Abstract
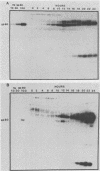
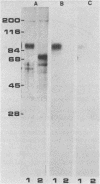
WE have raised a monoclonal antibody, designated E28D8, which reacts with an 80,000-dalton membrane glycoprotein (gp80) of Dictyostelium discoideum. gp80 has been implicated in the formation of the EDTA-resistant adhesions ("contact sites A") which appear during development. The monoclonal antibody reacted with other developmentally regulated proteins of D. discoideum, confirming previous results indicating the presence of common antigenic determinants recognized by polyclonal rabbit antibodies directed to gp80. Periodate sensitivity of the determinants suggests that carbohydrate may be necessary for reactivity. Thus, the determinant recognized by E28D8 may result from a posttranslational modification common to a number of proteins. Some of the proteins that carry the determinant were preferentially localized to posterior cells in slugs. Monoclonal antibody E28D8 did not inhibit contact-sites-A-mediated intercellular adhesion. However, gp80 affinity purified on immobilized monoclonal antibody was able to neutralize the adhesion-blocking effect of rabbit antiserum to gp80. Although gp80 itself may not be essential for cell-cell adhesion, it appears to carry the determinants associated with adhesion.
Full text
PDF

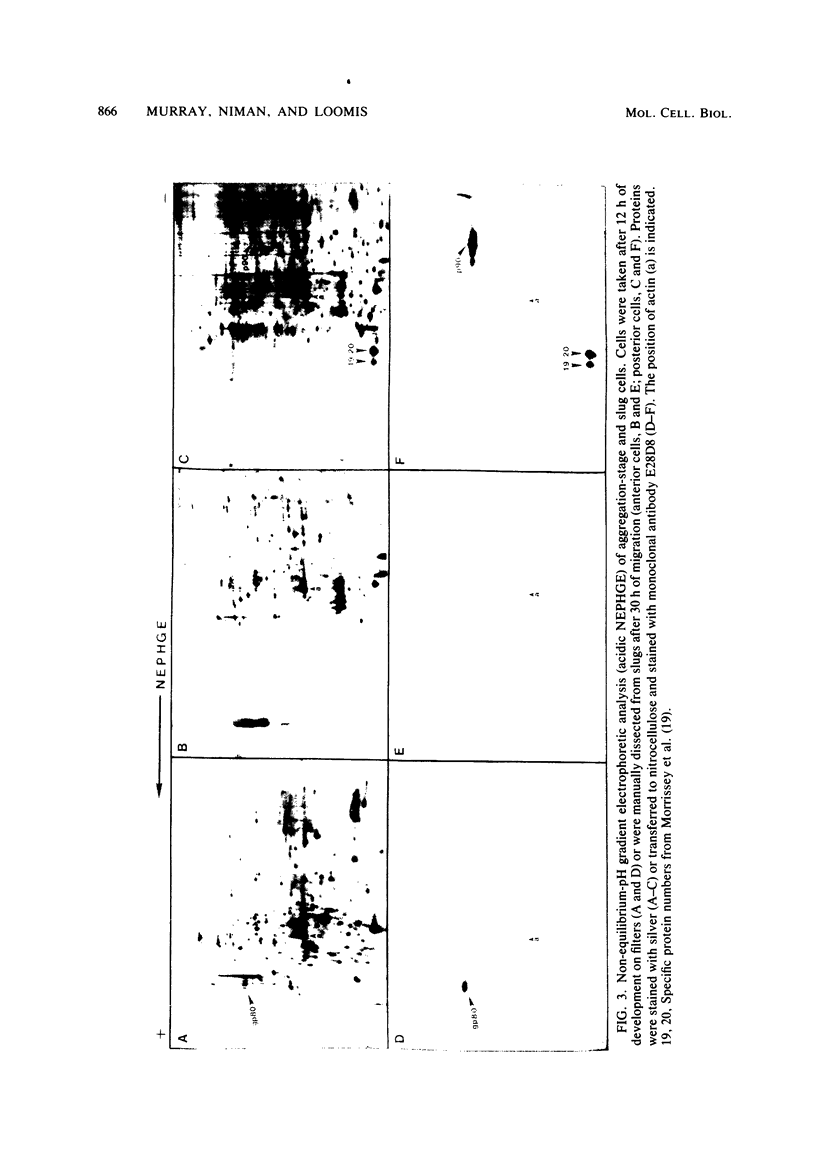

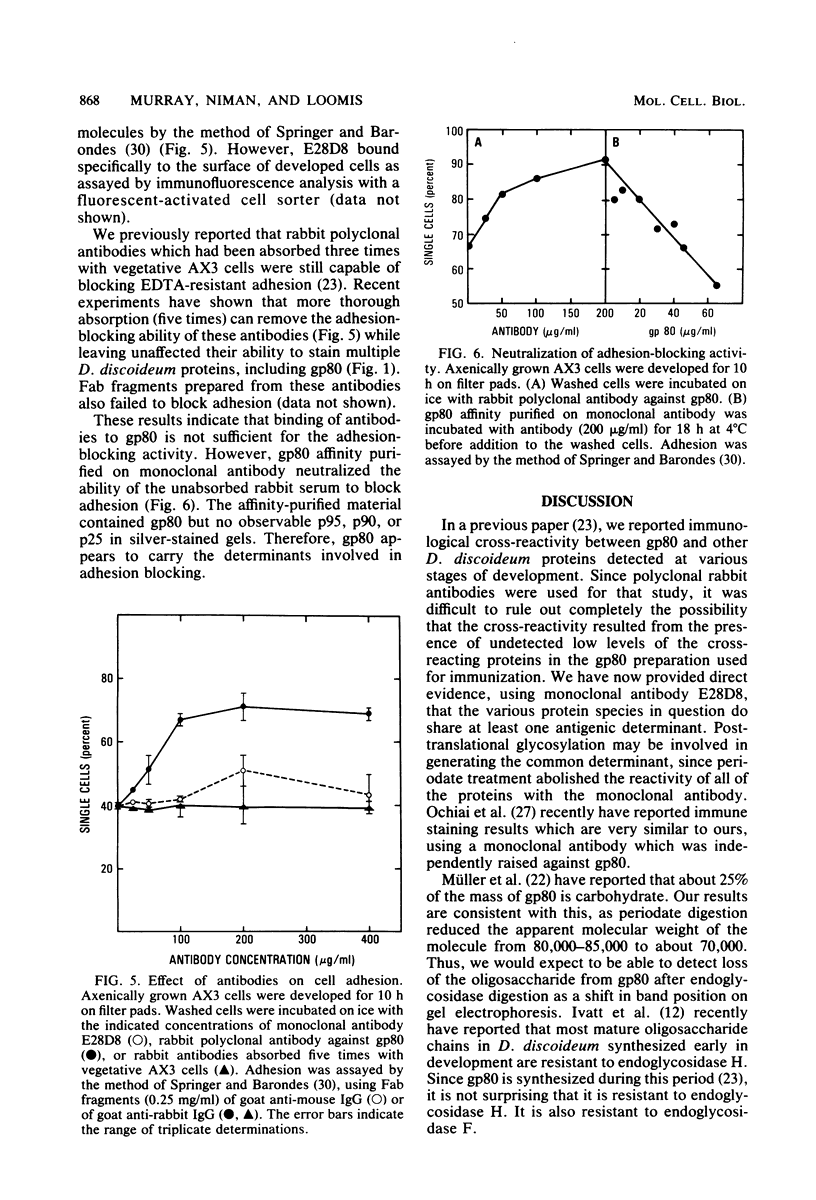


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., Gerisch G., Kempff S., Riedel V., Cremer G. Specific inhibition of cell contact formation in Dictyostelium by univalent antibodies. Exp Cell Res. 1970 Nov;63(1):147–158. doi: 10.1016/0014-4827(70)90343-5. [DOI] [PubMed] [Google Scholar]
- Beug H., Katz F. E., Gerisch G. Dynamics of antigenic membrane sites relating to cell aggregation in Dictyostelium discoideum. J Cell Biol. 1973 Mar;56(3):647–658. doi: 10.1083/jcb.56.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Katz F. E., Stein A., Gerisch G. Quantitation of membrane sites in aggregating Dictyostelium cells by use of tritiated univalent antibody. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3150–3154. doi: 10.1073/pnas.70.11.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman D. S., Leichtling B. H., Rickenberg H. V. Phosphoproteins in Dictyostelium discoideum. J Supramol Struct Cell Biochem. 1981;15(4):369–385. doi: 10.1002/jsscb.1981.380150407. [DOI] [PubMed] [Google Scholar]
- Devine K. M., Morrissey J. H., Loomis W. F. Differential synthesis of spore coat proteins in prespore and prestalk cells of Dictyostelium. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7361–7365. doi: 10.1073/pnas.79.23.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gersten D. M., Marchalonis J. J. A rapid, novel method for the solid-phase derivatization of IgG antibodies for immune-affinity chromatography. J Immunol Methods. 1978;24(3-4):305–309. doi: 10.1016/0022-1759(78)90133-3. [DOI] [PubMed] [Google Scholar]
- Ivatt R. J., Das O. P., Henderson E. J., Robbins P. W. Developmental regulation of glycoprotein biosynthesis in Dictyostelium. J Supramol Struct Cell Biochem. 1981;17(4):359–368. doi: 10.1002/jsscb.380170407. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Jr Sensitivity of Dictyostelium discoideum to nucleic acid analogues. Exp Cell Res. 1971 Feb;64(2):484–486. doi: 10.1016/0014-4827(71)90107-8. [DOI] [PubMed] [Google Scholar]
- Loomis W. F. The spatial pattern of cell-type differentiation in Dictyostelium. Dev Biol. 1982 Oct;93(2):279–284. doi: 10.1016/0012-1606(82)90117-8. [DOI] [PubMed] [Google Scholar]
- Mage M. G. Preparation of Fab fragments from IgGs of different animal species. Methods Enzymol. 1980;70(A):142–150. doi: 10.1016/s0076-6879(80)70045-9. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Farnsworth P. A., Loomis W. F. Pattern formation in Dictyostelium discoideum: an analysis of mutants altered in cell proportioning. Dev Biol. 1981 Apr 15;83(1):1–8. doi: 10.1016/s0012-1606(81)80002-4. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H., Wheeler S., Loomis W. F. New Loci in DICTYOSTELIUM DISCOIDEUM Determining Pigment Formation and Growth on BACILLUS SUBTILIS. Genetics. 1980 Sep;96(1):115–123. doi: 10.1093/genetics/96.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. A., Yee L. D., Loomis W. F. Immunological analysis of glycoprotein (contact sites A) involved in intercellular adhesion of Dictyostelium discoideum. J Supramol Struct Cell Biochem. 1981;17(3):197–211. doi: 10.1002/jsscb.380170302. [DOI] [PubMed] [Google Scholar]
- Müller K., Gerisch G., Fromme I., Mayer H., Tsugita A. A membrane glycoprotein of aggregating Dictyostelium cells with the properties of contact sites. Eur J Biochem. 1979 Sep;99(2):419–426. doi: 10.1111/j.1432-1033.1979.tb13271.x. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Elder J. H. Molecular dissection of Rauscher virus gp70 by using monoclonal antibodies: localization of acquired sequences of related envelope gene recombinants. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4524–4528. doi: 10.1073/pnas.77.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niman H. L., Elder J. H. Structural analysis of Rauscher virus Gp70 using monoclonal antibodies: sites of antigenicity and P15(E) linkage. Virology. 1982 Nov;123(1):187–205. doi: 10.1016/0042-6822(82)90305-1. [DOI] [PubMed] [Google Scholar]
- Ochiai H., Schwarz H., Merkl R., Wagle G., Gerisch G. Stage-specific antigens reacting with monoclonal antibodies against contact site A, a cell-surface glycoprotein of Dictyostelium discoideum. Cell Differ. 1982 Jan;11(1):1–13. doi: 10.1016/0045-6039(82)90011-2. [DOI] [PubMed] [Google Scholar]
- Parham P. Purification of immunologically active HLA-A and -B antigens by a series of monoclonal antibody columns. J Biol Chem. 1979 Sep 25;254(18):8709–8712. [PubMed] [Google Scholar]
- Schmidt J. A., Loomis W. F. Phosphorylation of the contact site A glycoprotein (gp80) of Dictyostelium discoideum. Dev Biol. 1982 Jun;91(2):296–304. doi: 10.1016/0012-1606(82)90036-7. [DOI] [PubMed] [Google Scholar]
- Springer W. R., Barondes S. H. Cell adhesion molecules: detection with univalent second antibody. J Cell Biol. 1980 Dec;87(3 Pt 1):703–707. doi: 10.1083/jcb.87.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]