Abstract
Giant unilamellar vesicles composed of a ternary mixture of phospholipids and cholesterol exhibit coexisting liquid phases over a range of temperatures and compositions. A significant fraction of lipids in biological membranes are charged. Here, we present phase diagrams of vesicles composed of phosphatidylcholine (PC) lipids, which are zwitterionic; phosphatidylglycerol (PG) lipids, which are anionic; and cholesterol (Chol). Specifically, we use DiPhyPG-DPPC-Chol and DiPhyPC-DPPG-Chol. We show that miscibility in membranes containing charged PG lipids occurs over similarly high temperatures and broad lipid compositions as in corresponding membranes containing only uncharged lipids, and that the presence of salt has a minimal effect. We verified our results in two ways. First, we used mass spectrometry to ensure that charged PC/PG/Chol vesicles formed by gentle hydration have the same composition as the lipid stocks from which they are made. Second, we repeated the experiments by substituting phosphatidylserine for PG as the charged lipid and observed similar phenomena. Our results consistently support the view that monovalent charged lipids have only a minimal effect on lipid miscibility phase behavior in our system.
Introduction
Roughly a dozen years ago, the first fluorescence micrographs of model vesicle membranes demixing into liquid-ordered (Lo) and liquid-disordered (Ld) phases were published (1). Since that time, biophysicists have amassed a wealth of phenomenological data. We know that vesicles that produce micron-scale coexisting liquid phases must, at minimum, be composed of a ternary lipid mixture. We know that the three components must be a lipid with a high melting temperature (Tm), a lipid with a low melting temperature, and a sterol such as cholesterol (2,3). We know that the acyl chains of lipids in the Lo phase have higher conformational order than of lipids in the Ld phase (4). A variety of physical parameters, such as bending rigidities, line tensions, and diffusion constants have been quantitatively mapped and measured (5). An exciting recent development in the field of membrane biophysics is that a sufficient number of miscibility phase diagrams have been compiled such that comparisons between different lipid systems and measurement methods can be made (5). These diagrams synthesize information for membranes of different compositions, namely their transition temperatures, tie lines, and critical points. However, to date, the majority of these phase diagrams have been mapped for systems that contain only lipids without an overall net charge. Here, we explore miscibility within membranes containing charged lipids.
Charged lipids introduce monopole electrostatic interactions into calculations of membrane miscibility phase behavior. In pure, bulk water, these electrostatic interactions are long range: the screening length (called the Debye length) is at least two orders of magnitude larger than the lipid-lipid distance. Electrostatic interactions between adjacent charged lipid headgroups in this scenario are strong, on the order of kBT. These interactions, in contrast to those that arise from lipid acyl chain packing or hydrogen bonding, are relatively well understood and straightforward to calculate (6,7), although mixed lipid systems present challenges (8,9). Consequently, introducing charged lipids into membranes and mapping phase diagrams at low salt conditions is a valuable method to benchmark the free energy scales that lead to demixing of liquid phases. Miscibility phase diagrams of charged lipids will be of particular relevance to test simulation methods (10–12).
In addition to their important physical properties, charged lipids are biologically relevant. They constitute a significant fraction of biological membranes, where they are asymmetrically distributed between the inner and outer leaflets. For example, in mammalian red blood cells, 15 mol % of lipids are charged, almost all of which reside in the cytoplasmic leaflet (13). Previously, it has been argued that repulsion between charged lipids may preclude heterogeneous lipid compositions in the inner leaflet (14) even though ionic strengths in biological systems correspond to ∼140 mM salt. Nevertheless, the clustering of anionic lipids is often associated with interactions between proteins and membranes (15–17). Charged lipids also play important roles in signaling. Multivalent phosphoinositides (e.g. PI, PIP, and PIP2) are the most extensively studied in this class of lipids (17–19).
Here, we map miscibility transition temperatures of two systems containing charged phosphatidylglycerol (PG) lipids. The PG lipids are mixed with uncharged phosphatidylcholine (PC) and cholesterol (Chol), to make either DiPhyPG-DPPC-Chol or DiPhyPC-DPPG-Chol. Structures of lipids are given in Fig. S1 in the Supporting Material. Diphytanoyl (as in DiPhyPG) was chosen for the chains of the low-Tm lipids and dipalmitoyl (as in DPPG) was chosen for the chains of the high-Tm lipids. These chains were chosen for two reasons. First, these choices facilitate comparison of our phase diagrams with previously published, extensively characterized diagrams of the corresponding uncharged system of DiPhyPC-DPPC-Chol (20). Second, neither lipid chain contains any double bonds, and hence all lipid membranes that we produce are minimally prone to artifacts of photooxidation (21). PG was chosen as the charged headgroup for three reasons. First, it has a relatively low pKa. Therefore, PG remains charged in unbuffered solutions, even when effects of salt concentration and local bilayer environment, both on the local pH and the pKa of the PG lipids, are taken into account (22–24). Second, the melting temperatures of PG lipids are similar to those of PC lipids with the same chains (e.g., those of DPPG and DPPC are both 41°C) (25). Likewise, binary mixtures of PC and PG lipids have similar melting temperatures to binary mixtures PC with the same acyl chains (24,26,27). Some correlation exists between lipid melting temperatures and membrane miscibility temperatures (2), and it is plausible that miscibility temperatures of all of the ternary systems that we study will be similar. Third, PG lipids are sometimes added as a minor component to neutral lipids of interest to increase vesicle yield (28).
In a separate set of experiments, we examine the phase diagram of charged membranes of DiPhyPG-DPPC-Chol in the presence of 10 mM monovalent salt, to determine the effect of screening the charges of the PG headgroup. The modest concentration of 10 mM is sufficient to shorten the Debye length to the same order of magnitude as the lipid-lipid spacing. Salt has been shown to reduce the effect of charge on membrane properties (29). The phase behavior of pure PG lipids has been shown to be strikingly different in pure water versus high salt conditions (30–32).
Materials and Methods
Lipids
1,2-diphytanoyl-sn-glycero-3-phosphocholine (DiPhyPC); 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC); 1,2-diphytanoyl-sn-glycero-3-phosphoglycerol (DiPhyPG); 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG); and 1,2-diphytanoyl-sn-glycero-3-phosphoserine (DiPhyPS) were obtained from Avanti Polar Lipids (Alabaster, AL). Cholesterol was obtained from Sigma (St. Louis, MO). All lipids were used without further purification and were stored in chloroform at −20°C until use. Texas Red 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (TR-DPPE, Invitrogen, Eugene, OR) was included at 0.8 mol % as a dye for contrast between phases in fluorescence experiments. 18 MΩ-cm water was produced by a Barnstead filtration system. All other chemicals were obtained from Sigma.
Making vesicles
We produced giant unilamellar vesicles (GUVs) by a gentle hydration protocol (33) modified for high yield. Briefly, 0.25 mg of lipids were mixed in chloroform and spread on a clean glass slide at 60°C, which is above the gel-liquid melting transition of all lipids used here. Slides were dried under vacuum for >30 min, and then hydrated overnight in 300 μL of aqueous solution at 60°C to produce vesicles. Vesicles were imaged within 4 h of production. In our studies, miscibility transition temperatures are reported within a window of all possible ratios of the three lipids in our membranes. This window is determined by vesicle yield and dye partitioning. Specifically, compositions that produced a sufficient yield of vesicles are constrained by an upper limit on cholesterol concentration set by the cholesterol solubility limit (34,35) and by a lower limit on charged lipids set by the gentle hydration method. Miscibility transition temperatures were measured only for samples that produced a high yield of vesicles. Dye partitioning constraints render the DPPG-cholesterol binary axis inaccessible, because the fluorescent probe used here does not preferentially partition into the Lo or solid phase, as has been previously reported (2). To control for salt leaching from glass, a sample made with only 18 MΩ-cm water was prepared as previously mentioned. At the end of the protocol, the conductivity of the water was measured using a HM Com-100 EC meter (Culver City, CA). Conductivity was measured to be 0.011 MΩ-cm, equivalent to a KCl concentration of 340 μM aqueous solution. We used gentle hydration rather than electroformation because we are unaware of an electroformation protocol that produces high yields of vesicles without introducing new components (e.g., sucrose) to vesicle solutions.
Imaging vesicles
To measure the miscibility transition temperature (Tmix), GUVs were observed by fluorescence microscopy as in Veatch et al. (2). Briefly, vesicle samples were diluted ∼5:1 in the appropriate solution and deposited between two coverslips. The coverslip assembly was sealed with vacuum grease and coupled with thermal paste (Omega Engineering, Stamford, CT) to a stage. Temperature control of the stage was achieved with a Wavelength controller connected to a Peltier device and a thermistor temperature probe with a manufacturer quoted accuracy of 0.02°C (Wavelength Electronics, Bozeman, MT). Epifluorescence microscopy was performed with a 40× objective on a Nikon microscope with either a Coolsnap HQ or QuantEM charge-coupled device camera (Photometrics, Tucson, AZ).
Only vesicles that appeared unilamellar and free from defects were included in measurements. Upon cooling, micron-scale domains nucleated in vesicle membranes. The sample was allowed to equilibrate for >2 min. at each temperature before vesicle phases were assessed. The transition temperature was recorded as the temperature at which half of the vesicles had visible domains. In most cases, all vesicles in a sample underwent phase separation within a range of 3°C, which is wider than for uncharged vesicles formed by electroformation (20), but nevertheless implies that lipid compositions did not vary drastically from vesicle to vesicle. All compositions were measured at least twice (i.e., reheated and cooled at least once) to ensure that domains were indicative of equilibrium phenomena. Liquid domains have round shapes and merge quickly upon collision with other liquid domains (36,37). Solid domains have static, typically noncircular shapes. Texas Red DHPE differentially labels the Ld phase, such that we distinguish Ld from Lo or solid phases, but not Lo from solid. Low illumination was used whenever possible to minimize the effect of light. Transition temperatures were constant over multiple measurements spanning >15 min, suggesting photooxidation was not significant, consistent with results with similar lipid mixtures (21).
Lipid extraction
Vesicles for mass spectrometry were prepared by the same method above, but without dye. Lipids extracted from vesicles were compared to lipids in stock solutions. Vesicle samples were first diluted in water to 0.5 mL, and then mixed with 3 mL 2:1 chloroform/methanol. Stock solutions in chloroform were diluted to 2 mL, and then mixed with 1.5 mL of 2:1 methanol/water. After this point, the vesicle and stock mixtures above were treated identically. The mixture was vigorously vortexed for >1 min. The mixture was spun on a bench top centrifuge for >10 min until separate organic and aqueous phases resolved. The organic phase was removed by syringe, dried under nitrogen, and placed under vacuum for >30 min. Dried lipids were stored at −20°C until analyzed by mass spectrometry.
Mass spectrometry
Electrospray mass spectrometry for PC versus PG lipids was performed using a Waters Quattro triple quadrupole mass spectrometer, a 2795 Alliance HT LC/autosampler system, and the QuanLynx software package (PC/PG). Spectrometry for Chol versus PC used a Bruker Esquire Liquid Chromatograph ion trap mass spectrometer (Chol/PC). Samples were dissolved in 300 μL [34.3]:[57.1]:[8.6] hexane/isopropyl alcohol/water with 10 mM ammonium acetate (PC/PG) or 300 μL [47.5]:[47.5]:[5] hexane/isopropyl alcohol/water (Chol/PC). PC/PG was determined in negative mode, Chol/PC in positive mode. Parent and fragment ion m/z values, cone voltages, and collision energies are in Table S1.
Mass spectrometry measures the current of daughter ions produced by a molecule of interest (i.e., PC, PG, or Chol). That current is integrated over a sample injection, and a background (solvent) value is subtracted to give a peak area. The area is assumed to be proportional to the initial concentration of the molecule of interest, with the constant of proportionality determined by the extraction and ionization efficiency. By comparing the peak areas of two different molecules, the relative abundance of ions produced can be compared without carefully controlling the total amount of sample. By comparing the peak area ratio for a sample to that of a stock solution of known concentrations under identical instrument conditions, we determined the relative abundance of parent molecules independent of ionization efficiency. With this method, internal standards are not necessary. Ion ratios were averaged for at least two samples. Run to run deviations in ion ratios were found to be similar for all samples of the same type (vesicle or stock). The uncertainty of each point was estimated to be the root mean squared of these deviations.
Results
This section details our four major results for systems of DiPhyPG-DPPC-Chol and DiPhyPC-DPPG-Chol, which are briefly summarized as follows: 1), Charged PG/PC/Chol vesicles formed by gentle hydration have the same composition as the lipid stocks from which they are made. 2), Phase diagrams of vesicles containing charged PG lipids are qualitatively similar to corresponding phase diagrams of vesicles with only neutral lipids, namely: 2a), charged membranes demix into two liquid phases at high temperatures (Tmix), and 2b), the charged lipids in our system strongly partition into one liquid phase. 3), The concentration of salt in solution of charged vesicles has only a small effect on membrane miscibility transition temperatures of charged vesicles. Specifically, using pure water versus 10 mM KCl in the production of charged vesicles has only small effects on Tmix over a broad range of lipid compositions. The same results are seen at 100 mM KCl, at least for one lipid composition. 4), Results 2 and 3 are valid over a range of experimental conditions, including the substitution of PS lipids for PG lipids.
Result 1: Charged PG/PC/Chol vesicles formed by gentle hydration have the same composition as the lipid stocks from which they are made
To accurately map the phase behavior of lipids in vesicles, it is first necessary to produce vesicles of known composition. Vesicle compositions are important to investigate because strong electrostatic interactions between charged lipids may result in some lipid species incorporating more readily into vesicles than others (38). To verify vesicle compositions, we used mass spectrometry of lipids from vesicles composed of ternary mixtures of DiPhyPG, DPPC, and cholesterol, without any fluorescently labeled lipids. As seen in Fig. 1, ion current ratios for lipids extracted from vesicles are indistinguishable within experimental uncertainty from ion ratios for lipids from stock solutions, both for cholesterol and phospholipids. The two ratios are sufficient to completely determine vesicle compositions. In addition, differences in the phospholipid ion ratio of 1:1 DiPhyPG/DPPC vesicles with 0, 20, and 40 mol % cholesterol were smaller than the sample to sample variation (data not shown). Taken together, these results show that the gentle hydration method produces vesicles with the same ternary ratios of DiPhyPG, DPPC, and cholesterol as in original stock solutions.
Figure 1.
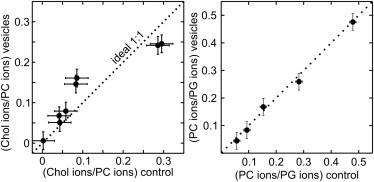
Integrated mass spectrometry ion currents for lipid solutions extracted from vesicles produced by gentle hydration (y axis) versus from lipid stock solutions (x axis), for cholesterol/PG (left) and PC/PG (right). The dotted line is the expected relationship for vesicles that incorporate lipids from stock solutions with perfect fidelity. All data fall close to this line. Uncertainty represents the root mean-squared deviation among three aliquots extracted from the same vesicle preparation, averaged over all compositions.
Two tests were conducted to verify that the presence of nonvesicle lipid aggregates does not skew mass spectrometry results. First, ion ratios were measured for vesicle samples that were determined to have high versus low vesicle yields and high versus low prevalence of aggregates. In all cases, ion ratios between vesicle and stock solutions agreed, as in Fig. 1 (data not shown). Second, a centrifugation step was added, which removed most aggregates. Ion ratios were indistinguishable for lipids extracted from vesicle solutions with and without the centrifugation step (data not shown).
Result 2: Phase diagrams of vesicles containing charged PG are qualitatively similar to the corresponding phase diagram of vesicles with only neutral lipids
Vesicles composed of the two different ternary lipid mixtures containing charged PG lipids studied here (DiPhyPG-DPPC-Chol and DiPhyPC-DPPG-Chol) phase separate over a wide range of compositions and temperatures, even in low-conductivity aqueous solutions created here by placing 18 MΩ-cm water into glass chambers (Fig. 2, Fig. 3, Fig. S2). In all membrane systems, micron-scale liquid-liquid phase separation is observed only when at least three lipid types are present: a lipid with a high melting temperature, a lipid with a low melting temperature, and a sterol such as cholesterol. Vesicles with compositions that fall on the binary axis of 0% cholesterol exhibit phase separation between solid (gel) and liquid phases. An unusual feature of the DiPhyPC-DPPG-Chol system is that solid-liquid coexistence is observed above the melting temperature of both DiPhyPC and DPPG, possibly due to widening of the transition in solutions of low ionic strengths (32). However, it is difficult to quantitatively compare our results to those from calorimetry experiments, which are performed at significantly higher concentrations of lipids (and their counterions).
Figure 2.
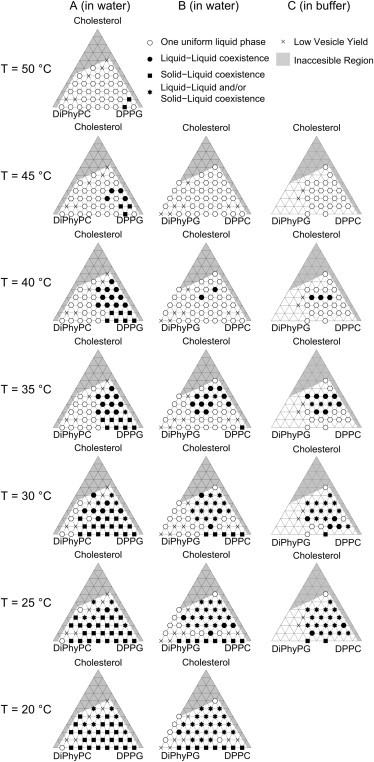
Miscibility phase diagrams at different temperatures for (A) DiPhyPC-DPPG-Chol vesicles prepared in water, (B) DiPhyPG-DPPC-Chol vesicles prepared in water, and (C) DiPhyPG-DPPC-Chol vesicles prepared in 10 mM KCl, 5 mM TRIS, and 0.5 mM EDTA. Regions shaded in gray are inaccessible by our method of gentle hydration and fluorescence microscopy. Star symbols represent phase separation that is coexistence of Lo/Ld, solid/Ld, or solid/Lo/Ld phases. This ambiguity arises because Lo and solid phases can be challenging to distinguish by fluorescence microscopy of vesicles, especially when three phases (Ld, Lo, and solid) coexist in the same vesicle, and/or when a temperature quench from the Ld–Lo coexistence region nucleates solid domains.
Figure 3.
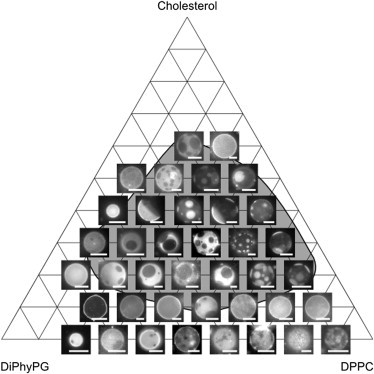
Representative micrographs of DiPhyPG-DPPC-Chol vesicles at 25°C, including cases of low vesicle yield noted in Fig. 3. The shaded region encloses all compositions investigated for which vesicle membranes undergo a liquid-liquid transition temperature above 25°C. The fraction of dark, Lo phase increases strongly from left to right in the phase diagram (as DPPC replaces DiPhyPG) and weakly from bottom to top (as cholesterol fraction increases). All scale bars are 15 μm.
Miscibility transition temperatures for both of the charged ternary membrane systems studied here qualitatively mimic those of the corresponding uncharged system DiPhyPC-DPPC-Chol (Fig. 4). In particular, all three systems feature high miscibility temperatures and broad composition ranges over which phase separation is observed. This result clearly shows that in our system, monovalent lipids only weakly influence the miscibility of a membrane, even in low-conductivity aqueous solutions. Lipid acyl chain identities are far more important. This result is consistent with the observation that binary mixtures of PC and PG lipids have similar melting temperatures as binary mixtures of corresponding PC mixtures with the same acyl chains (24,26,27).
Figure 4.
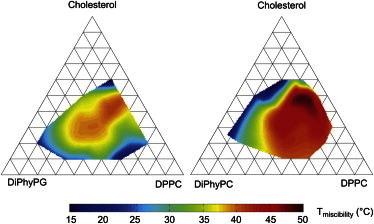
(Color online) Compilation of data from Fig. 2 to show approximate miscibility transition temperatures of gentle hydration GUVs composed of the charged system DiPhyPG-DPPC-Chol (left) and electroformed GUVs composed of the uncharged system DiPhyPC-DPPC-Chol (right, from Veatch et al. (18)). In both cases, the highest transition temperatures appear near 20 mol % DiPhyPG/PC, 40% DPPC, and 40% cholesterol.
Result 2a: Charged membranes demix into two liquid phases at high temperatures
As in the uncharged system DiPhyPC-DPPC-Chol, there are compositions within both of the charged systems studied here (DiPhyPG-DPPC-Chol and DiPhyPC-DPPG-Chol) that phase separate at temperatures above 50°C, even in solutions with low conductivity. This high temperature, which is higher than the melting temperature of either DPPC or DPPG, implies that favorable interactions between like lipid species are significantly stronger than repulsive interactions between charged headgroups.
Result 2b: The charged lipids in our system strongly partition into one of the two liquid phases
In both the charged and the uncharged vesicle systems studied here, the two liquid phases differ mostly in their phospholipid content, or, visually, the tie lines are more horizontal than vertical. An equivalent statement is that changes in the fraction of membrane area covered by Ld versus Lo phases are more strongly correlated with changes in phospholipid ratio than with changes in cholesterol content (Fig. 3). Specifically, vesicles composed of higher concentrations of the lipid with a high melting temperature (with dipalmitoyl chains) have higher area fractions of the dark, ordered phase. This observation is highlighted in Fig. 5 in panels A–F. Another way to come to the same conclusion is to note that the lipid compositions that produce vesicles whose surface area is approximately half covered by bright, Ld phase and half by dark, Lo phase are located within an approximately vertical stripe in the middle of the phase diagram, ranging from low to high cholesterol. In other words, the cholesterol content is only weakly correlated with the area fraction, unlike the phospholipid ratio. This observation is highlighted in Fig. 5 in panels G–I.
Figure 5.
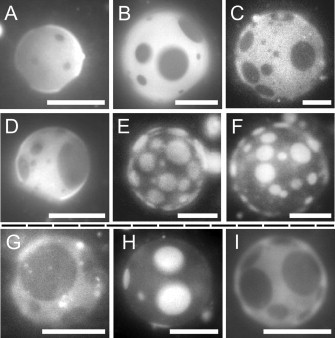
The fraction of membrane area covered by Lo (dark) phase strongly correlates with changes in the ratio of PG/PC lipids. Representative fluorescence micrographs are shown for GUVs composed of DiPhyPG-DPPC-Chol. Panels A–F are at T = 20°C with 20 mol % cholesterol, and molar percentages of DiPhyPG and DPPC of A: 60/20, B: 50/30, C: 40/40, D: 30/50, E: 20/60, F: 10/70. The area fraction of Lo phase increases dramatically from panel A to F. Panels G–I are at T = 25°C with 1:1 DiPhyPC/DPPC and mol % cholesterol of G: 20%, H: 40%, I: 60%. Panels G to I do not show a monotonic change in the Lo area fraction. All scale bars are 20 μm.
Because the coexistence regions in the phase diagrams of Fig. 2 and Fig. 3 are large, the tie-line endpoints are far apart, and the two phases have very different compositions. Because the main difference in composition between the two phases is the phospholipid content, they must have a large contrast in phospholipid content. Therefore, charged lipids partition strongly into one phase based on the packing properties of their acyl chains. This strong partitioning occurs despite the energetic penalty due to concentrating like charges. Exact directions of tie lines are difficult to determine without other methods such as NMR (20) or EPR (39). Quantitative tie lines can be determined by comparing area fractions of the Ld versus Lo phase only by purposefully neglecting lipid areal densities in the two phases as in recent work (40). Those densities likely differ by less than a factor of two, such that visual inspection of area fractions can be used to estimate tie lines. These estimates are consistent with quantitative tie lines determined by NMR, within experimental uncertainty (20).
Result 3: The concentration of salt in solution has only a small effect on membrane miscibility transition temperatures of charged vesicles
Salt in solution acts to screen electrostatic interactions and shortens the Debye length. This reduces the energetic cost of concentrating charge, and so would be expected to raise transition temperatures. To gauge the contribution of electrostatic interactions, we prepared charged DiPhyPG/DPPC/Chol vesicles in a solution of 10 mM KCl, which shortens the Debye length to ∼2.8 nm, 5 mM TRIS, and 0.5 mM EDTA. KCl served as a biologically relevant monovalent salt, TRIS stabilized pH to 7.4, and EDTA chelated any divalent cations. Preparing vesicles in salt solutions versus water did not have a major effect on miscibility phase behavior; transition temperatures of DiPhyPG-DPPC-Chol vesicles prepared in salt solutions versus water differed by ≤ 5°C (Fig. 6). For the small changes in transition temperatures that were seen, Tmix increased for the majority of lipid compositions, in agreement with our expectations. The small decrease in transition temperatures seen in a minority of lipid compositions is inconsistent with a purely electrostatic interaction, so must involve other interactions (e.g., ions sterically disrupting the packing of the ordered phase). For one such composition, the experiment was repeated by measuring Tmix after adding salt to vesicles formed in pure water. Tmix also decreased in this case, implying that the effect is not an artifact of salt interfering with vesicle preparation. Fig. 6 presents changes in Tmix for most but not all possible compositions because vesicles prepared in high salt solutions had significantly lower yields; some compositions had yields so low that no statistically significant transition temperatures could be determined.
Figure 6.
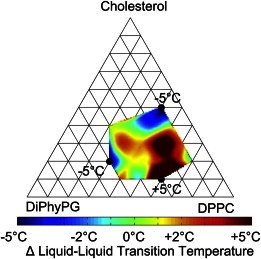
(Color online) Change in miscibility transition temperature for charged GUVs of DiPhyPG-DPPC-Chol prepared in a solution of 10 mM KCl, 5 mM TRIS, and 0.5 mM EDTA versus in water. Positive values indicate a higher transition temperature in the presence of salt.
Result 4: Results 2 and 3 are valid over a range of experimental conditions
Table 1 details additional tests in which vesicles were prepared in solutions of various pH, salt concentration (including 100 mM KCl), and EDTA concentration; all support the previous conclusions. The only experimental condition that resulted in a significant shift in miscibility temperature (larger than the shift upon changing lipid species in the previous section) was preparation of vesicles in KCl solution without EDTA, presumably due to divalent impurities in the KCl. Divalent cations are known to have a large effect on many physical properties of anionic bilayers, including miscibility transition temperature (41–44).
Table 1.
Shift in membrane miscibility temperature (ΔTmix) for charged vesicles of 50:30:20 DiPhyPG/DPPC/Chol prepared in various buffers and/or salt solutions versus in water (control)
| Trial | KCl | TRIS (pH) | EDTA | ΔTmix (°C) |
|---|---|---|---|---|
| Control Tmix = 36.5°C | – | – | – | 0 |
| 1. | 10 mM | 5 mM; pH 7.4 | 0.5 mM | −5 |
| 2. | 100 mM | 5 mM; pH 7.4 | 0.5 mM | +2 |
| 3. | 10 mM | – | – | +8 |
| 4. | 10 mM | – | 0.5 mM | −1 |
| 5. | – | – | 0.5 mM | −2 |
| 6. | – | 10 mM; pH 8.5 | – | +1 |
To verify that our main results are general rather than due to specific interactions of PG headgroups, we mapped the boundaries of the liquid-liquid coexistence region for a system incorporating PS, a different monovalent lipid (Fig. 7). We found the same results for ternary mixtures containing DiPhyPS as for mixtures containing DiPhyPG. Specifically, we found that immiscible liquid phases persist over a wide range of temperatures and composition in membranes of DiPhyPS-DPPC-Chol, and that the charged lipids strongly partition into one of the two liquid phases.
Figure 7.
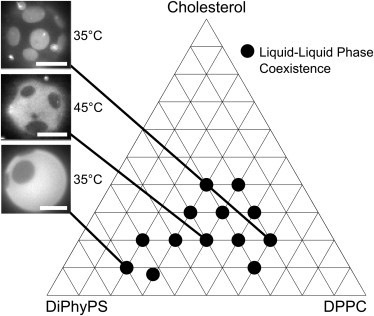
Compositions of DiPhyPS-DPPC-Chol observed to undergo liquid-liquid phase separation within the range of 10–50°C, with representative micrographs for three different compositions. A similar region of observable phase separation exists for membranes containing PG lipids. This region is bounded by both the window where gentle hydration fails to produce sufficient vesicles for characterization and by the compositions that do not separate into liquid phases. As in membranes containing PG lipids, the amount of Ld (dark) phase is correlated with the ratio of high-Tm/low-Tm lipids. All scale bars are 15 μm.
Discussion
Our overall result is that replacing PC lipids with PG lipids in ternary lipid membranes results in only small changes in the membranes’ miscibility phase behavior, at least in the systems we studied. In other words, coexisting liquid phases are observed over wide ranges of temperatures and compositions in membranes of the uncharged reference system DiPhyPC-DPPC-Chol and in membranes for which PG lipids are substituted for either of the phospholipids. This result is surprising for vesicles grown by gentle hydration in water; the Debye length of the aqueous solution is ∼15 nm, more than an order of magnitude longer than the lipid-lipid spacing of ∼0.5 nm. Neither the long tie lines nor the high miscibility transition temperatures are significantly perturbed by the presence of salt concentrations that are high enough to lower the Debye length to ∼2.8 nm (at 10 mM KCl). Both point to a relatively minor role for charge in miscibility. Increasing KCl concentrations further to 100 mM does not significantly alter miscibility transition temperatures.
We know of only two other systematic studies of liquid-liquid phase separation involving charged lipids, both of which reported a more pronounced effect of lipid charge. Comparison with these studies, which are by Vequi-Suplicy et al. and Shimokawa et al., is not straightforward. Vequi-Suplicy et al. (45) found that replacing all of the PC lipids by PG lipids in 1:1:1 DOPC-eggSM-Chol depressed the miscibility transition temperature by > 25°C. Here, DOPC is dioleoylphosphatidylcholine, which has one double bond in each tail, and eggSM is egg-sphingomyelin.
The system of Vequi-Suplicy et al. differs from ours in both the method of preparation and the types of lipids studied. The more important of the two is the method of preparation. They produced vesicles by electroformation in 0.2 M sugar (sucrose inside and glucose outside). We find that vesicles formed in this way have significantly lower transition temperatures than vesicles of the same composition formed by gentle hydration (Fig. S3). For example, we find that Tmix for 50:30:20 DiPhyPG/DPPC/Chol is 36.5°C for vesicles made by gentle hydration versus 24°C for vesicles made by electroformation in 0.2 M glucose. The shift is similar for 30:50:20 DOPG/eggSM/chol. This shift is larger than the shift in Tmix upon forming vesicle solutions in either 10 mM monovalent salt or 0.5 mM divalent cations. The shift due to the electroformation of vesicles with charged lipids in sugar explains most if not all of the qualitative difference between our results and those of Vequi-Suplicy et al. Although discovery of the mechanism of the large shift in Tmix seen by Vequi-Suplicy et al. is beyond the scope of our work here, we speculate that sugars interact significantly with charged lipids in electroformed membranes. Sugars have previously been found to interact with lipid headgroups (46,47). Interactions between sugars and uncharged lipids appear to be less important; inclusion of sucrose during electroformation of uncharged vesicles of DiPhyPC-DPPC-chol shifts Tmix by only ∼1°C (Fig. S3). We were unable to construct experimental conditions that isolated the effect of sugar. Namely, in our hands, vesicle yields were too low to analyze for charged GUVs produced either by gentle hydration in the presence of sugar or by electroformation in the absence of sugar.
Sugars may affect lipid vesicles in ways besides direct interaction with charged headgroups, but we suspect that the resulting shifts in Tmix are small. For example, the use of slightly different concentrations of sucrose and glucose can lead to a difference in osmotic pressure between the inside and outside of the vesicle. A difference in osmotic pressure would alter the membrane tension, which would result in a small change in Tmix (48). Furthermore, when sucrose is used to sink vesicles to a glass substrate, interactions may arise between the vesicle and the charged substrate (49) and fluctuations may be suppressed (50). Finally, there remains a possibility that electroformed vesicles of charged lipids are not composed of the same lipid ratios as their stock solutions. The report by Vequi-Suplicy et al. of immiscible phases and of a dependence on mixing composition implies that charged lipids were indeed present at significant concentrations in their vesicles as the authors intended.
The system of Vequi-Suplicy also differed from ours in the lipids studied. Namely, their system was pseudoternary (containing a mixture of PC lipids extracted from egg), and we chose to use diphytanoyl lipids instead of monounsaturated chains as in their system. In Fig. S3 we repeat experiments’ using the same lipids as Vequi-Suplicy et al. to show that lipid composition does not explain major differences between our results and those of Vequi-Suplicy et al. The minor differences in Tmix trends observed in Fig. S3 between vesicles of DiPhyPG-DPPC-chol (used here) and DOPG-eggSM-chol (used by Vequi-Suplicy et al.) may be due to the structure of the phytanoyl chains, which have four methyl groups. Lipids with phytanoyl chains are expected to pack poorly; there is no main chain transition temperature above −120°C (51). The particular structure of diphytanoyl lipids may well play a role in the membrane’s miscibility behavior. Nevertheless, replacing monounsaturated lipid chains in a membrane with phytanoyl chains does not fundamentally alter the membrane’s miscibility phase diagram (20). The differences that are observed upon the replacement are as follows: coexisting liquid phases persist in vesicles of DiPhyPC-DPPC-Chol over wider composition ranges and to higher temperatures than in vesicles of analogous mixtures such as DOPC-DPPC-Chol (20). These wide ranges and high temperatures render membranes containing diphytanoyl lipids to be highly amenable for study at laboratory temperatures. A lipid with four unsaturated bonds, as opposed to four methyl groups as in diphytanoyl, would be prohibitively sensitive to photooxidation.
The gentle hydration membranes of Shimokawa et al. (41) also differ from ours in several important aspects. 1), The charged species was phosphatidylserine (PS) instead of PG. We find qualitatively similar results for membranes containing PS lipids (Fig. 7) as PG lipids (Figs. 2 and 3). Nevertheless, it is worth keeping in mind that PS headgroups have three charges (two negative and one positive), with a pKa ∼1 pH unit higher than PG. Therefore, under equivalent experimental conditions, a PS headgroup may not be charged when a PG headgroup is charged (22). In a low salt solution like that used by Shimokawa et al., the pKa of PS approaches 7 and a significant amount of PS would be uncharged (52). This implies a quaternary system (DPPC, DOPS(−), DOPS+H, and Chol). 2), As noted in the previous paragraph, the role of lipid structures (here, of phytanoyl versus oleoyl chains) may also play a role in membrane miscibility. 3), In some experiments, Shimokawa et al. added CaCl2, a salt containing a divalent cation. Divalent cations have a qualitatively different electrostatic interaction with charged surfaces than monovalent cations (7) and have been shown to interact strongly with anionic lipids, becoming an integral part of the membrane (53). The studies by both Vequi-Suplicy et al. and Shimokawa et al. were conducted at only one temperature. Here, we have provided phase diagrams over a wide range of temperatures to aid future comparison with data from membranes held at any temperature.
A variety of theoretical approaches and conclusions appear in the literature regarding how charge might affect membrane miscibility. Work by Mengistu et al. (6,54) uses the Poisson-Boltzmann theory to estimate the free energetic cost of phase separation in a mixed anionic-zwitterionic system. For our system, given the lowest energy assumptions about tie-line endpoints and salt content, their model predicts an energetic cost of ∼2 kBT/lipid (∼1.3 kcal/mol). This is much larger than the interaction energies between lipids that lead to phase separation (55). However, this model makes the mean-field assumption inherent to the Poisson-Boltzmann framework, which ignores ion condensation and fluctuation effects, both of which would decrease the electrostatic penalty.
Work by Lau et al. (56,57) predicts that in high charge regimes, a significant fraction of counterions are condensed at the membrane surface, effectively renormalizing the charge density. This model has been used to explain experimental observation of attractive forces between like-charged surfaces (58,59). In our system, the membrane phase containing the most charged lipids has a surface charge density of ≳ 1 e/nm2, or approximately one charge per square Bjerrum length, which is the cutoff for the highly charged regime at which nonlinear effects become important. An extension of the model of Lau et al. that accounts for the zwitterionic headgroup of PC molecules could shed further light on our results.
Finally, it has been proposed that PG lipids may be capable of hydrogen bonding, unlike PC lipids (11,24,60–62). This interaction, or any other short range attraction between PG lipids, could, along with packing considerations, explain why membranes containing charged PG lipids have similar phase behavior as membranes containing only uncharged PC lipids.
Conclusion
All results that we present are consistent with the conclusion that replacing uncharged lipids with monovalent charged PG lipids within a ternary lipid membrane of DiPhyPC-DPPC-Chol has only a minimal effect on the membrane’s miscibility phase behavior. This is supported by observations of the membranes’ high temperatures and wide liquid-liquid coexistence regions, whether in the presence or the absence of solutions with significant salt concentrations. These results raise questions about the energetics of liquid-liquid phase separation in lipid bilayers and distribution of counter ions in the vicinity of the membrane. These questions echo concerns raised in measurements of the membrane potential in mixed lipid systems (8,9) and suggest that new research thrusts that provide more direct measurements of ion distributions associated with a phase separated membrane, either experimentally or in simulation, would be fruitful (63). Biological ramifications of our results follow from suggestions that lipids in the plasma membrane organize in a way similar to phase separation (64). If so, then our results imply that charged lipids are capable of organizing in the same way.
Acknowledgments
We thank Martin Sadilek and Jim Bollinger for assistance with mass spectrometry. We thank the reviewers for their comments.
This research was supported by funds from the National Science Foundation (MCB -0744852). M.C.B. was supported by National Institutes of Health (NIH) T32 GM008268. C.W.T. was supported by an NSF REU through MCB-0744852.
Supporting Material
References
- 1.Dietrich C., Bagatolli L.A., Gratton E. Lipid rafts reconstituted in model membranes. Biophys. J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veatch S.L., Keller S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie M.E., Veatch S.L., Keller S.L. Sterol structure determines miscibility versus melting transitions in lipid vesicles. Biophys. J. 2005;89:1760–1768. doi: 10.1529/biophysj.104.049635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polozov I.V., Gawrisch K. Characterization of the liquid-ordered state by proton MAS NMR. Biophys. J. 2006;90:2051–2061. doi: 10.1529/biophysj.105.070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh D. Cholesterol-induced fluid membrane domains: a compendium of lipid-raft ternary phase diagrams. Biochim. Biophys. Acta. 2009;1788:2114–2123. doi: 10.1016/j.bbamem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Mengistu D.H., May S. Nonlinear Poisson-Boltzmann model of charged lipid membranes: accounting for the presence of zwitterionic lipids. J. Chem. Phys. 2008;129:121105. doi: 10.1063/1.2990746. [DOI] [PubMed] [Google Scholar]
- 7.Lau A.W.C., Lukatsky D.B., Safran S.A. Charge fluctuations and counterion condensation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002;65:051502. doi: 10.1103/PhysRevE.65.051502. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty H., Sarkar M. Interaction of piroxicam and meloxicam with DMPG/DMPC mixed vesicles: anomalous partitioning behavior. Biophys. Chem. 2007;125:306–313. doi: 10.1016/j.bpc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Voinov M.A., Rivera-Rivera I., Smirnov A.I. Surface electrostatics of lipid bilayers by EPR of a pH-sensitive spin-labeled lipid. Biophys. J. 2013;104:106–116. doi: 10.1016/j.bpj.2012.11.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascales J.J.L., de la Torre J.G., Berendsen H.J.C. Molecular dynamics simulation of a charged biological membrane. J. Chem. Phys. 1996;104:2713–2720. [Google Scholar]
- 11.Zhao W., Róg T., Karttunen M. Atomic-scale structure and electrostatics of anionic palmitoyloleoylphosphatidylglycerol lipid bilayers with Na+ counterions. Biophys. J. 2007;92:1114–1124. doi: 10.1529/biophysj.106.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay P., Monticelli L., Tieleman D.P. Molecular dynamics simulation of a palmitoyl-oleoyl phosphatidylserine bilayer with Na+ counterions and NaCl. Biophys. J. 2004;86:1601–1609. doi: 10.1016/S0006-3495(04)74227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaux P.F., Zachowski A. Maintenance and consequences of membrane phospholipid asymmetry. Chem. Phys. Lipids. 1994;73:107–120. [Google Scholar]
- 14.Wang T.-Y., Silvius J.R. Cholesterol does not induce segregation of liquid-ordered domains in bilayers modeling the inner leaflet of the plasma membrane. Biophys. J. 2001;81:2762–2773. doi: 10.1016/S0006-3495(01)75919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinderliter A., Almeida P.F.F., Biltonen R.L. Domain formation in a fluid mixed lipid bilayer modulated through binding of the C2 protein motif. Biochemistry. 2001;40:4181–4191. doi: 10.1021/bi0024299. [DOI] [PubMed] [Google Scholar]
- 16.Mbamala E.C., Ben-Shaul A., May S. Domain formation induced by the adsorption of charged proteins on mixed lipid membranes. Biophys. J. 2005;88:1702–1714. doi: 10.1529/biophysj.104.048132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin S., Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 18.Gambhir A., Hangyás-Mihályné G., McLaughlin S. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys. J. 2004;86:2188–2207. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graber Z.T., Jiang Z., Kooijman E.E. Phosphatidylinositol-4,5-bisphosphate ionization and domain formation in the presence of lipids with hydrogen bond donor capabilities. Chem. Phys. Lipids. 2012;165:696–704. doi: 10.1016/j.chemphyslip.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Veatch S.L., Gawrisch K., Keller S.L. Closed-loop miscibility gap and quantitative tie-lines in ternary membranes containing diphytanoyl PC. Biophys. J. 2006;90:4428–4436. doi: 10.1529/biophysj.105.080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honerkamp-Smith A.R., Cicuta P., Keller S.L. Line tensions, correlation lengths, and critical exponents in lipid membranes near critical points. Biophys. J. 2008;95:236–246. doi: 10.1529/biophysj.107.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Dijck P.W.M., de Kruijff B., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim. Biophys. Acta. 1978;512:84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]
- 23.Watts A., Harlos K., Marsh D. Control of the structure and fluidity of phosphatidylglycerol bilayers by pH titration. Biochim. Biophys. Acta. 1978;510:63–74. doi: 10.1016/0005-2736(78)90130-x. [DOI] [PubMed] [Google Scholar]
- 24.Garidel P., Johann C., Blume A. The mixing behavior of pseudobinary phosphatidylcholine phosphatidylglycerol mixtures as a function of pH and chain length. Eur. Biophys. J. 1997;26:447–459. [Google Scholar]
- 25.Silvius J.R. Lipid-Protein Interactions. John Wiley; New York: 1982. Thermotropic phase transitions of pure lipids in model membranes and their modifications by membrane proteins; pp. 239–281. [Google Scholar]
- 26.Findlay E.J., Barton P.G. Phase behavior of synthetic phosphatidylglycerols and binary mixtures with phosphatidylcholines in the presence and absence of calcium ions. Biochemistry. 1978;17:2400–2405. doi: 10.1021/bi00605a023. [DOI] [PubMed] [Google Scholar]
- 27.Wiedmann T., Salmon A., Wong V. Phase behavior of mixtures of DPPC and POPG. Biochim. Biophys. Acta. 1993;1167:114–120. doi: 10.1016/0005-2760(93)90150-8. [DOI] [PubMed] [Google Scholar]
- 28.Feigenson G.W. Phase diagrams and lipid domains in multicomponent lipid bilayer mixtures. Biochim. Biophys. Acta. 2009;1788:47–52. doi: 10.1016/j.bbamem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filippov A., Orädd G., Lindblom G. Effect of NaCl and CaCl(2) on the lateral diffusion of zwitterionic and anionic lipids in bilayers. Chem. Phys. Lipids. 2009;159:81–87. doi: 10.1016/j.chemphyslip.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Epand R.M., Hui S.W. Effect of electrostatic repulsion on the morphology and thermotropic transitions of anionic phospholipids. FEBS Lett. 1986;209:257–260. doi: 10.1016/0014-5793(86)81123-1. [DOI] [PubMed] [Google Scholar]
- 31.Hauser H. Effect of inorganic cations on phase transitions. Chem. Phys. Lipids. 1991;57:309–325. doi: 10.1016/0009-3084(91)90083-n. [DOI] [PubMed] [Google Scholar]
- 32.Schneider M.F., Marsh D., Heimburg T. Network formation of lipid membranes: triggering structural transitions by chain melting. Proc. Natl. Acad. Sci. USA. 1999;96:14312–14317. doi: 10.1073/pnas.96.25.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akashi K., Miyata H., Kinosita K., Jr. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys. J. 1996;71:3242–3250. doi: 10.1016/S0006-3495(96)79517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens M.M., Honerkamp-Smith A.R., Keller S.L. Solubility limits of cholesterol, lanosterol, ergosterol, stigmasterol, and beta-sitosterol in electroformed lipid vesicles. Soft Matter. 2010;6:5882–5890. doi: 10.1039/c0sm00373e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J.Y., Buboltz J.T., Feigenson G.W. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta. 1999;1417:89–100. doi: 10.1016/s0005-2736(98)00260-0. [DOI] [PubMed] [Google Scholar]
- 36.Cicuta P., Keller S.L., Veatch S.L. Diffusion of liquid domains in lipid bilayer membranes. J. Phys. Chem. B. 2007;111:3328–3331. doi: 10.1021/jp0702088. [DOI] [PubMed] [Google Scholar]
- 37.Stanich, C. A., A. R.Honerkamp-Smith, …, S. L.Keller. Coarsening dynamics of domains in lipid membranes. Biophys. J. In press. [DOI] [PMC free article] [PubMed]
- 38.Angelova, M. I. 1988. Lipid swelling and liposome formation in electric fields. PhD thesis. Institute of Biophysics, Bulgarian Academy of Sciences, Sofia, Bulgaria.
- 39.Smith A.K., Freed J.H. Determination of tie-line fields for coexisting lipid phases: an ESR study. J. Phys. Chem. B. 2009;113:3957–3971. doi: 10.1021/jp808412x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husen P., Arriaga L.R., Bagatolli L.A. Morphometric image analysis of giant vesicles: a new tool for quantitative thermodynamics studies of phase separation in lipid membranes. Biophys. J. 2012;103:2304–2310. doi: 10.1016/j.bpj.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimokawa N., Hishida M., Yoshikawa K. Phase separation of a mixture of charged and neutral lipids on a giant vesicle induced by small cations. Chem. Phys. Lett. 2010;496:59–63. [Google Scholar]
- 42.Boggs J.M., Rangaraj G. Investigation of the metastable phase-behavior of phosphatidylglycerol with divalent-cations by calorimetry and manganese ion binding measurements. Biochemistry. 1983;22:5425–5435. [Google Scholar]
- 43.Mittler-Neher S., Knoll W. Ca(2+)-induced lateral phase separation in black lipid membranes and its coupling to the ion translocation by gramicidin. Biochim. Biophys. Acta. 1993;1152:259–269. doi: 10.1016/0005-2736(93)90257-z. [DOI] [PubMed] [Google Scholar]
- 44.Riske K.A., Dobereiner H.G., Lamy-Freund M.T. Comment on “Gel-Fluid transition in dilute versus concentrated DMPG aqueous dispersions”. J. Phys. Chem. B. 2003;107:5391–5392. [Google Scholar]
- 45.Vequi-Suplicy C.C., Riske K.A., Dimova R. Vesicles with charged domains. Biochim. Biophys. Acta. 2010;1798:1338–1347. doi: 10.1016/j.bbamem.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Pereira C.S., Hünenberger P.H. Interaction of the sugars trehalose, maltose and glucose with a phospholipid bilayer: a comparative molecular dynamics study. J. Phys. Chem. B. 2006;110:15572–15581. doi: 10.1021/jp060789l. [DOI] [PubMed] [Google Scholar]
- 47.Crowe J.H., Hoekstra F.A., Crowe L.M. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta. 1996;1280:187–196. doi: 10.1016/0005-2736(95)00287-1. [DOI] [PubMed] [Google Scholar]
- 48.Portet T., Gordon S.E., Keller S.L. Increasing membrane tension decreases miscibility temperatures; an experimental demonstration via micropipette aspiration. Biophys. J. 2012;103:L35–L37. doi: 10.1016/j.bpj.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parthasarathy R., Cripe P.A., Groves J.T. Electrostatically driven spatial patterns in lipid membrane composition. Phys. Rev. Lett. 2005;95:048104. doi: 10.1103/PhysRevLett.95.048101. [DOI] [PubMed] [Google Scholar]
- 50.Gordon V.D., Deserno M., Poon W.C.K. Adhesion promotes phase separation in mixed-lipid membranes. EPL. 2008;84:48003. [Google Scholar]
- 51.Lindsey H., Petersen N.O., Chan S.I. Physicochemical characterization of 1,2-diphytanoyl-sn-glycero-3-phosphocholine in model membrane systems. Biochim. Biophys. Acta. 1979;555:147–167. doi: 10.1016/0005-2736(79)90079-8. [DOI] [PubMed] [Google Scholar]
- 52.Tokutomi S., Ohki K., Ohnishi S. Proton-induced phase separation in phosphatidylserine/phosphatidylcholine membranes. Biochim. Biophys. Acta. 1980;596:192–200. doi: 10.1016/0005-2736(80)90354-5. [DOI] [PubMed] [Google Scholar]
- 53.Pedersen U.R., Leidy C., Peters G.H. The effect of calcium on the properties of charged phospholipid bilayers. Biochim. Biophys. Acta. 2006;1758:573–582. doi: 10.1016/j.bbamem.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 54.Mengistu D.H., May S. Debye-Hückel theory of mixed charged-zwitterionic lipid layers. Eur. Phys. J. E. Soft Matter. 2008;26:251–260. doi: 10.1140/epje/i2007-10319-8. [DOI] [PubMed] [Google Scholar]
- 55.Almeida P.F.F. Thermodynamics of lipid interactions in complex bilayers. Biochim. Biophys. Acta. 2009;1788:72–85. doi: 10.1016/j.bbamem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Lau A.W.C., Pincus P. Counterion condensation and fluctuation-induced attraction. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002;66:041501. doi: 10.1103/PhysRevE.66.041501. [DOI] [PubMed] [Google Scholar]
- 57.Lau A.W.C. Fluctuation and correlation effects in a charged surface immersed in an electrolyte solution. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2008;77:011502. doi: 10.1103/PhysRevE.77.011502. [DOI] [PubMed] [Google Scholar]
- 58.Kjellander R., Marcelja S., Quirk J.P. Attractive double-layer interactions between calcium clay particles. J. Colloid Interface Sci. 1988;126:194–211. [Google Scholar]
- 59.Larsen A.E., Grier D.G. Like-charge attractions in metastable colloidal crystallites. Nature. 1997;385:230–233. [Google Scholar]
- 60.Pascher I., Sundell S., Eibl H. Conformation and packing properties of membrane lipids: the crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim. Biophys. Acta. 1987;896:77–88. doi: 10.1016/0005-2736(87)90358-0. [DOI] [PubMed] [Google Scholar]
- 61.Nagle J.F. Theory of lipid monolayer and bilayer phase transitions: effect of headgroup interactions. J. Membr. Biol. 1976;27:233–250. doi: 10.1007/BF01869138. [DOI] [PubMed] [Google Scholar]
- 62.Garidel P., Blume A. Miscibility of phosphatidylethanolamine-phosphatidylglycerol mixtures as a function of pH and acyl chain length. Eur. Biophys. J. 2000;28:629–638. doi: 10.1007/s002490050003. [DOI] [PubMed] [Google Scholar]
- 63.Laanait N., Mihaylov M., Schlossman M.L. Tuning ion correlations at an electrified soft interface. Proc. Natl. Acad. Sci. USA. 2012;109:20326–20331. doi: 10.1073/pnas.1214204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


