Abstract
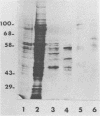
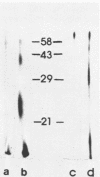
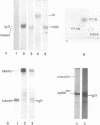
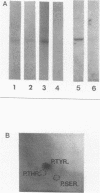
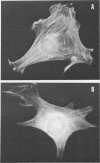
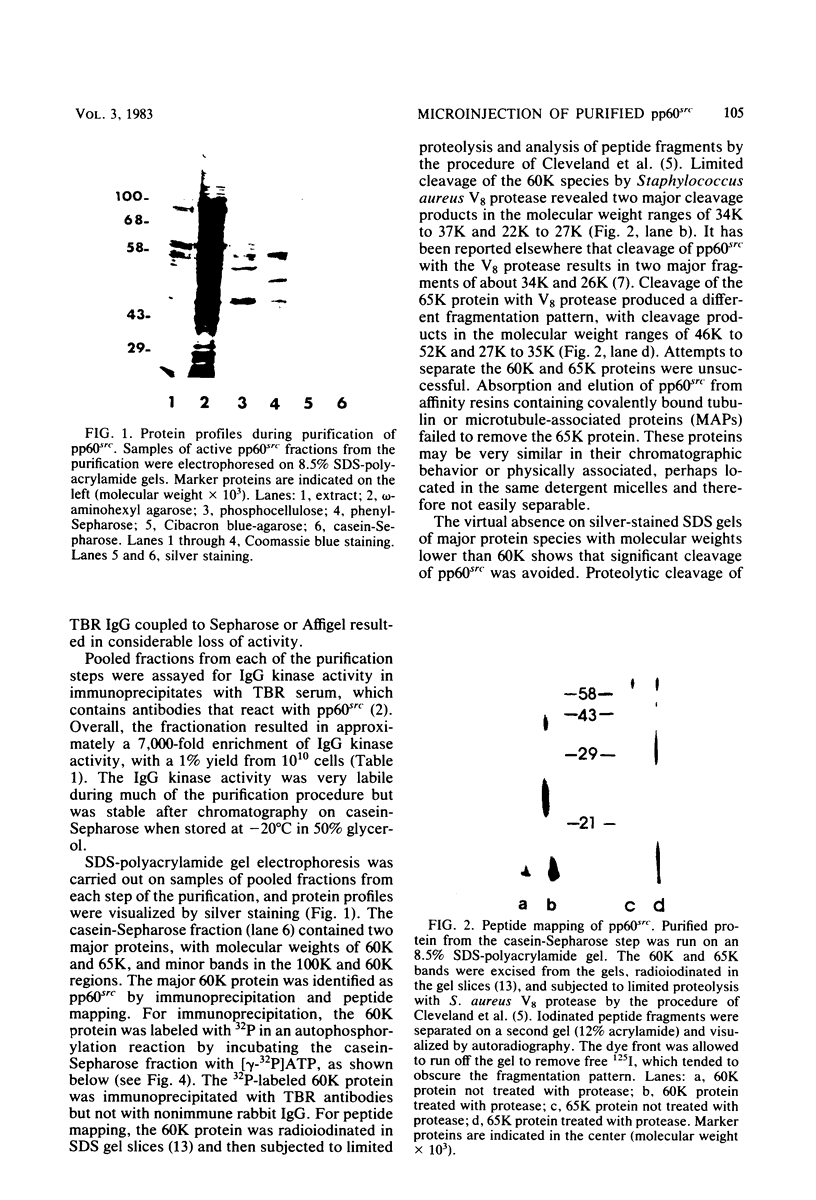
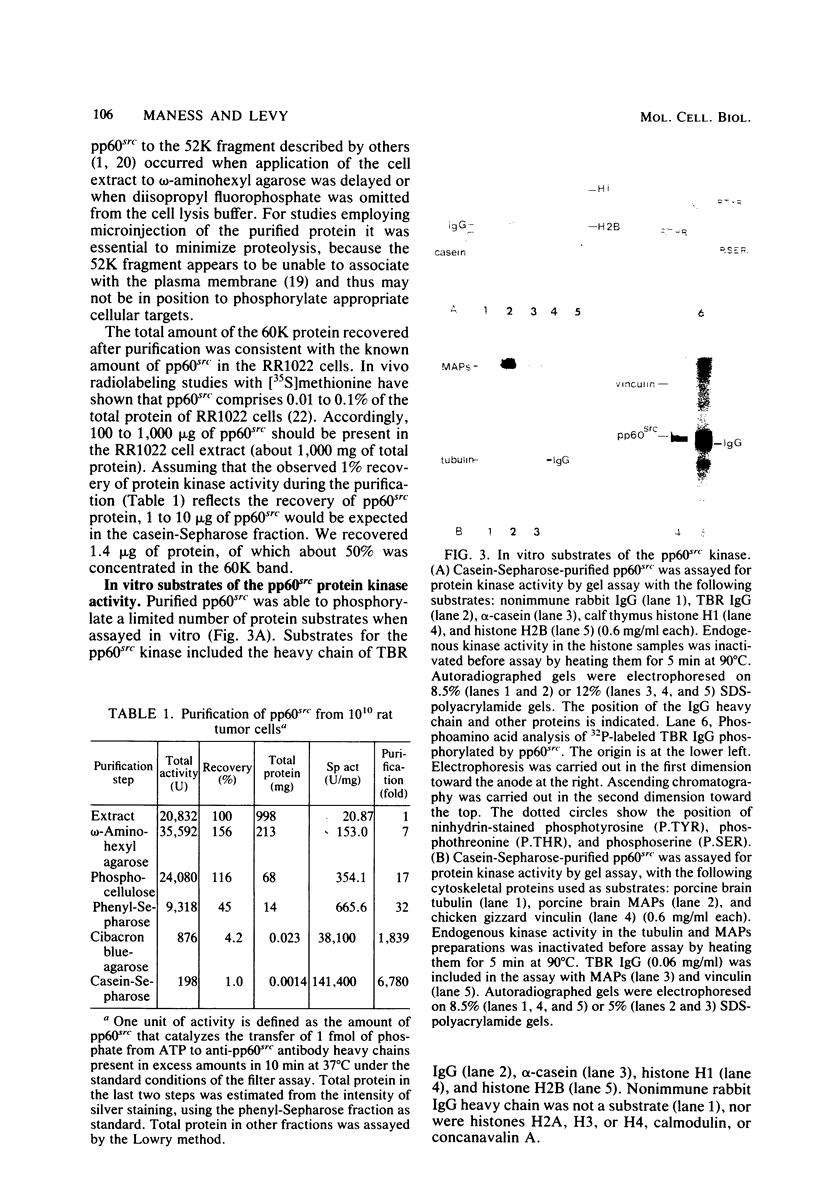
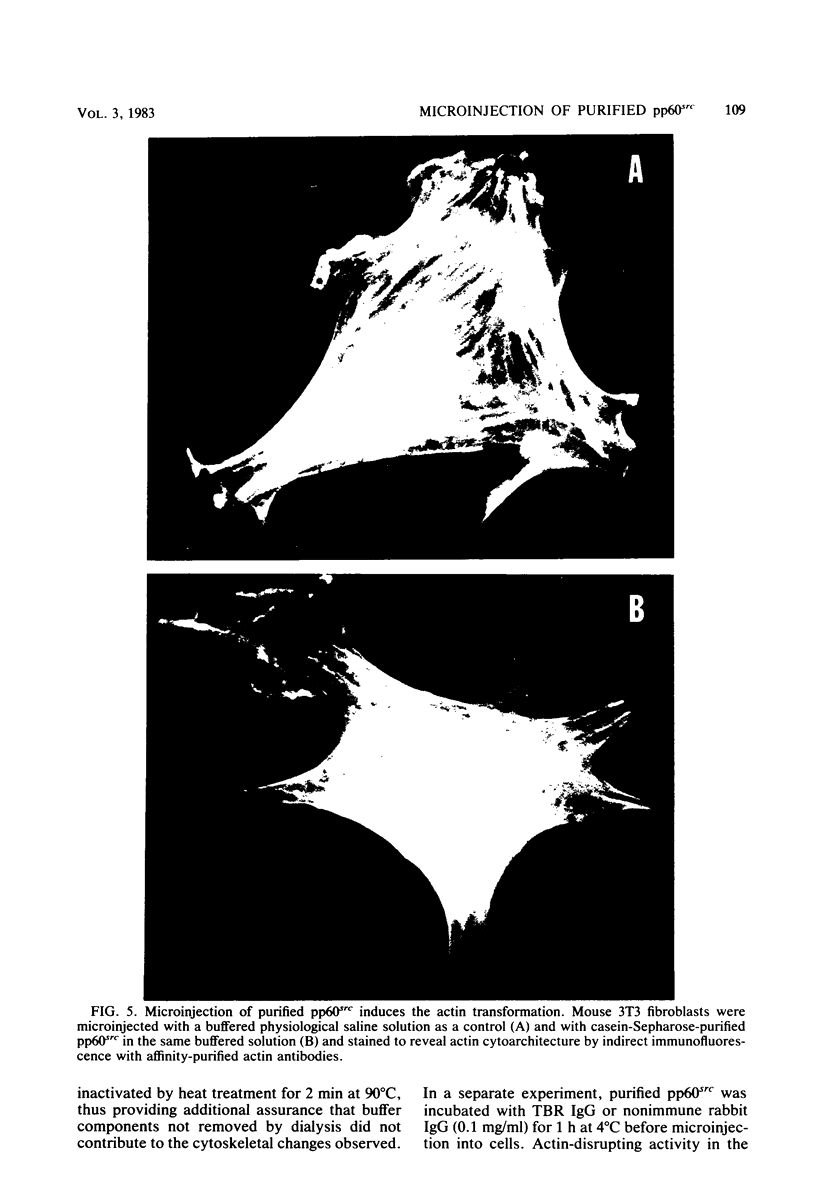
The src gene product of Rous sarcoma virus (pp60src) was highly purified from a rat tumor cell line and shown to have physiological actin transformation activity in a cellular microinjection assay that measures the dissolution of actin microfilament bundles in vivo. The purified pp60src fraction consisted of two major proteins, seen on silver-stained sodium dodecyl sulfate-polyacrylamide gels: a 60,000-dalton (60K) protein, identified as pp60src by immunoprecipitation with tumor-bearing rabbit immunoglobulin G (IgG) and peptide mapping, and an unrelated 65K protein. There was no evidence for proteolytic cleavage of pp60src. A 7,000-fold purification of the tyrosine-specific protein kinase activity of pp60src was achieved by this procedure. Purified pp60src phosphorylated tumor-bearing rabbit IgG heavy chains, casein, histones H1 and H2B, tubulin, and microtubule-associated proteins when assayed in vitro. When incubated with [γ-32P]ATP in the absence of exogenous phosphoacceptor substrates, purified pp60src became labeled with 32P at the tyrosine residues exclusively. Phosphatase and cyclic AMP-dependent protein kinase activities were undetectable in the purified fraction. Microinjection of highly purified pp60src into the cytoplasm of normal Swiss 3T3 mouse fibroblasts caused rapid and reversible dissolution of actin stress fibers, as visualized by indirect immunofluorescence with actin antibodies. The actin-disrupting activity was thermolabile and sensitive to inhibition by coinjection of tumor-bearing rabbit IgG, and purified to about the same extent (8,000-fold) as did the IgG kinase activity of pp60src, thus implicating pp60src as the active agent. Examination of actin-associated proteins as substrates for the pp60src kinase in vitro showed that vinculin was phosphorylated directly by pp60src, although to a small extent. Actin, myosin, and tropomyosin were not phosphorylated. Thus, pp60src purified by this procedure retains native functional properties and provides a useful probe for analyzing transformation-dependent changes in actin cytoarchitecture.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blithe D. L., Richert N. D., Pastan I. H. Purification of a tyrosine-specific protein kinase from Rous sarcoma virus-induced rat tumor. J Biol Chem. 1982 Jun 25;257(12):7135–7142. [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980 Mar;19(3):587–595. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Bunte T., Owada M. K., Moelling K. Biochemical characterization of pp60src-associated protein kinase from avian sarcoma virus Schmidt-Ruppin strain. J Biol Chem. 1981 Aug 25;256(16):8786–8794. [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979 Sep;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Rous sarcoma virus transforming protein, p60src, expressed in E. coli, functions as a protein kinase. Nature. 1981 Dec 24;294(5843):771–773. doi: 10.1038/294771a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Goldberg A. R. Evidence that the src gene product of Rous sarcoma virus is membrane associated. Virology. 1980 Feb;101(1):25–40. doi: 10.1016/0042-6822(80)90480-8. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Varmus H. E., Bishop J. M. The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. J Biol Chem. 1980 Dec 25;255(24):11973–11980. [PubMed] [Google Scholar]
- Maness P. F., Engeser H., Greenberg M. E., O'Farrell M., Gall W. E., Edelman G. M. Activities of the src-gene product of avian sarcoma virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):949–958. doi: 10.1101/sqb.1980.044.01.102. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Engeser H., Greenberg M. E., O'Farrell M., Gall W. E., Edelman G. M. Characterization of the protein kinase activity of avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5028–5032. doi: 10.1073/pnas.76.10.5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R., Nakamura K. D., Weber M. J. Identification of phosphotyrosine-containing proteins in untransformed and Rous sarcoma virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1982 Jun;2(6):653–665. doi: 10.1128/mcb.2.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain D. A., Maness P. F., Edelman G. M. Assay for early cytoplasmic effects of the src gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2750–2754. doi: 10.1073/pnas.75.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Levinson A. D. Bacterial expression of an enzymatically active protein encoded by RSV src gene. Nature. 1982 Feb 4;295(5848):423–425. doi: 10.1038/295423a0. [DOI] [PubMed] [Google Scholar]
- Purchio A. F. Evidence the pp60src, the product of the Rous sarcoma virus src gene, undergoes autophosphorylation. J Virol. 1982 Jan;41(1):1–7. doi: 10.1128/jvi.41.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert N. D., Blithe D. L., Pastan I. Properties of the src kinase purified from Rous sarcoma virus-induced rat tumors. J Biol Chem. 1982 Jun 25;257(12):7143–7150. [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Patschinsky T., Berdot C., Hunter T., Elliott T. Phosphorylation and metabolism of the transforming protein of Rous sarcoma virus. J Virol. 1982 Mar;41(3):813–820. doi: 10.1128/jvi.41.3.813-820.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver K., Rohrschneider L. Organization of pp60src and selected cytoskeletal proteins within adhesion plaques and junctions of Rous sarcoma virus-transformed rat cells. J Cell Biol. 1981 Jun;89(3):525–535. doi: 10.1083/jcb.89.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G. Microinjection studies of duck globin messenger RNA translation in human and avian cells. Cell. 1976 Dec;9(4 Pt 2):725–732. doi: 10.1016/0092-8674(76)90136-7. [DOI] [PubMed] [Google Scholar]
- Wang E., Goldberg A. R. Changes in microfilament organization and surface topogrophy upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4065–4069. doi: 10.1073/pnas.73.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]