Abstract
In this article, we provide the results of experimental studies demonstrating that corneal avascularity is an active process involving the production of anti-angiogenic factors, which counterbalance the proangiogenic/lymphangiogenic factors that are upregulated during wound healing. We also summarize pertinent published reports regarding corneal neovascularization (NV), corneal lymphangiogenesis and corneal angiogenic/lymphangiogenic privilege. We outline the clinical causes of corneal NV, and discuss the angiogenic proteins (VEGF and bFGF) and angiogenesis regulatory proteins. We also describe the role of matrix metalloproteinases MMP-2, -7, and MT1-MMP, anti-angiogenic factors, and lymphangiogenic regulatory proteins during corneal wound healing. Established and potential new therapies for the treatment of corneal neovascularization are also discussed.
1. Introduction
Angiogenesis is the process by which new blood vessels derive from pre-existing ones. First termed in 1787 (Folkman, 2008), angiogenesis remains an incompletely understood process that involves the interaction of multiple cell types, including endothelial cells, pericytes, and circulating cells, as well as parenchymal cells and stromal cells (Penn et al., 2008). It was not until three decades ago that major in vivo angiogenesis models were developed for testing potential therapeutic drugs. Derived from the word “cornu”, the cornea was first characterized as a hard structure etymologically related to an animal horn. The transparent and seemingly delicate anterior surface of the eye has contributed to major discoveries in the field of angiogenesis and, more recently, lymphangiogenesis (Alitalo et al., 2005; Lohela et al., 2009, 2003) (Table 1).
Table 1.
Milestones in corneal angiogenesis/lymphangiogenesis research.
| 1627 | First description of lymphatic vasculature | (Asellius, 1627) |
| 1787 | First use of the term angiogenesis | (Hunter, 1787) |
| 1939 | Laboratory studies of angiogenesis | (Ide et al., 1939) |
| 1971 | Hypothesis of angiogenesis and anti-angiogenesis | (Folkman, 1971) |
| 1974 | First experimental model of corneal angiogenesis | (Gimbrone et al., 1974) |
| 1976 | First use of micropocket pellet assay of corneal angiogenesis | (Langer and Folkman, 1976) |
| 1989 | Vascular endothelial growth factor sequenced | (Leung et al., 1989) |
| 1994 | Angiostatin | (O'Reilly et al., 1994) |
| 1995 | First lymphatic endothelial cell marker (FLT4/VEGFR-3) | (Kaipainen et al., 1995) |
| 1997 | Endostatin | (O'Reilly et al., 1997) |
| 1999 | Discover lymphatic vessel hyaluronan (HA) receptor-1 (LYVE-1) marker | (Banerji et al., 1999) |
| 2002 | Corneal lymphangiogenesis model to dissociate from angiogenesis | (Chang et al., 2002) |
| 2006 | Corneal angiogenic privilege | (Azar, 2006) |
| 2006 | VEGF trap hypothesis for corneal avascularity | (Ambati et al., 2006; Cursiefen et al., 2006a) |
Judah Folkman proposed the hypothesis that the growth of cancerous tumors depends on angiogenesis (Folkman, 1971). His proposal of anti-angiogenesis cancer therapies in 1971 led to major discoveries of angiogenesis inhibitors. His group described the first experimental corneal angiogenesis model demonstrating that tumors implanted into the stromal layers at various distances from the limbus of the rabbit cornea can induce neovascularization, as opposed to merely inducing vessel dilation (Gimbrone et al., 1974). These experiments were followed by the micropocket pellet assays used to influence specific molecules/proteins involved in angiogenesis (Langer and Folkman, 1976) and corneal chemical and suture induced injury, which more closely mimic the complex nature of human diseases (Montezuma et al., 2009; Norrby, 2006; Rogers et al., 2007).
The maintenance of corneal avascularity has recently been termed `angiogenic privilege' (Azar, 2006). This terminology mirrors the special protection the cornea enjoys against the immune rejection of grafted tissues, called `immune privilege.' Just as most parts of the body do not have special protection against immune rejection of foreign antigens, the `angiogenic privilege' designation implies that the absence of blood vessels in the corneal stroma is atypical. This designation also applies to other ocular tissues devoid of blood vessels, such as the lens, where the mechanisms contributing to angiogenic privilege may be shared or distinct. The use of the corneal angiogenic/lymphangiogenic privilege terminology implies that corneal avascularity represents an active process involving the production of anti-angiogenic factors that counterbalance the pro-angiogenic/lymphangiogenic factors that are upregulated after wound healing (even in the absence of new vessels) (Azar, 2006; Chang et al., 2001).
Unlike corneal angiogenesis, corneal lymphangiogenesis is neither clinically nor histologically distinct. Collin (1970) detected corneal lymphangiogenesis in an animal model using electron micrography and by monitoring the drainage of 131-I albumin from the vascular cornea into the lymph node (Collin, 1970). The field of lymphatic research had been neglected for a long time due to the challenging clinical invisibility of lymphangiogenesis, the lack of specific lymphatic markers and growth factors, and the lack of suitable in vitro and in vivo models of lymphangiogenesis. It was not until the last decade of the twentieth century that lymphangiogenesis research started to gain momentum. The discovery of specific markers (such as VEGFR-3, Prox-1, LYVE-1 and Podoplanin) has allowed lymph vessels to be detected in the human cornea during neovascularization (NV) (Banerji et al., 1999; Kaipainen et al., 1995). Cursiefen et al. (2000) have detected lymphatic vessels in human corneas with vascularization secondary to keratitis, graft rejection, limbal stem cell deficiency, and chemical burns. A mouse model was developed in Judah Folkman's laboratory to study lymphangiogenesis dissociated from angiogenesis (Chang et al., 2002). This model was used to identify bFGF as a potent lymphangiogenic factor. The formation of lymphatic vessels is induced early in the course of corneal NV, and these vessels are associated with stromal inflammatory cells. Schneider et al. (2006) have found that lymphatic capillaries develop de novo by differentiation of lymphatic endothelium from lymphangioblasts and that they sprout from pre-existing veins (Schneider et al., 2006). To date, several lymphangiogenic growth factors have been identified, with VEGF-C and VEGF-D being the best characterized (Cueni and Detmar, 2008a,b).
In this article, we present a review of the published literature pertaining to corneal NV, corneal lymphangiogenesis and corneal angiogenic/lymphangiogenic privilege. We also present new findings regarding the factors involved in maintaining corneal angiogenic/lymphangiogenic privilege, as well as established and potential new therapies for the treatment of corneal NV. New studies, presenting new findings, were performed with appropriate IACUC approval and Animal Care Committee protocols.
2. Clinical causes of corneal neovascularization
Angiogenesis forms the pathophysiological basis of diseases that affect nearly two billion people worldwide (Cho et al., 2009), including cancers, cardiovascular disease, blindness, arthritis, complications of AIDS, diabetes, Alzheimer's disease, and more than 70 other major health conditions affecting children and adults, in developed and developing nations.
Neovascular and infectious diseases of the cornea represent a major public health burden, almost always impair visual function, and may ultimately lead to blindness. A normal cornea is avascular; however, under certain conditions capillaries invade from the limbal vascular plexus causing corneal NV. A wide variety of insults can tip the balance between angiogenic and anti-angiogenic factors in favor of angiogenesis and cause various patterns of NV. Processes of corneal NV can be caused by inflammatory disorders (Fig. 1), corneal graft rejection (Fig. 2), infectious keratitis (Fig. 3), contact lens-related hypoxia (Fig. 4), alkali burns, neurotrophic ulceration (Fig. 5), aniridia, and limbal stem cell deficiency (Fig. 6) (Sellami et al., 2007). NV patterns can generally be grouped into three clinical entities: (1) deep NV overlying Descemet's membrane, as seen in herpetic and luetic interstitial keratitis (Fig. 3B), (2) stromal NV, which is mainly associated with stromal keratitis (Figs. 1B and 5), and (3) vascular pannus composed of connective tissue proliferating in the superficial corneal periphery and mainly associated with ocular surface disorders (Figs. 1A, 2 and 3A, 4 and 6) (Azar, 2006). Permanent scarring may result from vascular pannus, which can form when an insult is sustained for a long period of time. Deep stromal vascularization has been mainly seen in conjunction with scleritis, serious anterior segment injuries, tuberculosis, and syphilis (Lee et al., 1998).
Fig. 1.
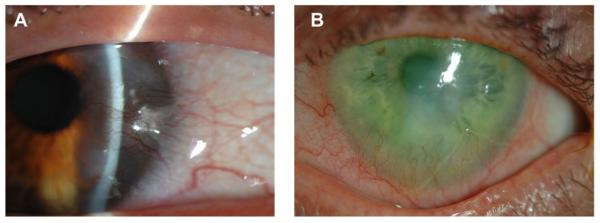
Clinical appearance of corneal NV in inflammatory disorders. A: Corneal NV in Salzmann's nodular degeneration. B: Corneal NV due to Rosacea. Dilated vessels at the limbus advance into the cornea predominantly inferiorly.
Fig. 2.
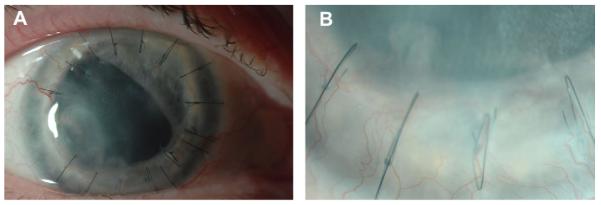
Clinical appearance of corneal NV post-keratoplasty (A) and its magnification (B).
Fig. 3.
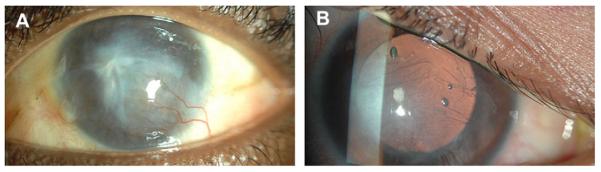
Clinical appearance of corneal NV in corneal infections. A: Superficial corneal vessel growth associated with Acanthamoeba Keratitis infection. The vast majority of reported cases of Acanthamoeba keratitis have been associated with contact lens use. Vessels normally grow inferiorly into the central cornea. This amebic infection has rarely been seen to spread beyond the cornea to affect the perilimbal and posterior ocular structures. However, a recent report shows that some patients had unexpected histopathologic findings of diffuse neuroretinal ischemia and perivascular lymphocytic infiltrates, some with vascular thrombosis, and chronic chorioretinal inflammation (Awwad et al., 2007). B: Retro-illumination image of an eye with deep stromal corneal NV due to interstitial keratitis. Patients with interstitial keratitis experience pain, photophobia, increased tearing, blepharospasm, and decreased vision. Neovascularization progresses centrally over time until the vessels coalesce in the central cornea.
Fig. 4.
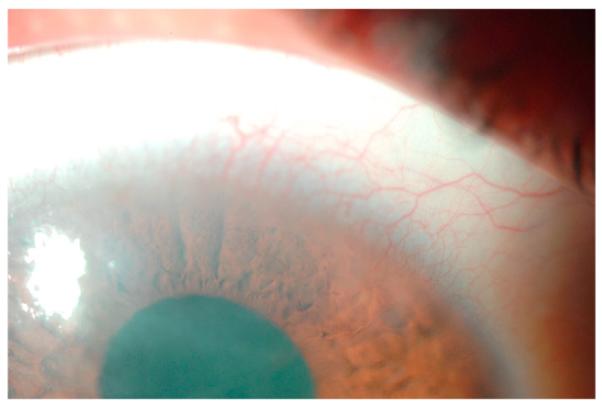
Corneal NV in contact lens-related hypoxia.
Fig. 5.
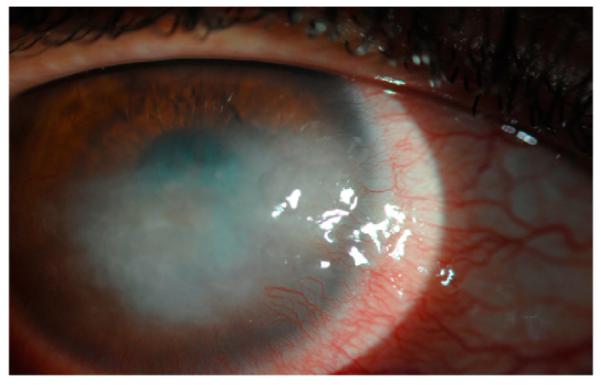
Superficial and mid stromal corneal NV due to neurotrophic ulceration.
Fig. 6.
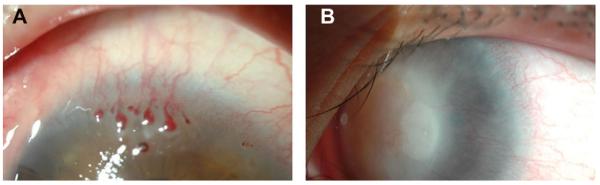
Corneal inflammation and neovascularization associated with limbal stem cell deficiency (A and B). Corneal NV associated with this condition is clinically challenging in that it persists long after the insult, and may not improve without transplantation of limbal stem cells.
A limited number of studies have illustrated the incidence and prevalence of corneal NV in the general population. By observing the number of patients presenting with corneal NV to a single general eye service and applying that number to the total number of visits for eye care in the US, Lee et al. estimated the incidence rate in the United States to be 1.4 million (Azar, 2006; Lee et al., 1998). In the following paragraphs, we present estimates of corneal NV in cases of infection, contact lens wear, corneal graft rejection, and trauma.
Corneal infections can provide us with a close estimate for the worldwide incidence of corneal NV. The reported incidence of ulcerative keratitis in a defined population was reported to be 11 per 100,000 person-years in the United States in 1993 (Erie et al., 1993). Trachoma, an infection caused by Chlamydia trachomatis, is one of the leading causes of preventable blindness in the world. It affects about 84 million people, of whom about 8 million are visually impaired. Chlamydia trachomatis was once endemic in 55 countries, primarily of Africa and Asia. At present, it is responsible for more than 3% blindness worldwide (WHO, 2009). A global initiative to eliminate trachoma as a blinding disease, entitled GET 2020 (Global Elimination of Trachoma), was launched under the World Health Organization's leadership in 1997. Other significant infections include Onchocerciasis and herpes simplex keratitis. It has recently been shown that HSV-1 infection disrupts the normal equilibrium between angiogenic and anti-angiogenic stimuli, leading to vascularization (Kaye and Choudhary, 2006). It is one of the most frequent causes of blindness in the United States, with 500,000 people experiencing HSV-related ocular disease (Lee et al., 1998; Wang et al., 2007), and approximately 20,000 new cases and more than 28,000 reactivations of ocular HSV occur in the United States annually.
The prevalence of contact lens-associated corneal angiogenesis is estimated to be within the range of 11–23% of contact lens wearers (Cursiefen and Kruse, 2006b). In a defined population in the United States, cases of ulcerative keratitis associated with contact lens wear increased from 0% in the 1950s and 1960s to 32% in the 1970s and 52% in the 1980s (Erie et al., 1993). Contact lens-related NV is mainly associated with soft-hydrogel lenses, especially with extended wear. Based on data from three studies, the annualized incidence of microbial keratitis with extended-wear silicone hydrogel contact lenses has been reported to be 19.8, 18.2, and 19.3/10,000 in Manchester, the United States and Australia, respectively (Keay et al., 2007). In the Netherlands, the estimated annualized incidence of microbial keratitis in 1999 was 1.1 per 10,000 users of daily-wear rigid gas-permeable lenses, 3.5 per 10,000 users of daily-wear soft lenses, and 20.0 per 10,000 users of extended-wear soft lenses (Cheng et al., 1999). Hydrogel, hard, and rigid gas-permeable contact lenses stimulate NV either by mechanically irritating the limbal sulcus or by creating corneal hypoxia, which leads to limbal inflammation, epithelial erosion, or hypertrophy, and hence, angiogenic mediator release. However, the prevalence of corneal NV is negligible (Cursiefen and Kruse, 2006b; Lee et al.,1998).
Post-keratoplasty, corneal angiogenesis occurs in about 50% of patients following low-risk keratoplasty in which the pre-operative recipient beds are avascular (Cursiefen and Kruse, 2006b). Recent reports indicate that lymphatic neovessels in the cornea may be as, or even more, important because these vessels provide alloantigen-bearing antigen-presenting cells effective access to regional lymph nodes (Chung et al., 2009).
Trauma is another cause of corneal NV. In the United States, it is estimated that there are 2.5 million new eye injuries each year and 40,000–60,000 of these patients suffer from trauma-related blindness (Broocker et al., 2007). Chemical burns represent 7.7–18% of ocular trauma (Broocker et al., 2007). Both acids and alkalis are capable of causing significant damage to the eye, but alkalis tend to cause the more severe damage due to its ability to penetrate the cornea in an extreme and rapid way. The worst-case estimate for the number of NV cases per year in the United States from alkali and other chemical burns was reported to approximately be 37,000 (Lee et al., 1998).
3. Corneal avascularity and angiogenic/lymphangiogenic privilege
The cornea is a highly specialized tissue that refracts and transmits light. Therefore, corneal clarity and corneal avascularity are important for the proper optical performance of the cornea. Approximately 1 mm thick peripherally and 0.5 mm centrally, the cornea comprises an outer stratified squamous nonkeratinized epithelium, an inner connective tissue stroma with resident keratocytes and, bordering the anterior chamber, a low cuboidal endothelium (Gipson et al., 2005). The cornea, with its unique avascular structure, has been used as an in vivo model for angiogenic and anti-angiogenic molecules. Recent investigations have focused on understanding the mechanisms that maintain corneal avascularity under homeostatic conditions and in avascular wound healing (Azar, 2006; Cho et al., 2009; Cursiefen, 2007). These studies suggest that corneal angiogenic privilege involves several active cascades and is not a passive process. The balance between angiogenic and anti-angiogenic factors in the corneal epithelium plays an important role in the avascularity of the cornea and its angiogenic privilege. The limbus has also been shown to act as a barrier for corneal angiogenesis; however, to date, the mechanism by how limbal stem cells maintain corneal avascularity is not fully understood.
3.1. The balance of angiogenic and anti-angiogenic factors in the cornea
Corneal NV in response to tissue injury, resulting from trauma, infection, and inflammatory or degenerative disorders, involves the dual invasion of blood (hemangiogenesis) and lymphatic (lymphangiogenesis) vessels (Fig. 7). More often, corneal wound healing occurs in the absence of NV. In this situation, the balance between angiogenic factors, such as fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF), and anti-angiogenic molecules, such as angiostatin, endostatin, or pigment epithelium-derived factor (PEDF), is tilted towards anti-angiogenesis. Corneal angiogenic privilege and maintenance of corneal avascularity not only occur as a result of the upregulation of anti-angiogenic factors, but also from the downregulation of pro-angiogenic factors (Azar, 2006).
Fig. 7.
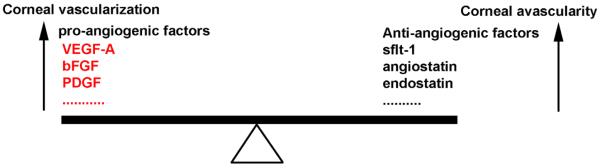
A diagram depicting corneal angiogenesis and avascularity. The normally quiescent vasculature (corneal avascularity) can be activated to sprout new capillaries (corneal vascularization), a process controlled by an angiogenic switch mechanism. In some pathologies not causing corneal angiogenesis, the absence of angiogenic inducers may preserve corneal avascularity, while in others the angiogenic inducers are present but held in check by higher levels of angiogenic inhibitors. An increase in the levels of pro-angiogenic factors in the cornea tilts the balance towards corneal vascularization. An increase in the levels of anti-angiogenic factors in the cornea tilts the balance towards corneal avascularity (Modified from Hanahan and Folkman, 1996).
A number of anti-angiogenic factors have been identified as being involved in the cornea during wound healing. These endogenous anti-angiogenic factors may play an important role in the regulation of angiogenesis during corneal wound repair and chronic inflammation. Gain-of-function or loss-of-function mutants of proand anti-angiogenic factors, created via knockout, transgenic, and siRNA-mediated targeting approaches, have been characterized in in vitro and in vivo angiogenesis assays to determine their roles in maintaining corneal NV. Table 2 lists the pro-angiogenic factors that are increased in various corneal neovascular diseases. For example, Mastyugin et al. (2001) have demonstrated that long-term contact lens wear causes VEGF and cytochrome p450 upregulation and extensive corneal angiogenesis; Hayashi et al. (2009) and Biswas et al. (2005) have shown that VEGF, MMP-2, -9, and cyclooxygenase-2 factors are enhanced in herpes virus corneal infection; Dana (2007) and Wallace et al. (2004) have shown that extensive interleukin and VEGF upregulation occur in corneal graft rejection; and Chui et al. (2007) and Jin et al. (2003) have shown an upregulation of VEGF and substance P in pterygium.
Table 2.
Expression of pro-angiogenic factors in corneal diseases.
| Corneal diseases | Increased in pro-angiogenic factor | References |
|---|---|---|
| Ocular cicatricial pemphigoid | TGF-β1 and 3 | (Elder et al., 1997) |
| Graft rejection | IL-1, TNF-α, VEGF-A, Chemokines, MIPs, MCP-1, IP-10, lymphotactin, fractalkine, RANTES, eotaxin, MIG, MIF and others | (Dana, 2007; Wallace et al., 2004) |
| Herpes simplex | VEGF and MMP-9, cyclooxygenase-2 (COX-2) | (Biswas et al., 2005; Hayashi et al., 2009) |
| Pseudomonas | IL-6, IL-8, and GRO | (Sack et al., 2009) |
| Onchocerciasis | O. volvulus activation associated secreted protein-1 (Ov-asp-1) | (Tawe et al., 2000) |
| Pterygium | VEGF and substance P | (Chui et al., 2007; Jin et al., 2003) |
| Contact lens wear | VEGF and cytochrome P450 4B1 | (Mastyugin et al., 2001) |
| Alkali burns | alpha(1), alpha(2), alpha(5), and beta(5) integrins and MMP-2 and MT1-MMP | (Zhang et al., 2002a) |
| Stem cells deficiency | TGF-beta | (Ma et al., 2006) |
In healthy corneas after minor injury, the upregulation of anti-angiogenic factors tilts the balance towards vessel regression. Following corneal wound healing newly-formed vessels do not invade the cornea, which maintains corneal avascularity. This can be attributed to the upregulation of anti-angiogenic factors and/or to a decrease in angiogenic factors, resulting in a shift towards vessel regression. While not much research has been published on this topic, vessel regression following corneal wound healing is likely due to a combination of anti-angiogenic factor upregulation and angiogenic factor downregulation. Table 3 lists the factors that are involved in the regulation of corneal angiogenesis. Many of the listed factors may be involved in maintaining the delicate balance required for maintaining corneal avascularity (Azar, 2006; Folkman and D'Amore, 1996; Huang et al., 2005).
Table 3.
Factors involved in regulating angiogenesis.
| Pro-angiogenic factors | Anti-angiogenic factors | Pro-/Anti-angiogenic factors |
|---|---|---|
| Fibroblast growth factor (FGF) (Cao et al., 2004; Chang et al., 2004) | Endostatin (Lai et al., 2007) Angiostatin (Cheng et al., 2007) | Transforming growth factor-β (TGF-β) (Friling et al., 1996; Sakamoto et al., 2000) |
| Vascular endothelial growth factor (VEGF) (Cao et al., 2004; Chen et al., 2008) | Prolactin (Duenas et al., 1999; Ueda et al., 2006) | Placenta growth factor (PIGF) (Cao et al., 1996; Eriksson et al., 2002) |
| Transforming growth factor-α (TGF-α) (Cursiefen et al., 2000; Yamamoto et al., 1994) | Thrombospondin (Panigrahy et al., 2008; Simantov et al., 2005) | Interleukins (Kim et al., 2005; Nakao et al., 2007) |
| Insulin-like growth factor (IGF) (Yamada et al., 2006) | Arresten (Mundel and Kalluri, 2007; Nyberg et al., 2008) | Matrix metalloproteinases (MMPs) (Azar, 2006; Kure et al., 2003; Ma et al., 2006) |
| Leptin (Park et al., 2001) | Canstatin (Magnon et al., 2007; Mundel and Kalluri, 2007) | Tumor necrosis factor α (TNF-α) (Chen et al., 2004; Saika, 2007; Ueda et al., 1998) |
| Integrins (Muether et al., 2007) | Tumstatin (Goto et al., 2008; Mundel and Kalluri, 2007) | |
| Platelet-derived growth factors (PDGF) (Dell et al., 2006) | Pigment epithelium-derived factor (PEDF) (Abdiu and Van Setten, 2008) | |
| Angiogenin (Crabtree et al., 2007) | Fibulin (Xie et al., 2008) | |
| Hepatocyte growth factor/scatter factor (HGF/SF) (Grierson et al., 2000) | Endorepellin (Woodall et al., 2008) | |
| Connective tissue growth factor (CTGF) (Babic et al., 1999) | Antithrombin (Schedin-Weiss et al., 2008) | |
| Monocyte chemoattractant protein-1 (MCP-1) (Yoshida et al., 2003) | Plasminogen activator inhibitor (PAI) (Vogten et al., 2003) | |
| Platelet activating factor (PAF) (Ma et al., 2004) | Vasostatin (Wu et al., 2005) | |
| Activin-A (Poulaki et al., 2004) | Neostatin-7 (Kojima et al., 2008) | |
| Thrombin (Hu et al., 2008) | IFN-γ (Kommineni et al., 2008) |
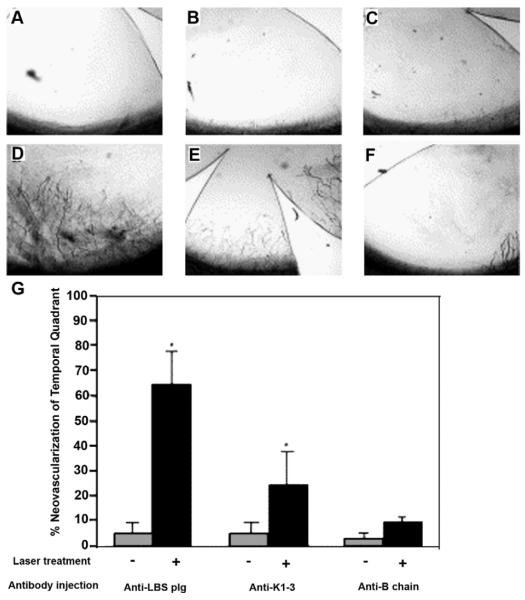
A role for the corneal epithelium in the maintenance of corneal angiogenic privilege has been suggested but not fully elucidated. Several naturally occurring anti-angiogenic factors have been characterized in the corneal epithelium and have been shown to regulate corneal avascularity and corneal angiogenic privilege. Some of these factors inhibit corneal NV (solubleVEGFR-1, VEGFR-3, and Neostatins), while others inhibit corneal NV through proteolytic action (MMP-7 and MMP-14) (Ambati et al., 2006; Azar, 2006; Cursiefen et al., 2006a). The corneal epithelium expresses soluble forms of VEGF receptor-1 and ectopic VEGFR-3, which act as endogenous VEGF traps (Ambati et al., 2006, 2007; Cursiefen et al., 2006a), thereby serving as a control mechanism for the proangiogenic properties of the VEGF family.
Ambati et al. (2006) have demonstrated the presence of soluble VEGFR-1 (sVEGFR-1 or sFlt-1) in the cornea. They have also demonstrated that upon suppression of this endogenous VEGF-A trap (sFlt-1) with neutralizing antibodies, RNA interference, or Cre-lox-mediated gene disruption, mice develop extensive corneal NV. Furthermore, they have compared the expression levels of sVEGFR-1 in normal and neovascularized human corneas (Ambati et al., 2007) to show that normal human corneas strongly express sVEGFR-1 in the corneal epithelium, that VEGF is bound by sVEGFR-1 in the normal human cornea, and that neovascularized human corneas have greatly reduced expression of sVEGFR-1 with significantly less VEGF bound to sVEGFR-1 (Fig. 8).
Fig. 8.
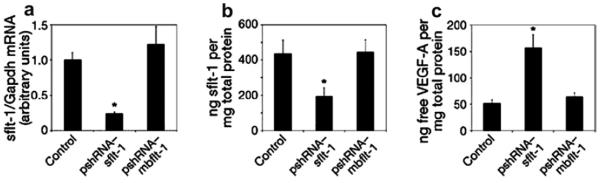
Real-time RT-PCR reveals reduced levels of sflt-1 mRNA (a) and an enzyme-linked immunosorbent assay (ELISA) reveals reduced levels of sflt-1 protein (b) and increased free VEGF-A protein (c) in WT mouse corneas 3 days after injection of pshRNA-sflt-1, but not pshRNA-mbflt-1. The asterisk denotes P < 0.05, Bonferroni corrected Mann–Whitney U-test. n = 8–12. Error bars depict s.e.m. (reprinted with permission from Ambati et al., 2006).
Non-vascular VEGF receptor-3 (VEGFR-3) is also expressed in the corneal epithelium (Cursiefen et al., 2006a). Using immunohistochemistry and RT-PCR, Cursiefen and colleagues have shown that VEGFR-3, normally present on proliferating blood vascular endothelium, is strongly expressed by the corneal epithelium. They also used in vivo models of corneal NV and epithelium removal, along with VEGFR-3 analysis, to demonstrate that corneal epithelium VEGFR-3 may be mechanistically responsible for suppressing inflammatory corneal angiogenesis (Fig. 9).
Fig. 9.
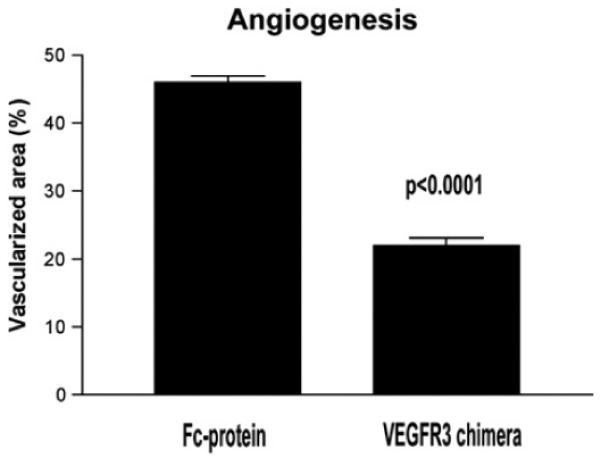
Diminished corneal vessels after administration of a VEGFR-3 chimera to wounded corneas. The neovascular response after cautery of de-epithelialized corneas is significantly diminished when a VEGFR-3 chimeric protein is administered locally (reprinted with permission from Cursiefen et al., 2006a).
VEGFR-3 has also been shown to control the development and growth of the lymphatic system. VEGFR-3 knockout embryos die early in development due to cardiovascular failure, which suggests that this receptor is essential in the formation of the primary cardiovascular network before the emergence of lymphatic vessels (Karkkainen et al., 2000). VEGFR-3 is not only expressed in the lymphatic endothelium, but also in tumor blood vessels during NV and in some fenestrated endothelia. Later in development, VEGFR-3 expression is restricted mainly to lymphatic vessels.
Two VEGFR-3 proteins, VEGFR-3s (short) and VEGFR-3l (long), differ in their carboxyl termini as a result of alternative mRNA splicing (Pajusola et al., 1993). VEGFR-3 forms homodimers or heterodimers with VEGFR-2 in response to the addition of VEGF-C (Alam et al., 2004; Dixelius et al., 2003). These heterodimeric receptors may be present in lymphatic endothelial cells, such as fenestrated capillaries, which express both receptors. VEGF-C and VEGF-D bind to VEGFR-3 and induce the tyrosine phosphorylation of VEGFR-3 to activate kinase activity. Phosphorylation of VEGFR-3 is required for the association with the Shc–Grb2 complex (Fournier et al., 1999). Additionally, activated VEGFR-3 induces the rapid tyrosine phosphorylation of Shc and the activation of MAPK. The VEGFR-3 signal transduction pathways increase cell motility, actin reorganization, and further induce cell proliferation (Wang et al., 2001). The integral role that VEGF-A, VEGF-C, VEGF-D, and endothelial VEGF receptors play in the induction of angiogenesis and lymphangiogenesis can explain why VEGF traps (sVEGFR-1 and non-vascular VEGFR-3) may be an invaluable control mechanism to maintain corneal avascularity.
Membrane-type 1 metalloproteinase (MT1-MMP) is another molecule that regulates corneal avascularity. It is expressed in the corneal basal epithelium and corneal stromal keratocytes in unwounded corneas. MT1-MMP has been shown to have anti-angiogenic and pro-angiogenic properties in the cornea (Azar, 2006; Azar et al., 2008). The pro-angiogenic role of MT1-MMP has been documented in MT1-MMP knockout mice that exhibit impaired bFGF-induced corneal NV (Zhou et al., 2000). MT1-MMP cleaves proMMP-2 to active MMP-2, which in turn contributes to extracellular matrix (ECM) remodeling. MT1-MMP also directly degrades ECM macromolecules such as gelatin, type I collagen, and fibronectin, leading to ECM remodeling (Azar, 2006).
We have recently observed that in contrast to its pro-angiogenic role in vascular endothelial cells and stromal fibroblasts, MT1-MMP has an anti-angiogenic role in corneal epithelial cells, which may be a contributing factor to the anti-angiogenic role of epithelial cells in the cornea. We and others have demonstrated that MMPs expressed in the corneal epithelium generate the anti-angiogenic factors, angiostatin and neostatin, through proteolytic activity against plasminogen and collagen XVIII, respectively (Chang et al., 2005; Gabison et al., 2004; Kure et al., 2003). We have also shown that MT1-MMP is expressed in corneal epithelial cells, primarily in the basal epithelium in unwounded corneas, and after keratectomy wounds in vivo (Ye et al., 2000). In a recent study, we have generated MT1-MMP knockout corneal epithelial cell lines and wild type (WT) MT1-MMP knockin and catalytically inactivated MT1-MMP (E240A) knockin epithelial cells. We compared the angiogenic potential of WT MT1-MMP with over expression of its mutant counterparts in MT1-MMP knockout corneal epithelial cells. Calf-pulmonary arterial endothelial cell (CPAE) proliferation and migration was significantly enhanced by the addition of conditioned media from MT1-MMP knockout corneal epithelial cells, suggesting that MT1-MMP contributes to anti-angiogenesis. In these cells, when WT MT1-MMP or mutated catalytically-inactive MT1-MMP (MT1-MMP-E240A) was overex-pressed, CPAE proliferation and migration reverted to levels similar to WT MT1-MMP. These data suggest that MT1-MMP plays an anti-angiogenic role in cultured corneal epithelial cells and that this effect is independent of MT1-MMP catalytic activity (Azar et al., in press). The anti-angiogenic role of MMPs can be explained by the ability of MMPs to generate anti-angiogenic fragments (e.g., neostatin-7 and -14) from precursors, which prevent bFGF-induced corneal NV (Chang et al., 2005; Kojima et al., 2008; Lamoreaux et al., 1998).
Cultured corneal epithelial cells have been shown to possess anti-angiogenic potential. Sekiyama et al. used immunohistochemical staining to show that anti-angiogenic factors Thrombospondin-1 (TSP-1), pigment epithelium-derived factor (PEDF), endostatin, and angiostatin are expressed in cultured corneal epithelial cells. Several recent studies have shown that conditioned media modulates vascular endothelial cell proliferation, migration, and/or tube formation. Pollina et al. (2008) have demonstrated that the balance between the angiogenesis inducers and inhibitors secreted in the microenvironment controls the rate of new blood vessel formation.
3.2. The limbus and the limbal barrier: questionable role in corneal angiogenic privilege
The limbus is the transition zone forming the border between the opaque sclera and the transparent cornea. However, there are no definite, reliable boundaries for the limbus. Various anatomic definitions of the limbus have been offered by anatomists, pathologists, histologists, and surgeons (Jakobiec and Ozanics, 1982; Van Buskirk, 1989). The broadest definition of the limbus is the zone between the termini of Bowman's layer and the posterior end of Schlemm's canal. For example, a line drawn between the termini of Bowman's layer and Descemet's membrane would form the anterior border of the limbus; and a parallel line approximately 1 mm posterior to the anterior line, passing through the posterior end of Schlemm's canal, would form the posterior border (Gipson and Joyce, 1999).
Limbal function has been a controversial topic since the mid 19th century (1859) (Azar, 2006). Over the following century, the limbus was believed to serve several functions (including acting as a gland) but studies failed to demonstrate any purpose for the limbus (Duke-Elder, 1958). It was not until 1971 that the concept of the limbus playing a role in corneal epithelial renewal was first discussed (Davanger and Evensen, 1971).
The limbal epithelium shares many features with the corneal epithelium. It is a stratified, squamous, non-keratinizing epithelium whose cell junctions have apical and basal specializations similar to those of the cornea (Gipson, 1989). The basal layer of the limbus appears unique and is believed to be the location of corneal epithelial stem cells responsible for renewing damaged corneal epithelium (Davanger and Evensen, 1971; Schermer et al., 1986).
In addition to playing a role in corneal epithelium renewal, the limbus is also believed to play an integral role in preventing corneal NV and maintaining corneal avascularity (Ma et al., 2006). This concept has been demonstrated over the past several years by the great increase in corneal NV and inflammation seen in pathological limbal stem cell deficiency and experimental limbal damage (Espana et al., 2002, 2003; Grueterich et al., 2003); and by the considerable improvement of corneal NV following limbal stem cell transplantation (Kenyon and Tseng, 1989; Tsai and Tseng, 1994). However, even though a role for the limbus in the maintenance of corneal avascularity is well accepted, little is known about the molecular mechanisms behind the limbal anti-angiogenic effect. The limbal barrier hypothesis is one of the most well-accepted explanations for the mechanism behind the limbal anti-angiogenic effect and the severe NV seen in limbal deficiency and damage (Friedenwald, 1951; Huang and Tseng, 1991; Tseng, 1989). This hypothesis describes the constant renewal (proliferation, migration, and differentiation) of corneal epithelial cells by the limbus as acting as a physical barrier to prevent conjunctival and vessel outgrowth in the cornea. However, the concept of the limbus acting as a physical barrier to prevent corneal NV has been recently questioned (Azar, 2006).
We have used a bFGF pellet corneal NV model to demonstrate that the limbus may not necessarily function as a true physical barrier to NV. bFGF-induced corneal NV was evaluated in WT mice after removal of half of the limbal and corneal epithelium (Figs. 10 and 11). The corneal NV pattern was also evaluated in the same manner in different MMPs and collagen XVIII knockout mice. A Hamilton needle was used to remove half of the limbal epithelium and half of the corneal epithelium. The debrided half of the cornea was swabbed with 70% alcohol for few seconds to remove all remaining epithelial cells. Following limbal and epithelial removal, bFGF pellets were implanted in the cornea to induce corneal NV. Corneas were routinely examined and photographed in en face, superior, inferior, nasal, and temporal positions with a slit-lamp biomicroscope on days 2, 5 and 7 post-pellet implantation.
Fig. 10.
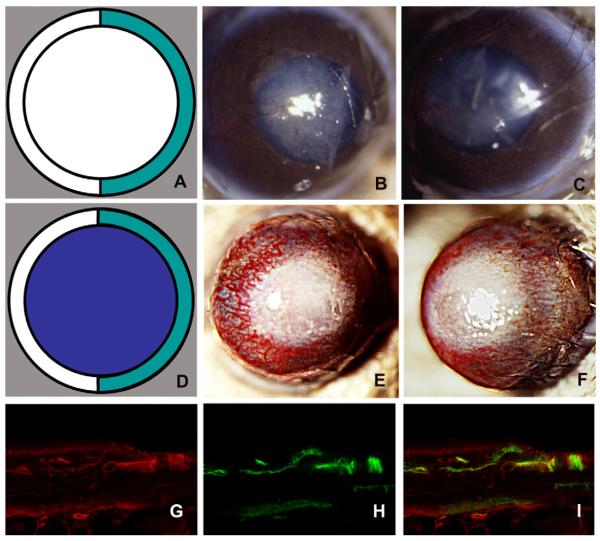
Hemilimbal deficiency: a model for injury-induced corneal NV. The diagrams depict limbal injury (A; green) and limbal plus epithelial removal (D; purple). The nasal limbi of WT mouse corneas were removed and photographed at day 7 after surgery (B, C). The nasal limbus and the epithelium of WT mouse corneas were removed and the corneas photographed at day 7 after surgery (E, F). Vascularized vessels were immunostained with anti-type IV collagen (G), anti-CD31 antibodies (H), and double staining (I) (reprinted with permission Azar, 2006).
Fig. 11.
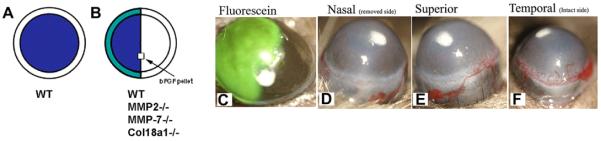
A diagram of the hemilimbal barrier model. Removal of the corneal epithelial layer by #15 blade (A) and hemilimbal (B; half of the limbal and corneal epithelium with bFGF-pellet implantation). Corneas of hemilimbal wounding were stained with fluorescein (C). Corneal NV at day 5 post-intrastromal bFGF-pellet implantation and hemilimbal debridement (n = 5). The debrided nasal side has no corneal NV (D), corneal NV were developed from the temporal unwounded side (F).
In the WT mouse corneas, NV began at day 3 post-intrastromal bFGF-pellet implantation and progressed until day 10. More importantly, NV was considerably more prevalent on the temporal side with the intact limbus, and was nearly absent on the nasal side that had the limbus and epithelium removed. Additionally, immunostaining demonstrated an enhanced corneal VEGF-A expression in the temporal side of the unwounded cornea when compared to that of wounded (nasal side) of the cornea (Fig. 12). One explanation is while that the keratocytes underneath the non-debrided side were intact, while the stroma underneath the debrided epithelium was deprived of keratocytes due to apoptosis. This may have resulted in bFGF-induced VEGF expression from the non-apoptotic keratocyte explaining the appearance of corneal NV from the intact side.
Fig. 12.
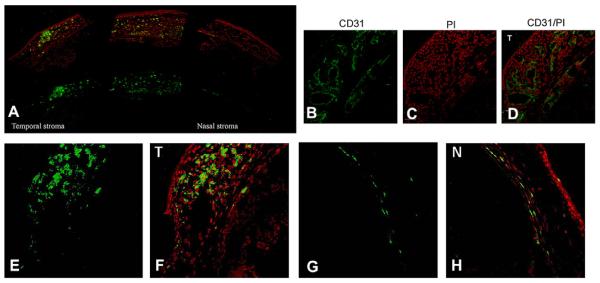
Enhanced corneal CD31 and VEGF-A expression in hemilimbal wounded corneas. Wounded corneas were immunostained with anti-CD31 (B) and VEGF A (E; temporal side, G; nasal side) antibodies. Enhanced CD31 immunostaining in the temporal side of the unwounded corneas (B), PI staining (C), and merged images (D). Enhanced corneal VEGF-A expression in the temporal side of the unwounded cornea (E) compared to the wounded side (nasal side) of the cornea (bar = 50 μm).
Our results show that removal of half of the limbus (hemilimbal deficiency) leads to corneal NV from the opposite side of the cornea where the limbus was intact; this observation questions the role of the limbus and whether it truly acts as a physical barrier to corneal NV. Since it has long been believed that the role of the limbus in corneal NV was to act as a true physical barrier, inhibiting the growth of vessels, additional studies in this area are required to elucidate the role of the limbus in corneal angiogenic privilege.
4. Molecular and cellular mechanisms of corneal NV
Angiogenesis (Hemangiogenesis/Lymphangiogenesis) is one of the most essential biological processes encountered in mammalian organisms. Hemangiogenesis is the sprouting and budding of endothelial cells from pre-existing vessels, usually the post-capillary and small terminal venules of the microvascular apparatus. Lymphatic endothelial cells, on the other hand, have been shown to arise from primitive veins, from local lymphangioblasts, or from bone marrow-derived progenitor cells (Cueni and Detmar, 2008b). Both processes are regulated by growth factors, proangiogenic cytokines, and inhibitors of neovascularization. The cornea with its angiogenic privilege has been used as a model to study the biological processes of hemangiogenesis/lymphangiogenesis. Recent studies have specifically focused on identifying lymphatic vessel formation, and blood vessel formation versus lymphatic vessel formation in the cornea. The identification of several molecules specifically expressed on either lymphatic or blood vascular endothelium has enabled the isolation of these two cell types. In addition, much has been learned about the stimulators and inhibitors of hemangiogenesis/lymphangiogenesis, and members of the VEGF family have emerged as prime mediators of both processes.
4.1. Corneal angiogenesis (hemangiogenesis)
Corneal NV usually extends between the collagen lamellae into the substantia propria of the cornea, but it is also a component of a fibrovascular sheet between the corneal epithelium and Bowman's layer (a pannus) (Kenyon et al., 1977). The anterior segment blood supply may be thought of as arising from several circular ring like systems that surround the cornea and communicate with each other (Carmichael, 2006). The process by which blood vessels grow into the cornea can be divided into several phases. Upon injury, the cornea releases growth factors which bind to specific receptors located on the vascular endothelial cells of nearby pre-existing blood vessels promoting proliferation. The endothelial cells degrade and migrate through their basement membrane and extracellular matrix. In preparation for movement away from the parent venule, activated endothelial cells undergo alterations in the expression of cell–cell and cell–matrix adhesive molecules, exhibit reorganization of cytoskeletal elements, and express cell surface adhesion molecules such as integrins, selectins, and components of the extracellular matrix. These “activated” endothelial cells also generate proteolytic enzymes that enable them to degrade their extracellular matrix and migrate away from the parent vessel (Polverini, 1995).
Several angiogenic molecules expressed during wound healing have been identified, including VEGF and bFGF. VEGF-A is upregulated in inflamed and vascularized corneas in animal models (Amano et al., 1998; Kvanta et al., 2000; Mastyugin et al., 2001). Several studies have identified members of the VEGF family that bind to different receptors stimulating hemangiogenesis. For example, VEGF-A has emerged as the main family member responsible for normal vasculogenesis and hemangiogenesis (Azar, 2006; Cursiefen, 2007). VEGF-A binds to VEGFR-1 and VEGFR-2 (Carmeliet, 2005). Additionally, VEGF-C and VEGF-D also exhibit hemangiogenesis activities through their binding to VEGFR-2 (Cao et al., 1998; Cursiefen et al., 2004; Rissanen et al., 2003). bFGF is another potent angiogenic factor that has been shown to play a role in hemangiogenesis. Recent studies have focused on identifying an interplay between FGF and VEGF signaling during angiogenesis, suggesting that bFGF binds to FGFR stimulating VEGF production (Onguchi et al., 2009; Shi et al., 2005).
4.2. Corneal lymphangiogenesis
Lymphatic endothelial cells (LECs) have been shown to have a venous origin. This has been supported by studies in Prox-1 deficient mice, revealing the role of this homeobox protein in lymphatic development (Wigle et al., 2002). Zebrafish studies have also shown that LECs of the thoracic duct originate from primitive veins. Using corneal models, bone marrow-derived cells have been shown to incorporate into newly-formed lymphatic vessels in FGF-2 treated corneas of chimeric mice. It has been suggested that these lymphatic endothelial progenitors may be macrophages, which can transdifferentiate into LECs (Cueni and Detmar, 2008b; Maruyama et al., 2005).
The cellular differentiation events in lymphangiogenesis utilize several of the same families of signaling molecules involved in hemangiogenesis, which involves the VEGF family. Prox-1 upregulates VEGFR-3 and through the expression of VEGF-C and VEGF-D and their binding to VEGFR-3 induces endothelial cells to begin differentiation into lymphangioblasts from the venous endothelial cells, they undergo further differentiation to form a lymphatic plexus that will eventually form the lymphatic capillaries. Several studies have shown that VEGF-C and VEGF-D are required for lymphangiogenesis (Alitalo et al., 2005; Karkkainen et al., 2004; Patel and Dana, 2009).
Recent studies include novel corneal models to study hemangiogenesis separately from lymphangiogenesis. Cursiefen et al. (2004) evaluated the role of VEGF-A in promoting lymphangio-genesis as well as hemangiogenesis through inducing these two biological processes and administering a VEGF Trap to neutralize VEGF-A. They observed that both hemangiogenesis and the outgrowth of lymphatic vessels were completely inhibited following injury. They also demonstrated that the VEGF-A recruitment of macrophages plays a crucial role in inducing inflammatory neovascularization by supplying signals essential for pathological hemangiogenesis and lymphangiogenesis (Cursiefen et al., 2004). Chung et al. (2009) used bFGF micropocket pellet implantation in BALB/c mice. They found that a hemangiogenesis-dominant corneal phenotype can be obtained 2 weeks after bFGF-pellet implantation with VEGFR-3 blockade compared to 3 weeks for lymphangiogenesis after bFGF-pellet implantation with no supplementary modulating agents.
5. Major angiogenic proteins of the cornea
5.1. Vascular endothelial growth factor (VEGF-A)
VEGF was initially identified as a stimulator of vascular permeability (called VPF, for vascular permeability factor) and was subsequently demonstrated to be an endothelial cell-specific mitogen and angiogenic factor. After VEGF was discovered, several additional family members were characterized which have been called VEGF-B, VEGF-C, and VEGF-D; the parent form is now called VEGF-A. VEGF-A expression has been correlated with embryonic, physiologic, and pathologic blood vessel growth, in vivo (Breier, 2000; Darland and D'Amore, 2001; Ferrara, 2001). VEGF-A is produced by many different cells, including pericytes, fibroblasts, macrophages, T-cells, retinal pigment epithelial cells, astrocytes, and smooth muscle cells (Witmer et al., 2003). The spatial and temporal expression patterns of VEGF-A and its tyrosine kinase receptors, flt-1 and flk-1/KDR, in several systems suggest that VEGF-A is a key mediator of vasculogenic and angiogenic events associated with a wide range of biological processes (Masuda et al., 2001; Ng et al., 2001a,b). The overall mechanism by which VEGF-A stimulates angiogenesis is through the increase of endothelial cell proliferation, migration, proteolytic activity, and capillary tube formation (Penn et al., 2008). VEGF-A also acts to significantly increase vascular permeability (Penn et al., 2008). Local and systemic signals (responsible for orchestrating the growth and regression of new blood vessels) regulate VEGF gene expression, including cAMP, steroid hormones, protein kinase C agonists, polypeptide growth factors, oxygen, free radicals, glucose, cobalt, and iron. Many of these agents (protein kinase C agonist polypeptide growth factor, oxygen, free radicals) modulate bFGF gene expression via transcriptional regulation through transcription activator protein-1 including AP-1, AP-2, p53, and NFκB (Bjorndahl et al., 2005; Pal et al., 2001; Shima et al., 1996).
Five isoforms of VEGF-A (VEGF115, VEGF121, VEGF165, VEGF189, and VEGF206) can be generated from the alternative splicing of a single gene (Sugihara et al., 1998). The longer isoforms (VEGF189 and 206) are matrix-bound, whereas the shorter isoforms (VEGF121 and 165) are freely diffusible. The shorter VEGF isoforms exhibit distinct functions when secreted. For example, all isoforms increase vascular permeability, but only VEGF121 and VEGF165 possess mitogenic activity. Furthermore, VEGF121 has greater angiogenic activity than VEGF165 or VEGF189. On the other hand, VEGF165 is more potent than VEGF121 in the induction of inflammation, ICAM-1 expression in endothelial cells, and the chemotaxis of monocytes. Thus, the alternative splicing of VEGF-A RNA can produce polypeptides with strikingly different secretion patterns, which suggests multiple physiological roles for this protein (Zhang et al., 2000; Zheng et al., 2001).
The expression of VEGF-A is tightly regulated. Enhanced VEGF-A production has been observed in hypoxia and during the inflammatory response. The overproduction of VEGF-A has been implicated in tumor cell proliferation. In addition, the induction of VEGF-A expression is associated with the malignant transformation of cultured cells (Carmeliet, 2005). Similarly, several reports demonstrate the upregulation of VEGF-A in vascularized corneas. VEGF-A expression is seen in corneal epithelial cells, corneal endothelial cells, vascular endothelial cells of limbal vessels, and keratocytes. In addition, VEGF-A expression is markedly increased in the epithelial cells of inflamed corneas, vascular endothelial cells, macrophage infiltrates, and fibroblasts in corneal scar tissue. VEGF-A concentrations are significantly higher in vascularized corneas than in normal control corneas (Zheng et al., 2001).
VEGF-A promotes several steps of angiogenesis, including proteolytic activities (dissolution of the membrane of the original vessel), vascular endothelial cell proliferation, migration, and capillary tube formation. The importance of VEGF-A in corneal angiogenesis was demonstrated in a rat model by the inhibition of NV after stromal implantation of an anti-VEGF-A blocking antibody. This result has been reproduced using VEGF-A-blocking peptides in a rabbit corneal model (Binetruy-Tournaire et al., 2000; Schlaeppi et al., 1999). Currently, anti-VEGF-A therapy is a mainstay for treatment of pathological corneal NV.
Other VEGF-family members, VEGF-B, VEGF-C, and VEGF-D, have been shown to bind differentially to VEGF receptors and to regulate angiogenesis and lymphangiogenesis (Cao, 2005; Li and Eriksson, 2001; Olofsson et al., 1999; Tammela et al., 2005a; Zawieja, 2005). VEGF-B is an inefficient vascular endothelial cell mitogen. It binds to the receptor VEGFR-1, but not to VEGFR-2 or -3. VEGF-C and -D are mitogenic for vascular endothelial cells. They activate VEGFR-3 and are involved in the regulation of the growth and/or differentiation of lymphatic and blood vessel endothelium. VEGF-C also binds to VEGFR-2 and VEGFR-3 (flt-4, which is predominantly expressed in lymphatic endothelial cells in adult tissues) (Hamada et al., 2000).
5.2. Basic fibroblast growth factor (bFGF)
bFGF is a member of the FGF family, which includes 23 structurally related heparin-binding peptides widely expressed in developing and adult tissues during cellular differentiation, angiogenesis, mitogenesis, and wound repair. bFGF is upregulated after tissue injury and in stromal fibroblast/vascular endothelial cell co-cultures (Zhou et al., 2000). The functions of FGFs are mediated through peptide–receptor interactions with FGF receptors (FGFR) -1, -2, -3, and -4. The repertoire of potential FGF-mediated intracellular signaling events has significantly increased, and the different FGFR isoforms display distinct biological functions (Mohammadi et al., 1998). In addition, tissue-specific FGFR expression reflects the diversity of its biological response, which is regulated through differences in ligand specificity and function. The regulation of growth factor receptor activity plays an important role in the orchestration of complex physiological processes (Jeffers et al., 2001).
In addition to the FGFR isoforms, the individual FGFs also contribute to the diversity of their functions. For example, FGF-1 is expressed in the normal corneal epithelium, and FGF-2 is upregulated after injury and during keratocyte-vascular endothelial cell coculture. Interestingly, bFGF binds to Bowman's (BM) and Descemet's membranes in normal corneas and vascular basement membranes in neovascularized corneas (Adamis et al., 1991). In fact, some believe that the BM actually acts as a reservoir for bFGF (and VEGF), sequestering these potent angiogenic factors and acting as an anti-angiogenic control mechanism (Bikfalvi et al., 1997). Additionally, the level of FGF binding is related to the stage of maturation of new vessels, as differential FGF binding has been demonstrated. The difference in binding observed between normal limbal vessels and newly-formed corneal vessels is probably due to the differential expression of heparan sulfate proteoglycans. This emphasizes the role of ECM components in the regulation of corneal angiogenesis (Soubrane et al., 1990). Using the alkali wounding model, we have demonstrated that FGF-1, 2, 3, 7, and 22 expression are enhanced at days 7 and 14 after wounding (Fig. 13).
Fig. 13.
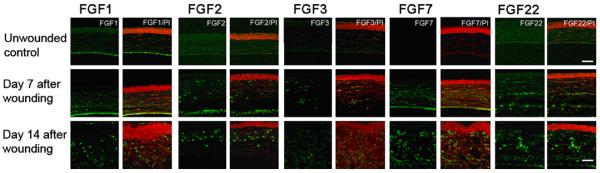
Enhanced expression of FGF1, 2, 3, 7, and 22 was detected in alkali wounded corneas. Corneas were treated with 1 N NaOH for 1 min and harvested at days 7 and 14 (n = 5). Corneal sections were immunostained with anti-FGF, 1, 2, 3, 7, and 22 antibodies (bar = 50 μm).
The function of bFGF in promoting corneal angiogenesis may be mediated through its effects on VEGF-A, -C, and -D production. bFGF is a potent angiogenic factor. Recently, bFGF has also been demonstrated to promote lymphangiogenesis: The induction of corneal lymphangiogenesis by bFGF may be due to the upregulation of VEGF-C and -D expression. As shown in Fig. 14, the upregulation of VEGF-C and -D was observed (potent inducers of lymphangiogenesis) following bFGF corneal pellet implantation.
Fig. 14.
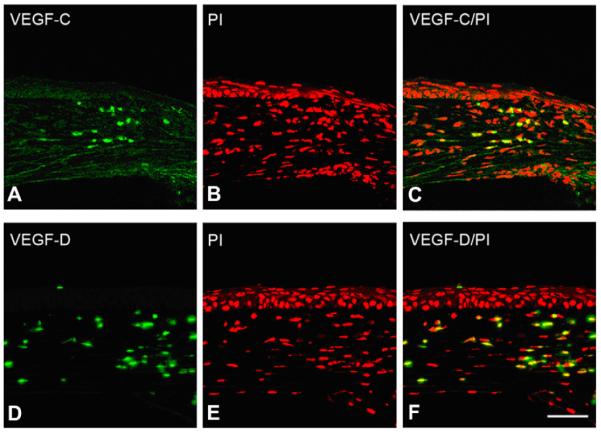
bFGF induces corneal VEGF-C/-D expression. Mouse corneas were implanted with bFGF pellets and corneal sections were immunostained with anti-VEGF-C and -D antibodies. VEGF-C/-D expression was shown to be enhanced after bFGF-pellet implantation (bar = 50 μm).
5.3. Angiogenic pathways (VEGF and bFGF): distinct but intersecting
During the past decade, much research has focused on the characterization of interactions between multiple membrane-bound receptors. These results have led to the hypothesis that, instead of transmitting signals across the membrane individually, membrane-anchored receptors associate and coordinate with other adjacent membrane-bound receptors to cooperatively induce an array of intracellular signaling cascades (Eliceiri, 2001; Schneller et al., 1997; Weis et al., 2007). Recently, an interplay between FGF and VEGF signaling has been observed in the maintenance of endothelial junctions and thus, vascular integrity, during angiogenesis (Komarova and Malik, 2008; Murakami and Simons, 2008). An alternative signaling pathway has been proposed in which c-Abl is a downstream mediator of FAK in the bFGF-induced signaling cascade, whereas in VEGF signaling, Src but not c-Abl is the essential factor in the activation of the VEGF-induced signaling. It has been suggested that an FGF-VEGF signaling balance may lie at the center of the regulation of permeability and angiogenesis. Both VEGF and FGF families are potent angiogenic growth factors. However, several functional differences in angiogenesis induced by VEGF and FGF factors have been reported (Table 4).
Table 4.
Functional differences in angiogenesis induced by VEGF and bFGF.
| VEGF | bFGF | |
|---|---|---|
| Endothelial cell migration (Yoshida et al., 1997) | Chemotaxis (directional migration) | Chemokinetic (random migration) |
| Cell survival transcriptional factors (Alavi et al., 2003) | Raf-1 (TYR-340 and TYR-341 phosphorylation) MEK1 | Raf-1 (Ser-338 and Ser-339 phosphorylation) PAK-1 |
| Integrin activity for angiogenesis (Brooks et al., 1994; Friedlander et al., 1995) | α v β 5 | α v β 3 |
| Src kinase activity (Eliceiri et al., 1999) | Dependent | Independent |
| C-Abl activity (Yan et al., 2008) | Independent | Dependent |
| Capillary endothelial permeability (Cao et al., 2004) | Leaky | Non-leaky |
| VE-cadherin/p120-catenin complex (for endothelial barrier function) (Murakami and Simons, 2008) | Disassembly | Assembly |
Once activated by VEGF, VEGFR-2 (named Flk-1/KDR) undergoes autophosphorylation on specific tyrosine residues, followed by the addition of Tyr(P) residues on adapter and signaling proteins that contain the Src homology domain 2 (SH2) (Guo et al., 1995). Subsequently, these adapter and receptor complexes activate multiple downstream effectors, including focal adhesion kinase (FAK) and mitogen-activated protein kinase (MAPK) kinases (Erk, p38, and/or JNK kinase; Abedi and Zachary, 1997; Wheeler-Jones et al., 1997). Flk-1/KDR also has the ability to trigger the activation of several other signaling cascades, including phospholipase C-γ (PLC γ) and PI3K-dependent Akt/PKB (Sun et al., 2005; Zeng et al., 2006). The Flk-1/KDR-mediated intracellular signaling appears to be similar to the signaling pathway involving bFGF; however, there is evidence to suggest that bFGF-induced angiogenesis is independent of Src kinase activity (Eliceiri et al., 1999, 2002) whereas VEGF signaling is not. Although bFGF- and VEGF-induced angiogenesis have been extensively investigated, the distinct intracellular signaling pathways that respond to each growth factor to induce angiogenesis are not completely understood. In particular, the interactions between these pathways and the molecular regulators of these interactions have not been well documented.
We have recently suggested that MT1-MMP may be one of several factors involved in linking the two pro-angiogenic pathways (VEGF and FGF signaling). We have demonstrated that MT1-MMP synergistically increases bFGF-induced VEGF upregulation and corneal NV in mice (Onguchi et al., 2009). In addition, MT1-MMP increases bFGF-induced VEGF upregulation in enzymatically inactive MT1-MMP corneal stromal fibroblasts. These data suggest that MT1-MMP enzymatic activity may play a role in linking the VEGF and FGF signaling pathways.
6. Additional angiogenesis regulatory proteins of the cornea
As detailed above, the regulation of corneal NV is a very delicate process that requires several individual mechanisms to maintain corneal angiogenic privilege and avascularity. Many of the primary regulatory mechanisms and proteins are described in other sections of this review. This section focuses on regulatory proteins (both anti- and pro-angiogenic) that are not described in other sections.
6.1. Decorin
Decorin belongs to the small leucine-rich proteoglycan (SLRP) family and consists of a protein core containing leucine repeats with aglycosaminoglycan (GAG) chain of either chondroitin sulfate or dermatan sulfate. SLRPs belong to a family of multifunctional molecules with diverse functions, such as the regulation of collagen fibrillogenesis, binding and inactivation of cytokines, and the direct modulation of cell behavior (Iozzo, 1997). SLRPs interact with a variety of extracellular matrix proteins and have been implicated in the regulation of various stages of collagen fibril assembly. For example, decorin has two separate binding domains for collagen type I and can also interact with collagen type VI (Wiberg et al., 2001). We and others have demonstrated that decorin may regulate corneal angiogenesis. Schönherr et al. (2004) have extensively studied the effects of decorin, biglycan, and fibromodulin on corneal angiogenesis in mice. In their studies, they used chemical cauterization to demonstrate that in decorin-deficient mice, the growth of corneal vessels is significantly diminished compared to wild type. Corneal NV does not significantly change in biglycan-and fibromodulin-deficient corneas.
In a recent study, we observed bFGF-induced MT1-MMP expression and diminished decorin expression around bFGF-pellet implanted areas in vascularized mouse corneas (Mimura et al., 2009). We showed that MT1-MMP cleaves decorin in vitro and that cell lysates from MT1-MMP-deficient keratocytes lose their decorin-processing activity. In addition, purified decorin has been shown to inhibit vascular tube formation in an aortic ring assay and the addition of recombinant active MT1-MMP reverses the decorin-mediated inhibition (Mimura et al., 2009). Although our data suggest a role for decorin in the MT1-MMP-mediated angiogenesis pathways, additional anti-angiogenic factors may also be involved in MT1-MMP-mediated corneal NV.
6.2. Ephrins and Eph receptors
Several cytokine–receptor complexes, including the VEGF/VEGF receptor, angiopoietin/Tie2, and ephrin/Eph have been shown to play a role in angiogenesis (Gale and Yancopoulos, 1999; Tallquist et al., 1999; Yancopoulos et al., 2000). The Eph/ephrin complex is the largest known family of receptor tyrosine kinases (RTK) so far identified. The family is made up of at least 14 receptors and 8 ligands, and the members are subdivided into class A and class B, according to the structure and ligand-binding characteristics of the receptor (Klein, 2004). EphA receptor kinases are made up of EphA1–8 and EphA10, and ephrinA ligands are made up of ephrinA1-A5. EphB receptors include EphB1–4 and B6, and ephrinB ligands include ephrinB1–B3. In general, EphA receptors bind to glycosyl phosphatidylinositol (GPI)-anchored ephrinA ligands, and EphB receptors bind to ephrinB ligands, which have a transmembrane domain (Cheng et al., 1999). Unlike other families of RTKs, which bind to soluble ligands, Eph receptors interact with cell membrane-bound ephrin ligands (Davis et al., 1994). Moreover, these receptor–ligand interactions activate bidirectional signaling pathways through both Eph receptors and ephrinB ligands (Poliakov et al., 2004). The Eph/ephrinA family was first studied for its role in axonal guidance. Guidance in the visual system is believed to depend heavily on the EphA receptors that are expressed along a gradient in the retina. The EphA receptor expression gradient in the retina projects to its target in the brain, the superior colliculus, which expresses ephrinA ligands in a gradient. The progressively higher concentration of ephrinA ligand eventually induces the repulsion of EphA-bearing axons, resulting in a stop-grow signal that matches the concentration of the Eph receptor on the axon (Kullander and Klein, 2002; O'Leary and Wilkinson, 1999). The Eph/ephrinA family also plays a role in the regulation of tumor angiogenesis. EphrinA1-EphA2 signaling is closely related to postnatal angiogenesis, particularly during tumorigenesis.
The Eph/ephrinB family plays a role in the development of the embryonic vascular system. EphrinB2 is an early marker of the formation of arterial endothelial cells, where one of its receptors, EphB4, is expressed reciprocally in venous endothelial cells. EphrinB2 and EphB4 knockout mice are lethal and defective in vessel remodeling and sprouting (Gerety et al., 1999; Wang et al., 1998). EphrinB families are also highly involved in postnatal angiogenesis. EphB1–B4 and ephrinB1 and B2 are expressed in several vascular endothelial cells (Adams et al., 1999; Daniel et al., 1996; Wang et al., 1998). EphB1 and ephrinB2 induce corneal angiogenesis in adult mice, (Huynh-Do et al., 2002; Maekawa et al., 2003) and ephrinB1 induces vascular endothelial cell (VEC) migration, assembly, and adhesion (Nagashima et al., 2002; Stein et al., 1998).
Recently, our laboratory characterized a role for ephrins and Eph receptors in corneal angiogenesis (Kojima et al., 2007a,b). We were able to demonstrate a pro-angiogenic role for ephrinB1/EphB1 in bFGF-induced corneal angiogenesis. Immunohistochemical studies were used to demonstrate that ephrinB1 and EphB1 are expressed in basic fibroblast growth factor (bFGF)-induced vascularized corneas (Fig. 15). EphB1 has also been shown to specifically co-localize with vascular endothelial marker CD31 surrounded by type IV collagen (Fig. 16, Kojima et al., 2007a). EphrinB1 is expressed in corneal-resident keratocytes and neutrophils. Recombinant ephrinB1-Fc, which induces EphB receptor activation, was found to enhance bFGF-induced tube formation in an in vitro aortic ring assay and to promote bFGF-induced corneal angiogenesis in vivo in a corneal pocket assay. Synergistically enhanced and sustained activation of extracellular signal-regulated kinase was noted in vascular endothelial cell lines after stimulation with ephrinB1 and bFGF combinations. These results suggest that ephrinB1 plays a synergistic role in corneal NV.
Fig. 15.
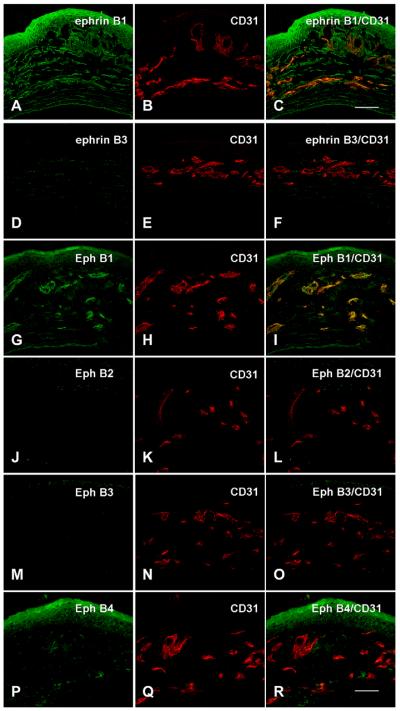
Expression of Eph and ephrin in bFGF-induced corneas. Corneas were harvested at day 7 after bFGF-pellet implantation (n = 3). Corneal sections were double immunostained with anti-ephrinB1, B3, EphB1, B2, B3, B4, and with CD31 antibodies. Notably, EphB1 is co-localized with CD31 in the corneal vessels after bFGF implantation (bar = 50 μm).
Fig. 16.
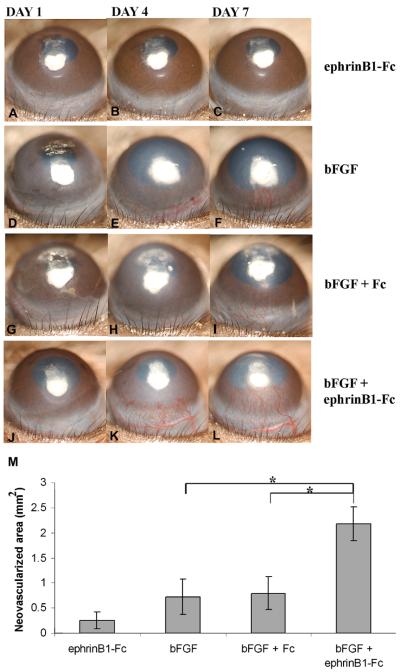
Effect of ephrinB1-Fc on corneal pocket assay. The pellet containing ephrinB1-Fc (A–C), bFGF (50 ng/pellet) (D–), bFGF + Fc (G–I), and ephrinB1-Fc + bFGF (J–L) was inserted into corneal stromal pocket. Photographs were taken on days 1, 4, and 7 after implantation. bFGF-induced corneal NV was significantly enhanced by ephrinB1-Fc, in vivo. M: Seven days after pellet insertion, the area of corneal NV was calculated using NIH ImageJ software. Results are representative of at least three independent experiments and represent the mean ± SEM. *P < 0.05 (reprinted with permission from Kojima et al., 2007a).
We also compared ephrinA/EphA expression to ephrinB/EphB expression in vascularized corneas. bFGF pellets were implanted to induce corneal NV. The eyes of WT, ephrinB2tlacZ/+, and EphB4tlacZ/+ heterozygous mice were harvested and sectioned 7 days after pellet implantation. Confocal immunohistochemistry was performed to compare the expression of the Eph/ephrinA family and Eph/ephrinB family. EphA1, EphA3, ephrinA1, ephrinA2, EphB1, EphB4, ephrinB1, and ephrinB2 were detected in WT mouse corneal epithelial cells and keratocytes. EphA2 was immunolocalized only in epithelial cells. In addition, EphA3, ephrinA1, EphB1, EphB4, and ephrinB1 were immunolocalized to the corneal epithelium and stroma. In the vascularized corneas, ephrinB1 was mainly immunolocalized to the keratocytes around the vessels, and ephrinB2, EphB1, and EphB4 were mainly co-localized with CD31 in the vascular endothelial cells. These studies further demonstrate that the Eph/ephrin family of receptor tyrosine kinases and their ligands may play a role in the regulation of corneal angiogenesis.
6.3. Activin receptor-like kinase
Activin Receptor-Like Kinase-1 (ALK-1) is one of the seven type I receptors recognizing transforming growth factor beta (TGF-β) family proteins (Massague, 1998). Its expression has been detected in endothelial cells of highly vascularized tissues (lungs and placenta), normal and neoplastic pituitary cells, anaplastic large cell lymphoma, inflammatory myofibroblastic tumors, and central nervous system cells. ALK-1 transduces the TGF-β1 signal by phosphorylating Smad1, Smad5, or Smad8 (ten Dijke and Hill, 2004). Upon phosphorylation by the receptors, Smad complexes translocate into the nucleus where they cooperate with sequence-specific transcription factors to regulate gene expression. This functional and physical interaction confers both specificity and complexity to transcriptional responses to TGF-β family ligands.
Lamouille et al. (2002) have implicated ALK-1 in the maturation phase of angiogenesis. They have shown that the transfection of a constitutively active form of ALK-1 (which results in constitutive ligand-independent receptor activation) inhibits both endothelial cell proliferation at the G1 phase of the cell cycle and endothelial cell migration through a modification of the dynamics of the endothelial cell cytoskeleton (Nespoli et al., 2006). Consistent with these results, a zebrafish ALK-1 mutant, vgb, whose vessel dilation phenotype is reminiscent of ALK-1−/− mice, shows an increased number of endothelial cells within the affected vessels, suggesting a role for ALK-1 in the inhibition of endothelial cell proliferation. Seki et al. (2004, 2003) have shown that ALK-1 is almost undetectable in the numerous capillaries that form in the area surrounding a wound.
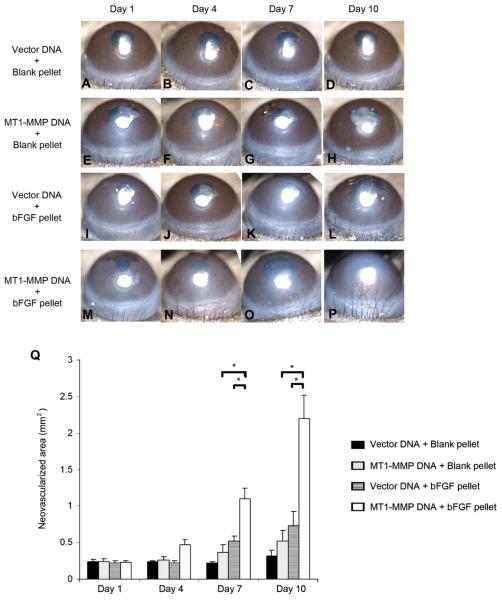
Using the pellet-induced corneal NV model, we have demonstrated that over expression of ALK-1 in the mouse cornea (through naked DNA injection) does not induce corneal NV and can prevent the growth of new bFGF-induced stromal vessels (Fig. 17). Our data and those of Seki and colleagues suggest that the expression of ALK-1 plays an important role in angiogenesis.
Fig. 17.
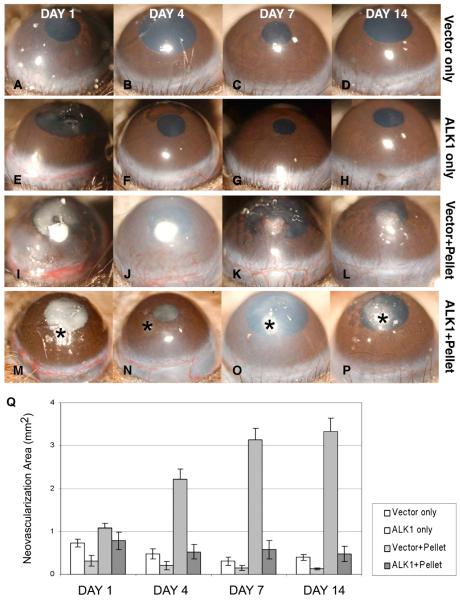
bFGF-induced corneal NV is inhibited by naked ALK-1 DNA injection, in vivo. No-pellet controls are shown in A–H: Injection of naked DNA [ALK-1 (E–H) and vector only (A–D)] did not induce corneal NV. The vector plus pellet positive controls are shown in I–L: Development of NV in the corneal stroma was evident by day 4 (J); new vessels continued to grow in the direction of the pellet on days 7 and 14. None of the mice in the ALK-1 and pellet groups (M–P) showed development of corneal NV on days 1, 4, 7, and 14 after pellet implantation. Asterisk (*) indicates pellet implantation. The area of corneal NV of the four groups at days 1–14 is shown (Q) (reprinted with permission from Albe et al., 2005).
Recently, we have described a proteomic approach to investigate the differential protein expression patterns and identify the physiologically relevant angiogenic and anti-angiogenic factors involved in the hyaloid vascular system regression. Differentially expressed proteins were identified using two-dimensional gel electrophoresis from the lens and vitreous of P1 and P16 mice (Fig. 18) followed by nanoflow chromatography coupled with tandem mass spectrometry (Albe et al., 2008). Using this approach, the following factors expressed at P16 may be involved in angiogenesis: Tumor necrosis factor-α (TNF-α), hepatoma-derived growth factor (HDGF), fibroblast growth factor-22, and kininogen. TNF-α is mainly secreted by macrophages and can induce the cell death of certain tumor cell lines. Under certain conditions, it can stimulate cell proliferation and induce cell differentiation. HDGF is involved in proliferative, angiogenic, and neurotrophic activity. FGF-22 is the mouse homologue of human FGF-10, a factor required for embryonic epidermal morphogenesis and also implicated as a primary factor in the wound-healing process (Beer et al., 2005). FGF-22 also induces angiogenesis and stabilizes the endothelial barriers protecting the microvascular and epithelial tissues against mild injuries, and it speeds their repair after major damage (Beyer et al., 2003). Kininogen is a plasma protein that plays important roles in fibrinolysis, thrombosis, and inflammation (Albe et al., 2008).
Fig. 18.
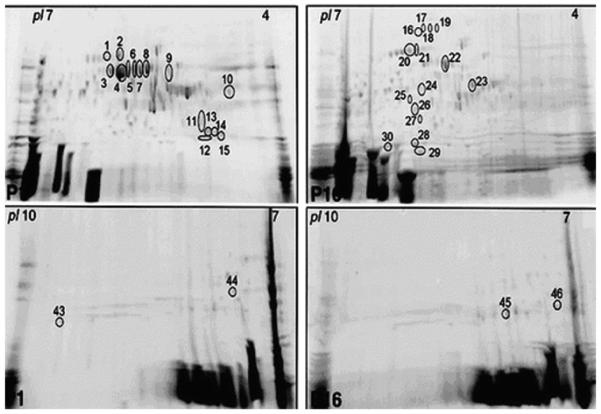
Differential HVS protein expression in postnatal days 1 and 16. Representative 2-DE gels of proteins obtained from the lens and vitreous of P1 mouse and P16 mouse using IPG strips with pH range 4–7 and 7–10. The proteins excised for analysis and identification by MS are marked with numbers from 1 to 46 (reprinted with permission from Albe et al., 2008).
6.4. Integrins
Integrins are a major family of type I transmembrane cell surface receptors. A total of 18 individual α subunits and 8 β subunits have been identified (Takada et al., 2007). Integrins are heterodimers that are composed of one α and one β subunit. The heterodimerization of different αβ subunits into 24 different integrins has been observed in humans; monomeric integrins are not processed or presented on the cell surface. The binding of integrins to the ECM serves as a transmembrane linker between extracellular ligands and the cytoskeleton (Han et al., 2006). In conjunction with growth factor receptors, integrins transmit cellular responses such as migration, survival, differentiation, and motility. The signal processes are dependent on the cytoplasmic tails of the integrins. The binding of integrins to the ECM induces conformational changes in their extracellular domains, modulating their cytoplasmic tails and causing cell signaling and activation. Both integrin α5 and αv are expressed on resting and activated lymphatic vessels, in vivo, and block the outgrowth of new lymphatic vessels after wounding. During angiogenesis, a significant upregulation of αvβ3 and α5β1 has been observed on activated vascular endothelium. α5 integrins play a key role during the development of the vascular system (Ruegg and Mariotti, 2003) (Okazaki et al., 2009). Genetic ablation of integrin α5 leads to severe vascular abnormalities. Like its extracellular ligand fibronectin, which is able to provide proliferative signals to vascular cells, α5β1 integrin is also upregulated in tumor blood vessels and plays a role in tumor angiogenesis and growth. In addition, integrin αvβ3 and αvβ5 antagonists have been shown to inhibit angiogenesis, in vitro and in vivo. Animals treated systemically with an α5β1-inhibiting small molecule showed a significant inhibition and regression of corneal NV. Combining small molecule inhibitors to integrin αv and α5 does not significantly increase the anti-lymphangiogenic effect, in vivo (Lohela et al., 2003; Vlahakis et al., 2007).
7. Matrix metalloproteinases in the cornea
Corneal extracellular matrix (ECM) remodeling by MMPs has also been implicated in corneal angiogenesis and in the maintenance of corneal avascularity. MMPs are a group of zinc-binding proteolytic enzymes that participate in ECM remodeling, NV, and lymphangiogenesis. They are produced as proenzymes and are activated by a variety of proteinases including MMPs and serine proteases. Among 25 MMPs already described, at least 15 have been identified in the cornea, including collagenases (MMP-1, -8 and -13), gelatinases A and B (MMP-2 and -9), stromelysins (MMP-3, -10, -11), matrilysin (MMP-7), macrophage metalloelastase (MMP-12), and membrane type (MT)-MMPs (MMP-14, -15, -17, -24, -25) (Berman, 1994; Dong et al., 2000, 2001; Dushku et al., 2001; Itoh et al., 1998; Kato et al., 2001; Kure et al., 2003; Li et al., 2003; Lu et al., 1999; Maguen et al., 2002; Mahajan et al., 2002; O'Brien et al., 2001; Reed et al., 2000; Saghizadeh et al., 2001; Sternlicht et al., 2000; Tao et al., 1995; Ye and Azar, 1998; Zhou et al., 2000). Table 5 lists the localization and properties of the MMPs of the cornea.
Table 5.
Localization and properties of matrix metalloproteinases in the cornea.
| MMP No. | Enzyme name | Corneal locations | Angiogenic properties | References |
|---|---|---|---|---|
| MMP-1 | Interstitial collagenase-1 | Epithelium, ant stroma, fibroblasts | (Berman, 1994; Maguen et al., 2002; Reed et al., 2000; Tao et al., 1995) | |
| MMP-2 | Gelatinase A | Epithelium and stroma (normal), basal epithelium superficial stroma (wounded) | Pro-angiogenic | (Itoh et al., 1998; Kato et al., 2001; Maguen et al., 2002; Ye and Azar, 1998) |
| MMP-3 | Stromelysin-1 | Epithelium, basement membrane, stroma (diabetics) | (Saghizadeh et al., 2001; Sternlicht et al., 2000) | |
| MMP-7 | Matrilysin | Epithelium | Anti-angiogenic | (Kure et al., 2003; Lu et al., 1999) |
| MMP-8 | Neutrophil Collagenase-2 | Epithelium | (O'Brien et al., 2001) | |
| MMP-9 | Gelatinase B | Basement membrane and superficial stroma | Pro-angiogenic | (Ye and Azar, 1998) |
| MMP-10 | Stromelysin-2 | Epithelium | (Li et al., 2003) | |
| MMP-11 | Stromelysin-3 | Epithelium | (Li et al., 2003) | |
| MMP-12 | Macrophage metalloelastase | Corneal fibroblasts (in vitro) | Pro-angiogenic | (Mahajan et al., 2002) |
| MMP-13 | Collagenase-3 | Deep stroma | (Lu et al., 1999) | |
| MMP-14 | MT1-MMP | Basal epithelial cells and stromal keratocytes | Pro-angiogenic | (Dong et al., 2000; Ye and Azar, 1998; Zhou et al., 2000) |
| MMP-15 | MT2-MMP | Epithelium | (Dushku et al., 2001) | |
| MMP-17 | MT4-MMP | Not present in normal, Substantia propia (infected cornea) | (Dong et al., 2001) | |
| MMP-24 | MT5-MMP | Epithelium (normal), substantia propia (infected cornea) | (Dong et al., 2001) | |
| MMP-25 | MT6-MMP Leukolysin | Infiltrating leukocytes | (Dong et al., 2001) |
MMPs were originally thought to function exclusively as enzymes that degrade structural components of the ECM. Additionally, MMP-mediated proteolysis is now known to induce several distinct biological functions (Page-McCaw, 2008; Page-McCaw et al., 2007). These include: (i) converting structural matrix proteins to signaling molecules (e.g., Collagen XVIII has an NC1 domain (endostatin), which is anti-angiogenic and present in the cornea); (ii) structural changes to the matrix proteins (e.g., cleavage of perlecan and decorin-corneal ECM proteoglycans); (iii) changes in tissue architecture (e.g., cleavage of E-cadherin); (iv) chemo-attraction (e.g., changes in chemotaxis: gradients form by shedding of syndecan); (v) proliferation (e.g., epidermal-growth-factor receptor (EGFR) ligand processing); (vi) cell survival (e.g., neuronal survival factor: stromal-cell derived factor-1); (vii) activation of latent signaling molecules (e.g., tumor necrosis factor-α (TNF-α) shedding and cleavage of insulin-growth-factor (IGF)-binding protein); (viii) changes in the range of action of a signaling molecule (e.g., VEGF: change in range of diffusion); and (ix) differentiation (e.g., adipocyte maturation).
The upregulation of MMPs has been clearly demonstrated to occur during corneal angiogenesis (Ma et al., 2006; Saika et al., 2007). However, their definitive roles in the regulation of angiogenesis are ambiguous because the same molecule can simultaneously act as a pro-angiogenic and an anti-angiogenic factor. The dual function of MMPs during angiogenesis may be explained by their ability to degrade the ECM, allowing tissue invasion by MMP-bearing endothelial cells, and to generate or release anti-angiogenic fragments from their precursors (Lin et al., 2001; Maeshima et al., 2000, 2001a,b; Pepper, 2001). In the following sections, we discuss and provide additional information on the roles of MMP-2, MMP-7, and MT1-MMP in corneal angiogenesis.
7.1. Matrix metalloproteinase-2 (MMP-2)
MMP-2 (gelatinase A) has long been associated with angiogenesis. It has been shown to be involved in vascular invasion by direct matrix degradation or through the release of matrix-bound cytokines or growth factors (Azar, 2006). We have previously shown that MMP-2 is immunolocalized to the epithelium and stroma of normal corneas and is predominant in the basal epithelium and superficial stroma at 3 and 7 days after wounding (Ye and Azar, 1998). In situ hybridization confirmed MMP-2 expression by epithelial cells and stromal keratocytes (Ye and Azar, 1998). We have also defined the physiological role of MMP-2 in the process of angiogenesis (Kato et al., 2001). This was performed by examining corneal angiogenesis induced by bFGF in mice deficient in MMP-2 in vivo, to determine whether a null mutation in MMP-2 gene leads to the suppression of angiogenetic response. Additionally, we prepared aortic rings from MMP-2-deficient mice to determine the role of MMP-2 in vascular endothelial cell migration and tube formation in vitro (Kato et al., 2001). Results demonstrated that the angiogenetic response induced by bFGF is markedly reduced in mice lacking a functional MMP-2 gene compared to wild type animals (Kato et al., 2001). The use of MMP-deficient mice is potentially more advantageous because the distinct activities of a specific MMP are eliminated. In addition, the non-specific inhibition of ECM components and of other MMPs is minimized in these mice. Our data using MMP-2-deficient mice provide more striking evidence for a critical role for this enzyme in angiogenesis.
We have also observed that endothelial cells from MMP-2-deficient mice fail to display normal outgrowth in the presence of 5 ng/ml bFGF suggesting that the differences in bFGF-induced angiogenesis between MMP-2-deficient mice and WT mice may be related to differences in the vascular endothelial cells. It may be difficult for the endothelial cells lacking a functional MMP-2 to traverse the basement membrane.
The MMP-2-null mice develop almost normally, and bFGF induces corneal angiogenesis even in the MMP-2-mutant mice, clearly indicating that the angiogenetic process is not totally dependent on MMP-2. Zhou et al. have performed bFGF micro-pocket assays and showed complete absence of corneal angiogenesis in MT1-MMP-deficient mice (Zhou et al., 2000). MT1-MMP contains a transmembrane domain which we hypothesize facilitates the cell-mediated activation of MMP-2. These data show that activation of MMP-2 by MT1-MMP is likely an important mechanism for the regulation of angiogenesis. Unlike MT1-MMP null mice, MMP-2-null mice still have the ability to show angiogenesis. This suggests the possibility that MT1-MMP enzymatic activity by itself may play a critical role in angiogenesis process. Further research is needed to explain the angiogenic discrepancy between MMP-2-null mice and MT1-MMP null mice.
MMPs have been shown to modulate VEGF bioavailability through intramolecular processing. Specifically, Lee et al. showed that a subset of MMPs can cleave matrix-bound isoforms of VEGF, releasing soluble fragments to promote capillary dilation of existent vessels (Dean et al., 2007; Lee et al., 2005). In a recent study, Dean et al. used isotope mass tagging quantitative proteomics to study the effects of proteolysis on the secretome of MMP-2 transfected cells. They discovered that heparin affin regulatory peptide (HARP) and connective tissue growth factor (CTGF) are novel MMP-2 substrates that are cleaved and inactivated upon proteolysis. By cleaving these angiogenic and mitogenic cytokine inhibitors in complex with VEGF, MMP-2 releases VEGF. As a result, MMP-2 possesses potential pro-angiogenic activity by mobilizing intact VEGF from HARP or CTGF cytokine-inhibitory complexes (Dean et al., 2007).
7.2. Matrix metalloproteinase-7 (MMP-7)
MMP-7 is the designated name of matrilysin. The zymogen of MMP-7 has a molecular mass of 28 kDa. When cleaved, the 19-kDa catalytic form is generated. MMP-7 is expressed in epithelial-derived dividing cells such as menstrual endometrial epithelial cells and adenocarcinomas of stroma and liver. MMP-7 is also expressed in basal epithelial cells during the migration and proliferation phases of corneal wound healing after excimer keratectomy (Lu et al., 1999; Ye and Azar, 1998). MMP-7 possesses catalytic activities against a broad range of extracellular matrix substrates such as fibronectin, gelatins (types I, III, IV, and V), collagen type IV, laminin, entactin-nidogen, and elastin. MMP-7 also cleaves factors that modulate angiogenesis including CTGF, sVEGFR-1, plasminogen and collagen XVIII.
Di Girolamo et al. (2001) have demonstrated positive MMP-7 staining in the basal epithelial cells of pterygium specimens (Di Girolamo et al., 2001) implicating its potential function in the pathogenesis of the disease and angiogenesis in pterygium. MMP-7 also plays a role in maintaining corneal avascularity. It has been shown that MMP-7 cleaves corneal collagen XVIII to generate a 28-kDa fragment (Lin et al., 2001). This MMP-7-generated fragment contains the endostatin domain of collagen XVIII which has a potent anti-angiogenic function.
Our previously published data demonstrated the upregulation of MMP-7 in WT animal wounding models and an increased vascular response in MMP-7-deficient littermates (Kure et al., 2003). A substantially higher level of corneal NV develops in MMP-7 KO mice after excimer keratectomy wounding than in age-matched WT mice littermates. The presence of vessels was confirmed by India ink perfusion, electron microscopy, and immunohistochemical localization of type IV collagen and CD31. In MMP-7 KO mice, a decrease in the levels of antiangiogenic factors in the keratectomy wounding model tilts the balance towards corneal angiogenesis.
Most research on corneal vascularization has focused on the upregulation of angiogenic factors in diseased corneas, and data are consistent with current views of tumor-related angiogenesis. It has been suggested that the induction of new vessels involves not only the activation of angiogenic factors such as VEGF and bFGF but also the suppression of anti-angiogenic factors. In MMP-7 KO mice, we did not find elevated levels of bFGF and VEGF in the corneal epithelial cells.
Since the involvement of MMP-2 and MMP-7 in corneal NV has been demonstrated after wounding, limbal deficiency models were further being used to assay the vessel growth. In Fig. 19, we showed that the area containing extended corneal vessels was significantly larger in the undebrided temporal side of the cornea when compared to the debrided nasal side at post-operative day 7 (p < 0.01). Mean surface areas of vascularization in the temporal undebrided and nasal debrided sides at post-operative day 7 were 3.59 mm2 and 1.75 mm2 for MMP-2−/− mice, 4.01 mm2 and 0.81 mm2 for MMP-7−/− mice, 2.73 mm2 and 1.72 mm2 for col18a1−/− mice, and 2.75 mm2 and 1.81 mm2 for wild type mice, respectively (Fig. 20).
Fig. 19.
Enhanced bFGF-induced corneal angiogenesis and lymphangiogenesis in MMP-7−/− mice. A bFGF pellet was implanted into WT and MMP-7−/− mice for 7 days. Three mouse corneas were whole-mounted and double immunostained with anti-LYVE (A, D) and CD31 (B, E) antibodies. The areas of corneal angiogenic and lymphangiogenic vessels were determined using ImageJ. The levels of corneal lymphangiogenesis in MMP-7−/− are significantly higher than WT mice.
Fig. 20.
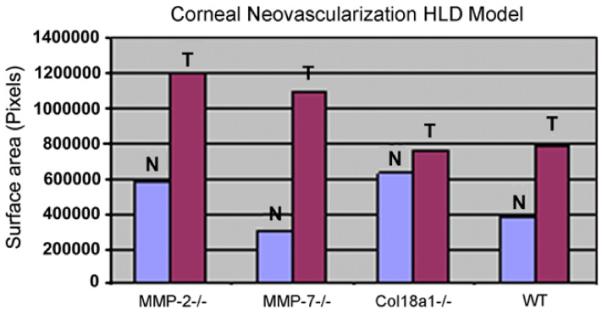
The total surface area of corneal NV in MMP-2−/− , MMP-7−/−, Col18a1−/− (collagen XVIII), or WT (wild type) mice in the hemilimbal deficiency model. At day 7 after injury, total areas of corneal NV in temporal side (T) and nasal side (N) of the wounded corneas. (HLD: Hemilimbal Deficiency).
Vascular vessel formation is regulated by the balance of anti-angiogenic and angiogenic factors. Anti-angiogenic factors, such as angiostatin and endostatin, are derived from proteolytic cleavage of precursor molecules, which generates functional fragments. Angiostatin and endostatin are effective in blocking vascular endothelial cell proliferation and may contribute to regression in the cornea. The fact that MMP-7 has been shown to cleave plasminogen and collagen XVIII in vitro to generate angiostatin and endostatin, respectively, leads us to hypothesize that the reduction of MMP-7-derived angiostatin and/or endostatin in the cornea may contribute to corneal NV after excimer laser keratectomy in MMP-7 KO animals.
Our hypothesis is that MMP-7-derived, endostatin-containing fragments (including neostatin-7) may be among the factors that prevent new vascular and lymphatic vessel formation after wounding. Use of the cornea as a model has allowed the study of lymphangiogenic and anti-lymphangiogenic molecules in vivo. Although the involvement of neostatin-7 has not been proven in the corneal model of the enhanced effect of injury-induced corneal lymphangiogenesis in collagen XVIII KO mice, our injury-induced corneal lymphangiogenesis model in collagen XVIII KO mice has shown that collagen XVIII or one of its degradation products is involved in the regulation of corneal lymphangiogenesis during wound healing. Our data also show that enhanced corneal lymphangiogenesis and VEGF-C expression were present in keratectomy-treated col18a1−/− mice. In addition, reduced bFGF-induced corneal lymphangiogenesis by neostatin-7 was demonstrated by administration of recombinant GST-neostatin-7 and bFGF in corneal pellet implantation. The novel observation of neostatin-7 binding to VEGFR-3 in vitro suggests a role for neostatin-7 in the regulation of corneal lymphangiogenesis (Kojima et al., 2008). Endostatin has already been approved by the FDA for cancer-related NV and can perhaps be used as an additional treatment for corneal NV and lymphangiogenesis.
7.3. Membrane-type 1 metalloproteinase (MT1-MMP)
Perhaps the most strongly implicated (and most important) MMP in angiogenesis is the membrane-type MMP, MT1-MMP. In the cornea, expression of MT1-MMP has been detected in the epithelium and stromal keratocytes during wound healing (Ye and Azar, 1998). The upregulation of MT1-MMP in fibroblasts grown in relaxed collagen lattices suggests that MT1-MMP synthesis may also be regulated by the cytoskeleton and mediated by the lack of stress fibers in those cells (Tomasek et al., 1997). MT1-MMP also plays a major role in the activation of proMMP-2. For example, MT1-MMP is important in ECM remodeling through activation of proMMP-2 and direct cleavage of some ECM macromolecules such as gelatin, type I collagen, and fibronectin. The importance of MT1-MMP is further demonstrated by the fact that to date, genetic KO of MT1-MMP is the only lethal knockout of all tested MMPs. MT1-MMP knockout mice die at three weeks to one month of age. In addition, MT1-MMP knockout mice show delayed vascular development and impaired corneal NV by bFGF (Zhou et al., 2000). To further study MT1-MMP's role in corneal NV, we and others have generated antibodies against mouse MT1-MMP, cultured MT1-MMP KO keratocytes and epithelial cells and animal models to better understand the mechanism and function by which MT1-MMP induces corneal NV. Using corneal wounding models, we have detected enhanced MT1-MMP expression in alkali wounded corneal NV (Fig. 21). We also used Real-time PCR to evaluate the expression of growth factor receptors including platelet-derived growth factor alpha (PDGFα), PDGFβ, VEGFR-1, and epidermal-growth-factor receptor EGFR, in WT, MT1-MMP KO, and MT1- MMP KI mouse cornea stromal fibroblasts. Our results demonstrated that there is no significant difference in the expression patterns of PDGFα, PDGFβ, and VEGFR-1 between the WT, MT1-MMP KO, and MT1-MMP KI cells (Fig. 22). However, the expression of EGFR was decreased in the MT1-MMP KO cells when compared to the WT and MT1-MMP KI cells. These data suggest that MT1-MMP may play a role in the regulation of EGFR expression. This may be an additional mechanism by which MT1-MMP is pro-angiogenic, as EGFR is a regulator of fibroblast cell proliferation and migration (Kajanne et al., 2007). The primers utilized for amplification of genes are listed in Table 6. Although some of our preliminary data implicate the expression of EGFR as a potential mechanism by which MT1-MMP regulates corneal angiogenesis, much of the critical role that MT1-MMP contributes to regulating corneal angiogenesis may be due to its interactions with major angiogenic proteins, bFGF and VEGF. Fig. 23 dissects many aspects of the role of MT1-MMP in corneal NV.
Fig. 21.
Enhanced corneal MT1-MMP and CD31 expression after alkali wounding. A mouse was anesthetized by intraperitoneal injection of ketamine HC1 (50 mg/kg) and xylazine (10 mg/kg). Topical tetracaine was applied to the cornea for 1 min before alkali wounding. A Whatman #3 filter cut by a trephine of 2-mm diameter was presoaked with 2 μL of 1 N sodium hydroxide solution, and placed on the central cornea of left eye for 1 min. Wounded surfaces were immediately washed by physiologic saline. Day 7 alkali wounded corneas were immunostained with four different anti-MT1-MMP (N1, N2, La, Cb), CD31, and PI. Enhanced MT1-MMP (E, I, M and Q) and CD31 (F, J, N and R) immunostaining were detected in alkali wounded corneas when compared to unwounded control (A and B) (bar = 50 μm).
Fig. 22.
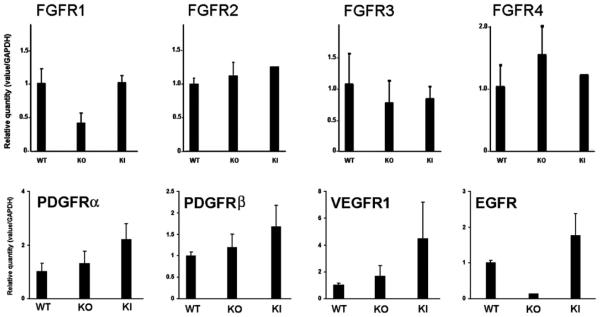
Diminished FGFR-1 and EGFR in MT1-MMP knockout keratocyte cell lines. Total RNA was extracted from WT, KO, and KI corneal keratocyte cells and quantitative real-time PCR was performed. Diminished FGFR-1 and EGFR expression were detected in MT1-MMP knockout keratocytes and recovered in MT1-MMP knockin keratocytes (Onguchi et al., 2009).
Table 6.
Primers used for quantitative RT-PCR.
| mRNA | Forward primer (5′–3′) | Reverse primer (5′–3′) | Amplification |
|---|---|---|---|
| PD GF a | CAAACCCTGAGACCACAATG | TCCCCCAACAGTAATCCAAG | 235 bp |
| PDGF-b | TGCCTCAGCCAAATGTCACC | TGCTCACCACCTCGTATTCC | 159 bp |
| VEGFR-1 | AGTGTGAACGGCTGCCCTAT | GCCAAATGCAGAGGCTTGAA | 118 bp |
| EGFR | CAAGTAACAGGCTCACCCAACTG | CAAGTTCCCAAGGACCACTTCA | 94 bp |
Fig. 23.
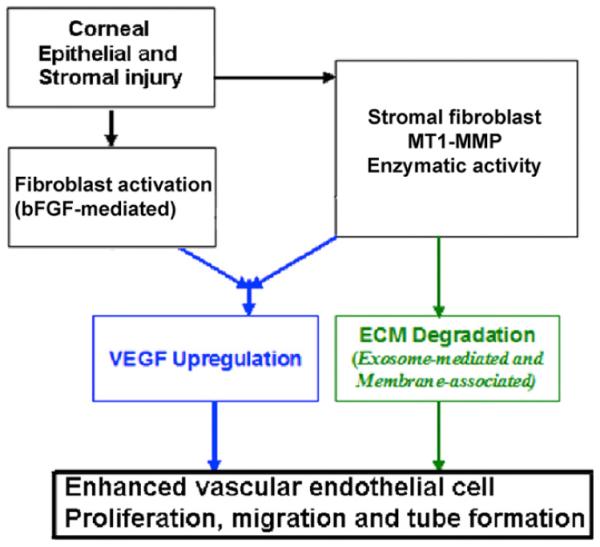
The diagram depicts MT1-MMP functions during corneal wounding. Corneal kertocyte MT1-MMP upregulates VEGF production, MMP-2 activation, and cleavage of ECM proteins after bFGF-pellet implantation and alkali wounding.
7.4. MT1-MMP interactions with VEGF and FGF
We have shown that MT1-MMP may link the VEGF and FGF signaling pathways (Onguchi et al., 2009). However, the role of MT1-MMP in linking these two pathways remains unclear. The MT1-MMP pro-angiogenic effect has been reported to be mediated at least in part by the upregulation of VEGF at both mRNA and protein levels (Sounni et al., 2002). There is also evidence that MT1-MMP and VEGF are functionally linked in tumoral angiogenesis (Sounni et al., 2003). Such a link between the expression of VEGF and MT1-MMP has been confirmed by immunohistochemical and RT-PCR analysis of human glioma tissue samples (Munaut et al., 2003). In further support of a functional link, hypoxia-induced upregulation of MT1-MMP in murine bone marrow-derived stromal cells correlates with the stimulation of VEGF (Annabi et al., 2003). Although the activation of the MAPK/ERK kinase, p38 MAPK, or phosphatidylinositol 3-kinase pathways are not required for VEGF upregulation (Sounni et al., 2004), protein kinase D1 (PKD1)-dependent histone deacetylase (HDAC7) phosphorylation stimulated by VEGF is involved in MT1-MMP expression, endothelial cell migration, tube formation, and microvessel sprouting (Ha et al., 2008). Additionally, the pathway through which MT1-MMP regulates VEGF is distinct from that implicated in the induction of cell migration which involves extracellular signal-regulated protein kinase (ERK) (Gingras et al., 2001). This evidence suggests that interactions between the signaling pathways of MT1-MMP and VEGF may play a role in regulating corneal angiogenesis.
The interaction between MT1-MMP and FGF has also been well documented. It has been reported that FGF-1 induces MT1-MMP transcription in LNCaP prostate carcinoma cells and that FGFR-1 and STAT3 are involved in FGF-1 mediated MT1-MMP expression (Udayakumar et al., 2004). Additionally, FGF-10 induces the upregulation of MT1-MMP expression in pancreatic cancer cells (Nomura et al., 2008). In our laboratory, we have demonstrated that bFGF-induced corneal NV is enhanced when the bFGF pellet is used in combination with naked MT1-MMP DNA plasmid injection (Fig. 24). We have demonstrated the interplay between MT1-MMP, VEGF, and bFGF by experiments in which VEGF and MT1-MMP expression was enhanced following bFGF-pellet implantation in murine cornea (Fig. 25).
Fig. 24.
Enhanced bFGF-induced corneal NV after a combination of bFGF-pellet implantation and naked MT1-MMP DNA plasmid injection. The blank pellets were implanted immediately after MT1-MMP DNA (E–H) or vector control DNA (A–D) was injected into corneal stroma. Likewise, the bFGF pellets were implanted immediately after MT1-MMP DNA (M–P) or vector control DNA (I–L) was injected into corneal stroma. Photographs were taken on days 1, 4, 7, and 10 after surgery. Q: Graphic representation of at least five independent experiments (mean ± SEM, *P < 0.05) (reprinted with permission from Onguchi et al., 2009).
Fig. 25.
Enhanced MT1-MMP expression in central and peripheral corneas at day 4 after bFGF implantation. bFGF-induced vessels were detected at peripheral cornea, but not in the central cornea at day 4 and 14. Enhanced VEGF expression was detected at day 4 and 14 after bFGF-pellet implantation (reprinted with modification from Azar, 2006).
8. Inhibitors of angiogenesis in the cornea
The production and functions of several potent anti-angiogenic factors are involved in the maintenance of corneal angiogenic privilege. Several anti-angiogenic molecules have either been detected or tested in the cornea. They are either derived from larger precursors by proteolytic cleavage or directly produced in their active forms (Narasaki et al., 2005). Angiostatin, a 38 kDa proteolytic fragment of plasminogen, is a potent anti-angiogenic factor (O'Reilly et al., 1994). Implantation of angiostatin and angiostatin-like fragments in the eye has been shown to inhibit corneal NV (Cao et al., 1999; Shin et al., 2000). Endostatin, another anti-angiogenic factor, is a 20 kDa proteolytic fragment of collagen XVIII (O'Reilly et al., 1997). Endostatin has been isolated from the conditioned medium of a murine hemangioendothelioma cell line; it inhibits bFGF and VEGF-induced vascular endothelial cell migration and proliferation in vitro and reduces tumor progression in mice (O'Reilly et al., 1997). Addition of endostatin or other anti-angiogenic molecules into the pellets significantly suppresses bFGF-induced angiogenesis in corneal pocket assays (Chang et al., 2005; Gabison et al., 2004; Vazquez et al., 1999). Other potent anti-angiogenic factors important for corneal angiogenic privilege include restin, arresten, canstatin, tumstatin, and pigment epithelial-derived factor (PEDF)(Azar, 2006).
8.1. Basement membrane-derived inhibitors of angiogenesis
8.1.1. Endostatin, neostatin, restin
8.1.1.1. Endostatin
Collagen XVIII has been identified as a heparin sulfate proteoglycan and belongs to a family of collagen-like proteins (multiplexins) that are mainly localized in perivascular positions. Collagen XVIII is expressed in the basement membrane (BM) in developing and postnatal eyes. Mice lacking collagen XVIII develop normally and without evidence of abnormal vascular morphogenesis. However, these collagen XVIII-deficient mice do develop some ocular abnormalities, similar to Knobloch syndrome (Morimoto et al., 2002; Tomono et al., 2002).
Endostatin, a 20 kDa cleavage fragment of carboxyl-terminal 183 amino acids in the NC1 domain of collagen XVIII, was first identified in the conditioned medium of hemangioendothelioma cells and has since then been isolated from circulating serum. This fragment has been shown to possess anti-angiogenic properties, and effectively inhibits bFGF-induced corneal NV, in vivo, and VEGF-induced endothelial cell proliferation and migration, in vitro (Eriksson et al., 2003).
Endostatin and endostatin-containing peptides have been isolated from tissue extracts and circulating blood, suggesting that these fragments are physiological cleavage products. The hinge region of the NC1 domain of collagen XVIII contains more than one potential cleavage site for MMPs and cathepsin L. Several MMPs (MMP-3, -7, -9, -12, -13, and -20) have been shown to cleave within the hinge region of the NC1 domain. It has been proposed that the resulting endostatin-containing fragments are further processed by cathepsin L to generate mature endostatin (Chang et al., 2005; Felbor et al., 2000; Ferreras et al., 2000; Lin et al., 2001; Wen et al., 1999).
Endostatin associates with tropomyosins, integrins, VEGF receptors, MMPs, and glypicans to produce antimigratory and anti-proliferative effects on vascular endothelial cells. Endostatin causes endothelial cell cycle arrest in G1. The binding of endostatin to tropomyosins may be important for a variety of cellular functions, including contraction, cytokinesis, intracellular transport, secretion, motility, morphogenesis, and cell transformation. Endostatin blocks VEGF-induced tyrosine phosphorylation of KDR/Flk-1, resulting in a downstream signaling effect that inactivates ERK, p38 MAPK, and p125FAK, which are signaling molecules involved in the mitogenic responses induced by VEGF in vascular endothelial cells. Endostatin inhibits the binding of VEGF to vascular endothelial cells and to its cell surface receptor, KDR/Flk-1. The binding of endostatin to KDR/Flk-1, but not to VEGF, suggests that endostatin directly binds KDR/Flk-1, which blocks the VEGF binding site on vascular endothelial cells (Kim et al., 2002). In addition, endostatin treatment increases the activity of the intracellular protease, caspase 3, enhancing vascular endothelial cell apoptosis (Dhanabal et al., 1999).
Endostatin has been shown to affect lymphangiogenesis. Shao and Chi (2005) have demonstrated that recombinant endostatin inhibits the proliferation and migration of lymphatic endothelial cells, in vitro (Shao and Chi, 2005). Fukumoto et al. (2005) have shown that endostatin inhibits lymphangiogenesis and lymph expansion by down-regulating VEGF-C expression in cultured cells (Fukumoto et al., 2005). In an animal tumor model of lymphangiogenesis, endostatin overproduction significantly reduced the number of tumor lymphatic LYVE-1-positive vessels and also prevented tumor cell dissemination into the lymph nodes. This may be due to the ability of endostatin to inhibit the distribution of VEGF-C-producing tumor-associated inflammatory mast and to induce the apoptosis of VEGFR-3 expressing cells (Brideau et al., 2007).
8.1.1.2. Neostatin-7/-14
Another process by which corneal epithelium produces its anti-angiogenic effect is through MMP-7's cleavage of Collagen XVIII, to produce another anti-angiogenic molecule, neostatin-7. As mentioned above, endostatin is from the C-terminal domain of collagen XVIII and is a strong anti-angiogenic substance. In addition to neostatin-7, we have also demonstrated that neostatin-14, the product of MT1-MMP-mediated cleavage of collagen XVIII also has anti-angiogenic activity (Fig. 26) (Chang et al., 2005). In addition, collagen XVIII is actively secreted by corneal epithelial cells (Lin et al., 2001), and MMP-7 and MT1-MMP are both expressed by corneal epithelial cells (Lu et al., 1999). Furthermore, recombinant neostatin-7 blocks bFGF-induced corneal angiogenesis and lymphangiogenesis (Fig. 27) (Kojima et al., 2008). Taken together, these data suggest that one of the mechanisms by which corneal epithelium inhibits corneal NV may be through the production of neostatin-7, and -14 by MMP-7 and MT1-MMP-mediated cleavage of corneal epithelial collagen XVIII.
Fig. 26.
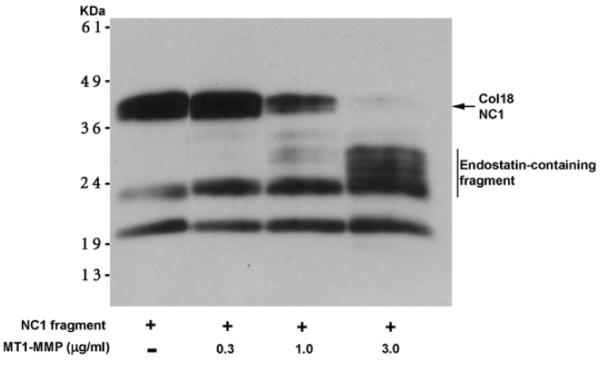
MT1-MMP cleaves a recombinant collagen NC1 fragment. MT1-MMP cleaves a recombinant NC1 (Chang et al., 2005) fragment of collagen XVIII to generate endostatin-containing fragments. In an in vitro assay, the addition of higher concentrations of MT1-MMP enhanced the production of endostatin-containing fragments detected by anti-endostatin antibody (Chang et al., 2005).
Fig. 27.
GST-neostatin-7 reduced the bFGF-induced corneal hem- and lymphangiogenesis. Mouse corneas were implanted with bFGF (80 ng/pellet) plus either GST (500 ng/pellet) (A) or GST-neostatin-7 protein (500 ng/pellet) (B). Corneal NV images were taken by slit-lamp microscope 7 days after pellet implantation. The pellet containing GST-neostatin-7 significantly reduced bFGF-induced NV (I). Corneal lymphangiogenesis was visualized by whole-mount immunohistochemical staining using anti-LYVE-1 antibody on day 7 after pellet implantation. Enhanced corneal lymphangiogenesis was visualized in GST plus bFGF implanted corneas (B); however, diminished corneal lymphatic vessels were observed in GST-neostatin-7 plus bFGF implanted corneas (F). Overlay images of corneal angiogenesis and lymphangiogenesis are shown in (D) and (H), respectively. Quantification of lymphatic vessels showed a reduction in corneal lymphatic vessels in GST-neostatin-7 implanted corneas (J). *Represents P < 0.05 (reprinted with permission from Kojima et al., 2008).
Recent evidence strongly suggests an important role for the corneal epithelium in maintaining corneal angiogenic privilege. In this review, we have extensively detailed the effects of corneal epithelial VEGFR-1, VEGFR-3, collagen XVIII (Neostatin-7), and MT1-MMP. It is quite possible that the corneal epithelium has several other anti-angiogenic/anti-lymphangiogenic properties that have not yet been characterized. Additional research is necessary to fully understand the role of the corneal epithelium in maintaining corneal angiogenic/lymphangiogenic privilege.
8.1.1.3. Restin
Like endostatin, restin is a collagen-derived (collagen XV), anti-angiogenic molecule. Collagen XV has been identified as a chondroitin sulfate proteoglycan and belongs to a family of collagen-like proteins (multiplexins) that are mainly localized in perivascular positions. Collagen XV is primarily expressed in the heart, skeletal muscle, placenta, and kidneys. Collagen XV-deficient mice tend to display a high propensity for exercise-induced muscle injury and progressive degeneration of skeletal muscle with collapsed capillaries. Restin is derived from the carboxyl terminus of collagen XV (Ramchandran et al., 1999; Tomono et al., 2002).
8.1.2. Arresten, canstatin, and tumstatin
Arresten, canstatin, and tumstatin are three type IV collagen-derived proteins that have also been shown to possess anti-angiogenic activity (Magnon et al., 2005; Pasco et al., 2005; Sudhakar et al., 2005; Sund et al., 2005). Type IV collagen is the major component of all basal lamina (BL). The BL is a thin, sheet-like, highly specialized structure of the ECM that separates epithelial cells from stroma. There are different isoforms of type IV collagen proteins expressed in BLs which display stage- and position-specific distribution during development. Type IV collagen is composed of six distinct polypeptide chains (α1–α6). These BL proteins act as regulators of specific biological functions, such as cellular growth, differentiation, repair, and migration, as well as modulators of pathological events, such as tumor cell differentiation, invasion, and metastasis (Maeshima et al., 2000, 2001a,b, 2002).
Type IV collagen promotes cell adhesion, migration, differentiation, and growth and, through these functions, may play a crucial role in angiogenesis. Molecular defects in type IV collagen have been linked to Goodpasture's syndrome, an autoimmune disease characterized by glomerulonephritis and pulmonary hemorrhages; and Alport's syndrome, a genetic disease with progressive glomerulonephritis, and diffuse esophageal leiomyomatosis, characterized by benign proliferation of smooth muscle (Turner and Rees, 1996).
Loss of BL components is a hallmark of invasive lesions, and disrupted collagen IV labeling of BL has been shown to precede tumor invasion in lung cancers. The disruption of BL components that occurs during tumor progression may be due to their degradation by proteolytic enzymes. Enzymatic degradation of type IV collagen α1 in BMs triggers cell motility and enhances local tumor progression. In lung cancers, stromal cells are the principal source of synthesis of α1 chains, and interaction between the tumor cells and the ECM could potentially modulate their invasive capacity (Colorado et al., 2000).
Type IV collagen-derived proteins have also been shown to possess considerable anti-angiogenic effects. Arresten is a 26-kDa protein derived from the non-collagenous (NC1) domain of the type IV collagen α1 chain. Arresten's functions include suppression of tumor growth, inhibition of endothelial cell proliferation and migration, and induction of endothelial cell apoptosis (Mundel and Kalluri, 2007). It has been shown that arresten can successfully inhibit bFGF-induced proliferation, migration, and tube formation of cultured endothelial cells (Colorado et al., 2000; Sudhakar et al., 2005). The receptor that mediates arresten's activity is believed to be the α1β1 integrin (Nyberg et al., 2008).
Canstatin is a 24 kDa protein derived from the non-collagenous domain of the type IV collagen α2 chain. Canstatin's functions include suppression of tumor growth, inhibition of endothelial cell proliferation and migration, and induction of endothelial cell apoptosis (Magnon et al., 2005; Nyberg et al., 2008). The receptors believed to mediate canstatin's activity are α3β1, ανβ3, and ανβ5 integrins.
Tumstatin is a 28 kDa protein derived from the non-collagenous domain of the type IV collagen α3 chain. Tumstatin's functions include suppression of tumor growth, inhibition of endothelial cell proliferation and migration, and induction of endothelial cell apoptosis (Mundel and Kalluri, 2007; Sudhakar and Boosani, 2008). The receptors that mediate tumstatin's activity are believed to be the ανβ3 and α6β1 integrins. Tumstatin binds to ανβ3 integrin in an RGD-independent manner, and this binding is essential for its anti-angiogenic activity. Tumstatin peptides inhibit protein synthesis by inhibiting the phosphorylation of FAK, induced in endothelial cells by attachment to vitronectin, and by inhibiting the activation of PI3-kinase through ανβ3 binding (Maeshima et al., 2000, 2001a,b, 2002).
8.2. Plasminogen-derived and serine protease inhibitors of angiogenesis
8.2.1. Angiostatin
Angiostatin is an example of a molecule generated by primary tumors to inhibit both primary and secondary tumor growth. Recombinant angiostatin has been used successfully to suppress tumor growth and metastasis in animal model systems (Ambati et al., 2002; Dell'Eva et al., 2002; Hajitou et al., 2002; Kisker et al., 2001; Perri et al., 2005; Shin et al., 2000). Initially, plasminogen is converted to an A chain (N-terminal kringle domains) and a B chain (serine protease plasmin). The A chain of plasminogen is then further processed by several MMPs and cathepsin to generate kringle-containing fragments (further demonstrating that MMPs have both pro-angiogenic and anti-angiogenic characteristics). One of the enzymes responsible for the generation of angiostatin in Lewis lung carcinoma has been identified as a macrophage-derived metalloelastase (MMP-12) (Acuff et al., 2006; Houghton et al., 2006), but human matrilysin (MMP-7) and neutrophil gelatinase B (MMP-9) also convert plasminogen to angiostatin fragments (Patterson and Sang, 1997). The cleavage sites in plasminogen by MMP-7 and MMP-9 are located between the 4th and 5th kringle domains. In addition, an angiostatin-like fragment (containing kringles 1–4) can be generated from plasminogen with stromelysin-1 (MMP-3). MMP-3 hydrolyzes three peptide bonds (Glu59-Asn60, Pro447-Val448, and Pro544-Ser545) in plasminogen, yielding a 55 kDa NH2-terminal fragment comprised of kringles 1 through 4 (Cornelius et al., 1998; Lijnen et al., 1998).
We have demonstrated the involvement of angiostatin in corneal avascularity after wounding (Gabison et al., 2004). We have confirmed that angiostatin-like molecules are expressed in the corneal epithelium and in cultured corneal epithelial cells. Western blotting after incubation of scraped corneal epithelial cell lysate to detect plasminogen showed a reduction of the size of the plasminogen bands at 6, 12, and 24 h, respectively. We performed experiments to determine whether this balance is tilted towards angiogenesis in the presence of angiostatin blocking antibodies. Corneal NV was observed after excimer laser keratectomy when anti-angiostatin antibodies were injected into the cornea. Corneal segmental NV was noted after anti-angiostatin (anti-LBS or anti-K1–3) antibody injection and excimer laser keratectomy. NV was significantly higher with injection of anti-angiostatin antibodies than with injection of plasmin B chain antibodies. Corneal segmental NV was determined by Indian ink injection and areas of vessel invasion were determined. The percentage of NV after laser wounds were determined and the differences between anti-LBS and plasmin B chain antibodies groups were statistically significant (p < 0.05). These studies suggest that angiostatin may contribute to the maintenance of corneal avascularity after excimer laser keratectomy (Fig. 28).
Fig. 28.
Corneal injection of anti-angiostatin antibody enhanced corneal vascularization after excimer laser wounding. Intrastromal injection of anti-plasminogen (LBS), anti-angiostatin, and anti-plasmin B chain antibodies in unwounded (A–C) and wounded (D–E) rat corneas. Corneal NV was visualized after India ink perfusion (flat mounted corneas). Quotidian injection (for 5 days) of anti-plasminogen (LBS; A, D) and anti K-1–3 (B, E) antibodies induced corneal NV after wounding (D, E), but not in unwounded corneas (A, B). In contrast, the injection of an anti-plasmin B chain showed no significant increase of vascularization in unwounded (C) and wounded (F) corneas. (G) Quantification of corneal NV and statistical analysis (unpaired t-test). (*, statistically significant) (reprinted with permission from Gabison et al., 2004).
The effects of angiostatin function to inhibit both angiogenesis during wound repair, as well as developmental NV. Angiostatin works by down-regulating endothelial cell migration and proliferation, and inducing endothelial cell apoptosis. Angiostatin binds to ATP synthase, causing a decrease in endothelial cell ATP production, resulting in the downregulation of cell proliferation and migration (Moser et al., 1999). Angiostatin also binds to integrin αvβ3, affecting both angiogenesis and developmental NV. Disruption of integrin αvβ3 ligation with neutralizing antibody LM609 or peptide antagonists of integrin αvβ3 has been shown to affect blood vessel formation. In addition, plasmin specifically binds to integrin αvβ3 through its kringle domains (like angiostatin) and induces vascular endothelial cell migration. The induced vascular endothelial cell migration can be blocked by anti-integrin αvβ3 agents (i.e., angiostatin) and a serine protease inhibitor (Tarui et al., 2001).
Angiostatin also induces vascular endothelial cell apoptosis, and cells arrest at the G2/M transition interface in the presence of angiostatin (Griscelli et al., 1998). Administration of angiostatin to tumor-bearing mice has not resulted in detectable systemic cytotoxicity; only angiogenic proliferation appears to be inhibited. Therefore, angiostatin, appears to be an effective and non-toxic inhibitor of NV (Shepard et al., 2000).
Similarly, angiostatin inhibits lymphangiogenesis, in vitro. Treatment of the lymphatic endothelial (LE) cells isolated from pig thoracic duct cells with angiostatin results in a decrease in the rate of cell proliferation in a dose-dependent manner, as assessed by MTT assays. The cell migration rate of LE cells was also significantly inhibited by angiostatin in a dose-dependent fashion, compared to controls. Treatment of LE cells with angiostatin results in an increase in apoptosis (Shao and Chi, 2005).
8.2.2. Pigment epithelium-derived factor (PEDF)
PEDF is a potent anti-angiogenic factor that has been immunolocalized to the corneal epithelium and endothelium (Karakousis et al., 2001). PEDF belongs to the serine protease inhibitor family. It has previously been demonstrated that PEDF-blocking antibodies implanted in the cornea facilitate corneal NV (Meyer et al., 2002), and that pre-clearing of human corneal stromal extracts with anti-PEDF antibodies abrogates the inhibition of vascular endothelial cell migration normally caused by these extracts. Furthermore, it has been demonstrated that recombinant PEDF inhibits bFGF-induced corneal NV (Dawson et al., 1999). The recombinant PEDF can be cleaved by MT1-MMP, but not by MMP-2, in vitro (Fig. 29).
Fig. 29.
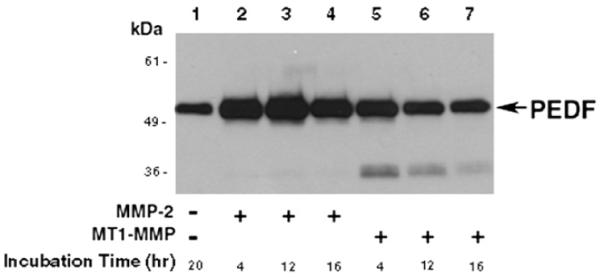
In vitro degradation of PEDF by MT1-MMP. Recombinant PEDF was incubated with active MMP-2 or MT1-MMP. The degradation products of PEDF by MT1-MMP were detected by western blot analysis using anti-PEDF antibodies.
These findings are all consistent with an essential role for PEDF in the exclusion of vessels from the cornea, vitreous, and retina. The molecular mechanism of PEDF depends on its interaction with receptors on the cell surface that activate the necessary signal transduction cascade. Several PEDF-binding molecules have been characterized, including glycosaminoglycans and collagen I (Meyer et al., 2002).
In the eye, there are several large compartments from which blood vessels are completely excluded: the vitreous, the aqueous humor that fills the anterior chamber, and the cornea. PEDF is an essential contributor to the maintenance of avascularity in these ocular tissues. Given its effectiveness against multiple inducers of angiogenesis, including VEGF and interleukin-8 (IL-8), PEDF is a good candidate for drug development for the pharmacologic inhibition of ocular angiogenesis.
9. Lymphangiogenesis regulatory proteins of the cornea
Some of the primary regulatory mechanisms and proteins involved in the regulation of corneal lymphangiogenesis have been previously described in other sections of this review. This section will focus on the regulatory proteins that have not been described in other sections. While we attempt to provide detailed information on several lymphangiogenic regulatory proteins, a comprehensive list is not within the scope of this review.
9.1. VEGF-C/-D
VEGF-C is mainly expressed in the heart, small intestine, placenta, ovaries, and thyroid. VEGF-C stimulates mitosis and the migration of endothelial cells, in vitro, and induces lymphangiogenesis in transgenic mouse skin, in vivo. In addition to its effects on lymphangiogenesis, recombinant VEGF-C also promotes angiogenesis in the chorioallantoic membrane of chicks, in mouse corneas, and in the ischemic hind limbs of rabbits. VEGF-C is produced, secreted, bound together by disulfide bonds, and proteolyzed by plasmin and other proteases to generate the final product, which has a high affinity for both VEGFR-2 and VEGFR-3. The mature form of VEGF-C induces the mitogenesis, migration, and survival of endothelial cells. During development, VEGF-C is expressed along with its receptor, VEGFR-3, predominantly in regions near lymphatic vessels. Manipulation of cellular VEGF-C gene expression through knockout or over expression influences the growth of functional lymphatic vessels in several animal models. Karkkainen et al. (2004) have demonstrated that VEGF-C-deficient mice fail to develop lymphatic vessels, and thus succumb to tissue edema at E15.5–E17.5. Over expression of VEGF-C in pancreatic islet cell tumors induces lymphangiogenesis and promotes lymph node metastasis. Over expression of a soluble VEGFR-3 (VEGFR-3-Fc) inhibits lymphangiogenesis and lymph node metastasis (Cueni and Detmar, 2008b; He et al., 2004a,b, 2002). We have also shown that VEGF-D strongly induces corneal lymphangiogenesis in col18a1−/− mice. It has also been demonstrated that the induction of lymphangiogenesis is greater in collagen XVIII knockout mice compared to WT mice (discussed in greater detail in other sections of this review).
Structurally, VEGF-D is 48% identical to VEGF-C. VEGF-D is expressed in many adult tissues, including the vascular endothelium, heart, skeletal muscle, lung, and both small and large intestines. VEGF-D is proteolytically processed at its N- and C-termini. It binds to and activates VEGFR-2 and VEGFR-3, and activates hemangiogenesis, as well as lymphangiogenesis, in endothelial cells, in vivo. Mouse VEGF-D binds only to VEGFR-3, indicating a distinct role for VEGF-D in mice. In experimental tumor models, over expression of VEGF-D also increases lymphatic vessel growth and lymphatic metastasis (Cueni and Detmar, 2008b; He et al., 2004a,b, 2002). Recent publications have shown that VEGF-A, -C, and -D induce hemangiogenesis and lymphangiogenesis (Karpanen and Alitalo, 2008a,b). Thus, it will be difficult to dissect their roles in hemangiogenesis or lymphangiogenesis. However, mouse VEGF-D differs from its human counterpart and from VEGF-C in that it does not bind to the major angiogenic receptor VEGFR-2, which has been shown to cooperate with VEGFR-3 in lymphatic endothelial cell migration and proliferation (Karpanen and Alitalo, 2008a,b).
VEGF-C is not detected in the normal cornea but is present during corneal wounding. The application of exogenous VEGF-C to the cornea leads to the growth of both blood vessels and lymphatic vessels. Thus, the endogenous VEGF-C detected in injured corneas may account for the growth of blood vessels, as well as lymphatic vessels during wound healing. In our hands, VEGF-C was not detected in the normal cornea but was present during corneal wound healing. Additionally, VEGF-D enhanced corneal NV and lymphangiogenesis in collagen XVIII−/− mice (Fig. 30). Our findings are consistent with previous research indicating that VEGF-C and -D are induced after bFGF-induced corneal lymphangiogenesis (Kojima et al., 2008).
Fig. 30.
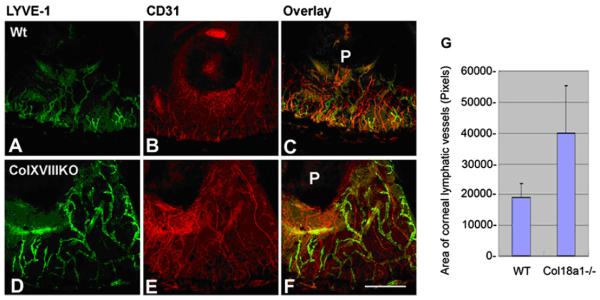
VEGF-D enhances corneal lymphangiogenesis in col18a1−/− mice. Corneas from WT and col18a1−/− mouse were implanted with VEGF-D pellets (160 ng/pellet). Corneas were harvested 7 days later and processed for whole-mount immunostaining. LYVE-1 (A and D) and CD31 immunostaining (B and E) are shown. Quantification of LYVE-1 staining revealed that more lymphatic vessels developed in the pellet-implanted side of col18a1 knockout mouse corneas than in the pellet-implanted side of WT corneas (G) (bar = 50 μm).
The downstream signaling pathways of VEGFs are well-established and are mediated by MAP kinase (MAPK) and PI3-kinase (P13-K). The PI3-K pathway is linked to mitogenesis, which is involved in the activation of serine–threonine kinase Akt and which regulates the cell survival pathway (protein kinase B pathway). The MAPK and PI3-K pathways in cell survival are regulated through the post-translational modification of pro-survival gene products and by the modulation of cell death machinery (Graells et al., 2004).
9.2. Endothelin-1
Endothelin-1 has been shown to play a crucial role in angiogenesis, tumorigenesis and lymphangiogenesis (Bek and McMillen, 2000; Kuhlmann et al., 2005; Spinella et al., 2009). In an in vitro study, ET-1 has been shown to promote proliferation, invasiveness, vascular-like structure formation, and phosphorylation of AKT and p42/44 mitogen-activated protein kinase. In addition, endothelin-1 is also able to upregulate the expression of vascular endothelial growth factor (VEGF)-C, VEGF receptor-3, and VEGF-A, and to stimulate hypoxia-inducible factor (HIF)-1α expression (Spinella et al., 2009; Wulfing et al., 2005).
9.3. Angiopoietin-1
The Angiopoietin/Tie system acts as a vascular specific ligand/receptor system to control endothelial cell survival and vascular maturation. The Angiopoietin family includes four ligands (Ang-1, -2, -3 and -4) and two corresponding tyrosine kinase receptors (Tie1 and Tie2) (Thomas and Augustin, 2009). Ang-1 is primarily expressed by mesenchymal cells and acts in a paracrine manner on the endothelium through the receptor tyrosine kinase Tie2, which is expressed almost exclusively on the surface of endothelial cells. Ang-1 has also been shown to regulate the formation and stabilization of the blood vessel network during embryogenesis. In adults, Ang-1 is associated with blood vessel stabilization and recruitment of perivascular cells (London et al., 2009).
Tammela et al. showed that overexpression of Ang-1 activates lymphatic vessel endothelial proliferation, vessel enlargement and leads to new sprouts (Tammela et al., 2005a,b, 2008). Ang-1 stimulates lymphatic cells resulting in upregulation of VEGFR-3, and lymphatic sprouting can be inhibited by the administration of soluble VEGFR-3. Morisada et al. also demonstrated that Ang-1 acts on in vivo lymphatic angiogenesis and in vitro growth of lymphatic endothelial cells (Morisada et al., 2005). A chimeric form of Ang-1 promotes lymphatic angiogenesis in mouse cornea in vivo and stimulates lymphatic endothelial cell colony formation in vitro. The Ang-1-induced in vivo and in vitro effects on lymphatic endothelial cells are also inhibited by exogenous soluble Tie2 receptor.
10. Clinical therapy of corneal angiogenesis and lymphangiogenesis
The identification and adequate treatment of the underlying cause of corneal NV is critical. Therapies for corneal NV range from antimicrobial therapy for infectious keratitis to systemic immunosuppression for autoimmune diseases, like ocular cicatricial pemphigoid. Over the years, many different medical and surgical treatments have been used for the treatment of corneal NV, or assessed as potential anti-angiogenic molecules on various models of corneal NV (Table 7). Currently, several different modalities of both medical and surgical treatment have been shown to be effective in decreasing corneal NV in various pathological diseases.
Table 7.
Treatments for angiogenesis and lymphangiogenesis.
| Treatment | Animal studies |
Human trial | Reference | |
|---|---|---|---|---|
| Animal model | Injury model | |||
Anti-VEGF therapy
|
Rabbit, rat | Alkali Burn, chemical cauterization | Yes | (Barros and Belfort, 2007; Bock et al., 2008a, 2007; Hosseini et al., 2007; Uy et al., 2008; Yoeruek et al., 2008) |
Steroids
|
Rabbit, rat | Sulfur Mustard, Alkali Burn, Pellet Implantation | Yes | (Akpek et al., 2004; BenEzra et al., 1997; Dan et al., 2008; Kadar et al., 2008; McNatt et al., 1999) |
NSAID
|
Rabbit, rat mouse | Sulfur Mustard, bFGF, VEGF, Chemical Burn | Yes | (Frucht and Zauberman, 1984; Jung et al., 2007; Kadar et al., 2008; Masferrer et al., 2000; Pakneshan et al., 2008; Srinivasan and Kulkarni, 1989) |
| Cyclosporin | Rat, mouse | Transplantation, chemical cauterization, VEGF | Yes | (Benelli et al., 1997; Bourges et al., 2006; Hernandez et al., 2001; Sonmez et al., 2007) |
| Angiostatin | Rat | Alkali Burn | – | (Cheng et al., 2007) |
| PAF antagonist | Rabbit | Silk Suture, Transplantation | – | (Cohen et al., 1994) |
| Methotrexate (MTX) | Rabbit | bFGF pellet | – | (Hirata et al., 1989; Joussen et al., 1999) |
| Rapamycin | Mouse, rabbit | Alkali Burn, bFGF, Transplantation | – | (Kwon et al., 2005; Kwon and Kim, 2006; Shi et al., 2006) |
| FK-506 | Rat, rabbit | Transplantation, Silk Suture | – | (Benelli et al., 1996; Mills et al., 1995) |
| Thalidomide | Rabbit | Alkali Burn, VEGF pellet | – | (Abbas et al., 2002; Kruse et al., 1998) |
| Prolactin/Prolactin-like mol. | Rat | bFGF | – | (Duenas et al., 1999) |
| Curcumin | Rat, mouse | Alkali Burn, bFGF | – | (Arbiser et al., 1998; Bian et al., 2008) |
| 1–25(OH)D3 | Mouse | Nylon Sutures | – | (Suzuki et al., 2000a) |
| Farnesyltransferase inhibitors (FTI) | Mouse | VEGF pellet | – | (Gu et al., 1999) |
| IL-1 antagonist (IL-1Ra) | Mice | Electrocautery | – | (Biswas et al., 2004; Dana, 2007) |
| Soluble TNF-α receptor | Mice | Electrocautery | – | (Dana, 2007) |
| Octreotide | Mice | LCI-D20 micropocket | – | (Jia et al., 2003) |
| Spironolactone | Rat | Transplantation | (Otasevic et al., 2007) | |
| Surgical treatment Argon laser | Rabbit | Alkali burn | Yes | (Gerten, 2008; Gordon et al., 2002; Luengo Gimeno et al., 2007) |
| Electrocoagulation | – | – | Yes | (Wertheim et al., 2007) (Pillai et al., 2000) |
| Photodynamic therapy | Mice, rabbit | Nylon suture, silk suture | Yes | (Framme et al., 2006; Sugisaki et al., 2008; Yoon et al., 2006, 2007) |
| Limbal (stem cell) transplantation | Rabbit | Alkali burn | Yes | (Luengo Gimeno et al., 2007; Kawashima et al., 2007) |
| Amniotic membrane (AM) transplantation | Rabbit | Epithelium/Limbus removal | Yes | (Burman et al., 2004; Kheirkhah et al., 2008; Kim and Tseng, 1995) |
| Conjunctival (cell) transplantation | Rabbit | Limbal deficiency | Yes | (Kwitko et al., 1995; Ono et al., 2007) |
10.1. Clinically-approved medical therapies
10.1.1. Anti-inflammatory agents
Anti-inflammatory compounds, such as steroids, have long been used for the suppression of inflammation and the associated angiogenesis (Akpek et al., 2004; BenEzra et al., 1997; Dan et al., 2008; Kadar et al., 2008; McNatt et al., 1999). The anti-angiogenic effects of steroid treatment are likely secondary to their anti-inflammatory actions and include the inhibition of chemotaxis and cytokine synthesis. Steroids have also been shown to inhibit vascular EC proliferation and migration. Steroid therapies have been used successfully for many years to manage pathological corneal NV. However, the extensive side effects of these compounds make long-term administration difficult for many patients. Additionally, while their efficacy in treating inflammatory mediated corneal NV is quite high, steroid therapy for the management of non-inflammatory mediated corneal NV remains limited.
Non-steroidal anti-inflammatory agents have also been extensively used in the management of ocular surface disorders. Two types of COX enzymes have been found in the cornea, the constitutive COX-1 and the inducible COX-2. Treatment with selective inhibitors has been used to assess their differential significance in corneal angiogenesis. Selective inhibition of COX-2 has a 20% inhibition rate of basal prostaglandin E2 (PGE2) corneal synthesis. This rate of inhibition rises to 80% after wounding (Yamada et al., 1999). Furthermore, selective COX-2 inhibition significantly inhibits corneal NV with a similar anti-angiogenic effect to indometacin, a nonselective COX-1 and -2 inhibitor. Various other research molecules have shown anti-angiogenic activity in corneal NV, including topical application of IL-1 receptor antagonist (Biswas et al., 2004; Dana, 2007), octreotide (a long-acting somatostatin analogue) (Jia et al., 2003), cyclosporine A (Sonmez et al., 2007), angiostatin (Cheng et al., 2007), spironolactone (Otasevic et al., 2007), thalidomide (Abbas et al., 2002), curcumin (Bian et al., 2008), and PAF antagonist (Cohen et al., 1994). Table 7 details several different molecules that have been shown to have anti-angiogenic effects in the cornea. However, because medical treatment is currently most efficient only in actively growing NV, there is still much ongoing investigation regarding the advancement of medical as well as surgical treatments for corneal NV.
10.1.2. Bevacizumab
Recent advances in the understanding of the mechanisms underlying ocular NV have led to the identification of new pharmacologic targets. Given the key role of VEGF in NV of the eye (Grisanti and Tatar, 2008), attention has been directed to developing drugs that will counteract VEGF activity. Bevacizumab® is an anti-VEGF antibody that binds to all VEGF isoforms (Bock et al., 2007; Uy et al., 2008). This molecule inhibits VEGF/VEGFR interactions and, in this way, blocks all VEGF activity. Bevacizumab® is currently approved by the FDA to treat metastatic colorectal cancer. It has also been tested for the treatment of wet (neovascular) age-related macular degeneration (AMD). Ranibizumab® is another anti-VEGF antibody that has been approved for use in the eye to treat wet AMD (Dadgostar and Waheed, 2008). Subconjunctival injection, as well as topical application of this molecule, have been used with promising results to treat HSV keratitis, recurrent pterygia, rejection of corneal grafts, and Stevens Johnson syndrome (Barros and Belfort, 2007; Bock et al., 2008a, 2007; Hosseini et al., 2007; Uy et al., 2008; Yoeruek et al., 2008). However, data on these treatments remain limited and controversial. Table 8 outlines 10 patient-based studies regarding the treatment of corneal NV with Bevacizumab.
Table 8.
Patient studies using bevacizumab.
| Author & year | Study design | No. of patient (eyes) | Presenting diagnosis (cause of NV) | Administration | Results | Adverse effects |
|---|---|---|---|---|---|---|
| (Mackenzie et al., 2009) | Case Study | 1 (1) | Failed corneal graft | Topical (through light shield) | No regression | NA |
| (Wu et al., 2009) | Case Study | 1 (1) | Recurrent Pterygium | Topical | “Prominent regression” | None observed |
| (Gerten, 2008) | Case series | 2 (2) | herpes simplex/Injury | Subconj injection (& argon laser) | “Marked regression” | NA |
| (Qian et al., 2008) | Case Series | 2 (2) | Sclerokeratitis/Terrien marginal degeneration | Subconj injection (& superficial keratectomy) | “Significant regression” | None observed |
| (Doctor et al., 2008) | Retrospective | 7 (8) | Various Causes | Subconj injection | All eyes showed regression | None observed |
| (Carrasco, 2008) | Case study | 1 (1) | Herpetic stromal keratitis | Subconj injection | “Rapid regression” | None observed |
| (Kim et al., 2008) | Prospective case series | 7 (10) | Various Causes | Topical | Regression in 7/10 eyes | Epitheliopathy 6/10 eyes |
| (Uy et al., 2008) | Retrospective | 2 (3) | Stevens-Johnson | Topical | All eyes showed regression | None observed |
| (Bahar et al., 2008a) | Retrospective | 10 (10) | Various causes | Subconj injection | “Partial regression” 7/10 eyes | None observed |
| (Bahar et al., 2008b) | Retrospective | 5 (5) | Recurrent pterygium | Subconj injection | “No Significant Regression” | None observed |
While anti-VEGF therapy has been shown by some to be effective for inhibiting and regressing corneal NV, adverse reactions, such as loss of epithelial integrity and progression of thinning may occur. One mechanism believed to play a role in the adverse effects of anti-VEGF therapy on corneal integrity is that VEGF mediates corneal nerve repair. Yu et al. (2008) have used in vitro and in vivo experiments to demonstrate that VEGF mediates corneal nerve repair. They have demonstrated that VEGF and VEGF receptors are present in the trigeminal ganglia and that inhibition of VEGF signaling reduces nerve growth, both in vivo and in vitro. Perhaps, reduction in corneal nerve growth and repair caused by anti-VEGF therapy is one mechanism by which corneal damage following treatment can occur. The concept that anti-VEGF therapy has adverse effects on corneal integrity and wound healing remains controversial and under investigation (Bock et al., 2009; Chiang et al., 2008; Yu et al., 2008). Further research will be required to establish efficacy, adequate dosing, and safety in the different clinical scenarios that present with corneal NV.
Other forms of anti-VEGF therapy are currently undergoing clinical trials. One example is VEGF TRAP, a high-affinity VEGF antagonist designed to bind and neutralize VEGF in the circulation and within tissues. It binds to all isoforms of VEGF and to placental growth factor, which is a related pro-angiogenic factor. SIRNA-027, another anti-VEGF therapy, is a small interfering RNA designed to down-regulate VEGFR-1 expression. PKC412 is an orally administered tyrosine kinase inhibitor that binds to the intracellular, enzymatically active domain of the VEGF receptor and prevents phosphorylation and activation of the VEGF signaling cascade. Some of these compounds may be available for use in the near future.
10.2. Alternative medical therapies
Several natural health products have been shown to have a high degree of anti-angiogenic activity. Some of these products also possess anti-tumor and anti-metastasis activities. Given the multiple effects of these natural health agents, quality assurance of appropriate extracts, and an understanding of the mechanisms of action and toxicities of these compounds are essential before they are used in the treatment of corneal NV.
10.2.1. Propolis extract
Propolis is a resinous mixture that honey bees collect from tree buds, sap flows, or other botanical sources. Propolis possesses anti-inflammatory and anticancer properties. It has been reported that ethanol extracts of Brazilian propolis (EEBP) suppresses tumor-induced angiogenesis, in vivo, and tube formation of endothelial cells, in vitro, through the induction of apoptosis (Ahn et al., 2007). Studies have shown that propolis extract and its components can treat corneal NV by inhibiting critical steps in angiogenesis, such as proliferation, migration, tube formation, and inhibition of VEGF, MMP-2, and MMP-9 secretion (Keshavarz et al., 2009).
10.2.2. Epigallocatechin-3-gallate
Green tea contains epicatechin-derived polyphenolic components, which include (–)-epicatechin (EC), (–)-epigallocatechin (EGC), (–)-epicatechingallate (ECG), and (–)-epigallocatechin-3-gallate (EGCG). EGCG, the main polyphenolic constituent of green tea has been shown to effectively inhibit several angiogenic processes (Ahmad et al., 1997). For example, EGCG inhibits endothelial cell growth, VEGF expression and binding to its receptor, VEGFR expression and phosphorylation, and STAT3 activation (Stephanou, 2004). In addition, the local administration of a nutrient mixture (NM) containing lysine, proline, ascorbic acid, and green tea extract is an effective corneal NV treatment. The effect of the green tea extract is the reduced secretion of major stimulatory factors involved in cell proliferation and angiogenesis, especially VEGF and MMP-2 and -9 (Sen et al., 2009).
10.2.3. Resveratrol
Resveratrol is a polyphenol compound enriched in red wine and other grape products (Martin et al., 2004). Resveratrol has been reported to have cancer chemopreventive activity in animals with both carcinogen-induced and implanted tumors. Its anti-tumor activity has been observed in several tumor models and appears to be independent of cell type. In vitro investigations have indicated that resveratrol inhibits several key events of the angiogenic process, such as proliferation and migration of endothelial cells and vascular smooth muscle cells, and the expression of two major proangiogenic factors, vascular endothelial growth factor (VEGF) and MMP-2 (Gagliano et al., 2005).
10.3. Vitamin D
In 1990, Schwartz and Hulka hypothesized that vitamin D deficiency is a risk factor for prostate cancer (Schwartz and Hulka, 1990). Their hypothesis is based on the epidemiological data that vitamin D maintains the normal phenotype of prostatic cells and that decreased vitamin D exposure increases the risk for clinical prostate cancer. Vitamin D is a hormone that is produced from 7-dehydrocholesterol by a series of reactions that culminates in the most active metabolite of vitamin D, 1α,25(OH)2D3, also known as calcitriol (Holick et al., 1980). Currently, it is known that 1α,25 (OH)2D3 inhibits the proliferation of endothelial cells, in vitro, and reduces angiogenesis, in vivo. Schwartz and Hulka have proposed that vitamin D interrupts IL-8 signaling and leads to the inhibition of endothelial cell migration and tube formation. In addition, a significant inhibition of metastasis is observed in prostate and lung murine models treated with 1α,25(OH)2D3 (Campbell et al., 2000).
In the corneal angiogenesis model, topical administration of 1α,25(OH)2D3 inhibits Langerhans cell migration and corneal NV when sutures are placed in the center of a mouse cornea (Suzuki et al., 2000a,b). A lower concentration of 1α,25(OH)2D3 was enough to inhibit Langerhans cell migration, whereas only a high concentration effectively suppressed corneal NV (Riachy et al., 2006). These data are in agreement with Dam et al. who showed that topical administration of 1α,25(OH)2D3 suppresses the number and antigen-presenting function of Langerhans cells in human skin, both in vitro and in vivo (Dam et al., 1996). The mechanism of 1α,25 (OH)2D3 on the immobilization of Langerhans cells may be directly mediated by their receptors and may also act on corneal epithelial cells and inhibit the production of cytokines, such as interleukin (IL)-1α and 1β, and granulocyte–macrophage colony-stimulating factor (GM-CSF), known to induce Langerhans cell migration. Anti-angiogenic effects of the systemic administration of 1α,25(OH)2D3 in mice have been reported. Most of these experiments have shown a 30–60% suppression of vessel formation in mice treated with 1α,25(OH)2D3 compared with control mice.
Similarly, 1α,25(OH)2D3 addition to control cells or to Pseudomonas aeruginosa-colonized cells alters gene and protein expression of IL-1β, IL-6, and IL-8. 1α,25(OH)2D3 significantly inhibits the expression of IL-1β, IL-6, and IL-8 protein in HCE cells colonized with P. aeruginosa. These results suggest that 1α,25(OH)2D3, when administered at the appropriate concentration, inhibits the host inflammatory response through the inhibition of the expression of pro-inflammatory cytokines and chemokines during P. aeruginosa ocular infection (Xue et al., 2002).
10.4. Promising potential medical therapies
10.4.1. Endostatins and neostatins
Endostatin overproduction in keratinocytes significantly reduced the number of tumor lymphatics in transgenic J4 mice and also prevented tumor cell dissemination into lymph nodes, possibly by inhibiting the recruitment of VEGF-C-producing mast cells. In addition, recombinant endostatin inhibits the proliferation and migration of lymphatic endothelial cells, in vitro, and inhibits lymphangiogenesis and lymph expansion by down-regulating VEGF-C expression in cultured squamous carcinoma cells. The reduced bFGF-induced corneal lymphangiogenesis by neostatin-7 was demonstrated by the administration of recombinant GST-neostatin-7 and bFGF in corneal micropellet implantation.
10.4.2. Anti-VEGFR-3 antibody
Until now, there has been no effective treatment for high-risk corneal transplant rejection. However, there are data that show that surgical lymphadenectomy leads to improved corneal graft survival. Surgical lymphadenectomy may not be practical for enhancing corneal transplant survival. Thus, inhibition of lymphangiogenesis may provide a way to treat lymphangiogenesis-related corneal disorders (Regenfuss et al., 2008). Various agonists and other reagents, including soluble VEGFR-3, anti-VEGFR-3 antibody, anti-VEGFR-2 antibody, anti-VEGF-A antibody, or VEGF TRAP are currently under investigation to evaluate their efficacy in preventing corneal lymphangiogenesis. In the experimental animal model, anti-VEGF receptor-3 has been shown to effectively block tumor angiogenesis and lymphangiogenesis. In the corneal bFGF pellet lymphangiogenesis model, treatment with AFL4, a rat monoclonal antibody (mAb) with specificity for murine VEGFR-3, decreases bFGF-induced lymphangiogenesis, but does not completely eliminate it (Matsumura et al., 2003). AFL4 has also been shown to inhibit blood angiogenesis in tumors. Other monoclonal antibodies (mF4-31C1) that block VEGFR-3 activation have been reported to inhibit the growth of glioblastoma or prostatic carcinoma cells in nude mice. In an inflammatory corneal NV model, mF4-31C1 inhibits highly specific inflammatory lymphangiogenesis in the cornea. mF4-31C1 inhibits the outgrowth of actively developing lymphatic vessels (Burton et al., 2008; Bock et al., 2008b; Goldman et al., 2007; Pytowski et al., 2005). However, an incomplete inhibition of lymphangiogenesis on the established and matured lymphatic vessels (after 2 weeks of pellet implantation) was found with mF4-31C1 treatment. Thus, blocking VEGFR-3 completely and specifically prevents both physiologically normal and tumor VEGF-C-enhanced lymphangiogenesis in the adult mouse, but has no effect on either hemangiogenesis or matured lymphatic vessels. These results suggest that targeting VEGFR-3 with anti-VEGFR-3 antibody may only block new lymphatic growth.
10.4.3. “Non-bevacizumab” anti-VEGF-A antibody
As mentioned above, VEGF plays a key role in angiogenesis. Additionally, VEGF also possesses a pro-lymphangiogenic role in wound healing, corneal injury and tumorigenesis. Blocking VEGF-A using anti-VEGF antibody has been shown to suppress tumor lymphangiogenesis and metastasis in breast carcinoma MDA-MB-435 and MDA-MB-231 models. The Anti-VEGF-A treatment is associated with significant downregulation of VEGFR-3 protein on residual tumor lymphatic vasculature. The mechanism of the reduced angiogenesis and metastasis after anti-VEGF-A treatment is regulated by several pathways, including i) inhibition of VEGF-A-induced angiogenesis in primary tumors, ii) inhibition of tumor lymphangiogenesis, and iii) blocking macrophage recruitment. Anti-lymphangiogenic effect of anti-VEGF-A 2C3 antibody is mediated by blocking macrophage recruitment that supplied VEGF-C and VEGF-D factors and reduce the expression of VEGFR-3 in lymphatic endothelium (Zhang et al., 2002b; Whitehurst et al., 2007).
10.4.4. Anti-VEGFR-2 antibody
DC101, which is the inhibitory antibody against VEGFR-2, potently inhibited the growth of a variety of human tumor xenografts in mouse models. DC101 also inhibited lymphangiogenesis within the primary tumor of VEGF-C-overexpressing MDA-MB-435 cells. Partial suppression of lymphangiogenesis by blocking the VEGFR-2 receptor using DC101 has also been reported, with the DC101 treatment being less efficacious than blocking the VEGFR-3 receptor (Burton et al., 2008; Goldman et al., 2007).
10.4.5. Soluble VEGFR-3
Many tumors metastasize through the lymphatic vessels. VEGF-C and/or VEGF-D expression in tumor cells has been linked to lymphangiogenesis associated with tumors, invasion of cancer cells into the lymphatic vessels, and lymph node metastasis. VEGFR-3 levels are increased in the vascular endothelia of various types of solid tumors and VEGF-C has been detected in tumor cells; two indications that VEGF-C may stimulate tumor angiogenesis and/or lymphangiogenesis. In the MCF-7 breast carcinoma model, administration of soluble VEGFR-3 by an adenovirus slightly affected angiogenesis, but totally inhibited tumor lymphangiogenesis. Transgenic mice expressing soluble VEGFR-3 prevented the formation of lymphatic vessels for the first 4 weeks postnatal, but lymphatics regenerated after postnatal 4 weeks (Jeon et al., 2008).
In the animal models, the disruption of VEGFR-3 signaling by the soluble VEGFR-3 protein can completely destroy the lymphatic network and lead to a lymphedema-like phenotype (Makinen et al., 2001). Soluble VEGFR-3 is shown to be highly specific for lymphatic vessels without detectable effects on the blood vascular endothelium. sVEGFR-3 has been shown to bind VEGF-C and VEGF-D with the same efficiency as the full-length receptor. Thus, inhibition of VEGF-C and/or VEGF-D binding to VEGFR-3 indicates that continuous VEGFR-3 signaling is required for the survival of the lymphatic endothelial cells. VEGFR-3-Fc causes regression of the LECs but did not seem to affect the blood vessels.
10.4.6. VEGFR-3 inhibitor
As mentioned above, the VEGF-C/VEGF-R3 signal directly promoted invasion of cancer cells and increased both lymph node and lung metastases of human lung adenocarcinoma cells in mice. The combinative treatment against VEGF-R2 and anti-VEGF-R3 is more effective against lymph node and lung metastases than treatment with anti-VEGF-R2 antibody alone. Dual inhibition of both VEGF-R2 and VEGF-R3 proved to be a better strategy for suppressing metastases of VEGF-C-overexpressing tumors. E7080, a novel multi-kinase inhibitor, inhibitor of VEGF-R2 and VEGF-R3 kinases in in vitro and in vivo assays. E7080 significantly inhibited both lymph nodes and lung metastasis in MDA-MB-231 models (Matsui et al., 2008). Among several multiple kinase inhibitors tested, E7080 is one of the most potent dual inhibitors of VEGF-R2 and VEGF-R3 kinases in cell-free kinase assays and E7080 showed stronger inhibitory activity than sunitinib (SU-11248, small molecule, Sutent-inhibitor of receptor protein-tyrosine kinases including, VEGFR-3) in cell phosphorylation assays using HUVEC. E7080 may show more potent anti-lymphangiogenic and anti-angiogenic activity in comparison to similar inhibitors of the same class of drugs (Matsui et al., 2008).
10.4.7. Inhibitors of VEGFR-2 and VEGFR-3 signal pathways
Both VEGF receptor-2 and VEGF receptor-3 stimulation activates eNOS in lymphatic endothelial cells. l-NMMA, an eNOS inhibitor, blocks the regeneration of lymphatic vessels. Genetic deletion of eNOS in the host also leads to a decrease in T241 tumor cell dissemination to the lymph nodes and the macroscopic lymph node metastasis of B16F10 melanoma. These findings indicate that eNOS mediates VEGF-C-induced lymphangiogenesis and, consequently, plays a critical role in lymphatic metastasis (Lahdenranta et al., 2009).
10.4.8. Platelet derived growth factor-B (PDGF-B)
The PDGF family consists of four ligands, PDGFA-D, and two receptors, PDGFRα and PDGFRβ. All PDGF ligands (A–D) can dimerize. The receptors α and β can homo- and hetero-dimerize, upon ligand binding, into αα, αβ, and ββ combinations. Phosphorylation and phosphorylated receptors serve as docking sites for multiple protein complexes that function as transducer signaling cascades.
PDGF plays several roles in myofibroblasts, macrophages, and tumor cells. It is generally regarded as a potent mitogen and chemoattractant for myofibroblasts and macrophages, Also, PDGFs activate PDGF-receptors and further induce VEGF production in tumor and perivascular cells. Blockage of PDGF has been shown to be effective in vessel regression. Inhibition of PDGF-B signaling by an anti-PDGFR-β antibody causes disruption of the endothelial/mural cell association and destabilization of the developing vessels.
Although anti-VEGF therapy has been extensively used in the treatment of angiogenesis-related diseases, anti-VEGF alone may not be sufficient to cause vessel regression in advanced stages of aberrant angiogenesis. Recent studies have shown that anti-PDGFR-β antibody significantly enhances anti-tumor and anti-angiogenic activities of anti-VEGFR-2 (DC101) antibody, in pancreatic (BxPC-3) and tumor xenograft models (Shen et al., 2007). In addition, the response of blood vessels to anti-VEGF therapy is influenced by vessel maturation that may be attributed to the presence of vascular mural cells (pericytes and smooth muscle cells). Mural cells are required for normal vascular stability and function. The recruitment of mural cells to endothelial cells requires platelet-derived growth factor-B (PDGF-B) and signaling through the PDGF receptor-type β (PDGFR-β). Jo et al. have further demonstrated that the effectiveness of anti-VEGF treatment in causing vessel regression decreased over time in animal models of corneal NV. In addition, systemic administration of an anti-mouse PDGFR-β antibody completely blocked mural cell recruitment to blood vessels in the neonatal mouse retina. The specific targeting of both VEGF-A and PDGF-B signaling pathways is more effective at preventing and regressing pathological ocular NV than targeting VEGF-A or PDGF-B signaling alone (Jo et al., 2006).
10.5. Surgical interventions for corneal angiogenesis and lymphangiogenesis
In addition to the various mainstream medical treatments and alternative medical treatments discussed above, surgical treatments can also be used for the treatment of corneal angiogenesis and lymphangiogenesis, if needed. Surgical treatments for corneal angiogenesis and lymphangiogenesis include, argon laser (Gerten, 2008), electrocoagulation (Wertheim et al., 2007), fine-needle diathermy (Pillai et al., 2000), photodynamic therapy (Sugisaki et al., 2008), limbal (stem cell) transplantation (Luengo Gimeno et al., 2007), amniotic membrane (AM) transplantation (Kheirkhah et al., 2008), and conjunctival (cell) transplantation (Ono et al., 2007).
Argon laser has long been a staple in the treatment of corneal angiogenesis and lymphangiogenesis, particularly prior to penetrative keratoplasty (PK). The use of argon laser can induce vessel coagulation, thereby potentially causing vessel regression. Laser treatment involves low-power light activation of a photosensitized dye to cause the localized photochemical thrombosis of vessels. The use of laser treatment seems to be relatively safe and has shown some promising results in both animal and human studies. In 2002, Gordon et al. conducted a comprehensive study of 15 patients suffering from pathological corneal NV being treated with argon laser treatment. In their study, they assessed both subjective and objective improvement of the corneal NV and concluded that argon laser therapy for corneal NV, edema, and lipid keratopathy resulted in a significant reduction in symptoms and improved quality of life for 14 of 15 patients (Gordon et al., 2002). Although the use of argon laser treatment seems relatively safe, the benefit of laser therapy prior to high-risk keratoplasty remains unclear, and laser therapy does not appear to be useful for treating extensive corneal NV. One potential limitation to photocoagulation recently discussed is that the process of destroying vessels through coagulation may activate an inflammatory cascade and lead to the upregulation of VEGF. Some promising evidence has been reported using a combination of argon laser treatment and anti-VEGF therapy (Gerten, 2008).
The use of electrocoagulation by way of electrolysis-needle cauterization has been described by Wertheim et al. (2007). In a study using electrolysis-needle cauterization on three patients with lipid keratopathy and associated corneal vessels, they concluded that in all three patients the vessels remained occluded for 8 months after the procedure. Another procedure, similar to electrolysis-needle cauterization is fine-needle diathermy (FND). In FND, a stainless-steel 3/8 single-armed needle attached to a 10–0 monofilament black nylon suture is inserted close to the limbus next to the vessel to be occluded. The unipolar diathermy probe is then brought into contact with the 10–0 needle to produce the coagulation (Pillai et al., 2000). Since these procedures are believed to be fairly safe, their effectiveness alone appears to be limited and may produce better results when used concomitantly with medical treatment.
Transplantation is another approach that has been used with mixed results for the treatment of corneal angiogenesis and lymphangiogenesis. Limbal (stem cell) transplantation, amniotic membrane (AM) transplantation, and conjunctival (cell) transplantation have all been studied with regards to the treatment of corneal NV (Kheirkhah et al., 2008; Luengo Gimeno et al., 2007; Ono et al., 2007). Limbal, AM, and conjuctival transplantation are often used as a last resort and may be required to restore the ocular surface. These procedures have been shown in various studies to decrease corneal NV. However, surgical complications, as well as the various complications that often occur with transplantation are a real concern. Transplantation should be reserved as a last resort and, like other surgical treatments, may show the best results when used in conjunction with medical treatment.
11. Future directions
Although much significant progress has been made, a comprehensive understanding of the complex processes governing corneal angiogenic/lymphangiogenic privilege remains out of reach. It is clear at this time that the maintenance of corneal avascularity is an active process, which requires a delicate balance between angiogenic and anti-angiogenic mechanisms. It is also becoming increasingly clear that there are distinct as well as intersecting downstream pathways that affect angiogenesis. Future research will elucidate the interplay between the various molecular players and their signal transduction pathways that affect angiogenesis and lymphangiogenesis either simultaneously or separately.
Acknowledgement
Supported by: EY01792 (DTA), EY14048 (JHC), EY10101 (DTA), and an unrestricted grant from Research to Prevent Blindness, New York, NY.
References
- Abbas A, Khan B, Feroze AH, Hyman GF. Thalidomide prevents donor corneal graft neovascularization in an alkali burn model of corneal angiogenesis. J. Pak. Med. Assoc. 2002;52:476–482. [PubMed] [Google Scholar]
- Abdiu O, Van Setten G. Antiangiogenic activity in tears: presence of pigment-epithelium-derived factor. New insights and preliminary results. Ophthalmic Res. 2008;40:16–18. doi: 10.1159/000111153. [DOI] [PubMed] [Google Scholar]
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, Pozzi A, Carbone DP, Schwartz DR, Moin K, Sloane BF, Matrisian LM. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66:7968–7975. doi: 10.1158/0008-5472.CAN-05-4279. [DOI] [PubMed] [Google Scholar]
- Adamis AP, Meklir B, Joyce NC. In situ injury-induced release of basic-fibroblast growth factor from corneal epithelial cells. Am. J. Pathol. 1991;139:961–967. [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- Ahn MR, Kunimasa K, Ohta T, Kumazawa S, Kamihira M, Kaji K, Uto Y, Hori H, Nagasawa H, Nakayama T. Suppression of tumor-induced angiogenesis by Brazilian propolis: major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Lett. 2007;252:235–243. doi: 10.1016/j.canlet.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Akpek EK, Dart JK, Watson S, Christen W, Dursun D, Yoo S, O'Brien TP, Schein OD, Gottsch JD. A randomized trial of topical cyclosporin 0.05% in topical steroid-resistant atopic keratoconjunctivitis. Ophthalmology. 2004;111:476–482. doi: 10.1016/j.ophtha.2003.05.035. [DOI] [PubMed] [Google Scholar]
- Alam A, Herault JP, Barron P, Favier B, Fons P, Delesque-Touchard N, Senegas I, Laboudie P, Bonnin J, Cassan C, Savi P, Ruggeri B, Carmeliet P, Bono F, Herbert JM. Heterodimerization with vascular endothelial growth factor receptor-2 (VEGFR-2) is necessary for VEGFR-3 activity. Biochem. Biophys. Res. Commun. 2004;324:909–915. doi: 10.1016/j.bbrc.2004.08.237. [DOI] [PubMed] [Google Scholar]
- Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–96. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- Albe E, Escalona E, Rajagopal R, Javier JA, Chang JH, Azar DT. Proteomic identification of activin receptor-like kinase-1 as a differentially expressed protein during hyaloid vascular system regression. FEBS Lett. 2005;579(25):5481–5486. doi: 10.1016/j.febslet.2005.08.078. [DOI] [PubMed] [Google Scholar]
- Albe E, Chang JH, Azar NF, Ivanov AR, Azar DT. Proteomic analysis of the hyaloid vascular system regression during ocular development. J. Proteome Res. 2008;7(11):4904–4913. doi: 10.1021/pr800551m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- Ambati BK, Joussen AM, Ambati J, Moromizato Y, Guha C, Javaherian K, Gillies S, O'Reilly MS, Adamis AP. Angiostatin inhibits and regresses corneal neovascularization. Arch. Ophthalmol. 2002;120:1063–1068. doi: 10.1001/archopht.120.8.1063. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443(7114):993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati BK, Patterson E, Jani P, Jenkins C, Higgins E, Singh N, Suthar T, Vira N, Smith K, Caldwell R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br. J. Ophthalmol. 2007;91:505–508. doi: 10.1136/bjo.2006.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi B, Thibeault S, Lee YT, Bousquet-Gagnon N, Eliopoulos N, Barrette S, Galipeau J, Beliveau R. Matrix metalloproteinase regulation of sphingosine-1-phosphate-induced angiogenic properties of bone marrow stromal cells. Exp. Hematol. 2003;31:640–649. doi: 10.1016/s0301-472x(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Klauber N, Rohan R, van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol. Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- Asellius G. Asellii Cremonensis Antomici Ticiensis Qua Sententiae Anatomicae multae, nel perperam receptae illustrantur, De Lacteibus sive lacteis venis Quarto Vasorum Mesaroicum genere novo invente Gasp. Mediolani; Milan: 1627. [Google Scholar]
- Awwad ST, Heilman M, Hogan RN, Parmar DN, Petroll WM, McCulley JP, Cavanagh HD. Severe reactive ischemic posterior segment inflammation in Acanthamoeba keratitis: a new potentially blinding syndrome. Ophthalmology. 2007;114:313–320. doi: 10.1016/j.ophtha.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans. Am. Ophthalmol. Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- Azar DT, Casanova FH, Mimura T, Jain S, Chang JH. Effect of MT1-MMP deficiency and overexpression in corneal keratocytes on vascular endothelial cell migration and proliferation. Curr. Eye Res. 2008;33:954–962. doi: 10.1080/02713680802461106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar D, Casanova F, Mimura T, Jain S, Han K, Chang J. Corneal epithelial MT1-MMP inhibits vascular endothelial cell proliferation and migration. Cornea. doi: 10.1097/ICO.0b013e3181b1165d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphav-beta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol. Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar I, Kaiserman I, McAllum P, Rootman D, Slomovic A. Subconjunctival bevacizumab injection for corneal neovascularization. Cornea. 2008a;27:142–147. doi: 10.1097/ICO.0b013e318159019f. [DOI] [PubMed] [Google Scholar]
- Bahar I, Kaiserman I, McAllum P, Rootman D, Slomovic A. Subconjunctival bevacizumab injection for corneal neovascularization in recurrent pterygium. Curr. Eye Res. 2008b;33:23–28. doi: 10.1080/02713680701799101. [DOI] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros LF, Belfort R., Jr. The effects of the subconjunctival injection of bevacizumab (avastin) on angiogenesis in the rat cornea. An. Acad. Bras. Cienc. 2007;79:389–394. doi: 10.1590/s0001-37652007000300004. [DOI] [PubMed] [Google Scholar]
- Beer HD, Bittner M, Niklaus G, Munding C, Max N, Goppelt A, Werner S. The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: implications for epithelial repair. Oncogene. 2005;24:5269–5277. doi: 10.1038/sj.onc.1208560. [DOI] [PubMed] [Google Scholar]
- Bek EL, McMillen MA. Endothelins are angiogenic. J. Cardiovasc. Pharmacol. 2000;36:S135–S139. doi: 10.1097/00005344-200036051-00043. [DOI] [PubMed] [Google Scholar]
- Benelli U, Lepri A, Del Tacca M, Nardi M. FK-506 delays corneal graft rejection in a model of corneal xenotransplantation. J. Ocul. Pharmacol. Ther. 1996;12:425–431. doi: 10.1089/jop.1996.12.425. [DOI] [PubMed] [Google Scholar]
- Benelli U, Ross JR, Nardi M, Klintworth GK. Corneal neovascularization induced by xenografts or chemical cautery. Inhibition by cyclosporin A. Invest. Ophthalmol. Vis. Sci. 1997;38:274–282. [PubMed] [Google Scholar]
- BenEzra D, Griffin BW, Maftzir G, Sharif NA, Clark AF. Topical formulations of novel angiostatic steroids inhibit rabbit corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 1997;38:1954–1962. [PubMed] [Google Scholar]
- Berman MB. Regulation of corneal fibroblast MMP-1 secretion by cytochalasins. Cornea. 1994;13:51–57. doi: 10.1097/00003226-199401000-00009. [DOI] [PubMed] [Google Scholar]
- Beyer TA, Werner S, Dickson C, Grose R. Fibroblast growth factor 22 and its potential role during skin development and repair. Exp. Cell Res. 2003;287:228–236. doi: 10.1016/s0014-4827(03)00139-3. [DOI] [PubMed] [Google Scholar]
- Bian F, Zhang MC, Zhu Y. Inhibitory effect of curcumin on corneal neovascularization in vitro and in vivo. Ophthalmologica. 2008;222:178–186. doi: 10.1159/000126081. [DOI] [PubMed] [Google Scholar]
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr. Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- Binetruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, Plouet J, Derbin C, Perret G, Mazie JC. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. Embo J. 2000;19:1525–1533. doi: 10.1093/emboj/19.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas PS, Banerjee K, Zheng M, Rouse BT. Counteracting corneal immunoinflammatory lesion with interleukin-1 receptor antagonist protein. J. Leukoc. Biol. 2004;76:868–875. doi: 10.1189/jlb.0504280. [DOI] [PubMed] [Google Scholar]
- Biswas PS, Banerjee K, Kim B, Kinchington PR, Rouse BT. Role of inflammatory cytokine-induced cyclooxygenase 2 in the ocular immunopathologic disease herpetic stromal keratitis. J. Virol. 2005;79:10589–10600. doi: 10.1128/JVI.79.16.10589-10600.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, Wu L, Cao Y. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261–9268. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M, Zahn G, Cursiefen C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 2007;48:2545–2552. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- Bock F, Konig Y, Kruse F, Baier M, Cursiefen C. Bevacizumab (avastin) eye drops inhibit corneal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 2008a;246:281–284. doi: 10.1007/s00417-007-0684-4. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Dietrich T, Bachmann B, Pytowski B, Cursiefen C. Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch. Clin. Exp. Ophthalmol. 2008b;246:115–119. doi: 10.1007/s00417-007-0683-5. [DOI] [PubMed] [Google Scholar]
- Bock F, Onderka J, Rummelt C, Dietrich T, Bachmann B, Kruse FE, Schlotzer-Schrehardt UM, Cursiefen C. Safety profile of topical VEGF-neutralization at the cornea. Invest. Ophthalmol. Vis. Sci. 2009 doi: 10.1167/iovs.07-1129. [DOI] [PubMed] [Google Scholar]
- Bourges JL, Lallemand F, Agla E, Besseghir K, Dumont JM, BenEzra D, Gurny R, Behar-Cohen F. Evaluation of a topical cyclosporine A prodrug on corneal graft rejection in rats. Mol. Vis. 2006;12:1461–1466. [PubMed] [Google Scholar]
- Breier G. Angiogenesis in embryonic development–a review. Placenta. 2000;21(Suppl. A):S11–S15. doi: 10.1053/plac.1999.0525. [DOI] [PubMed] [Google Scholar]
- Brideau G, Makinen MJ, Elamaa H, Tu H, Nilsson G, Alitalo K, Pihlajaniemi T, Heljasvaara R. Endostatin overexpression inhibits lymphangiogenesis and lymph node metastasis in mice. Cancer Res. 2007;67:11528–11535. doi: 10.1158/0008-5472.CAN-07-1458. [DOI] [PubMed] [Google Scholar]
- Broocker G, Aaron M, Kim J. Injury to the eye. In: Feliciano DV, Mattox KL, Moore EE, editors. Trauma. sixth ed. McGraw Hill Professional; 2007. pp. 419–435. [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Burman S, Tejwani S, Vemuganti GK, Gopinathan U, Sangwan VS. Ophthalmic applications of preserved human amniotic membrane: a review of current indications. Cell Tissue Bank. 2004;5:161–175. doi: 10.1023/B:CATB.0000046067.25057.0a. [DOI] [PubMed] [Google Scholar]
- Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, Alitalo K, Wu L. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res. 2008;68:7828–7837. doi: 10.1158/0008-5472.CAN-08-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, Gombart AF, Kwok SH, Park S, Koeffler HP. The anti-proliferative effects of 1alpha,25(OH)2D3 on breast and prostate cancer cells are associated with induction of BRCA1 gene expression. Oncogene. 2000;19:5091–5097. doi: 10.1038/sj.onc.1203888. [DOI] [PubMed] [Google Scholar]
- Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat. Rev. Cancer. 2005;5:735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- Cao Y, Linden P, Shima D, Browne F, Folkman J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J. Clin. Invest. 1996;98:2507–2511. doi: 10.1172/JCI119069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Tombran-Tink J, Chen W, Mrazek D, Elias R, McGinnis JF. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. J. Neurosci. Res. 1999;57:789–800. [PubMed] [Google Scholar]
- Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res. 2004;94:664–670. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl. 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- Carmichael T. Corneal angiogenesis. In: Tombran-Tink J, Barnstable CJ, editors. Ocular Angiogenesis: Diseases, Mechanisms, and Therapeutics. Humana Press; 2006. pp. 50–71. [Google Scholar]
- Carrasco MA. Subconjunctival bevacizumab for corneal neovascularization in herpetic stromal keratitis. Cornea. 2008;27:743–745. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr. Opin. Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Chang L, Kaipainen A, Folkman J. Lymphangiogenesis new mechanisms. Ann. N. Y. Acad. Sci. 2002;979:111–119. doi: 10.1111/j.1749-6632.2002.tb04872.x. [DOI] [PubMed] [Google Scholar]
- Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Javier JA, Chang GY, Oliveira HB, Azar DT. Functional characterization of neostatins, the MMP-derived, enzymatic cleavage products of type XVIII collagen. FEBS Lett. 2005;579:3601–3606. doi: 10.1016/j.febslet.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Chen JX, Chen Y, DeBusk L, Lin W, Lin PC. Dual functional roles of Tie-2/angiopoietin in TNF-alpha-mediated angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H187–H195. doi: 10.1152/ajpheart.01058.2003. [DOI] [PubMed] [Google Scholar]
- Chen WL, Lin CT, Lin NT, Tu IH, Li JW, Chow LP, Liu KR, Hu FR. Subconjunctival injection of bevacizumab (avastin) inhibits corneal neovascularization in different rabbit models of corneal angiogenesis. Invest. Ophthalmol. Vis. Sci. 2008 doi: 10.1167/iovs.08-1997. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PG, Geerards AJ, Kijlstra A. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–185. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- Cheng HC, Yeh SI, Tsao YP, Kuo PC. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol. Vis. 2007;13:2344–2352. [PubMed] [Google Scholar]
- Chiang CC, Chen WL, Lin JM, Tsai YY. Effect of bevacizumab on human corneal endothelial cells: a six-month follow-up study. Am. J. Ophthalmol. 2008;146:688–691. doi: 10.1016/j.ajo.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Cho WG, Albuquerque RJ, Kleinman ME, Tarallo V, Greco A, Nozaki M, Green MG, Baffi JZ, Ambati BK, De Falco M, Alexander JS, Brunetti A, De Falco S, Ambati J. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui J, Di Girolamo N, Coroneo MT, Wakefield D. The role of substance P in the pathogenesis of pterygia. Invest. Ophthalmol. Vis. Sci. 2007;48:4482–4489. doi: 10.1167/iovs.07-0123. [DOI] [PubMed] [Google Scholar]
- Chung ES, Saban DR, Chauhan SK, Dana R. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Invest. Ophthalmol. Vis. Sci. 2009;50:1613–1618. doi: 10.1167/iovs.08-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Gebhardt BM, Bazan NG. A platelet-activating factor antagonist reduces corneal allograft inflammation and neovascularization. Curr. Eye Res. 1994;13:139–144. doi: 10.3109/02713689409042408. [DOI] [PubMed] [Google Scholar]
- Collin HB. Lymphatic drainage of 131-I-albumin from the vascularized cornea. Invest. Ophthalmol. 1970;9:146–155. [PubMed] [Google Scholar]
- Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J. Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- Crabtree B, Holloway DE, Baker MD, Acharya KR, Subramanian V. Biological and structural features of murine angiogenin-4, an angiogenic protein. Biochemistry. 2007;46:2431–2443. doi: 10.1021/bi062158n. [DOI] [PubMed] [Google Scholar]
- Cueni L, Detmar M. Lymphatic vascular system and lymphangiogenesis. In: Figg WD, Folkman J, editors. Angiogenesis: an Integrative Approach from Science to Medicine. Springer; 2008a. pp. 505–516. [Google Scholar]
- Cueni LN, Detmar M. The lymphatic system in health and disease. Lymphat Res. Biol. 2008b;6:109–122. doi: 10.1089/lrb.2008.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy. 2007;92:50–57. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Rummelt C, Kuchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea. 2000;19:526–533. doi: 10.1097/00003226-200007000-00025. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, Kruse FE, Wiegand SJ, Dana MR, Streilein JW. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest. Ophthalmol. Vis. Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Saint-Geniez M, Hamrah P, Jin Y, Rashid S, Pytowski B, Persaud K, Wu Y, Streilein JW, Dana R. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. U. S. A. 2006a;103(30):11405–11410. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Kruse F. New aspects of angiogenesis in the cornea. Essentials Ophthalmol. 2006b:83–99. [Google Scholar]
- Dadgostar H, Waheed N. The evolving role of vascular endothelial growth factor inhibitors in the treatment of neovascular age-related macular degeneration. Eye. 2008;22:761–767. doi: 10.1038/eye.2008.86. [DOI] [PubMed] [Google Scholar]
- Dam TN, Moller B, Hindkjaer J, Kragballe K. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J. Investig. Dermatol. Symp. Proc. 1996;1:72–77. [PubMed] [Google Scholar]
- Dan L, Shi-long Y, Miao-li L, Yong-ping L, Hong-jie M, Ying Z, Xiang-gui W. Inhibitory effect of oral doxycycline on neovascularization in a rat corneal alkali burn model of angiogenesis. Curr. Eye Res. 2008;33:653–660. doi: 10.1080/02713680802245772. [DOI] [PubMed] [Google Scholar]
- Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation, neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans. Am. Ophthalmol. Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stein E, Cerretti DP, St John PL, Robert B, Abrahamson DR. ELK and LERK-2 in developing kidney and microvascular endothelial assembly. Kidney Int. Suppl. 1996;57:S73–S81. [PubMed] [Google Scholar]
- Darland DC, D'Amore PA. Cell–cell interactions in vascular development. Curr. Top. Dev. Biol. 2001;52:107–149. doi: 10.1016/s0070-2153(01)52010-4. [DOI] [PubMed] [Google Scholar]
- Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- Dean RA, Butler GS, Hamma-Kourbali Y, Delbe J, Brigstock DR, Courty J, Overall CM. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol. Cell. Biol. 2007;27:8454–8465. doi: 10.1128/MCB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell S, Peters S, Muther P, Kociok N, Joussen AM. The role of PDGF receptor inhibitors and PI3-kinase signaling in the pathogenesis of corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 2006;47:1928–1937. doi: 10.1167/iovs.05-1071. [DOI] [PubMed] [Google Scholar]
- Dell'Eva R, Pfeffer U, Indraccolo S, Albini A, Noonan D. Inhibition of tumor angiogenesis by angiostatin: from recombinant protein to gene therapy. Endothelium. 2002;9:3–10. doi: 10.1080/10623320210712. [DOI] [PubMed] [Google Scholar]
- Dhanabal M, Ramchandran R, Waterman MJ, Lu H, Knebelmann B, Segal M, Sukhatme VP. Endostatin induces endothelial cell apoptosis. J. Biol. Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Coroneo MT, Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest. Ophthalmol. Vis. Sci. 2001;42:1963–1968. [PubMed] [Google Scholar]
- Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J. Biol. Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- Doctor PP, Bhat PV, Foster CS. Subconjunctival bevacizumab for corneal neovascularization. Cornea. 2008;27:992–995. doi: 10.1097/ICO.0b013e31817786ad. [DOI] [PubMed] [Google Scholar]
- Dong Z, Ghabrial M, Katar M, Fridman R, Berk RS. Membrane-type matrix metalloproteinases in mice intracorneally infected with Pseudomonas aeruginosa. Invest. Ophthalmol. Vis. Sci. 2000;41:4189–4194. [PubMed] [Google Scholar]
- Dong Z, Katar M, Alousi S, Berk RS. Expression of membrane-type matrix metalloproteinases 4, 5, and 6 in mouse corneas infected with P. aeruginosa. Invest. Ophthalmol. Vis. Sci. 2001;42:3223–3227. [PubMed] [Google Scholar]
- Duenas Z, Torner L, Corbacho AM, Ochoa A, Gutierrez-Ospina G, Lopez-Barrera F, Barrios FA, Berger P, Martinez de la Escalera G, Clapp C. Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest. Ophthalmol. Vis. Sci. 1999;40:2498–2505. [PubMed] [Google Scholar]
- Duke-Elder S. Corneal Wound Healing. Henry Klimpton; London: 1958. [Google Scholar]
- Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch. Ophthalmol. 2001;119:695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- Elder MJ, Dart JK, Lightman S. Conjunctival fibrosis in ocular cicatricial pemphigoid – the role of cytokines. Exp. Eye Res. 1997;65:165–176. doi: 10.1006/exer.1997.0311. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP. Integrin and growth factor receptor crosstalk. Circ. Res. 2001;89:1104–1110. doi: 10.1161/hh2401.101084. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch. Ophthalmol. 1993;111:1665–1671. doi: 10.1001/archopht.1993.01090120087027. [DOI] [PubMed] [Google Scholar]
- Eriksson A, Cao R, Pawliuk R, Berg SM, Tsang M, Zhou D, Fleet C, Tritsaris K, Dissing S, Leboulch P, Cao Y. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1:99–108. doi: 10.1016/s1535-6108(02)00028-4. [DOI] [PubMed] [Google Scholar]
- Eriksson K, Magnusson P, Dixelius J, Claesson-Welsh L, Cross MJ. Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett. 2003;536:19–24. doi: 10.1016/s0014-5793(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Espana EM, Grueterich M, Romano AC, Touhami A, Tseng SC. Idiopathic limbal stem cell deficiency. Ophthalmology. 2002;109:2004–2010. doi: 10.1016/s0161-6420(02)01250-2. [DOI] [PubMed] [Google Scholar]
- Espana EM, Ti SE, Grueterich M, Touhami A, Tseng SC. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. Br. J. Ophthalmol. 2003;87:1509–1514. doi: 10.1136/bjo.87.12.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. Embo J. 2000;19:1187–1194. doi: 10.1093/emboj/19.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001;280:C1358–C1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. History of angiogenesis. In: Figg WD, Folkman J, editors. Angiogenesis: an Integrative Approach from Science to Medicine. Springer; 2008. pp. 1–14. [Google Scholar]
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Fournier E, Blaikie P, Rosnet O, Margolis B, Birnbaum D, Borg JP. Role of tyrosine residues and protein interaction domains of SHC adaptor in VEGF receptor 3 signaling. Oncogene. 1999;18:507–514. doi: 10.1038/sj.onc.1202315. [DOI] [PubMed] [Google Scholar]
- Framme C, Flucke B, Birngruber R. Comparison of reduced and standard light application in photodynamic therapy of the eye in two rabbit models. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:773–781. doi: 10.1007/s00417-005-0221-2. [DOI] [PubMed] [Google Scholar]
- Friedenwald JS. Growth pressure and metaplasia of conjunctival and corneal epithelium. Doc. Ophthalmol. 1951;5–6:184–192. doi: 10.1007/BF00143661. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Friling R, Yassur Y, Levy R, Kost J, Schwartz B, Mikhailowsky R, Lamprecht SA. A role of transforming growth factor-beta 1 in the control of corneal neovascularization. In Vivo. 1996;10:59–64. [PubMed] [Google Scholar]
- Frucht J, Zauberman H. Topical indomethacin effect on neovascularisation of the cornea and on prostaglandin E2 levels. Br. J. Ophthalmol. 1984;68:656–659. doi: 10.1136/bjo.68.9.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Morifuji M, Katakura Y, Ohishi M, Nakamura S. Endostatin inhibits lymph node metastasis by a down-regulation of the vascular endothelial growth factor C expression in tumor cells. Clin. Exp. Metastasis. 2005;22:31–38. doi: 10.1007/s10585-005-3973-5. [DOI] [PubMed] [Google Scholar]
- Gabison E, Chang JH, Hernandez-Quintela E, Javier J, Lu PC, Ye H, Kure T, Kato T, Azar DT. Anti-angiogenic role of angiostatin during corneal wound healing. Exp. Eye Res. 2004;78(3):579–589. doi: 10.1016/j.exer.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Gagliano N, Moscheni C, Torri C, Magnani I, Bertelli AA, Gioia M. Effect of resveratrol on matrix metalloproteinase-2 (MMP-2) and secreted protein acidic and rich in cysteine (SPARC) on human cultured glioblastoma cells. Biomed. Pharmacother. 2005;59:359–364. doi: 10.1016/j.biopha.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol. Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Gerten G. Bevacizumab (avastin) and argon laser to treat neovascularization in corneal transplant surgery. Cornea. 2008;27:1195–1199. doi: 10.1097/ICO.0b013e318180e50f. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Jr., Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J. Natl. Cancer Inst. 1974;52:413–427. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Gingras D, Bousquet-Gagnon N, Langlois S, Lachambre MP, Annabi B, Beliveau R. Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP) FEBS Lett. 2001;507:231–236. doi: 10.1016/s0014-5793(01)02985-4. [DOI] [PubMed] [Google Scholar]
- Gipson IK. The epithelial basement membrane zone of the limbus. Eye. 1989;3(Pt 2):132–140. doi: 10.1038/eye.1989.21. [DOI] [PubMed] [Google Scholar]
- Gipson I, Joyce N, Zieske J. The anatomy and cell biology of the human cornea, limbus, conjunctiva. In: Foster SC, Azar DT, Dohlman CH, editors. Smolin and Thoft's the Cornea: Scientific Foundations and Clinical Practice. fourth ed. Lippincott Williams and Wilkins; 2005. pp. 1–35. [Google Scholar]
- Gipson IK, Joyce NC. Anatomy and cell biology of the cornea, superficial limbus and conjunctiva. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. WB Saunders; Philadelphia: 1999. pp. 612–629. [Google Scholar]
- Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmokel HG, Willey S, Hicklin DJ, Pytowski B, Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. Faseb J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- Gordon YJ, Mann RK, Mah TS, Gorin MB. Fluorescein-potentiated argon laser therapy improves symptoms and appearance of corneal neovascularization. Cornea. 2002;21:770–773. doi: 10.1097/00003226-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Goto T, Ishikawa H, Matsumoto K, Nishimura D, Kusaba M, Taura N, Shibata H, Miyaaki H, Ichikawa T, Hamasaki K, Nakao K, Maeshima Y, Eguchi K. Tum-1, a tumstatin fragment, gene delivery into hepatocellular carcinoma suppresses tumor growth through inhibiting angiogenesis. Int. J. Oncol. 2008;33:33–40. [PubMed] [Google Scholar]
- Graells J, Vinyals A, Figueras A, Llorens A, Moreno A, Marcoval J, Gonzalez FJ, Fabra A. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J. Invest. Dermatol. 2004;123:1151–1161. doi: 10.1111/j.0022-202X.2004.23460.x. [DOI] [PubMed] [Google Scholar]
- Grierson I, Heathcote L, Hiscott P, Hogg P, Briggs M, Hagan S. Hepatocyte growth factor/scatter factor in the eye. Prog. Retin. Eye Res. 2000;19:779–802. doi: 10.1016/s1350-9462(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog. Retin. Eye Res. 2008;27:372–390. doi: 10.1016/j.preteyeres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C, Perricaudet M, Yeh P, Lu H. Angiostatin gene transfer: inhibition of tumor growth in vivo by blockage of endothelial cell proliferation associated with a mitosis arrest. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6367–6372. doi: 10.1073/pnas.95.11.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv. Ophthalmol. 2003;48:631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Gu WZ, Tahir SK, Wang YC, Zhang HC, Cherian SP, O'Connor S, Leal JA, Rosenberg SH, Ng SC. Effect of novel CAAX peptidomimetic farnesyltransferase inhibitor on angiogenesis in vitro and in vivo. Eur. J. Cancer. 1999;35:1394–1401. doi: 10.1016/s0959-8049(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Guo D, Jia Q, Song HY, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. J. Biol. Chem. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- Ha CH, Jhun BS, Kao HY, Jin ZG. VEGF stimulates HDAC7 phosphorylation and cytoplasmic accumulation modulating matrix metalloproteinase expression and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1782–1788. doi: 10.1161/ATVBAHA.108.172528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajitou A, Grignet C, Devy L, Berndt S, Blacher S, Deroanne CF, Bajou K, Fong T, Chiang Y, Foidart JM, Noel A. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. Faseb J. 2002;16:1802–1804. doi: 10.1096/fj.02-0109fje. [DOI] [PubMed] [Google Scholar]
- Hamada K, Oike Y, Takakura N, Ito Y, Jussila L, Dumont DJ, Alitalo K, Suda T. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood. 2000;96:3793–3800. [PubMed] [Google Scholar]
- Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr. Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Hooper LC, Detrick B, Hooks JJ. HSV immune complex (HSV-IgG: IC) and HSV-DNA elicit the production of angiogenic factor VEGF and MMP-9. Arch. Virol. 2009;154:219–226. doi: 10.1007/s00705-008-0303-7. [DOI] [PubMed] [Google Scholar]
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim. Biophys. Acta. 2004a;1654:3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004b;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J. Exp. Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum. 1989;32:1065–1073. doi: 10.1002/anr.1780320903. [DOI] [PubMed] [Google Scholar]
- Holick MF, Uskokovic M, Henley JW, MacLaughlin J, Holick SA, Potts JT., Jr. The photoproduction of 1 alpha,25-dihydroxyvitamin D3 in skin: an approach to the therapy of vitamin-D-resistant syndromes. N. Engl. J. Med. 1980;303:349–354. doi: 10.1056/NEJM198008143030701. [DOI] [PubMed] [Google Scholar]
- Hosseini H, Nejabat M, Mehryar M, Yazdchi T, Sedaghat A, Noori F. Bevacizumab inhibits corneal neovascularization in an alkali burn induced model of corneal angiogenesis. Clin. Exp. Ophthalmol. 2007;35:745–748. doi: 10.1111/j.1442-9071.2007.01572.x. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- Hu L, Roth JM, Brooks P, Luty J, Karpatkin S. Thrombin up-regulates cathepsin D which enhances angiogenesis, growth, and metastasis. Cancer Res. 2008;68:4666–4673. doi: 10.1158/0008-5472.CAN-07-6276. [DOI] [PubMed] [Google Scholar]
- Huang AJ, Li DQ, Li CH, Shang TY, Hernandez E. Modulation of corneal vascularization. Ocul. Surf. 2005;3:S190–S193. doi: 10.1016/s1542-0124(12)70253-7. [DOI] [PubMed] [Google Scholar]
- Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest. Ophthalmol. Vis. Sci. 1991;32:96–105. [PubMed] [Google Scholar]
- Hunter J. Lectures on the principles of surgery. In: Palmer J, editor. The Works of John Hunter. 1787. [Google Scholar]
- Huynh-Do U, Vindis C, Liu H, Cerretti DP, McGrew JT, Enriquez M, Chen J, Daniel TO. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J. Cell Sci. 2002;115:3073–3081. doi: 10.1242/jcs.115.15.3073. [DOI] [PubMed] [Google Scholar]
- Ide A, Baker N, Warren S. Vascularization of the brown Pearce rabbit epithelioma transplant as seen in the transparent ear chamber. Am. J. Roentgenol. 1939;42:891–899. [Google Scholar]
- Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit. Rev. Biochem. Mol. Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- Jakobiec FA, Ozanics V. General topographic anatomy of the eye. In: Jakobiec FA, editor. Ocular Anatomy, Embryology, Teratology. Harper & Row; Philadelphia: 1982. [Google Scholar]
- Jeffers M, Shimkets R, Prayaga S, Boldog F, Yang M, Burgess C, Fernandes E, Rittman B, Shimkets J, LaRochelle WJ, Lichenstein HS. Identification of a novel human fibroblast growth factor and characterization of its role in oncogenesis. Cancer Res. 2001;61:3131–3138. [PubMed] [Google Scholar]
- Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K, Cha HJ, Schwendener RA, Jang KY, Kim KS, Alitalo K, Koh GY. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 2008;68:1100–1109. doi: 10.1158/0008-5472.CAN-07-2572. [DOI] [PubMed] [Google Scholar]
- Jia WD, Xu GL, Sun HC, Wang L, Xu RN, Xue Q. Effect of octreotide on angiogenesis induced by hepatocellular carcinoma in vivo. Hepatobiliary Pancreat. Dis. Int. 2003;2:404–409. [PubMed] [Google Scholar]
- Jin J, Guan M, Sima J, Gao G, Zhang M, Liu Z, Fant J, Ma JX. Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 2003;22:473–477. doi: 10.1097/00003226-200307000-00015. [DOI] [PubMed] [Google Scholar]
- Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am. J. Pathol. 2006;168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Kruse FE, Volcker HE, Kirchhof B. Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 1999;237:920–927. doi: 10.1007/s004170050387. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Shim JS, Suh YG, Kim YM, Ono M, Kwon HJ. Potent inhibition of in vivo angiogenesis and tumor growth by a novel cyclooxygenase-2 inhibitor, enoic acanthoic acid. Cancer Sci. 2007;98:1943–1948. doi: 10.1111/j.1349-7006.2007.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, Turetz J, Gutman H, Buch H, Brandeis R, Horwitz V, Solomon A, Amir A. Ocular injuries following sulfur mustard exposure-pathological mechanism and potential therapy. Toxicology. 2008 doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajanne R, Miettinen P, Mehlem A, Leivonen SK, Birrer M, Foschi M, Kahari VM, Leppa S. EGF-R regulates MMP function in fibroblasts through MAPK and AP-1 pathways. J. Cell Physiol. 2007;212:489–497. doi: 10.1002/jcp.21041. [DOI] [PubMed] [Google Scholar]
- Karakousis PC, John SK, Behling KC, Surace EM, Smith JE, Hendrickson A, Tang WX, Bennett J, Milam AH. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol. Vis. 2001;7:154–163. [PubMed] [Google Scholar]
- Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 2000;25:153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu. Rev. Pathol. 2008a;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Alitalo K. VEGF-D: a modifier of embryonic lymphangiogenesis. Blood. 2008b;112:1547–1548. doi: 10.1182/blood-2008-06-159343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Kure T, Chang JH, Gabison EE, Itoh T, Itohara S, Azar DT. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508:187–190. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- Kawashima M, Kawakita T, Satake Y, Higa K, Shimazaki J. Phenotypic study after cultivated limbal epithelial transplantation for limbal stem cell deficiency. Arch. Ophthalmol. 2007;125:1337–1344. doi: 10.1001/archopht.125.10.1337. [DOI] [PubMed] [Google Scholar]
- Kaye S, Choudhary A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Keay L, Radford C, Dart JK, Edwards K, Stapleton F. Perspective on 15 years of research: reduced risk of microbial keratitis with frequent-replacement contact lenses. Eye Contact Lens. 2007;33:167–168. doi: 10.1097/01.icl.0000248157.94115.7c. [DOI] [PubMed] [Google Scholar]
- Kenyon KR, Fogle JA, Stone DL, Stark WJ. Regeneration of corneal epithelial basement membrane following thermal cauterization. Invest. Ophthalmol. Vis. Sci. 1977;16:292–301. [PubMed] [Google Scholar]
- Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. discussion 722–703. [DOI] [PubMed] [Google Scholar]
- Keshavarz M, Mostafaie A, Mansouri K, Shakiba Y, Motlagh HR. Inhibition of corneal neovascularization with propolis extract. Arch. Med. Res. 2009;40:59–61. doi: 10.1016/j.arcmed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kheirkhah A, Johnson DA, Paranjpe DR, Raju VK, Casas V, Tseng SC. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch. Ophthalmol. 2008;126:1059–1066. doi: 10.1001/archopht.126.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Hwang S, Kim YM, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J. Biol. Chem. 2002;277:27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- Kim B, Lee S, Suvas S, Rouse BT. Application of plasmid DNA encoding IL-18 diminishes development of herpetic stromal keratitis by antiangiogenic effects. J. Immunol. 2005;175:509–516. doi: 10.4049/jimmunol.175.1.509. [DOI] [PubMed] [Google Scholar]
- Kim SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115:e33–e38. doi: 10.1016/j.ophtha.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–484. [PubMed] [Google Scholar]
- Kisker O, Onizuka S, Banyard J, Komiyama T, Becker CM, Achilles EG, Barnes CM, O'Reilly MS, Folkman J, Pirie-Shepherd SR. Generation of multiple angiogenesis inhibitors by human pancreatic cancer. Cancer Res. 2001;61:7298–7304. [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr. Opin. Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kojima T, Chang JH, Azar DT. Proangiogenic role of ephrinB1/EphB1 in basic fibroblast growth factor-induced corneal angiogenesis. Am. J. Pathol. 2007a;170(2):764–773. doi: 10.2353/ajpath.2007.060487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Chung TY, Chang JH, Sayegh R, Casanova FH, Azar DT. Comparison of EphA receptor tyrosine kinases and ephrinA ligand expression to EphB-ephrinB in vascularized corneas. Cornea. 2007b;26:569–578. doi: 10.1097/ICO.0b013e3180335526. [DOI] [PubMed] [Google Scholar]
- Kojima T, Azar DT, Chang JH. Neostatin-7 regulates bFGF-induced corneal lymphangiogenesis. FEBS Lett. 2008;582(17):2515–2520. doi: 10.1016/j.febslet.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y, Malik AB. FGF signaling preserves the integrity of endothelial adherens junctions. Dev. Cell. 2008;15:335–336. doi: 10.1016/j.devcel.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommineni VK, Nagineni CN, William A, Detrick B, Hooks JJ. IFN-gamma acts as anti-angiogenic cytokine in the human cornea by regulating the expression of VEGF-A and sVEGF-R1. Biochem. Biophys. Res. Commun. 2008;374:479–484. doi: 10.1016/j.bbrc.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse FE, Joussen AM, Rohrschneider K, Becker MD, Volcker HE. Thalidomide inhibits corneal angiogenesis induced by vascular endothelial growth factor. Graefes Arch. Clin. Exp. Ophthalmol. 1998;236:461–466. doi: 10.1007/s004170050106. [DOI] [PubMed] [Google Scholar]
- Kuhlmann CR, Most AK, Li F, Munz BM, Schaefer CA, Walther S, Raedle-Hurst T, Waldecker B, Piper HM, Tillmanns H, Wiecha J. Endothelin-1-induced proliferation of human endothelial cells depends on activation of K+channels and Ca+ influx. Acta Physiol. Scand. 2005;183:161–169. doi: 10.1111/j.1365-201X.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Kure T, Chang JH, Kato T, Hernandez-Quintela E, Ye H, Lu PC, Matrisian LM, Gatinel D, Shapiro S, Gosheh F, Azar DT. Corneal neovascularization after excimer keratectomy wounds in matrilysin-deficient mice. Invest. Ophthalmol. Vis. Sci. 2003;44:137–144. doi: 10.1167/iovs.01-1058. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Sarman S, Fagerholm P, Seregard S, Steen B. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Exp. Eye Res. 2000;70:419–428. doi: 10.1006/exer.1999.0790. [DOI] [PubMed] [Google Scholar]
- Kwitko S, Marinho D, Barcaro S, Bocaccio F, Rymer S, Fernandes S, Neumann J. Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology. 1995;102:1020–1025. doi: 10.1016/s0161-6420(95)30918-9. [DOI] [PubMed] [Google Scholar]
- Kwon YS, Hong HS, Kim JC, Shin JS, Son Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2005;46:454–460. doi: 10.1167/iovs.04-0753. [DOI] [PubMed] [Google Scholar]
- Kwon YS, Kim JC. Inhibition of corneal neovascularization by rapamycin. Exp. Mol. Med. 2006;38:173–179. doi: 10.1038/emm.2006.21. [DOI] [PubMed] [Google Scholar]
- Lahdenranta J, Hagendoorn J, Padera TP, Hoshida T, Nelson G, Kashiwagi S, Jain RK, Fukumura D. Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res. 2009;69:2801–2808. doi: 10.1158/0008-5472.CAN-08-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai LJ, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J. Biomed. Sci. 2007;14:313–322. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- Lamoreaux WJ, Fitzgerald ME, Reiner A, Hasty KA, Charles ST. Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc. Res. 1998;55:29–42. doi: 10.1006/mvre.1997.2056. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Mallet C, Feige JJ, Bailly S. Activin receptor-like kinase 1 is implicated in the maturation phase of angiogenesis. Blood. 2002;100:4495–4501. doi: 10.1182/blood.V100.13.4495. [DOI] [PubMed] [Google Scholar]
- Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263:797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv. Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int. J. Biochem. Cell Biol. 2001;33:421–426. doi: 10.1016/s1357-2725(01)00027-9. [DOI] [PubMed] [Google Scholar]
- Li DQ, Shang TY, Kim HS, Solomon A, Lokeshwar BL, Pflugfelder SC. Regulated expression of collagenases MMP-1, -8, and -13 and stromelysins MMP-3, -10, and -11 by human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2003;44:2928–2936. doi: 10.1167/iovs.02-0874. [DOI] [PubMed] [Google Scholar]
- Lijnen HR, Ugwu F, Bini A, Collen D. Generation of an angiostatin-like fragment from plasminogen by stromelysin-1 (MMP-3) Biochemistry. 1998;37:4699–4702. doi: 10.1021/bi9731798. [DOI] [PubMed] [Google Scholar]
- Lin HC, Chang JH, Jain S, Gabison EE, Kure T, Kato T, Fukai N, Azar DT. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest. Ophthalmol. Vis. Sci. 2001;42:2517–2524. [PubMed] [Google Scholar]
- Lohela M, Saaristo A, Veikkola T, Alitalo K. Lymphangiogenic growth factors, receptors and therapies. Thromb. Haemost. 2003;90:167–184. doi: 10.1160/TH03-04-0200. [DOI] [PubMed] [Google Scholar]
- Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- London NR, Whitehead KJ, Li DY. Endogenous endothelial cell signaling systems maintain vascular stability. Angiogenesis. 2009 doi: 10.1007/s10456-009-9130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PC, Ye H, Maeda M, Azar DT. Immunolocalization and gene expression of matrilysin during corneal wound healing. Invest. Ophthalmol. Vis. Sci. 1999;40:20–27. [PubMed] [Google Scholar]
- Luengo Gimeno F, Lavigne V, Gatto S, Croxatto JO, Correa L, Gallo JE. Advances in corneal stem-cell transplantation in rabbits with severe ocular alkali burns. J. Cataract Refract. Surg. 2007;33:1958–1965. doi: 10.1016/j.jcrs.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Ma X, Ottino P, Bazan HE, Bazan NG. Platelet-activating factor (PAF) induces corneal neovascularization and upregulates VEGF expression in endothelial cells. Invest. Ophthalmol. Vis. Sci. 2004;45:2915–2921. doi: 10.1167/iovs.04-0128. [DOI] [PubMed] [Google Scholar]
- Ma DH, Chen JK, Zhang F, Lin KY, Yao JY, Yu JS. Regulation of corneal angiogenesis in limbal stem cell deficiency. Prog. Retin. Eye Res. 2006;25:563–590. doi: 10.1016/j.preteyeres.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mackenzie SE, Tucker WR, Poole TR. Bevacizumab (avastin) for corneal neovascularization – corneal light shield soaked application. Cornea. 2009;28:246–247. doi: 10.1097/ICO.0b013e3181861cc9. [DOI] [PubMed] [Google Scholar]
- Maekawa H, Oike Y, Kanda S, Ito Y, Yamada Y, Kurihara H, Nagai R, Suda T. Ephrin-B2 induces migration of endothelial cells through the phosphatidylinositol-3 kinase pathway and promotes angiogenesis in adult vasculature. Arterioscler. Thromb. Vasc. Biol. 2003;23:2008–2014. doi: 10.1161/01.ATV.0000096655.56262.56. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Colorado PC, Kalluri R. Two RGD-independent alpha vbeta 3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J. Biol. Chem. 2000;275:23745–23750. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR, Kharbanda S, Kalluri R. Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. J. Biol. Chem. 2001a;276:15240–15248. doi: 10.1074/jbc.M007764200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Yerramalla UL, Dhanabal M, Holthaus KA, Barbashov S, Kharbanda S, Reimer C, Manfredi M, Dickerson WM, Kalluri R. Extracellular matrix-derived peptide binds to alpha(v)beta(3) integrin and inhibits angiogenesis. J. Biol. Chem. 2001b;276:31959–31968. doi: 10.1074/jbc.M103024200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Sudhakar A, Lively JC, Ueki K, Kharbanda S, Kahn CR, Sonenberg N, Hynes RO, Kalluri R. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science. 2002;295:140–143. doi: 10.1126/science.1065298. [DOI] [PubMed] [Google Scholar]
- Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, Griscelli F, Opolon P, Perricaudet M. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Res. 2005;65:4353–4361. doi: 10.1158/0008-5472.CAN-04-3536. [DOI] [PubMed] [Google Scholar]
- Magnon C, Opolon P, Ricard M, Connault E, Ardouin P, Galaup A, Metivier D, Bidart JM, Germain S, Perricaudet M, Schlumberger M. Radiation and inhibition of angiogenesis by canstatin synergize to induce HIF-1alpha-mediated tumor apoptotic switch. J. Clin. Invest. 2007;117:1844–1855. doi: 10.1172/JCI30269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguen E, Zorapapel NC, Zieske JD, Ninomiya Y, Sado Y, Kenney MC, Ljubimov AV. Extracellular matrix and matrix metalloproteinase changes in human corneas after complicated laser-assisted in situ keratomileusis (LASIK) Cornea. 2002;21:95–100. doi: 10.1097/00003226-200201000-00020. [DOI] [PubMed] [Google Scholar]
- Mahajan VB, Wei C, McDonnell PJ., 3rd Microarray analysis of corneal fibroblast gene expression after interleukin-1 treatment. Invest. Ophthalmol. Vis. Sci. 2002;43:2143–2151. [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Martin AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004;67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Mastyugin V, Mosaed S, Bonazzi A, Dunn MW, Schwartzman ML. Corneal epithelial VEGF and cytochrome P450 4B1 expression in a rabbit model of closed eye contact lens wear. Curr. Eye Res. 2001;23:1–10. doi: 10.1076/ceyr.23.1.1.5422. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, Ohashi R, Ishizaki M, Asano G, Sugisaki Y, Yamanaka N. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am. J. Pathol. 2001;159:599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 2008;14:5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Hirashima M, Ogawa M, Kubo H, Hisatsune H, Kondo N, Nishikawa S, Chiba T, Nishikawa S. Modulation of VEGFR-2-mediated endothelial-cell activity by VEGF-C/VEGFR-3. Blood. 2003;101:1367–1374. doi: 10.1182/blood-2002-05-1329. [DOI] [PubMed] [Google Scholar]
- McNatt LG, Weimer L, Yanni J, Clark AF. Angiostatic activity of steroids in the chick embryo CAM and rabbit cornea models of neovascularization. J. Ocul. Pharmacol. Ther. 1999;15:413–423. doi: 10.1089/jop.1999.15.413. [DOI] [PubMed] [Google Scholar]
- Meyer C, Notari L, Becerra SP. Mapping the type I collagen-binding site on pigment epithelium-derived factor. Implications for its antiangiogenic activity. J. Biol. Chem. 2002;277:45400–45407. doi: 10.1074/jbc.M208339200. [DOI] [PubMed] [Google Scholar]
- Mills RA, Jones DB, Winkler CR, Wallace GW, Wilhelmus KR. Topical FK-506 prevents experimental corneal allograft rejection. Cornea. 1995;14:157–160. [PubMed] [Google Scholar]
- Mimura T, Han KY, Onguchi T, Chang JH, Kim TI, Kojima T, Zhou Z, Azar DT. MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009;46:541–550. doi: 10.1159/000226222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, Eliseenkova AV, Green D, Schlessinger J, Hubbard SR. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. Embo J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montezuma SR, Vavvas D, Miller JW. Review of the ocular angiogenesis animal models. Semin. Ophthalmol. 2009;24:52–61. doi: 10.1080/08820530902800017. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Aoyagi M, Tamaki M, Yoshino Y, Hori H, Duan L, Yano T, Shibata M, Ohno K, Hirakawa K, Yamaguchi N. Increased levels of tissue endostatin in human malignant gliomas. Clin. Cancer Res. 2002;8:2933–2938. [PubMed] [Google Scholar]
- Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K, Suda T. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muether PS, Dell S, Kociok N, Zahn G, Stragies R, Vossmeyer D, Joussen AM. The role of integrin alpha5beta1 in the regulation of corneal neovascularization. Exp. Eye Res. 2007;85:356–365. doi: 10.1016/j.exer.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Munaut C, Noel A, Hougrand O, Foidart JM, Boniver J, Deprez M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int. J. Cancer. 2003;106:848–855. doi: 10.1002/ijc.11313. [DOI] [PubMed] [Google Scholar]
- Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc. Res. 2007;74:85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol. 2008;15:215–220. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Endo A, Ogita H, Kawana A, Yamagishi A, Kitabatake A, Matsuda M, Mochizuki N. Adaptor protein Crk is required for ephrin-B1-induced membrane ruffling and focal complex assembly of human aortic endothelial cells. Mol. Biol. Cell. 2002;13:4231–4242. doi: 10.1091/mbc.E02-04-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S, Hata Y, Miura M, Noda K, Kimura YN, Kawahara S, Kita T, Hisatomi T, Nakazawa T, Jin Y, Dana MR, Kuwano M, Ono M, Ishibashi T, Hafezi-Moghadam A. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am. J. Pathol. 2007;171:1058–1065. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaki R, Kuribayashi H, Shimizu K, Imamura D, Sato T, Hasumi K. Bacillolysin MA, a novel bacterial metalloproteinase that produces angiostatin-like fragments from plasminogen and activates protease zymogens in the coagulation and fibrinolysis systems. J. Biol. Chem. 2005;280:14278–14287. doi: 10.1074/jbc.M500241200. [DOI] [PubMed] [Google Scholar]
- Nespoli A, Vercillo R, di Nola L, Diani L, Giannattasio M, Plevani P, Muzi-Falconi M. Alk1 and Alk2 are two new cell cycle-regulated haspin-like proteins in budding yeast. Cell Cycle. 2006;5:1464–1471. doi: 10.4161/cc.5.13.2914. [DOI] [PubMed] [Google Scholar]
- Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am. J. Clin. Pathol. 2001a;116:838–845. doi: 10.1309/FXNL-QTN1-94FH-AB3A. [DOI] [PubMed] [Google Scholar]
- Ng YS, Rohan R, Sunday ME, Demello DE, D'Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev. Dyn. 2001b;220:112–121. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nomura S, Yoshitomi H, Takano S, Shida T, Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Miyazaki M. FGF10/FGFR2 signal induces cell migration and invasion in pancreatic cancer. Br. J. Cancer. 2008;99:305–313. doi: 10.1038/sj.bjc.6604473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby K. In vivo models of angiogenesis. J. Cell Mol. Med. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg P, Xie L, Sugimoto H, Colorado P, Sund M, Holthaus K, Sudhakar A, Salo T, Kalluri R. Characterization of the anti-angiogenic properties of arresten, an alpha1beta1 integrin-dependent collagen-derived tumor suppressor. Exp. Cell Res. 2008;314:3292–3305. doi: 10.1016/j.yexcr.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien TP, Li QJ, Sauerburger F, Reviglio VE, Rana T, Ashraf MF. The role of matrix metalloproteinases in ulcerative keratolysis associated with perioperative diclofenac use. Ophthalmology. 2001;108:656–659. doi: 10.1016/s0161-6420(00)00590-x. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Wilkinson DG. Eph receptors and ephrins in neural development. Curr. Opin. Neurobiol. 1999;9:65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Ni A, Ayeni OA, Baluk P, Yao LC, Vossmeyer D, Zischinsky G, Zahn G, Knolle J, Christner C, McDonald DM. {alpha}5{beta}1 integrin blockade inhibits lymphangiogenesis in airway inflammation. Am. J. Pathol. 2009 doi: 10.2353/ajpath.2009.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B, Jeltsch M, Eriksson U, Alitalo K. Current biology of VEGF-B and VEGF-C. Curr. Opin. Biotechnol. 1999;10:528–535. doi: 10.1016/s0958-1669(99)00024-5. [DOI] [PubMed] [Google Scholar]
- Onguchi T, Han KY, Chang JH, Azar DT. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am. J. Pathol. 2009;174(4):1564–1571. doi: 10.2353/ajpath.2009.080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Yokoo S, Mimura T, Usui T, Miyata K, Araie M, Yamagami S, Amano S. Autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane in a rabbit model. Mol. Vis. 2007;13:1138–1143. [PMC free article] [PubMed] [Google Scholar]
- Otasevic L, Gong N, Ritter T, Mergler S, Pleyer U. Effects of spironolactone on corneal allograft survival in the rat. Ophthalmic Res. 2007;39:325–329. doi: 10.1159/000109988. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A. Remodeling the model organism: matrix metalloproteinase functions in invertebrates. Semin. Cell Dev. Biol. 2008;19:14–23. doi: 10.1016/j.semcdb.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajusola K, Aprelikova O, Armstrong E, Morris S, Alitalo K. Two human FLT4 receptor tyrosine kinase isoforms with distinct carboxy terminal tails are produced by alternative processing of primary transcripts. Oncogene. 1993;8:2931–2937. [PubMed] [Google Scholar]
- Pakneshan P, Birsner AE, Adini I, Becker CM, D'Amato RJ. Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs. Invest. Ophthalmol. Vis. Sci. 2008;49:3909–3913. doi: 10.1167/iovs.07-1527. [DOI] [PubMed] [Google Scholar]
- Pal S, Datta K, Mukhopadhyay D. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 2001;61:6952–6957. [PubMed] [Google Scholar]
- Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnes CM, Fannon M, Laforme AM, Chaponis DM, Folkman J, Kieran MW. PPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc. Natl. Acad. Sci. U. S. A. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- Pasco S, Brassart B, Ramont L, Maquart FX, Monboisse JC. Control of melanoma cell invasion by type IV collagen. Cancer Detect. Prev. 2005;29:260–266. doi: 10.1016/j.cdp.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Patel SP, Dana R. Corneal lymphangiogenesis: implications in immunity. Semin. Ophthalmol. 2009;24:135–138. doi: 10.1080/08820530902801320. [DOI] [PubMed] [Google Scholar]
- Patterson BC, Sang QA. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9) J. Biol. Chem. 1997;272:28823–28825. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS. Extracellular proteolysis and angiogenesis. Thromb. Haemost. 2001;86:346–355. [PubMed] [Google Scholar]
- Perri SR, Nalbantoglu J, Annabi B, Koty Z, Lejeune L, Francois M, Di Falco MR, Beliveau R, Galipeau J. Plasminogen kringle 5-engineered glioma cells block migration of tumor-associated macrophages and suppress tumor vascularization and progression. Cancer Res. 2005;65:8359–8365. doi: 10.1158/0008-5472.CAN-05-0508. [DOI] [PubMed] [Google Scholar]
- Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest. Ophthalmol. Vis. Sci. 2000;41:2148–2153. [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Pollina EA, Legesse-Miller A, Haley EM, Goodpaster T, Randolph-Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7:2056–2070. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverini PJ. The pathophysiology of angiogenesis. Crit. Rev. Oral Biol. Med. 1995;6:230–247. doi: 10.1177/10454411950060030501. [DOI] [PubMed] [Google Scholar]
- Poulaki V, Mitsiades N, Kruse FE, Radetzky S, Iliaki E, Kirchhof B, Joussen AM. Activin a in the regulation of corneal neovascularization and vascular endothelial growth factor expression. Am. J. Pathol. 2004;164:1293–1302. doi: 10.1016/S0002-9440(10)63216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J. Natl. Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- Qian CX, Bahar I, Levinger E, Rootman D. Combined use of superficial keratectomy and subconjunctival bevacizumab injection for corneal neovascularization. Cornea. 2008;27:1090–1092. doi: 10.1097/ICO.0b013e31817c41e3. [DOI] [PubMed] [Google Scholar]
- Ramchandran R, Dhanabal M, Volk R, Waterman MJ, Segal M, Lu H, Knebelmann B, Sukhatme VP. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem. Biophys. Res. Commun. 1999;255:735–739. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Corsa AC, Kudravi SA, McCormick RS, Arthur WT. A deficit in collagenase activity contributes to impaired migration of aged microvascular endothelial cells. J. Cell Biochem. 2000;77:116–126. [PubMed] [Google Scholar]
- Regenfuss B, Bock F, Parthasarathy A, Cursiefen C. Corneal (lymph) angiogenesis – from bedside to bench and back: a tribute to Judah Folkman. Lymphat. Res. Biol. 2008;6:191–201. doi: 10.1089/lrb.2008.6348. [DOI] [PubMed] [Google Scholar]
- Riachy R, Vandewalle B, Moerman E, Belaich S, Lukowiak B, Gmyr V, Muharram G, Kerr Conte J, Pattou F. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. Apoptosis. 2006;11:151–159. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholova I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Yla-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ. Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- Rogers MS, Birsner AE, D'Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat. Protoc. 2007;2:2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
- Ruegg C, Mariotti A. Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cell Mol. Life Sci. 2003;60:1135–1157. doi: 10.1007/s00018-003-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R, Sathe S, Beaton AR, McNamara N, Fleiszig S, Ni M. Protein array characterization of bioactive proteins secreted by immortalized human corneal epithelium in response to pseudomonas constituents. Curr. Eye Res. 2009;34:92–98. doi: 10.1080/02713680802539869. [DOI] [PubMed] [Google Scholar]
- Saghizadeh M, Brown DJ, Castellon R, Chwa M, Huang GH, Ljubimova JY, Rosenberg S, Spirin KS, Stolitenko RB, Adachi W, Kinoshita S, Murphy G, Windsor LJ, Kenney MC, Ljubimov AV. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: a possible mechanism of basement membrane and integrin alterations. Am. J. Pathol. 2001;158:723–734. doi: 10.1016/S0002-9440(10)64015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S. Yin and yang in cytokine regulation of corneal wound healing: roles of TNF-alpha. Cornea. 2007;26:S70–S74. doi: 10.1097/ICO.0b013e31812f6d14. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Okada Y, Miyamoto T, Kitano A, Flanders KC, Ohnishi Y, Nakajima Y, Kao WW, Ikeda K. Effect of overexpression of PPARgamma on the healing process of corneal alkali burn in mice. Am. J. Physiol. Cell Physiol. 2007;293:C75–C86. doi: 10.1152/ajpcell.00332.2006. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Ueno H, Sonoda K, Hisatomi T, Shimizu K, Ohashi H, Inomata H. Blockade of TGF-beta by in vivo gene transfer of a soluble TGF-beta type II receptor in the muscle inhibits corneal opacification, edema and angiogenesis. Gene Ther. 2000;7:1915–1924. doi: 10.1038/sj.gt.3301320. [DOI] [PubMed] [Google Scholar]
- Schedin-Weiss S, Richard B, Hjelm R, Olson ST. Antiangiogenic forms of antithrombin specifically bind to the anticoagulant heparin sequence. Biochemistry. 2008;47:13610–13619. doi: 10.1021/bi801656u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeppi JM, Siemeister G, Weindel K, Schnell C, Wood J. Characterization of a new potent, in vivo neutralizing monoclonal antibody to human vascular endothelial growth factor. J. Cancer Res. Clin. Oncol. 1999;125:336–342. doi: 10.1007/s004320050283. [DOI] [PubMed] [Google Scholar]
- Schneider M, Buchler P, Giese N, Giese T, Wilting J, Buchler MW, Friess H. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int. J. Oncol. 2006;28:883–890. [PubMed] [Google Scholar]
- Schneller M, Vuori K, Ruoslahti E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. Embo J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr E, Sunderkötter C, Schaefer L, Thanos S, Grässel S, Oldberg A, Iozzo RV, Young MF, Kresse H. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc. Res. 2004;41:499–508. doi: 10.1159/000081806. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer Res. 1990;10:1307–1311. [PubMed] [Google Scholar]
- Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ. Res. 2003;93:682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- Seki T, Hong KH, Yun J, Kim SJ, Oh SP. Isolation of a regulatory region of activin receptor-like kinase 1 gene sufficient for arterial endothelium-specific expression. Circ. Res. 2004;94:e72–e77. doi: 10.1161/01.RES.0000127048.81744.31. [DOI] [PubMed] [Google Scholar]
- Sellami D, Abid S, Bouaouaja G, Ben Amor S, Kammoun B, Masmoudi M, Dabbeche K, Boumoud H, Ben Zina Z, Feki J. Epidemiology and risk factors for corneal graft rejection. Transplant. Proc. 2007;39:2609–2611. doi: 10.1016/j.transproceed.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Sen T, Moulik S, Dutta A, Choudhury PR, Banerji A, Das S, Roy M, Chatterjee A. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009;84:194–204. doi: 10.1016/j.lfs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Shao XJ, Chi XY. Influence of angiostatin and thalidomide on lymphangiogenesis. Lymphology. 2005;38:146–155. [PubMed] [Google Scholar]
- Shen J, Vil MD, Zhang H, Tonra JR, Rong LL, Damoci C, Prewett M, Deevi DS, Kearney J, Surguladze D, Jimenez X, Iacolina M, Bassi R, Zhou K, Balderes P, Mangalampalli VR, Loizos N, Ludwig DL, Zhu Z. An antibody directed against PDGF receptor beta enhances the antitumor and the anti-angiogenic activities of an anti-VEGF receptor 2 antibody. Biochem. Biophys. Res. Commun. 2007;357:1142–1147. doi: 10.1016/j.bbrc.2007.04.075. [DOI] [PubMed] [Google Scholar]
- Shepard SR, Boucher R, Johnston J, Boerner R, Koch G, Madsen JW, Grella D, Sim BK, Schrimsher JL. Large-scale purification of recombinant human angiostatin. Protein Expr. Purif. 2000;20:216–227. doi: 10.1006/prep.2000.1276. [DOI] [PubMed] [Google Scholar]
- Shi YH, Wang YX, Bingle L, Gong LH, Heng WJ, Li Y, Fang WG. In vitro study of HIF-1 activation and VEGF release by bFGF in the T47D breast cancer cell line under normoxic conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. J. Pathol. 2005;205:530–536. doi: 10.1002/path.1734. [DOI] [PubMed] [Google Scholar]
- Shi W, Gao H, Xie L, Wang S. Sustained intraocular rapamycin delivery effectively prevents high-risk corneal allograft rejection and neovascularization in rabbits. Invest. Ophthalmol. Vis. Sci. 2006;47:3339–3344. doi: 10.1167/iovs.05-1425. [DOI] [PubMed] [Google Scholar]
- Shima DT, Kuroki M, Deutsch U, Ng YS, Adamis AP, D'Amore PA. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J. Biol. Chem. 1996;271:3877–3883. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- Shin SH, Kim JC, Chang SI, Lee H, Chung SI. Recombinant kringle 1–3 of plasminogen inhibits rabbit corneal angiogenesis induced by angiogenin. Cornea. 2000;19:212–217. doi: 10.1097/00003226-200003000-00016. [DOI] [PubMed] [Google Scholar]
- Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005;24:27–34. doi: 10.1016/j.matbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Sonmez B, Beden U, Erkan D. Regression of severe corneal stromal neovascularization with topical cyclosporine 0.05% after penetrating keratoplasty for fungal corneal ulcer. Int. Ophthalmol. 2007 doi: 10.1007/s10792-007-9180-4. [DOI] [PubMed] [Google Scholar]
- Soubrane G, Jerdan J, Karpouzas I, Fayein NA, Glaser B, Coscas G, Courtois Y, Jeanny JC. Binding of basic fibroblast growth factor to normal and neovascularized rabbit cornea. Invest. Ophthalmol. Vis. Sci. 1990;31:323–333. [PubMed] [Google Scholar]
- Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM, Noel A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. Faseb J. 2002;16:555–564. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Janssen M, Foidart JM, Noel A. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix Biol. 2003;22:55–61. doi: 10.1016/s0945-053x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Roghi C, Chabottaux V, Janssen M, Munaut C, Maquoi E, Galvez BG, Gilles C, Frankenne F, Murphy G, Foidart JM, Noel A. Up-regulation of vascular endothelial growth factor-A by active membrane-type 1 matrix metalloproteinase through activation of Src-tyrosine kinases. J. Biol. Chem. 2004;279:13564–13574. doi: 10.1074/jbc.M307688200. [DOI] [PubMed] [Google Scholar]
- Spinella F, Garrafa E, Di Castro V, Rosano L, Nicotra MR, Caruso A, Natali PG, Bagnato A. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69:2669–2676. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- Srinivasan BD, Kulkarni PS. Inhibitors of the arachidonic acid cascade in the management of ocular inflammation. Prog. Clin. Biol. Res. 1989;312:229–249. [PubMed] [Google Scholar]
- Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, Daniel TO. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou A. Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J. Cell Mol. Med. 2004;8:519–525. doi: 10.1111/j.1582-4934.2004.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar A, Boosani CS. Inhibition of tumor angiogenesis by tumstatin: insights into signaling mechanisms and implications in cancer regression. Pharm. Res. 2008;25:2731–2739. doi: 10.1007/s11095-008-9634-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, Cosgrove D, Kalluri R. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J. Clin. Invest. 2005;115:2801–2810. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sugihara T, Wadhwa R, Kaul SC, Mitsui Y. A novel alternatively spliced form of murine vascular endothelial growth factor, VEGF 115. J. Biol. Chem. 1998;273:3033–3038. doi: 10.1074/jbc.273.5.3033. [DOI] [PubMed] [Google Scholar]
- Sugisaki K, Usui T, Nishiyama N, Jang WD, Yanagi Y, Yamagami S, Amano S, Kataoka K. Photodynamic therapy for corneal neovascularization using polymeric micelles encapsulating dendrimer porphyrins. Invest. Ophthalmol. Vis. Sci. 2008;49:894–899. doi: 10.1167/iovs.07-0389. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang DA, Jain RK, Carie A, Paquette S, Ennis E, Blaskovich MA, Baldini L, Coppola D, Hamilton AD, Sebti SM. Inhibiting angiogenesis and tumorigenesis by a synthetic molecule that blocks binding of both VEGF and PDGF to their receptors. Oncogene. 2005;24:4701–4709. doi: 10.1038/sj.onc.1208391. [DOI] [PubMed] [Google Scholar]
- Sund M, Hamano Y, Sugimoto H, Sudhakar A, Soubasakos M, Yerramalla U, Benjamin LE, Lawler J, Kieran M, Shah A, Kalluri R. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2934–2939. doi: 10.1073/pnas.0500180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sano Y, Kinoshita S. Effects of 1alpha,25-dihydroxyvitamin D3 on Langerhans cell migration and corneal neovascularization in mice. Invest. Ophthalmol. Vis. Sci. 2000a;41:154–158. [PubMed] [Google Scholar]
- Suzuki T, Sano Y, Sotozono C, Kinoshita S. Regulatory effects of 1alpha,25-dihydroxyvitamin D(3) on cytokine production by human corneal epithelial cells. Curr. Eye Res. 2000b;20:127–130. [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P, Klinghoffer RA. Growth factor signaling pathways in vascular development. Oncogene. 1999;18:7917–7932. doi: 10.1038/sj.onc.1203216. [DOI] [PubMed] [Google Scholar]
- Tammela T, Petrova TV, Alitalo K. Molecular lymphangiogenesis: new players. Trends Cell Biol. 2005a;15:434–441. doi: 10.1016/j.tcb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005b;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- Tao Y, Bazan HE, Bazan NG. Platelet-activating factor induces the expression of metalloproteinases-1 and -9, but not -2 or -3, in the corneal epithelium. Invest. Ophthalmol. Vis. Sci. 1995;36:345–354. [PubMed] [Google Scholar]
- Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J. Biol. Chem. 2001;276:39562–39568. doi: 10.1074/jbc.M101815200. [DOI] [PubMed] [Google Scholar]
- Tawe W, Pearlman E, Unnasch TR, Lustigman S. Angiogenic activity of Onchocerca volvulus recombinant proteins similar to vespid venom antigen 5. Mol. Biochem. Parasitol. 2000;109:91–99. doi: 10.1016/s0166-6851(00)00231-0. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem. Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis. 2009 doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Halliday NL, Updike DL, Ahern-Moore JS, Vu TK, Liu RW, Howard EW. Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. J. Biol. Chem. 1997;272:7482–7487. doi: 10.1074/jbc.272.11.7482. [DOI] [PubMed] [Google Scholar]
- Tomono Y, Naito I, Ando K, Yonezawa T, Sado Y, Hirakawa S, Arata J, Okigaki T, Ninomiya Y. Epitope-defined monoclonal antibodies against multiplexin collagens demonstrate that type XV and XVIII collagens are expressed in specialized basement membranes. Cell Struct. Funct. 2002;27:9–20. doi: 10.1247/csf.27.9. [DOI] [PubMed] [Google Scholar]
- Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389–400. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3(Pt 2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- Turner AN, Rees AJ. Goodpasture's disease and Alport's syndromes. Annu. Rev. Med. 1996;47:377–386. doi: 10.1146/annurev.med.47.1.377. [DOI] [PubMed] [Google Scholar]
- Udayakumar TS, Nagle RB, Bowden GT. Fibroblast growth factor-1 transcriptionally induces membrane type-1 matrix metalloproteinase expression in prostate carcinoma cell line. Prostate. 2004;58:66–75. doi: 10.1002/pros.10293. [DOI] [PubMed] [Google Scholar]
- Ueda T, Ueda T, Fukuda S, Browne R, Jenis E, Spengler R, Chou R, Buch P, Aljada A, Dandona P, Sasisekharan R, Dorey CK, Armstrong D. Lipid hydroperoxide-induced tumor necrosis factor (TNF)-alpha, vascular endothelial growth factor and neovascularization in the rabbit cornea: effect of TNF inhibition. Angiogenesis. 1998;1:174–184. doi: 10.1023/A:1018377621102. [DOI] [PubMed] [Google Scholar]
- Ueda E, Ozerdem U, Chen YH, Yao M, Huang KT, Sun H, Martins-Green M, Bartolini P, Walker AM. A molecular mimic demonstrates that phosphorylated human prolactin is a potent anti-angiogenic hormone. Endocr. Relat. Cancer. 2006;13:95–111. doi: 10.1677/erc.1.01076. [DOI] [PubMed] [Google Scholar]
- Uy HS, Chan PS, Ang RE. Topical bevacizumab and ocular surface neovascularization in patients with Stevens–Johnson syndrome. Cornea. 2008;27:70–73. doi: 10.1097/ICO.0b013e318158f6ad. [DOI] [PubMed] [Google Scholar]
- Van Buskirk EM. The anatomy of the limbus. Eye. 1989;3(Pt 2):101–108. doi: 10.1038/eye.1989.16. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J. Biol. Chem. 1999;274:23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, Sheppard D. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. J. Biol. Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- Vogten JM, Reijerkerk A, Meijers JC, Voest EE, Borel Rinkes IH, Gebbink MF. The role of the fibrinolytic system in corneal angiogenesis. Angiogenesis. 2003;6:311–316. doi: 10.1023/B:AGEN.0000029414.24060.fe. [DOI] [PubMed] [Google Scholar]
- Wallace GR, John Curnow S, Wloka K, Salmon M, Murray PI. The role of chemokines and their receptors in ocular disease. Prog. Retin. Eye Res. 2004;23:435–448. doi: 10.1016/j.preteyeres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang JF, Zhang XF, Groopman JE. Stimulation of beta 1 integrin induces tyrosine phosphorylation of vascular endothelial growth factor receptor-3 and modulates cell migration. J. Biol. Chem. 2001;276:41950–41957. doi: 10.1074/jbc.M101370200. [DOI] [PubMed] [Google Scholar]
- Wang K, Mahalingam G, Hoover SE, Mont EK, Holland SM, Cohen JI, Straus SE. Diverse herpes simplex virus type 1 thymidine kinase mutants in individual human neurons and ganglia. J. Virol. 2007;81:6817–6826. doi: 10.1128/JVI.00166-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Lindquist JN, Barnes LA, Lutu-Fuga KM, Cui J, Wood MR, Cheresh DA. Cooperation between VEGF and beta3 integrin during cardiac vascular development. Blood. 2007;109:1962–1970. doi: 10.1182/blood-2005-10-038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Moses MA, Wiederschain D, Arbiser JL, Folkman J. The generation of endostatin is mediated by elastase. Cancer Res. 1999;59:6052–6056. [PubMed] [Google Scholar]
- Wertheim MS, Cook SD, Knox-Cartwright NE, Van DL, Tole DM. Electrolysis-needle cauterization of corneal vessels in patients with lipid keratopathy. Cornea. 2007;26:230–231. doi: 10.1097/01.ico.0000248383.09272.ee. [DOI] [PubMed] [Google Scholar]
- Wheeler-Jones C, Abu-Ghazaleh R, Cospedal R, Houliston RA, Martin J, Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997;420:28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- Whitehurst B, Flister MJ, Bagaitkar J, Volk L, Bivens CM, Pickett B, Castro-Rivera E, Brekken RA, Gerard RD, Ran S. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int. J. Cancer. 2007;121:2181–2191. doi: 10.1002/ijc.22937. [DOI] [PubMed] [Google Scholar]
- WHO [(accessed 2.05.09)];Trachoma. 2009 http://wwwwhoint/blindness/causes/trachoma/en/indexhtml.
- Wiberg C, Hedbom E, Khairullina A, Lamande SR, Oldberg A, Timpl R, Morgelin M, Heinegard D. Biglycan and decorin bind close to the n-terminal region of the collagen VI triple helix. J. Biol. Chem. 2001;276:18947–18952. doi: 10.1074/jbc.M100625200. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. Embo J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Woodall BP, Nystrom A, Iozzo RA, Eble JA, Niland S, Krieg T, Eckes B, Pozzi A, Iozzo RV. Integrin alpha2beta1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem. 2008;283:2335–2343. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- Wu PC, Yang LC, Kuo HK, Huang CC, Tsai CL, Lin PR, Wu PC, Shin SJ, Tai MH. Inhibition of corneal angiogenesis by local application of vasostatin. Mol. Vis. 2005;11:28–35. [PubMed] [Google Scholar]
- Wu PC, Kuo HK, Tai MH, Shin SJ. Topical bevacizumab eyedrops for limbal–conjunctival neovascularization in impending recurrent pterygium. Cornea. 2009;28:103–104. doi: 10.1097/ICO.0b013e3181822615. [DOI] [PubMed] [Google Scholar]
- Wulfing P, Kersting C, Buerger H, Mattsson B, Mesters R, Gustmann C, Hinrichs B, Tio J, Bocker W, Kiesel L. Expression patterns of angiogenic and lymphangiogenic factors in ductal breast carcinoma in situ. Br. J. Cancer. 2005;92:1720–1728. doi: 10.1038/sj.bjc.6602567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Palmsten K, MacDonald B, Kieran MW, Potenta S, Vong S, Kalluri R. Basement membrane derived fibulin-1 and fibulin-5 function as angiogenesis inhibitors and suppress tumor growth. Exp. Biol. Med. (Maywood) 2008;233:155–162. doi: 10.3181/0706-RM-167. [DOI] [PubMed] [Google Scholar]
- Xue ML, Zhu H, Thakur A, Willcox M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol. Cell Biol. 2002;80:340–345. doi: 10.1046/j.1440-1711.80.4august.1.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Kawai M, Kawai Y, Mashima Y. The effect of selective cyclooxygenase-2 inhibitor on corneal angiogenesis in the rat. Curr. Eye Res. 1999;19:300–304. doi: 10.1076/ceyr.19.4.300.5301. [DOI] [PubMed] [Google Scholar]
- Yamada N, Yanai R, Kawamoto K, Nagano T, Nakamura M, Inui M, Nishida T. Promotion of corneal epithelial wound healing by a tetrapeptide (SSSR) derived from IGF-1. Invest. Ophthalmol. Vis. Sci. 2006;47:3286–3292. doi: 10.1167/iovs.05-1205. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Terada N, Nishizawa Y, Petrow V. Angiostatic activities of medroxyprogesterone acetate and its analogues. Int. J. Cancer. 1994;56:393–399. doi: 10.1002/ijc.2910560318. [DOI] [PubMed] [Google Scholar]
- Yan W, Bentley B, Shao R. Distinct angiogenic mediators are required for basic fibroblast growth factor – and vascular endothelial growth factor – induced angiogenesis: the role of cytoplasmic tyrosine kinase c-Abl in tumor angiogenesis. Mol. Biol. Cell. 2008;19:2278–2288. doi: 10.1091/mbc.E07-10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Ye HQ, Azar DT. Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound healing. Invest. Ophthalmol. Vis. Sci. 1998;39:913–921. [PubMed] [Google Scholar]
- Ye HQ, Maeda M, Yu FS, Azar DT. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest. Ophthalmol. Vis. Sci. 2000;41:2894–2899. [PubMed] [Google Scholar]
- Yoeruek E, Ziemssen F, Henke-Fahle S, Tatar O, Tura A, Grisanti S, Bartz-Schmidt KU, Szurman P. Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008;86:322–328. doi: 10.1111/j.1600-0420.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- Yoon KC, Ahn KY, Lee SE, Kim KK, Im SK, Oh HJ, Jeong IY, Park SW, Park YG, Nah HJ, Im WB. Experimental inhibition of corneal neovascularization by photodynamic therapy with verteporfin. Curr. Eye Res. 2006;31:215–224. doi: 10.1080/02713680600559564. [DOI] [PubMed] [Google Scholar]
- Yoon KC, You IC, Kang IS, Im SK, Ahn JK, Park YG, Ahn KY. Photodynamic therapy with verteporfin for corneal neovascularization. Am. J. Ophthalmol. 2007;144:390–395. doi: 10.1016/j.ajo.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Yoshida A, Matsui H, Takada Y, Ishibashi T. Involvement of macrophage chemotactic protein-1 and interleukin-1beta during inflammatory but not basic fibroblast growth factor-dependent neovascularization in the mouse cornea. Lab. Invest. 2003;83:927–938. doi: 10.1097/01.lab.0000075642.11787.83. [DOI] [PubMed] [Google Scholar]
- Yu CQ, Zhang M, Matis KI, Kim C, Rosenblatt MI. Vascular endothelial growth factor mediates corneal nerve repair. Invest. Ophthalmol. Vis. Sci. 2008;49:3870–3878. doi: 10.1167/iovs.07-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawieja D. Lymphatic biology and the microcirculation: past, present and future. Microcirculation. 2005;12:141–150. doi: 10.1080/10739680590900003. [DOI] [PubMed] [Google Scholar]
- Zeng L, Xiao Q, Margariti A, Zhang Z, Zampetaki A, Patel S, Capogrossi MC, Hu Y, Xu Q. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J. Cell Biol. 2006;174:1059–1069. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, Scott PA, Morbidelli L, Peak S, Moore J, Turley H, Harris AL, Ziche M, Bicknell R. The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br. J. Cancer. 2000;83:63–68. doi: 10.1054/bjoc.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li C, Baciu PC. Expression of integrins and MMPs during alkaline-burn-induced corneal angiogenesis. Invest. Ophthalmol. Vis. Sci. 2002a;43:955–962. [PubMed] [Google Scholar]
- Zhang W, Ran S, Sambade M, Huang X, Thorpe PE. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis. 2002b;5:35–44. doi: 10.1023/a:1021540120521. [DOI] [PubMed] [Google Scholar]
- Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]