Abstract
Background
Recent evidence suggests that antecedent packed red blood cell (PRBC) transfusions increase the risk for necrotizing enterocolitis (NEC), the most common gastrointestinal emergency encountered by very low birth weight (VLBW) infants. The underlying mechanism for this association is unknown. Altered oxygenation of the mesenteric vasculature during PRBC transfusion has been hypothesized to contribute to NEC development and was investigated in this study.
Study design and methods
Oxygenation patterns among four VLBW infants who developed transfusion-related NEC (TR-NEC) were compared to four VLBW infants with similar gestational age who were transfused but did not develop NEC (non-NEC). Cerebral and mesenteric patterns were recorded before, during and 48 hours subsequent to PRBC transfusion using near-infrared spectroscopy technology (NIRS). Percentage change from mean baseline regional saturation (rSO2) values and cerebro- splanchnic oxygenation ratio (CSOR) were analyzed.
Results
All TR-NEC infants (24–29 weeks gestation; 705–1080 grams) demonstrated greater variation in mesenteric oxygenation patterns surrounding transfusions than non-NEC infants (27.6–30 weeks gestation; 980–1210 grams). TR-NEC infants received larger mean volumes of total blood (27.75 ml/kg ± 8.77) than non-NEC infants (15.25ml/kg ± 0.5).
Conclusion
Wide fluctuation and decreases in mesenteric oxygenation patterns are more pronounced in TR-NEC infants, especially prior to TR-NEC onset, as compared to non-NEC infants. Greater total volume of infused blood was associated with TR-NEC in preterm infants. Using NIRS, larger prospective studies are needed to further evaluate potential risk factors for NEC in this high risk population.
Keywords: Transfusion-related NEC, necrotizing enterocolitis, near-infrared spectroscopy
Introduction
Necrotizing enterocolitis (NEC) is a major cause of neonatal morbidity and mortality; especially in very low birth weight (VLBW) infants weighing < 1500 grams.1 The pathogenesis of NEC is unclear. A leading hypothesis proposes that the development of NEC is characterized by mesenteric ischemia which results in an inflammatory cascade and eventual bowel necrosis.2 However, other hypotheses suggest a multi-factorial pathophysiology with specific causal factors yet to be identified. Current prevention strategies for NEC are ineffective, and consequently the incidence has remained unchanged over the last several decades. 2,3
Several recent studies suggest that packed red blood cell (PRBC) transfusions are temporally related with NEC onset, which tends to occur immediately and up to 48 hours post-transfusion (TR-NEC).4–12 The underlying mechanism of this relationship is unknown. Our prospective study endeavors to quantify mesenteric tissue oxygenation patterns, using Near Infrared Spectroscopy (NIRS) technology, of very low birth weight (VLBW) premature infants receiving PRBC transfusions. Prior studies have utilized NIRS technology to observe mesenteric oxygenation changes during and for the 12-hour period following PRBC transfusion; however, no occurrences of TR-NEC were analyzed. Transient improvement was seen during and immediately after PRBC infusion; however, levels began to decline 12 hours post transfusion.13 Therefore, we evaluated cerebral and mesenteric tissue oxygenation during and for 48 hours following PRBC transfusion to further examine tissue oxygenation trends at a time when VLBW infants are most likely to develop TR-NEC. This case series compares mesenteric tissue oxygenation patterns exhibited by a subset of VLBW infants, due to the development of TR-NEC, to tissue oxygenation patterns of four VLBW infants of similar gestational age in the same study who did not develop NEC subsequent to PRBC transfusion.
Materials and Methods
Study population
Premature infants were recruited into the Emory institutional review board approved study from November 30, 2010 to December 31, 2011. Recruited infants were preterm gestational age (GA) < 37 weeks, admitted to Emory level IIIB neonatal intensive care unit (NICU), who were to receive a PRBC transfusion. Infants with congenital anomalies, intraventricular hemorrhage Grade III or greater, hemodynamically significant patent ductus arteriosis, requiring vasopressor support and current or previous NEC were excluded. All routine care was recorded from nursing flow sheets. Transfusion administration (volume and duration) and enteral feeding continuation or cessation during the transfusion event was determined by the attending physician. All infants were followed until discharge, death or transfer for the development of NEC. NEC diagnosis was based on Bell’s Staging criteria.14 TR-NEC was defined as infants diagnosed with NEC within 48 hours post- PRBC transfusion. The timing of TR-NEC onset was determined based on medical record documentation of actual disease onset. Medical TR-NEC was defined as infants who were medically managed (antimicrobial therapy, bowel rest and decompression) and did not require surgical intervention due to complications associated with NEC progression. Surgical TR-NEC was defined as infants who required surgical intervention for complications directly resulting from TR-NEC onset and/or progression of disease.
As part of a larger ongoing study examining oxygenation patterns during and subsequent to PRBC transfusion using NIRS, this case series describes four VLBW infants who developed TR-NEC and were compared to four VLBW infants who received a transfusion that did not develop this complication. The non-NEC infants were selected from other VLBW infants on study with the closest corrected GA at the time of transfusion. Only 4 of the 19 infants enrolled in the larger study met this criterion.15
Packed Red Blood Cell Data and Characteristics
All PRBC units transfused to infants were stored in a citrate-phosphate-dextrose-adenine (CPDA-1) solution. In this case series, all infants received cytomegalovirus negative, irradiated, leukoreduced, group O Rh-negative PRBC units, except one infant who received group O Rh-positive PRBCs. Irradiation storage time and age of PRBCs were recorded. Infant hemoglobin values were recorded prior to each PRBC transfusion per routine NICU care prior to transfusion.
For each infant, the number of PRBC transfusion events received before, during and after the study transfusion event were recorded, as were the volume and duration of the study PRBC transfusion. Enteral feeding events were recorded during and after transfusion events including type, volume, duration, route, frequency, tolerance and timing related to the transfusion event.
Near-Infrared Spectroscopy Monitoring
Cerebral and mesenteric regional oxygen saturation (rSO2) values were measured using an INVOS 5100C Cerebral/Somatic Oximeter (Covidien, Boulder, CO), an FDA approved NIRS device. NIRS measures total oxygen bound to hemoglobin, which at the tissue level is a result of oxygen delivered, minus oxygen consumed by the tissue and is reported as the rSO2. This measure reflects overall tissue perfusion status.16 The range of rSO2 measurements of this NIRS device is 15–95%. Data were recorded every 30 seconds in real-time prior to, during and 48 hours subsequent to receiving a PRBC transfusion. NIRS sensor probes were placed on the forehead and lower abdomen to obtain cerebral and mesenteric measurements. Upon completion of the monitoring period, data were downloaded from the NIRS device and transferred to a research computer for data analysis.
Data Analysis
SAS statistical software (SAS/STAT Software, version 9.2 Cary, NC: Institute; 2000–2008) was used to calculate mean mesenteric and cerebral rSO2 values in 30-minute intervals for all infants. Baseline means were calculated from every 30 second rSO2 readings for the first 30-minute period for each initial transfusion event. All rSO2 values for the remainder of the monitoring period were averaged in 30- minute intervals for comparison as percentage change from baseline values. We chose this empiric approach to quantify fluctuations from mesenteric baseline means for pattern comparison. Cerebro-splanchnic ratios (CSOR) were calculated from raw rSO2 cerebral and mesenteric values by dividing mesenteric rSO2 by cerebral rSO2, and then 30-minute interval means were calculated. Cut-off values for CSOR raw and mean scores were set at 0.75 to depict altered perfusion.17 Mesenteric and CSOR mean rSO2 patterns were descriptively interpreted as they related to concurrent events during transfusions and over time subsequent to each transfusion event.
Results
Infant Characteristics
Characteristics of TR-NEC (n=4) and non-NEC (n=4) infants are listed in Table 1. Due to small sample size, p values were not computed to statistically evaluate the differences between TR-NEC and non-NEC groups. During the study period, five infants received 2 PRBC transfusions in a short period of time. Two infants received divided aliquots of equal volume separated by 12 hours, and 3 received 2 full volume PRBC transfusions (15–20ml/kg) in ≤67 hours. TR-NEC infants received larger volumes of PRBCs (27.75 ml/kg ± 8.77) compared to the non-NEC infants (15.25ml/kg ± 0.5). Hemoglobin levels were collected per routine NICU policy prior to the first transfusion event for all infants. All PRBC transfusion in this case series were administered for anemia of prematurity. All infants were receiving enteral feedings prior to the transfusion event. The decision to continue or hold feedings during transfusion events was made by attending physician independent of this study.
Table 1.
Infant Demographics and Clinical Data.
| TR-NEC group | Non-NEC group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Infant Number | 1 | 2 | 3 | 4 | Mean±SD | 5 | 6 | 7 | 8 | Mean±SD |
| GA Birth (weeks) | 29 | 27 | 24 | 26 | 26.5 ± 2.1 | 29 | 30 | 27.6 | 28 | 28.6 ± 1.07 |
| Birth weight (g) | 1080 | 1000 | 705 | 803 | 897.0 ± 173 | 1210 | 1160 | 980 | 1060 | 1102.5 ± 102.6 |
| PNA (days) | 31 | 22 | 11 | 8 | 18 ± 10.6 | 31 | 24 | 8 | 14 | 19.3 ± 10.2 |
| Mean mesenteric baseline (rSO2) | 40.8 | 47 | 48.1 | 16.1 | 38 ± 14.9 | 43.4 | 42.8 | 18.9 | 15.7 | 30.2 ± 15.0 |
| # transfusions received prior to study | 0 | 1 | 7 | 0 | 0 | 0 | 1 | 1 | ||
| Volume of 1st transfusion (ml/kg) | 7.5 | 15 | 20 | 15 | 14.4 ± 5.2 | 7.5 | 15 | 15 | 16 | 13.4 +± 3.9 |
| Volume of 2nd transfusion (ml/kg) | 7.5 | 15 | 15 | 16 | 13.4 ± 3.9 | 7.5 | - | - | - | - |
| Time between transfusions (hours) | 12 | 21 | 67 | 24 | 12 | - | - | - | - | |
| Time to TR-NEC Onset (hours) | 0.5 | 11.5 | 38.5 | 0 | 12.6 ± 18 | - | - | - | - | |
TR-NEC, Transfusion-related necrotizing enterocolitis; GA, gestational age at birth; PNA, postnatal age; rSO2; regional oxygenation saturation.
TR-NEC Onset
The onset of Bell’s Stage IA or greater as diagnosed and documented in the medical record by attending physician occurred within 48 hours of the second (or split volume) transfusion for all TR-NEC group infants (see Table 1). TR-NEC infants 1 and 2 were medically managed and infants 3 and 4 required surgical intervention. Infant 1 acutely developed medical TR-NEC within 30 minutes following the second split-volume transfusion, and infant 2 developed acute medical TR-NEC within 11.5 hours subsequent to the second full-volume transfusion event. Following mechanical ventilation for respiratory distress, antimicrobial therapy, nothing by mouth status and gastric decompression, both infants recovered without further problems. Infant 3 developed gastrointestinal perforation 38.5 hours subsequent to receiving a second full volume transfusion (15ml/kg). Peritoneal drains were placed with transient improvement in clinical status over the next 14 days; however, bowel resection and ileostomy were required for bowel necrosis two weeks subsequent to this event. Infant 4 developed symptoms of TR-NEC during the second full-volume transfusion event including abdominal distention, green gastric residuals, and dilated loops of bowel on abdominal radiograph without pneumatosis. Enteral feedings were held for 12 hours. Feeding intolerance re-developed and this infant was again placed on NEC precautions four days subsequent to the second transfusion event. Eight days later, pneumatosis was evident on abdominal radiograph confirming Bell’s Stage IIB NEC. Bowel resection related to stricture development was required five weeks after this event.
Near-Infrared Spectroscopy Data
Mean mesenteric baseline values calculated for the 30 minute period for every first or single transfusion received are listed in Table 1. TR-NEC group rSO2 mesenteric baselines were 41.5 ± 19.4 compared to 32.9 ± 15.6 for the non-NEC group.
rSO2 Pattern Comparison
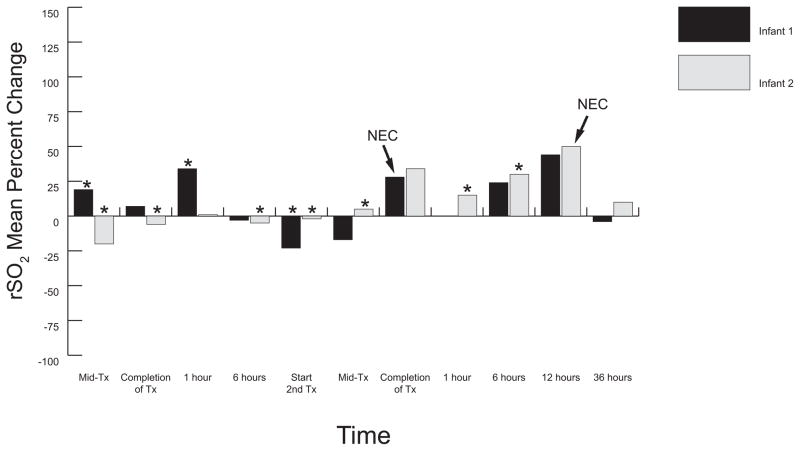
Figures 1 and 2 demonstrate rSO2 mean percent changes from baseline (increased or decreased) for TR-NEC infants; figure 3 demonstrates rSO2 patterns for all non-NEC infants. Mesenteric means for TR-NEC infants overall exhibited greater fluctuation above and below baselines than did the non-NEC infants. Medical TR-NEC infants (Figure 1) rSO2 means fell during enteral feedings following the first transfusion event, and remained below baseline prior to initiation of the second transfusion event. Increases in mean rSO2 values were not as great during the second transfusion event; however, means dramatically rose at the end of the second transfusion for both infants. This dramatic rise for infant 1 coincided with TR-NEC onset, which was clinically diagnosed 30 minutes post-transfusion. Mean rSO2 for infant 2 remained 17% above baseline and rose to 54% above baseline at the time of TR-NEC onset 12 hours following the end of the second transfusion.
Figure 1.
Mesenteric Mean Percentage Change from Baseline: Medical TR-NEC Infants. This graph illustrates the wide mesenteric oxygenation fluctuations above and below baseline measurements during and subsequent to each transfusion event and further reveals decreased oxygenation immediately prior to TR-NEC onset and subsequent increased patterns at the time of TR-NEC onset. Infant 1 received two half-volume PRBC transfusions (7.5ml/kg each) separated by 12 hours. Infant 2 received two full volume (15ml/kg) PRBC transfusions separated by 21 hours. Infant 1 had NIRS monitor removed during resuscitation and transfer to NICU (time point 1 hour after 2nd transfusion). Tx, transfusion; TR-NEC, transfusion-related necrotizing enterocolitis; Mid-Tx, time at which 50% of total volume had infused; * enteral feeding given during specified time frame; 0 = baseline; NEC, onset of TR-NEC.
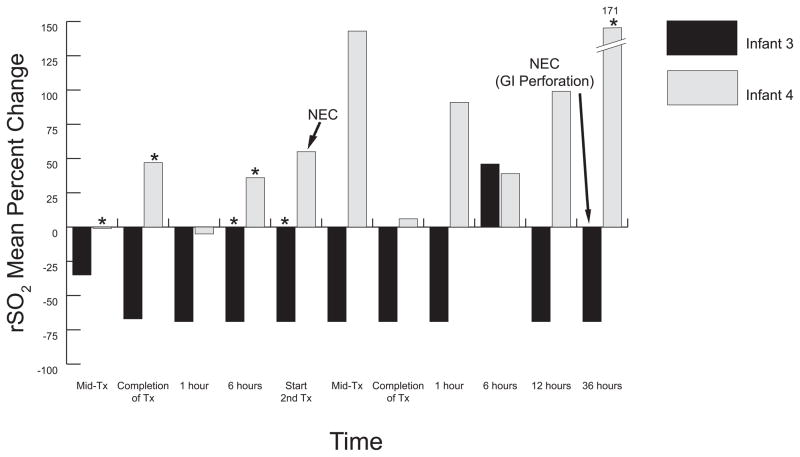
Figure 2.
Surgical TR-NEC Infants Percent Change from Baseline Means. This graph illustrates mesenteric oxygenation patterns of infants that developed surgical TR-NEC. Both infants received two full volume PRBC transfusions; infant 3 transfusions separated by 67 hours, and infant 4 separated by 24 hours. Infant 3 demonstrated an immediate large and persistent decline in oxygenation (−69%) immediately following the initiation of the 1st full volume transfusion (20ml/kg) which persisted until gastrointestinal perforation developed 38.5 hours after the conclusion of the second full volume (20ml/kg) PRBC transfusion. Wide fluctuations in mesenteric oxygenation were observed in infant 4 prior to and following the development of Bell’s Stage IA TR-NEC symptoms at the beginning of the 2nd full volume transfusion (15ml/kg). Enteral feedings were held for 18 hours and then resumed. Tx, transfusion; TR-NEC, transfusion-related necrotizing enterocolitis; Mid-Tx, time at which 50% of total volume had infused; *enteral feeding given during specified time frame; 0 = baseline.
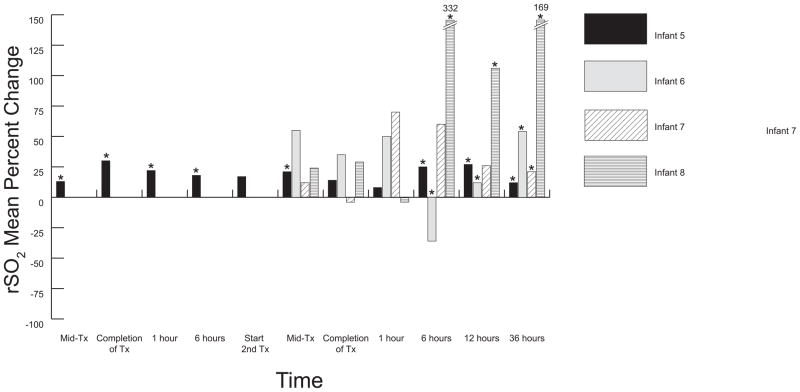
Figure 3.
Mesenteric percent change from baseline mean for Non-NEC Infants. Infant 5 received two half-volume PRBC transfusions (7.5ml. kg each) separated by 12 hours; all other infants received one full volume PRBC transfusion. Overall increased oxygenation in mesenteric oxygenation was prevalent among the non-NEC infants, although wide mean fluctuation was apparent in infant 6, 7 and 8 (closely resembling surgical TR-NEC infant 4). Six hours post-transfusion, infant 6 demonstrated a dramatic decline in oxygenation that coincided with severe bradycardic and apneic episodes, which improved following elective intubation. Tx, transfusion; Mid-Tx, time at which 50% of total volume had infused; *enteral feeding given during specified time frame; 0 = baseline.
Changes in rSO2 patterns for TR-NEC infants are shown in Figure 2. Infant 3 means fell to 69% below baseline immediately following initiation of the first full volume transfusion (15ml/kg) and persisted at this low level between transfusions and throughout the second full volume (15ml/kg) PRBC transfusion until gastrointestinal perforation developed 38.5 hours later. Following peritoneal drain placement, rSO2 means rose (data not shown) to 40% above baseline levels. Infant 4 demonstrated increased oxygenation change from baseline, yet highly variable with a large range in oxygenation (−7% to 178%) throughout the study. TR-NEC development (Bell’s stage IA) occurred during the second transfusion with slow progression until Stage IIB NEC was confirmed four days later. Surgical stricture repair and ileal resection was required five weeks following our study. Prior to TR-NEC onset and during periods of rSO2 fluctuations, all 4 infants remained normotensive with SpO2 readings > 92%.
Mean rSO2 patterns for the non-NEC group are shown in Figure 3. Overall, there was much less variability above and below baseline measurements in these patterns as compared to the TR-NEC infants. In general, rSO2 means remained higher than baseline values for non-NEC infants following each transfusion event. One large drop from baseline was seen 6 hours post-transfusion in infant 6 which coincided with enteral feeding intolerance, but no symptoms of NEC.
Cerebro-splanchnic Oxygenation Ratio (CSOR) Pattern Comparison
CSOR values (data not shown) among the TR-NEC group revealed greater fluctuation than non-NEC infants. However, infants from both groups demonstrated CSOR values below and above our assigned cutoff value of 0.75. During medical TR-NEC onset, CSOR values for were > 0.75 and ranged from 0.25 to 1.09 for surgical TR-NEC infants.
CSORs for non-NEC infants were less variable over time ranging from 0.25 to 1.15. During episodes of profound apnea, bradycardia and desaturation, raw CSOR values for non-NEC infant 7 rose (from 0.36 to 1.00) and mesenteric rSO2 signal drop out was frequently recorded with associated sharply decreased cerebral values (data not shown). This elevation in CSOR means was related to sharp decreases in both cerebral and mesenteric values. Once this infant was placed on mechanical ventilation, cerebral values returned to baseline, but mesenteric means remained low generating overall low CSOR values (0.19–0.24).
Discussion
This study demonstrated mesenteric tissue oxygenation pattern changes using NIRS technology during TR-NEC onset in VLBW infants. Although previous studies have used NIRS to prospectively examine tissue oxygenation patterns during and following PRBC transfusions, the occurrence of TR-NEC was not observed.13,18 Further, these previous studies did not observe or include the combined effect of enteral feedings and transfusions on mesenteric oxygenation pattern changes.
NIRS technology simultaneously measures real-time regional tissue oxygenation producing rSO2 values which reflect differential organ oxygenation. The actual rSO2 reading measures changes in tissue concentration of oxyhemoglobin and deoxyhemoglobin, or the balance of oxygen that is delivered minus the amount extracted at the tissue level.16 There are several reasons for decreased rSO2 values: increased consumption of oxygen at the tissue level, diminished or absent blood flow, or altered and/or decreased oxygen carrying capacity.16 A combination of any or all of these factors may also be present. Therefore, it is vital that infant factors be closely monitored for reasons contributing to low rSO2 measurements.
The results of this study demonstrate unique tissue oxygenation pattern variations between infants experiencing TR-NEC to those who did not. Oxygenation changes in TR-NEC infants showed greater variability between time points than non-NEC counterparts. Gastrointestinal immaturity may have played a substantial role in this pattern variation. 3 In the presence of impaired immune response and ineffective circulatory regulation, VLBW infants are vulnerable to the effects of impaired mesenteric blood flow.19,20 Studies have shown that sustained decreased blood flow in the superior mesenteric artery followed by reperfusion may disrupt mesenteric circulatory regulation mechanisms and increase susceptibility to intestinal barrier injury.21 The decreased rSO2 means with subsequent increases at the time of TR-NEC onset in the medical TR-NEC infants may have been related to perfusion-reperfusion injury.21,22 It is also possible that the increase in rSO2 following TR-NEC onset in these infants was the result of volume resuscitation.23 These findings are consistent with previous studies in which rSO2 pattern variability was associated with low to high rSO2 readings preceding NEC development.24 Moreover, these abrupt changes in rSO2 values were not reflected in routine physiologic monitoring, as SpO2 values in all TR-NEC infants remained > 92% until actual disease onset.
The sharp decline in mesenteric oxygenation in TR-NEC infant 3 who developed pneumoperitoneum may have resulted from distortion of infrared light path length, ischemic bowel or absence of mesenteric perfusion. The highly fluctuant patterns exhibited in infant 4 may have been related to perfusion-reperfusion injury.21,25,26 the consequence of enteral feedings immediately post-transfusion, 27 or combined effect. However, non-NEC infant 8 also demonstrated wide swings in the immediate post-transfusion period, but subsequently stabilized with increased oxygenation levels. Larger studies are needed for further evaluation of tissue oxygenation pattern changes relative to PRBC transfusion and NEC development and should include the effects of enteral feeding continuation during and post-transfusion.
Tissue oxygenation patterns of the non-NEC infants in this case series demonstrate overall improvement in tissue oxygenation during and following PRBC transfusions. Although point-to-point mesenteric variability from baseline measurements was fairly wide, 30-minute rSO2 means were fairly stable throughout our study period.
Studies have also evaluated rSO2 values in CSOR format which is calculated as mesenteric rSO2/cerebral rSO2.17,18,28 Fortune et al found NEC occurred in preterm infants when CSOR values were < 0.75.17 Furthermore, Bailey et al reported recently that infants with pre-transfusion CSOR values < 0.73 are more likely to demonstrate clinical improvement following a PRBC transfusion than those infants with beginning CSOR values > 0.73.18 Our study findings related to CSOR values were not in agreement with these previous studies. We support the concept that CSOR values are beneficial when cerebral autoregulation is intact as the change in value directly reflects mesenteric changes. However, we postulate that if cerebral autoregulation is lost or impaired which is common in critically ill VLBW infants, 29,30 an improvement in the CSOR value may be reflective of decreased cerebral tissue oxygenation with little or no change in mesenteric values. We posit that it is necessary to evaluate absolute cerebral and mesenteric rSO2 values to ensure an improved CSOR value reflects mesenteric, not cerebral changes. For these reasons, we chose to evaluate absolute mesenteric measurements as a percentage of increased or decreased fluctuations from baseline mean to describe the effects of related PRBC transfusion. We then analyzed CSOR means within the context of absolute rSO2 pattern changes. Our study demonstrates that CSOR values < 0.75 are not always associated with ischemic bowel and NEC development. We further illustrate that infants who develop TR-NEC may exhibit CSOR values > 0.75. To increase the generalizability of our study findings, we did not exclude infants with confirmed or suspected sepsis as did the Bailey study.18
Previous retrospective studies suggest that PRBC transfusions are an independent risk factor for TR-NEC,12 with a 25–35% incidence in VLBW preterm infants.4,7–9,12,31 Furthermore, TR-NEC occurs immediately and up to 48 hours post PRBC transfusion in VLBW infants.4–6,8,11,12, Compared to previous studies, 21% (4/19) of the VLBW infants in our larger study developed Bell’s Stage IA NEC or greater following a PRBC transfusion, with all cases occurring in < 48 hours subsequent to a transfusion event.15
There were several limitations to this study, the first major limitation being a small sample size. We recognize our comparison group differed maturationally as compared to the TR-NEC group, and these differences may have influenced the risk factor for disease development, feeding intolerance and mesenteric tissue oxygenation changes. Additionally, pre-transfusion baseline means for all infants in this case series were not obtained which limited direct comparison of post-transfusion oxygenation changes. NIRS technology can be associated with probe displacement which interferes with continuous trend monitoring, and ability to calculate CSOR values if cerebral and/or mesenteric values are missing. Continuous mesenteric monitoring is challenging, given the large surface area of the intestine, peristalsis, and increased infrared path length in the presence of pneumoperitoneum, abdominal distention or increased fluid/gas surfaces. These limitations may lead to “signal drop out” which was observed on our infant with pneumoperitoneum. However, it is more likely that persistent low rSO2 readings coupled with frequent signal drop is associated with a substantial decrease in tissue oxygenation.24 Finally, this study was limited by the inability to quantify decreased percentage changes from baseline values when beginning measurements are extremely low. Because the lowest measurement capable of NIRS technology is 15%, infants with baseline values at or near this level exhibited a “floor” effect. Therefore, in these cases, it is crucial to evaluate if oxygenation improves or remains persistently low signifying potential perfusion impairment.
In conclusion, this prospective observational case series demonstrates actual changes in mesenteric tissue oxygenation patterns in infants who developed TR-NEC. Further distinct differences in these patterns were demonstrated in the TR-NEC infants as compared to similar infants that did not develop NEC. The major differences between our groups were greater fluctuations above and below beginning baseline values demonstrated by our TR-NEC infants than infants who did not develop NEC. We demonstrate that analyzing percent changes from baseline quantifies the magnitude of baseline changes, and may be more beneficial when used in conjunction with CSOR values, rather than CSOR values alone.
We recognize that establishing rSO2 patterns during enteral feedings prior to the transfusion event would strengthen our understanding of pattern changes during and subsequent to transfusions, illustrating the need for further research. This study demonstrates that severe and sudden decreases in mesenteric tissue oxygenation patterns may increase the risk for TR-NEC onset, especially if low readings persist. Enteral feedings may have a compounded impact on mesenteric oxygenation and perfusion during and following PRBC transfusions; however, further analysis of this possibility are needed. NIRS is a useful diagnostic tool to directly observe tissue bed oxygenation in real-time without interrupting routine bedside care and may elucidate compromised mesenteric perfusion before changes in routine physiologic monitoring are evident, primarily SpO2 measurements. Future studies utilizing this technology to analyze mesenteric oxygenation pattern changes relating to risk factors for TR-NEC development seems promising and feasible with the capability to improve prediction and prevention strategies especially when modifiable risk factors are present.
Acknowledgments
Sources of support: The manuscript is supported in part by National Institute of Health Pediatric Transfusion Medicine Academic Career Award HL086773-01; Florida Association of Neonatal Nurse Practitioners; Sigma Theta Tau International Honor Society of Nursing
Footnotes
Reprints will not be available from the author
Conflict of Interest Statement: The authors declare that they have no conflicts of interest relevant to the manuscript submitted to Transfusion.
References
- 1.Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 2.Schnabl KL, Van Aerde JE, Thomson AB, Clandinin MT. Necrotizing enterocolitis: a multifactorial disease with no cure. World J Gastroenterol. 2008;14:2142–2161. doi: 10.3748/wjg.14.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Josephson CD, Wesolowski A, Bao G, et al. Do red cell transfusions increase the risk of necrotizing enterocolitis in premature infants? J Pediatr. 2010;156:972–978. doi: 10.1016/j.jpeds.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh R, Visintainer PF, Frantz ID, 3rd, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 2011;31:176–182. doi: 10.1038/jp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Dib M, Narang S, Lee E, Massaro AN, Aly H. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol. 2011;31:183–187. doi: 10.1038/jp.2010.157. [DOI] [PubMed] [Google Scholar]
- 7.Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127:635–641. doi: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 8.Mally P, Golombek SG, Mishra R, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol. 2006;23:451–458. doi: 10.1055/s-2006-951300. [DOI] [PubMed] [Google Scholar]
- 9.Christensen RD, Lambert DK, Henry E, et al. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion. 2010 doi: 10.1111/j.1537-2995.2009.02542.x. [DOI] [PubMed] [Google Scholar]
- 10.McGrady GA, Rettig PJ, Istre GR, Jason JM, Holman RC, Evatt BL. An outbreak of necrotizing enterocolitis. Association with transfusions of packed red blood cells. Am J Epidemiol. 1987;126:1165–1172. doi: 10.1093/oxfordjournals.aje.a114754. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129:529–540. doi: 10.1542/peds.2011-2872. [DOI] [PubMed] [Google Scholar]
- 12.Stritzke AI, Smyth J, Synnes A, Lee SK, Shah PS. Transfusion-associated necrotising enterocolitis in neonates. Arch Dis Child Fetal Neonatal Ed. 2012 doi: 10.1136/fetalneonatal-2011-301282. [DOI] [PubMed] [Google Scholar]
- 13.Bailey SM, Hendricks-Munoz KD, Wells JT, Mally P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am J Perinatol. 2010;27:455–453. doi: 10.1055/s-0030-1247598. [DOI] [PubMed] [Google Scholar]
- 14.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin T. Mesenteric perfusion pattern changes as the result of packed red blood cell transfusions in preterm infants. Doctoral Dissertation. 2012 Retrieved from Emory Electronic Theses and Dissertations Repository. http://holden.library.emory.edu/ark:/25593/bpbht.
- 16.Marin T, Moore J. Understanding near-infrared spectroscopy. Adv Neonatal Care. 2011;11:382–388. doi: 10.1097/ANC.0b013e3182337ebb. [DOI] [PubMed] [Google Scholar]
- 17.Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. 2001;27:1401–1407. doi: 10.1007/s001340100994. [DOI] [PubMed] [Google Scholar]
- 18.Bailey SM, Hendricks-Muñoz KD, Mally P. Splanchnic-cerebral oxygenation ratio as a marker of preterm infant blood transfusion needs. Transfusion. 2012;52:252–260. doi: 10.1111/j.1537-2995.2011.03263.x. [DOI] [PubMed] [Google Scholar]
- 19.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32:83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiology and pathophysiology. Clin Perinatol. 2002;29:23–39. doi: 10.1016/s0095-5108(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 21.Nowicki PT. Effects of sustained flow reduction on postnatal intestinal circulation. Am J Physiol Gastrointest Liver Physiol. 1998;275:G758–G768. doi: 10.1152/ajpgi.1998.275.4.G758. [DOI] [PubMed] [Google Scholar]
- 22.Nowicki PT. Ischemia and necrotizing enterocolitis: Where, when, and how. Semin Pediatr Surg. 2005;14:152–158. doi: 10.1053/j.sempedsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Cohn SM, Varela JE, Giannotti GD, et al. Splanchnic perfusion evaluation during hemorrhage and resuscitation with gastric near-infrared spectroscopy. J Trauma. 2001;50:629–634. doi: 10.1097/00005373-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J Matern Fetal Neonatal Med. 2011;24:574–582. doi: 10.3109/14767058.2010.511335. [DOI] [PubMed] [Google Scholar]
- 25.Young CM, Kingma SDK, Neu J. Ischemia-reperfusion and neonatal intestinal injury. J Pediatr. 2011;158:e25–e28. doi: 10.1016/j.jpeds.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Papparella A, Deluca FG, Oyer CE, Pinar H, Stonestreet BS. Ischemia-reperfusion injury in the intestines of newborn pigs. Pediatr Res. 1997;42:180–188. doi: 10.1203/00006450-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Krimmel GA, Baker R, Yanowitz TD. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. Am J Perinatol. 2009;26:99–105. doi: 10.1055/s-0028-1090595. [DOI] [PubMed] [Google Scholar]
- 28.Dave V, Brion LP, Campbell DE, Scheiner M, Raab C, Nafday SM. Splanchnic tissue oxygenation, but not brain tissue oxygenation, increases after feeds in stable preterm neonates tolerating full bolus orogastric feeding. J Perinatol. 2009;29:213–218. doi: 10.1038/jp.2008.189. [DOI] [PubMed] [Google Scholar]
- 29.Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–73. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 30.Limperopoulos C, Gauvreau KK, O’Leary H, et al. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics. 2008;122:e1006–1013. doi: 10.1542/peds.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr. 2011;158:403–409. doi: 10.1016/j.jpeds.2010.09.015. [DOI] [PubMed] [Google Scholar]