Abstract
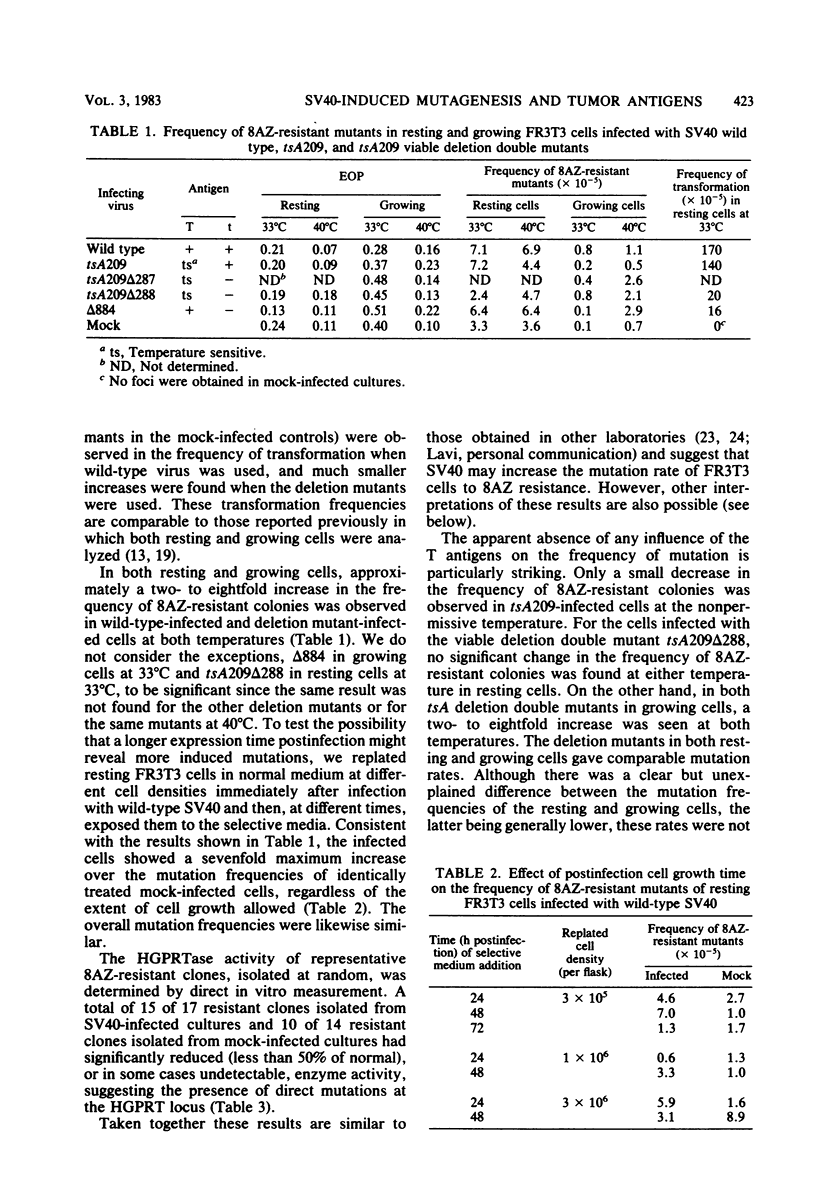
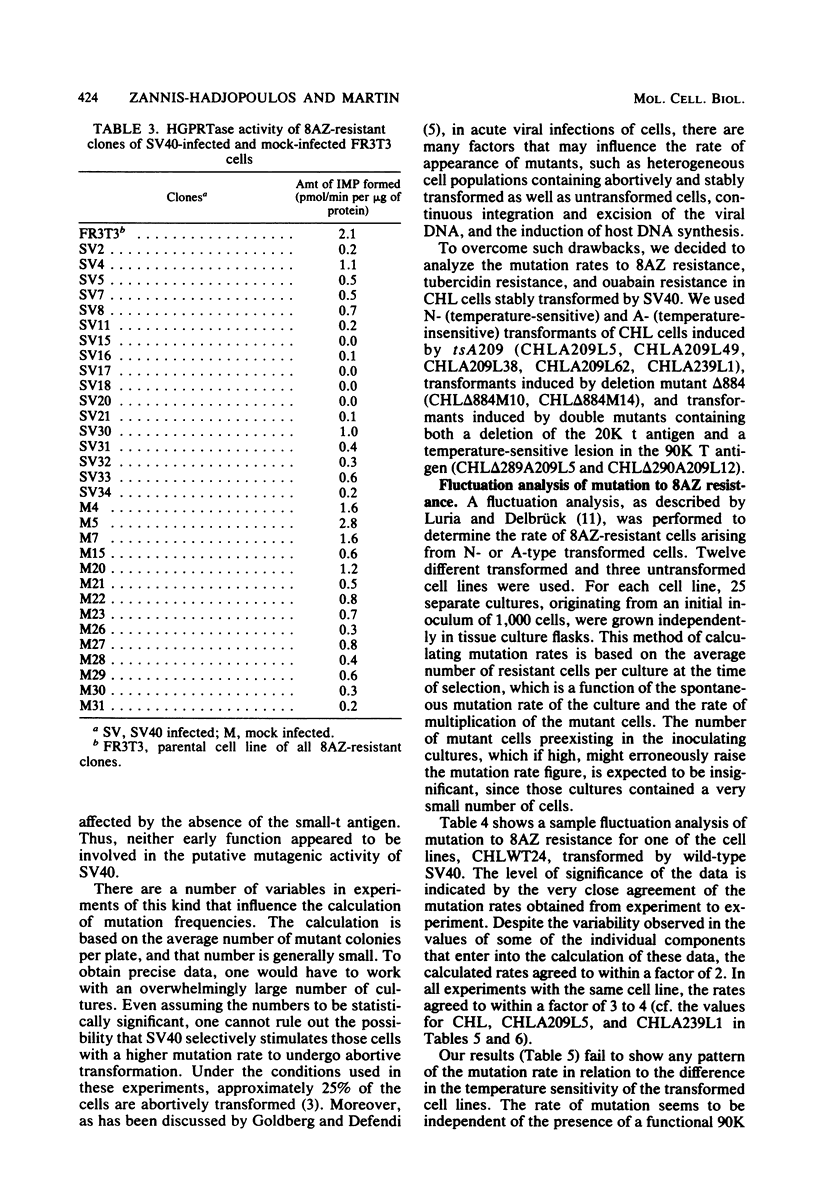
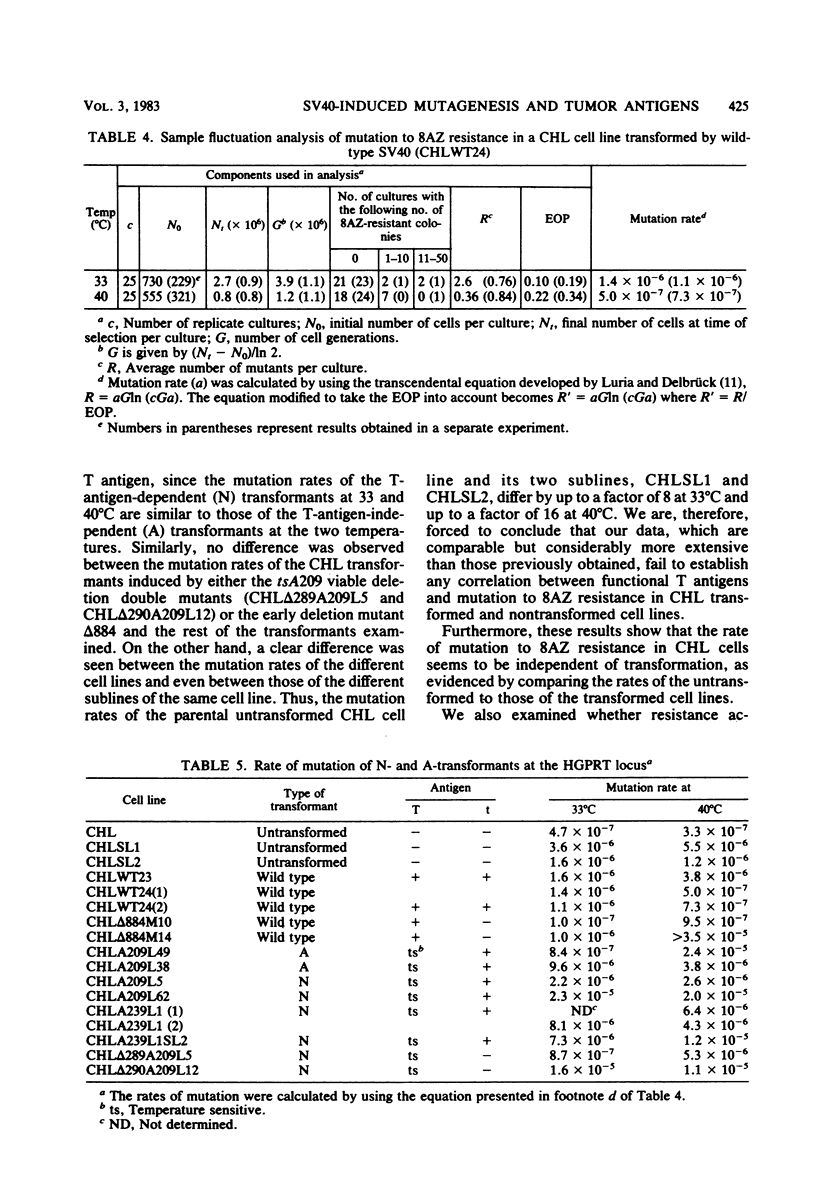
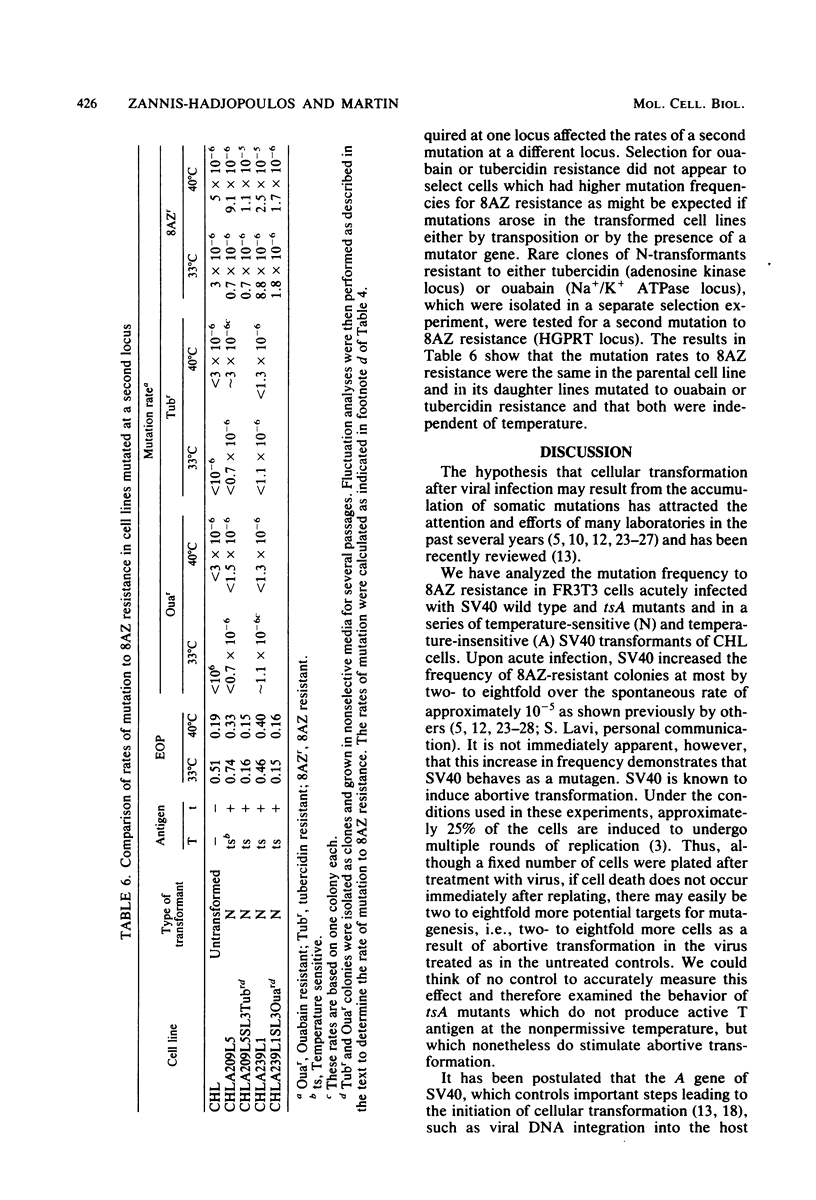
We analyzed the mutation frequency to 8-azaguanine (8AZ) resistance in rat FR3T3 cells acutely infected with simian virus 40 wild type and tsA and early deletion mutants and in a series of temperature-sensitive (N) and temperature-insensitive (A) transformants derived from Chinese hamster lung (CHL) cells. Upon acute infection, the frequency of mutation to 8AZ resistance was raised at most by two- to eightfold over the spontaneous frequency, and it was independent of the presence of a functional 90,000-molecular-weight T antigen or 20,000-molecular-weight t antigen or both. Similarly, in the stable transformants of CHL cells, no correlation was found between functional T antigens and mutation to 8AZ resistance. It therefore seems unlikely that simian virus 40-induced transformation results from any mutagenic activity of this virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chepelinsky A. B., Seif R., Martin R. G. Integration of the simian virus 40 genome into cellular DNA in temperature-sensitive (N) and temperature-insensitive (A) transformants of 3T3 rat and Chinese hamster lung cells. J Virol. 1980 Jul;35(1):184–193. doi: 10.1128/jvi.35.1.184-193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. From the molecular biology of oncogenic DNA viruses to cancer. Science. 1976 Apr 30;192(4238):437–440. doi: 10.1126/science.1257779. [DOI] [PubMed] [Google Scholar]
- Fluck M. M., Benjamin T. L. Comparisons of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979 Jul 15;96(1):205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S., Defendi V. Increased mutation rates in doubly viral transformed Chinese hamster cells. Somatic Cell Genet. 1979 Nov;5(6):887–895. doi: 10.1007/BF01542648. [DOI] [PubMed] [Google Scholar]
- Hirai K., Defendi V. The effects of serum concentration on the level of integration of simian virus (SV40) genome and the transformation frequency in SV40-infected Chinese hamster cells. Virology. 1976 Jan;69(1):229–236. doi: 10.1016/0042-6822(76)90209-9. [DOI] [PubMed] [Google Scholar]
- Horan P. K., Jett J. H., Romero A., Lehman J. M. Flow microfluorometry analysis of DNA content in Chinese hamster cells following infection with simian virus 40. Int J Cancer. 1974 Oct 15;14(4):514–521. doi: 10.1002/ijc.2910140411. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Defendi V. Changes in deoxyribonucleic acid synthesis regulation in Chinese hamster cells infected with simian virus 40. J Virol. 1970 Dec;6(6):738–749. doi: 10.1128/jvi.6.6.738-749.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. M. Early chromosome changes in diploid Chinese hamster cells after infection with Simian virus 40. Int J Cancer. 1974 Feb 15;13(2):164–172. doi: 10.1002/ijc.2910130203. [DOI] [PubMed] [Google Scholar]
- Lukash L. L., Buzhievskaya T. I., Varshaver N. B., Shapiro N. I. Oncogenic adenovirus as mutagen for chinese hamster cells in vitro. Somatic Cell Genet. 1981 Mar;7(2):133–146. doi: 10.1007/BF01567653. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak M. I., Varshaver N. B., Shapiro N. I. Induction of gene mutations and chromosomal aberrations by simian virus 40 in cultured mammalian cells. Mutat Res. 1975 Dec;30(3):383–396. [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A. Roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells: studies with simian virus 40 double mutants. J Virol. 1979 Sep;31(3):596–607. doi: 10.1128/jvi.31.3.596-607.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Martin R. G. The transformation of cell growth and transmogrification of DNA synthesis by simian virus 40. Adv Cancer Res. 1981;34:1–68. doi: 10.1016/s0065-230x(08)60238-9. [DOI] [PubMed] [Google Scholar]
- Pollack R., Vogel A. Isolation and characterization of revertant cell lines. II. Growth control of a polyploid revertant line derived from SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Aug;82(1):93–100. doi: 10.1002/jcp.1040820111. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Seif R., Cuzin F. Conditions leading to the establishment of the N (a gene dependent) and A (a gene independent) transformed states after polyoma virus infection of rat fibroblasts. J Virol. 1978 Nov;28(2):421–426. doi: 10.1128/jvi.28.2.421-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. The transforming genes of SV40 and polyoma. Nature. 1979 Dec 20;282(5741):781–784. doi: 10.1038/282781a0. [DOI] [PubMed] [Google Scholar]
- San R. H., Stich H. F. DNA repair and chromatid anomalies in mammalian cells exposed to 4-nitroquinoline I-oxide. Mutat Res. 1970 Oct;10(4):389–404. doi: 10.1016/0027-5107(70)90051-5. [DOI] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Growth state of the cell early after infection with simian virus 40 determines whether the maintenance of transformation will be A-gene dependent or independent. J Virol. 1979 Aug;31(2):350–359. doi: 10.1128/jvi.31.2.350-359.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow V. P., Persico-Dilauro M., Edwards C. A., Martin R. G. The isolation of SV40 tsA/deletion, double mutants and the induction of host DNA synthesis. Virology. 1980 Feb;101(1):250–260. doi: 10.1016/0042-6822(80)90500-0. [DOI] [PubMed] [Google Scholar]
- Theile M., Scherneck S., Geissler E. Mutagenesis by simian virus 40. I. detection of mutations in Chinese hamster cell lines using different resistance markers. Mutat Res. 1976 Oct;37(1):111–123. doi: 10.1016/0027-5107(76)90059-2. [DOI] [PubMed] [Google Scholar]
- Theile M., Scherneck S., Greissler E. DNA of simian virus 40 mutates Chinese hamster cells. Arch Virol. 1980;65(3-4):293–309. doi: 10.1007/BF01314545. [DOI] [PubMed] [Google Scholar]
- Theile M., Strauss M., Luebbe L., Scherneck S., Krause H., Geissler E. SV40-induced somatic mutations: possible relevance to viral transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):377–382. doi: 10.1101/sqb.1980.044.01.042. [DOI] [PubMed] [Google Scholar]
- Theile M., Strauss M. Mutagenesis by simian virus 40. II. Changes in substrate affinities in mutant hypoxanthine-guanine phosphoribosyl transferase enzymes at different pH values. Mutat Res. 1977 Oct;45(1):111–123. doi: 10.1016/0027-5107(77)90049-5. [DOI] [PubMed] [Google Scholar]
- WOLMAN S. R., HIRSCHHORN K., TODARO G. J. EARLY CHROMOSOMAL CHANGES IN SV40-INFECTED HUMAN FIBROBLAST CULTURES. Cytogenetics. 1964;3:45–61. doi: 10.1159/000129797. [DOI] [PubMed] [Google Scholar]
- Zuna R. E., Lehman J. M. Heterogeneity of karyotype and growth potential in simian virus 40-transformed Chinese hamster cell clones. J Natl Cancer Inst. 1977 May;58(5):1463–1472. doi: 10.1093/jnci/58.5.1463. [DOI] [PubMed] [Google Scholar]


