Abstract
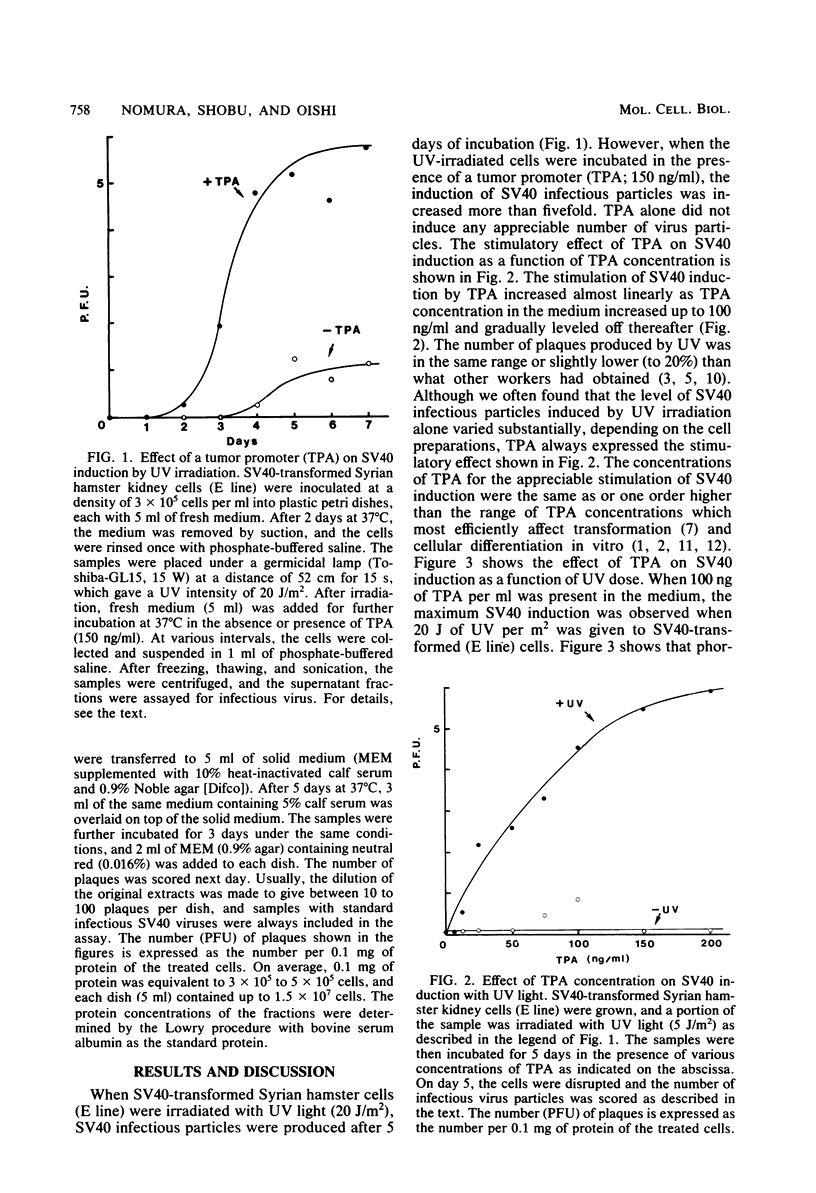
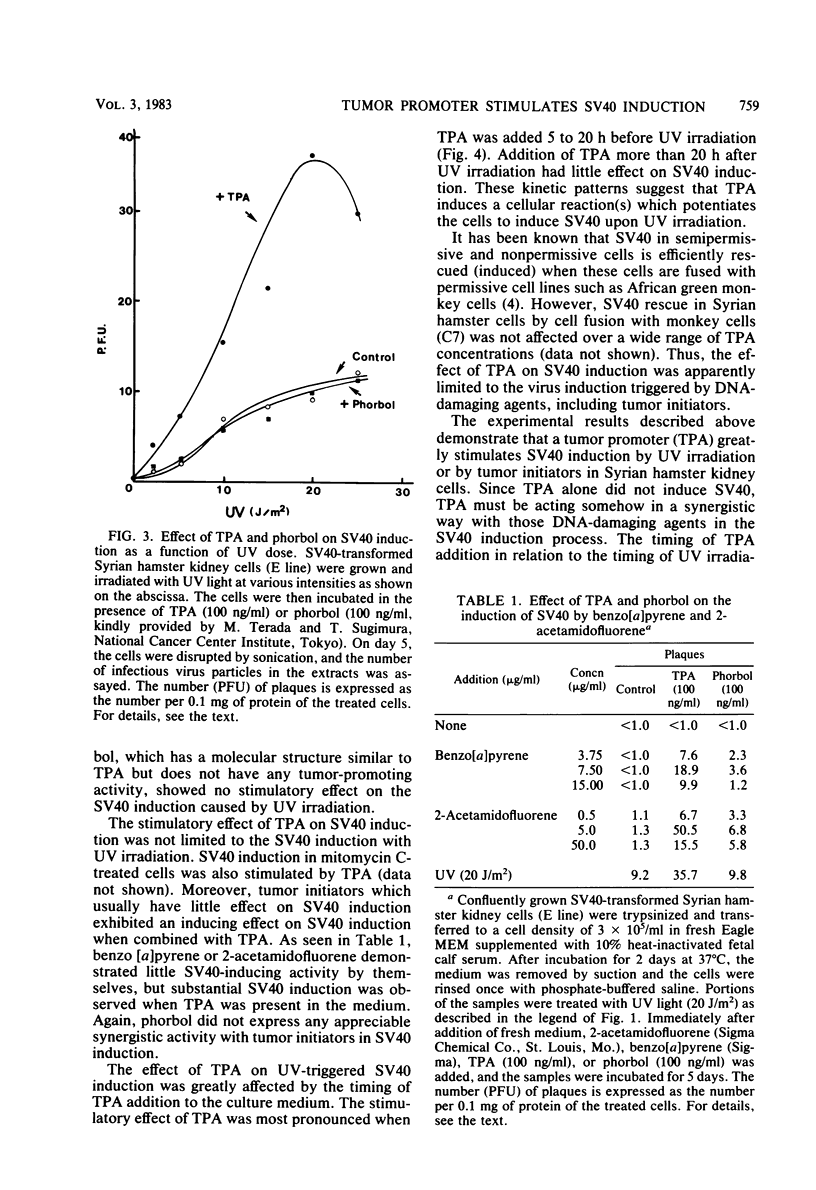
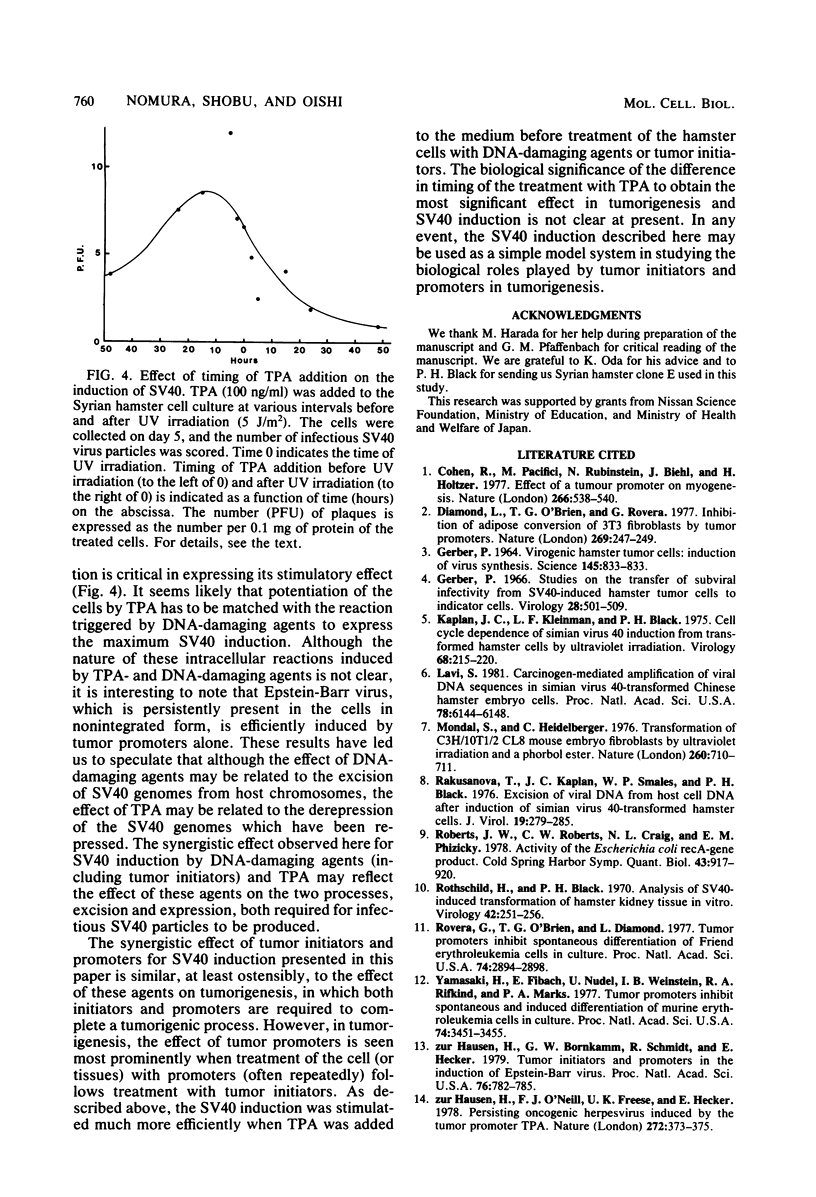
Simian virus 40 (SV40)-transformed Syrian hamster kidney cells produce infectious SV40 virus particles after treatments which damage DNA, such as UV irradiation or mitomycin C treatment. We have found that the induction of SV40 by DNA-damaging agents is greatly stimulated when a typical tumor promoter, 12-O-tetradecanoylphorbol 13-acetate (TPA), is present in the medium. Phorbol, which has a molecular structure similar to TPA but does not have any tumor-promoting activity, showed no such stimulatory effect on SV40 induction. This apparent synergistic effect of DNA-damaging agents and tumor promoter (TPA) was more pronounced when a tumor initiator, benzo [a]pyrene or 2-acetamido-fluorene, was combined with TPA. The effect of TPA on UV-triggered SV40 induction was greatly influenced by the timing of TPA addition to the culture medium, which was most efficient when addition of TPA was 5 to 20 h before UV irradiation. The effect of TPA, however, was not observed in SV40 rescue from hamster cells by cell fusion with permissive monkey (C7) cells.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen R., Pacifici M., Rubinstein N., Biehl J., Holtzer H. Effect of a tumour promoter on myogenesis. Nature. 1977 Apr 7;266(5602):538–540. doi: 10.1038/266538a0. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- GERBER P. VIROGENIC HAMSTER TUMOR CELLS: INDUCTION OF VIRUS SYNTHESIS. Science. 1964 Aug 21;145(3634):833–833. doi: 10.1126/science.145.3634.833. [DOI] [PubMed] [Google Scholar]
- Gerber P. Studies on the transfer of subviral infectivity from SV40-induced hamster tumor cells to indicator cells. Virology. 1966 Apr;28(4):501–509. doi: 10.1016/0042-6822(66)90234-0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Kleinman L. F., Black P. H. Cell cycle dependence of simian virus 40 induction from transformed hamster cells by ultraviolet irradiation. Virology. 1975 Nov;68(1):215–220. doi: 10.1016/0042-6822(75)90162-2. [DOI] [PubMed] [Google Scholar]
- Lavi S. Carcinogen-mediated amplification of viral DNA sequences in simian virus 40-transformed Chinese hamster embryo cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6144–6148. doi: 10.1073/pnas.78.10.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S., Heidelberger C. Transformation of C3H/10T1/2CL8 mouse embryo fibroblasts by ultraviolet irradiation and a phorbol ester. Nature. 1976 Apr 22;260(5553):710–711. doi: 10.1038/260710a0. [DOI] [PubMed] [Google Scholar]
- Rakusanova T., Kaplan J. C., Smales W. P., Black P. H. Excision of viral DNA from host cell DNA after induction of simian virus 40-transformed hamster cells. J Virol. 1976 Jul;19(1):279–285. doi: 10.1128/jvi.19.1.279-285.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L., Phizicky E. M. Activity of the Escherichia coli recA-gene product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):917–920. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- Rothschild H., Black P. H. Analysis of SV40-induced transformation of hamster kidney tissue in vitro. VII. Induction of SV40 virus from transformed hamster cell clones by various agents. Virology. 1970 Sep;42(1):251–256. doi: 10.1016/0042-6822(70)90264-3. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Tumor promoters inhibit spontaneous differentiation of Friend erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2894–2898. doi: 10.1073/pnas.74.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Bornkamm G. W., Schmidt R., Hecker E. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):782–785. doi: 10.1073/pnas.76.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]


