Abstract
Objectives
To ascertain whether the use of oral glucosamine influences symptoms or functional outcomes in patients with chronic low back pain (LBP) thought to be related to spinal osteoarthritis (OA).
Design
Systematic review of randomised control trials. Searches were performed up to March 2011 on Medline, AMED, CINHAL, Cochrane and EMBASE with subsequent reference screening of retrieved studies. In addition, the grey literature was searched via opensigle. Included studies were required to incorporate at least one of the Cochrane Back Pain Review Group's outcome measures as part of their design. Trials with participants over 18 years with a minimum of 12 weeks of back pain, in combination with radiographic changes of OA in the spine, were included. Studies were rated for risk-of-bias and graded for quality.
Results
148 studies were identified after screening and meeting eligibility requirements, and three randomised controlled trials (n=309) were included in the quantitative synthesis. The review found that there was low quality but generally no evidence of an effect from glucosamine on function, with no change in the Roland-Morris Disability Questionnaire score in all studies. Conflicting evidence was demonstrated with pain scores with two studies showing no difference and one study with a high risk-of-bias showing both a statistically and clinically significant improvement from taking glucosamine.
Conclusions
On the basis of the current research, any clinical benefit of oral glucosamine for patients with chronic LBP and radiographic changes of spinal OA can neither be demonstrated nor excluded based on insufficient data and the low quality of existing studies.
Keywords: glucosamine, facet joint osteoarthritis, spinal osteoarthritis
Article summary.
Article focus
The current study examines and evaluates the evidence for the use of glucosamine in back pain.
Key messages
There is insufficient evidence to either confidently demonstrate or exclude a clinical benefit of glucosamine for spinal osteoarthritis.
The use of more objective outcome measures, longer follow-up periods and a clearer understanding of the possible biochemical model for glucosamine's mode of action in back pain may enable more definitive conclusions to be drawn.
Strengths and limitations of this study
The review incorporated several databases and utilised systematic and vigorous search strategy. Limitations included the exclusion of articles not published in English and the attempted comparison of studies with participants with different demographics such as age.
Introduction
Rationale
Low back pain (LBP) affects around one-third of UK adults each year.1 2 Around 20% will consult their general practitioner (GP), making it one of the commonest presentations seen in primary care.3 Additionally, there are considerable financial consequences associated with back pain, with previous estimates of direct healthcare costs in the UK amounting to over £1.6 billion and indirect costs from informal care and loss of productivity to the economy of £10.7 billion.4
Osteoarthritis (OA) is a highly prevalent degenerative joint condition that the WHO Scientific Group on Rheumatic Diseases estimates is the cause of significant clinical problems in at least 10% of patients who are 60 years or older.5
OA can affect several parts of the body including the spine. Within the spine, OA affects the vertebral facet joints6 and may occur with or without the presence of LBP.7
Borenstein8 suggested that OA may cause LBP; however, this relationship is complex and controversial. Some of the evidence supporting a link between spinal OA and LBP comes from early studies which showed improved back pain following intra-articular or peri-articular joint injections.9––11 However, it is apparent that not all patients with LBP will have symptoms that correlate with severity of radiographic OA changes on imaging.7
A further degenerative process can be found in the spine in the form of intervertebral degenerative disc disease (DDD). A recent twin study demonstrated the presence of lumbar degenerative discs on MRI to be a major determinate feature of patients with LBP.12 Although the prevalence of DDD and facet joint OA correlates,13 it is unclear whether they are independent of one another or whether they are different ends of the spectrum of the same pathological process.
Pharmacological therapies are the most frequently used intervention for LBP;14 however, serious side effects associated with the long-term use of some medications such as non-steroidal anti-inflammatory drugs (NSAIDS) has led patients to seek alternative medicines such as glucosamine.
Glucosamine is available to purchase as a food supplement and is gaining popularity among patients in the UK for the relief of knee and hip pain associated with osteoarthritis; however, more than 25% of patients have tried glucosamine for LBP.15
Glucosamine is a naturally occurring amino monosaccharide and is a precursor for glycosaminoglycans, a major component of joint cartilage and synovial fluid,16 and this forms the basis of the rationale for its use in OA. Glucosamine is available in over 50 different preparations most commonly in the form of glucosamine sulfate and hydrochloride.17
Glucosamine hydrochloride (Alateris) is the only preparation licensed for medical use in the UK and the license is restricted to the symptomatic relief of mild to moderate knee OA. Despite its being a licensed drug, there is less evidence for its use compared with glucosamine sulfate and neither are currently recommended by the National Institute for Health and Clinical Excellence (NICE).18
Several trials and systematic reviews have looked into the use of glucosamine in knee and hip arthritis. A Cochrane review identified 16 double-blind randomised controlled trials (RCTs) and concluded that there was good evidence that glucosamine is both effective and safe in treating OA, but this did not assess spinal OA19 This review was updated and failed to show a uniformly positive conclusion, if only high-quality studies were included.20 Analysis restricted to studies with adequate allocation concealment failed to show any benefit of glucosamine for pain, function and stiffness based on Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) used to assess pain, stiffness and function in patients with hip and knee OA. However, the review also assessed pain and function on the Lequesne index, which did reveal an improvement after glucosamine when compared with placebo. The disparity between these findings remains unexplained by the authors; however, a study that compared and tested the validity of WOMAC and the Lequesne index found that although both measures show internal validity when assessing function in hip and knee OA, only WOMAC is consistently reliable when assessing symptoms such as pain.21
Given the lack of conclusive evidence regarding an improvement in LBP from glucosamine and, at present, no recommendations from NICE, the indications for using glucosamine remain controversial for clinicians and patients.
The reviews so far have focused on trials looking at the use of glucosamine in hip and knee OA.22 The current study has been undertaken to provide an up-to-date systematic review of the evidence for the use of glucosamine in LBP.
Objective
To systematically search and assess the quality of the evidence of the efficacy of glucosamine on LBP symptoms in patients diagnosed with spinal facet joint OA or DDD.
Methods
Criteria for considering studies for this review
Types of studies
Only RCTs were considered for this review as randomisation ensures that patients in the treatment and control groups are comparable from the start. In the hierarchy of study designs, RCTs and systematic reviews are considered the highest level of evidence.23 At least 1 day of follow-up was required to ascertain any effect of an intervention. RCTs were included if they: (1) evaluated the efficacy and toxicity of glucosamine in OA, (2) were placebo-based or comparative studies, (3) were open-label, single-blinded or double-blinded, (4) evaluated glucosamine-only or combination preparations, (5) utilised oral administration of glucosamine as this is the route which will be used by the majority of patients.
Types of participants
Participant inclusion criteria for this review included: adult participants (≥18 years), with chronic back pain (≥12 weeks) and signs of spinal OA. As there are no consensus guidelines about what constitutes a diagnosis of OA in the spine, any radiographic changes consistent with OA were included. A variety of radiographic grading systems have been proposed, but there is no single global staging system suitable for the assessment of OA at all sites.24
The exclusion criteria were: trials that included subjects with specific LBP caused by other pathologies such as vertebral canal stenosis, ankylosing spondylitis, scoliosis and coccydinia and trials that looked at OA at multiple sites but did not separate the data from the different sites, making conclusions regarding changes in spinal symptoms difficult.
Types of interventions
Both placebo-controlled trials and comparative studies were eligible. The types of comparison considered appropriate were conventional therapies used for OA such as physical therapy, analgesics and anti-inflammatories.
Types of outcome measures
For inclusion, at least one of the following outcome measures recommended by the Cochrane Back Review Group (CBRG) had to be observed: (1) pain intensity, for example, a visual analogue scale, (2) reliable and valid measure of functional status or disability, for example, the Roland-Morris Disability Questionnaire (RMDQ),25 26 (3) perceived recovery, (4) return-to-work status, (5) structural benefits measured by radiography, (6) adverse effects. The primary outcomes for this review were pain and functional status. The timing of the measured outcomes had to be explicitly described.
Search methods for identification of studies
All the three authors are practicing clinicians in the UK and have either completed or are undertaking higher research degrees.
The search strategy was formulated jointly by the first two named authors. Retrieval of searches, reference screening and subsequent data synthesis was subsequently performed independently. Differences were resolved after discussion with the third author. The search was conducted up to March 2011 and included the grey literature, searched via opensigle. Papers not published in English were excluded. By searching MEDLINE (medical, nursing and biomedical journals), it was anticipated that approximately half of the available RCTs would be identified; therefore, a subsequent search of EMBASE (biochemical and pharmaceutical journals) would ensure a comprehensive search as there is little overlap between these databases and in the field of LBP, EMBASE has been shown to retrieve more clinical trials.27 Searching AMED and CINHAL would cover complementary medicine and allied health journals, while including Cochrane enabled high-quality evidence from RCTs and systematic reviews to be included. References of relevant studies were screened to identify additional studies.
The electronic search strategy outlined in online supplementary appendix 1 was developed in MEDLINE and adapted for the other databases. The search was developed by reviewing relevant articles in the area of back pain and OA and combining search terms used in these studies.
Risk-of-bias assessment and quality
The risk-of-bias was assessed using the criteria advised by CBRG.27 Each criterion was scored as yes, unclear or no, where yes indicated that the criterion had been met. Studies are rated as having a low ‘risk-of-bias’ when at least 6 of the 12 CBRG criteria have been met with no serious flaws.
Data extraction
Data were recorded onto a standardised form and described the main trial characteristics, patient demographics, interventions, comparisons, outcomes, analysis, results and assessment of trial quality (tables 1 and 2).
Table 1.
Characteristics of studies included
| Methods | Participants | Interventions | Outcome measures | Notes | |
|---|---|---|---|---|---|
| Wilkens et al30 | RCT Double-blind Single centre | Outpatients (N=250) Country—Norway Mean age 48.5, 48.4% female Inclusion criteria: Chronic LBP >6 months, MRI findings indicating degenerative lumbar OA, age >25 | 1500 mg glucosamine sulphate versus placebo for 6 months | Primary outcome: disability—RMDQ Secondary outcomes: pain at rest and during activity (11-point scale), quality of life (QOL): EQ-5D and EQ-VAS, global perception of effect (7 point scale) Adverse effects | Sponsored by Pharma Nord |
| Tant et al31 | RCT Open-label Single centre | Outpatients (N=36) Country—Belgium Mean age 64, 43.8% female Inclusion criteria: LBP >12 weeks with associated signs of lumbar arthrosis on radiography, pain score on VAS >3 mm | Conventional treatment (CT) (anti-inflammatory and physical therapy) plus glucosamine complex (containing equivalent: 1500 mg glucosamine, 200 mg of Ribes nigrum, 2000 mg methylsulfonylmethane and 100 mg colloidal silicon) for 12 weeks vs CT alone | Primary outcome: pain at rest on VAS Secondary outcomes: lumbar stiffness on VAS, 2 QOL questionnaires—ODI and RMDQ, global assessment of treatment (satisfied or not) Adverse effects |
Sponsored by Pierre Fabre Sante |
| Leffler et al32 | RCT (cross-over) Double-blind Single centre | Outpatients (N=34, 23 back patients) Country—USA Mean age 43.5 100% male Inclusion Criteria: chronic knee or low back pain on most days for at least 3 months and radiographic evidence of degenerative joint disease | 16 weeks (8 weeks each arm). 1500 mg glucosamine hydrochloride, 1200 mg chondroitin sulphate, 228 g manganese ascorbate vs placebo | Pain (VAS scores), Function: Lequesne Index ( knee), RMDQ (back), patient assessment of handicap, physician assessment of severity and physical examination scores | Patients from US Navy diving and special warfare community Mixed population of knee and back pain (21—knee OA, 23—spinal degenerative joint disease Data separated by site for analysis Sponsored by Nutramax |
Table 2.
Methodological quality assessment and risk-of-bias
| Randomisation adequate? | Allocation concealed? | Groups similar at baseline? | Patient blinded? | Care provider blinded? | Outcome ssessor blinded? | Dropout rate described and acceptable? | Intention to treat analysis? | Co-interventions avoided or similar? | Compliance acceptable? | Timing outcome assessment similar? | Report free of selective outcome reporting | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wilkens et al30 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 12 |
| Tant et al31 | Yes | No | Yes | No | No | No | Yes | No | Yes | Unclear | Yes | Yes | 6 |
| Leffler et al32 | unclear | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 10 |
Risk-of-bias assessed using criteria from the CBRG.27 Studies rated as having a ‘low risk-of-bias’ when at least 6 of the 12 CBRG criteria have been met and it has no serious flaws.
Quality of the evidence
Grades of Recommendation, Assessment, Development and Evaluation criteria were used to evaluate the overall quality of the evidence. This is recommended by the Cochrane Handbook to rate the quality of evidence for each important patient-centred outcome as it goes beyond the reporting of quantitative analysis. The quality of evidence was based on five domains: limitations of the study design, inconsistency, indirectness (inability to generalise), imprecision (insufficient or imprecise data) of results and publication bias across all studies that measure that particular outcome.28 The overall quality was considered to be high when at least 75% of RCTs with no limitations of study design had consistent findings, direct and precise data and no known or suspected publication biases.27 The grades of quality of evidence are outlined in online supplementary appendix 2.29
Results
Description of studies
Study selection
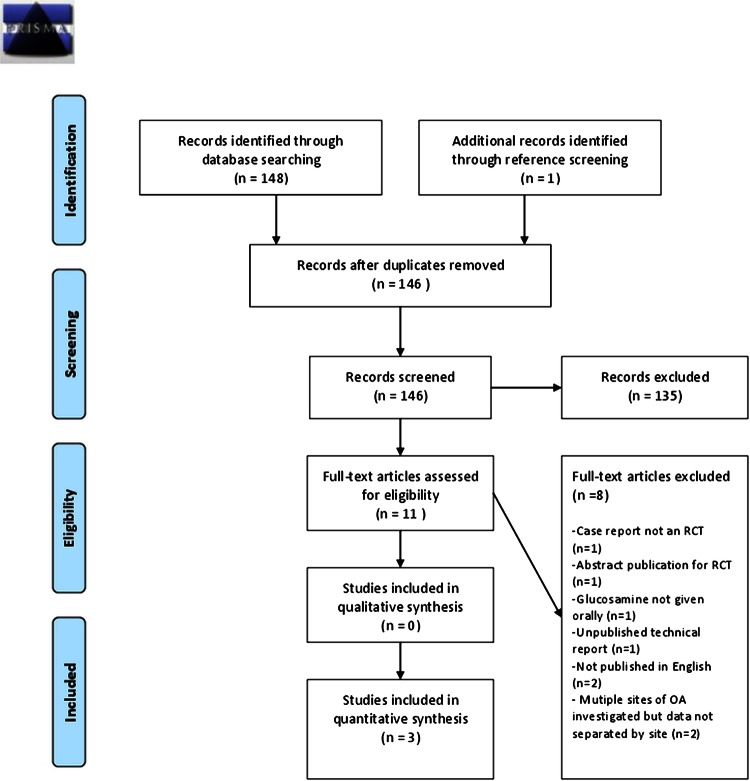
Studies were identified through the following databases: MEDLINE (11), EMBASE (53), Cochrane library (84) (Cochrane reviews (10), other reviews (7), clinical trials (67)). Three studies were included (table 1). Reasons for exclusion are outlined in figure 1. Owing to differences in the study design of included trials, meta-analysis was not attempted.
Figure 1.
Flow diagram of inclusion and exclusion of articles for glucosamine use in spinal OA (PRISMA 2009 Flow Diagram)40
Risk-of-bias in included studies
The risk-of-bias assessment is shown in table 2. Although all studies were described as randomised, only two described adequate randomisation.30 31 One trial was open-label and did not report compliance.31 One trial had a 20% dropout rate.32 All trials had similar groups at baseline, timings of outcome assessments and cointerventions in both groups.
From this assessment, two studies have been rated as having a low risk-of-bias. The one study rated as a high risk-of-bias scored 6, but its open-label design was considered to be a significant methodological flaw.
Effects of intervention
Tables 3–6 summarise the findings with respect to the main outcomes measured. For pain, the two studies with a low risk-of-bias failed to show any significant improvement with glucosamine compared with placebo, while the one study with a high risk-of-bias did show a significant difference with glucosamine compared with no glucosamine.
Table 3.
Key findings: effect of glucosamine on back pain outcomes
| Study | Risk-of-bias | Method of assessment | Key findings | Notes |
|---|---|---|---|---|
| Wilkens et al30 | Low | Low back and leg pain intensities during activity and rest measured by 11-point numeric pain rating scale (NRS) Patients assessed at baseline, 6 weeks, 3 months, 6 months, 1 year |
Baseline NRS LBP at rest for the glucosamine group was 3.7 (95% CI 3.3 to 4.1) and 3.9 (95% CI 3.5 to 4.3) for placebo. The 6-month NRS score was 2.5 (95% CI 2.1 to 2.9) for glucosamine and 2.4 (95% CI 2.0 to 2.8) for placebo. No statistical difference in change between the two groups found at 6 months (p=0.91) for LBP at rest and (p=0.97) for LBP during activity | No significant difference between glucosamine and placebo |
| Tant et al31 | High | VAS for pain at rest and on movement (0–10 cm) measured every 4 weeks | At week 4, mean change from baseline VAS scores for pain at rest was significantly greater in the glucosamine group compared with control group (−2.18 vs +0.13, p<0.001). Difference also significant at 8 and 12 weeks (both p<0.01). The between-group difference in mean VAS scores for pain on movement was only significant at week 12 (2.08) in glucosamine group vs (4.00) in control group; (p=0.029) | Significant difference between CT+glucosamine and CT |
| Leffler et al32 | Low | VAS for pain recorded at clinic visits (0–10 cm) VAS for pain recorded in a daily diary by patients (VAS 0–7 cm). Assessed after weeks 7&8 Knee and back data were separated in later analysis |
Back: The VAS for pain showed a mean change of -28.0% when medication was compared to placebo during the clinic visit (p>0.06) and −21.0% in the diary data (p>0.06). No CI | No significant effect on back pain between glucosamine and placebo |
Table 4.
Key findings: effect of glucosamine on function outcomes
| Study | Risk-of-bias | Method of function assessment | Key findings | Notes |
|---|---|---|---|---|
| Wilkens et al30 | Low | RMDQ | At baseline, mean RMDQ scores were 9.2 (95% CI 8.4 to 10.0) for glucosamine and 9.7 (95% CI 8.9 to 10.5) for the placebo group. At 6 months, the mean RMDQ score was the same for the glucosamine and placebo groups (5.0; 95% CI 4.2 to 5.8). No statistically significant difference in change between the groups found when assessed at 6 months and 1 year (p=0.72) | No significant difference between placebo and glucosamine |
| Tant et al31 | High | RMDQ and ODI | Mean score on the ODI significantly improved from baseline at weeks 4, 8 and 12 in the glucosamine group (all p<0.001). In the control group no significant improvement in score until week 12 (p<0.001). At 12 weeks: significant difference in ODI score between the 2 groups (p=0.028) At baseline, mean RMDQ scores were 9.76 for glucosamine and 7.86 for placebo group. Mean RMDQ scores significantly improved from baseline at weeks 4, 8 and 12 in both groups (all p<0.001) but no significant between-group differences found |
No significant difference between CT+glucosamine and CT for RMDQ but significant difference for ODI |
| Leffler et al32 | Low | RMDQ | Back: Mean baseline RMDQ score was 6.9 with a mean change of −13.7% when medication was compared to placebo (p>0.06) No CI | No significance difference between placebo and glucosamine |
Table 5.
Key findings: adverse effects
| Study | Risk-of-bias | Monitoring | Adverse effects | Notes |
|---|---|---|---|---|
| Wilkens et al30 | Low | Adverse events, blood pressure (bp) monitored every visit Fasting blood glucose, cholesterol levels before and following intervention |
Adverse events (n=86), 40 in glucosamine group, 46 in placebo group. ∼30% of patients had adverse events 10 patients withdrew due to adverse events Adverse events: mild gastrointestinal and dermatological symptoms. All self-limiting Fasting blood glucose, cholesterol and bp did not alter |
1 patient died in glucosamine group 1 participant in each group developed a disc herniation requiring surgery, events not considered study related |
| Tant et al31 | High | Patients interviewed at clinic visit regarding undesirable effects | Abdominal discomfort reported at 8 weeks by 1 patient in the glucosamine group and 1in the control group None of the patients discontinued treatment due to an adverse event |
Adverse effect may have been due to analgesic/anti-inflammatory treatment instead of glucosamine as abdominal discomfort also occurred in 1 patient not receiving glucosamine |
| Leffler et al32 | Low | Patient survey of toxicity symptoms and faecal occult blood testing at end of each phase Bp and pulse measured 21 patients had blood count and coagulation studies carried out |
No patients reported symptoms requiring termination of the study Symptom frequency on medication was similar to that at baseline Vital signs, occult blood testing and haematological parameters did not change significantly from placebo to medication |
Table 6.
GRADE evidence profile
| Quality assessment | |||||||
|---|---|---|---|---|---|---|---|
| Number of studies | Design | Limitations | Inconsistency | Indirectness | Imprecision | Other considerations | Quality |
| Pain measured on VAS, follow up (4 weeks–1 year) | |||||||
| 3 | Randomised trials | Serious* | Serious† | Serious* | No serious imprecision | None | Very low |
| Function/disability measured on RMDQ, follow-up (4 weeks–1 year) | |||||||
| 3 | Randomised trials | Serious* | No serious inconsistency | Serious‡ | No serious imprecision | None | Low |
| Adverse effects | |||||||
| 3 | Randomised trials | Serious* | No serious inconsistency | Serious‡ | No serious imprecision | None | Low |
*One study was open-label.31 There were limitations regarding unclear randomisation in another trial.32 One trial did not clearly employ an intention to treat analysis and compliance was also unclear.31
†Two trials with a low risk-of-bias failed to show any significant decrease in pain levels,30 32 whereas one trial with a high risk-of-bias31 showed a significant effect of glucosamine on back pain.
‡One trial used male patients from US Navy special warfare community with a history of high activity levels and unique occupational exposures; hence the results may not be generalisable.32 One study used a mixed population of both knee and back pain patients and some patients had both, although data was separated by site.32
Back function/disability was measured by the Owestry Disability Index (ODI) and RMDQ, both validated tools26 33; there was no significant difference in the RMDQ scores with glucosamine as an intervention. The study with a high risk-of-bias demonstrated a statistically significant difference in the ODI score reduction for the glucosamine group, although this difference was small.31
With respect to adverse effects, one trial revealed ∼30% of participants experienced adverse effects irrespective of whether they were in the placebo or glucosamine group.30
Other outcomes that were considered but not across all trials included an assessment of quality-of-life measured by the Euro-Qol-5 Dimensions index and overall health status measured by EQ-VAS.30 There was no significant difference found between glucosamine and placebo with these outcomes.
One study used several assessments that were totalled to provide an overall summary score.32 In addition to measuring pain and function, physical examination scores and running times were assessed. There were no statistically significant changes in the LBP group when considering the overall summary score or individual outcome measures.
None of the studies looked at radiographic changes in association with glucosamine use.
Discussion
In this review, three RCTs were included that evaluated the effectiveness of glucosamine as an intervention for chronic LBP associated with spinal OA.30–32
Overall, the review found that the limited number of studies had methodological deficiencies. The studies did not demonstrate a clear beneficial effect of using glucosamine for LBP due to OA.30 32 One study, however, showed a statistically significant difference in the ODI score reduction for the glucosamine group,31 although this difference was small and does not appear to reach the minimally clinical important difference (MCID) alluded to in previous research.34
There was conflicting evidence regarding the effect of glucosamine on pain scores. Two studies showed no statistically significant difference on pain scores between the intervention and placebo groups.30 32 One study did show a statistical and clinically significant reduction in pain scores for those taking glucosamine.31 While this study had significant methodological shortcomings that are discussed in the next section, this alone may not completely explain differences when compared with the two other studies. A possible reason was that the study recruited older patients with a mean age of 64 compared with a much younger demographic in the remaining two studies. Facet-joint OA is known to become more prevalent with age,13 and therefore the proportion of patients with pain related to facet joints as opposed to discogenic pain may have been higher. This, combined with a theoretical possibility that glucosamine may predominantly affect articular cartilage, as opposed to intervertebral discs, may lead to an under-representation of the effect of glucosamine in studies with a younger cohort.
Methodological considerations
There were several factors that contributed to the very low-quality or low-quality assessment for the main outcomes measured in the trials. The results of one study31 in particular, which found positive effects of glucosamine on both pain and function, appear to contradict the findings of the other two; however, this may be partly explained by its limitations. A key limitation was its open-label design. Since the participants and clinicians were aware of the group allocation, bias was introduced.
Another study had unclear details about its randomisation.32 Blinding and randomisation decrease the likelihood of selection and performance bias, which would affect the internal validity of the study.35 This same RCT employed a crossover design, which may intrinsically have introduced bias if the 5-week washout period employed was too short.
To minimise the attrition bias, the dropout rate should be described and be acceptable with all the randomised patients analysed in the group to which they were allocated, by an intention-to-treat analysis (ITT).35 One trial did not employ an ITT analysis and compliance was unclear.31
There are difficulties in how the trials can be directly applied to the general population and this adversely affects their relevance to practice and external validity. One trial used patients from the US Navy diving and special warfare community who have a history of high-activity levels and unique occupational exposures. They were also all men; hence, the results may not be generalisable.32 This study used a mixed population of both knee and back-pain patients, with some participants having both knee and back pain. The proportion of patients in each group was described and the data were separated.
Despite the fact that the risk-of-bias was low in two studies, the studies collectively showed flaws regarding the concealment of treatment allocation, adequate randomisation, compliance and dropout rates. The review findings were significantly influenced by these shortcomings despite the fact that the study by Wilkens et al was of a high quality and well designed. The quality of future RCTs needs to be improved to reduce bias in future reviews.
Review strengths and limitations
The selection procedure and literature search utilised in this review may have introduced bias.
The selection criteria did not place limits on the ages of participants and as the pathology of LBP may change with age, direct comparisons between studies of patients with different patient demographics need to be taken with caution.
Relevant, unpublished trials may have been omitted and as these are likely to be small studies without positive results, this may lead to publication bias. Studies not published in English were excluded and may also have introduced bias. Utilising references of the included trials to identify other studies may have also led to an over-representation of positive studies.
The search strategy, however, was vigorous with several databases utilised, in addition to reference screening of included studies, which ensured that the omission of relevant studies was minimised.
Implications for health practice
LBP is extremely prevalent with considerable financial consequences.4 OA accounts for a significant proportion of LBP seen by GPs and secondary care clinicians. Current treatment options such as NSAIDs and surgery have some potentially serious adverse effects .Therefore, alternative treatments such as glucosamine, which may provide a possible solution to this problem, seem attractive.
Global sales of glucosamine reached almost £1.3billion in 2008.36 Currently in the UK, glucosamine is available as a food supplement and can be prescribed for knee OA. The evidence for its use in back pain is conflicting; it is therefore imperative that a consensus based on sound clinical evidence is reached to justify this immense cost to the public.
This review helps to clarify the existing evidence for the use of glucosamine in back pain, which will be of particular relevance to patients and clinicians considering using glucosamine.
The current review has demonstrated that if only the studies with a low risk-of-bias are considered, there is no evidence of a significant difference between glucosamine and placebo for pain or pain-related disability associated with OA in the lower back.
The mechanism by which glucosamine may exert its effect is poorly understood. Wilkens et al, previously proposed that glucosamine may reduce LBP by inhibiting interleukin (IL)-1β, which is present in lumbar discs and facet joints. This mechanism is purely theoretical with no conclusive evidence demonstrating a direct pharmacological effect on the spine. The lack of a sound scientific rationale for the use of glucosamine in LBP makes it difficult to successfully design a study to prove any clinical benefit that it may have. In addition, there is much debate as to the relationship between LBP and spinal OA findings. Not all patients with LBP have spinal OA and vice versa; however, most studies assume they are correlated.
All the studies included in the review had limitations. All were single centre trials and two had small sample sizes. There were methodological differences in randomisation, blinding, allocation concealment and varying outcome measures. Inclusion criteria varied between trials; some looked at both facet joint OA and DDD. These two conditions do not necessarily represent the same pathological process. In addition, the method for diagnosing OA differed as there is no existing consensus or criteria for diagnosis. Back pain is complex and while spinal OA may cause LBP, several other structures may be responsible and pathologies may coexist.
It is possible that glucosamine may work better in more severe disease, as has been suggested with knee OA.37 All the studies reviewed had varying severities of OA symptoms required for inclusion. This limits the conclusions that can be made as the studies did not separate the data for different levels of severity in the analysis.
OA is a chronic disease and patients taking supplements such as glucosamine may do so for several years. Follow-up periods for the trials varied from 8 weeks to 1 year. Glucosamine may take longer than this to have an apparent affect. A case report revealed an improvement in the structural quality of disc cartilage on MRI in a patient taking glucosamine over a 2-year period.38 The patient's symptoms only began to improve at 6 months and continued until the end of the study period. None of the studies in this review looked at objective radiographic changes as an outcome and while there are obvious limitations to drawing any broad conclusions from a single case report, this provides an argument for a longer follow-up RCT and more objective outcome measures.
A strength of this review is that it contained several placebo-controlled RCTs. One especially well-designed study clearly showed that patients treated with glucosamine for 1 year, who had a combination of chronic LBP and either or both facet-joint OA and DDD, had no difference in pain or disability when compared with placebo.
An important factor to consider when assessing the relevance of trial data to everyday practice is the generalisability or external validity of the studies. The current review included one study which used a relatively young cohort of male patients who were from a US Navy diving and special warfare community with a history of high-activity levels and unique occupational exposures. This is not the profile of a typical OA patient a doctor would see in general practice.
An important distinction is between statistical significance and clinical relevance of findings. One study showed a statistically significant difference in pain-related disability on ODI; however, the difference was very small and may not have represented a clinically relevant change.31 Currently, there is consensus regarding minimal clinically important changes for pain and function (measured by RMDQ, not ODI) in back pain.27 For LBP, 30% on VAS/NRS for pain is considered to be clinically significant and 2–3 points (8–12%) on the RMDQ for function is also considered as clinically significant.39
Glucosamine may be viewed as a relatively safe medication; however, the current review nonetheless highlights a high incidence of adverse effects and although these were mild, it is an important consideration when recommending it.
On based on the current evidence explored in this review, there is insufficient evidence to either demonstrate or exclude a clinical benefit of glucosamine for spinal OA. Using more objective measures such as radiography to look at any change in OA progression, refining the study inclusion criteria, providing longer follow-up periods and trying to establish a clear biochemical model for glucosamine may enable more definitive conclusions to be drawn so that clinicians can confidently advise their patients based on the best available evidence.
Supplementary Material
Footnotes
Contributors: All the listed authors fulfil the ICMJE guidelines for authorship. The first author (RS) conceptually designed the study, performed the searches and data synthesis and provided the initial draft for publication. NS performed the database searches and also aided the data synthesis and prepared the manuscript for publication. MA contributed to the data analysis and revised the manuscript prior to submission. All authors have read and approved the final manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Macfarlane GJ, Beasley M, Jones EA, et al. The prevalence and management of low back pain across adulthood: results from a population-based cross-sectional study (the MUSICIAN study). Pain 2012;153:27–32 [DOI] [PubMed] [Google Scholar]
- 2.Papageorgiou AC, Croft PR, Thomas E, et al. Influence of previous pain experience on the episode incidence of low back pain: results from the South Manchester Back Pain Study. Pain 1996;66:181–5 [DOI] [PubMed] [Google Scholar]
- 3.Papageorgiou AC, Rigby AS. Review of UK data on the rheumatic diseases—7. Low back pain. Br J Rheumatol 1991;30:208–10 [DOI] [PubMed] [Google Scholar]
- 4.Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain 2000;84:95–103 [DOI] [PubMed] [Google Scholar]
- 5.Murray CJL, Lopez AD. The global burden of disease. A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Cambridge, MA: Harvard School of Public Health on behalf of the World Health Organization and The World Bank, 1996 [Google Scholar]
- 6.Kalichman L, Hunter DJ. Lumbar facet joint osteoarthritis: a review. Semin Arthritis Rheum 2007;37:69–80 [DOI] [PubMed] [Google Scholar]
- 7.Kalichman L, Li L, Kim D, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine 2008;33:2560–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borenstein D. Does osteoarthritis of the lumbar spine cause chronic low back pain? Curr Pain Headache Rep 2004;8:512–17 [DOI] [PubMed] [Google Scholar]
- 9.Goldthwait J. The lumbosacral articulation. An explanation of many cases of lumbago, sciatica, and paraplegia. Boston Med and Surg J 1911;164:365–72 [Google Scholar]
- 10.Schwarzer AC, Aprill C, Derby R. Clinical features of patients with pain stemming from the lumbar zygapophyseal joints. Is the lumbar facet syndrome a clinical entity? Spine 1994;10:1132–7 [DOI] [PubMed] [Google Scholar]
- 11.Dreyer SJ, Dreyfuss PH. Low back pain and the zygapophysial (facet) joints. Arch Phys Med Rehabil 1996;77:290–300 [DOI] [PubMed] [Google Scholar]
- 12.Livshits G, Popham M, Malkin I, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis 2011;70:1740–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Muehleman C, Abe Y, et al. Prevalence of facet joint degeneration in association with intervertebral joint degeneration in a sample of organ donors. J Orthop Res 2011;29:1267–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuijpers T, Van Middelkoop M, Rbinstein S, et al. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low-back pain. Eur Spine J 2011;20:40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avins A. Glucosamine and the ongoing enigma of chronic low back pain. JAMA 2010;304:1. [DOI] [PubMed] [Google Scholar]
- 16.Arendt-Nielsen L, Weidner M, Bartholin D, et al. A double-blind randomized placebo controlled parallel group study evaluation the effects of Ibuprofen and glucosamine sulphate on exercise induced muscle soreness. J Musculoskelet Pain 2007;15:21–8 [Google Scholar]
- 17.C+D Data, 2008. The Product File [online]. http://www.cddata.co.uk [Product search for “glucosamine”: 20 October 2008].
- 18. NICE Guideline CG59 Osteoarthritis the care and management of osteoarthritis in adults. http://www.nice.org.uk/guidance/index (accessed Feb 2008)
- 19.Towheed TE, Anastassiades TP, Shea B, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;(1):CD002946. [DOI] [PubMed] [Google Scholar]
- 20.Towheed T, Maxwell L, Anastassiades T, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2005;(2):CD002946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stucki G, Sangha O, Stucki S, et al. Comparison of the WOMAC (Western Ontario and McMaster Universities) osteoarthritis index and a self-report format of the self-administered Lequesne-Algofunctional index in patients with knee and hip osteoarthritis. Osteoarthritis Cartilage 1998;6:79–86 [DOI] [PubMed] [Google Scholar]
- 22.Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010;16:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines for the Scottish Intercollegiate Guidelines Network Grading Review Group. (SIGN) BMJ 2001;323:334–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roland W, Moskowitz M, Roy D, et al. Osteoarthrits: diagnosis and medical/surgical management. 4th edn Lippincott: Willliams and Wilkens, 2006 [Google Scholar]
- 25.Roland M, Morris RA. Study of the natural history of backpain: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983;8:141–4 [DOI] [PubMed] [Google Scholar]
- 26.Stratford PW, Binkley JM, Riddle DL, et al. Sensitivity to change the Roland–Morris back pain Questionnaire:Part 1. Phys Ther 1998;78:1186–96 [DOI] [PubMed] [Google Scholar]
- 27.Furlan A, Pennick V, Bombardier C, et al. Updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34:1929–41 [DOI] [PubMed] [Google Scholar]
- 28.Atkins D, Best D, Briss P. GRADE Working Group Grading quality of evidence and strength of recommendations. BMJ 2006;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Vist G, et al. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkens P, Scheel I, Grundes O, et al. Effect of glucosamine on pain-related disability in patients with chronic low back pain and degenerative lumbar osteoarthritis. JAMA 2010;304:45–52 [DOI] [PubMed] [Google Scholar]
- 31.Tant L, Gillard B, Appelboom T. Open-label, randomized, controlled pilot study of the effects of a glucosamine complex on low back pain. Curr Ther Res 2005;66:511–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leffler C, Philippi A, Leffler S, et al. Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo controlled pilot study. Mil Med 1999;164:85–91 [PubMed] [Google Scholar]
- 33.Fairbank J, Couper J, Davies J, et al. The Owestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3 [PubMed] [Google Scholar]
- 34.Lauridsen HH, Manniche C, Korsholm L, et al. What is an acceptable outcome of treatment before it begins? Methodological considerations and implications for patients with chronic low back pain. Eur Spine J 2009;18:1858–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hart A. Making sense of statistics in health care. Radcliffe Medical Press, 2001 [Google Scholar]
- 36.Heller L. US glucosamine grows slow, lags global sales. 2009. http://www.nutraingredients-usa.com/Consumer-Trends/US-glucosamine-grows-slow-lags-global-sales
- 37.Clegg D, Reda D, Harris C. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006;354:795–808 [DOI] [PubMed] [Google Scholar]
- 38.Van Blitterswijk W, van de Nes J, Wuisman P. Glucosamine and chondroitin sulphate supplementation to treat symptomatic disc degeneration: biochemical rationale and case report. BMC Complement Altern Med 2003;3:2;1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostelo R, Deyo R. Stratford P interpreting change scores for pain and function status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33:90–4 [DOI] [PubMed] [Google Scholar]
- 40.Moher D, Liberati A, Tetzlaff J, et al. ; The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.