Abstract
Current functional MRI techniques measure hemodynamic changes induced by neural activity. Alternative measurement of signals originated from tissue is desirable and may be achieved using T1ρ, the spin-lattice relaxation time in the rotating-frame, which is measured by spin-lock MRI. Functional T1ρ changes in the brain can have contributions from vascular dilation, tissue acidosis, and potentially other contributions. When the blood contributions were suppressed with a contrast agent at 9.4 T, a small tissue-originated T1ρ change was consistently observed at the middle cortical layers of cat visual cortex during visual stimulation, which had different dynamic characteristics compared to hemodynamic fMRI such as a faster response and no post-stimulus undershoot. Functional tissue T1ρ is highly dependent on the magnetic field strength and experimental parameters such as the power of the spin-locking pulse. With a 500 Hz spin-locking pulse, the tissue T1ρ without the blood contribution increased during visual stimulation, but decreased during acidosis-inducing hypercapnia and global ischemia, indicating different signal origins. Phantom studies suggest that it may have contribution from concentration decrease in metabolites. Even though the sensitivity is much weaker than BOLD and its exact interpretation needs further investigation, our results show that non-hemodynamic functional signal can be consistently observed by spin-lock fMRI.
Keywords: tissue-specific, functional MRI, non-hemodynamics, spin-lock, metabolism
Introduction
The ability of functional magnetic resonance imaging (fMRI) to detect brain function noninvasively with high spatial resolution opens great opportunities to advance our understanding of brain organization and function, and may potentially provide new methods to help the diagnosis and treatment of neurological diseases. Elevated neuronal activity increases oxygen and glucose metabolism in tissue and modulates hemodynamics including blood flow, volume and oxygenation level. Currently, almost all fMRI techniques are based on hemodynamic responses thus their spatial and temporal resolutions are intrinsically limited by vasculature density and regulation (Kim and Ogawa, 2012). Moreover, the exact correlation between hemodynamic-based fMRI and neuronal activation is still not fully understood and remains a concern for many neuroscientists (Editorial, 2009). There has been wide interest to investigate tissue-originated fMRI signals that are not based on hemodynamics and have closer correlation with the neuronal activities. However, despite some encouraging results in phantom, in vitro and non-brain studies, in vivo brain imaging of neuronal activity directly or a change in neuronal cell microstructure has been challenging, and the robustness of detection is also controversial (Bandettini et al., 2005; Chu et al., 2004; Jin and Kim, 2008b; Le Bihan et al., 2006; Miller et al., 2007; Park et al., 2004; Parkes et al., 2007; Tang et al., 2008; Truong and Song, 2006; Xiong et al., 2003).
Functional changes in the brain tissue microenvironment/microstructure may be detected with a T1ρ contrast. When a B1 spin-locking (SL) pulse is applied along the direction of transverse magnetization immediately after a non-selective 90° excitation on the resonance frequency of water, the magnetization is “locked” by the B1 pulse and decays with a time constant T1ρ, which is named as the spin-lattice relaxation time in the rotating-frame. The nutation frequency of the SL pulse, ω1 = γB1, is usually several kHz or less for in vivo studies, and T1ρ relaxation is sensitive to the water fluctuations caused by physical processes with correlation time τ close to 1/2πω1. Recent studies have demonstrated that proton exchange between bulk water and labile protons of protein or metabolites is an important contributor for the low-frequency T1ρ dispersion (the dependency of T1ρ on ω1) in biological tissue (Duvvuri et al., 2001; Jin et al., 2011; Makela et al., 2001). Due to its sensitiveness to the local tissue microenvironment and microstructure such as the pH level, the protein density and composition, T1ρ contrast has been applied in many pathological studies such as cerebral ischemia (Grohn et al., 2000; Kettunen et al., 2001), and neurodegenerative diseases (Borthakur et al., 2006; Michaeli et al., 2007).
Functional increases in T1ρ have been observed during visual stimulation in humans (Hulvershorn et al., 2005; Magnotta et al., 2012). Very recently, Magnotta et al. showed that activation-induced increase of T1ρ is better localized to tissue than conventional BOLD fMRI, suggesting that T1ρ is an attractive fMRI contrast. The source of T1ρ increase has been attributed to local tissue acidosis because T1ρ is sensitive to tissue pH via chemical exchange processes (Magnotta et al., 2012). However, earlier studies suggested that a local increase in cerebral blood volume (CBV) is the dominant factor of functional T1ρ increase (Hulvershorn et al., 2005) because blood T1ρ is much longer than tissue T1ρ (Hulvershorn et al., 2005; Kettunen et al., 2002; Magnotta et al., 2012). Since T1ρ is affected by the blood oxygenation (Hulvershorn et al., 2005; Kettunen et al., 2002), intravascular T1ρ change may also contribute. Additionally, other changes, such as a functional change in the cerebrospinal fluid (CSF) volume fraction (Jin and Kim, 2010), or a decrease in the concentration of metabolites, may also affect functional T1ρ signal. Since the utility of T1ρ fMRI is closely dependent on its signal origin, it is critical to untangle multiple potential sources.
This study aims to address two issues regarding T1ρ fMRI signal: 1) whether the signal originated from tissue, not blood can be detected, and 2) if detectable, what might be its possible source. The task-induced T1ρ response was evaluated in three steps. First, to separate the contributions of tissue versus blood and CSF to functional T1ρ response and to examine the detectability of tissue-originated T1ρ fMRI signal, high-resolution functional studies were performed on the cat visual cortex without and with suppression of the blood signal by using an intravascular contrast agent. Then, temporal and spatial properties of the T1ρ response were assessed. Second, to investigate potential sources of functional tissue T1ρ change, T1ρ experiments were performed in rats with the suppression of blood signals during hyperoxia, pH-decreasing hypercapnia and global ischemia, and susceptibility variations induced by i.v. injection of iron oxide. Third, to better understand possible source of in vivo T1ρ change, T1ρ was measured in protein and metabolite phantoms with different pH and metabolite concentrations. Since T1ρ of tissue water measured with different SL frequencies are sensitive to molecular motion with different correlation times (Duvvuri et al., 2001; Makela et al., 2001), two or more ω1 values were studied to shed light on the underlying mechanism. Throughout the whole text, we will use “tissue T1ρ” to indicate T1ρ measured after the suppression of the blood signal. Preliminary findings were reported as meeting abstracts previously (Jin and Kim, 2009, 2011).
Materials and Methods
Theoretical basis of spin-lock fMRI
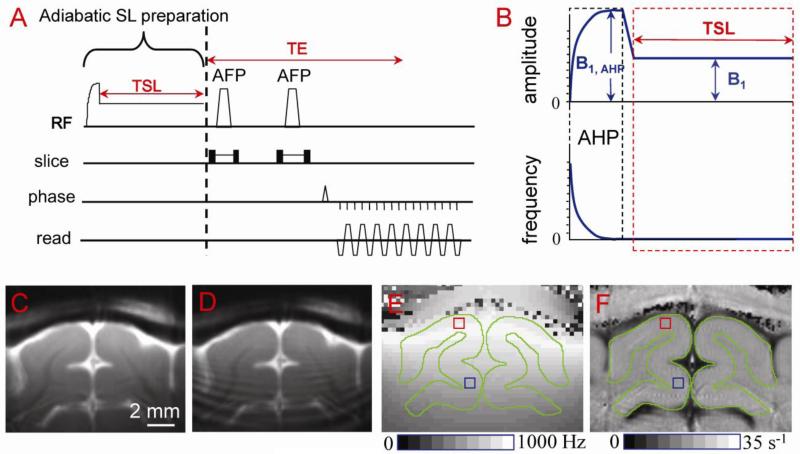
The pulse sequence for the SL experiment was a double spin-echo echo-planar imaging (EPI) sequence with a non-selective adiabatic SL preparation (Fig. 1A), where the amplitude and frequency modulation of the adiabatic SL preparation is illustrated in Fig. 1B. Namely, a 2-ms adiabatic half passage pulse was followed by a ramp of 0.5 ms, during which the amplitude of radiofrequency (RF) pulse was decreased to the desired SL field (B1) and then held constant for the spin-locking time (TSL) (Grohn et al., 2005; Jin and Kim, 2010). Following the spin locking preparation, transverse spins were refocused using two adiabatic full-passage RF pulses with slice-selection gradients, therefore, T1ρ-weighted images are additionally weighted by T2 during the echo time (TE).
Fig. 1. Surface-coil T1ρ pulse sequence (A, B) and applications to cat primary visual cortex studies (C-F).
The pulse sequence (A) is a double spin-echo EPI acquisition with a non-selective spin-locking (SL) preparation (B). Adiabatic half passage (AHP, 2 ms in length with amplitude B1, AHP) ensures that spins nutate into the transverse plane over a relatively large volume of B1 inhomogeneity. The RF amplitude is then immediately ramped to the desired SL level (B1) and held constant for the spin-lock time (TSL); the transmit frequency of the pulse remains the same during the short ramp and TSL. Following the spin-lock preparation, transverse spins are refocused by two adiabatic full-passage (AFP) pulses with slice-selection gradients. High quality T1ρ-weighted image (ω1 = 500 Hz and TSL = 40 ms) can be obtained when adiabatic SL condition is satisfied (C), but signal oscillations appear otherwise (D). (E) The spin-locking nutation frequency ω1 map shows B1 inhomogeneity. For our conditions, ω1 at an area proximal to the coil (red square) is ~600 Hz, while at a distal area (blue square) it is ~ 400 Hz. Gray matter areas are outlined in green. (F) The map of R1ρ (=1/T1ρ) shows little spatial variations within visual cortex because the T1ρ dispersion is very small within the narrow ω1 range (e.g., 400-600 Hz).
If a voxel contains multiple compartments such as blood, tissue and CSF, the TSL-dependent MR signal can be expressed as
| [1] |
where i = arterial blood, venous blood, tissue water and CSF, and V and M are the volume fraction and the water proton magnetization of each compartment at TSL and TE = 0, respectively. In order to determine T1ρ, T1ρ-weighted images are generally acquired with multiple TSL values and then fitted by a mono-exponential function of TSL. The apparent T1ρ obtained will have contributions from all compartments, thus the data interpretation is complex. In particular, the apparent T1ρ may be modulated by a change of T1ρ value in any compartment or a change of any one of the weighting functions (e.g., V, M, or T2).
When the blood signal is suppressed, the signal in parenchyma (without CSF) would reduce to a single compartment:
| [2] |
Hence, the tissue T1ρ can be extracted by multiple TSL measurements from high-resolution fMRI data without CSF. The functional change in total T1ρ obtained with Eq. [1] may surrogate the change in tissue T1ρ only if the contributions from other compartments are small and do not change significantly during activation.
R1ρ (=1/T1ρ) equals R2,0 + Rex where Rex is the chemical exchange-mediated relaxation rate, and R2,0 is the transverse relaxation rate in the absence of chemical exchange. For a simple two-pool SL model with asymmetric population approximation (Jin et al., 2011; Trott and Palmer, 2002), Rex can be described as
| [3] |
or normalized as
| [4] |
where p is the relative population of the labile proton which is assumed to be much smaller than water (i.e., p << 1), and k and δ are the exchange rate and the chemical shift between the labile proton and water, respectively. For a given ω1/δ value, maximum Rex occurs when . Thus, Rex at very low ω1 is optimized for intermediate exchanges (k/δ ~ 1). To understand the contribution of different chemical exchange rates to R1ρ, Rex as a function of ω1/δ was calculated from Eq. [4].
Animal preparation and stimulation
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Female adolescent cats weighing 1.1 - 1.7 kg were used for visual stimulation studies, while Sprague-Dawley rats weighing 318 - 492 g were used for global modulation studies. Cats were treated with atropine sulfate (0.05 mg/kg, I.M.) and initially anesthetized with a cocktail of ketamine (10-25 mg/kg, I.M.) and xylazine (2.5 mg/kg, I.M.), while rats were initially anesthetized with 5% isoflurane. Then, both species were intubated and mechanically ventilated, and 2.0-2.2% isoflurane in a mixture of 70% N2 and 30% oxygen was used during surgery. The femoral vein was cannulated to deliver maintenance fluid with pancuronium bromide (0.2 mg/kg per hour) and to inject contrast agent, and the femoral artery was catheterized to monitor the arterial blood pressure and to obtain blood samples for arterial blood gas measurements. During MRI experiments, the isoflurane level was maintained at 1.1 ± 0.2% isoflurane for cats and 1.5% for rats. End-tidal CO2 level was kept within 3.5 ± 0.5% and the rectal temperature was controlled at 38.5 ± 0.5°C using a water circulating pad. The visual stimuli for cat functional studies were binocular, full-field, black and white, square-wave drifting gratings (spatial frequency 0.15 cycle/degree and temporal frequency 2 cycles/s). A gray screen was presented for the control. For gas challenge studies, a home-built electronic gas switching system was used to switch between two different gas mixtures.
MR experiments
All MRI experiments were performed on a 9.4 T MR system (Varian, Palo Alto, California, USA). For phantom experiments, a 3.8-cm diameter volume coil (Rapid Biomedical, Ohio) was used for both transmission and reception. For in vivo experiments, a custom-made single-loop surface coil with diameter of 1.6 cm was chosen to achieve high spatial resolution and high sensitivity, and also to reduce power deposition induced by a spin-locking pulse. A surface coil setup has been used in many T1ρ studies (Borthakur et al., 2004; Grohn et al., 2005; Hakumaki et al., 2002; Koskinen et al., 2006; Makela et al., 2004; Xu et al., 2008), where the adiabatic SL pulse is essential to nutate all spins within our region of interest (ROI) to the transverse plane. The adiabatic SL condition was determined from simulations of the Bloch Equations, and calibrated to ensure that such condition was achieved for most of the primary visual area. This is further confirmed by evaluating the quality of T1ρ-weighted images (Fig. 1C vs. 1D), such that no ringing or stripe artifact was observed. The T1ρ measurement with the adiabatic SL sequence using a surface coil had been validated previously by phantom studies with a conventional SL sequence using a homogeneous coil (data not shown).
To evaluate the spatial heterogeneity of RF fields, the B1 map was measured using a square pulse (to replace the SL preparation pulse in Fig. 1), where the pulse length was incremented such that signal intensity oscillated for several cycles in each imaging voxel, and the nutation frequency of this oscillation (ω1) was obtained (Jin and Kim, 2010). The spatial heterogeneity of B1 within our ROIs was negligible for rat studies (see Fig. 5A inset below), but significant for cat experiments where the ROI were much larger. In a representative ω1 map of cat brain (Fig. 1E), the ω1 frequency was about 600 Hz at a region close to the coil (red square) and 400 Hz at a region distal from the coil (blue square). Nevertheless, the R1ρ maps showed very small spatial variance within the cat dorsal cortical area because the R1ρ dispersion is small in this low frequency range (Fig. 1F). To ensure similar ω1 was applied to all animals, the middle cortical ROI (see below) was first determined from anatomical images, then the transmit power was adjusted so that the averaged ω1 during TSL reached the targeted value, e.g., 500 or 2000 Hz. The averaged ω1 and R1ρ values on each ROI were reported in texts.
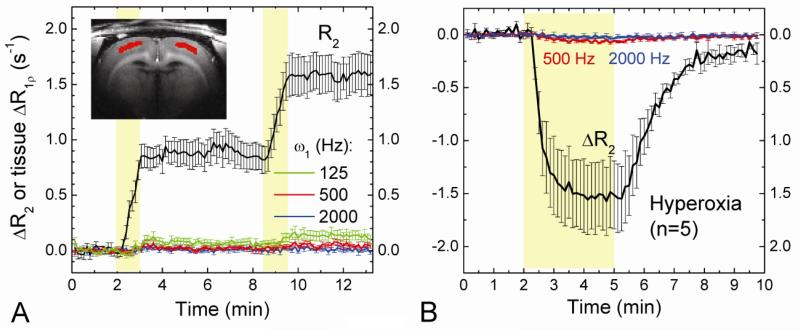
Fig. 5. Effects of intravascular susceptibility variation and hyperoxia challenge on extravascular water R1ρ.
In order to detect the contribution of intravascular susceptibility changes to tissue R1ρ, the blood signal was suppressed with the injection of 5 mg/kg MION before experiments. Dynamic changes in tissue R2 and R1ρ were obtained during two injections of 1 mg/kg MION (A, n = 4 rats) and 3 minutes inhalation of 60% O2 (B, n = 5 rats) indicated by the yellow shaded regions. Time courses were obtained from the red pixels within the cortex (Inset). When spin-locking frequencies were measured at ≥500 Hz, a variation in intravascular susceptibility does not contribute to tissue R1ρ measurements.
In vivo experiments
FLASH or T1-weighted spin-echo EPI was used to obtain anatomic images with 128 × 128 matrix. Single slice was chosen for in vivo functional studies. In cats, multi-slice scout fMRI was used for the selection of the imaging slice. To suppress the intravascular signal, dextran-coated monocrystalline iron oxide nanoparticles (MION) were injected i.v. to greatly shorten blood T2. Five sets of in vivo experiments were performed to measure R1ρ (= 1/T1ρ) changes: 1) R1ρ with and without blood contributions during cat visual stimulation, 2) tissue R1ρ (without blood contributions) at two ω1 values during cat visual stimulation, 3) tissue R1ρ at four ω1 values during two injections of MION, 4) tissue R1ρ at two ω1 values during hypercapnia and hyperoxia, and 5) tissue R1ρ at multiple ω1 values during global ischemia. Specifically, the experimental designs were:
Expt. I: T1ρ fMRI (n = 6 cats) was performed with ω1 = 500 Hz on the visual cortex before and after the injection of 5 mg/kg of MION. The averaged SL frequency was ω1 = 492 ± 26 Hz at a middle cortical ROI (see Fig. 2B below). After the T1ρ fMRI experiments in 5 out of the 6 animals, the CBV response was measured with injection of 10-13 mg/kg MION using double spin-echo EPI with TE of 40 ms to compare the temporal and spatial characteristics. Each fMRI run consisted of 40 s control, 40 s stimulation, and 60 s control; and there was ~1 minute resting time between each run. Twenty to forty runs were averaged for T1ρ fMRI experiments and ~10 runs averaged for CBV experiments.
Expt. II: To examine the effect of SL frequency ω1, tissue T1ρ responses were measured after the injection of 5 mg/kg of MION during cat visual stimulation at two ω1 values of 500 and 2000 Hz (n = 6 cats). The stimulation paradigm for cat visual stimulation was 20 s control, followed by 20 s stimulation and then 30 s control.
Expt. III: To confirm that tissue R1ρ measurement is insensitive to the intravascular susceptibility with ω1 values applied in our studies, 2 mg/kg of MION were initially injected to suppress the blood signal. Then, dynamic changes of tissue R2 and R1ρ with ω1 of 125, 250, 500, and 2000 Hz (n = 4 rats) were measured during two separate injections of 1 mg/kg MION.
Expt. IV: Tissue T1ρ responses were measured after the injection of 5 mg/kg of MION during hyperoxic (60% O2, n = 5 rats) and hypercapnic stimulations (8% CO2, n = 5 rats) at ω1 values of 500 and 2000 Hz. Hyperoxic and hypercapnic stimulations were achieved by inhalation of 60% of O2 for 3 minutes and 8% CO2 for 6 minutes, respectively. Hyperoxia challenge induces a drop in cerebral blood flow (CBF) and CBV (Lu et al., 2009), as well as an increase in the intravascular and extravascular oxygen level. On the other hand, hypercapnia challenge induces an increase of CBF and CBV, and a decrease in tissue pH.
Expt. V: Tissue T1ρ responses were measured after the injection of 1-5 mg/kg of MION during global ischemia induced by KCl injection, with ω1 values of 250, 500, 1000, 2000 and 4000 Hz (n = 6 rats).
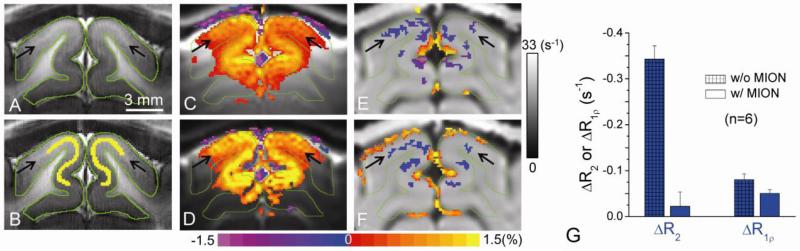
Fig. 2. Intravascular and extravascular contributions to functional R1ρ change in the cat visual cortex.
A slightly bright band (indicated by black arrows) within the gray matter (outlined in green) in high-resolution T1-weighted EPI image (A) indicates cortical layer IV (Kim and Kim, 2011). Pixels along the white band (yellow) were chosen for quantitative analyses of cat's functional studies (B). All active pixels were overlaid in color on their respective baseline images (C-F), and the vertical grayscale bar indicates the baseline R1ρ values (for E and F). Functional percentage signal change maps were obtained by T2-weighted (TSL = 0, C) and T1ρ-weighted fMRI (TSL = 50 ms and ω1 = ~500 Hz, D) without MION during visual stimulation. Higher changes were observed in T1ρ-weighted fMRI, indicating an increase in T1ρ (decrease in R1ρ). To determine the blood contribution, R1ρ change maps obtained without (E) and with (F) 5 mg/kg MION were compared. An increase in R1ρ at the surface of the cortex is due to a volume fraction change between tissue and CSF, while a decrease in R1ρ is located at the middle of the cortex. (G) At the middle cortical ROI (yellow pixels in B), the functional R2 change (ΔR2) is nearly suppressed with MION injection, whereas ΔR1ρ only decreases slightly.
Although measurement of more TSL values would allow better accuracy for T1ρ quantification, it increases scan time and reduces the temporal resolution; therefore, only two TSL values were chosen for dynamic functional studies. In each fMRI experiment, TSL = 0 and TSL = 50 ms images for each ω1 value were acquired in an interleaved manner. For experiments with multiple runs (Expt. I, II, and IV), the order of the images was alternated for different runs. Imaging data were acquired using a 2 × 2 cm2 field of view and a 2 mm slice thickness for cat visual stimulation (Experiment I and II) and rat hypercapnic experiments (Experiment IV), and a 2.56 × 2.56 cm2 field of view and a 4 mm slice thickness for rat hyperoxia (Experiment IV) and global ischemia experiments (Experiment V). The spin-echo time was TE = 25 ms, and the repetition time (TR) was 2.5 s for global ischemia and 2 s for all other experiments. Although 1-2 mg/kg of MION is already sufficient to suppress the blood signal (Kim and Kim, 2005), in fMRI experiments a higher dose of 5 mg/kg were chosen so that the BOLD effect would be roughly canceled by an increase in the MION susceptibility due to vessel dilation (Lu et al., 2007), minimizing any residue effect from susceptibility change to our tissue R1ρ measurement. The intravascular half-life for MION was measured to be 2.5 to 6 hour for cat while ~10 hours for rat. Thus, in all cat experiments with MION injection, the susceptibility effect was frequently monitored by measuring parenchyma T2 values, and additional doses were injected when necessary to maintain a relatively stable MION level.
Phantom experiments
At SL frequencies usually applied for in vivo studies, R1ρ relaxation is highly sensitive to chemical exchange process between water and the labile non-water protons of protein and metabolites (Duvvuri et al., 2001; Jin et al., 2011; Makela et al., 2001). An acidosis-induced T1ρ increase has been suggested by Magnotta et al. as the dominant contributor to the functional signal, which was also illustrated in pH-varied protein phantoms and explained by a slowdown of chemical exchange between amide protons of protein backbone and water (Magnotta et al., 2012). It should be noted that R1ρ relaxation is not specific and has contributions from all relaxation pathways including chemical exchange processes from all labile protons. Besides proteins, T1ρ is also very sensitive to metabolites which may have different pH-dependence on SL frequencies. For example, we have shown that T1ρ is much more sensitive to labile protons with intermediate exchange rate, e.g., amines or hydroxyls groups, as compared to the slow exchanging amides (Jin et al., 2011). In particular, the amine-water proton exchanges from free metabolites are likely much faster than the overall chemical exchange rate of proteins (Zong et al., 2013).
Since chemical exchange contribution to R1ρ relaxation is dependent on pH, to further examine the possible sources of in vivo R1ρ change, three different pH phantoms were studied at 37°C:
#1.1. 8% of native bovine serum albumin (BSA) with four pH values of 6.0, 6.5, 7.0, and 7.5,
#1.2. 4% native egg-white albumin (EWA) with three pH values of 6.2, 6.8, and 7.4, and
#1.3. 4% EWA and 30 mM glutamate (Glu) with three pH of 6.2, 6.8, and 7.4.
All Phantoms above were dissolved in phosphate buffer saline (PBS) containing 10 mM of phosphate with 0.1 mM MnCl2.
The purpose of Phantoms #1.1-#1.3 experiments is to qualitatively compare the pH-dependence of protein-only phantom versus protein phantom containing faster chemical exchanging metabolites. Because there are many amine-containing metabolites in brain, 30 mM Glu was chosen to roughly represent the total amine concentration from free metabolites.
The chemical exchange contributions to R1ρ from metabolites are mostly arisen from amine and hydroxyl protons. In order to separate the contributions of amine vs. hydroxyl proton exchanges to R1ρ, two concentration-dependent phantom experiments were performed at 37°C as
#2.1. Glu with amine protons of 0, 10 and 20 mM in 2.2% agarose with 0.07 mM of MnCl2 at pH = 7.0, and
#2.2. glucose (Glc) with hydroxyl protons of 0, 5, 10, 20 mM in PBS with 0.1 mM MnCl2 at pH = 7.0.
The R1ρ dispersion properties of metabolites with hydroxyl versus amine protons will be compared to determine whether they can be differentiated by R1ρ measurements at 500 and 2000 Hz. The exchange-mediated relaxation rate Rex is linearly proportional to the metabolite concentration (Jin et al., 2011). Thus, instead of measuring a small concentration of metabolites directly, where the accuracy may be limited because of the small Rex compared to a much larger intrinsic R2,0 in our phantoms with agarose or MnCl2, we chose an alternative approach to obtain Rex per mM of metabolite by fitting to data with three to four different concentrations.
In all phantoms, agarose and/or MnCl2 was added to decrease the T2 of water closer to in vivo values, but without changing the chemical exchange contributions to R1ρ (Jin and Kim, 2012). For each phantom, R1ρ values were measured for eleven ω1 values of 125 Hz to 4000 Hz, by eleven TSL values (the range was varied for different phantoms, from 0 ms to approximately two T2 values).
Data analysis
Data was analyzed with in-house Matlab® programs and STIMULATE software (Strupp, 1996). For functional experiments, images were first zero-filled to 128 × 128 and smoothed with a Gaussian filter with a full-width-half-maximum of 3 pixels. The EPI images were then compared with the anatomical image, if in-plane motions were more than 1 pixel, then those runs were excluded for signal averaging. Runs with same ω1 and TSL values were grouped together and averaged. Then, in vivo R1ρ images for each time point were calculated from mono-exponential fitting of signal intensities on the two TSL-values (0 and 50 ms), as R1ρ = ln(STSL=0/STSL=50ms)/50ms, and ΔR1ρ was calculated from the difference between the R1ρ values averaged over the activation and baseline periods. In each experiment, T2-weighted BOLD with TSL of 0 ms, T1ρ-weighted fMRI with TSL of 50 ms, and R1ρ fMRI runs were obtained. For Expt. III, the ρR2 time course was also calculated from the linear relationship between the relative signal change and TE as: ΔR2 = −[(S − S0)/S0]/TE (Ogawa et al., 1993), where the baseline signal S0 was defined as the averaged signal before the MION injection.
For fMRI, the Student's t-test was performed on a pixel-by-pixel basis to detect the activated area. First, a p-value threshold was chosen (p < 0.01 for experiment I - III, and p < 0.05 for experiment IV, uncorrected for multiple comparisons), and a minimal cluster size of eight pixels was applied. To calculate the CBV fMRI response, a correction of BOLD effect was performed (Zhao et al., 2006). Quantitative analyses were performed on the ROI determined from the anatomic images, regardless of whether these pixels pass the statistical threshold. Two ROIs were chosen at the middle and surface of the visual cortex for cat studies, respectively, while one ROI was selected at the middle of the cortex for rat studies. To measure the rising times, the time courses of BOLD, CBV, and R1ρ before and after MION for each animal were interpolated to 200 ms temporal resolution, and the time from stimulus onset to 50% of the peak change was obtained, and paired Student's t-test was performed to evaluate the difference in rising time of the BOLD, CBV, and R1ρ responses. The averaged data were reported by mean and standard deviation (SD), and standard error of mean (SEM) for all time courses in the figures.
Results
Functional change of the T1ρ without and with suppression of blood signals
Since an imaging voxel contains blood, extravascular tissue and CSF with different T1ρ values, the change in the relative composition and/or the T1ρ of these compartments will modulate the measured T1ρ value (see Eq. [1]), and it has been reported in human visual stimulation and rat hypercapnia challenge studies that vessel dilation will lengthen T1ρ (Hulvershorn et al., 2005; Kettunen et al., 2002). Thus, it is critical to separate functional T1ρ changes originating from blood and tissue. To address this issue, we performed fMRI studies on six animals with and without suppression of blood signals by MION injection (Experiment I). A well-established layer model of the cat visual cortex was employed to examine whether functional T1ρ changes are localized to the most active sites, as suggested by Magnotta et al. (Magnotta et al., 2012). The middle cortical layer (layer IV) is known to have the highest change in neuronal activities and in metabolic rate during stimulation (Price, 1983; Tieman and Tumosa, 1983) and can be visualized as a slightly bright band (indicated by arrows) in the cat visual cortex (outlined by green contours) in T1-weighted inversion recovery echo-planar images (Fig. 2A) (Jin and Kim, 2008c; Kim and Kim, 2011).
The T2-weighted fMRI map before MION injection shows significant stimulation-induced signal increase in gray matter (outlined in green) within the visual cortex (Fig. 2C) as expected from BOLD contrast (Zhao et al., 2006). When T1ρ-weighting with ω1 of 500 Hz was applied (Fig. 2D), a higher percentage signal change was detected within the parenchyma. Functional R1ρ maps calculated from T2-weighted and T1ρ-weighted fMRI show a decrease in R1ρ (i.e., increase in T1ρ) in the parenchyma for without (Fig. 2E) and with blood suppression (Fig. 2F), indicating that extravascular tissue R1ρ change indeed exists. Note that negative fMRI signal changes (blue/purple pixels in Fig. 2C and 2D) and R1ρ increases (red/yellow pixels in Fig. 2E and 2F) were observed at the boundary of the cortical surface and CSF. This is most likely due to a reduction of CSF partial volume caused by functional vessel dilation (Jin and Kim, 2010; Piechnik et al., 2009; Scouten and Constable, 2008) because R2 and R1ρ values of CSF are much smaller than those of tissue water (e.g., R1ρ of about 2.2 s-1 vs. 21 s-1 at 9.4 T (Jin and Kim, 2010)).
Fig. 2G compared the functional ΔR2 and ΔR1ρ from an ROI drawn at the middle cortical layer disregarding the statistical thresholds for activation (Fig. 2B). Large difference in the functional ΔR2 before and after MION (-0.343 ± 0.029 s-1 vs. -0.022 ± 0.031 s-1) were observed, because for the latter case the BOLD effect was nearly canceled by the vessel dilation-induced increase of MION concentration (Lu et al., 2007). In contrast ~63% of the functional ΔR1ρ remains after the blood signal is suppressed (0.081 ± 0.013 s-1 vs. 0.051 ± 0.008 s-1), indicating that even though intravascular signals contribute to functional ΔR1ρ, tissue R1ρ fMRI signals dominate during visual stimulation at 9.4 T.
Temporal properties of T1ρ fMRI signal at the middle cortical layer
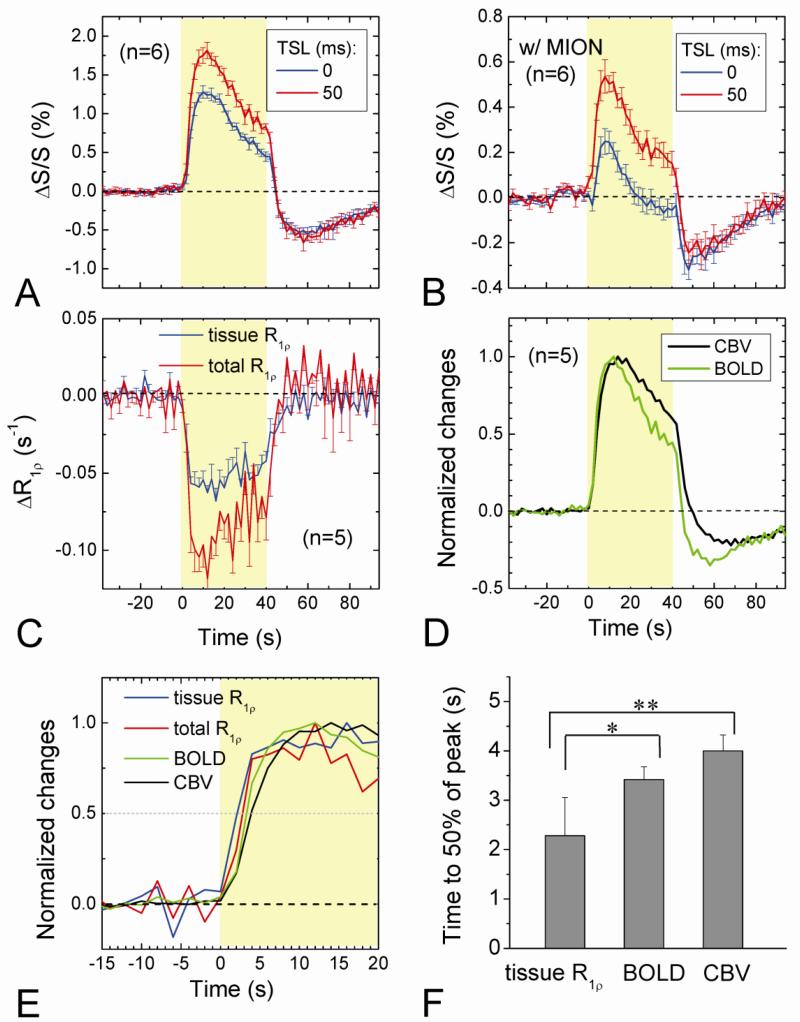
Characteristics of dynamic functional changes can provide insights into the source of R1ρ fMRI. The averaged time courses (n = 6 animals) of the fMRI response at the middle cortical ROI (see Fig. 2B) were shown for both conditions without (Fig. 3A) and with MION (Fig. 3B). In both cases the runs with TSL = 50 ms and ω1 of 500 Hz (red) have larger positive signal changes than those with TSL = 0 (blue), whereas the post-stimulus undershoots are similar. To compare with R1ρ and BOLD fMRI time courses, CBV fMRI studies were also performed in five animals after an intravascular MION injection of 10-13 mg/kg. During the 40-s stimulation period, R1ρ without MION, BOLD and CBV responses decreased gradually following its peak (Fig. 3D), while tissue R1ρ with MION almost remained on a plateau (Fig. 3C). After the stimulus offset, the post-stimulus undershoot was prominent in BOLD and CBV (Fig. 3D), but not in tissue R1ρ with MION (Fig. 3C). To further evaluate their temporal behavior, the normalized time courses were compared for the SE-BOLD, CBV, and R1ρ before and after MION (Fig. 3E). The time to 50% of the peak occurs in the order of tissue R1ρ, R1ρ (without MION), BOLD, and CBV (dotted line). The rising time of tissue R1ρ is 1.1 s and 1.7 s faster than the BOLD (n = 5, p < 0.05) and CBV (p < 0.01) responses, respectively (Fig. 3F). These temporal characteristics suggest that the tissue R1ρ change induced by stimulation has origins different from hemodynamics as in BOLD and CBV fMRI.
Fig. 3. Dynamic changes in BOLD, T1ρ-weighted fMRI, CBV, and R1ρ without and with MION during visual stimulation.
Averaged time courses of T2-weighted (TSL = 0), T1ρ-weighted fMRI (TSL = 50 ms), and R1ρ change were obtained from the middle cortical ROI during visual stimulation without and with blood signal suppression with MION (A-C). The normalized time courses of BOLD and CBV show a significant undershoot after the stimulus offset (D), unlike those time courses of the two R1ρ responses (C). The BOLD, CBV, total R1ρ (without MION) and tissue R1ρ responses were normalized and their initial rising periods during stimulation were compared in (E). The rising time, defined as the time from the stimulus onset to 50% of the peak change, is faster for the tissue R1ρ than BOLD (p < 0.05, n = 5) and CBV (p < 0.01) responses (F).
SL frequency dependence of tissue R1ρ changes during visual stimulation
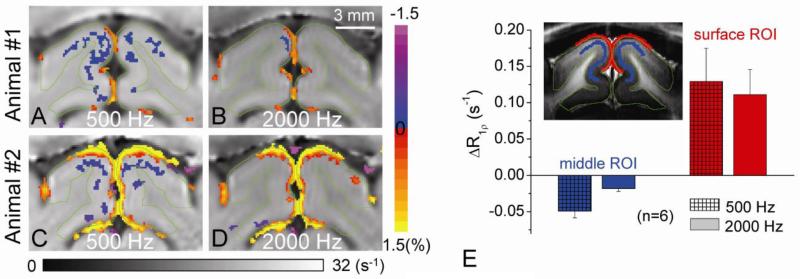
Since T1ρ is sensitive to molecular motions with correlation time τ close to 1/2πω1, SL frequency dependency studies may provide insights into functional R1ρ changes. Thus, T1ρ fMRI studies were performed with ω1 of 500 and 2000 Hz after the suppression of blood signals with MION during 40-s visual stimulation (n = 6 cats; Expt. II). The baseline R1ρ values at the middle cortical ROI were 21.0 ± 0.14 s-1 and 17.55 ± 0.13 s-1 for ω1 = 500 and 2000 Hz, respectively, similar to previous values measured at the rat cortex (Makela et al., 2004). At ω1 = 500 Hz, the temporal variation of R1ρ in the baseline period is 0.012 ± 0.002 s-1 (n = 6). When ω1 increases from 500 Hz to 2000 Hz for visual stimulation studies, the increase of R1ρ remains similar at the boundary of the cortical surface and CSF, while the decrease of R1ρ at the middle of the cortex almost disappears (Fig. 4A and 4C for 500 Hz vs. 4B and 4D for 2000 Hz). Since the R1ρ change at the cortical surface is dominated by changes to the CSF volume fraction, it is to be expected that it is insensitive to SL frequencies. However, the functional R1ρ decrease at the middle cortical ROI for 500 Hz is 0.049 s-1 and 2.7 times larger (p < 0.005) than at 2000 Hz (Fig. 4E). This experiment indicates tissue R1ρ change is highly dependent on SL frequency, suggesting its source is from the slow molecular motion of tissue water rather than vascular response.
Fig 4. Functional tissue R1ρ changes at two spin-locking frequencies.
Visual stimulation-induced tissue R1ρ changes were measured after 5 mg/kg MION for two SL frequencies ω1 = 500 and 2000 Hz. Tissue R1ρ change maps of two representative animals were shown for ω1 = 500 Hz (A, C) and 2000 Hz (B, D), where the horizontal grayscale bar indicates the baseline R1ρ values and the vertical color bar indicates the functional change. The R1ρ decrease at the parenchyma is significantly reduced for ω1 = 2000 Hz, whereas the R1ρ increasing pixels at the surface of the cortex are similar for the two frequencies. The averaged change (n = 6 cats) in tissue R1ρ at ω1 = 500 Hz is 2.7 times larger than that at 2000 Hz (E).
Tissue T1ρ is insensitive to intravascular susceptibility changes
Functional R1ρ change may be influenced by blood susceptibility effects due to insufficient spin locking. To achieve effective spin locking, the B1 field should be much higher than the local magnetic field inhomogeneity. If B1 is close to 0, the transverse spins are not locked and would relax by diffusion across the inhomogeneous field; therefore, T1ρ approaches T2. With high spin-locking frequency (ω1 = 2500 Hz), it has been reported that the T1ρ of extravascular water is almost unaffected by intravascular susceptibility effects (Kettunen et al., 2002), such as from a variation of paramagnetic deoxyhemoglobin, i.e., the BOLD effect. However, this may not be true for lower ω1 values usually applied for in vivo studies due to the limitation of RF power deposition. To assess whether a change of intravascular susceptibility may affect T1ρ of tissue water for our imaging parameters, T1ρ was measured in the rat cortex under two intravascular injections of 1 mg Fe/kg MION (Expt. III, n = 4 rats). Before each experiment, 2 mg Fe/kg MION was injected i.v. to fully suppressed the intravascular signal. In the extravascular tissue, the susceptibility change induced a large modulation in R2 but much smaller change in R1ρ with ω1 = 125 Hz (Fig. 5A), and the change in R1ρ is minimal for ω1 ≥ 500 Hz, indicating that tissue R1ρ is insensitive to changes in blood susceptibility for SL frequencies applied in our functional T1ρ experiments.
Tissue T1ρ is insensitive to hyperoxia-induced hemodynamic changes
In addition to a change of vascular susceptibility, hemodynamics also induces small change in tissue microstructure/microenvironment such as a modulation of the extracellular oxygenation level, perfusion, and local cellular structural distortion caused by vessel volume change. Whether these vascular-originated effects will alter extravascular T1ρ is examined by a hyperoxia challenge with the suppression of blood (Exp. IV, n = 5 rats). During inhalation of 60% O2, a large decrease of tissue R2 (i.e., an increase in BOLD signal) was observed due to reduction of intravascular susceptibility (Fig. 5B). In contrast, the change in tissue R1ρ change is minimal for both ω1 of 500 and 2000 Hz. This experiment suggested that in addition to intravascular susceptibility, the tissue R1ρ should also be insensitive to modulations of extracellular oxygenation level, perfusion, and local cellular structural distortion caused by vessel volume change.
Tissue R1ρ changes during hypercapnic challenge and global ischemia
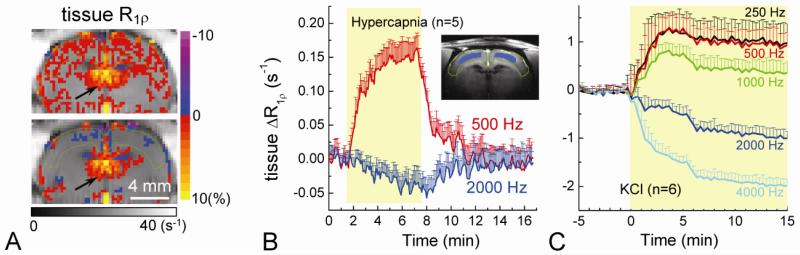
Tissue R1ρ decreases (i.e., T1ρ increases) may be caused by a change of chemical exchange between water and exchangeable protons resulting from a suggested drop in pH during stimulation; a similar change was observed during pH-decreasing hypercapnic challenge at 3 T (Magnotta et al., 2012). However, similar hemodynamic changes, i.e., increase of CBV and CBF, occur for both cases, potentially leading to similar R1ρ changes if the intravascular contribution is significant (Hulvershorn et al., 2005). Thus, it is important to compare tissue R1ρ changes induced by visual stimulation and hypercapnic challenge, after the suppression of intravascular contribution. Unlike the observation during visual stimulation in our studies (Figs. 2 – 4), during hypercapnic challenge the tissue R1ρ increases (i.e., T1ρ decreases) at the rat cortex for ω1 of 500 Hz, but slightly decreases for ω1 of 2000 Hz at 9.4 T (Fig. 6A). The averaged time course of R1ρ obtained from the middle cortical ROI shows a large positive change for ω1 = 500 Hz, but a small negative change for 2000 Hz (Fig. 6B).
Fig. 6. Spin-locking frequency-dependent tissue R1ρ change during tissue acidosis.
Tissue R1ρ changes were measured during hypercapnia and global ischemia after 5 mg/kg MION injection. Tissue R1ρ change maps for ω1 = 500 Hz and 2000 Hz are shown for hypercapnic challenge in one rat (A). Green contour indicates the cortical area. Unlike visual stimulation, an increase in tissue R1ρ was observed for ω1 = 500 Hz. Note that the increases of R1ρ near the ventricle area are similar in the two maps and can be attributed to a change of CSF volume fraction (arrows). The averaged time course (n = 5 rats) of the tissue R1ρ response obtained the cortical ROI (Inset) shows a significant increase for 500 Hz whereas a small decrease for 2000 Hz (B). The averaged tissue R1ρ responses (n = 6 rats) for ω1 from 250 to 4000 Hz during KCl injection (C), which induces tissue acidosis, show spin-locking frequency-dependent changes. These are qualitatively similar to hypercapnia.
While an ω1-dependence of R1ρ was expected and had been reported in previous ischemia studies at 4.7 T (Grohn et al., 2000; Kettunen et al., 2002), the observation of opposite polarity of R1ρ change for the two ω1 values of 500 and 2000 Hz is surprising. To examine whether this is a distinct property for tissue pH decrease at our high field of 9.4 T, we measured the tissue R1ρ response for more ω1 values during the initial period of rat global ischemia induced by potassium chloride injection which is well-known to cause tissue acidosis (n = 6 animals; Experiment V) (Kettunen et al., 2002). Tissue R1ρ increases rapidly after KCl injection for ω1 ≤ 1000 Hz and decreases for ω1 ≥ 2000 Hz, similar to the results for hypercapnic challenge, albeit of much larger magnitudes. The change in tissue R1ρ reached a maximum at ~ 3 minutes post-KCl then showed a similar rate of decrease for all the ω1 values in following 15 minutes or so. These observations are quite different from visual stimulation studies, in particular, tissue R1ρ change with ω1 = 500 Hz is negative for visual stimulation (Fig. 3C), but positive for tissue acidosis (global ischemia and hypercapnia) (Fig. 6B-C), indicating that neural stimulation-induced tissue R1ρ decrease is not due to tissue acidosis.
T1ρ measurements of protein and metabolite phantoms
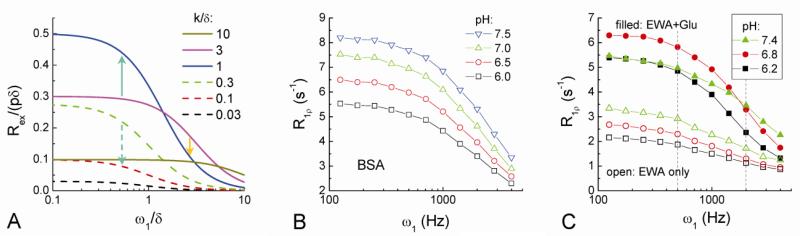
To gain more insight into the difference of tissue R1ρ changes during tissue acidosis and neural stimulation, ω1-dependent phantom experiments were performed and compared to theoretical models. Using Eq. [4], Rex/(pδ) was plotted against ω1/δ at different exchange rates (k/δ) (Fig. 7A). Note that an increase in Rex leads to an increase in R1ρ. In the case of tissue acidosis (i.e., a decrease of k/δ), if k/δ decreases from intermediate exchange to slow exchange (k/δ << 1), Rex would decrease for all ω1 values (Fig. 7A, e.g., dashed green downward arrow). On contrary, if k/δ decreases from fast exchange (k/δ >> 1) to intermediate exchange, the Rex measured at a small ω1 would increase (solid green upward arrow), and Rex measured at a large ω1 would decrease (solid orange downward arrow), exhibiting an opposite polarity of R1ρ change.
Fig. 7. Calculated Rex dispersion (A) and the measured R1ρ dispersion of pH-dependent phantoms (B-C).
Rex dispersion was calculated with Eq. [4] as a function of exchange rate k. Upward and downward arrows indicate changes in Rex when the exchange between labile protons and water is slow down due to pH decrease (see texts). The R1ρ dispersion of 8% bovine serum albumin (BSA) decreases with pH values (B). The R1ρ dispersion of 4% egg white albumin (EWA) only (open symbols) and 4% EWA with 30 mM of glutamate (filled symbols) were both measured for three pH values (C). Vertical dashed lines (C) indicate spin-locking frequencies of 500 and 2000 Hz used for in vivo studies. The addition of glutamate (Glu) changes the pH-dependence of R1ρ dispersion.
The R1ρ dispersions of BSA and EWA decrease similarly with pH in the ω1 range of 125 to 4000 Hz (Fig. 7B and 7C). While proteins have many different labile protons with different exchange rates, these pH-dependence results suggest that the overall proton exchange of these proteins can be considered as in the slow to intermediate exchange regime at 9.4 T. The addition of Glu to EWA enhances R1ρ dispersion significantly and also changes its pH-dependence (Fig. 7C), suggesting that the amine-water proton exchange from Glu are much faster than those from proteins and falls into the intermediate to fast exchange category. When pH is decreased from 7.4 to 6.8, R1ρ increases for ω1 ≤ 1414 Hz whereas R1ρ decreases for ω1 ≥ 2000 Hz. This pH-dependent R1ρ change at 500 vs. 2000 Hz is qualitatively similar to hypercapnia (Fig. 6B) and early ischemia results (Fig. 6C), but is different from the neural stimulation-induced R1ρ changes (Fig. 4E).
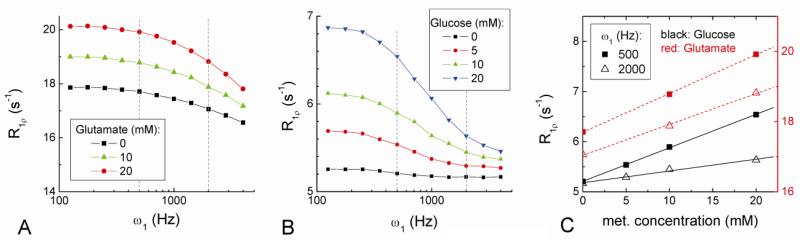
Since R1ρ is also sensitive to the concentration of labile protons (Eq. [3]), the small decrease of R1ρ observed for neural stimulation might be caused by a reduction in the concentration of metabolites. In Fig. 8A and 8B, the R1ρ of both amine-containing Glu and hydroxyl-containing Glc phantoms increases linearly with concentration and decreases with ω1, but their relative sensitivity at 500 vs. 2000 Hz is different due to the different chemical shifts and exchange rates of amine and hydroxyl protons (Jin et al., 2011; Liepinsh and Otting, 1996; van Zijl and Yadav, 2011). Although the concentrations of these metabolites are quite different from in vivo conditions, possible contributions from amine- or hydroxyl containing metabolite can be roughly estimated from 1) the linear-dependence of Rex (and hence R1ρ) on metabolite concentration (Eq. [3]), and 2) the difference of R1ρ for the two ω1 values (500 and 2000 Hz) which is correlated with the exchange rate. The slope of R1ρ vs. Glu and Glc concentration is 0.11 and 0.067 s-1 · mM-1 for ω1 of 500 Hz and 0.088 and 0.024 s-1 · mM-1 for ω1 of 2000 Hz, respectively (Fig. 8C). The high sensitivity of R1ρ on metabolites indicates that the observed tissue R1ρ change of 0.051 s-1 at 500 Hz during visual stimulation could be explained by only sub-millimolar decrease of these metabolites. The ratio of slopes at ω1 of 500 Hz to 2000 Hz is only 1.25 for Glu, but 2.8 for Glc which is very similar to the ratio of 2.7 observed in the visual stimulation studies. These results suggest that sub-millimolar decreases in metabolite concentrations, especially hydroxyl-containing metabolites such as glucose, potentially give rise to a plausible source of functional R1ρ decrease.
Fig. 8. R1ρ dispersions of concentration-dependent amine and hydroxyl metabolite phantoms.
Three amine-containing glutamate concentrations in agarose with 0.07 mM MnCl2 (A), and four hydroxyl-containing glucose (Glc) concentrations in PBS with 0.1 mM MnCl2 (B) were used. R1ρ is linearly dependent on Glu and Glc concentration, and the slope for ω1 = 500 Hz is only slightly larger than that for ω1 = 2000 Hz for Glu, but is much larger for Glc (C). The ratio of R1ρ at ω1 = 500 Hz to 2000 Hz is similar for glucose phantom (2.8) and for in vivo functional response (2.7).
Discussions
Our results show that the source of activation-induced T1ρ change is complex and has both vascular and tissue originated components. The vascular-originated component is mostly due to a local volume redistribution effect in an imaging voxel, because there is a large difference between the T1ρ values of blood, tissue and CSF water. Specifically, vessel dilation would lengthen the apparent T1ρ in the parenchyma but would shorten it at the boundary of cortex and CSF. After the suppression of vascular signal, the observed tissue T1ρ signal in parenchyma is likely caused by an activation-induced change of tissue metabolism, based on following reasons. Tissue T1ρ response shows (i) a better localization to the middle cortical layer than BOLD response, is (ii) sensitive to the SL frequency, is (iii) faster than both BOLD and CBV, and has (iv) no post-stimulus undershoot unlike BOLD and CBV. Its non-hemodynamic origin is also supported by the observation that tissue R1ρ is unaffected by tissue oxygenation, CBF and CBV changes induced by hyperoxia shown in Fig. 5B (Lu et al., 2009). Even though there have been intense efforts to develop non-hemodynamic tissue-specific fMRI methods for the last two decades (Bandettini et al., 2005; Chu et al., 2004; Jin and Kim, 2008b; Le Bihan et al., 2006; Miller et al., 2007; Parkes et al., 2007; Tang et al., 2008; Xiong et al., 2003), our results showed for the first time that with complete suppression of blood, a tissue-originated fMRI signal, while the sensitivity is much weaker than BOLD (Fig. 2G), can be consistently detected in brain in vivo using spin-lock fMRI.
T1ρ relaxation is highly dependent on experimental condition and experimental parameters such as B0. Since the difference in T1ρ between the blood, tissue and CSF is dependent on magnetic field strength (B0), the vascular contribution to functional change of T1ρ would also be B0-dependent. Similarly, a functional change in tissue T1ρ, if it is mainly caused by the proton-exchange effect, would also be B0-dependent because a high field increases the chemical shifts between labile protons and water, and consequently the exchange-mediated relaxation rate (Rex increases with δ in Eq. [3]). Thus, we postulate a larger tissue-originated T1ρ signal in the parenchyma at a higher B0. Our results at 9.4 T showed that the vascular contribution to activation-induced T1ρ change is about 37% with ω1 = 500 Hz at the brain parenchyma. At 3 T, an increase in end-tidal CO2 (e.g., hypercapnia) decreases tissue pH and increases T1ρ value measured with ω1 of 400 Hz, and it was recently suggested by Magnotta et al. that in vivo T1ρ increase during human visual stimulation is also due to tissue acidosis (Magnotta et al., 2012). While a correlation between tissue acidosis and T1ρ increase was established for hypercapnia, there was no evidence that the increase of T1ρ is mostly from the tissue compartment. Importantly, both visual stimulation and hypercapnia increase CBV, which will consequently lengthen the measured T1ρ due to longer T1ρ of blood relative to tissue. Indeed, the vascular contribution with ω1 = 500 Hz was estimated to be dominant (> 90%) in a human T1ρ fMRI study at 3 T according to the estimation of Hulvershorn et al. (Hulvershorn et al., 2005). In our case with suppression of blood signal, tissue T1ρ at 500 Hz increases during visual stimulation but decreases for tissue acidosis, disagree with the interpretation of functional T1ρ change by Magnotta et al. (Magnotta et al., 2012).
T1ρ relaxation is also highly dependent on ω1, and T1ρ dispersion studies with more than one ω1 value would be necessary to shed light on the underlying mechanisms. During rat global ischemia at 4.7 T, Kettunen et al. reported an increase of T1ρ at ω1 ≥ 2500 Hz, but marginal T1ρ change for ω1 of ~850 Hz (Kettunen et al., 2001). In our experiments with suppression of blood signals at 9.4 T, T1ρ values during hypercapnia and the initial period of global ischemia decrease for ω1 ≤ 1000 Hz, while increase for ω1 ≥ 2000 Hz. This opposite polarity of T1ρ changes for low and high ω1, especially the increase of R1ρ at a small ω1 with decreasing pH, can be qualitatively explained by a proton-exchange mechanism where k is much faster than δ of contributing exchangeable protons relative to water at normal physiological pH, and becomes closer to δ during tissue acidosis (e.g., k/δ decreases from 3 to 1 in Fig. 7A). From phantom experiments (Fig. 7C), contributing protons with fast to intermediate exchanges can be mainly from metabolites, such as free amino acids. In our recent rat focal brain ischemia study (Zong et al., 2013), we have investigated the properties of amine-water proton exchange using off-resonance SL technique measured at a frequency offset 2.5 ppm from water. An increase in the off-resonance R1ρ with ω1 of 500 Hz was observed due to slowdown of chemical exchanges during stroke, and the change of R1ρ is strongly correlated with pH and the concentration of glutamate and γ-aminobutyric acid (GABA). These findings are in good agreement with the on-resonance R1ρ results observed in current pH-dependent studies (Fig. 7C).
While a small local tissue acidosis might occur during visual stimulation (Lin et al., 2012; Magnotta et al., 2012), the difference in tissue T1ρ responses during visual stimulation versus hypercapnic challenge and global ischemia indicated that tissue acidosis is unlikely the major contributor to the neuronal activation-induced T1ρ signal. One plausible mechanism may be a local drop in metabolites concentration due to elevated consumption. For example, a decreased glucose concentration ranging from 0.13 to 0.51 μmol·g-1 has been reported in human magnetic resonance spectroscopy (MRS) studies during sustained visual stimulation of several minutes or more (Chen et al., 1993; Frahm et al., 1996; Lin et al., 2012; Mangia et al., 2007). The averaged change in T1ρ during our cat visual stimulation studies would correspond to a reduction in glucose concentration of ~0.6 μmol·g-1 (considering 0.83 g water/g tissue) for the middle cortical ROI, and ~0.3 μmol·g-1 for a larger ROI including part of white matter area (not shown). This concentration decrease estimated from functional T1ρ change falls well into the range of MRS results whose voxel should also have significant partial volume effect. Besides chemical exchange, there may be other contributions to the T1ρ fMRI signal. For example, the tissue component itself might also be sub-compartmentalized into different T1ρ pools, hence, a relative volume change between sub-compartments or variation in the rate of exchange in these sub-compartments, which has been suggested for the T1ρ contrast during ischemia (Jokivarsi et al., 2009), may occur as a results of neuronal cell-swelling and alter the observed T1ρ. Further investigations are needed to determine the exact source of tissue-originated signals.
Conclusion
Functional T1ρ responses can be contributed by changes in blood, CSF and/or tissue signals. In order to detect tissue-originated functional responses, it is critical to remove the CSF contamination, and also to separate contributions of blood and tissue signals. While T1ρ fMRI with blood contribution has already been shown in human studies to offer better tissue-localization than the BOLD response with large contributions from draining veins (Hulvershorn et al., 2005; Magnotta et al., 2012), its utility may not be better than CBF and CBV-based fMRI which are also more specific to neural active sites than BOLD fMRI (Jin and Kim, 2008a; Zhao et al., 2006). In addition to the difficulty of fully removing the intravascular signal as well as the CSF contamination, the application of tissue T1ρ to human studies may be challenging due to the limitation on specific adsorption rate which increases quadratically with magnetic field strength, and also due to the reduced sensitivity compared to BOLD. On the other hand, functional tissue T1ρ change, albeit the sensitivity is still much lower than BOLD, can be consistently observed at 9.4 T. Tissue-originated T1ρ signal is faster than hemodynamic responses, has no post-stimulus undershoot unlike BOLD and CBV fMRI, and shows high specificity to middle cortical areas. Therefore, tissue T1ρ fMRI may potentially be exploited as a non-hemodynamic imaging tool for animal research.
Highlights.
Activation-induced change of T1ρ has both vascular- and tissue-originated components.
T1ρ fMRI signal is dependent on magnetic field strength and spin-locking pulse parameters.
Functional tissue T1ρ change shows distinct non-hemodyanmic characteristics.
Tissue T1ρ fMRI signal may be caused by a change of metabolite concentration rather than pH.
Acknowledgments
We thank Ping Wang for animal preparation, Kristy Hendrich for maintaining the 9.4 T system, Dr. Risto Kauppinen and Joonas Autio for helpful discussions, and Hunter Mehrens for proof reading. This work is supported by NIH grants EB003324, EB003375, and NS44589.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Applied Magnetic Resonance. 2005;29:65–88. [Google Scholar]
- Borthakur A, Charagundla SR, Wheaton A, Reddy R. T-1ho-weighted MRI using a surface coil to transmit spin-lock pulses. Journal of Magnetic Resonance. 2004;167:306–316. doi: 10.1016/j.jmr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Borthakur A, Gur T, Wheaton AJ, Corbo M, Trojanowski JQ, Lee VMY, Reddy R. In vivo measurement of plaque burden in a mouse model of Alzheimer's disease. Journal of Magnetic Resonance Imaging. 2006;24:1011–1017. doi: 10.1002/jmri.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Novotny E, Zhu X-H, Rothman D, Shulman RG. Localized 1H NMR measurement of glucose consumption in human brain during visual stimulation. Proc Natl Acad Sci USA. 1993;90:9896–9900. doi: 10.1073/pnas.90.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu RN, de Zwart JA, van Gelderen P, Fukunaga M, Kellman P, Holroyd T, Duyn JH. Hunting for neuronal currents: absence of rapid MRI signal changes during visual-evoked response. Neuroimage. 2004;23:1059–1067. doi: 10.1016/j.neuroimage.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Duvvuri U, Goldberg AD, Kranz JK, Hoang L, Reddy R, Wehrli FW, Wand AJ, Englander SW, Leigh JS. Water magnetic relaxation dispersion in biological systems: The contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12479–12484. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial Connecting the dots. Nature Neuroscience. 2009;12:99–99. doi: 10.1038/nn0209-99. [DOI] [PubMed] [Google Scholar]
- Frahm J, Kruger KD, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med. 1996;35:143–148. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Grohn HI, Michaeli S, Garwood M, Kauppinen RA, Grohn OHJ. Quantitative T-1rho and adiabatic Carr-Purcell T-2 magnetic resonance imaging of human occipital lobe at 4 T. Magnetic Resonance in Medicine. 2005;54:14–19. doi: 10.1002/mrm.20536. [DOI] [PubMed] [Google Scholar]
- Grohn OHJ, Kettunen MI, Makela HI, Penttonen M, Pitkanen A, Lukkarinen JA, Kauppinen RA. Early detection of irreversible cerebral ischemia in the rat using dispersion of the magnetic resonance imaging relaxation time, T1rho. Journal of Cerebral Blood Flow and Metabolism. 2000;20:1457–1466. doi: 10.1097/00004647-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Hakumaki JM, Grohn OHJ, Tyynela K, Valonen P, Yla-Herttuala S, Kauppinen RA. Early gene therapy-induced apoptotic response in BT4C gliomas by magnetic resonance relaxation contrast T-1 in the rotating frame. Cancer Gene Therapy. 2002;9:338–345. doi: 10.1038/sj.cgt.7700450. [DOI] [PubMed] [Google Scholar]
- Hulvershorn J, Borthakur A, Bloy L, Gualtieri EE, Reddy R, Leigh JS, Elliott MA. T-1 rho contrast in functional magnetic resonance imaging. Magnetic Resonance in Medicine. 2005;54:1155–1162. doi: 10.1002/mrm.20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Autio J, Obata T, Kim SG. Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons. Magnetic Resonance in Medicine. 2011;65:1448–1460. doi: 10.1002/mrm.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim S-G. Change of the cerebrospinal fluid volume during brain activation investigated by T1ρ-weighted fMRI. Neuroimag. 2010;51:1378–1383. doi: 10.1016/j.neuroimage.2010.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim SG. Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage. 2008a;43:1–9. doi: 10.1016/j.neuroimage.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim SG. Functional changes of apparent diffusion coefficient during visual stimulation investigated by diffusion-weighted gradient-echo fMRI. Neuroimage. 2008b;41:801–812. doi: 10.1016/j.neuroimage.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim SG. Improved cortical-layer specificity of vascular space occupancy fMRI with slab inversion relative to spin-echo BOLD at 9.4 T. Neuroimage. 2008c;40:59–67. doi: 10.1016/j.neuroimage.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Kim SG. Separation of the vascular and tissue contributions to the T1rho change induced by brain activation.. Proc 19th ISMRM Annual Meeting. Proc 19th ISMRM Annual Meeting; Honolulu, Hawaii. 2009. [Google Scholar]
- Jin T, Kim SG. Dynamic changes in the tissue microenvironment induced by hypercapnia and hyperoxia: a T1rho dispersion study at 9.4 T.. Proc 19th ISMRM Annual Meeting. Proc 19th ISMRM Annual Meeting; Montreal, Canada. 2011. [Google Scholar]
- Jin T, Kim SG. Quantitative Chemical Exchange Sensitive MRI Using Irradiation with Toggling Inversion Preparation. Magn Reson Med. 2012;68:1056–1064. doi: 10.1002/mrm.24449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokivarsi KT, Niskanen JP, Michaeli S, Grohn H, Garwood M, Kauppinen RA, Grohn OH. Quantitative assessment of water pools by T 1 rho and T 2 rho MRI in acute cerebral ischemia of the rat. J Cereb Blood Flow Metab. 2009;29:206–216. doi: 10.1038/jcbfm.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen MI, Grohn OHJ, Penttonen M, Kauppinen RA. Cerebral T-1 rho relaxation time increases immediately upon global ischemia in the rat independently of blood glucose and anoxic depolarization. Magnetic Resonance in Medicine. 2001;46:565–572. doi: 10.1002/mrm.1228. [DOI] [PubMed] [Google Scholar]
- Kettunen MI, Grohn OHJ, Silvennoinen MJ, Penttonen M, Kauppinen RA. Effects of intracellular pH, blood, and tissue oxygen tension on T-1 rho relaxation in rat brain. Magnetic Resonance in Medicine. 2002;48:470–477. doi: 10.1002/mrm.10233. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ogawa S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow and Metab. 2012;32:1188–1206. doi: 10.1038/jcbfm.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Kim SG. Quantification of Cerebral Arterial Blood Volume and Cerebral Blood Flow Using MRI With Modulation of Tissue and Vessel (MOTIVE) Signals. Magnetic Resonance in Medicine. 2005;54:333–342. doi: 10.1002/mrm.20550. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim SG. Temporal dynamics and spatial specificity of arterial and venous blood volume changes during visual stimulation: implication for BOLD quantification. J Cereb Blood Flow and Metab. 2011;31:1211–1222. doi: 10.1038/jcbfm.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen SK, Niemi PT, Kajander SA, Komu MES. T-1 rho Dispersion profile of rat tissues in vitro at very low locking fields. Magnetic Resonance Imaging. 2006;24:295–299. doi: 10.1016/j.mri.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Urayama S, Aso T, Hanakawa T, Fukuyama H. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8263–8268. doi: 10.1073/pnas.0600644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh E, Otting G. Proton exchange rates from amino acid side chains - Implications for image contrast. Magnetic Resonance in Medicine. 1996;35:30–42. doi: 10.1002/mrm.1910350106. [DOI] [PubMed] [Google Scholar]
- Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG. Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. J Cereb Blood Flow Metab. 2012;32:1484–1495. doi: 10.1038/jcbfm.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HB, Scholl CA, Zuo YT, Stein EA, Yang YH. Quantifying the Blood Oxygenation Level Dependent Effect in Cerebral Blood Volume–Weighted Functional MRI at 9.4T. Magn Reson Med. 2007;58:616–621. doi: 10.1002/mrm.21354. [DOI] [PubMed] [Google Scholar]
- Lu J, Dai GP, Egi Y, Huang S, Kwon SJ, Lo EH, Kim YR. Characterization of cerebrovascular responses to hyperoxia and hypercapnia using MRI in rat. Neuroimag. 2009;45:1126–1134. doi: 10.1016/j.neuroimage.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Heo H-Y, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proc Nat Acad Sci. 2012;109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela HI, De Vita E, Grohn OHJ, Kettunen MI, Kavec M, Lythgoe M, Garwood M, Ordidge R, Kauppinen RA. B-0 dependence of the on-resonance longitudinal relaxation time in the rotating frame (T-1p) in protein phantoms and rat brain in vivo. Magnetic Resonance in Medicine. 2004;51:4–8. doi: 10.1002/mrm.10669. [DOI] [PubMed] [Google Scholar]
- Makela HI, Grohn OHJ, Kettunen MI, Kauppinen RA. Proton exchange as a relaxation mechanism for T-1 in the rotating frame in native and immobilized protein solutions. Biochemical and Biophysical Research Communications. 2001;289:813–818. doi: 10.1006/bbrc.2001.6058. [DOI] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from H-1 NMR spectroscopy in the human visual cortex. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Michaeli S, Oz G, Sorce DJ, Garwood M, Ugurbil K, Majestic S, Tuite P. Assessment of brain iron and neuronal integrity in patients with Parkinson's disease using novel MRI contrasts. Movement Disorders. 2007;22:334–340. doi: 10.1002/mds.21227. [DOI] [PubMed] [Google Scholar]
- Miller KL, Bulte DP, Delvlin H, Robson MD, Wise RG, Woolrich MW, Jezzard P, Behrens TE. Evidence for a vascular contribution to diffusion fMRI at high b value. Proc Nat Acad Sci. 2007;104:20967–20973. doi: 10.1073/pnas.0707257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim S-G, Merkle H, Ellermann JM, Ugurbil K. Functional Brain Mapping by Blood Oxygenation Level-Dependent Contrast Magnetic Resonance Imaging. Biophys J. 1993;64:800–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Lee SY, Park JH. Effect of nerve cell currents on MRI images in snail ganglia. Neuroreport. 2004;15:2783–2786. [PubMed] [Google Scholar]
- Parkes LM, de Lange FP, Fries P, Toni I, Norris DG. Inability to directly detect magnetic field changes associated with neuronal activity. Magnetic Resonance in Medicine. 2007;57:411–416. doi: 10.1002/mrm.21129. [DOI] [PubMed] [Google Scholar]
- Piechnik SK, Evans J, Bary LH, Wise RG, Jezzard P. Functional Changes in CSF Volume Estimated Using Measurement of Water T-2 Relaxation. Magnetic Resonance in Medicine. 2009;61:579–586. doi: 10.1002/mrm.21897. [DOI] [PubMed] [Google Scholar]
- Price DJ. Patterns of cytochrome oxidase activity in areas 17, 18, and 19 of the visual cortex of cats and kittens. Exp Brain Res. 1983;58:125–133. doi: 10.1007/BF00238960. [DOI] [PubMed] [Google Scholar]
- Scouten A, Constable RT. VASO-based calculations of CBV change: accounting for the dynamic CSF volume. Magnetic Resonance in Medicine. 2008;58:308–315. doi: 10.1002/mrm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp JP. Stimulate: A GUI based fMRI analysis software package. NeuroImage. 1996;3:S607. [Google Scholar]
- Tang L, Avison MJ, Gatenby JC, Gore JC. Failure to direct detect magnetic field-dephasing corresponding to ERP generation. Magnetic Resonance Imaging. 2008;26:484–489. doi: 10.1016/j.mri.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman SB, Tumosa N. [14C]-2-Deoxyglucose demonstration of the organization of ocular dominance in areas 17 and 18 of normal cat. Brain Res. 1983;267:35–46. doi: 10.1016/0006-8993(83)91037-5. [DOI] [PubMed] [Google Scholar]
- Trott O, Palmer AG. R-1 rho relaxation outside of the fast-exchange limit. Journal of Magnetic Resonance. 2002;154:157–160. doi: 10.1006/jmre.2001.2466. [DOI] [PubMed] [Google Scholar]
- Truong TK, Song AW. Finding neuroelectric activity under magnetic-field oscillations (NAMO) with magnetic resonance imaging in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12598–12601. doi: 10.1073/pnas.0605486103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl PCM, Yadav NN. Chemical Exchange Saturation Transfer (CEST): what is in a name and what isn't? Mag Reson Med. 2011;65:927–948. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JH, Fox PT, Gao JH. Directly mapping magnetic field effects of neuronal activity by magnetic resonance imaging. Human Brain Mapping. 2003;20:41–49. doi: 10.1002/hbm.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Yang J, Shen J. In vivo T1rho-weighted MR imaging of rat brain using a surface coil at 11.7T.. Proceedings of 16th ISMRM, Proceedings of 16th ISMRM; Toronto, Canada. 2008. p. 3077. [Google Scholar]
- Zhao F, Wang P, Hendrich K, Ugurbil K, Kim S-G. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: Insights into hemodynamic regulation. NeuroImage. 2006;30:1149–1160. doi: 10.1016/j.neuroimage.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Zong X, Wang P, Kim S-G, Jin T. Sensitivity and Source of Amine-Proton Exchange and Amide-Proton Transfer Magnetic Resonance Imaging in Cerebral Ischemia. Magnetic Resonance in Medicine. 2013 doi: 10.1002/mrm.24639. DOI: 10.1002/mrm.24639. [DOI] [PMC free article] [PubMed] [Google Scholar]