Abstract
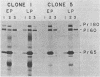
Some molecular changes which correlate with the tumorigenic progression of neoplastic cells can best be studied with in vitro cell lines that represent each stage in the progression. Lymphoid cells infected by Abelson murine leukemia virus exhibit a wide range of growth potential in vitro and in vivo. Uncloned populations that are poorly oncogenic early after infection become progressively more oncogenic with successive passages of the cells in culture. In such mass cultures, it is difficult to evaluate whether a rare subpopulation of highly oncogenic cells becomes dominant in the culture or whether the individual cells progress in oncogenic phenotype. To examine this latter possibility, Abelson virus-infected lymphoid cells were cloned by limiting-dilution culture 10 days postinfection. We isolated two clones that grew poorly in agar, required feeder layers of adherent bone marrow cells for growth in liquid culture, and were extremely slow to form tumors in syngeneic animals. Both clones, after passage in the presence of adherent feeder layers for 3 months, grew well in liquid and agar-containing cultures in the absence of feeder layers and formed tumors in animals at a rapid rate. The progression of these clonal cell lines to a more malignant growth phenotype occurred in the absence of detectable changes in the concentration, half-life, phosphorylation, in vitro kinase activity, or cell localization of the Abelson virus-encoded transforming protein. No change in the concentration or arrangement of integrated Abelson viral DNA sequences was detected in either clone. Thus, perhaps changes in the expression of cellular genes would appear to alter the growth properties of lymphoid cells after their initial transformation by Abelson virus. Such cellular changes could complement the activity of the Abelson virus transforming protein in producing the fully malignant growth phenotype.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. E., Majumdar S. K., Wivell J. A., Terry R. W. Tumorigenicity of Friend murine erythroleukemia cell lines differing in spontaneous differentiation rates. Int J Cancer. 1980 Dec 15;26(6):799–804. doi: 10.1002/ijc.2910260614. [DOI] [PubMed] [Google Scholar]
- Boss M. A., Dreyfuss G., Baltimore D. Localization of the Abelson murine leukemia virus protein in a detergent-insoluble subcellular matrix: architecture of the protein. J Virol. 1981 Nov;40(2):472–481. doi: 10.1128/jvi.40.2.472-481.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980 Dec;56(6):947–958. [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Paraskeva C. A study to determine the reasons for differences in the tumorigenicity of rat cell lines transformed by adenovirus 2 and adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):703–713. doi: 10.1101/sqb.1980.044.01.075. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Witte O. N., Gilboa E., Rosenberg N., Baltimore D. Genome structure of Abelson murine leukemia virus variants: proviruses in fibroblasts and lymphoid cells. J Virol. 1981 May;38(2):460–468. doi: 10.1128/jvi.38.2.460-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimura T., Gonzalez R., Nicolson G. L. Effects of tunicamycin on B16 metastatic melanoma cell surface glycoproteins and blood-borne arrest and survival properties. Cancer Res. 1981 Sep;41(9 Pt 1):3411–3418. [PubMed] [Google Scholar]
- Lane M. A., Sainten A., Cooper G. M. Stage-specific transforming genes of human and mouse B- and T-lymphocyte neoplasms. Cell. 1982 Apr;28(4):873–880. doi: 10.1016/0092-8674(82)90066-6. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Tumors; a mixed bag of cells. Science. 1982 Jan 15;215(4530):275–277. doi: 10.1126/science.7053575. [DOI] [PubMed] [Google Scholar]
- Mintz B. Malignancy vs. normal differentiation of stem cells as analyzed in genetically mosaic animals. Adv Pathobiol. 1977;(6):153–157. [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Patek P. Q., Collins J. L., Cohn M. Transformed cell lines susceptible or resistant to in vivo surveillance against tumorigenesis. Nature. 1978 Nov 30;276(5687):510–511. doi: 10.1038/276510a0. [DOI] [PubMed] [Google Scholar]
- Pollack R., Lo A., Steinberg B., Smith K., Shure H., Blanck G., Verderame M. SV40 and cellular gene expression in the maintenance of the tumorigenic syndrome. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):681–688. doi: 10.1101/sqb.1980.044.01.072. [DOI] [PubMed] [Google Scholar]
- Ponticelli A. S., Whitlock C. A., Rosenberg N., Witte O. N. In vivo tyrosine phosphorylations of the Abelson virus transforming protein are absent in its normal cellular homolog. Cell. 1982 Jul;29(3):953–960. doi: 10.1016/0092-8674(82)90458-5. [DOI] [PubMed] [Google Scholar]
- Poste G., Doll J., Hart I. R., Fidler I. J. In vitro selection of murine B16 melanoma variants with enhanced tissue-invasive properties. Cancer Res. 1980 May;40(5):1636–1644. [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Reading C. L., Brunson K. W., Torrianni M., Nicolson G. L. Malignancies of metastatic murine lymphosarcoma cell lines and clones correlate with decreased cell surface display of RNA tumor virus envelope glycoprotein gp70. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5943–5947. doi: 10.1073/pnas.77.10.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter V., Boss M. A., Baltimore D. Increased concentration of an apparently identical cellular protein in cells transformed by either Abelson murine leukemia virus or other transforming agents. J Virol. 1981 Apr;38(1):336–346. doi: 10.1128/jvi.38.1.336-346.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Control of cell differentiation in normal hematopoietic and leukemic cells. Adv Pathobiol. 1977;(6):124–140. [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Burton R. C., Holland J. M., Kripke M. L., Schreiber H. Mechanisms of syngeneic tumor rejection. Susceptibility of host-selected progressor variants to various immunological effector cells. J Exp Med. 1982 Feb 1;155(2):557–573. doi: 10.1084/jem.155.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke R. A., Slavin S., Coffman R. L., Butcher E. C., Knapp M. R., Strober S., Weissman I. L. The pathology and homing of a transplantable murine B cell leukemia (BCL1). J Immunol. 1979 Sep;123(3):1181–1188. [PubMed] [Google Scholar]
- Wendling F., Moreau-Gachelin F., Tambourin P. Emergence of tumorigenic cells during the course of Friend virus leukemias. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3614–3618. doi: 10.1073/pnas.78.6.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Abelson virus-infected cells can exhibit restricted in vitro growth and low oncogenic potential. J Virol. 1981 Nov;40(2):577–584. doi: 10.1128/jvi.40.2.577-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Whitlock C. A., Goff S. P., Gifford A., Witte O. N. Lethal effect of the Abelson murine leukemia virus transforming gene product. Cell. 1981 Dec;27(3 Pt 2):477–486. doi: 10.1016/0092-8674(81)90389-5. [DOI] [PubMed] [Google Scholar]