Summary
Subcortical dopamine system dysregulation has been suggested to underlie the positive symptoms of schizophrenia. Recent preclinical investigations and human imaging studies have proposed that the augmented dopamine system function observed in schizophrenia patients may be secondary to aberrant hippocampal activity. Thus, we posit that the hippocampus represents a novel therapeutic target for the treatment of schizophrenia. Here we provide evidence of the effectiveness of a unique approach aimed at decreasing hippocampal function in a rodent model of schizophrenia. Specifically, in a rodent model of schizophrenia, we demonstrate that ventral hippocampal (vHipp) deep brain stimulation (DBS) can normalize aberrant dopamine neuron activity and behaviors associated with positive symptoms. In addition, we provide evidence that this approach may also be effective in restoring deficits in cognitive function, often left unaltered by conventional antipsychotic medications. Therefore, we have provided initial preclinical evidence demonstrating the feasibility of hippocampal DBS as a potential novel approach for the treatment of schizophrenia.
Keywords: Deep Brain Stimulation, Schizophrenia, MAM rat, Hippocampus, Dopamine, Cognition
Introduction
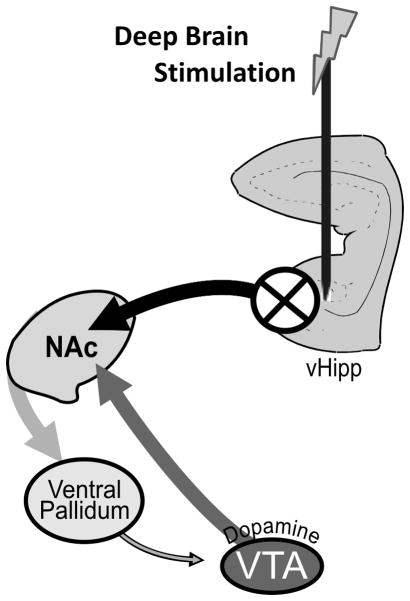
Schizophrenia is a debilitating disease with a lifetime prevalence of about 1% (Stilo and Murray, 2010). Aberrant sub-cortical dopamine signaling has been consistently associated with positive symptoms of schizophrenia based on multiple observations, including imaging studies, and the efficacy of antipsychotic medications in treating the disease (Abi-Dargham, 2004; Creese et al., 1976; Laruelle and Abi-Dargham, 1999; Seeman et al., 1975). Given that no primary pathology exists within the midbrain dopamine system of schizophrenia patients, it has been suggested that aberrant dopamine signaling may be secondary to pathology within cortical and hippocampal regions, which are known to display progressive structural and neurochemical alterations in schizophrenia. We have recently demonstrated, in a rodent model of schizophrenia (for review see: (Lodge and Grace, 2009)), that aberrant dopamine signaling and associated behavioral hyper-responsivity to psychomotor stimulants are due to hyperactivity within the vHipp (Lodge and Grace, 2007; Lodge and Grace, 2011). This is in accord with a growing literature demonstrating that activity within distinct hippocampal subfields is increased, at rest, in schizophrenia patients (Heckers et al., 1998; Lewandowski et al., 2005; Malaspina et al., 1999; Medoff et al., 2001; Molina et al., 2003; Schobel et al., 2009; Tamminga et al., 2010). Furthermore, these increases in hippocampal activity have been directly correlated with clinical measures of psychosis (Molina et al., 2003; Schobel et al., 2009). Thus, we suggest that aberrant hippocampal activity is the source of the dopamine system dysregulation in schizophrenia and, furthermore, that a novel therapeutic approach for the treatment of psychosis may be achieved by directly targeting hippocampal function (See Figure 1).
Figure 1.
DBS decreases aberrant vHipp activity, thus restoring dopamine system function. Our hypothesis of schizophrenia suggests that aberrant hippocampal activity drives the nucleus accumbens (NAc) that, in turn, inhibits the tonic activity within the VP. A decrease in GABAergic transmission from the VP results in an increased dopamine neuron population activity (adapted from (Grace et al., 2007); for review see (Lodge and Grace, 2011)).
DBS is being increasingly utilized for the treatment of psychiatric conditions such as depression, as well as, for disorders including Parkinson’s Disease, and epilepsy (Krack et al., 2010). The mechanism of action of DBS has yet to be conclusively demonstrated and likely differs depending on the brain region examined. Based on clinical (Boon et al., 2007) and preclinical studies (Wyckhuys et al., 2010), DBS of the anterior hippocampus has been purported to decrease hippocampal activity. Given our hypothesis that augmented hippocampal activity underlies dopamine dependant psychosis in schizophrenia (Lodge and Grace, 2011), we posit that DBS of the vHipp will decrease hippocampal output and subsequently normalize aberrant dopamine neuron activity and behavioral deficits in an established rodent model schizophrenia. Interestingly, long-term DBS of the hippocampus has been previously investigated clinically as a treatment for epilepsy and appears to be well tolerated with no observable side effects, albeit in a pathological condition (Boon et al., 2007; Velasco et al., 2007). Here we examined the feasibility of vHipp DBS as a therapeutic approach to reverse behavioral deficits in a rodent model of schizophrenia.
Methods
All experiments were performed in accordance with the guidelines outlined in the USPHS Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center.
Animals
Methylazoxymethanol acetate (MAM) treatments were performed as described previously (Moore et al., 2006). In brief, timed pregnant female Sprague Dawley rats were obtained from Harlan Laboratories at GD16 and housed individually in plastic housing tubs. MAM (diluted in saline, 22mg/kg, i.p.) was administered on gestational day (GD) 17. Control rats received injections of saline (1ml/kg, i.p). Male pups were weaned on postnatal day 21 and housed in groups of 2–3 with litter mates until adulthood (>PN 60) at which time they were used for physiological or behavioral studies. All experiments were performed on multiple litters of MAM and saline-treated rats.
VTA Dopamine Neuron Extracellular Recordings
All rats used for electrophysiology had not undergone any behavioral testing. Male offspring (250–400g) were anesthetized with chloral hydrate (400 mg/kg, i.p.), as this anesthetic does not significantly depress dopamine neuron activity (Hyland et al., 2002), and placed in a stereotaxic apparatus. Anesthesia was maintained by supplemental administration of chloral hydrate as required to maintain suppression of limb compression withdrawal reflex. A core body temperature of 37°C was sustained by a thermostatically controlled heating pad (KOPF – TCAT-2LV). Bipolar concentric stimulating electrodes (KOPF/Rhodes NEX100) were implanted into the right vHipp (A/P +5.3, M/L +5.0, D/V −7.0 mm from bregma). Extracellular glass microelectrodes (impedance 6–14MΩ) were lowered into the right ventral tegmental area (VTA; A/P −5.3, M/L +0.6 mm from bregma and −6.5 to −9.0 mm ventral of brain surface) using a hydraulic micropositioner (KOPF - Model 640). The activity of the population of dopamine neurons was determined by counting the number of spontaneously active dopamine neurons encountered while making multiple vertical passes (typically 6), separated by 200μm, in a predetermined pattern to sample equivalent regions of the VTA. Spontaneously active dopamine neurons were identified with open filter settings (low pass: 50Hz, high pass: 16kHz) using previously established electrophysiological criteria (Grace and Bunney, 1983) and once isolated, activity was recorded for 2–3 mins. High frequency DBS (isolated current pulses: 130Hz, 0.3mA, 0.1ms pulse duration were provided by a Grass S88X stimulator connected to a PSIU6X photoelectric isolation unit) was applied 10 mins prior to starting electrophysiological recordings and continued throughout the duration of the experiment, typically <3 hours. Sham rats were treated identically with electrodes implanted and connected; however, the stimulator remained off.
High frequency stimulation results in numerous stimulus artifacts which presents a challenge when recording spontaneously active neurons, thus, to confirm that increases in population activity could be observed during DBS, we chemically inactivated the ventral pallidum (VP), with baclofen and muscimol (0.2 μg each/0.5μl of Dulbecco’s PBS). Given the tonic GABAergic drive from the VP, inactivation of this region should increase VTA dopamine neuron activity independent of hippocampal manipulations, such as DBS (see Figure 1). A further difficulty, associated with the high number of stimulus artifacts, occurs when analyzing firing rate and burst firing of dopamine neurons. Given that we have previously demonstrated hippocampal manipulations (both activation (Floresco et al., 2003; Lodge and Grace, 2006) and inactivation (Lodge and Grace, 2007; Lodge and Grace, 2008)) do not alter these parameters, we focused this study on dopamine neuron population activity which is known to be altered in MAM-treated rats and reversed by hippocampal manipulations (Gill et al., 2011; Lodge and Grace, 2007). Electrophysiology data were analyzed by a Two Way ANOVA (MAM and DBS as factors), followed by a Holm-Sidak post-hoc test. The effects of VP manipulation were only performed in saline-treated rats and analyzed by a One Way ANOVA.
Survival Surgeries
All survival surgical procedures were performed under general anesthesia in a semi-sterile environment. Briefly, male rats were anesthetized with pentobarbital (60mg/kg, i.p.) and placed in a stereotaxic apparatus using blunt atraumatic ear bars. Bipolar twisted platinum stimulating electrodes (Plastics1: MS303/6-B/SPC) were implanted bilaterally in the vHipp (A/P −5.3, M/L ±5.3, D/V −7.5 mm from bregma), fixed in place with dental cement and four anchor screws. Once the cement was completely solid, the wound was sutured, the rat removed from the stereotaxic frame, and monitored closely until conscious. Sham rats received identical electrode implants.
Amphetamine-Induced Hyper-Locomotion
All rats were connected to an 8-channel stimulus generator (ALA Scientific Instruments: STG4008) via a 4 Channel Commutator (plastics1: SL2+2C). DBS (isolated current pulses: 130Hz, 0.3mA, 0.1ms pulse duration) was initiated immediately prior to placing rats in an open field arena (Med Associates) where spontaneous locomotor activity in the X-Y plane was determined for 40 mins by beam breaks and recorded with Open Field Activity Software (Med Associates). Sham rats were treated identically, but the leads from the commutator to the stimulator were not connected. Following the baseline period, all rats were injected with D-amphetamine sulfate (0.5mg/kg, i.p.) and locomotor activity recorded for 40 mins. Lastly, rats received an additional injection of D-amphetamine sulfate (2.0 mg/kg, i.p.) and locomotor activity recorded for an additional 40 mins. DBS was administered continuously for the entire duration of the behavioral experiment. Locomotor data were analyzed by three separate 3-way ANOVAs (MAM, DBS and time as factors), one for each of the relevant time periods (spontaneous, 0.5 mg/kg, 2.0 mg/kg), followed by a Holm-Sidak post-hoc test.
Attentional Set Shifting
The attentional set shifting task (AST) was performed using a method adapted was from (Lapiz and Morilak, 2006). Rats examined for cognitive flexibility had been previously examined for locomotor activity or saccharine preference (data not shown). The testing apparatus was a rectangular arena divided into three quadrants. One was the start box, while the other two contained pots defined by a pair of cues along two stimulus dimensions: digging medium and odor. A Cheerio was placed at the bottom of the “positive” pot and buried with the digging medium. During habituation rats were trained to reliably dig in the pots to obtain a food reward. The following day, rats were trained on a series of simple discriminations (SD), to reach a criterion of six consecutive correct trials. Finally, on testing day MAM- and saline- treated rats were exposed to DBS (isolated current pulses: 130Hz, 0.3mA, 0.1ms pulse duration) for the duration of testing (typically 3–8 hours). Initially, rats were trained in a SD task of odor. Once the criterion of 6 consecutive trials was achieved, rats were then tested in a number of increasingly difficult tasks to include: a compound discrimination (CD), an intra-dimension shift (ID), a second reversal (R2), an extra-dimensional shift (ED) and a third reversal (R3). It should be noted that during AST a subset of rats (4 MAM and 6 saline) treated with DBS displayed seizure-like activity following long term administration of DBS and were subsequently removed from the trial. Only data preceding the seizure were included, resulting in varying number of animals for the later stages of the task. We do not believe this to be a significant problem with the interpretation of the data since there was sufficient statistical power to identify deficits at the later stages of AST (i.e. extra-dimensional set shifting). Given that vHipp DBS is reported as an effective treatment for epilepsy, this likely reflects the need to optimize stimulus parameters. AST data were analyzed by a Three Way RM ANOVA (MAM, DBS and task as factors), followed by a LSD post-hoc test.
Histology
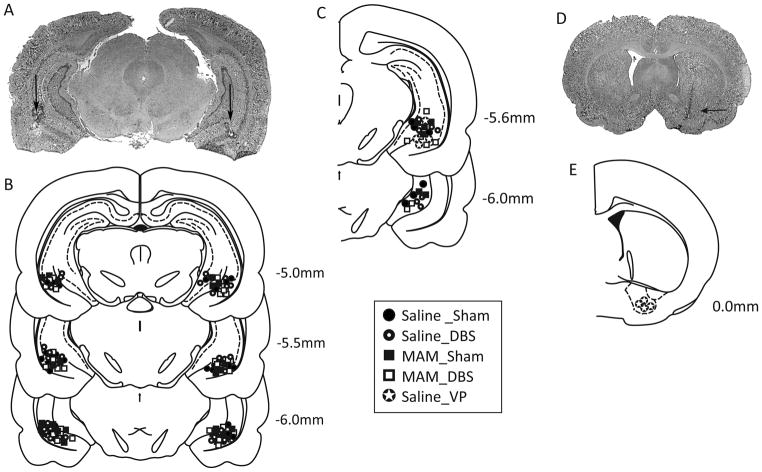
For acute/electrophysiological studies, rats were killed by an overdose of anesthetic (chloral hydrate, additional 400 mg/kg, i.p.), whereas for chronic/behavioral studies rats were killed by a lethal dose of pentobarbital (120mg/kg, i.p.). All rats were decapitated, brains removed, and fixed for at least 48 hours (8% w/v paraformaldehyde in phosphate buffered saline containing potassium ferrocyanide), and cryoprotected (25% w/v sucrose in PBS) until saturated. Brains were sectioned (25μm coronal sections), mounted onto gelatin-chrom alum coated slides, and processed with a Nissl stain for histochemical verification of electrode sites (Fig 2). All histology was performed with reference to a stereotaxic atlas (Paxinos and Watson, 1986). It should be noted that we did not examine any histological effects of MAM administration as this has been performed previously with reported decreases in hippocampal area of about 15% (Moore et al., 2006).
Figure 2.
Histological localization of electrode placements. A representative photomicrograph depicting bilateral electrode locations within the vHipp is depicted in A. The group data for bilateral (B) and unilateral (C) implantations are included schematically. A representative photomicrograph depicting cannula location within the VP is shown in D while the group data are below (E).
Analysis
Electrophysiological analysis of single unit neuron activity was performed using commercial computer software (LabChart Pro – ADInstruments), whilst locomotor behavior was recorded using Open Field Activity Software (Med Associates). All data are represented as the mean ± standard error of the mean (SEM) unless otherwise stated, with n-values depicting the number of animals per experimental group. Statistics were calculated using either SigmaPlot (Systat Software Inc) or Statistica (StatSoft Inc).
Materials
Methylazoxymethanol acetate (MAM) was purchased from Midwest Research Institute (Kansas City, MO). Pentobarbital sodium (USP) was obtained from Lundbeck (Deerfield, IL). Chloral Hydrate, Pentobarbital sodium (non-USP), Dulbecco’s phosphate buffered saline, and D-Amphetamine sulfate were all purchased from Sigma (St Louis, MO). All other chemicals and reagents were of either analytical or laboratory grade and purchased from various suppliers.
Results
Dopamine Neuron Electrophysiology
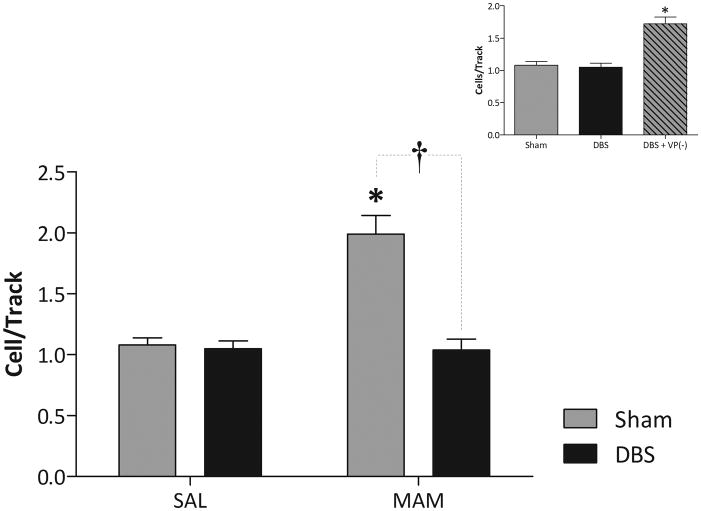
Sham rats (vHipp electrodes implanted but not stimulated) that received GD17 saline injections (n = 6 rats) exhibited an average of 1.08 ± 0.06 spontaneously active dopamine neurons per electrode track, consistent with previous findings in untreated rats (Lodge and Grace, 2007). Sham rats administered MAM prenatally (for review see (Lodge and Grace, 2009)) at GD17 (n = 6 rats) exhibited significantly greater (~2-fold) dopamine neuron population activity (1.99 ± 0.15 cell/track; 2-way ANOVA; F(MAM)=21.484, F(DBS)=25.807, F(MAMxDBS)=22.533; Holm-Sidak; t = 6.514; p<0.05; Fig 3), again consistent with our previous findings (Lodge and Grace, 2007). DBS of the vHipp (130Hz, 0.3mA, 0.1ms duration) (n = 6 rats) completely normalized the aberrant dopamine neuron population activity observed in MAM-treated rats to a level not significantly different from saline-treated rats (1.04 ± 0.09 cells/track; 2-way ANOVA; Holm-Sidak; t = 6.823; p<0.05), while vHipp DBS did not significantly alter dopamine neuron population activity in saline-treated rats (n = 7 rats: 1.05 ± 0.07 cells/track; 2-way ANOVA; Holm-Sidak; t = 0.240; p>0.05; Fig 3).
Figure 3.
vHipp DBS normalizes aberrant dopamine neuron activity in the MAM model of schizophrenia (main graph). * Represents significant difference from saline-sham whereas † represents significant difference from MAM-sham p<0.05 two-way ANOVA, n=6–7 rats/group). VP inactivation still produces an increase in dopamine neuron population activity during DBS (inset). * Represents significant difference from both sham and DBS only, saline-treated animals, n=4–7 rats/group).
To confirm that increases in population activity could be observed during DBS, we chemically inactivated the VP in saline-treated rats. Consistent with previous observations (Floresco et al., 2003), VP inhibition significantly increased dopamine neuron activity (n = 4 rats: 1.72 ± 0.10 cells/track; 1-way ANOVA; F=22.495; Holm-Sidak; p<0.05: Fig 3 inset) in DBS-treated rats when compared to both sham (t = 5.777, p<0.05) and DBS only, saline-treated rats (t = 6.250, p<0.05).
Amphetamine-Induced Hyper-Locomotion
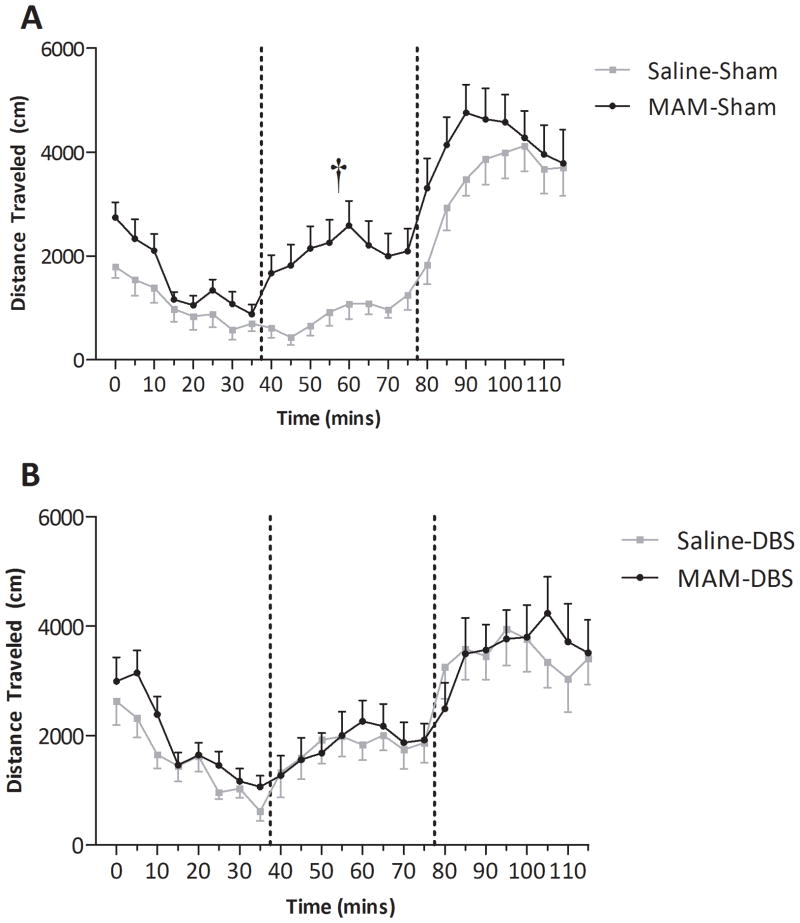
Sham, MAM-treated rats displayed a significantly enhanced locomotor response to low dose (0.5mg/kg, i.p.) amphetamine administration when compared to sham, saline-treated controls (3-way ANOVA of 0.5mg/kg dose; F(MAM)=23.462, F(DBS)=6.136, F(MAMxDBS)=19.233; Holm-Sidak t=6.450, p<0.05; Fig 4), without changes in baseline activity (3-way ANOVA of baseline; F(MAMxDBS)=0.372; p>0.05) or in response to the higher dose (3-way ANOVA of 2.0mg/kg dose; F(MAMxDBS)= 2.551; p>0.05) consistent with previous observations (Lodge and Grace, 2007). This augmented locomotor activity was only observed with low dose amphetamine administration, as this is likely more sensitive to changes in impulse-dependent dopamine neuron activity. Consistent with the effects on dopamine neuron activity (Fig 3), MAM-treated rats administered vHipp DBS no longer demonstrated an enhanced locomotor response to amphetamine when compared to saline-treated rats administered DBS (3-way ANOVA of 0.5mg/kg dose; Holm-Sidak t= 0.328; p>0.05; Fig 4). These data confirm that the beneficial effect of DBS on dopamine neuron activity translates into a normalization of aberrant dopamine-mediated behaviors in a rodent model of schizophrenia. It should be noted that DBS augmented amphetamine-induced locomotor activity in saline-treated rats following the 0.5mg/kg dose only (3-way ANOVA; Holm-Sidak t=4.629 ; p<0.05; Fig 4).
Figure 4.
vHipp DBS normalizes the augmented locomotor response to amphetamine. MAM-treated rats display a significantly greater locomotor response to low-dose (0.5mg/kg 1st dashed line), but not high-dose (2.0mg/kg 2nd dashed line) when compare to saline-treated rats (A). This increased locomotor response was not observed following vHipp DBS (B). † Represents significant difference from saline-sham following the 0.5mg/kg dose (three-way ANOVA, n=11–14 rats/group).
Attentional Set Shifting
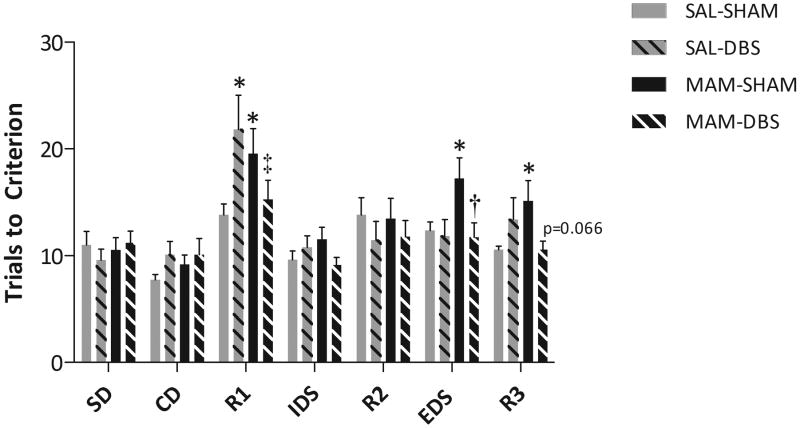
MAM-treated rats displayed deficits in reversal learning and extra-dimensional set shifting when compared to saline-treated rats, consistent with previous observations (Featherstone et al., 2007; Gastambide et al., 2011) (3-way RM-ANOVA; F(Task)=12.888, F(TaskxDBS)=2.646, F(TaskxMAMxDBS)=3.432; LSD test; p<0.05; Fig 5). Saline-treated rats administered DBS displayed aberrant reversal learning as demonstrated by an increase in the number of trials to meet criteria during the first reversal task (3-way RM-ANOVA; LSD test; p<0.05; Fig 5). Interestingly, the same treatment that disturbed function in saline-treated animals actually normalized reversal learning in MAM-treated rats. Thus, MAM-treated rats with vHipp DBS displayed similar cognitive flexibility as saline-treated rats, with a complete reversal of the extra-dimensional set shifting deficits and a similar trend to normalize deficits in reversal learning (3-way RM-ANOVA; LSD test; p<0.05; Fig 5). Taken together these data suggest that the benefits of vHipp DBS appear to be not simply limited to attenuating positive symptoms, but may also be effective at treating cognitive dysfunction.
Figure 5.
vHipp DBS restored deficits in cognitive flexibility. MAM-treated rats display a deficit in reversal learning (R1 & R3) and extra-dimensional set shifting (ED) when compare to saline-treated rats. The deficit in ED was completely reversed by vHipp DBS while a similar trend was observed for reversal learning. * Represents significant difference from saline-sham, † represents significant difference from MAM-sham, and ‡ represents significant difference from saline-DBS (three-way RM-ANOVA).
Discussion
Increasing evidence from clinical and preclinical studies suggests that the augmented dopamine system function in schizophrenia may be secondary to aberrant hippocampal activity (Heckers and Konradi, 2010; Lodge and Grace, 2007; Lodge and Grace, 2011; Medoff et al., 2001; Schobel et al., 2009). Thus, we suggest that the hippocampus represents a potential novel therapeutic target for the treatment of psychosis in schizophrenia patients (Lodge and Grace, 2011). To examine this hypothesis, we employ a rodent model of schizophrenia, namely MAM GD17, that displays deficits consistent with those observed in schizophrenia patients, including anatomical changes (deficits in parvalbumin expression and subtle reductions in the volume of medial prefrontal cortex & hippocampus), behavioral deficits (decreased prepulse inhibition of startle, stimulant induced hyperlocomotion & deficits in cognitive flexibility) and altered neuronal information processing (hippocampal and dopamine neuron hyperactivity) – for review see (Lodge and Grace, 2009). Here we utilize this model to demonstrate that DBS of the vHipp can normalize aberrant dopamine neuron activity and associated behaviors. Furthermore, the beneficial effects of DBS are not limited to behaviors associated with positive symptoms, but also appear to normalize aberrant cognitive flexibility. To the best of our knowledge this is the first experimental evidence examining the feasibility of DBS as a potential novel therapeutic approach for the treatment of schizophrenia.
As detailed above, augmented dopamine system function in schizophrenia is thought to be mediated by aberrant hippocampal activity (Lodge and Grace, 2007; Lodge and Grace, 2011). We have previously demonstrated that the vHipp can selectively regulate the number of spontaneously active dopamine neurons in the VTA, thought to regulate the gain of the dopamine system (Floresco et al., 2001; Floresco et al., 2003; Lodge and Grace, 2006). Specifically, activation of the vHipp leads to an increase in the number of spontaneously active dopamine neurons without altering their firing rate or burst firing pattern (Floresco et al., 2001; Floresco et al., 2003; Lodge and Grace, 2006). This effect is likely mediated by a pathway including the nucleus accumbens (NAc) and VP (Figure 1), as it is blocked by accumbal glutamatergic antagonist administration and mimicked by VP inactivation (Floresco et al., 2001; Floresco et al., 2003). Similarly, MAM-treated rats display an increase in the number of spontaneously active dopamine neurons (Gill et al., 2011; Lodge and Grace, 2007). We have previously demonstrated that this is attributable to aberrant hippocampal activity, as it can be completely normalized by inactivation of the vHipp (Gill et al., 2011; Lodge and Grace, 2007). Clinical and preclinical data report that DBS can attenuate hippocampal activity (Boon et al., 2007; Wyckhuys et al., 2010), thus we posit that DBS may provide a novel therapeutic approach for the treatment of schizophrenia. Indeed, here we provide initial evidence that vHipp DBS can normalize aberrant dopamine neuron population activity in MAM-treated rats without observable effects on dopamine system function in saline-treated animals. The finding that vHipp DBS did not alter dopamine neuron activity in control animals is consistent with our previous observations that tetrodotoxin-inactivation of the vHipp does not alter dopamine system function (Lodge and Grace, 2007; Lodge and Grace, 2008) and likely reflects the low-spontaneous activity of vHipp pyramidal neurons under ‘normal’ conditions (Jung et al., 1994; Lodge and Grace, 2007).
Given the technical challenges associated with recording spontaneously active neurons during high frequency electrical stimulation (i.e. 130 stimulus artifacts per second) it is possible that that the differences seen with DBS may be due to technical limitations rather than due to a therapeutic effect. We do not believe this to be the case as dopamine neuron population activity was unchanged by DBS in saline-treated animals (Fig 3) and dopamine neurons can be clearly isolated during DBS (Supplementary Movie 1). Nonetheless, to confirm that increases in population activity could be observed during DBS, we chemically inactivated the VP, a manipulation that increases dopamine neuron activity downstream of the hippocampus (Floresco et al., 2003) and should therefore not be affected by DBS (Fig 1). VP inhibition was able to significantly increase dopamine neuron activity in saline-treated rats receiving vHipp DBS. These data were included, not to inform on the mechanisms underlying the effects of DBS, but rather to demonstrate that the effects of DBS on dopamine neuron population activity are not simply associated with potential technical limitations of the study.
As a correlate for the beneficial effects of vHipp DBS on dopamine neuron activity we examined whether DBS also reversed behavioral deficits analogous to those observed in schizophrenia patients. To assess the effectiveness of DBS against positive symptoms, we examined the hyper-responsivity to psychomotor stimulants, which is consistently observed in both animal models and schizophrenia patients alike (Laruelle et al., 1996; Lodge and Grace, 2007; Moore et al., 2006). It has been suggested that an enhanced sensitivity to psychomotor stimulants is associated with an enhanced baseline dopamine neuron population activity secondary to vHipp hyperactivity (Lodge and Grace, 2007; Lodge and Grace, 2011). Thus, an increase in dopamine neuron activity would be expected to augment impulse-dependent dopamine release induced by amphetamine-mediated inhibition of the dopamine transporter. Consistent with this hypothesis, MAM-treated rats display an enhanced response to low-dose amphetamine (Lodge and Grace, 2007; Moore et al., 2006). This augmented response was abolished by DBS such that there were no significant differences in the response to amphetamine in saline- or MAM-treated rats. It should be noted that DBS augmented amphetamine-induced locomotor activity in saline-treated rats, an effect that was not observed in MAM-treated rats. DBS alone did not alter dopamine neuron activity, therefore the effects of DBS in saline-treated rats may be mediated by inputs to the NAc arising from regions such as the prefrontal cortex; however, this requires further investigation. Taken together, these results demonstrate that vHipp DBS effectively normalizes aberrant dopamine neuron activity and the hyper-responsivity to psychomotor stimulants in a rodent model of schizophrenia.
Given that long-term hippocampal DBS is well tolerated in human studies (Boon et al., 2007; Velasco et al., 2007), our preliminary evidence suggests that DBS may provide a novel therapeutic approach to treat the positive symptoms of schizophrenia. It is important to note, however, that schizophrenia patients not only display positive symptoms, but also cognitive symptoms that are arguably as debilitating and not as effectively treated with conventional antipsychotics (Meltzer and McGurk, 1999). The hippocampus also regulates prefrontal cortical function (Thierry et al., 2000), therefore it is also possible that hippocampal pathology may also contribute to cognitive deficits observed in patients (Meltzer and McGurk, 1999). Thus, we examined the effects of DBS on cognitive symptoms using an AST paradigm (Lapiz and Morilak, 2006).
Consistent with previous observations, MAM-treated rats display deficits in both reversal learning and extra-dimensional set shifting. Hippocampal DBS was able to completely reverse these deficits in MAM-treated rats, suggesting that DBS may also be effective at treating cognitive deficits in schizophrenia patients. Extra-dimensional set shifting is known to be dependent on medial prefrontal cortical function (mPFC) in rodents (Birrell and Brown, 2000). Thus, the deficits in extra-dimensional set shifting observed in the MAM model likely reflect aberrant mPFC function. This could be attributable to either a direct pathology within the mPFC (for example, a loss of parvalbumin (Lodge et al., 2009)), or aberrant activity of afferent inputs (i.e. from the vHipp (Lodge and Grace, 2007)). Moreover, extensive literature demonstrating a relationship between dopamine and PFC performance (Floresco and Magyar, 2006; Vijayraghavan et al., 2007), suggesting that the aberrant dopamine transmission observed in MAM-treated rats may also contribute to deficits in extra-dimensional set-shifting. Thus, the ability of vHipp DBS to reverse deficits in extra-dimensional set-shifting are likely due to either attenuation of aberrant vHipp-mPFC transmission or restoration of dopamine system function; however, the exact mechanisms remain to be elucidated.
While extra-dimensional set shifting likely involves mPFC regions, reversal learning is more often associated with orbitofrontal cortex function (OFC) (McAlonan and Brown, 2003). Interestingly, the OFC does not receive a strong hippocampal projection, suggesting that the deficits in reversal learning observed in the MAM-treated rats are likely associated with either a direct pathology within the OFC or upstream alterations in OFC transmission (i.e. aberrant dopamine system function). It should be noted that the OFC receives reciprocal projections from the entorhinal cortex (Ongur and Price, 2000) and given that DBS can activate axonal fibers, combined with the considerable input to the hippocampus from the entorhinal cortex, it is possible that DBS may indirectly alter OFC activity. Indeed, vHipp DBS produced a deleterious effect in saline-treated rats with a significant impairment of reversal learning observed. This deficit was only observed during the first reversal task, as this task appears to be more sensitive to manipulation (Lapiz-Bluhm et al., 2009). In contrast, the opposite response was observed in MAM-treated rats, (i.e. a beneficial effect of DBS on reversal learning) possibly attributable to a normalization of aberrant dopamine neuron activity.
Taken as a whole, here we provide the first experimental evidence demonstrating the feasibility of ventral hippocampal DBS to reverse aberrant dopamine neuron activity and behaviors associated with positive symptoms in a rodent model of schizophrenia. In addition, we provide evidence that this approach may also be effective in restoring deficits in cognitive function, often left unaltered by conventional antipsychotic medications. Given that long-term DBS of the anterior hippocampus has been previously investigated in human patients and is tolerated with minimal side effects (Boon et al., 2007; Velasco et al., 2007), we provide initial evidence that DBS may present as a novel therapeutic approach for the treatment of schizophrenia. Moreover, we provide rationale for the specific targeting of the anterior hippocampus in this disease.
Supplementary Material
Spontaneously active dopamine neuron electrophysiology during 130Hz DBS of the vHipp. The identification of spontaneously active dopamine neurons during DBS is potentially confounded by the repeated stimulus artifacts occurring 130 times per second. This video demonstrates that spontaneously activity dopamine neurons can indeed be recorded during high frequency stimulation. Note the stimulus artifacts in the video appear superimposed on the left hand side of the oscilloscope. The time-base on the oscilloscope is 0.5ms/division.
Acknowledgments
The authors would like to thank Dr. David Morilak and Julianne Jett for their help and expertise with the attentional set shifting task. This work was supported by the NIH (R01: MH090067) and a NARSAD award from the Maltz Family Foundation.
Footnotes
Conflict of Interest
The authors have no competing financial interests in relation to the work described in this manuscript. Dr. Lodge discloses receiving consulting fees from Dey Pharmaceuticals, while Perez, Shah and Asher do not have any conflicts of interest to report.
References
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon P, Vonck K, De Herdt V, Van Dycke A, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48(8):1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptors and average clinical doses. Science. 1976;194(4264):546. doi: 10.1126/science.194.4264.546. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Nobrega JN, Kapur S, et al. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: Parallels to schizophrenia. Neuropsychopharmacology. 2007;32(2):483–492. doi: 10.1038/sj.npp.1301223. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: Beyond working memory. Psychopharmacology (Berl) 2006;188(4):567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, et al. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Cotel MC, Gilmour G, O’Neill MJ, et al. Selective Remediation of Reversal Learning Deficits in the Neurodevelopmental MAM Model of Schizophrenia by a Novel mGlu5 Positive Allosteric Modulator. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, et al. A novel α5GABAAR-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36(9):1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10(2):301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Current topics in behavioral neurosciences. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JNJ, Hay J, Perk CG, et al. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114(2):475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. Journal of Neuroscience. 1994;14(12):7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krack P, Hariz MI, Baunez C, Guridi J, et al. Deep brain stimulation: From neurology to psychiatry? Trends in Neurosciences. 2010;33(10):474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MDS, Soto-Piña AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology (Berl) 2009;202(1–3):329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137(3):1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Van Dyck CH, Gil R, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Science. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski NM, Schobel SS, Wu WE, Corcoran C, et al. Isolating hippocampal subregions most vulnerable to schizophrenia. Annual Society for Neuroscience Meeting; 2005. Program number: 443.1. [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of neuroscience : the official journalof the Society for Neuroscience. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31(7):1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28(31):7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: A developmental disruption model of schizophrenia. Behav Brain Res. 2009;204(2):306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends in Pharmacological Sciences. 2011;32(9):507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, et al. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry. 1999;46(1):89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146(1–2):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia Bulletin. 1999;25(2):233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Pascau J, Sanz J, et al. Anatomical and functional cerebral variables associated with basal symptoms but not risperidone response in minimally treated schizophrenia. Psychiatry Research-Neuroimag ing. 2003;124(3):163–175. doi: 10.1016/s0925-4927(03)00107-0. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, et al. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60(3 ):253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press Australia; 1986. [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Chau Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci U S A. 1975;72(11):4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilo SA, Murray RM. The epidemiology of schizophrenia: Replacing dogma with knowledge. Dialogues Clin Neurosci. 2010;12(3):305–315. doi: 10.31887/DCNS.2010.12.3/sstilo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. American Journal of Psychiatry. 2010;167(10):1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Dégénétais E, Glowinski J. Hippocampo-prefrontal cortex pathway: Anatomical and electrophysiological characteristics. Hippocampus. 2000;10(4):411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Velasco AL, Velasco F, Velasco M, Trejo D, et al. Electrical stimulation of the hippocampal epileptic foci for seizure control: A double-blind, long-term follow-up study. Epilepsia. 2007;48(10):1895–1903. doi: 10.1111/j.1528-1167.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wyckhuys T, Staelens S, Van Nieuwenhuyse B, Deleye S, et al. Hippocampal deep brain stimulation induces decreased rCBF in the hippocampal formation of the rat. NeuroImage. 2010;52(1):55–61. doi: 10.1016/j.neuroimage.2010.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spontaneously active dopamine neuron electrophysiology during 130Hz DBS of the vHipp. The identification of spontaneously active dopamine neurons during DBS is potentially confounded by the repeated stimulus artifacts occurring 130 times per second. This video demonstrates that spontaneously activity dopamine neurons can indeed be recorded during high frequency stimulation. Note the stimulus artifacts in the video appear superimposed on the left hand side of the oscilloscope. The time-base on the oscilloscope is 0.5ms/division.