Abstract
Objective
Trefoil factor (TFF) peptides are expressed in gastric tissues, where they are part of the epithelial defences. To complement previous in vitro work, the goal of the present study was to examine directly if TFF2 was essential for gastric restitution in vivo during the recovery from microscopic damage.
Design
TFF2 mutant (KO) mice were examined to study the epithelial repair process in vivo after laser-induced photodamage (LPD). Using two-photon laser energy absorption (710 nm), LPD was imposed on an ~3–5 cell region of surface epithelium in anaesthetised mouse stomach. Responses to damage were evaluated during confocal time-lapse microscopy; including area of damage and the extracellular pH adjacent to the damaged surface (Cl-NERF pH sensor).
Results
In control (TFF2+/+ and TFF2+/−) mice, damaged cells were exfoliated and the damaged epithelium was repaired by indomethacin. The resting surface pH was similar between control and TFF2-KO animals, but the post-LPD alkalisation of surface pH observed in control mice (ΔpH 0.3±0.05, n=21) was attenuated in the TFF2-KO stomach (ΔpH −0.08±0.09, n=18). Recobinant rat TFF3 partially rescued the attenuated surface pH change in TFF2-KO stomach, in the presence or absence of indomethacin.
Conclusions
In the gastric epithelium in vivo, TFFs promote epithelial restitution via a mechanism that does not require cyclooxygenase activation. A novel role for TFFs to affect gastric surface pH is observed.
INTRODUCTION
The stomach confronts a wide variety of intrinsic and exogenous stress factors, including luminal irritants and pathogens. In response to weakening or disruption of the gastric epithelial layer, the rapid restoration of epithelial defences is an indispensable step for protecting the stomach from penetrance of noxious materials into the body.1–5 Disruption of epithelial defences is a central feature of gastric disorders, including injury by non-steroidal anti-inflammatory drugs and peptic ulcers.6
In the gastrointestinal tract, epithelial integrity depends in part on the trefoil factor (TFF) gene family of small protease-resistant peptides.5,7,8 TFF peptides are most abundant in mucinsecretory cells, with the secreted peptide being detected in mucus layers overlying the tissue.9–11 In the healthy gastrointestinal epithelium, three isoforms of the peptide have been identified. TFF1 and TFF2 are abundant in mucus cells within the pit and mucus neck region of the gastric mucosa, while TFF3 is found in both small and large intestinal goblet cells.9–13 The three isoforms of trefoil peptide share a common motif of six cysteine residues with three interchain disulfide bonds yielding the signature ‘trefoil’ structure, also termed as the P domain. There is ~50% amino acid sequence identity among the three TFF isoforms14,15 and, although the receptor(s) for the peptides remain unknown, it appears that there is some redundancy of function among the peptides.8,16
In various epithelial cell culture models, TFFs promote healing after injury through motogenic (cell migratory) and antiapoptotic activities.17 In these models, the intracellular mechanism of TFFs promoting gastric epithelial restitution has been proposed as being linked to ras-dependent mitogen-activated protein (MAP) kinase activation and to redistributing E-cadherin from the cell surface to the intracellular domain.8 Less is known in vivo about the roles of TFFs in the stomach. Most evidence for the function of TFFs in vivo is extrapolated from observing altered expression of the TFF peptides in experimental injury or disease models.10,18,19 Stronger in vivo information comes from observations of TFF-knockout (KO) mice. TFF2-KO mice exhibited modest gastric abnormalities, and, in contrast to the increased proliferation rate of mucosal cells in TFF1-KO mice, TFF2-KO mice had decreased thickness of the gastric mucosa, increased amounts of gastric acid and increased susceptibility to indomethacin-induced gastric ulcerations compared with wild-type mice.20 These latter findings of improved long-term repair suggest that TFF2 has a direct protective effect on the gastric mucosa. Further, addition of exogenous TFF peptides can rescue the TFF-null phenotype.21,22 Information is lacking about the ability to TFFs to facilitate gastric epithelial repair in the short term, since methods have not been available to monitor the gastric healing process in living animals in real time. This has limited the ability to compare in vitro outcomes with those in vivo. For this reason, we have applied the photodamage model reported by our group previously, which produces microlesions in mouse stomach and allows continuous monitoring of epithelial repair.23 Our goal was to examine the physiological function of TFF2 by investigating gastric epithelial restitution after laser-induced photodamage (LPD) in TFF2-KO mice.
One hallmark of acute gastric damage is the alkalinisation of luminal (extracellular) pH in the microdomain adjacent to the gastric surface, a process regulated in healthy tissue by the bicarbonate secreted from surface epithelium cells.24,25 Since bicarbonate secretion and surface pH are regulated by cyclooxygenases (COXs) in both normal and injured tissues, it is perhaps not surprising that application of indomethacin or use of COX-1-deficient mice result in a lowered rate of epithelial repair coincident with an attenuated alkali surge.23 It was proposed that COX-derived products thromboxane A2 and prostaglandin (PG)H2 are involved as downstream effectors activated by TFFs during cellular invasion.26 Therefore, in the present study we ask if TFF also requires COX activity during the injury/repair cycle.
METHODS
Animal husbandry and surgery
TFF2-KO mice were backcrossed onto a C57BL/6J (Jackson Lab, Bar Harbor, Maine, USA) background until >90% of genomic microsatellite markers were from C57BL/6J (assessed by the Beier lab in the Division of Genetics, Harvard Medical School, Boston, Massachusetts, USA). Subsequently, heterozygous mutant breeding pairs were used to generate animals for experiments. Pups were genotyped by genomic PCR as described,27 and used for experimentation at 2–4 months of age. In preliminary experiments, the post-LPD restitution rate in TFF2+/+ (wild type) and TFF2+/− (heterozygous) animals was not significantly different (data not shown), so outcomes from these two groups were combined as the control group to compare versus the TFF2-KO genotype.
The surgical preparation of animals has been previously described.23,24 Brieflky, mice were anaesthetised with inactin (10 mg/kg intraperitoneally, Sigma, St Louis, Missouri, USA) and ketamine (50 mg/kg intraperitoneally, Phoenix, St Joseph, Missouri, USA), then the stomachs of anaesthetised mice were exteriorised and everted to expose the gastric mucosa. The mouse was placed in the prone position such that a portion of the exposed mucosa protruded into a perfusion chamber on the stage of an inverted confocal/two-photon microscope (Zeiss LSM 510 NLO), with the microscope stage enclosed and heated to keep the animal’s body temperature at ~37°C. The mucosal surface was superfused at 0.2 ml/min with a pH 3 solution containing 150 mM NaCl, 1 mM homo-PIPES (Research Organics, Cleveland, Ohio, USA) and a fluorescent pH sensor (10 μM Cl-NERF, Invitrogen, Carlsbad, California, USA). All experimental procedures were approved by the Animal Care and Use Committee of the University of Cincinnati.
Live tissue quantitative imaging
Using an anaesthetised animal equilibrated for 20 min on the microscope stage, the mucosal surface of the gastric corpus was observed using a Zeiss C-Apo ×40 objective and data were collected in a time series. The interval between each imaging time point was 2–30 s. As described below, four images were collected for each time point. A tissue autofluorescence image (two-photon excitation 710 nm, emission 435–485 nm) was collected simultaneously with a confocal reflectance image (reflecting 710 nm light to show cell/tissue structure). Rapidly thereafter, images of 550–600 nm Cl-NERF fluorescence were collected in response to alternating excitation at 514 or 458 nm. Imaging regions were selected in which perfusion solutions could access the epithelial layer freely, and outcomes were not quantified from regions with any artefactual fluorescence due to dye binding to luminal mucus.
Laser-induced microlesion in superficial gastric epithelium
The method of causing microscopic photodamage in the gastric surface epithelium with a two-photon laser has been described previously.23 Briefly, after collecting a set of control images using minimal 710 nm laser power, a small rectangular region (~200 μm2) of gastric mucosa was repetitively scanned at 350 mW average laser power for 100 iterations. The resultant photobleaching of endogenous fluorophores (putative NAD(P) H) led to a sequence of epithelial damage and repair that was recorded by time course imaging.23 The damage-repair cycle was measured independently several times per animal in different locations of the corpus, and outcomes from at least three animals were compiled for each experimental protocol. In some experiments, the rat TFF3 peptide (rTFF3; 2 mg/ml in saline, solubilised on the day of the experiment) was topically applied to the exposed gastric surface 2–3 times within 20 min. Due to limited reagent, rTFF3 was not included in the perfusion solution, but all data were collected within 2 h after administering rTFF3. As performed previously, some experiments also used the COX inhibitor indomethacin (0.5 mg/ml solubilised in saline with minimal Tween-80) applied subcutaneously (5 mg/kg) 1 h prior to analysis of damage repair.
Image analysis
All image analysis was performed with Metamorph software (ver. 6.3, Molecular Devices). As described previously, the damaged area was defined by cellular loss of NAD(P)H auto-fluorescence (see left panel of figure 1A) and/or by cellular uptake of Cl-NERF, in comparison with simultaneously recorded confocal reflectance images that confirmed the location of cellular structures.23 In the time course series of images collected from each experiment, measured values of the area of damage were used to report the maximum observed area of damage (Amax), and the time to attain the maximal damage (Tmax). The changing size of the damaged area over time was used to estimate rates of epithelial restitution, by fitting the 10 min of data collected following Tmax to a single exponential decay curve (see right panel of figure 1A). Best fit values of the rate constant (κ) were used as estimates of the rate of restitution (estimate of the fractional recovery per time, in units of s−1), and were used to estimate the half-time (T1/2) of restitution.
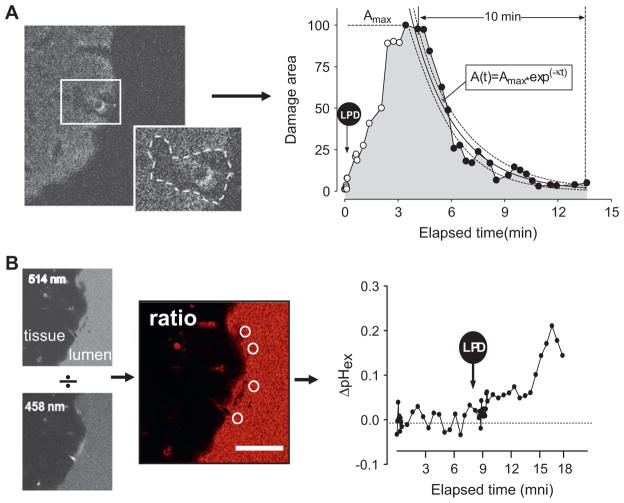
Figure 1.
Data analysis procedures during two-photon laser-induced photodamage (LPD) of the mouse gastric epithelial surface. (A) Analysis of the damaged area. Left: sample image of NAD(P)H autofluorescence collected from the mouse gastric epithelial surface ~5 min post-LPD. Autofluorescence is reduced markedly after photodamage in the region bounded by the dotted line in the insert image, and the included area is used to estimate damage size. Right: the graph shows normalised representative data points measuring the damaged area over time in a control mouse. Time of maximal damage (Tmax) and area of maximal damage (Amax) are estimated from individual data points. The rate constant of area recovery (κ, s−1) is calculated by curve fitting the data between Amax and the following 10 min (black dots in trace) to a single exponential decay equation. Dotted lines in the graph show the 95% CI on the curve fit. (B) Analysis of the extracellular pH at the gastric surface. Left: Cl-NERF added to the perfusate allows sequential collection of confocal images of juxtamucosal fluorescence at 514 and 458 nm excitation. White circles show areas used for measuring the surface pH from 514/458 ratio images. Right: the graph shows representative surface pH measurements in control mouse stomach before and after LPD (arrow). Scale bar=50 μm.
Extracellular pH near the gastric surface was measured from Cl-NERF images.23 As described previously, the background-corrected 514/458 nm ratio image was calculated, and normalised for daily instrument settings prior to estimation of pH by reference to a ratio calibration curve. The extracellular luminal pH values adjacent to the site of damage (<20 μm distance from the gastric surface) were obtained by averaging 3–5 circular regions (diameter 16 μm) from every ratio image (see figure 1B).
Western blots
In 4°C fresh phosphate-buffered saline, mouse corpus was blunt dissected under a stereo microscope to separate gastric mucosa from muscle layer. Then 700 μl of RIPA buffer (Sigma, St Louis, Missouri, USA) with protease inhibitor (1:100, Roche Applied Science, Mannheim, Germany) was added to isolated mucosa (100–300 mg), and tissue homogenised. Protein concentration was determined using a BCA Protein Assay Kit (Pierce, Rockford, Illinois, USA). Protein (20 μg) was separated on a 7.5% acryl-amide gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore Corporation, Billerica, Massachusetts, USA). Membranes were incubated for 1 h with Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, Nebraska, USA) and then incubated overnight at 4°C with rabbit polyclonal antimurine COX-1 (1:1000 dilution; Cayman Chemical, Ann Arbor, Michigan, USA). Membranes were then washed and incubated for 1 h in goat antirabbit secondary antibody (Alex fluor 680, 1:1000 dilution; Invitrogen). Blots were quantified using an Odyssey infrared imaging system (Li-Cor Biosciences).
Determination of PGE2 content
PGE2 levels were determined in the stomach tissue. In anaesthetised mice, stomachs were exteriorised and everted to expose the gastric mucosa. If required, indomethacin (5 mg/kg) was given subcutaneously 1 h before any subsequent treatment. The exposed mucosa was then bathed for 20 min with saline in the absence or presence of rTFF3 (2 mg/ml). At the end of this incubation period, the corpus was excised, weighed, and put in a tube containing 100% methanol plus 0.1 mM indomethacin.28 The samples were minced with scissors, homogenised, and centrifuged at 4°C for 10 min at 12 000 rpm. The supernatant of each sample was used for the determination of PGE2 by enzyme immunoassay using a PGE2 Direct BiotrakAssay (GE Healthcare, Piscataway, New Jersey, USA) according to the manufacturer’s directions. Data were expressed as ng/g tissue.
Statistical analysis
All values are reported from representative experiments as the mean±SEM from multiple experiments. The number of repetitions is reported as the number of independent damage-repair cycles (n) analysed, and the number of different animals used is also indicated. All results were reproduced in at least three animals. Statistical significance was determined using unpaired Student t test, or one-way analysis of variance (ANOVA). A p value of <0.05 was considered significant.
RESULTS
We have developed methods for the study of the surgically exposed gastric mucosa in anaesthetised mice, such that the epithelial and juxtamucosal luminal space can be imaged in vivo with confocal and two-photon microscopy. Using this preparation, we previously reported that the gastric epithelium is promptly repaired in response to microscopic damage of 3–5 cells caused by rapid LPD.23 Images in figure 2A qualitatively confirm previous findings in control mice, demonstrating creation and expansion of damage followed by exfoliation of cells and simultaneous restoration of a continuous epithelium within ~15 min. After quantifying the size of damage from such images, as described in the Methods section, outcomes were compiled from multiple experiments and are shown in the graph of figure 2A.
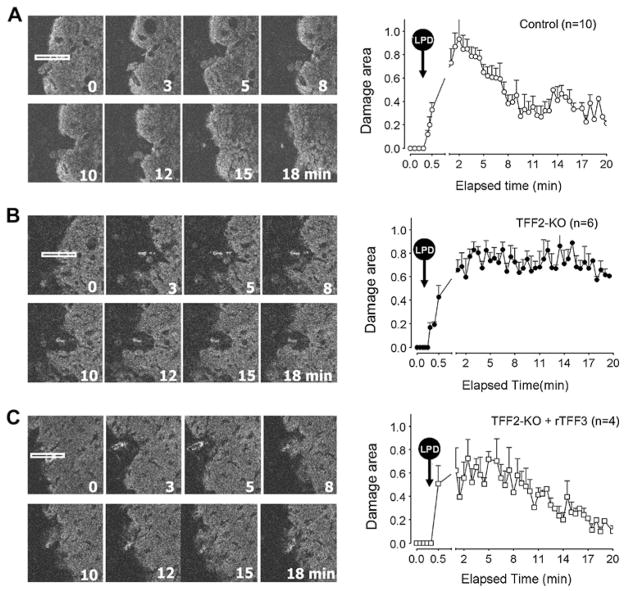
Figure 2.
Time course of changes in damaged area after laser-induced photodamage (LPD) in (A) control mice, (B) trefoil factor 2 knockout (TFF2-KO) mice and (C) TFF2-KO mice after topical addition of recombinant rat TFF3 (rTFF3), as described in the Methods section. In each panel, eight images from a representative time course experiment are shown on the left. In those images, the white rectangle in the first image of the series shows the gastric surface region exposed to LPD (70×4 μm2) at time zero, and all other images indicate the time of imaging the same tissue location after LPD. On the right of each panel, results are compiled for the indicated number of experiments (each collected from at least three animals) with values presented as mean±SEM.
Role of TFF peptides in epithelial restitution
In contrast to the findings in figure 2A, both the images and compiled data in figure 2B show qualitatively that TFF2-KO mice had a similar magnitude of laser-induced damage to controls, but a delayed repair from damage. The TFF2-KO mice have been reported as having a more acidic stomach and a thinner gastric wall20; however, no macroscopic abnormalities including shape, size, colour or spontaneous ulceration were observed in the stomachs of the TFF2-KO mice prior to photo-damage. Some variability in outcomes was observed. Most TFF2-KO gastric mucosa underwent some reduction in damage size within the first 10 min post-LPD, and then the restitution stayed at a very slow rate until the end of the experiment. The compiled results shown in the graph of figure 2B reflect that in most experiments, the damaged area of TFF2-KO stomach did not exfoliate cells and repair the epithelium even 30 min post-LPD (data not shown).
Results in gene knockout mice may or may not be due to direct effects of the deleted gene, as compensatory effects may drive observed phenotypes.29 To help verify the role of TFFs as the necessary agent to promote epithelial restitution after LPD, peptide rescue experiments were used. Of the three TFF isoforms, we only had access to recombinant TFF3 from rat small intestine (rTFF3). Fortunately, rTFF3 has been shown previously to be effective to promote gastric repair in other models,16 suggesting it may be promiscuous on the (as yet unidentified) TFF receptors. As shown qualitatively in figure 2C, 20 min preincubation with topical rTFF3 (2 mg/ml in saline) improved the healing process in TFF2-KO stomach.
Quantifying microlesion repair
Based on the results of experiments performed as shown in figure 2, measurements were compiled to evaluate maximal damage size (figure 3A), the time required to reach maximal damage (figure 3B), and the restitution rate (κ) and half-time for repair (T1/2) (figure 3C,D, respectively). The rates of repair were determined by curve fitting of the early repairing phase after maximal damage; as shown in figure 1A and described in the Methods section.
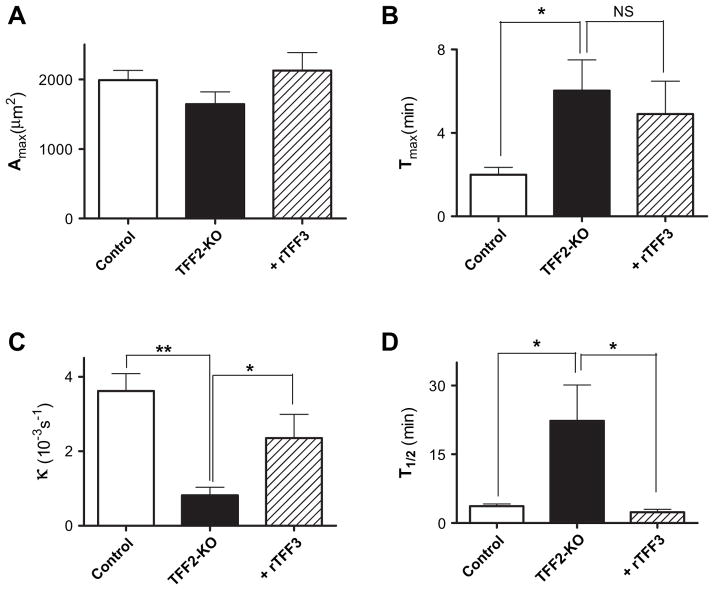
Figure 3.
Damage recovery parameters from control mice, trefoil factor 2 knockout (TFF2-KO) mice and TFF2-KO mice after topical addition of recombinant rat TFF3 (+rTFF3). As described in the Methods section and figure 1A, parameters were extracted from individual experiments performed as in figure 2 and then results were compiled. The parameters were (A) maximum damaged area (Amax), (B) the time to attain the maximal damaged area after laser-induced photodamage (LPD) at time zero (Tmax), (C) the restitution rate constant (κ) and (D) the time required to repair half of the maximal damage (T1/2). Results are presented as the mean±SEM of n=4–10 experiments (each condition performed in at least three animals). *p<0.05, **p<0.001 in unpaired t test versus values from the TFF2-KO animals. NS, no significance.
Results in figure 3 demonstrate that although damage size is comparable in all conditions, the absence of TFF2 slows the time to reach maximal damage as well as the time to repair the induced damage. After addition of rTFF3 to TFF2-KO mice, there was no significant change in the time to reach maximal damage, but the time to repair of the damage was accelerated. For example, the rate constant for repair of the TFF2-KO mucosa (0.0005±0.0002 s−1, n=6 was accelerated fourfold with rTFF3 (0.0023±0.0005 s−1, n=4, p<0.05).
Gastric epithelial surface pH alterations in control and TFF2-KO stomach
Extracellular pH is a critical factor in cell migration,30 and gastric acid is well recognised as limiting gastric repair.31 Since the microdomain of pH adjacent to the gastric epithelium is raised in response to damage,23 we questioned if lack of TFF2 altered gastric surface pH regulation. We employed an extracellular pH-sensitive (ratiometric) dye (Cl-NERF) to image surface pH, as described previously.23
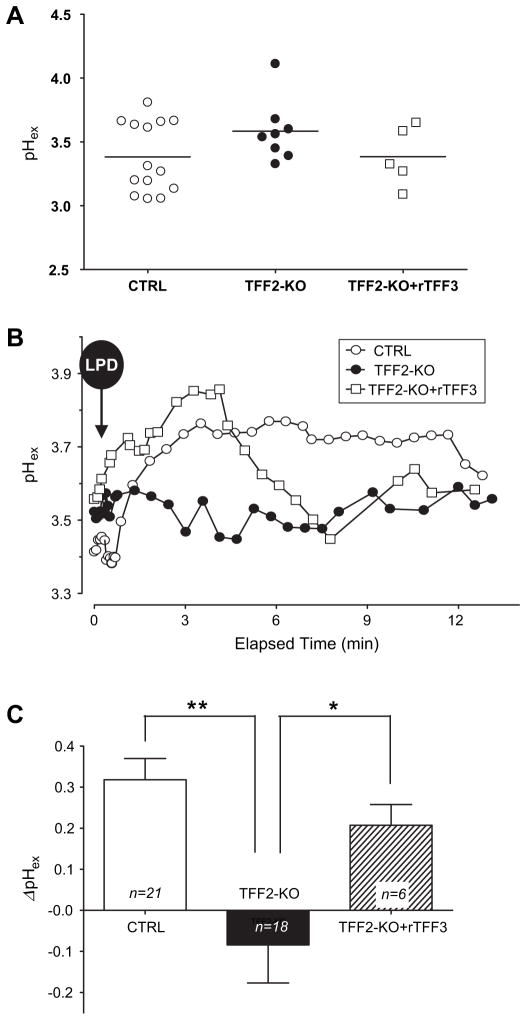
As shown in figure 4A, the resting surface pH prior to any photodamage was modestly more alkaline than the pH 3 perfusion solution, an effect shown previously to be due to COX-1-regulated bicarbonate secretion.32,33 Since TFF2-KO mice have been shown previously to have altered acid secretion,20 some compensatory mechanisms may be in place to regulate resting surface pH. In contrast to the invariable resting gastric surface pH in these different conditions, differences were noted after photodamage. As shown in the representative experiments of figure 4B, and the compiled outcomes in figure 4C, LPD caused a distinct pH increase in control mice as reported previously.23 However, in TFF2-KO mice, LPD caused an attenuated pH increase. In some individual experiments, this actually reversed to a pH acidification immediately after LPD. Interestingly, addition of rTFF3 to the TFF2-KO mice restored a significant surface pH alkalinisation after photodamage (p<0.05 compared with the TFF2-KO without rTFF3, figure 4C), possibly due to the proton permeation regulation effect of TFF2 in the epithelial layer, which has been verified in vivo and in vitro.34
Figure 4.
Gastric epithelial surface pH values from control mice, trefoil factor 2 knockout (TFF2-KO) mice and TFF2-KO mice + recombinant rat TFF3 (rTFF3). As described in the Methods section and figure 1B, extracellular pH was measured adjacent to the gastric surface (pHex) using confocal microscopy of Cl-NERF fluorescence. (A) Resting surface pH collected prior to laser-induced photodamage (LPD). Every pH value is the average value of 6–8 measurements from an individual animal. No significant difference of resting pH among conditions (p=0.87, one-way analysis of variance; SDs were not significantly different). (B) Representative time course of pHex following LPD in control mice (open circles), TFF2-KO mice (filled circles) and TFF2-KO mice after topical application of rTFF3 (open squares). The initial pHex values before LPD were 3.44, 3.52 and 3.61 in control, TFF2-KO and TFF2-KO+rTFF3, respectively. (C) The maximal surface pH change after LPD (ΔpH) was calculated relative to the pre-LPD surface pH in individual experiments, and then values were compiled. The results are presented as the mean±SEM for n experiments. *p<0.05, **p<0.001 in unpaired t test versus values from the TFF2-KO animals.
Effects in TFF2-KO mice with inhibition of prostaglandin generation
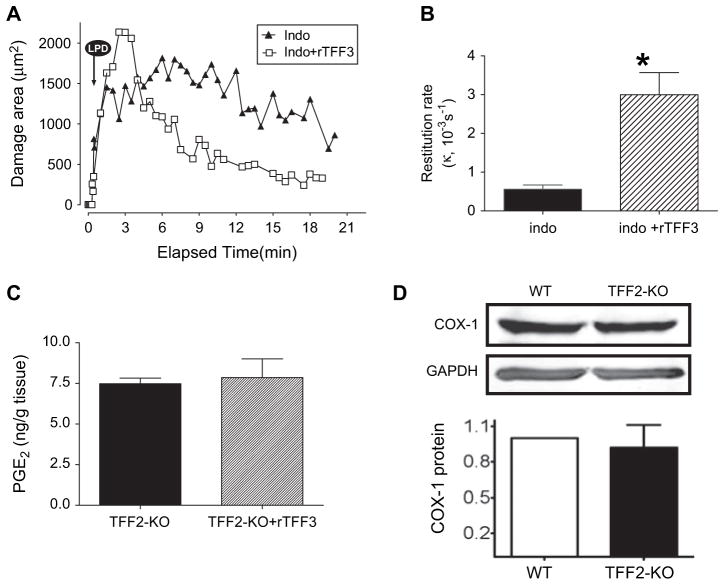
Both PGs and TFFs have been proposed to play a role in defending injured tissue, and have effects in promoting cell migration.7,35,36 In order to test if the effects of the endogenous TFF required COX activity, the effect of rTFF3 was examined when COX activity was inhibited by 1 h pretreatment with indomethacin (5 mg/kg subcutaneoulsy). Direct measurement of PGE2 content confirmed that PGE2 levels in the gastric tissue of control mice (11.3±1.4 ng/g tissue, n=5) were reduced after this pretreatment with indomethacin (to 2.7±0.6 ng/g tissue, n=5, p<0.05). In TFF2-KO mice, indomethacin caused no change in gastric restitution rates (κ=0.0006±0.0001 s−1, n=3) versus the absence of indomethacin (p>0.05 vs values compiled in figure 3). However, in the presence of indomethacin the impaired restitution of TFF2-KO mice was markedly improved when exogenous rTFF3 was added (κ=0.0037±0.00032, n=5, p<0.001 vs absence of rTFF3 in indomethacin-pretreated mice). Results are shown in a representative experiment in figure 5A (performed in the same indomethacin-treated TFF2-KO mouse before and after rTFF3), and in compiled values of figure 5B. Figure 5C shows that addition of rTFF3 to the TFF2-KO mouse (in the absence of indomethacin) did not result in any increase in PGE2 levels within the gastric tissue. Further, in the presence of indomethacin, the tissue levels of PGE2 in control mice (given above; 2.7±0.6 ng/g tissue) were not changed upon addition of exogenous rTFF3 (2.9±0.2, n=5).
Figure 5.
Role of cyclooxygenase (COX) in gastric restitution mediated by trefoil factor 2 knockout (TFF2-KO) mice. (A) Representative experiments examining the damaged area in response to laser-induced photodamage (LPD) imposed on TFF2-KO mice after 1 h indomethacin pretreatment (5 mg/kg subcutaneously), in either the absence (filled triangles) or presence (open squares) of recombinant rat TFF3 (rTFF3). (B) Values of the restitution rate compiled from experiments performed as in A (n=3, indo.; n=5, indo+rTFF3). In an unpaired t test, values in the presence of indomethacin were not different compared with rates in the absence of indomethacin in figure 3C. In the presence of indomethacin, rTFF3 significantly accelerated the restitution rate (p<0.05). (C) Prostaglandin E2 (PGE2) levels (measured by enzyme immunoassay; see the Methods section) in TFF2-KO animals in the absence or presence of rTFF3 (D) Upper panel: western blot analysis qualitatively reports COX-1 expression in wild type (+/+) and TFF2-KO mice gastric mucosa, using glyceraldehyde phosphate dehydrogenase (GAPDH) as loading control in the same blots. Lower panel: densitometry of COX-1 blots, normalised to GAPDH and then standardised to the response in wild-type (WT) animals, shows no significant difference in COX-1 protein between phenotypes (n=4).
Measurements of surface pH identified that in the presence of indomethacin, the resting surface pH of TFF2-KO mice was comparable before and after exogenous rTFF3 (3.5±0.2 n=5 and 3.4±0.1 n=4, respectively). However, while the response of TFF2-KO mice to LPD was a surface pH acidification of −0.23±0.01 (n=3 experiments in two animals), after addition of exogenous rTFF3 the TFF2-KO mice responded to a second LPD with a surface pH alkalinisation of 0.21±0.07 (ΔpH, n=4 experiments in three animals). The results suggest that rTFF3 acted via a signalling pathway that is independent of COX activity, and that rapidly regulates gastric acid/base secretions.
As the COX-1-KO mouse also exhibited impaired epithelial restitution and altered surface pH regulation,23,24 we performed experiments to examine COX-1 protein expression in the TFF2-KO gastric mucosa. Western blot results in figure 5D confirmed that results were not influenced by changes in COX-1 protein expression. Further, preliminary Western blot results with affinity-purified anti-COX-2 antisera (Cayman Chemical) also showed no increase in COX-2 protein level in the TFF2-KO gastric mucosa (data not shown).
DISCUSSION
TFFs have been extensively studied in vivo and in vitro, with most data suggesting that these small protease-resistant peptides promote epithelial repair in the gastrointestinal (GI) tract and other systems.5,7,17,37,38 In the proximal and distal GI tract, it is recognised that TFF expression is increased in long-term injury or ulcer models.10,18,19 In complementary studies, administration of exogenous trefoil peptides to acutely wounded monolayers of intestinal epithelial cell cultures increases the rate of cell migration, resulting in epithelial restitution.37,39 The present study for the first time examines the in vivo requirement of the gastric epithelium for TFF2 during the rapid repair of acute microscopic lesions. By continuously studying the entire damage-repair cycle in vivo, outcomes extend previous results.
Laser-induced cellular damage is used for some clinical applications (photodynamic therapy, cellular ablation), and is highly advantageous as an experimental model for tracking damage and repair. It should be recognised that the mechanisms of laser-induced cellular damage may, or may not, have conventional pathophysiological correlates, and this has not been evaluated in our experiments. However, the method has been exploited by our group and others in studies of cell and tissue repair in stomach, intestine, embryos and pancreatic islets.40–42 Advantages include producing a microscopic lesion in seconds, with the ability to control initial damage size and site of damage at the gastric mucosal surface.23 Combined with the ability to perform confocal time-lapse microscopy to quantify the progression of epithelial repair with high time and spatial resolution in vivo, the model provides unique advantages. In this report, we introduce use of curve fitting of the repairing damaged area as a way impartially to evaluate rates of epithelial restitution. Curve fits to a single exponential decay function provided good fits to these data (in 48 experiments, R2=0.78±0.02, range 0.62–0.96). It should be noted that the model also has disadvantages, and in this report the most striking one is the difficulty of performing analyses that report on biochemical events when the damage site only consists of 20–30 cells. Due to technical limitations, we have not been successful in identifying the site of damage for immunohistochemistry or microdissection.
In the normal mouse, TFF2 is expressed in the mucus neck cells and at the gastric surface mucus layer.9 Our results show a severely impaired restitution of the gastric surface epithelium in TFF2-deficient mouse stomach. This outcome suggests that TFF2 is, directly or indirectly, an indispensable factor in promoting prompt epithelial restitution after acute and modest gastric injury. It should be noted that in TFF2-KO mice, the stomach expresses both TFF1 and TFF3. In the TFF2-KO mouse, expression of TFF1 (at or near the gastric surface in the corpus) is unchanged compared with wild-type mice,20 but TFF3 expression is increased at the pylorus-duodenal junction.27 To test whether lack of trefoil peptide was the deficit leading to impaired repair (vs an indirect adaptive effect caused by constitutive loss of TFF2 in this mutant mouse model), we tested the effect of adding exogenous trefoil peptide to the TFF2-KO stomach. We could not obtain recombinant TFF2, and so utilised rTFF3 in these experiments. Our use of TFF3 in these rescue experiments limits interpretation since we cannot be confident that the actions of this peptide would mirror those after addition of exogenous TFF2. Fortunately, previous results have shown that TFF peptide function can be redundant among isoforms, potentially because of their high sequence homology and/or acting in an additive fashion through complementary mechanisms.16,43 Since acute addition of exogenous rTFF3 rescued epithelial restitution in the TFF2-KO mouse stomach, the results suggest (1) signalling pathways for TFFs remained intact in the TFF2-KO animal and (2) the simplest explanation for slow restitution in the TFF2-KO mouse stomach is inadequate levels of TFFs acting at the site of repair. This conclusion is consistent with observations that TFF2 is the quick responding and high concentration (~10 μM in mucus gel) TFF in the stomach.12
The products of COX activity, especially prostaglandins, are well established as protective factors in the gastric epithelium through their actions in regulating blood flow as well as increasing bicarbonate and mucin secretion.35,36 Interestingly, it was reported that activation of cell invasion by TFF1 and TFF3 in a colon epithelial cell model is mediated by COX-dependent signalling.26 Since we had previously shown that COX-1 activity is required for prompt epithelial repair of the gastric epithelium,23 we asked if COX-1 protein was induced in the TFF2-KO (it was not). We also asked if a COX inhibitor, indomethacin, blocked the rescue of TFF2-KO gastric repair caused by rTFF3. Assays of PGE2 showed that rTFF3 did not raise PGE2 levels in TFF2-KO gastric tissue, and that indomethacin effectively lowered PGE2 levels in control tissues. Since rTFF3 still restored rapid restitution rates in indomethacin-pretreated TFF2-KO mouse stomach, the results strongly suggested that the TFFs regulate gastric epithelial restitution by a pathway that does not require COX activity.
We and others have previously observed that one feature of gastric epithelial damage/repair is an increase in extracellular pH adjacent to the gastric surface (surface pH).23–25 Since extra-cellular pH is an important factor to influence cell migration (via remodelling of the focal adhesion complex to affect cell-cell and cell-substratum adhesion)30 and TFF2 is a known motogen that promotes cell migration, we asked if the TFF2-KO mouse had altered surface pH regulation. The results showed that while the TFF2-KO surface pH was the same as that of control mice in an unperturbed epithelium, there was no significant surface pH increase in the TFF2-KO stomach following damage. In the TFF2-KO mice, the surface pH response was restored by exogenous TFF peptide (rTFF3). Although COX-1 activity has been shown to be a major regulator of surface pH in mouse,23,28 addition of indomethacin did not eliminate the ability of rTFF3 to rescue surface pH increases after damage. This is consistent with the lack of involvement of COX activity in rTFF3 effects, as discussed earlier. The results suggest that this novel effect of TFFs to regulate surface pH is via a COX-independent mechanism, but further experimentation will be needed to determine whether the change in surface pH has any impact on the rate of restitution.
In summary, this study in a microscopic damage model indicates that TFF2 plays a role in restitution after acute gastric epithelial injury, and the effect of exogenous trefoil peptides to promote acute wound repair does not require COX activity. We report the novel finding that TFFs affect gastric surface pH regulation, but can only speculate that this may have an impact on the ability of the peptides to promote epithelial restitution/migration. Further experimentation will be needed to test the causal link between changes in surface pH and cell migration in models of gastric damage.
Significance of this study.
What is already known about this subject?
In cultured cells, TFF2 promotes cell migration.
In cultured cells, TFF-induced cell invasion has been proposed to act via eicosanoids.
TFF2-KO mice have increased sensitivity to indomethacin-induced gastric damage.
What are the new findings?
TFF2 accelerates gastric lesion repair in vivo.
TFF2’s ability to repair damage in vivo is independent of COX activity.
TFF2 and COX act independently to regulate gastric surface pH after damage.
How might the work impact on clinical practice in the foreseeable future?
The results suggest that the action of recombinant TFF2 to accelerate gastric repair may work synergistically with COX activity, offering a rationale for new therapeutic targets and treatment modalities of gastric damage.
Acknowledgments
We thank Andrea Matthis PhD for breeding and maintaining the TFF2 mutant mice colony, and Chet Closson for his critical confocal microscopy technical support.
Funding This study was supported by National Institutes of Health Grant DK54940 (to MHM).
Footnotes
Competing interests DKB holds equity in ‘The GI Company’ which has licensed rights to TFF3, and DKB is an inventor on patents relevant to trefoil factors. No other authors have competing interests relevant to the work in this manuscript.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Kvietys PR, Specian RD, Grisham MB, et al. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol. 1991;261:G384–91. doi: 10.1152/ajpgi.1991.261.3.G384. [DOI] [PubMed] [Google Scholar]
- 2.Lacy ER. Epithelial restitution in the gastrointestinal tract. J Clin Gastroenterol. 1988;10(Suppl 1):S72–7. doi: 10.1097/00004836-198812001-00012. [DOI] [PubMed] [Google Scholar]
- 3.Lacy ER, Ito S. Rapid epithelial restitution of the rat gastric mucosa after ethanol injury. Lab Invest. 1984;51:573–83. [PubMed] [Google Scholar]
- 4.Silen W, Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol. 1985;47:217–29. doi: 10.1146/annurev.ph.47.030185.001245. [DOI] [PubMed] [Google Scholar]
- 5.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–32. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 6.Dieckgraefe BK, Santoro SA, Alpers DH. Immunolocalization of alpha-integrin subunits and extracellular matrix components during human colonic organogenesis. Gastroenterology. 1996;110:58–71. doi: 10.1053/gast.1996.v110.pm8536889. [DOI] [PubMed] [Google Scholar]
- 7.Kjellev S. The trefoil factor family—small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66:1350–69. doi: 10.1007/s00018-008-8646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podolsky DK. Mechanisms of regulatory peptide action in the gastrointestinal tract: trefoil peptides. J Gastroenterol. 2000;35(Suppl 12):69–74. [PubMed] [Google Scholar]
- 9.Hanby AM, Poulsom R, Elia G, et al. The expression of the trefoil peptides pS2 and human spasmolytic polypeptide (hSP) in ‘gastric metaplasia’ of the proximal duodenum: implications for the nature of ‘gastric metaplasia’. J Pathol. 1993;169:355–60. doi: 10.1002/path.1711690313. [DOI] [PubMed] [Google Scholar]
- 10.Podolsky DK, Lynch-Devaney K, Stow JL, et al. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:12230. [PubMed] [Google Scholar]
- 11.Tomasetto C, Rio MC, Gautier C, et al. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990;9:407–14. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffrey GP, Oates PS, Wang TC, et al. Spasmolytic polypeptide: a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology. 1994;106:336–45. doi: 10.1016/0016-5085(94)90590-8. [DOI] [PubMed] [Google Scholar]
- 13.Madsen J, Nielsen O, Tornøe I, et al. Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem. 2007;55:505–13. doi: 10.1369/jhc.6A7100.2007. [DOI] [PubMed] [Google Scholar]
- 14.Itoh H, Tomita M, Uchino H, et al. cDNA cloning of rat pS2 peptide and expression of trefoil peptides in acetic acid-induced colitis. Biochem J. 1996;318:939–44. doi: 10.1042/bj3180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre O, Wolf C, Kédinger M, et al. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J Cell Biol. 1993;122:191–8. doi: 10.1083/jcb.122.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babyatsky MW, deBeaumont M, Thim L, et al. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489–97. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci. 2005;62:2932–8. doi: 10.1007/s00018-005-5481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanby AM, Poulsom R, Singh S, et al. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993;105:1110–16. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- 19.Wright NA, Poulsom R, Stamp GW, et al. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990;162:279–84. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- 20.Farrell JJ, Taupin D, Koh TJ, et al. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193–204. doi: 10.1172/JCI12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mashimo H, Wu DC, Podolsky DK, et al. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–5. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 22.Paulsen FP, Woon CW, Varoga D, et al. Intestinal trefoil factor/TFF3 promotes re-epithelialization of corneal wounds. J Biol Chem. 2008;283:13418–27. doi: 10.1074/jbc.M800177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starodub OT, Demitrack ES, Baumgartner HK, et al. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol. 2008;294:C223–32. doi: 10.1152/ajpcell.00395.2006. [DOI] [PubMed] [Google Scholar]
- 24.Baumgartner HK, Kirbiyik U, Coskun T, et al. Endogenous cyclooxygenase activity regulates mouse gastric surface pH. J Physiol. 2002;544:871–82. doi: 10.1113/jphysiol.2002.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu S, Tanaka S, Kaunitz JD, et al. Dynamic regulation of gastric surface pH by luminal pH. J Clin Invest. 1999;103:605–12. doi: 10.1172/JCI5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues S, Nguyen QD, Faivre S, et al. Activation of cellular invasion by trefoil peptides and src is mediated by cyclooxygenase- and thromboxane A2 receptor-dependent signaling pathways. FASEB J. 2001;15:1517–28. doi: 10.1096/fj.00-0802com. [DOI] [PubMed] [Google Scholar]
- 27.Baus-Loncar M, Schmid J, Lalani el-N, et al. Trefoil factor 2 (TFF2) deficiency in murine digestive tract influences the immune system. Cell Physiol Biochem. 2005;16:31–42. doi: 10.1159/000087729. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi K, Aihara E, Sasaki Y, et al. Involvement of cyclooxygenase-1, prostaglandin E2 and EP1 receptors in acid-induced. J Physiol Pharmacol. 2006;57:661–76. [PubMed] [Google Scholar]
- 29.Guan Y, Dong J, Tackett L, et al. NHE2 is the main apical NHE in mouse colonic crypts but an alternative Na+-dependent acid extrusion mechanism is upregulated in NHE2-null mice. Am J Physiol Gastrointest Liver Physiol. 2006;291:G689–99. doi: 10.1152/ajpgi.00342.2005. [DOI] [PubMed] [Google Scholar]
- 30.Stock C, Gassner B, Hauck CR, et al. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J Physiol. 2005;567:225–38. doi: 10.1113/jphysiol.2005.088344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace JL, Whittle BJ. Role of mucus in the repair of gastric epithelial damage in the rat. Inhibition of epithelial recovery by mucolytic agents. Gastroenterology. 1986;91:603–11. doi: 10.1016/0016-5085(86)90629-3. [DOI] [PubMed] [Google Scholar]
- 32.Baumgartner HK, Starodub OT, Joehl JS, et al. Cyclooxygenase 1 is required for pH control at the mouse gastric surface. Gut. 2004;53:1751–7. doi: 10.1136/gut.2004.040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeeda M, Yamato M, Kato S, et al. Cyclooxygenase isozymes involved in adaptive functional responses in rat stomach after barrier disruption. J Pharmacol Exp Ther. 2003;307:713–19. doi: 10.1124/jpet.103.054973. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka S, Podolsky DK, Engel E, et al. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997;272:G1473–80. doi: 10.1152/ajpgi.1997.272.6.G1473. [DOI] [PubMed] [Google Scholar]
- 35.Peskar BM. Role of cyclooxygenase isoforms in gastric mucosal defense and ulcer healing. Inflammopharmacology. 2005;13:15–26. doi: 10.1163/156856005774423809. [DOI] [PubMed] [Google Scholar]
- 36.Peskar BM, Maricic N. Role of prostaglandins in gastroprotection. Dig Dis Sci. 1998;43(9 Suppl):23S–9S. [PubMed] [Google Scholar]
- 37.Playford RJ, Marchbank T, Chinery R, et al. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995;108:108–16. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 38.Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut. 1999;44:890–5. doi: 10.1136/gut.44.6.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dignass A, Lynch-Devaney K, Kindon H, et al. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–83. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark AG, Miller AL, Vaughan E, et al. Integration of single and multicellular wound responses. Curr Biol. 2009;19:1389–95. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocheleau JV, Head WS, Piston DW. Quantitative NAD(P)H/flavoprotein autofluorescence imaging reveals metabolic mechanisms of pancreatic islet pyruvate response. J Biol Chem. 2004;279:31780–7. doi: 10.1074/jbc.M314005200. [DOI] [PubMed] [Google Scholar]
- 42.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–95. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinery R, Playford RJ. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond) 1995;88:401–3. doi: 10.1042/cs0880401. [DOI] [PubMed] [Google Scholar]