Abstract
Cl channels in the basolateral membrane play a key role in Cl absorption in the thick ascending limb (TAL). The patch clamp experiments were performed to test whether AngII increases Cl absorption in the TAL by stimulating the basolateral 10 pS Cl channels. AngII (1-100 nM) stimulated the 10 pS Cl channel in the TAL, an effect that was blocked by losartan (AT1R antagonist) but not by PD123319 (AT2R antagonist). Inhibition of phospholipase C (PLC) or protein kinase C (PKC) also abolished the stimulatory effect of AngII on Cl channels. Moreover, stimulation of PKC with phorbol 12-myristate 13-acetate (PMA) mimicked the effect of AngII and increased Cl channel activity. However, the stimulatory effect of AngII on Cl channels was absent in the TAL pretreated with DPI, an inhibitor of NADPH-dependent oxidase (NOX). Moreover, treatment of the TAL with DPI also blocked the effect of PMA on the 10 pS Cl channel. Western blotting demonstrated that incubation of isolated TAL with AngII increased phosphorylation of P47phox at Ser304, suggesting that AngII stimulates the basolateral Cl channels by increasing NOX-dependent superoxide generation. This notion was also supported by the observation that H2O2 significantly increased 10 pS Cl channel activity in the TAL. We conclude that stimulation of AT1R increased the basolateral Cl channels by activating the PKC-dependent NOX pathway. The stimulatory effect of AngII on the basolateral Cl channel may contribute to AngII-induced increases in NaCl reabsorption in the TAL and AngII-infuse-induced hypertension.
Keywords: Angiotensin II receptor, PKC, NADPH oxidase, ClC-kb, hypertension
Introduction
The TAL is responsible for reabsorption of 25% filtered Na and Cl load and plays a key role in urinary concentrating mechanisms 1-3. The transepithelial Cl transport in the TAL is a two-step process: Cl enters the cell through type II Na-K-Cl cotransporters (NKCC2) in the apical membrane 4 and exits the basolateral membrane through either Cl channels or KCl cotransporters 5. Although type 1 KCl cotransporter (KCC1) has been shown to be expressed in the basolateral membrane of the TAL6, the role of KCl cotransporter in mediating Cl exit is not understood. Therefore, it is generally accepted that Cl channels in the basolateral membrane of the TAL play an important role in mediating Cl exit and regulating transepithelial Cl absorption. Patch-clamp experiments have identified a 10 pS Cl channel as the main type of Cl channels in the basolateral membrane of the TAL. Moreover, ClC-K2 is most likely the pore-containing component of the 10 pS Cl channel 7-9. A large body of evidence supports the role of AngII in modulating renal Na transport in different nephron segments. In the proximal tubule, AngII at low dose has been shown to stimulate fluid and bicarbonate absorption by activating Na/H exchangers 10,11. Microperfusion studies have shown that AngII stimulated Na, bicarbonate and fluid absorption in the early distal nephron of rat kidney 12. AngII infusion has been shown to increase furosemide-sensitive oxygen consumption in the TAL of rat kidneys, an indication of augmented NaCl absorption in the TAL13. However, the NaCl absorption in the TAL requires the involvement of several ion transporters such as Na-K-ATPase, NKCC2, ROMK and ClCK2. The aim of the present study is to examine the hypothesis that AngII-induced stimulation of NaCl transport is partially achieved by activating the basolateral Cl channels in the outer medullary TAL (mTAL).
METHODS
Preparation of the TAL
Sprague-Dawley rats of either sex (<90 g) were purchased from the animal facility of the Second Affiliated Hospital of Harbin Medical University (Harbin, China). The animals were kept on a normal rat chow with free access to water. We removed both kidneys after the animal was killed by cervical dislocation. We followed the methods described by Guinamard et al. 7 for the preparation of the TAL. The kidney was cut into 1 mm thick slices with a razor blade and the kidney slices were incubated in a HEPES buffer solution containing collagenase type 1A (1 mg/ml; Sigma, St. Louis, MO) at 37°C for 45-60 min. After the collagenase treatment, the kidney slices were gently rinsed with a HEPES-buffered solution containing (in mM): 140 NaCl, 5 KCl, 1.8 MgCl2, 1.8 CaCl2, and 10 HEPES (pH=7.4) at 4°C. A single TAL was dissected from the outer stripe of the outer medulla for the experiments as described previously 14. The animal protocol was approved by the animal care and use committee of Harbin Medical University.
Patch-clamp technique
The method for the patch-clamp experiments has been described previously 14 and the pipette solution contains (in mM): 140 NaCl, 1.8 MgCl2, and 10 HEPES (pH=7.4).. Channel activities were defined as NPo, a product of channel open probability (Po) and channel number (N). The NPo was calculated from data samples of 60s duration in the steady state as follows:
in which ti is the fractional open time spent at each of the observed current levels. The slope conductance of the channel was determined by measuring Cl currents at several holding potentials.
Western blot
Equal amounts of protein (80 μg) extracted from isolated mTAL were separated by electrophoresis using 12% SDS-PAGE and transferred to pure nitrocellulose blotting membranes (Pall Life Sciences). After blocking in 0.1% Tween-Tris-buffered saline (TBS-T) containing 5% nonfat dry milk, the membranes were incubated overnight at 4°C with the corresponding primary antibody. The membranes were washed four times (10 min for each wash) with TBS-T, followed by incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 hour. Protein bounds were detected using the ECL detection system (Thermo Fisher Scientific Inc.) and quantified by densitometry using Quantity One software (Bio-Rad).
Chemicals and antibodies
Anti-phospho-p47phox (p-P47phox)at Ser304 and anti-p47phox antibodies were obtained from Sigma (St Louis, MO). Angiotensin II, PMA, DPI, apocynin, calphostin C, U73122, Losartan and PD123319 were obtained from Sigma (St Louis, MO). U73122, phorbol-12-myristate-13-acetate (PMA), calphostin C, apocynin and diphenyleneiodonium sulfate (DPI) were dissolved in DMSO. The final concentration of DMSO in the bath was less than 0.1% which had no significant effect on channel activity.
Statistic
Data are shown as means ±SEM. We used paired Student’s t-tests or one way ANOVA test to determine the significance of the difference between the control and experimental groups. Statistical significance was taken as P<0.05.
Results
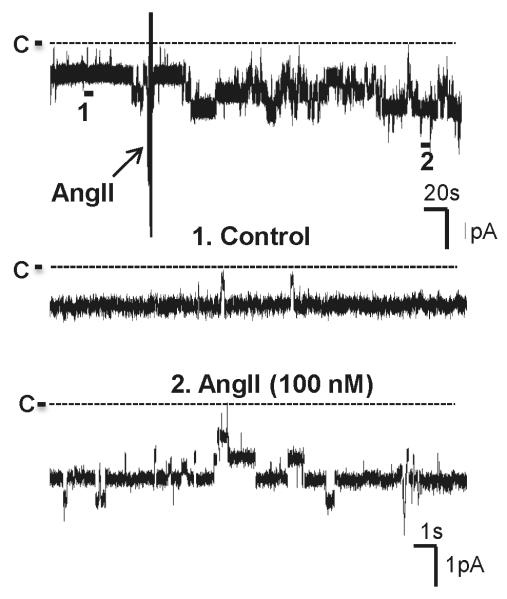
Previous patch-clamp experiments demonstrated the presence of two types of Cl channels, a 10 pS and a 20-40 pS, in the basolateral membrane of the TAL 7. Moreover, the 10 pS Cl channel was a main type of Cl channels expressed in the basolateral membrane. We confirmed the previous finding and further examined the effect of AngII on basolateral 10 pS Cl channels in the TAL. Fig. 1 is a channel recording made in a cell-attached patch showing that the addition of AngII (100 nM) stimulated basolateral 10 pS Cl channels and increased channel activity, as defined by NPo, from 1.01±0.05 to 2.1±0.12 (n=12). Fig. 2A is a dose-response curve of AngII’s effect showing that 1 nM AngII and 10 nM AngII significantly increased channel activity to 1.34±0.11 (n=12) and 1.5±0.1 (n=7), respectively. Since 100 nM AngII had a robust effect on the 10 pS Cl channels, we used 100 nM AngII throughout the experiments.
Fig. 1. AngII stimulates the basolateral 10 pS Cl channels in the TAL.
A channel recording shows the effect of 100 nM AngII on the basolateral 10-pS Cl channels. The experiment was performed in a cell-attached patch and holding potential was −60 mV (hyperpolarization). The top trace shows the time course of the experiments and two parts of the traces indicated by numbers are extended to show the fast time resolution. The channel closed level was indicated by a dotted line and “C” and the arrow indicates the addition of AngII. The pipette solution contained 140 mM KCl, 1.8 mM MgCl2 and 10 mM HEPES (pH=7.4) and the bath solution was composed of 140 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1.8 mM MgCl2 and 10 mM HEPES (pH=7.4).
Fig.2. The effect of AngII is mediated by AT1R.
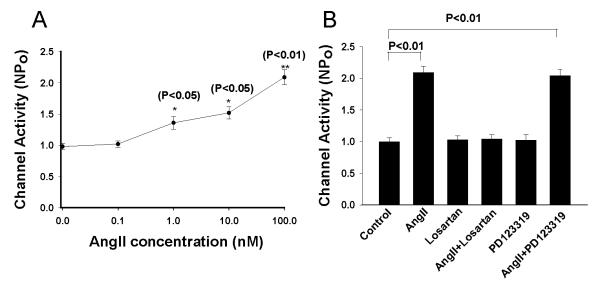
(A) A dose response curve of AngII’s effect on the 10 pS Cl channels. Asterisk indicates the significant difference between the control (no AngII) and AngII treatment. (B) A bar graph summarizes the experiments in which the effect of AngII on the 10 pS Cl channels was tested in the presence of 10 μM losartan or PD123319.
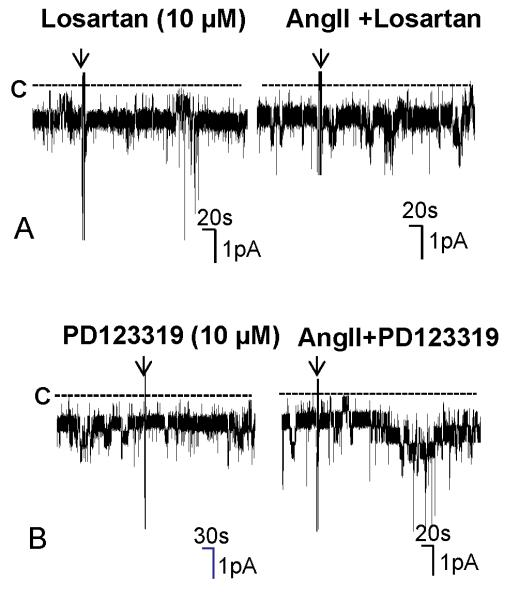
We next examined whether the effect of AngII on the Cl channels was mediated via AT1R or AT2R by examining the effect of AngII in the presence of losartan or PD123319. Inhibition of AT1R with 10 μM losartan did not significantly affect the Cl channel activity (Losartan, NPo=1.03±0.06, n=5) (Fig.2B). However, it completely abolished the effect of AngII on the 10 pS Cl channels. Fig. 3A is a channel recording made in a cell-attached patch showing that application of AngII failed to stimulate the Cl channels. Results from 7 experiments are summarized in Fig. 2B, demonstrating that NPo was 1.04±0.07 in the presence of 100 nM AngII in the TAL treated with losartan. In contrast, inhibition of AT2R failed to abolish the effect of AngII. Fig. 3B is a channel recording showing that AngII was still able to stimulate the Cl channels in the presence of PD123319. Results are summarized in Fig.2B showing that AngII increased channel activity from 1.02±0.1 (PD123319 alone) to 2.04±0.1 (AngII+PD123319) in the TAL treated with 10 μM PD123319 (n=7). Therefore, the results strongly suggested that AT1R was responsible for the stimulatory effect of AngII on the 10 pS Cl channels.
Fig. 3. Inhibition of AT1R but not AT2R abolishes the effect of AngII on the 10 pS Cl channels.
(A) A channel recording demonstrates the effect of AngII on the 10 pS Cl channels in the TAL treated with losartan. (B) A channel recording shows the effect of AngII on the 10 pS Cl channels in the TAL treated with 10 μM PD123319. The experiment was performed in a cell-attached patch at −60 mV and the left and right traces were recorded from the same patch. The unregularly downward deflections were electric noisy.
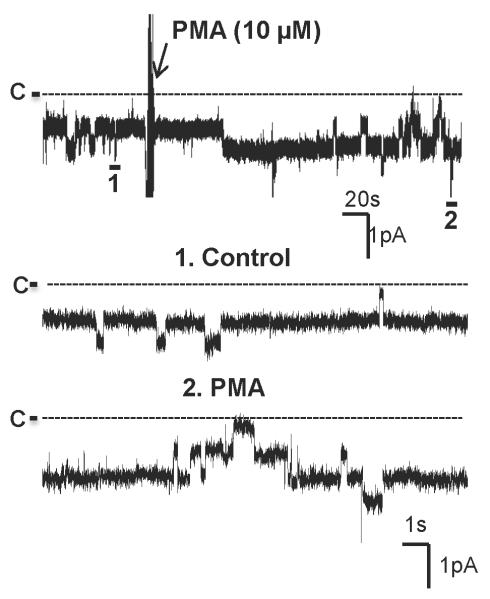
Stimulation of AT1R has been shown to activate phospholipase C (PLC) through the Gq protein 15. Thus, we examined the role of PLC in mediating the effect of AngII on the basolateral 10 pS Cl channels. The experiments were performed in cell-attached patches and the results are summarized in Fig. 4. Inhibition of PLC with 5 μM U73122 had no significant effect on the Cl channels (NPo=1.03±0.07, n=5). However, it blocked the stimulatory effect of AngII because channel activity in the presence of AngII (0.99±0.07, n=5) was not different from those in the absence of AngII. Next, we examined the role of PKC in mediating the effect of AngII on the Cl channels in the Cl channels by investigating the effect of AngII in the TAL treated with calphostin C. Inhibition of PKC did not significantly affect the Cl channel activity (NPo=1.01±0.05, n=4), however, it abolished the effect of AngII on the Cl channels. Results summarized in Fig. 4 demonstrate that AngII failed to increase the channel activity in the presence of calphostin C (NPo=1.02±0.05, n=4). The role of PKC in stimulating 10 pS Cl channels was also demonstrated by examining the effect of PMA on the Cl channels. Fig. 5 is a channel recording demonstrating that adding 10 μM PMA stimulated the 10 pS Cl channels in the TAL. Fig. 4 summarizes the results from 8 experiments showing that treatment of the TAL with PMA significantly increased channel activity to 1.82±0.1. Therefore, the results strongly suggest that AngII stimulates 10 pS Cl channels by PLC and PKC pathways.
Fig. 4. Inhibition of PLC or PKC abolishes the stimulatory effect of AngII on the 10 pS Cl channels.
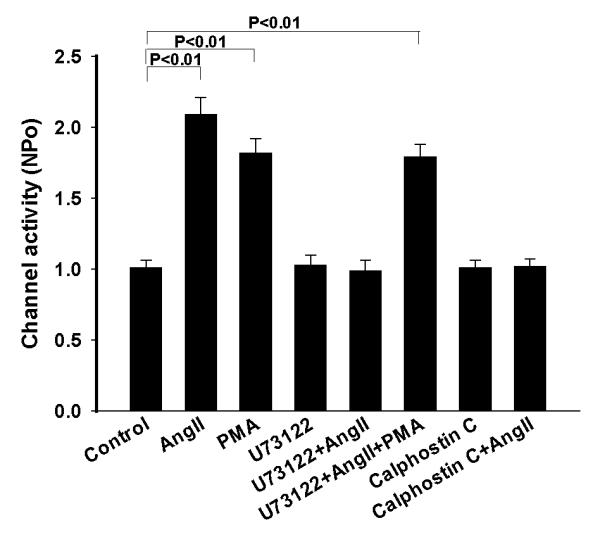
A bar graph summarizes the experiments in which the effect of AngII /PMA on the 10 pS Cl channels in the TAL was examined in the presence or in the absence of 5 μM U73122 or 100 nM calphostin C. All experiments were performed in cell-attached patches at −60 mV and the channel activity was determined at the steady state of each treatment.
Fig. 5. Stimulation of PKC increases the activity of the 10 pS Cl channels.
A channel recording shows the effect of PMA on the basolateral 10-pS Cl channels in the TAL. The experiment was performed in a cell-attached patch and holding potential was −60 mV (hyperpolarization). The top trace shows the time course of the experiments and two parts of the traces indicated by numbers are extended to show the fast time resolution. The channel closed level was indicated by a dotted line and “C”.
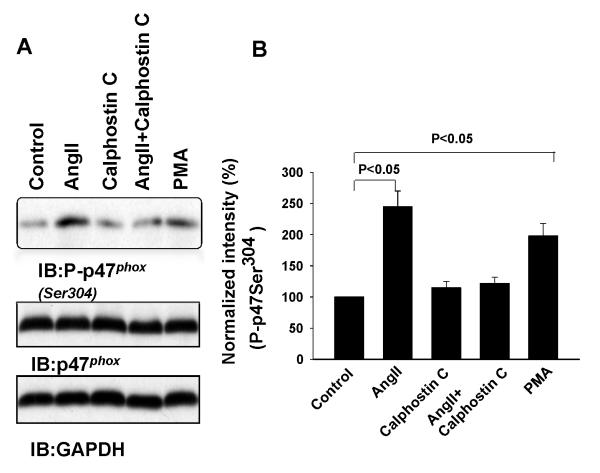
AngII has been shown to activate NADPH oxidase (NOX) by a PKC-dependent pathway16-18. Moreover, increases in superoxide anions play a role in stimulating Na transport in the TAL13. To test whether AngII stimulates the 10 pS Cl channels by activating NOX, we examined the effect of AngII on the phosphorylation of p47phox at Serine residue 304 (Ser304 ), which is a PKC phosphorylation site and serves as an indication of activation NOX 19. The isolated mTALs were incubated with 100 nM AngII for 5 min and the proteins from TAL lysates were resolved by SDS gel. Fig.6A is a Western blot showing that AngII incubation increased the phosphorylation of p47phox (P-p47phox ) by 145±25% (n=5), an effect that was abolished by calphostin C. Results summarized in Fig.6B show that calphostin C had no significant effect on the phosphorylation of p47phox (115±10% of the control). However, in the presence of calphostin C, AngII failed to increase the phosphorylation of p47phox (122±10% of the control). Moreover, incubation of the TAL with PMA also increased the phosphorylation of p47phox by 90±20% (n-5). Thus, AngII is able to stimulate NOX by increasing PKC-dependent phosphorylation of p47phox.
Fig. 6. AngII stimulates the phosphorylation of p47phox at Ser304.
(A) Western blot demonstrating the expression of P-p47phox, p47phox and GAPDH in the TAL treated with 100 nM AngII, 100 nM calphostin C, AngII+calphostin C and 10 μM PMA for 5 min. (B) A bar graph summarizes the results from 5 similar experiments shown in Fig. 6A.
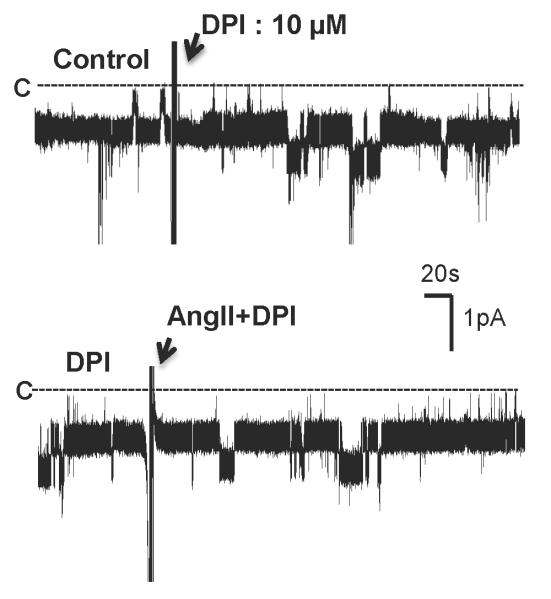
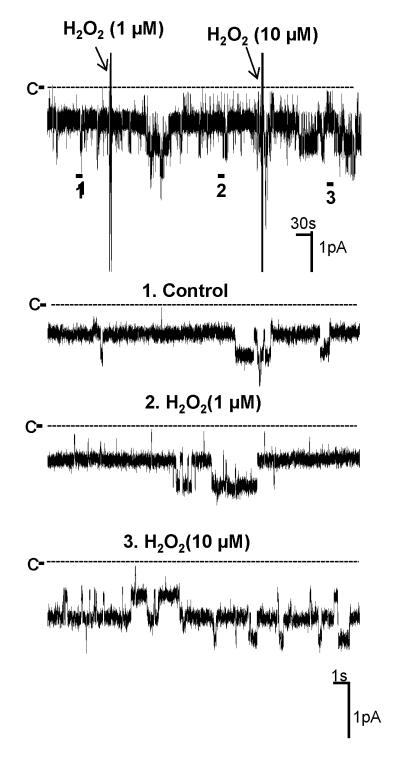
Next, we investigated whether the stimulatory effect of AngII on the Cl channels was induced by activating NOX by examining the effect of AngII on the Cl channels in the TAL treated with DPI, an inhibitor of NOX20. Fig. 7 is a recording showing that inhibition of NOX with 10 μM DPI did not significantly change the Cl channel activity. However, addition of AngII failed to stimulate the 10 pS Cl channels in the TAL treated with DPI. Results summarized in Fig. 8 show that unlike the effect of AngII on the Cl channels in the absence of DPI, AngII did not increase Cl channel activity (NPo=0.98±0.05, n=5) in the presence of DPI. Since DPI has been shown to have an effect other than inhibiting NOX 21, we also used apocynin, which has a different chemical structure and inhibits NOX, to examine the role of NOX in mediating the effect of AngII on the 10 pS Cl channels. Fig. 8 shows that inhibition of NOX with apocynin did not significantly affect the Cl channel activity (NPo=1.10±0.05, n=4) but it abolished the effect of AngII on the Cl channels in the TAL (NPo=0.99±0.1, n=4). Also, inhibition of NOX with DPI blocked the effect of PMA on the Cl channels (NPo=1.03±0.05, n=7) (Fig.8). Thus, results suggest that the stimulation of NOX by PKC plays an important role in mediating the stimulatory effect of AngII on the 10 pS Cl channels in the TAL. This notion was also supported by experiments in which the effect of H2O2 on the Cl channels was examined. Fig. 9 is a channel recording in a cell-attached patch showing that application of 10 μM H2O2 activated the 10 pS Cl channels and increased channel activity from 1.06±0.05 to 1.83±0.1 (n=9). Data suggest that superoxide anion-related products stimulate the basolateral Cl channel activity in the TAL.
Fig.7. Inhibition of NOX diminishes the effect of AngII on the 10 pS Cl channels.
A channel recording shows the effect of 100 nM AngII on the basolateral 10 pS Cl channels in the TAL treated with 10 μM DPI. The experiment was performed in a cell-attached patch and holding potential was −60 mV (hyperpolarization). The top and bottom traces were recorded from the same patch. The channel closed level is indicated by C and a dotted line.
Fig. 8. Inhibition of NADPH oxidase abolishes the effect of AngII and PMA on the 10 pS Cl channels.
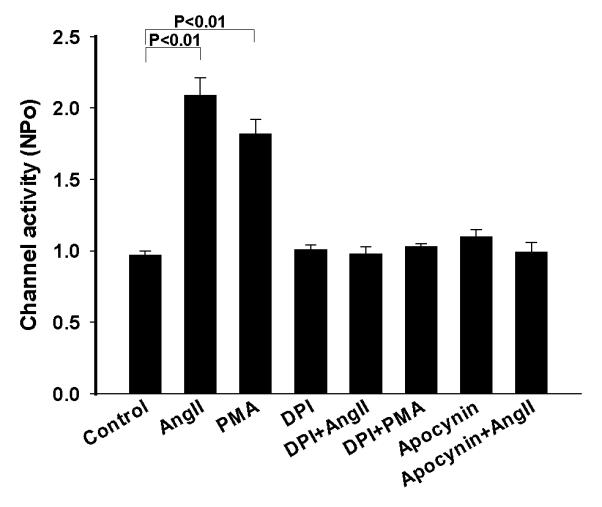
A bar graph summarizes the experiments in which the effect of AngII or PMA on the 10 pS Cl channels was examined in the TAL treated with DPI or apocynin. All experiments were performed in cell-attached patches at −60 mV and the channel activity was determined at the steady state of each treatment.
Fig. 9. Hydrogen peroxide stimulates the 10 pS Cl channel.
A channel recording shows the effect of hydrogen peroxide on the basolateral 10 pS Cl channels. The experiment was performed in a cell-attached patch and holding potential was −60 mV (hyperpolarization). The top trace shows the time course of the experiments and three parts of the traces indicated by numbers are extended to show the fast time resolution. The channel closed level was indicated by a dotted line and “C”.
Discussion
Basolateral 10 pS Cl channels play an important role in the regulation of transepithelial Cl absorption in the TAL because they provide the major pathway for Cl exit. It has been reported that 10 pS Cl channel activity was observed in over 80% patches in the basolateral membrane of forskolin-treated TALs 7. ClC-K2 is most likely to be the pore-forming component of the 10 pS Cl channel because it shares the biophysical properties of ClC-K2 22 and its regulatory mechanisms such as pH-sensitivity and stimulation by cAMP 8. Immunostaining and in situ hybridization confirmed that ClC-K2 was overwhelmingly expressed in the basolateral membrane of the TAL in the rat kidney 23 while ClC-K1 was mainly expressed in the thin ascending limb 24. The role of basolateral Cl channels in maintaining NaCl absorption in the TAL is best demonstrated by the observation that defective gene products encoding human basolateral Cl channel (ClC-Kb) and Barttin, a subunit of ClC-Kb, caused type III and IV Bartter’s syndrome, respectively 5. On the other hand, gain-of-function –mutations of ClC-Kb has been reported to have predisposition to hypetension25. Hence, the regulation of basolateral Cl channels is an important component for modulating epithelial transport in the TAL.
The main finding of the present study is that AngII stimulates the 10 pS Cl channels in the basolateral membrane of the TAL. We demonstrated that AngII, at concentration as low as 1 nM, significantly increased the 10 pS Cl channel activity, suggesting that AngII plays a role in stimulating basolateral Cl channels under physiological conditions. The effect of AngII on the Cl channels was mediated by AT1R rather than AT2R because losartan abolished the effect of AngII. Our previous study showed that AngII also stimulated apical ROMK channels in the TAL 26. Since stimulation of ROMK channels is expected to enhance the K recycling across the apical membrane thereby increasing NKCC2 activity, the observation that AngII stimulated the basolateral Cl channels in the TAL is consistent with the notion that AngII may stimulate transepithelial NaCl absorption. This is consistent with the report that AngII infusion enhanced furosemide-sensitive oxygen consumption in the TAL13. However, a flux study performed in inner stripe of the outer medullary TAL demonstrated that AngII inhibited Cl absorption 27. The reason causing discrepancies between these two studies was not clear. One possibility is that different segments of the mTAL were used (the inner stripe vs the outer stripe used in the present study).
Two lines of evidence suggest that AngII stimulates the 10 pS Cl channels by activating PKC-dependent pathways: 1) the effect of AngII on the Cl channels was abolished by calphostin C; 2) PMA mimicked the effect of AngII and stimulated the Cl channels. Although we could not exclude the possibility that PKC-mediated phosphorylation of the Cl channels was involved in stimulating Cl channel activity, the stimulatory effect of PKC on the 10 pS Cl channels was, at least partially, the result of stimulation of NOX. This notion was supported by the finding that AngII stimulated phosphorylation of p47phox . The phosphorylation of p47Phox is expected to facilitate the translocation of phosphorylated p47 Phox from cytosolic complexes to the plasma membrane 28,19 thereby interacting with gp91 Phox and p22 Phox complex and activating NOX 29,30. The role of PKC in mediating the stimulatory effect of AngII on NOX activity has been suggested in a variety of tissues 31. Moreover, two additional pieces of evidence supported the role of NOX in mediating the effect of AngII on the 10 pS Cl channels: 1) inhibition of NOX abolished the effect of AngII on the Cl channels; 2) addition of H2O2 activated the Cl channels. In addition to be an activator, however, PKC could also be a mediator for superoxide-induced effect 32. It has been suggested that superoxide anions or its related products could activate PKC by oxidizing N-terminal regulatory domain containing zink-binding, cysteine-rich motifs thereby stimulating PKC. It is possible that hydrogen peroxide-induced stimulation of Cl channel was the result of activating a different type of PKC which further stimulates the 10 pS Cl channel by phosphorylation.
AngII has been shown to stimulate the generation of superoxide anions in a variety of tissues such as heart, vascular tissue and kidney 33-36. AngII-induced increases in superoxide anions play an important role in AngII-mediated hypertension and organ damage 35-37. We speculate that AngII-induced stimulation of basolateral Cl channels in the TAL contributes to AngII-induced hypertension. Moreover, ours and others have demonstrated that the effect of AngII on superoxide generation is mediated by AT1R 35,36,38,39. It has been shown that NOXII and NOX4 are mainly expressed in the kidney 36. We have previously demonstrated that the generation of superoxide and related products was significantly attenuated in gp91phox (-/-) mice40, suggesting that NOXII plays a role in generating superoxide in the renal tubule. Accumulated evidences have indicated that superoxide anion-related products play a role in stimulating renal Na absorption 41. It has been reported that superoxide anion-related products stimulated luminal Na/H exchange and Na/K/2Cl cotransporter in the TAL 42,43. We now demonstrated that superoxide anion-related product stimulates the basolateral Cl channels. Although the mechanism by which hydrogen peroxide activates the Cl channel is not known, it could activate the Cl channels directly by oxidative modulation of the Cl channel protein or by activating PKC as discussed above. However, we could not speculate whether superoxide or hydrogen peroxide is the likely activator of Cl channel in vivo. Stimulation of Cl channels should facilitate the Cl exit across the basolateral membrane thereby decreasing the intracellular Cl concentration. A low intracellular Cl concentration has been shown to enhance the phosphorylation of NKCC2 by Ste20-related proline/alanine-rich kinase and to activate NKCC2 44. Therefore, it is conceivable that superoxide anions-induced stimulation of NaCl absorption in the TAL may result from stimulating both NKCC2 and the basolateral 10 pS Cl channels.
Perspectives
The physiological importance of the present study is to illustrate that AngII stimulated the 10 pS Cl channel in the basolateral membrane of the TAL. The AngII-induced increases in Cl channel activity may contribute to AngII-dependent salt-sensitive hypertension and may also play a role in augmenting NaCl absorption during Na-restriction. Fig. 10 is a cell scheme illustrating a possible mechanism by which AngII activates the basolateral Cl channels in the TAL. Stimulation of AT1R by AngII activates PKC which in turn activates NOX and increases superoxide anions. An increase in superoxide anions and related products activates Cl channels in the TAL. Thus, it is conceivable that enhanced salt absorption induced by high level of superoxide anions is partially achieved by stimulating basolateral Cl channels in the TAL.
Fig. 10.
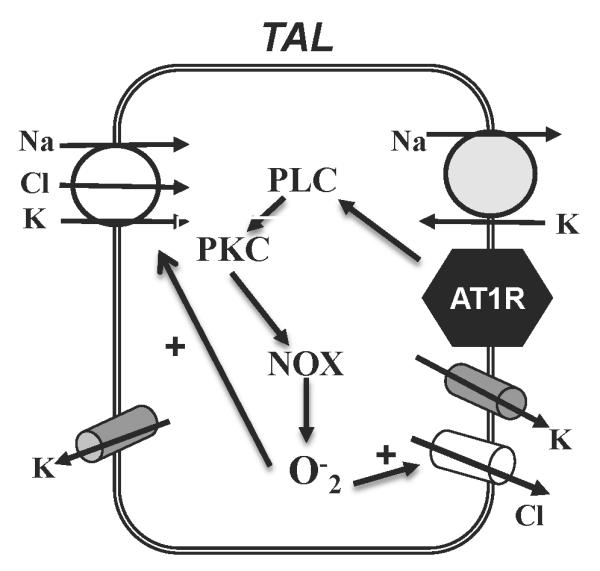
A cell scheme illustrates the mechanism by which AngII stimulates the 10 pS Cl channels in the TAL.
Novelty and Significance.
What is New
AngII stimulates the basolateral 10 pS Cl channels in the TAL.
The effect of AngII on the Cl channels is the result of increasing superoxide anions.
What is Relevant
The finding provides a mechanism by which AngII stimulates NaCl absorption in the TAL.
This mechanism should be relevant for AngII-induced hypertension.
Summary
We illustrate the mechanism by which stimulation of AT1R activates the basolateral Cl channels.
AngII-induced activation of NOX plays a role in stimulating basolateral Cl channels in the TAL.
Upregulated Cl channel activity in the TAL induced by AngII may play a role in AngII-dependent hypertension.
Acknowledgments
Sources of Funding The work is supported by Chinese National Natural Science Foundation #31171110 (RMG), #31071017 (RMG) and NIH grant HL34100 (WHW).
Footnotes
Disclosure None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 2.Greger R. Ion transport mechanisms in thick ascending limb of Henle’s loop of mammalian nephron. Physiological Reviews. 1985;65:760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- 3.Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol (Renal Fluid Electrolyte Physiol ) 1984;246:F745–F756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- 4.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee W-S, Hediger MA, Hebert SC. Molecular cloning, primary structure and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 5.Kramer BK, Bergler T, Stoelcker B, Waldegger S. Mechanisms of Disease: the kidney-specific chloride channels ClCKA and ClCKB, the Barttin subunit, and their clinical relevance. Nat Clin Pract Neph. 2008;4:38–46. doi: 10.1038/ncpneph0689. [DOI] [PubMed] [Google Scholar]
- 6.Di Stefano A, Jounier S, Wittner M. Evidence supporting a role for KCl cotransporter in the thick ascending limb of Henle’s loop. Kidney Int. 2001;60:1809–1823. doi: 10.1046/j.1523-1755.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- 7.Guinamard R, Chraibi A, Teulon J. A small-conductance Cl-channel in the mouse thick ascending limb that is activated by ATP and protein kinase A. J Physiol. 1995;485:97–112. doi: 10.1113/jphysiol.1995.sp020715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guinamard R, Paulais M, Teulon J. Inhibition of a small-conductance cAMP-dependent Cl- channel in the mouse thick ascending limb at low internal pH. J Physiol. 1996;490:759–765. doi: 10.1113/jphysiol.1996.sp021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marvao P, Jesus Ferreira MC, Bailly C, Paulais M, Bens M, Guinamard R, Moreau R, Vandewalle A, Teulon J. Cl− absorption across the thick ascending limb is not altered in cystic fibrosis mice. A role for a pseudo-CFTR Cl− channel. J Clin Invest. 1998;102:1986–1993. doi: 10.1172/JCI4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Chan YL. Mechanism of angiotensin II action on proximal tubular transport. J Pharmacol Exp Ther. 1989;252:689–695. [PubMed] [Google Scholar]
- 11.Wang T, Chan YL. Mechanism of angiotensin II action on proximal tubular transport. Journal of Pharmacology And Experimental Therapeutics. 1990;252:689–695. [PubMed] [Google Scholar]
- 12.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 13.Silva GB, Garvin JL. Angiotensin IIΓÇôDependent Hypertension Increases Na Transport-Related Oxygen Consumption by the Thick Ascending Limb. Hypertension. 2008;52:1091–1098. doi: 10.1161/HYPERTENSIONAHA.108.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu RM, Yang L, Zhang Y, Wang L, Kong S, Zhang C, Zhai Y, Wang M, Wu P, Liu L, Gu F, Zhang J, Wang WH. CYP-omega-hydroxylation-dependent metabolites of arachidonic acid inhibit the basolateral 10[thinsp]pS chloride channel in the rat thick ascending limb. Kidney Int. 2009;76:849–856. doi: 10.1038/ki.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Jayadev S, Escobedo JA. Identification of a Domain in the Angiotensin II Type 1 Receptor Determining Gq Coupling by the Use of Receptor Chimeras. J Biol Chem. 1995;270:16677–16682. doi: 10.1074/jbc.270.28.16677. [DOI] [PubMed] [Google Scholar]
- 16.Choi H, Leto TL, Hunyady L+, Catt KJ, Bae YS, Rhee SG. Mechanism of Angiotensin II-induced Superoxide Production in Cells Reconstituted with Angiotensin Type 1 Receptor and the Components of NADPH Oxidase. J Biol Chem. 2008;283:255–267. doi: 10.1074/jbc.M708000200. [DOI] [PubMed] [Google Scholar]
- 17.Massey KJ, Hong NJ, Garvin JL. Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4. American Journal of Physiology - Cell Physiology. 2012;303:C781–C789. doi: 10.1152/ajpcell.00457.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle’s loop in rat kidney. Am J Physiol Renal Physiol. 2002;282:F1111–F1119. doi: 10.1152/ajprenal.00218.2001. [DOI] [PubMed] [Google Scholar]
- 19.Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshumi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- 20.Riganti C, Gazzano E, Polimeni M, Costamagna C, Bosia A, Ghigo D. Diphenyleneiodonium Inhibits the Cell Redox Metabolism and Induces Oxidative Stress. J Biol Chem. 2004;279:47726–47731. doi: 10.1074/jbc.M406314200. [DOI] [PubMed] [Google Scholar]
- 21.Wind S, Beuerlein K, Eucker T, M++ller H, Scheurer P, Armitage ME, Ho H, Schmidt HHHW, Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nissant A, Paulais M, Lachheb S, Lourdel S, Teulon J. Similar chloride channels in the connecting tubule and cortical collecting duct of the mouse kidney. AJP - Renal Physiology. 2006;290:F1421–F1429. doi: 10.1152/ajprenal.00274.2005. [DOI] [PubMed] [Google Scholar]
- 23.Winters CJ, Zimniak L, Reeves WB, Andreoli TE. Cl- channels in basolateral renal medullary membranes XII. Anti-rbClC-Ka antibody blocks MTAL Cl- channels. AJP - Renal Physiology. 1997;273:F1030–F1038. doi: 10.1152/ajprenal.1997.273.6.F1030. [DOI] [PubMed] [Google Scholar]
- 24.Uchida S, Sasaki S, Nitta K, Uchida K, Horita S, Nihei H, Marumo F. Localization and functional characterization of rat kidney-specific chloride channel, ClC-K1. J Clin Invest. 1995;95:104–113. doi: 10.1172/JCI117626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeck N, Waldegger S, Lampert A, Boehmer C, Waldegger P, Lang PA, Wissinger B, Friedrich Br, Risler T, Moehle R, Lang UE, Zill P, Bondy B, Schaeffeler E, Asante-Poku S, Seyberth Hr, Schwab M, Lang F. Activating Mutation of the Renal Epithelial Chloride Channel ClC-Kb Predisposing to Hypertension. Hypertension. 2004;43:1175–1181. doi: 10.1161/01.HYP.0000129824.12959.f0. [DOI] [PubMed] [Google Scholar]
- 26.Lu M, Zhu Y, Balazy M, Reddy KM, Falck JR, Wang W. Effect of angiotensin II on the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol. 1996;108:537–547. doi: 10.1085/jgp.108.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerolle N, Bourgeois S, Leviel F, Lebrun Gt, Paillard M, Houillier P. Angiotensin II inhibits NaCl absorption in the rat medullary thick ascending limb. American Journal of Physiology - Renal Physiology. 2004;287:F404–F410. doi: 10.1152/ajprenal.00265.2003. [DOI] [PubMed] [Google Scholar]
- 28.Bedard K, Krause KH. The Nox family of ROS-generating NADPH oxidase: Physiology and Pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 29.Babior BM. NADPH oxidase: An update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 30.Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, Eguchi S. Angiotensin II signal transduction through small GTP-binding proteins: Mechanism and significance in vascular smooth muscle cells. Hypertension. 2006;48:534–540. doi: 10.1161/01.HYP.0000237975.90870.eb. [DOI] [PubMed] [Google Scholar]
- 32.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radical Biology and Medicine. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension Caused by Angiotensin II Infusion Involves Increased Superoxide Production in the Central Nervous System. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 34.Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of Superoxide in Modulating the Renal Effects of Angiotensin II. Hypertension. 2003;42:1150–1156. doi: 10.1161/01.HYP.0000101968.09376.79. [DOI] [PubMed] [Google Scholar]
- 35.Welch WJ. Angiotensin II-Dependent Superoxide: Effects on Hypertension and Vascular Dysfunction. Hypertension. 2008;52:51–56. [Google Scholar]
- 36.Sachse A, Wolf G. Angiotensin II Induced Reactive Oxygen Species and the Kidney. J Am Soc Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 37.Kopkan L, Castillo A, Navar LG, Majid DSA. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. AJP - Renal Physiology. 2006;290:F80–F86. doi: 10.1152/ajprenal.00090.2005. [DOI] [PubMed] [Google Scholar]
- 38.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- 39.Jin Y, Wang Y, Wang ZJ, Lin DH, Wang WH. Inhibition of angiotensin type 1 receptor impairs renal ability of K conservation in response to K restriction. AJP - Renal Physiology. 2009;296:F1179–F1184. doi: 10.1152/ajprenal.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox-Containing NADPH Oxidase in Mediating the Effect of K Restriction on ROMK Channels and Renal K Excretion. J Am Soc Nephrol. 2007;18:2037–2045. doi: 10.1681/ASN.2006121333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 42.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. AJP - Renal Physiology. 2005;288:F982–F987. doi: 10.1152/ajprenal.00348.2004. [DOI] [PubMed] [Google Scholar]
- 43.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol. 2006;290:R79–R83. doi: 10.1152/ajpregu.00447.2005. [DOI] [PubMed] [Google Scholar]
- 44.Ponce-Coria J, San Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, los Heros P, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proceedings of the National Academy of Sciences. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]