Abstract
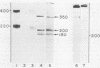
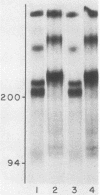
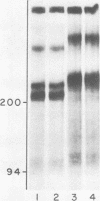
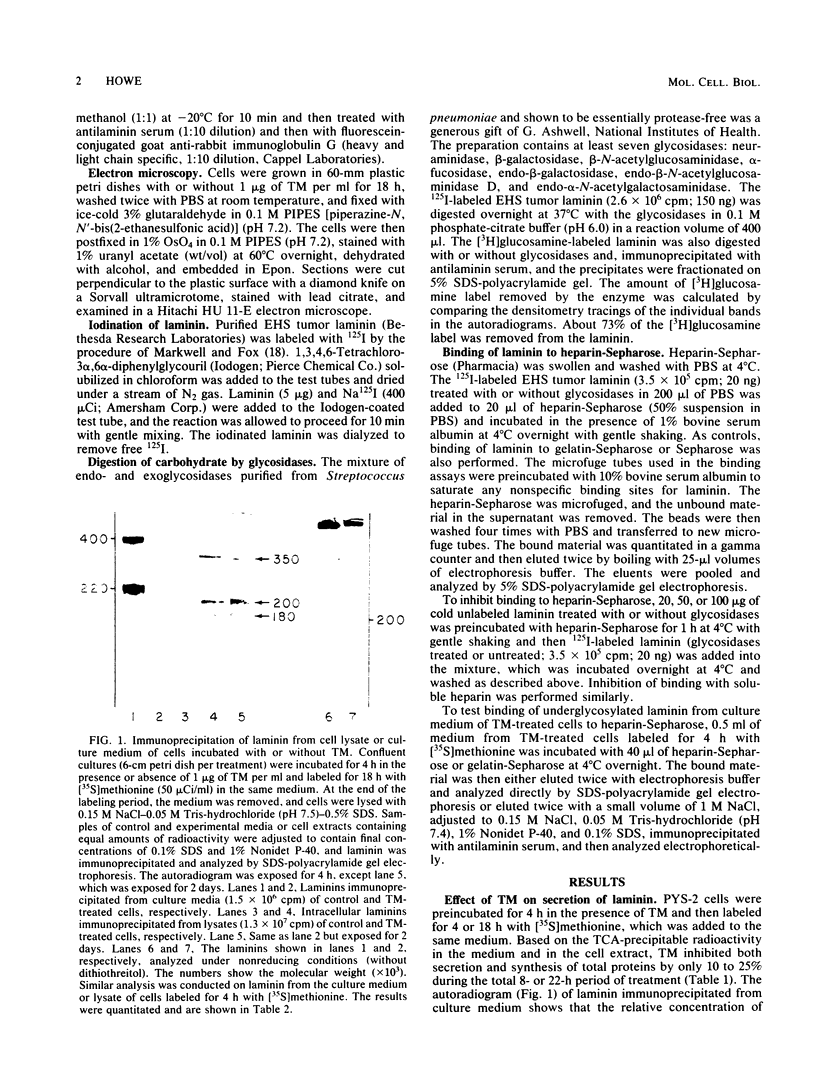
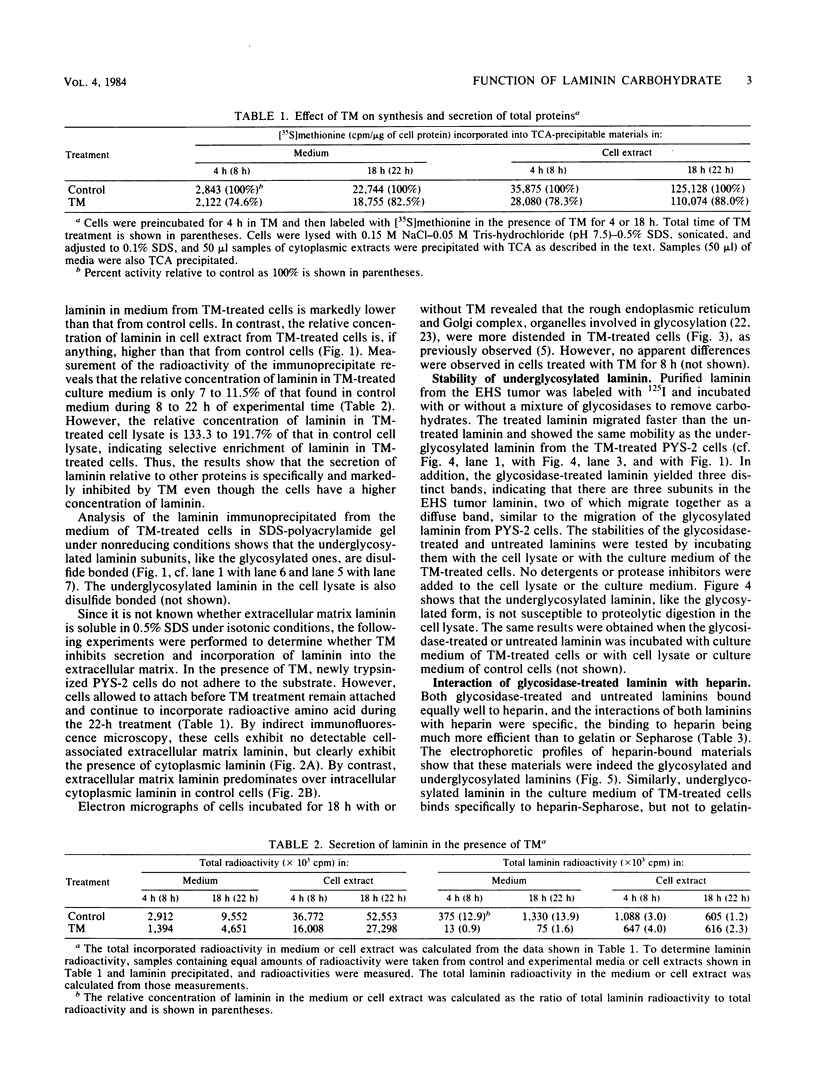
Previous work showed that tunicamycin suppresses glycosylation of laminin. In the present work, the role of glycosylation in the secretion of laminin and in the disulfide bonding of laminin subunits was studied, using tunicamycin to inhibit glycosylation. Tunicamycin inhibited extensively the secretion of laminin into culture medium and extracellular matrix even though the treated cells contained higher concentrations of laminin than the control cells. The laminin subunits synthesized in the presence of tunicamycin were disulfide bonded. Thus, suppression of glycosylation did not adversely affect disulfide bonding of the subunits, but did decrease the secretion of laminin. Glycosidases were also used to remove the carbohydrate of laminin to study the role of carbohydrate in the stability of laminin and in its interaction with another extracellular matrix component, heparin. The glycosidases removed about 73% of [3H]glucosamine. Both glycosidase-treated and untreated laminin were stable when incubated with cell lysate or culture medium. The glycosidase-treated laminin bound as efficiently as the untreated laminin to heparin. These results suggest that the presence of a carbohydrate moiety, at least at the level found in untreated laminin, is not essential in binding to heparin or in protecting laminin from proteolytic degradation in the cell or culture medium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atienza-Samols S. B., Pine P. R., Sherman M. I. Effects of tunicamycin upon glycoprotein synthesis and development of early mouse embryos. Dev Biol. 1980 Sep;79(1):19–32. doi: 10.1016/0012-1606(80)90070-6. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Engvall E., Freeman A., Ruoslahti E. Laminin and fibronectin in cell adhesion: enhanced adhesion of cells from regenerating liver to laminin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2403–2406. doi: 10.1073/pnas.78.4.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A. E., Jaffe R., Freeman I. L., Vergnes J. P., Braginski J. E., Carlin B. Properties of a basement membrane-related glycoprotein synthesized in culture by a mouse embryonal carcinoma-derived cell line. Cell. 1979 Feb;16(2):277–287. doi: 10.1016/0092-8674(79)90005-9. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., Kurkinen M., Taylor A., Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur J Biochem. 1981 Sep;119(1):189–197. doi: 10.1111/j.1432-1033.1981.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Levy-Benshimol A., Buck C. A., Warren L. Effect of tunicamycin on the synthesis, intracellular transport and shedding of membrane glycoproteins in BHK cells. Exp Cell Res. 1979 Mar 1;119(1):1–13. doi: 10.1016/0014-4827(79)90329-x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Bell M. L., Carlsson R. N., Miller E. J., Ruoslahti E. Nonhelical, fibronectin-binding basement-membrane collagen from endodermal cell culture. Cell. 1982 Jun;29(2):475–482. doi: 10.1016/0092-8674(82)90164-7. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Housley T. J., Rowland F. N., Ledger P. W., Kaplan J., Tanzer M. L. Effects of tunicamycin on the biosynthesis of procollagen by human fibroblasts. J Biol Chem. 1980 Jan 10;255(1):121–128. [PubMed] [Google Scholar]
- Howe C. C., Dietzschold B. Structural analysis of three subunits of laminin from teratocarcinoma-derived parietal endoderm cells. Dev Biol. 1983 Aug;98(2):385–391. doi: 10.1016/0012-1606(83)90367-6. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Solter D. Identification of noncollagenous basement membrane glycopolypeptides synthesized by mouse parietal entoderm and an entodermal cell line. Dev Biol. 1980 Jun 15;77(2):480–487. doi: 10.1016/0012-1606(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Johansson S., Kjellén L., Hök M., Timpl R. Substrate adhesion of rat hepatocytes: a comparison of laminin and fibronectin as attachment proteins. J Cell Biol. 1981 Jul;90(1):260–264. doi: 10.1083/jcb.90.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Leivo I., Vaheri A., Timpl R., Wartiovaara J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol. 1980 Apr;76(1):100–114. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Ott U., Odermatt E., Engel J., Furthmayr H., Timpl R. Protease resistance and conformation of laminin. Eur J Biochem. 1982 Mar;123(1):63–72. doi: 10.1111/j.1432-1033.1982.tb06499.x. [DOI] [PubMed] [Google Scholar]
- Pierce G. B. The development of basement membranes of the mouse embryo. Dev Biol. 1966 Apr;13(2):231–249. doi: 10.1016/0012-1606(66)90066-2. [DOI] [PubMed] [Google Scholar]
- Sakashita S., Engvall E., Ruoslahti E. Basement membrane glycoprotein laminin binds to heparin. FEBS Lett. 1980 Jul 28;116(2):243–246. doi: 10.1016/0014-5793(80)80654-5. [DOI] [PubMed] [Google Scholar]
- Surani M. A. Glycoprotein synthesis and inhibition of glycosylation by tunicamycin in preimplantation mouse embryos: compaction and trophoblast adhesion. Cell. 1979 Sep;18(1):217–227. doi: 10.1016/0092-8674(79)90370-2. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Thesleff I., Pratt R. M. Tunicamycin-induced alterations in basement membrane formation during odontoblast differentiation. Dev Biol. 1980 Nov;80(1):175–185. doi: 10.1016/0012-1606(80)90507-2. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]