M. (n = 6). Symbols represent different dosing conditions in different groups of mice. *Statistically significant difference from the vehicle controls (○, 0 μg) (P < 0.05).
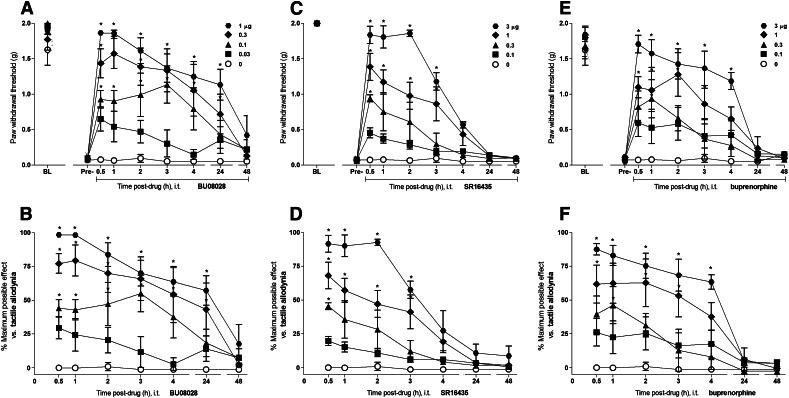
Figure 3 illustrates the time course and antiallodynic effects of intrathecal BU08028 (0.03–1 µg), SR16435 (0.1–3 µg), and buprenorphine (0.1–3 µg) on tactile allodynia in mice with chronic constriction injury. BU08028 caused an increase in the paw withdrawal thresholds and %MPE for its antiallodynic effects in dose-dependent [F(4,35) = 13.5, P < 0.05] and time-dependent [F(6,210) = 26, P < 0.05] manners. The peak effect (%MPE of 98 ± 1) of BU08028 at 1 μg was observed within 0.5 hours and lasted up to 24 hours. SR16435 also dose-dependently [F(4,25) = 32.9, P < 0.05] and time-dependently [F(6,150) = 69.2, P < 0.05] increased the paw withdrawal thresholds and %MPE for its antiallodynic effects. The peak effect (%MPE of 92 ± 6) of SR16435 at 3 μg was observed within 0.5 hours and lasted up to 3 hours. Similarly, buprenorphine increased paw withdrawal thresholds and %MPE for its antiallodynic effects in dose-dependent [F(4,25) = 23.9, P < 0.05] and time-dependent [F(6,150) = 21.2, P < 0.05] manners. The peak effect (%MPE of 88 ± 4) of buprenorphine at 3 μg was observed within 0.5 hours and lasted up to 4 hours.
Fig. 3.
Antiallodynic effects of intrathecal administration of BU08028, SR16435, and buprenorphine in mice with neuropathic pain. Effects of BU08028 (A), SR16435 (C), and buprenorphine (E) on paw withdrawal thresholds (g). Percentage of maximum possible effect of BU08028 (B), SR16435 (D), and buprenorphine (F) for attenuating tactile allodynia. BL, baseline values before induction of nerve injury. Pre-, predrug values before intrathecal administration of drugs. Behavioral responses were measured at 0.5, 1, 2, 3, 4, 24, and 48 hours after drug administration. Each value represents the mean ± S.E.M. (n = 8). Symbols represent different dosing conditions in different groups of mice. *Statistically significant difference from the vehicle controls (○, 0 μg) (P < 0.05).
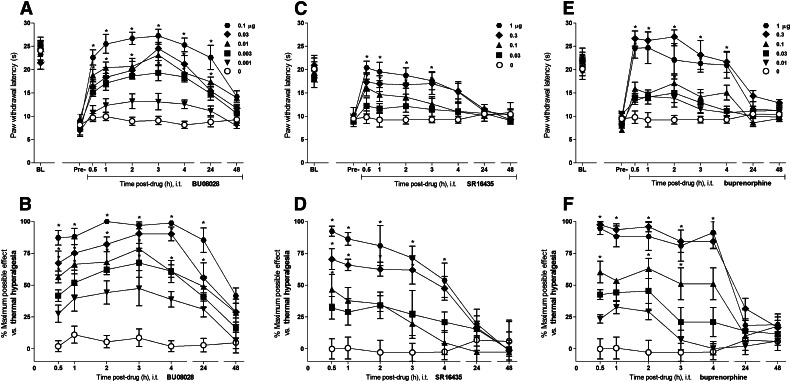
Figure 4 illustrates the time course and antihyperalgesic effects of intrathecal BU08028 (0.001–0.1 μg), SR16435 (0.03–1.0 μg), and buprenorphine (0.01–1.0 μg) on thermal hyperalgesia in mice with carrageenan-induced paw inflammation. BU08028 caused an increase in the paw withdrawal latencies and %MPE for its antihyperalgesic effects in dose-dependent [F(5,30) = 46.8, P < 0.05] and time-dependent [F(6,180) = 22.6, P < 0.05] manners. The peak effect (%MPE of 87 ± 5) of BU08028 at 0.1 μg was observed within 0.5 hours and lasted up to 24 hours. SR16435 also produced dose-dependent [F(4,25) = 16.7, P < 0.05] and time-dependent [F(6,150) = 17.5, P < 0.05] increase in the paw withdrawal latencies and %MPE for its antihyperalgesic effects. Peak effect (%MPE of 89 ± 6) of SR16435 at 1 μg was observed within 0.5 hours and lasted up to 4 hours. Buprenorphine caused an increase in the paw withdrawal latencies and %MPE for its antihyperalgesic effects in dose-dependent [F(6,29) = 35.7, P < 0.05] and time-dependent [F(6,174) = 30, P < 0.05] manners. The peak effect with %MPE of 98 ± 2 and 95 ± 5 was observed within 0.5 hours after the administration of 0.3 and 1 μg of buprenorphine, respectively. These effects lasted up to 4 hours.
Fig. 4.
Antihyperalgesic effects of intrathecal administration of BU08028, SR16435, and buprenorphine in mice with inflammatory pain. Effects of BU08028 (A), SR16435 (C), and buprenorphine (E) on paw withdrawal latencies (sec). The % maximum possible effect of BU08028 (B), SR16435 (D), and buprenorphine (F) for attenuating thermal hyperalgesia. BL, baseline values before induction of paw inflammation. Pre-, predrug values before intrathecal administration of drugs. Behavioral responses were measured at 0.5, 1, 2, 3, 4, 24, and 48 hours after drug administration. Each value represents the mean ± S.E.M. (n = 8). Symbols represent different dosing conditions in different groups of mice. *Statistically significant difference from the vehicle controls (○, 0 μg) (P < 0.05).
Figure 5 compares the antihypersensitive (antiallodynic and antihyperalgesic) potencies of intrathecally administered NOP- and MOP-related ligands in neuropathic and inflammatory pain. For both neuropathic and inflammatory pain modalities, SCH221510 and morphine showed similar potencies. BU08028, SR16435, and buprenorphine were more potent than SCH221510 and morphine in blocking neuropathic and inflammatory pain. In particular, all drugs were more potent for their antihyperalgesic effects against inflammatory pain as compared with their antiallodynic effects against neuropathic pain.
Fig. 5.
Comparison of the antinociceptive potencies of intrathecally administered NOP- and MOP-related ligands in neuropathic and inflammatory pain at 0.5 hours after drug administration. Dose-response curves for of BU08028, SR16435, buprenorphine, morphine, and SCH221510 for their antiallodynic effects against neuropathic pain (A) and antihyperalgesic effects against inflammatory pain (B). Different symbols represent different drugs. (C) Comparison of the antinociceptive effects as ED50 + 95% confidence interval values of BU08028, SR16435, buprenorphine, morphine, and SCH221510: ○ represents each drug’s ED50 against neuropathic pain, and □ represents each drug’s ED50 against inflammatory pain.
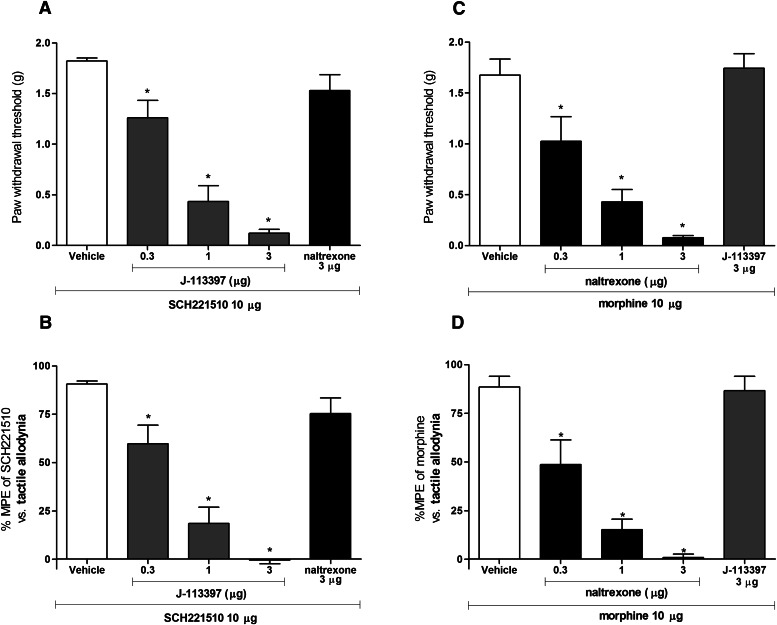
Figure 6 illustrates the effects of intrathecally administered NOP and MOP antagonists as a pretreatment on antiallodynic activity of SCH221510 (10 μg) and morphine (10 μg) at 0.5 hours in mice with neuropathic pain. NOP antagonist J-113397 (0.3–3.0 μg) attenuated the antiallodynic effects of SCH221510 in a dose-dependent [F(3,20) = 54.5, P < 0.05] manner. Complete inhibition of SCH221510-induced antiallodynia was observed at 3 μg of J-113397. MOP antagonist naltrexone at 3 μg did not significantly block the antiallodynic effects of SCH221510. Pretreatment with naltrexone (0.3–3.0 μg) dose-dependently [F(3,20) = 42.9, P < 0.05] attenuated the antiallodynic activity of morphine. Complete inhibition of morphine-induced antiallodynia was observed at 3 μg of naltrexone. J-113397 at 3 μg did not significantly block the antiallodynic effects of morphine.
Fig. 6.
Effects of NOP and MOP antagonists on antiallodynic effects of intrathecal SCH221510 (10 µg) and morphine (10 µg) in neuropathic pain. Antagonists were administered intrathecally 10 minutes before SCH221510 or morphine. Changes in SCH221510-induced increase in the paw withdrawal thresholds (A) and the percentage of maximum possible antiallodynic effect (B) after J-113397, naltrexone, or vehicle pretreatment at 0.5 hours after intrathecal SCH221510. Right panels: changes in morphine-induced increase in the paw withdrawal thresholds (B) and the percentage of maximum possible antiallodynic effect (D) after naltrexone, J-113397, or vehicle pretreatment at 0.5 hours after intrathecal morphine. *Statistically significant difference from the vehicle control (P < 0.05).
Figure 7 illustrates the effects of intrathecally administered naltrexone (3 μg), J-113397 (3 μg) or their coadministration (3 μg) on the antiallodynic activity of BU08028 (1 μg), SR16435 (3 μg) and buprenorphine (3 μg) at 0.5 hours in mice with neuropathic pain. There were statistically significant differences among the dosing conditions for BU08028 [F(3,23) = 32.6, P < 0.05], SR16435 [F(3,23) = 68.2, P < 0.05], and buprenorphine [(3,23) = 48.3, P < 0.05]. Pretreatment with naltrexone or J-113397 when administered individually partially but significantly blocked antiallodynic effects of BU08028 and SR16435. When naltrexone and J-113397 were coadministered, complete blockade of antiallodynic effects of BU08028 and SR16435 was observed. Naltrexone alone or coadministration of naltrexone and J-113397 significantly attenuated buprenorphine’s antiallodynic effects. However, J-113397 when administered individually failed to block antiallodynic effects of buprenorphine.
Fig. 7.
Effects of NOP and MOP antagonists on antiallodynic effects of intrathecal BU08028 (1 µg), SR16435 (3 µg) and buprenorphine (3 µg) in neuropathic pain at 0.5 hours. Antagonists J-113397 (3 µg), naltrexone (3 µg), or combination of naltrexone and J-113397 (3 µg) were intrathecally administered as a 10-minute pretreatment. Top panels: effects of antagonists on increased paw withdrawal thresholds (g) induced by BU08028 (A), SR16435 (C), and buprenorphine (E). Bottom panels: effects of antagonists on percentage of maximum possible antiallodynic effects of BU08028 (B), SR16435 (D), and buprenorphine (F). *Statistically significant difference from the vehicle control (P < 0.05).
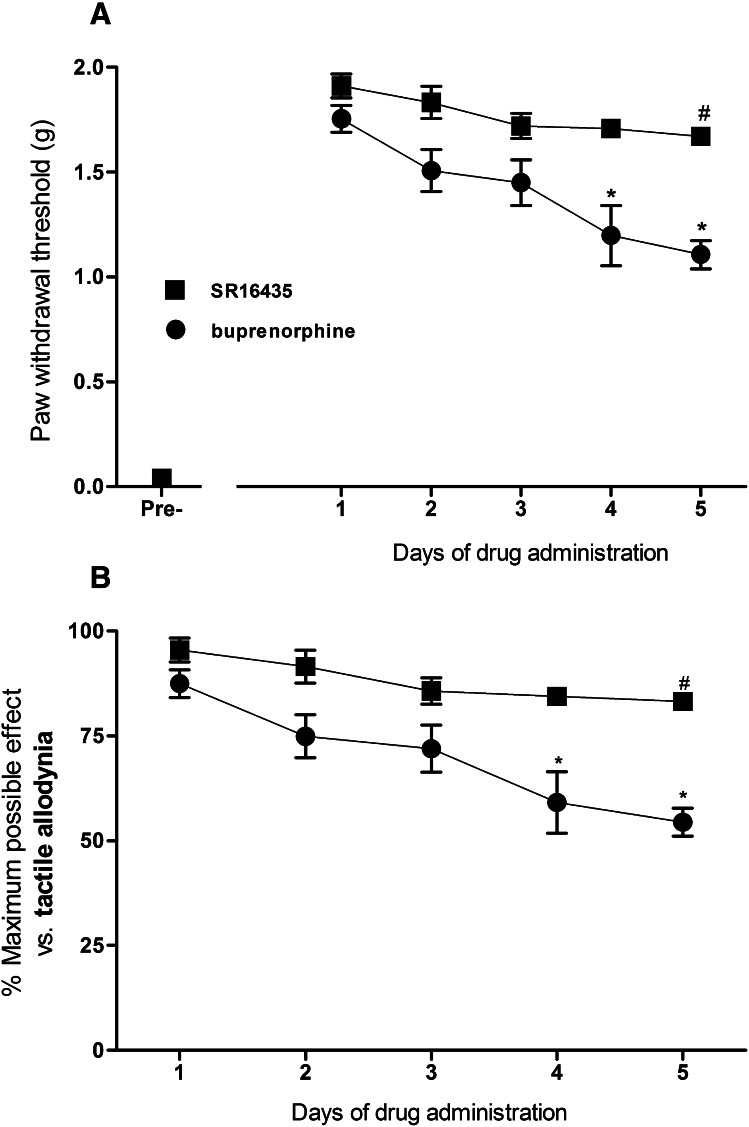
Figure 8 compares the effects of repeated intrathecal administration of SR16435 (3 μg) and buprenorphine (3 μg) on the development of tolerance to their antiallodynic effects in mice with neuropathic pain at 0.5 hours after their administration. There were statistically significant differences among the dosing conditions for SR16435 [F(4,25) = 3.2, P < 0.05] and buprenorphine [F(4,25) = 6.5, P < 0.05]. A small change of 12% in the %MPE of SR16435 was observed on day 5 compared with day 1. For buprenorphine, an approximately 35% change in %MPE was observed on day 5 compared with day 1.
Fig. 8.
Development of tolerance to the antiallodynic effects of repeated intrathecal administration of SR16435 (3 µg) or buprenorphine (3 µg) in mice with neuropathic pain. (A) Changes in SR16435 and buprenorphine-induced increase in paw withdrawal thresholds (g). (B) Changes in SR16435- and buprenorphine-induced percentage of maximum possible antiallodynic effects. Mice were tested each day at 0.5 hours after the drug administration. ▪ is SR16435; ● is buprenorphine. #Statistically significant difference from the antiallodynic effects of SR16435 on day 1 of the treatment. *Statistically difference from the antiallodynic effects of buprenorphine on day 1 (P < 0.05).
Discussion
The first part of the study demonstrated the antihypersensitive effects of spinally administered bifunctional NOP/MOP ligands in comparison with the selective NOP and MOP agonists in neuropathic and inflammatory pain modalities in mice (Figs. 1–5). Antihypersensitive effects of intrathecally administered NOP agonist SCH221510 and MOP agonist morphine were similar in terms of their potency, duration of action, and efficacy. Both SCH221510 and morphine effectively blocked nerve injury–induced tactile allodynia and inflammation-induced thermal hyperalgesia in dose-dependent manners. Neither SCH221510 nor morphine affected the motor function in mice at antihypersensitive doses, as similarly reported in neuropathic rats after intrathecally administered NOP agonist Ro 64-6198 [(1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one] (Obara et al., 2005). Despite differences in the ligand-binding specificities, NOP and MOP drive antinociception through similar mechanisms. Activation of NOP receptors blocks cAMP production and calcium currents and activates potassium currents, which ultimately inhibit the nociceptive neurotransmission (Meunier et al., 1995; Reinscheid et al., 1995).
Previous studies have reported that intrathecal administration of both NOP and MOP agonists can produce antinociceptive and antihypersensitive effects, indicating that NOP and MOP receptors in the spinal cord independently drive these effects. The present study is the first to demonstrate antihypersensitive effects of intrathecally administered bifunctional NOP/MOP ligands in neuropathic and inflammatory pain. Intrathecal BU08028 and SR16435, both of which bind to NOP and MOP with high affinity (Ki = 2–8 nM) and partial efficacy as measured by guanosine 5′-O-(3-[35S]thio)triphosphate functional assay (20–50% stimulation) (Khroyan et al., 2007, 2011a), were more potent than intrathecal SCH221510 or morphine for their antihypersensitive effects (Fig. 5). For instance, BU08028 was 10 to 20 times more potent than morphine in producing antiallodynic effects in neuropathic pain as per the ED50 values (0.09 versus 1.6 μg) or the dose required to produce full antiallodynia (1 versus 10 μg). The difference in potency was also observed against carrageenan-induced thermal hyperalgesia. BU08028 and SR16435 effectively produced antihyperalgesic effects at doses 3 to 10 times lower than those of morphine or SCH221510. Overall, these findings indicate that not only with improved potency, bifunctional ligands with partial agonist action are also as effective as selective full agonists.
The second part of the study determined the role of spinal NOP and MOP in modulating neuropathic pain (Figs. 6 and 7). Pretreatment with intrathecal administration of MOP antagonist naltrexone dose-dependently attenuated antiallodynic effects of morphine. Similarly, pretreatment with intrathecal administration of NOP antagonist J-113397 dose-dependently attenuated antiallodynic effects of SCH221510. However, the dose of naltrexone that completely abolished morphine’s antiallodynic effects did not attenuate the antiallodynic effects of SCH221510, indicating selective MOP inhibition of naltrexone. Similarly, the largest dose of J-113397 did not block antiallodynic activity of morphine, indicating the selective NOP inhibition of J-113397. When naltrexone (3 μg) and J-113397 (3 μg) were administered individually, an approximately 50% reduction in the antiallodynic effects of BU08028 or SR16435 was observed. More importantly, coadministration of naltrexone and J-113397 completely blocked the antiallodynic effects of BU08028 and SR16435 (Fig. 7). These data indicate that both NOP and MOP receptors in the spinal cord were activated to achieve antiallodynia induced by intrathecal administration of bifunctional NOP/MOP ligands. These findings are consistent with their in vitro profile, which indicates that BU08028 and SR16435 activate both NOP and MOP receptors when measured by the guanosine 5′-O-(3-[35S]thio)triphosphate functional assay (Khroyan et al., 2007, 2011a). However, previous studies in rodents have suggested that the antinociceptive effects of systemically administered BU08028 and SR16435 in the tail flick assay were mainly mediated by MOP receptors and that the pretreatment with systemic administration of NOP antagonists enhanced the ascending part of the dose-response curve for the antinociceptive effects of BU08028 and SR16435 (Khroyan et al., 2009, 2011a).
Such discrepancies in receptor mechanisms underlying antinociception after spinal versus systemic administration of these bifunctional ligands could be due to the differential effects of NOP activation depending on the site of action such as spinal versus supraspinal. In rodents, spinal NOP presumably mediates the analgesic effects, while the supraspinal NOP has antianalgesic effects (Meunier et al., 1995; Reinscheid et al., 1995). A possible explanation for the NOP antagonist-mediated enhancement in the antinociceptive effects of systemic BU08028 and SR16435 could be the inhibition of MOP-mediated antinociception by supraspinal NOP receptors (Pan et al., 2000). However, the neurobiological mechanisms by which the supraspinal NOP receptors modulate antinociception induced by spinal or systemic NOP or MOP agonists are not fully understood.
Buprenorphine is a speculated bifunctional ligand with relatively high binding affinity at MOP and low binding affinity at NOP with partial agonist activity at MOP and NOP receptors (Cowan et al., 1977; Huang et al., 2001; Clark et al., 2006). In humans, buprenorphine is a highly effective, long-acting opioid analgesic when administered via systemic, intrathecal, or epidural routes (Celleno and Capogna, 1989; Miwa et al., 1996; Pergolizzi et al., 2010), and it has a systemic potency of 20 to 70 times that of morphine (Kress, 2009; Pergolizzi et al., 2010; Kawamoto et al., 2011). Our studies have shown that intrathecal buprenorphine effectively produces antihypersensitive effects against neuropathic and inflammatory pain. The antiallodynic effects of buprenorphine are mainly mediated by MOP receptors as spinally administered NOP antagonist did not alter buprenorphine’s antiallodynic effects (Fig. 7). MOP-mediated antiallodynia may be expected due to its high binding affinity and intrinsic activity at MOP receptors as well as MOP-mediated antinociception in rodents (Schmauss and Yaksh, 1984; Bernatzky and Jurna, 1986; Tejwani and Rattan, 2002) and nonhuman primates (Cremeans et al., 2012).
However, it was recently documented in rodents that antinociception produced by systemic buprenorphine is potentiated in the presence of systemically administered NOP antagonists and in NOP-receptor knockout mice (Lutfy et al., 2003; Ding and Raffa, 2009; Khroyan et al., 2009). Given that buprenorphine has an extremely low binding affinity at NOP versus MOP (Ki = 285 versus 0.08 nM) and is much less potent in activating NOP receptors in vitro (EC50 = 35 versus 0.08 nM) (Huang et al., 2001), it seems unlikely that intrathecal buprenorphine activates the spinal NOP receptors at antiallodynic doses. In particular, with well-justified doses of MOP and NOP antagonists (Fig. 6), our data indicate that buprenorphine’s susceptibility to NOP versus MOP antagonism is different from the bifunctional NOP/MOP ligands BU08028 and SR16435 (Fig. 7).
In the clinical setting, development of tolerance to analgesia during long-term treatment with opioids is often reported and poses a major challenge in pain management. The final part of the study compared development of tolerance to the antiallodynic effects of SR16435 and buprenorphine after repeated intrathecal administration (Fig. 8). Our hypothesis was that a compound that simultaneously activates NOP and MOP receptors could have reduced or slower tolerance development as fewer receptors will be used to obtain antiallodynia, thus keeping more receptors available for the subsequent treatment. The antiallodynic effects of intrathecal buprenorphine declined relatively faster, with up to 35% reduction on day 5 compared with day 1 of the treatment. A smaller change (12%) in the antiallodynic effects of intrathecal SR16435 was observed on day 5 as compared with day 1 of the treatment. These differences between SR16435 and buprenorphine could therefore be attributed to the additional NOP agonist activity of SR16435. Whether a bifunctional ligand with full agonist activity at NOP and MOP will have different rate and degree of tolerance development than a partial NOP/MOP agonist for their antihypersensitive effects is currently unknown. Nevertheless, these findings indicate that bifunctional NOP/MOP agonists may provide effective spinal analgesia with a slower development of tolerance.
This study is the first to directly provide the pharmacological evidence that intrathecally administered bifunctional NOP/MOP ligands are not only as effective as but also more potent than the selective full NOP or MOP agonists for their antihypersensitive effects against neuropathic and inflammatory pain in mice. Studies in rodents and nonhuman primates have previously shown that activation of NOP potentiates MOP-mediated antinociception but not the MOP-associated side effects such as motor dysfunction, respiratory depression, and pruritus (Tian et al., 1997; Ko and Naughton, 2009; Lin and Ko, 2013; Sukhtankar and Ko, 2013). Analgesic drugs targeting both NOP and MOP receptors may therefore have the desired improved efficacy and wider therapeutic window. Importantly, our data also indicate that such compounds may have a slower tolerance development to their analgesic effects. These findings further highlight the importance of investigating bifunctional NOP/MOP ligands as a novel therapeutic strategy (Spagnolo et al., 2008; Toll et al., 2009; Cremeans et al., 2012; Molinari et al., 2013).
Discovery of the NOP receptor crystal structure has revealed the atomic details of ligand-receptor recognition (Thompson et al., 2012), which will facilitate the synthesis of novel compounds with optimum NOP/MOP-binding properties to achieve effective analgesia with reduced side effects and slower tolerance development. Together, our study demonstrates that at the spinal level in rodents NOP receptors mediate similar antihypersensitive effects as seen in nonhuman primates and thus provides a platform to further validate the antinociceptive properties of bifunctional NOP/MOP ligands in primate models. These preclinical studies therefore establish a translational bridge to the therapeutic profile of bifunctional NOP/MOP ligands as spinal analgesics in humans.
Acknowledgments
The authors thank Matthew Zaks, Colette Cremeans, and Erin Gruley for technical assistance with data collection.
Abbreviations
- BU08028
(2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol
- J-113397
1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one
- MOP
μ-opioid receptors
- %MPE
percentage of the maximum possible effect
- NOP
nociceptin/orphanin FQ receptors
- Ro 64-6198
(1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one
- SCH221510
3-endo-8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol
- SR16435
1-(1-(2,3,3α,4,5,6-hexahydro-1H-phenalen-1-yl)piperidin-4-yl)-indolin-2-one
Authorship Contributions
Participated in research design: Sukhtankar, Ko.
Conducted experiments: Sukhtankar.
Contributed new reagents: Zaveri, Husbands.
Performed data analysis: Sukhtankar, Ko.
Wrote or contributed to the writing of the manuscript: Sukhtankar, Zaveri, Husbands, Ko.
Footnotes
This study was supported by the National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant R01-AR-059193]; and by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA-032568]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Bennett GJ, Xie YK. (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107 [DOI] [PubMed] [Google Scholar]
- Bernatzky G, Jurna I. (1986) Intrathecal injection of codeine, buprenorphine, tilidine, tramadol and nefopam depresses the tail-flick response in rats. Eur J Pharmacol 120:75–80 [DOI] [PubMed] [Google Scholar]
- Briscini L, Corradini L, Ongini E, Bertorelli R. (2002) Up-regulation of ORL-1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur J Pharmacol 447:59–65 [DOI] [PubMed] [Google Scholar]
- Calo G, Guerrini R. (2013) Medicinal chemistry, pharmacology, and biological actions of peptide ligands selective for the NOP receptor, in Research and Development of Opioid-Related Analgesics (Ko MC, Husbands SM, eds) pp 275–325, ACS Books, Washington, DC [Google Scholar]
- Celleno D, Capogna G. (1989) Spinal buprenorphine for postoperative analgesia after caesarean section. Acta Anaesthesiol Scand 33:236–238 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63 [DOI] [PubMed] [Google Scholar]
- Choi SS, Lee HK, Shim EJ, Kwon MS, Seo YJ, Lee JY, Suh HW. (2004) Alterations of c-Fos mRNA expression in hypothalamic-pituitary-adrenal axis and various brain regions induced by intrathecal single and repeated substance P administrations in mice. Arch Pharm Res 27:863–866 [DOI] [PubMed] [Google Scholar]
- Clark MJ, Furman CA, Gilson TD, Traynor JR. (2006) Comparison of the relative efficacy and potency of mu-opioid agonists to activate Gα(i/o) proteins containing a pertussis toxin-insensitive mutation. J Pharmacol Exp Ther 317:858–864 [DOI] [PubMed] [Google Scholar]
- Courteix C, Coudoré-Civiale MA, Privat AM, Pélissier T, Eschalier A, Fialip J. (2004) Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain 110:236–245 [DOI] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. (1977) Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, Ko MC. (2012) Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther 343:72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Inturrisi CE. (1999) d-Methadone blocks morphine tolerance and N-methyl-d-aspartate-induced hyperalgesia. J Pharmacol Exp Ther 289:1048–1053 [PubMed] [Google Scholar]
- Ding Z, Raffa RB. (2009) Identification of an additional supraspinal component to the analgesic mechanism of action of buprenorphine. Br J Pharmacol 157:831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462 [DOI] [PubMed] [Google Scholar]
- Fairbanks CA. (2003) Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev 55:1007–1041 [DOI] [PubMed] [Google Scholar]
- Hao JX, Xu IS, Wiesenfeld-Hallin Z, Xu XJ. (1998) Anti-hyperalgesic and anti-allodynic effects of intrathecal nociceptin/orphanin FQ in rats after spinal cord injury, peripheral nerve injury and inflammation. Pain 76:385–393 [DOI] [PubMed] [Google Scholar]
- Hopkins E, Rossi G, Kest B. (2004) Sex differences in systemic morphine analgesic tolerance following intrathecal morphine injections. Brain Res 1014:244–246 [DOI] [PubMed] [Google Scholar]
- Hu E, Calò G, Guerrini R, Ko MC. (2010) Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain 148:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. (2001) Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 297:688–695 [PubMed] [Google Scholar]
- Jia Y, Linden DR, Serie JR, Seybold VS. (1998) Nociceptin/orphanin FQ binding increases in superficial laminae of the rat spinal cord during persistent peripheral inflammation. Neurosci Lett 250:21–24 [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Tatsumi K, Kataoka T, Kamikawa T, Yanagida T, Mandai R. (2011) Comparison of intrathecal morphine and buprenorphine for postoperative analgesia in cesarean delivery. [article in Japanese]. Masui 60:892–896 [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Cami-Kobeci G, Husbands SM, Zaveri NT, Toll L. (2011a) The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther 336:952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L. (2009) Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J Pharmacol Exp Ther 331:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Orduna J, Montenegro J, Jiang F, Zaveri NT, Toll L. (2011b) Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: studies with bifunctional NOP/μ receptor agonists in the sciatic nerve ligation chronic pain model in mice. J Pharmacol Exp Ther 339:687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, Toll L. (2007) SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther 320:934–943 [DOI] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. (2009) Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain 10:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. (2009) Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34:2088–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress HG. (2009) Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain 13:219–230 [DOI] [PubMed] [Google Scholar]
- Lambert DG. (2008) The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 7:694–710 [DOI] [PubMed] [Google Scholar]
- Largent-Milnes TM, Vanderah TW. (2010) Recently patented and promising ORL-1 ligands: where have we been and where are we going? Expert Opin Ther Pat 20:291–305 [DOI] [PubMed] [Google Scholar]
- Lewis JW. (1985) Buprenorphine. Drug Alcohol Depend 14:363–372 [DOI] [PubMed] [Google Scholar]
- Lin AP, Ko MC. (2013) The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci 4:214–224 DOI: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, et al. (2003) Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23:10331–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535 [DOI] [PubMed] [Google Scholar]
- Miwa Y, Yonemura E, Fukushima K. (1996) Epidural administered buprenorphine in the perioperative period. Can J Anaesth 43:907–913 [DOI] [PubMed] [Google Scholar]
- Molinari S, Camarda V, Rizzi A, Marzola G, Salvadori S, Marzola E, Molinari P, McDonald J, Ko MC, Lambert DG, et al. (2013) [Dmt1]N/OFQ(1-13)-NH2: a potent nociceptin/orphanin FQ and opioid receptor universal agonist. Br J Pharmacol 168:151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B. (2005) Spinal and local peripheral antiallodynic activity of Ro64-6198 in neuropathic pain in the rat. Pain 116:17–25 [DOI] [PubMed] [Google Scholar]
- Pan Z, Hirakawa N, Fields HL. (2000) A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron 26:515–522 [DOI] [PubMed] [Google Scholar]
- Pergolizzi J, Aloisi AM, Dahan A, Filitz J, Langford R, Likar R, Mercadante S, Morlion B, Raffa RB, Sabatowski R, et al. (2010) Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract 10:428–450 [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270:792–794 [DOI] [PubMed] [Google Scholar]
- Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM. (2008) Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice: comparison to morphine. Eur J Pharmacol 579:141–148 [DOI] [PubMed] [Google Scholar]
- Schmauss C, Yaksh TL. (1984) In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther 228:1–12 [PubMed] [Google Scholar]
- Sounvoravong S, Takahashi M, Nakashima MN, Nakashima K. (2004) Disability of development of tolerance to morphine and U-50,488H, a selective kappa-opioid receptor agonist, in neuropathic pain model mice. J Pharmacol Sci 94:305–312 [DOI] [PubMed] [Google Scholar]
- Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, Khroyan TV, Husbands SM, Lewis JW, Toll L, et al. (2008) Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol 153:609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhtankar DS, Ko MC. (2013) Pharmacological investigation of NOP-related ligands as analgesics without abuse liability, in Research and Development of Opioid-Related Analgesics (Ko MC, Husbands SM, eds) pp 393–416, ACS Books, Washington, DC [Google Scholar]
- Tejwani GA, Rattan AK. (2002) The role of spinal opioid receptors in antinociceptive effects produced by intrathecal administration of hydromorphone and buprenorphine in the rat. Anesth Analg 94:1542–1546 table of contents [DOI] [PubMed] [Google Scholar]
- Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, et al. (2012) Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 485:395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, Han JS. (1997) Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol 120:676–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O’Brien A, White A, et al. (1998) Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr 178:440–466 [PubMed] [Google Scholar]
- Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. (2009) Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther 331:954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]