Abstract
Platelet-derived growth factors (PDGFs) and their tyrosine kinase receptors play instrumental roles in embryonic organogenesis and diseases of adult organs. In particular, platelet-derived growth factor receptor-alpha (PDGFRα) is expressed by multipotent cardiovascular progenitors in mouse and human embryonic stem cell systems. Although cardiac PDGFRα expression has been studied in multiple species, little is known about its expression in the human heart. Using immunofluorescence, we analyzed PDGFRα expression in both human fetal and diseased adult hearts, finding strong expression in the interstitial cells of the epicardium, myocardium, and endocardium, as well as the coronary smooth muscle. Only rare endothelial cells and cardiomyocytes expressed PDGFRα. This pattern was consistent for both the fetal and adult diseased hearts, although more PDGFRα+ cardiomyocytes were noted in the latter. In vitro differentiation assays were then performed on the PDGFRα+ cell fraction isolated from the cardiomyocyte-depleted human fetal hearts. Protocols previously reported to direct differentiation to a cardiomyocyte (5-azacytidine), smooth muscle (PDGF-BB), or endothelial cell fates (vascular endothelial growth factor [VEGF]) were used. Although no significant cardiomyocyte differentiation was observed, PDGFRα+ cells generated significant numbers of smooth muscle cells (smooth muscle-α-actin+ and smooth muscle myosin+) and endothelial cells (CD31+). These data suggest that a subfraction of the cardiac PDGFRα+ populations are progenitors contributing predominantly to the vascular and mesenchymal compartments of the human heart. It may be possible to control the fate of these progenitors to promote vascularization or limit fibrosis in the injured heart.
Introduction
Platelet-derived growth factors (PDGFs) affect wide and varied cellular responses, including proliferation, differentiation, migration, and survival [1]. The biological effects of PDGFs are exerted by activation of two tyrosine kinases platelet-derived growth factor receptor (PDGFR)α and β. In particular, PDGFRα is instrumental during embryonic organogenesis and development by directing the differentiation, migration, and function of specialized mesenchymal cells [2,3]. Although expression of PDGFRα has been studied in the hearts of multiple species [4–6], relatively little is known about its expression in the human heart.
Recent evidence suggests that PDGFRα-expressing cells in both the murine heart [7,8] and in human embryonic stem cell systems [9,10] are important cardiovascular progenitors capable of multilineage differentiation. Currently, enormous global efforts are being made to generate stem cell therapies for cardiac diseases (reviewed in [11,12]). Therefore, increased understanding of human PDGFRα cardiac progenitors is important in this context. In the present study, we sought to analyze PDGFRα expression in both the human fetal and diseased adult hearts and to investigate the multipotency of the fetal cardiac PDGFRα+ population. We found that cardiac PDGFRα+ cells appeared to maintain the vascular and mesenchymal compartments of the human heart. Limited expression of PDGFRα in cardiomyocytes, coupled with limited ability of PDGFRα+ cells to upregulate cardiac proteins or transcription factors after in vitro differentiation, suggests a lesser role in regulating the cardiomyocyte compartment.
Materials and Methods
Immunofluorescence analysis of fetal and adult hearts
Fetal hearts of gestational age 93–105 days were obtained via the University of Washington Congenital Defects Laboratory under a program supported by the National Institutes of Health. The tissues were procured according to the conditions approved by the Institutional Review Board of the University of Washington. Adult heart tissue was obtained from the subjects who were undergoing cardiac transplantation or placement of left ventricular-assist device for end-stage heart disease. The hearts used in this study were affected by ischemic cardiomyopathy. The University of Washington Institutional Review Board approved the study protocols, and written informed consent was obtained from all participants.
For histological studies, the fetal and adult hearts were fixed in 4% paraformaldehyde before processing and embedding in paraffin. Then, 5-μm sections were cut and stained with the primary antibody overnight, followed by 1 h of secondary antibody incubation. For immunofluorescence, Alexa fluorphore-conjugated secondary antibodies were employed; the Hoechst (Sigma) counterstain was used to visualize the nuclei. The following primary antibodies were used: rabbit polyclonal anti-PDGFRα (Abcam; prediluted, 1:10), mouse monoclonal anti-human CD31 (Dako; 1:15), mouse monoclonal anti-cardiac troponin T (Developmental Studies Hybridoma Bank; 1:1000), mouse monoclonal anti-smooth muscle α-actin (Dako; 1:800), mouse monoclonal anti-c-Kit (Abcam; 1:100), mouse monoclonal anti-WT-1 (Novocastra; 1:50), goat anti-Nkx2-5 (R&D; 1:400), rabbit monoclonal anti-CD146 (Epitomics; 1:20), biotinylated donkey anti-rabbit IgG (Fab fragment; Jackson Immuno Research). Alexa 488- or 594-conjugated goat anti-mouse or horse anti-goat (Invitrogen; 1:100). For PDGFRα, signal amplification with HRP goat anti-rabbit and Alexa-488 tyramide (Invitrogen) was used as per the manufacturer's instructions.
Cell isolation and culture
PDGFRα+ cells were isolated from the fetal hearts (n=7) as per previously published methods with minor modifications [7]. Briefly, mononuclear cells were isolated from the dissected hearts by mincing the tissue before digestion in Liberase Blendzyme (Roche; 1:50) in PBS at 37°C for 30 min with triturating mechanically at 10-min intervals and removing the myocyte debris with a 40-μm filter. The resulting cells were then subjected to incubation with APC-conjugated mouse monoclonal anti-PDGFRα antibody (R&D; 1:10) and EasySep Human APC-Positive Selection magnetic bead separation (Stem Cell Technologies) according to the manufacturer's instructions. Isolated cells were plated on a tissue culture plastic and cultured in a basal medium (80% knockout Dulbecco's minimum essential medium [DMEM; Invitrogen], 10% fetal bovine serum [FBS; HyClone], 1 mM l-glutamine [Gibco], 0.1 mM β-mercaptoethanol, 1% nonessential amino acid stock [Gibco], penicillin G 100 U/mL [Cellgro], and streptomycin 100 mg/mL [Cellgro]) at 37°C and 5% CO2.
Flow cytometry
The atria and ventricles of the human fetal hearts (n=5) were minced and digested as described in the cell isolation methods above. Cells were stained with anti-PDGFRα antibody–APC (R&D; 1:10), anti-c-Kit-PE (BD Biosciences; 1:10), and anti-CD90-FITC (Abcam; 1:10) for >30 min. Control staining was performed simultaneously using isotype-matched, irrelevant antibodies directly conjugated to FITC, PE, or APC. The fluorescence intensity of the cells was analyzed by flow cytometry (BD FACS Canto I; BD Biosciences).
Differentiation assays
PDGFRα+ cells were plated onto 10-cm tissue culture dishes (for quantification assays) or two-well chamber slides (Nunc), with or without gelatin coating and grown to 70% confluency before the basal medium was removed and specific differentiation medium added as follows [7].
Cardiomyocyte differentiation—adult stem cell protocol l [13]
Cultured cells were exposed to 10 μM 5-azacytidine (Tocris) and 10 μg/L basic fibroblast growth factor (R&D) in DMEM high-glucose (DMEM-HG; Invitrogen) containing 10% FBS, for 48 h. They were then cultured without 5-azacydidine for 14 days.
Cardiomyocyte differentiation—pluripotent stem cell protocol [9,10]
We also tested the ability of PDGFRα+ cells to differentiate into cardiomyocytes using conditions that promote cardiogenesis in hESCs. We plated 0.5×106 P2 PDGFRα+ fetal cells into each well of a low-attachment six-well tissue culture dish in a basal medium and cultured at 37°C and 5% CO2 for 24 h to allow embryoid body-like formation. The medium was then replaced with the StemPro-34 medium (Invitrogen) with 10 ng/mL penicillin/streptomycin, 2 mM l-glutamine, 1 mM ascorbic acid, 4×10−4 M monothioglycerol (MTG; Sigma). To this medium, 10 ng/mL human-BMP4, 5 ng/mL human-basic Fibroblast growth factor (bFGF), and 6 ng/mL human-Activin A were added, and cells were cultured at 37°C and 5%CO2, 5% O2, and 90% N2 for 3 days. The medium was then changed to the basal StemPro-34 medium, supplemented with 150 ng/mL human-DKK1 and 10 ng/mL human vascular endothelial growth factor (VEGF; R&D Systems) and cultured at 37°C and 5% CO2 for 4 days. The medium was then changed to backbone and cultured at 37°C and 5% CO2 for further 16 days, with the medium changed every 4 days.
Smooth muscle cell differentiation [14]
Cells were cultured in the presence of 50 ng/mL PDGF-BB (Abcam) with 2% FCS in DMEM-HG.
Endothelial differentiation [7,15]
Cells were cultured in 1% FBS in Iscove's modified Dulbecco's medium (Invitrogen) containing 10 μg/mL bFGF and 10 ng/mL VEGF (R&D Systems). The medium was changed every 3–4 days, and the cells were fixed with methanol after 14 days of differentiation. Immunofluorescence was performed using the same antibodies or the following: mouse monoclonal anti-α-actinin (Sigma; 1:800), mouse monoclonal anti-smooth muscle myosin (Sigma; 1:800), and rabbit anti-von Willebrand Factor (Dako; 1:500).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Human fetal kidney and human fetal hearts were obtained from the University of Washington Congenital Defects Laboratory. Total RNA was isolated using the RNeasy Fibrous Tissue kit (Qiagen). Equal amounts of RNA (500 ng) were reverse transcribed using random primers and Superscript III (Invitrogen). For PCR, 2 μL of the RT reactions were amplified for 35 cycles at 60°C annealing temperature using GoTaq (Promega). The primer pairs were as follows: β-Actin, F-ATTGGCAATGAGCGGTTCCGC, R-CTCCTGCT TGCTGATCCACATC; GAPDH, F- CATCCATGACAACT TTGGTATC, R- CACCCTGTTGCTGTAGCCAA; PDGF-A, F-CCCCTGCCCATTCGGAGGAAGAG, R-TTGGCCACCT TGACGCTGCGGTG; PDGF-B, F-GATCCGCTCCTTTGATGATC, R-GTCTCACACTTGCATGCCAG; PDGF-C, F-TCTTG GCAAGGCTTTTGTTT, R-TGCTTGGGACACATTGA CAT; PDGFRα, F-ATCAATCAGCCCAGATGGAC, R-TTCACGGG CAGAAAGGTACT; PDGFRβ, F-AATGTCTCCAGCACCTT CGT, R-AGCGGATGTGGTAAGGCATA. Agarose gel electrophoresis (1.2%) was carried out using 10 μL of the 50-μL PCR reactions with ethidium bromide as a staining reagent.
PDGF-A ligand experiments
PDGFRα+ cells were isolated and cultured as described in cell isolation and culture methods above. 40,000 cells were plated into 10 cm tissue culture dishes with or without the addition of 110 ng/mL recombinant human (rh)PDGF-A (Prospec). Cells without rhPDGF-A were cultured in basal medium (80% DMEM, 10% FBS, 1mM l-glutamine, 0.1 mM β-mercaptoethanol, 1% non-essential amino acids, penicillin G 100 U/ml and streptomycin 100 mg/ml). Cells with rhPDGF-A were cultured in 2% FBS in DMEM/F12 (Invitrogen) with 1 mM l-glutamine (Gibco) and penicillin G 100 U/mL (Cellgro). The medium was changed every 3 days. After 10 days, the cells were harvested, counted, and prepared for quantification of smooth muscle and endothelial cell marker expression (see below).
Quantification of differentiation and statistical analysis
Differentiated cells were trypsinized, fixed in 2% paraformaldehyde or methyl Carnoy's solution, and then pelleted in Histogel (American Mastertech) as per the manufacturer's instructions. Pellets were processed and embedded in paraffin before cutting and primary antibody staining. Biotinylated goat anti-mouse IgG and biotinylated goat anti-rabbit IgG secondary antibodies (Jackson Labs; 1:500) were used, followed by a thirty-minute incubation in the enzyme-based ABC reagent (Vector Labs); the binding was visualized by DAB (Sigma) and followed by hematoxylin nuclear counterstain. Cells with hematoxylin and DAB signal were scored as positive and those with hematoxylin-only scored as negative. At least 300 cells per condition were counted and results expressed as mean±standard error of three independent experiments. Statistical analysis was used using GraphPad Prism 4.0. Paired t-tests with the threshold for significance were set at level P<0.05.
Microscopy and image preparation
Standard light micrographs were taken at room temperature using a Nikon Eclipse 80i microscope. All immunofluorescent images were collected by a Nikon A1 Confocal System attached to a Nikon Ti-E-inverted microscope platform and using water-immersion Nikon 60× CFI Plan Apo objective lens with 1.2 NA. Image were acquired at room temperature using Nikon NIS Elements 3.1 software to capture 12-bit raw files that were then rescaled to 16-bit images for further processing. All images were collected as a single scan with the pinhole adjusted to 1 Airy unit at a 1024×1024 pixel density. For figure preparation, images were exported into Photoshop CS3 (Adobe). If necessary, brightness and contrast were adjusted for the entire image, and the image was cropped.
Results
PDGFRα expression in the human fetal heart
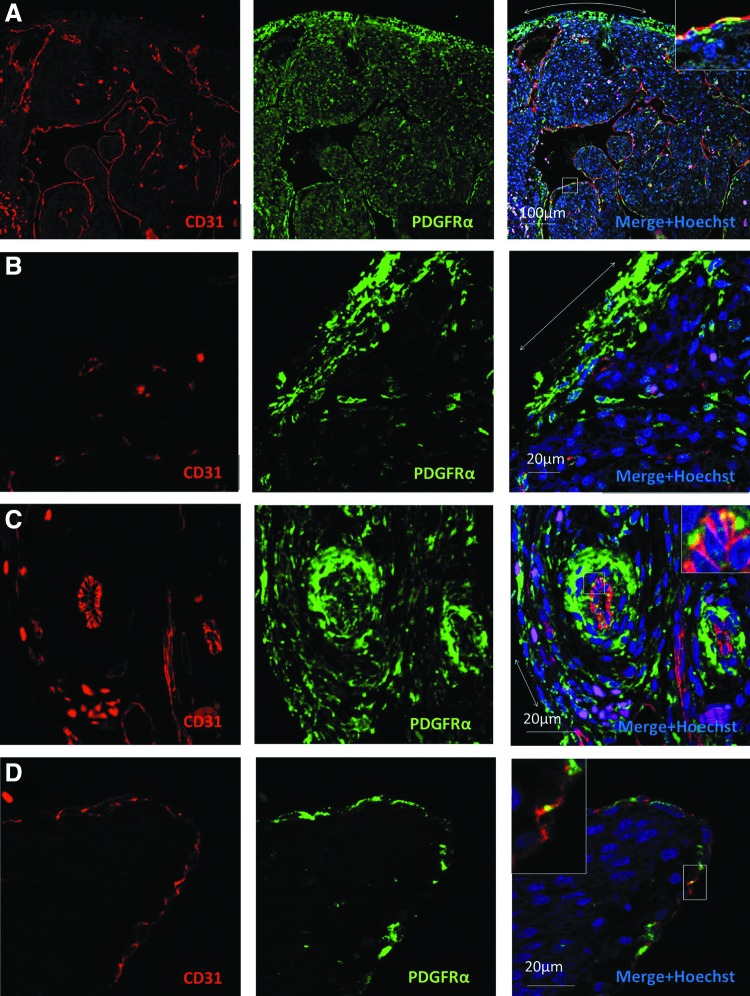
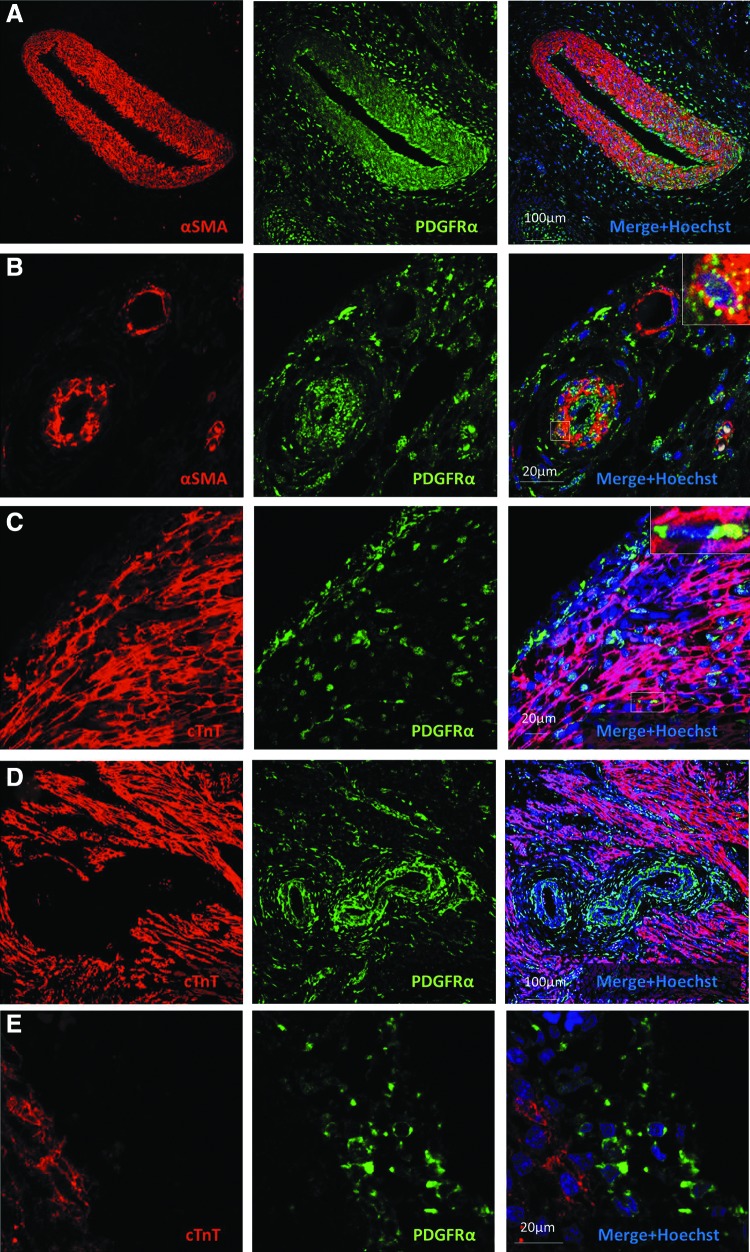
We examined the sections of human fetal hearts of gestational age 93–105 days by immunofluorescence. PDGFRα expression was predominantly seen in interstitial cells of the epicardium, endocardium, and myocardium of both the atria and ventricles (Fig 1A, B), as well as in the smooth muscle cells of medium and large coronary vessels (Fig. 1C). Endocardial expression of PDGFRα was more heterogeneous than in the epicardium (Fig. 1D). PDGFRα was largely distinct from CD31 expression (Fig 1A–D), indicating that endothelial cells do not significantly express this receptor, although occasional regions of possible coexpression (vs. cellular juxtaposition) were identified in endocardial endothelial cells (Fig 1A, D insets) and in the endothelium of larger epicardial vessels (Fig. 1C inset). PDGFRα was not seen in the endothelium of small vessels or capillaries. PDGFRα frequently colocalized with smooth muscle α-actin in the media of the coronary vessels (Fig 2A, B). Cardiomyocytes throughout the atria and ventricles (identified by cTnT staining) did not express PDGFRα, except in rare occasions (Fig 2C, D, E). Taken together, these findings suggest that PDGFRα+ cells may be important for the development and homeostasis of the coronary smooth muscle and the heart's interstitium, but this receptor is absent from cardiomyocytes and endothelium in human fetal hearts of 93–105-day gestational age.
FIG. 1.
Endothelial PDGFRα expression is rare in the human fetal heart. (A–D) PDGFRα-expressing cells are located predominantly in the interstitium of the epicardium (double-headed arrows), myocardium, endocardium, and coronary vascular media. Insets show a magnified view of boxed regions where a rare PDGFRα+/CD31+ endothelium is seen. (A) Low-power image of left ventricular cross-section. (B) High-power image of the left ventricular epicardium. Note that CD31+ capillary endothelium does not coexpress PDGFRα. (C) High-power image of epicardial vessels. (D) High-power image of ventricular endocardium. PDGFRα, platelet-derived growth factor receptor-alpha.
FIG. 2.
PDGFRα expression is common in smooth muscle, but not in cardiomyocytes within the human fetal heart. (A) Low-power image of the great cardiac vessel showing PDGFRα coexpression with α-smooth muscle actin. (B) High-power image of epicardial vessels with PDGFRα coexpression in the larger (likely arterial), but not smaller (likely venous) vessel. Inset shows a magnified view of boxed region. (C) High-power image of the left ventricular epicardium showing PDGFRα expression in subepicardium and interstitial cells, but not in most cardiac cTnT-expressing cardiomyocytes. Inset shows a boxed region with rare PDGFRα+/cTnT+ cardiomyocytes. (D) Large intramyocardial vessels with PDGFRα expression in the media and adventitia. (E) Higher-power view of membrane-bound PDGFRα-expressing cells. αSMA, alpha-smooth muscle actin, PDGFRα, platelet-derived growth factor receptor-alpha, cTnT, cardiac Troponin-T.
PDGFRα expression in the diseased adult human heart
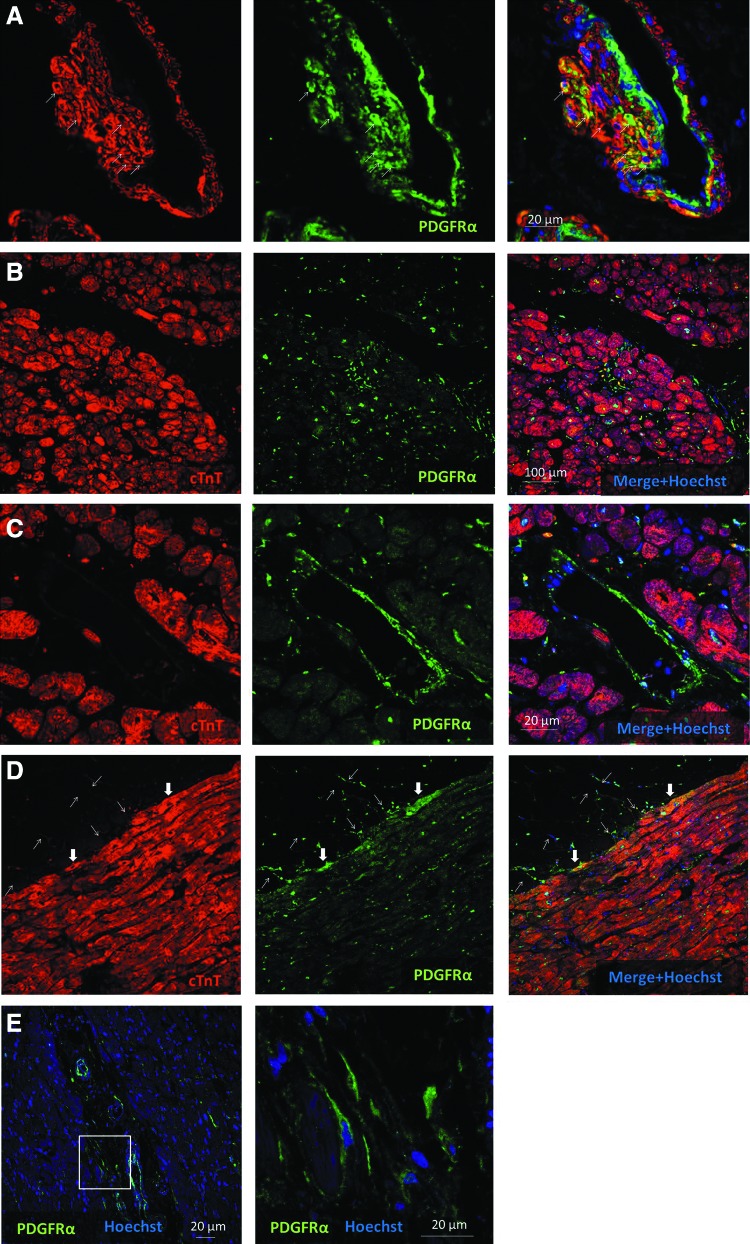
We next examined human adult hearts with ischemic cardiomyopathy by immunofluorescence. This revealed PDGFRα expression predominantly in the smooth muscle cells of the coronary media and intima, as well as interstitial cells throughout the ventricles (Fig 3A–C, E). This represents a similar pattern to that seen in fetal human hearts). PDGFRα coexpression with smooth muscle α-actin was again seen in the media of the coronary vessels (Fig. 3A). PDGFRα coexpression with cardiac troponin-T-expressing cardiomyocytes was seen more often in the diseased adult heart, as compared to the fetal heart. However, this was still rare and was predominantly seen at the epicardial surface (Fig. 3D). Interestingly, PDGFRα expression was observed in cells within the epicardial fat (Fig. 3D). However, these may be adipose fibroblasts rather than adipocytes. These findings suggest that PDGFRα+ cells are important for maintaining homeostasis of the cardiac mesenchymal and vascular compartments of the diseased adult heart.
FIG. 3.
PDGFRα expression in the diseased human adult heart. (A–D) Sections of the adult heart from patients with ischemic cardiomyopathy who subsequently underwent heart transplant or implantation of left ventricular-assist device for end-stage heart failure. (A) High-power image of the coronary vessel showing coexpression of PDGFRα and smooth muscle α-actin (arrows). Note that the artery has an eccentric smooth muscle-rich intimal proliferation, possibly related to thrombus organization (B–C) PDGFRα expression is seen in left ventricular interstitial cells and vessels, but not in cardiomyocytes. (D) PDGFRα expression in the epicardial fat layer (thin arrows) and occasional subepicardial cardiomyocytes (block arrows). (E) PDGFRα expression in interstitial cells. Inset displayed in higher magnification.
Coexpression of PDGF receptors and ligands in human fetal and diseased adult hearts
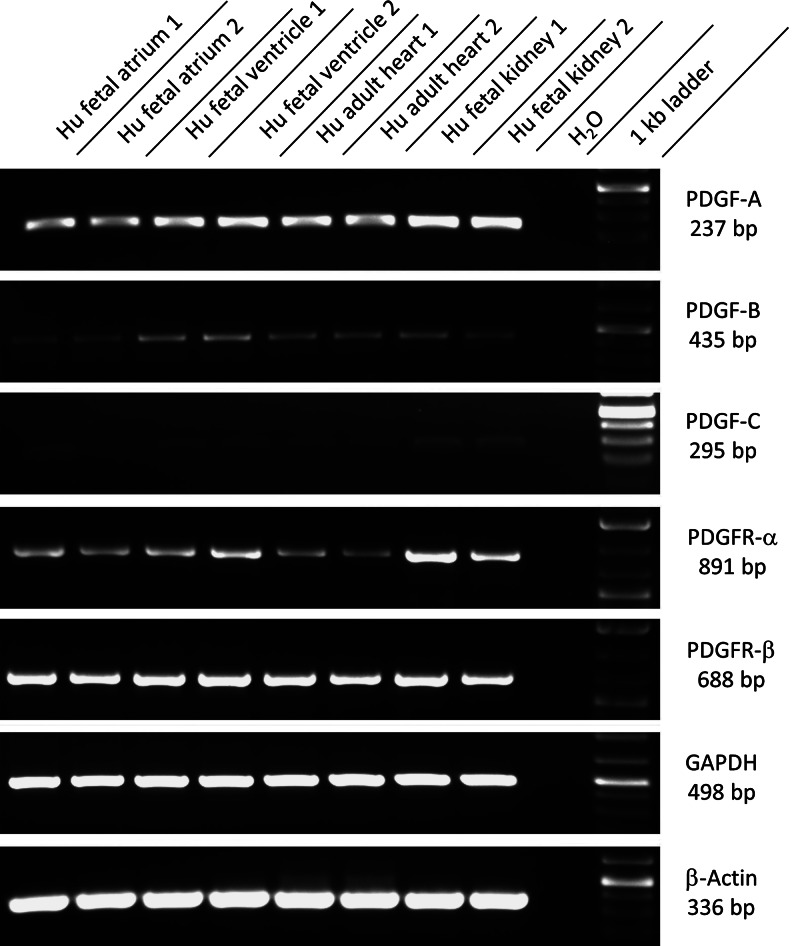
Although immunofluorescence analysis demonstrates robust expression of PDGFRα, demonstrating coexpression of known ligands for this receptor would strengthen evidence for a functional role. Therefore, we performed RT-PCR on RNA samples from human fetal atria, fetal ventricles, and adult diseased ventricles. Fetal kidney tissue was used as a positive control, and high expression of PDGFRα and both of its ligands, PDGF-A and PDGF-C, was found as previously reported [16]. In the hearts, we found the highest expression of the PDGFRα transcript in fetal ventricles, with expression in fetal atria higher than in adult diseased ventricles (Fig. 4). Robust expression of the PDGF-A transcript was seen in all samples, although only weak expression of PDGF-C was detected. We also detected the transcript of PDGFR-β and its ligand PDGF-B in all samples. Expression for PDGFR-β was higher than the comparative levels for PDGFRα. These data show that both PDGF ligands and receptors are expressed in fetal and diseased adult hearts.
FIG. 4.
Coexpression of PDGF receptors and ligands in the human fetal and diseased adult hearts. Reverse transcriptase–polymerase chain reactions were performed. Results from RNA samples of human fetal atria, ventricles, and adult diseased ventricles (from two independent organs) shown. Primers for PDGFRα, PDGFRβ, and their ligands PDGF-A, PDGF-B, and PDGF-C were used. Human fetal kidney tissue was used as a positive control. Robust expression of the PDGFRα transcript in all samples was seen with highest expression in fetal ventricles. Expression in the fetal atria was higher than in diseased adult ventricles. Robust expression of transcripts for PDGF-A and weak expression of PDGF-C were seen in all samples. Strong expression of the PDGFR-β transcript and weaker expression for its ligand PDGF-B were seen in all samples. HFA, human fetal atria; HFV, human fetal ventricle; HAV, human adult ventricle; HFK, human fetal kidney; H2O, water, PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; GAPDH, glycerol-3-phosphate dehydrogenase.
PDGFRα coexpression with cardiac progenitor markers in the fetal heart
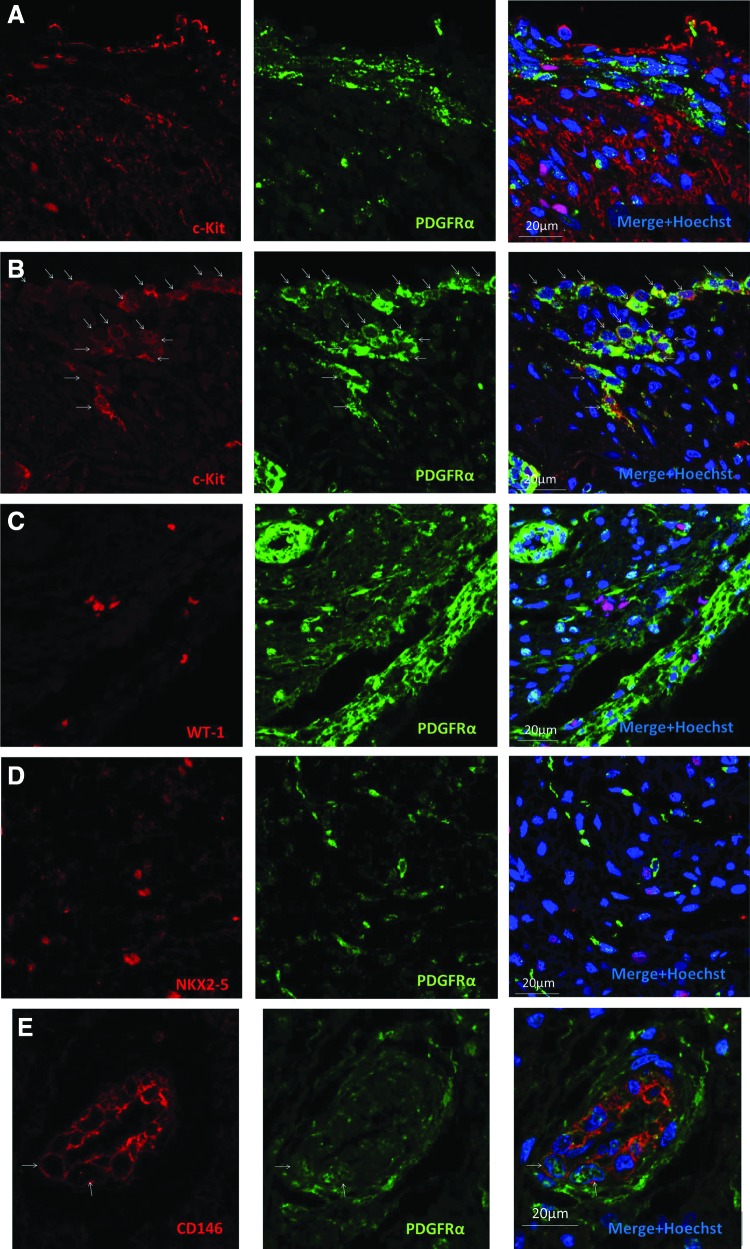
To investigate a potential progenitor phenotype of PDGFRα+ cells, we next asked if PDGFRα is coexpressed with the markers of cardiac stem/progenitor cells [12]. c-Kit is a tyrosine kinase receptor extensively studied in the hearts of multiple species, including humans. It marks a purported multipotent cardiac progenitor/stem cell that is currently under investigation as cell therapy for ischemic cardiomyopathy [17–19]. We found strong c-Kit expression in the ventricular epicardium with a reciprocal relationship to PDGFRα+ cells in the subepicardium (Fig. 5A). Patchy, weaker expression of c-Kit was also seen throughout the fetal ventricular myocardium, likely reflecting its known expression in immature cardiomyocytes [20]. In contrast to findings in the ventricle, atrial coexpression of PDGFRα and c-Kit was frequently seen in the epicardium, subepicardium, and myocardium (Fig. 5B). Flow cytometry performed on the fetal atrial or ventricular nonmyocytes showed that PDGFRα-dim cells comprised within the CD90-expressing population (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd). Rare c-Kit/PDGFRα double-positive cells were seen in both atria and ventricles.
FIG. 5.
PDGFRα coexpression with a c-Kit cardiac stem/progenitor cell marker in the human fetal heart. (A) High-power image of the left ventricle showing c-Kit expression in the epicardium and myocardium, but not in PDGFRα-expressing subepicardium and interstitial cells. (B) High-power image of the atria showing many c-Kit+/PDGFRα+ cells (arrows) in the epicardium, subepicardium, and myocardium. (C) High-power image of the left ventricle showing WT-1 progenitors without expression of PDGFRα. (D) High-power image of the deep region of the left ventricle showing Nkx2–5-expressing progenitors without PDGFRα expression. (E) Cardiac vessel showing CD146-expressing subendothelial cells with some coexpression of PDGFRα. Arrows show cells with clear coexpression of CD146 and PDGFRα. Note abundant CD146–/PDGFRα+ cells in the interstitium.
WT-1 is a zinc-finger transcription factor crucial for the normal development of several organs, including the heart, where it plays an important role in epithelial-to-mesenchymal transformation of the epicardium [21,22]. WT-1 has been reported to mark epicardium-derived multipotent cardiac progenitors [23,24]. We found no coexpression of WT-1 and PDGFRα in the ventricles or atria of the fetal hearts examined (Fig. 5C). Nkx2-5 is a homeobox transcription factor required for cardiac development that has been shown to mark multipotent cardiac progenitor cells [25,26]. We found no coexpression of PDGFRα and Nkx2-5 in the fetal hearts examined (Fig. 5D). CD146 is a membrane glycoprotein expressed on endothelial cells and may mark mesenchymal stromal cells with increased pluripotency [27,28]. We found CD146 expression in the subendothelial population with some minor coexpression of PDGFRα (Fig. 5E). The finding that PDGFRα is coexpressed with the known cardiac progenitor cell marker c-Kit suggests a potential progenitor role for PDGFRα in the human fetal heart.
Multilineage potential of fetal human PDGFRα+ cardiac progenitors in vitro
To further investigate possible progenitor cell functions of PDGFRα-expressing cells, we conducted in vitro experiments to propagate and differentiate PDGFRα+ cells isolated from human fetal hearts. We predicted that not all PDGFRα+ cells would function as progenitors. For example, in murine studies, only a subfraction of the PDGFRα+/Sca1+/CD31– cardiac cells possess a stem cell phenotype [7]. Further, of all the noncardiomyocytes in the mouse heart, it is only those within the PDGFRα+/Sca1+/CD31– fraction that are capable of propagation on tissue culture plastic. Since Sca1 is a murine protein with no known human ortholog, in this study, we used antibody-mediated magnetic bead cell sorting for PDGFRα expression alone before plastic adherence culture. This is a similar strategy to that adopted in early murine embryos where Sca1 is not expressed until mid-gestation.
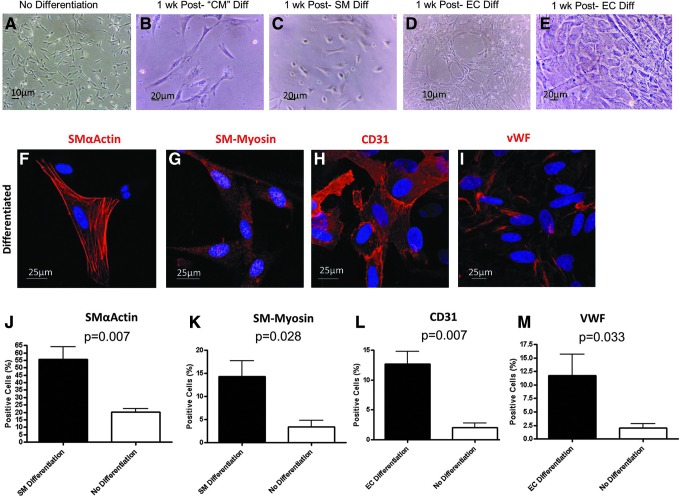
PDGFRα+ cells isolated from the fetal hearts were propagated for 80 days without interruption (split approximately every 4 days). Early- and late-passage cells cultured in a basal medium (without agents to induce differentiation) maintained a fibroblastic morphology with little change (Fig. 6A). In contrast, morphological changes were promptly observed after the baseline medium was changed to specific differentiation medium previously used to differentiate murine cardiac mesenchymal stromal cells and human bone marrow mesenchymal stromal cells (MSCs) [7,13,15]. One week after cardiomyocyte differentiation was induced with 5-azacytidine and bFGF, cultured cells appeared larger and had a more triangular shape (Fig. 6B). Similarly, 1 week after smooth muscle differentiation was induced with PDGF-BB [14], cultured cells appeared thickened with a more rectangular shape (Fig. 6C). Interestingly, despite the insignificant numbers of PDGFRα+ endothelial cells in intact hearts, cord-like structures (Fig. 6D) were seen 1 week after endothelial cell differentiation induced by VEGF and bFGF [7,15]. Although a typical cobblestone appearance was generally not seen, there were abundant areas of cells with a flattened and round morphology (Fig. 6E). Examination of cells after cardiomyocyte differentiation resulted in no expression of cardiac Troponin-T and Nkx2-5 in either differentiated cells or controls cultured in the basal medium alone (data not shown). Instead, the striated muscle marker α-actinin was seen after culture in these conditions (data not shown), suggesting skeletal myogenesis. As an alternative method of cardiomyocyte differentiation, we adapted a protocol for directed differentiation of human embryonic stem cells formed in aggregates known as embryoid bodies [9,10]. Human fetal cardiac PDGFRα+ cells formed embryoid body-like aggregates after 24 h of culture in low-attachment plates (data not shown). However, unlike hES cells, after sequential addition of bone morphogenic protein 4 (BMP4), Activin A, bFGF, VEGF, and dickkopf-related protein-1 (Dkk1), no spontaneous beating was observed out to 28 days.
FIG. 6.
In vitro multipotency of PDGFRα+ cells isolated from human fetal hearts. (A–D) Light micrographs of PDGFRα+ progenitors before and after exposure to in vitro differentiation assays. (A) PDGFRα+ progenitors have a fibroblastic morphology 24 h after isolation from human fetal hearts. (B) One week after culture in a cardiomyocyte differentiation medium, the cells appear thickened and triangular. (C) One week after culture in a smooth muscle differentiation medium, the cells adopt a rectangular or triangular shape. (D) One week after culture in an endothelial cell differentiation medium, tubular networks appear within some areas of confluent cells. (E) Flattened and round-shaped cells similar apparent after endothelial cell differentiation. (F–I) Confocal immunofluorescence of cells exposed to differentiation assays. (J–M) Quantification of cells differentiated by specific differentiation assays (black bars) or under basal conditions (white bars). Graphs show positive cells as the percentage of total cells counted (mean±S.E.M. of seven independent biological replicates). CM, cardiomyocyte; SM, smooth muscle; EC, endothelial cell; vWF, von Willebrand factor.
Next, we quantified PDGFRα+ cells for expression of smooth muscle markers after exposure to specific differentiation conditions. We found smooth muscle α-actin expressed in 56%±9% of differentiated cells compared to 20%±6% of nondifferentiated controls (P=0.007, Fig 6F, J). Smooth muscle myosin was expressed in 14%±3% of differentiated cells compared to 3%±1% in controls (P=0.028, Fig 6G, K). Finally, we examined for CD31 and von Willebrand Factor Factor (VWF) expression in cultures of PDGFRα+ cells exposed to endothelial-specific differentiation conditions. CD31 was expressed in 13%±5% of differentiated cells with 2%±1% in nondifferentiated controls (P=0.007, Fig 6H, L). VWF was expressed in 12%±4% of differentiated cells with 2%±1% in nondifferentiated controls (P=0.033, Fig 6I, M).
We also investigated the effects of PDGF-A ligand addition to fetal cardiac PDGFRα+ cells in vitro. There was a statistically insignificant trend for increased differentiation to smooth muscle phenotypes after the addition of rhPDGF-A (Supplementary Fig. S1B).
Taken together, these findings suggest that PDGFRα+ cells isolated from the human fetal heart are multipotent and may have an in vivo progenitor phenotype. The ability to produce smooth muscle and endothelial cells, but not cardiomyocytes, from the assays used suggests that PDGFRα progenitors may have a predisposition toward vascular and stromal phenotypes.
Discussion
We have found that in human fetal and diseased adult hearts, PDGFRα is expressed in interstitial cells of the epicardium, myocardium, and endocardium and also in the coronary vascular smooth muscle cells. In contrast, minimal expression of PDGFRα was observed in endothelial cells and cardiomyocytes. Further, a subset of the PDGFRα+ cells coexpressed either c-Kit or CD146, known markers of progenitors in the fetal heart [20,29]. These data, together with demonstrable in vitro multipotency of PDGFRα+ fetal cardiac cells, suggest that a subfraction of PDGFRα+ cells are progenitors that have the capacity to contribute to the vascular and mesenchymal compartments of the human heart.
To our knowledge, this study is the first to detail PDGFRα expression in the human fetal heart. However, embryonic cardiac PDGFRα expression has been studied in mice [4,5,7] and avian species [30]. These reports support our findings of robust epicardial PDGFRα expression. Further, in the murine studies, PDGFRα is expressed in fibroblasts and the coronary vasculature (particularly smooth muscle) as we have found in the human fetal heart.
Consistent with the findings in mice, we found that PDGFRα is not coexpressed with WT-1 or Nkx2-5 (markers that identify cardiac progenitors) [7]. In fetal mouse hearts, c-Kit expression has been linked to cardiomyocyte development and hyperplasia [31,32]. It is important to note that in any species, the relationship between various markers of cardiac stem/progenitor cells currently remains unclear. Although a detailed timecourse of expression for multiple markers would shed light on this issue, such systematic studies are better suited to small-animal species rather than human hearts.
The study of human specimens precludes the use of genetic techniques to fate map progenitors in situ. Nevertheless, the pattern of PDGFRα expression that we have found in human fetal hearts suggests migration of PDGFRα+ progenitors from the epicardium to form the interstitial and vascular cells of the developing human heart. This is supported by evidence from murine models. First, the genetic fate-mapping studies have shown that Sca1+/PDGFRα+ cardiac progenitor/stem cells are derived from epicardial derivatives [7]. Secondly, recent evidence using conditional epicardial deletion of PDGFRα has shown PDGFRα to be crucial for executing epithelial-to-mesenchymal transformation of epicardial progenitors [5]. Interestingly, in these studies, a loss of PDGFRα in the epicardium led to a deficit in cardiac fibroblast formation, whereas vascular smooth muscle was unaffected. A timecourse analysis of PDGFRα expression would help provide further insights into varying expression at different gestational ages. Due to limited gestational ages available in human fetal specimens, these experiments were not possible in this study.
In vitro experiments performed here show that PDGFRα+ progenitors isolated from the human fetal hearts can give rise to differentiated cells with smooth muscle and vascular endothelial cell phenotypes. This is consistent with the notion that PDGFRα+ cells are capable of multipotent differentiation, although our current data cannot rule out heterogeneity among the PDGFRα+ cells as an alternate explanation. In either case, the ability to generate endothelial and smooth muscle cells suggests a plasticity that would not be expected of mature/differentiated cells. Together with the PDGFRα expression pattern from immunofluorescence analysis, our findings suggest that a subset of PDGFRα+ cells are bona fide progenitors capable of adding to the various cell lineages in the developing and diseased human hearts. It is interesting that two different cardiomyocyte differentiation assays involving treatment with 5-azacytidine in monolayers or treatment of embryoid body-like aggregates with activin A and BMP4 did not generate cardiomyocytes. Interestingly, demethylation of DNA with 5-azacytidine yielded cells that expressed α-actinin, but did not express cardiac Troponin-T or Nkx2-5. This treatment, common in the adult cardiac progenitor cell field, appears to yield skeletal muscle cells, but not cardiac myocytes. Of note, bone marrow MSCs also differentiate into skeletal muscle and not cardiac muscle when treated with 5-azacytidine, suggesting that this is a common property of mesenchymal progenitors [33].
It is significant that murine PDGFRα+/Sca1+ progenitors are capable of a broad multilineage potency, including differentiation into cardiomyocytes and other mesodermal lineages [7]. The human hearts available for our study were more mature than these mouse hearts, which may account for a more restricted potential. The current study used bulk, cultured cells, and it was beyond the scope of this study to perform an in-depth clonal analysis of human PDGFRα+ cardiac progenitors. Such experiments may shed light on whether these differences are species specific or related to the state of differentiation of the input cell population studied.
The literature on PDGFRα expression in the adult human heart is largely limited to the analysis of adult cardiac allograft biopsies [34–36]. Here PDGFRα expression has been reported in smooth muscle, endothelial cells, interstitial cells, and cardiomyocytes, although detailed confocal microscopy is lacking. Interestingly, while PDGFRα expression is at very low levels in a normal heart graft before transplantation, expression significantly increases 1 week after transplantation before returning to baseline levels after 1 year [34]. This has been attributed to the strong role of PDGF in reparative processes (especially of vascular structures). PDGF-A, PDGF-B, and PDGF-C are known to activate PDGFRα. Studies investigating the PDGF ligands show that the expression pattern of PDGF after heart transplant is mainly confined to the vasculature [34,36,37]. Our findings in the diseased adult heart, where inflammatory signals are high, support these previous studies, although we found that cardiomyocyte expression of PDGFRα was rare. We also found PDGFRα expression in the epicardial fat layer of diseased hearts. This is interesting, since epicardial fat is known to be a paracrine and endocrine organ with important interactions with the underlying myocardium [38].
Characterization of the human cardiac progenitor population in this study is important to the current cardiac repair and regenerative efforts. The PDGFRα+ progenitor population described appears to preferentially contribute to the vascular and interstitial, rather than cardiomyocyte, compartments. Nevertheless, noncardiomyocyte lineages are crucial for vascular formation and cardiac matrix organization. Therefore, an increasing knowledge of key contributing factors to these processes is paramount. In conclusion, PDGFRα-expressing cells are abundant in the human fetal and adult heart. We propose that a subfraction of these cells may comprise a cardiac progenitor population that warrants further investigation with respect to therapies for cardiac repair.
Supplementary Material
Acknowledgments
We are very appreciative of the technical assistance provided by Veronica Muskheli, Dr. Julie Randolph-Habecker, and Tracy Goodpaster with immunofluorescence and immunohistochemistry. This work was supported by the NIH grants P01 HL094374 (to CEM and BTS), R01 HL084642 (to CEM), P01 GM081719 (to CEM), U01 HL100405 (to CEM), HL094384 (to ASO), HL099993 (to BTS and MI), and R01 HL64387. JC was supported by a National Health and Medical Research Council Australia Overseas Training and Australian-American Fulbright Commission Fellowships. The University of Washington Laboratory of Developmental Biology was supported by the NIH award number R24HD000836 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Author Disclosure Statement
None of the authors have any commercial conflict of interest in relation to this manuscript.
References
- 1.Andrae J. Gallini R. Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoch RV. Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 3.Tallquist MD. Soriano P. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development. 2003;130:507–518. doi: 10.1242/dev.00241. [DOI] [PubMed] [Google Scholar]
- 4.Kang J. Gu Y. Li P. Johnson BL. Sucov HM. Thomas PS. PDGF-A as an epicardial mitogen during heart development. Dev Dyn. 2008;237:692–701. doi: 10.1002/dvdy.21469. [DOI] [PubMed] [Google Scholar]
- 5.Smith CL. Baek ST. Sung CY. Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res. 2011;108:e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Den Akker NM. Lie-Venema H. Maas S. Eralp I. DeRuiter MC. Poelmann RE. Gittenberger-De Groot AC. Platelet-derived growth factors in the developing avian heart and maturating coronary vasculature. Dev Dyn. 2005;233:1579–1588. doi: 10.1002/dvdy.20476. [DOI] [PubMed] [Google Scholar]
- 7.Chong James JH. Chandrakanthan V. Xaymardan M. Asli NS. Li J. Ahmed I. Heffernan C. Menon MK. Scarlett CJ, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–540. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kattman SJ. Huber TL. Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Kattman SJ. Witty AD. Gagliardi M. Dubois NC. Niapour M. Hotta A. Ellis J. Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Paige SL. Thomas S. Stoick-Cooper CL. Wang H. Maves L. Sandstrom R. Pabon L. Reinecke H. Pratt G, et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell. 2012;151:221–232. doi: 10.1016/j.cell.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laflamme MA. Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong JJ. Cell therapy for left ventricular dysfunction: an overview for cardiac clinicians. Heart Lung Circ. 2012;21:532–542. doi: 10.1016/j.hlc.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Makino S. Fukuda K. Miyoshi S. Konishi F. Kodama H. Pan J. Sano M. Takahashi T. Hori S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita J. Itoh H. Hirashima M. Ogawa M. Nishikawa S. Yurugi T. Naito M. Nakao K. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 15.Yoon YS. Wecker A. Heyd L. Park JS. Tkebuchava T. Kusano K. Hanley A. Scadova H. Qin G, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X. Ponten A. Aase K. Karlsson L. Abramsson A. Uutela M. Backstrom G. Hellstrom M. Bostrom H, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 17.Bolli R. Chugh AR. D'Amario D. Loughran JH. Stoddard MF. Ikram S. Beache GM. Wagner SG. Leri A, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Beltrami AP. Barlucchi L. Torella D. Baker M. Limana F. Chimenti S. Kasahara H. Rota M. Musso E, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 19.Urbanek K. Torella D. Sheikh F. De Angelis A. Nurzynska D. Silvestri F. Beltrami CA. Bussani R. Beltrami AP, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jesty SA. Steffey MA. Lee FK. Breitbach M. Hesse M. Reining S. Lee JC. Doran RM. Nikitin AY. Fleischmann BK. Kotlikoff MI. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci U S A. 2012;109:13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Estrada OM. Lettice LA. Essafi A. Guadix JA. Slight J. Velecela V. Hall E. Reichmann J. Devenney PS, et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet. 2009;42:89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Gise A. Pu WT. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res. 2012;110:1628–1645. doi: 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smart N. Bollini S. Dube KN. Vieira JM. Zhou B. Davidson S. Yellon D. Riegler J. Price AN, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou B. Ma Q. Rajagopal S. Wu SM. Domian I. Rivera-Feliciano J. Jiang D. von Gise A. Ikeda S. Chien KR. Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prall OW. Menon MK. Solloway MJ. Watanabe Y. Zaffran S. Bajolle F. Biben C. McBride JJ. Robertson BR, et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu SM. Fujiwara Y. Cibulsky SM. Clapham DE. Lien CL. Schultheiss TM. Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 27.Russell KC. Phinney DG. Lacey MR. Barrilleaux BL. Meyertholen KE. O'Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 28.Covas DT. Panepucci RA. Fontes AM. Silva WA. Orellana MD. Freitas MC. Neder L. Santos AR. Peres LC. Jamur MC. Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Craven M. Kotlikoff MI. Nadworny AS. C-kit expression identifies cardiac precursor cells in neonatal mice. Methods Mol Biol. 2012;843:177–189. doi: 10.1007/978-1-61779-523-7_17. [DOI] [PubMed] [Google Scholar]
- 30.Bax NA. Lie-Venema H. Vicente-Steijn R. Bleyl SB. Van Den Akker NM. Maas S. Poelmann RE. Gittenberger-de Groot AC. Platelet-derived growth factor is involved in the differentiation of second heart field-derived cardiac structures in chicken embryos. Dev Dyn. 2009;238:2658–2669. doi: 10.1002/dvdy.22073. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira-Martins J. Ogorek B. Cappetta D. Matsuda A. Signore S. D'Amario D. Kostyla J. Steadman E. Ide-Iwata N, et al. Cardiomyogenesis in the developing heart is regulated by C-kit-positive cardiac stem cells. Circ Res. 2012;110:701–715. doi: 10.1161/CIRCRESAHA.111.259507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Li M. Naqvi N. Yahiro E. Liu K. Powell PC. Bradley WE. Martin DI. Graham RM. Dell'Italia LJ. Husain A. c-kit is required for cardiomyocyte terminal differentiation. Circ Res. 2008;102:677–685. doi: 10.1161/CIRCRESAHA.107.161737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakitani S. Saito T. Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 34.Koch A. Palchyk E. Gassler N. Dengler TJ. Remppis A. Pritsch M. Sack FU. Hagl S. Schnabel PA. Expression of platelet-derived growth factor and fibroblast growth factor in cryopreserved endomyocardial biopsies early and late after heart transplant. Ann Thorac Surg. 2006;81:1372–1378. doi: 10.1016/j.athoracsur.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 35.Koch A. Schmidt CI. Dengler TJ. Remppis A. Sack FU. Schirmacher P. Hagl S. Karck M. Schnabel PA. Differentiated expression patterns of growth factors in routine formalin-fixed endomyocardial biopsies in the early postoperative phase after heart transplantation. Transplant Proc. 2007;39:554–557. doi: 10.1016/j.transproceed.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Sack FU. Vielfort TJ. Koch A. Haass M. Taylor S. Otto HF. Hagl S. Schnabel PA. The role of platelet derived growth factor in endomyocardial biopsies shortly after heart transplantation in relation to postoperative course. Eur J Cardiothorac Surg. 2004;25:91–97. doi: 10.1016/j.ejcts.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Zhao XM. Yeoh TK. Frist WH. Porterfield DL. Miller GG. Induction of acidic fibroblast growth factor and full-length platelet-derived growth factor expression in human cardiac allografts. Analysis by PCR, in situ hybridization, and immunohistochemistry. Circulation. 1994;90:677–685. doi: 10.1161/01.cir.90.2.677. [DOI] [PubMed] [Google Scholar]
- 38.Iacobellis G. Malavazos AE. Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 2011;43:1651–1654. doi: 10.1016/j.biocel.2011.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.