Abstract
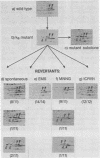
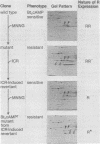
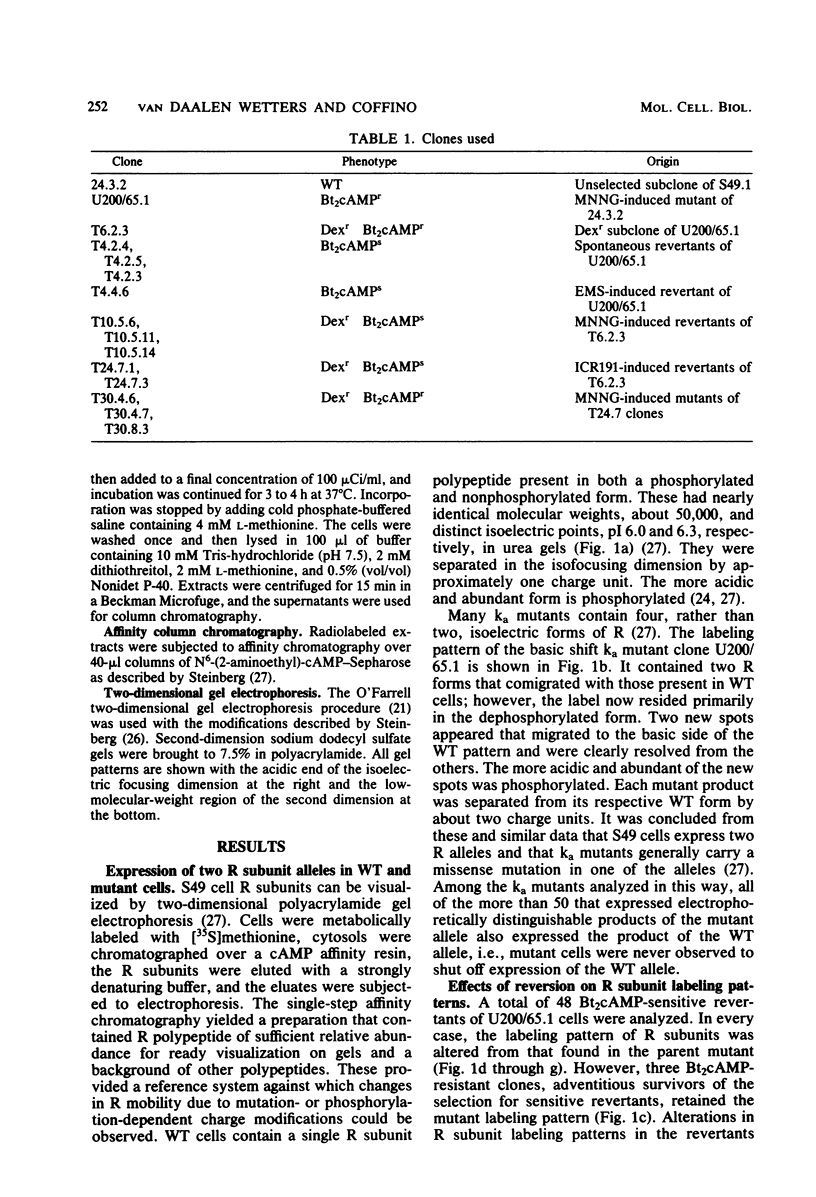
The regulatory subunits of cyclic AMP (cAMP)-dependent protein kinase from a dibutyryl cAMP-resistant S49 mouse lymphoma cell mutant, clone U200/65.1, and its revertants were visualized by two-dimensional polyacrylamide gel electrophoresis. Clone U200/65.1 co-expressed electrophoretically distinguishable mutant and wild-type subunits (Steinberg et al., Cell 10:381-391, 1977). In all 48 clones examined, reversion of the mutant to dibutyryl cAMP sensitivity was accompanied by alterations in regulatory subunit labeling patterns. Some spontaneous (3 of 11) and N-methyl-N'-nitro-N-nitrosoguanidine-induced (2 of 11) revertants retained mutant subunits, but these were altered in charge, degree of phosphorylation, or both. The charge alterations were consistent with single amino acid substitutions, suggesting that reversion was the result of second-site mutations in the mutant regulatory subunit allele that restored wild-type function, although not wild-type structure, to the gene product. The majority of spontaneous (8 of 11) and N-methyl-N'-nitro-N-nitrosoguanidine-induced (9 of 11) revertants and all of the revertants induced by ethyl methane sulfonate (14 of 14) and ICR191 (12 of 12) displayed only wild-type subunits. Dibutyryl cAMP-resistant mutants isolated from several of these revertants displayed new mutant but not wild-type subunits, suggesting that the revertant parent expresses only a single, functional regulatory subunit allele. The mutant regulatory subunit allele can, therefore, be modified in two general ways to produce revertant phenotypes: (i) by mutations that restore its wild-type function, and (ii) by mutations that eliminate its function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Whitfield H. J., Jr Frameshift mutagenesis in Salmonella. Cold Spring Harb Symp Quant Biol. 1966;31:221–225. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- Ayusawa D., Iwata K., Seno T., Koyama H. Conditional thymidine auxotrophic mutants of mouse FM3A cells due to thermosensitive thymidylate synthase and their prototrophic revertants. J Biol Chem. 1981 Dec 10;256(23):12005–12012. [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Friedrich U., Coffino P. Mutagenesis in S49 mouse lymphoma cells: induction of resistance to ouabain, 6-thioguanine, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1977 Feb;74(2):679–683. doi: 10.1073/pnas.74.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Genetic markers for quantitative mutagenesis studies in Chinese hamster ovary cells: characteristics of some recently developed selective systems. Mutat Res. 1980 Jan;69(1):113–126. doi: 10.1016/0027-5107(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Hochman J., Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. A structural gene mutation affecting the regulatory subunit of cyclic AMP-dependent protein kinase in mouse lymphoma cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5051–5055. doi: 10.1073/pnas.72.12.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Ingles C. J. Temperature-sensitive RNA polymerase II mutations in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):405–409. doi: 10.1073/pnas.75.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science. 1975 Nov 28;190(4217):896–898. doi: 10.1126/science.171770. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kruh G. D., Fenwick R. G., Jr, Caskey C. T. Structural analysis of mutant and revertant forms of Chinese hamster hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1981 Mar 25;256(6):2878–2886. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemaire I., Coffino P. Coexpression of mutant and wild type protein kinase in lymphoma cells resistant to dibutyryl cyclic AMP. J Cell Physiol. 1977 Sep;92(3):437–445. doi: 10.1002/jcp.1040920311. [DOI] [PubMed] [Google Scholar]
- MacInnes M. A., Friedrich U., van Daalen Wetters T., Coffino P. Quantitative forward-mutation specificity of mono-functional alkylating agents, ICR-191, and aflatoxin B1 in mouse lymphoma cells. Mutat Res. 1982 Aug;95(2-3):297–311. doi: 10.1016/0027-5107(82)90266-4. [DOI] [PubMed] [Google Scholar]
- Milman G., Krauss S. W., Olsen A. S. Tryptic peptide analysis of normal and mutant forms of hypoxanthine phosphoribosyltransferase from HeLa cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):926–930. doi: 10.1073/pnas.74.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G., Lee E., Ghangas G. S., McLaughlin J. R., George M., Jr Analysis of HeLa cell hypoxanthine phosphoribosyltransferase mutants and revertants by two-dimensional polyacrylamide gel electrophoresis: evidence for silent gene activation. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4589–4593. doi: 10.1073/pnas.73.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Agard D. A. Studies on the phosphorylation and synthesis of type I regulatory subunit of cyclic AMP-dependent protein kinase in intact S49 mouse lymphoma cells. J Biol Chem. 1981 Nov 10;256(21):11356–11364. [PubMed] [Google Scholar]
- Steinberg R. A., Agard D. A. Turnover of regulatory subunit of cyclic AMP-dependent protein kinase in S49 mouse lymphoma cells. Regulation by catalytic subunit and analogs of cyclic AMP. J Biol Chem. 1981 Nov 10;256(21):10731–10734. [PubMed] [Google Scholar]
- Steinberg R. A., Coffino P. Two-dimensional gel analysis of cyclic AMP effects in cultured S49 mouse lymphoma cells: protein modifications, inductions and repressions. Cell. 1979 Nov;18(3):719–733. doi: 10.1016/0092-8674(79)90126-0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., O'Farrell P. H., Friedrich U., Coffino P. Mutations causing charge alterations in regulatory subunits of the cAMP-dependent protein kinase of cultured S49 lymphoma cells. Cell. 1977 Mar;10(3):381–391. doi: 10.1016/0092-8674(77)90025-3. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Lofgren D. J., Adair G. M. Evidence for structural gene alterations affecting aminoacyl-tRNA synthetases in CHO cell mutants and revertants. Somatic Cell Genet. 1978 Jul;4(4):423–435. doi: 10.1007/BF01538864. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daalen Wetters T., Coffino P. Revertants of an S49 cell mutant that expresses altered cyclic AMP-dependent protein kinase. Mol Cell Biol. 1982 Oct;2(10):1229–1237. doi: 10.1128/mcb.2.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]