Abstract
Tumor progression is facilitated by regulatory T cells (Tregs) and restricted by effector T cells. In this study, we document parallel regulation of CD8+ T cells and Foxp3+ Tregs by programmed death-1 (PD-1, PDCD1). In addition, we identify an additional role of cytotoxic T lymphocyte antigen-4 (CTLA-4) inhibitory receptor in further promoting dysfunction of CD8+ T effector cells in tumor models (CT26 colon carcinoma and ID8-VEGF ovarian carcinoma). Two thirds of CD8+ tumor-infiltrating lymphocytes (TIL) expressed PD-1, while one third to half of CD8+ TIL co-expressed PD-1 and CTLA-4. Double-positive (PD-1+CTLA-4+) CD8+ TIL had characteristics of more severe dysfunction than single-positive (PD-1+ or CTLA-4+) TIL, including an inability to proliferate and secrete effector cytokines. Blockade of both PD-1 and CTLA-4 resulted in reversal of CD8+ TIL dysfunction and led to tumor rejection in two-thirds of mice. Double blockade was associated with increased proliferation of antigen-specific effector CD8+ and CD4+ T cells, antigen-specific cytokine release, inhibition of suppressive functions of Tregs, and upregulation of key signaling molecules critical for T cell function. When used in combination with GVAX vaccination (consisting of GM-CSF-expressing irradiated tumor cells), inhibitory pathway blockade induced rejection of CT26 tumors in 100% of mice and ID8-VEGF tumors in 75% of mice. Our study indicates that PD-1 signaling in tumors is required for both suppressing effector T cells and maintaining tumor Tregs, and that PD-1/PD-L1 pathway (CD274) blockade augments tumor inhibition by increasing effector T cell activity, while attenuating Treg cell suppression.
Introduction
An effective immune response that leads to meaningful anti-tumor effects requires not only an increase in immune activation but also reduction of suppressive or inhibitory elements of the immune system (1–3). Mechanisms regulating immune activation in cancer progression have been extensively investigated (4–16). There are multiple inhibitory mechanisms that suppress immune responses, including inhibitory receptors, secreted soluble inhibitors (IL-10 and TGF-β), and regulatory T cells (Tregs) (3, 16–17, 19–22). However, how these factors interact with each other in the tumor environment needs further study.
It is well established that tumors employ PD-1 and CTLA-4 inhibitory pathways to silence the immune system. Both these pathways are critical for physiological homeostasis. While PD-1 is broadly expressed on activated T cells and other hematopoietic cells (23–25), CTLA-4 is expressed on activated T cells including regulatory T cells (26). PD-1 binds two ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC). Upregulation of PD-L1 occurs on a wide variety of human tumors suggesting that cancer cells coopt the PD-1/PD-L1 inhibitory pathway to evade the host immune response (4–16). PD-L1 can also interact with B7.1, resulting in inhibition of T cell activation (24–25). Despite these similarities as terminators of T-cell activation, the difference in regulatory roles of the PD-1 and CTLA-4 pathways has been recognized (27–29). CD4+CD25+Foxp3+ T cells (Tregs) play critical roles in the control of anti-tumor immune responses (21–22, 30–38). Indeed, recent data have shown that blocking of PD-1 and CTLA-4 can modulate Treg functions and enhance antitumor responses (2, 35). However, the direct role and function of PD-1 in Tregs in the cancer environment remains unknown.
Immunoregulatory pathways involving PD-1, CTLA-4, and their ligands are highly complex. Co-expression of more than one receptor by CD8+ T cells has been correlated with a more severe exhausted phenotype (39–42). Antibody blockade of either PD-1 or CTLA-4 can enhance effector T-cell responses and induce T cell-mediated tumor rejection in mouse models (2, 4–15). However, the intricacies of each pathway and cross-talk between them make it unlikely that blockade of PD-1 or CTLA-4 would have identical effects. Hence, to optimally design the next generation of immunotherapeutic strategies, careful delineation of the individual contributions of PD-L1/PD-1, PD-L2/PD-1, CTLA-4/B7-1, and PD-L1/B7-1 interactions in vivo will be crucial.
In this study, we provide evidence that reversal of T cell dysfunction can be achieved by simultaneously targeting effector T cells and Tregs. First, we show that CTLA-4 is preferentially expressed by PD-1+ CD8+ T cells, and co-expression of both PD-1 and CTLA-4 is associated with marked dysfunction of antigen-specific T cells. Second, blockade of PD-1 and CTLA-4 pathways reversed T cell dysfunction. Blockade therapy with GVAX vaccination further enhanced tumor rejection in mice. Third, adoptive transfer of CD8+CTLA-4+PD-1+ TILs that had been pre-treated in vitro with αPD-1 and αCTLA-4 antibodies eliminated tumors in vivo. Fourth, blockade of PD-1/PD-L1 pathway in Tregs attenuates their suppressive function. Taken together, our study describes an approach involving vaccination and inhibitory receptor blockade to reverse T cell exhaustion, which we envision to be a promising anticancer immunotherapy.
Methods
Mice and cell lines
Six to 8 week-old female BALB/c or C57BL/6 mice (Jackson Laboratory) were used. CT26 colon carcinoma cell line was purchased from American Type Culture Collection (43). ID8-VEGF ovarian carcinoma cell line was developed previously from a mouse ovarian epithelial papillary serous adenocarcinoma cell line (ID8) obtained from Dr. Paul F. Terranova, University of Kansas, and used as described (44–46).
pMG-Lyt2 vector expressing murine GM-CSF was kindly provided by Dr. J.P. Allison, M.D. Anderson Cancer Center, Houston, TX (2). Development of CT26 or ID8-VEGF cells expressing murine GM-CSF (CT26/GVAX or ID8-VEGF/GVAX) was based on the methods described previously (2, 47). CT26/GVAX and ID8-VEGF/GVAX cells showed secretion of murine GM-CSF 365 ng/106 cells/48 h (95% confidence interval, 231–499) and 397 ng/106 cells/48 h (95% confidence interval, 275–519), respectively, by ELISA (R&D Systems, Minneapolis).
Tumor experiments and in vivo blockade
BALB/c or C57BL/6 mice were implanted subcutaneously (s.c.) on the right flank with either 5×105 CT26 or 5×106 ID8-VEGF tumor cells, respectively. Two hundred μg of rat α-mouse PD-1 (29F.1A12), PD-L1 (10F.9G2), PD-L2 (3.2) (24), or 100 μg of α-CTLA-4 (clone 9D9) were administered intraperitoneally (i.p.), either 3, 6, and 9 days following CT26 inoculation, or 10, 13, and 16 days following ID8-VEGF inoculation. In vaccination experiments, 106 irradiated (150Gy) CT26-GVAX or ID8-VEGF-GVAX were given on the contralateral flank intradermally (i.d.) once either day 3, or day 10 after CT26 or ID8-VEGF inoculations, respectively. CD8+ T cells and CD4+CD25+ T cells from tumor leucocytes were purified either on a MACS column or FACSAria.
Adoptive transfer experiments
CD8+CTLA-4+PD-1+ TILs from CT26 tumor (25 gms pooled from 80 mice) were sorted to more than 95% purity by FACS. Sorted TILs were treated in vitro with 10 μg/ml of α-PD-1 and α-CTLA-4 blocking antibodies and cultured in the presence of AH-1 peptide-loaded APCs for 7 days. The cells expanded an average of 10-fold in vitro in the week of culture. Following expansion, 5×106 purified CD8+ T cells per mouse were injected intratumorally (i.t) 5 times on alternate days starting from day 10 following s.c. CT26 tumor inoculation.
in vitro proliferation assay
PD-1+CTLA-4+ and PD1+CTLA-4− CD8+ TIL were sorted to more than 95% purity by FACS and co-cultured with splenocytes from naive CD45.1+ C57BL/6 mice in the presence of AH-1 (CT-26) or FR-α (ID8-VEGF) peptides for 3 days (48–49). Thymidine incorporation was measured after pulsing with 0.5 μCi/well [3H]-thymidine during the last 8 h.
in vitro Treg suppression and cytokine analysis
CFSE-labeled CD8+ T cells (5 × 104) were stimulated with 1.5 × 105 irradiated splenic CD45.1− APCs in the presence of αCD3. CD4+CD25+ Tregs (105) from CT26 TILs, along with αPD-L1, αCTLA-4, or control rat IgG were added to the culture as indicated. Four days later, in vitro Treg suppression was determined based on CFSE dilution by flowcytometry.
The whole tumor TILs were isolated and adjusted to 2 × 106/ml in 24-well plates and cultured with AH-1 peptide ± 10 μg/ml αPD-1, αPD-L1, αCTLA-4, or control IgG. After 3 days, supernatants were analyzed for secretion of TGF-β, IL-10 and IFN-γ by ELISA and cytokine bead array.
Results
PD-1, CTLA-4, and their ligands are highly expressed in tumor models
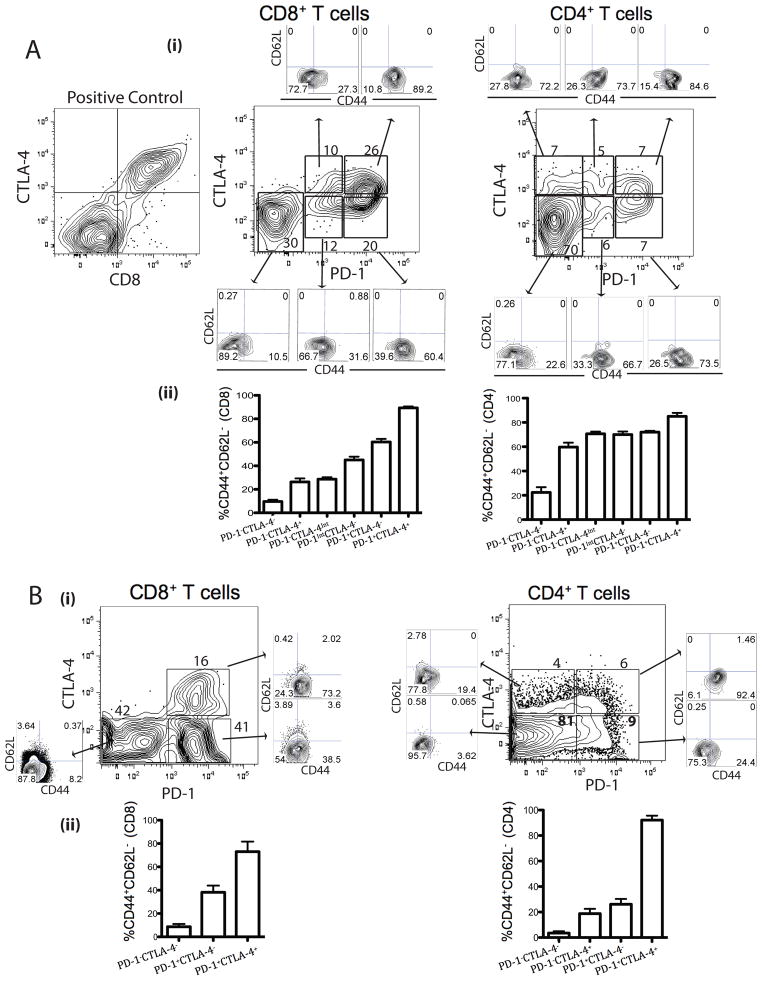
To examine the interplay between the PD-1 and CTLA-4 in cancer, we used the murine CT26 colon carcinoma and ID8-VEGF ovarian carcinoma models. In the CD8+ TIL population, two thirds expressed PD-1, and the majority of these (over two thirds) were PD-1hi (Fig. 1A and B). We also found expression of CTLA-4 on TIL, however CTLA-4 was expressed primarily in a subset of PD-1+CD8+ TIL, while PD-1−CD8+ cells were mostly CTLA-4−. About half of PD-1+CD8+ CT26 TIL and one third of PD-1+CD8+ ID8-VEGF TIL were CTLA-4+, while the remainder of PD-1+CD8+ TIL were CTLA-4−. CTLA-4 was expressed on PD-1hi as well as PD-1int cells in CT26 TILs, whereas CTLA-4 was expressed on PD-1+ cells in ID8-VEGF TILs. Taken together, among CD8+ TIL, one third co-expressed PD-1 and CTLA-4, one third expressed PD-1 only, and the remainder were negative for either receptor (Fig. 1A and B). Among the CD4+ TIL, only a third of the population expressed either of the receptors and approximately 10% were double-positive (Fig. 1A and B), while a quarter of splenic CD4+ T cells expressed CTLA-4, yet there were very few PD-1+ cells (Supplementary Fig. 1). We also found that CT26 and ID8-VEGF tumors upregulate PD-1 and CTLA-4 ligands on tumor and myeloid cells (Supplementary Fig. 2). Thus, CT26 and ID8-VEGF models are suitable for studying PD-1 and CTLA-4.
Figure 1. Phenotypic analysis of CT26 and ID8-VEGF TIL.
TIL isolated from CT26 and ID8-VEGF tumors were stained with antibodies to CD45, CD8, CD4, PD-1, CTLA-4, CD44, and CD62L as well as live/dead stain. Representative CT26 (A) and ID8-VEGF (B) tumor samples showing expression of PD-1 and CTLA-4 on CD8+CD45+ and CD4+CD45+ TIL with distinct cell populations, which are further analyzed for CD44 and CD62L. Summary data of CTLA-4 and PD-1 expression on TEM (CD44hiCD62Llo) CD8+ and CD4+ TIL. Data represent mean ± SD of n=6 mice per group and are representative of 4 independent analyses.
To begin defining the functional status of TIL, we examined their memory phenotype. Among CD8+ T cells, effector memory (TEM) cells were most prevalent in the double positive (PD-1hiCTLA-4+) population (approximately 90% of CT26 and 75% of ID8-VEGF cells), but also accounted for approximately 60% (CT26) and 40% (ID8-VEGF) of the PD-1hiCTLA-4− population and half of the rare PD-1−CTLA-4Int population (CT26). One third of the PD-1Int CT26 CD8+ T cells were TEM independently of their CTLA-4 status. Both single- and double-positive CT26 CD4+ T cells had a TEM phenotype, whereas only PD-1+CTLA-4+ ID8-VEGF CD4+ T cells had a TEM phenotype (Fig. 1A and C). Thus, a significant fraction of TILs upregulated PD-1, and many of these cells were TEM. A proportion of PD-1hi TILs also upregulated CTLA-4, and these double-positive cells were mostly TEM.
Co-expression of PD-1 and CTLA-4 correlates with more severe dysfunction of tumor-specific CD8+ T cells
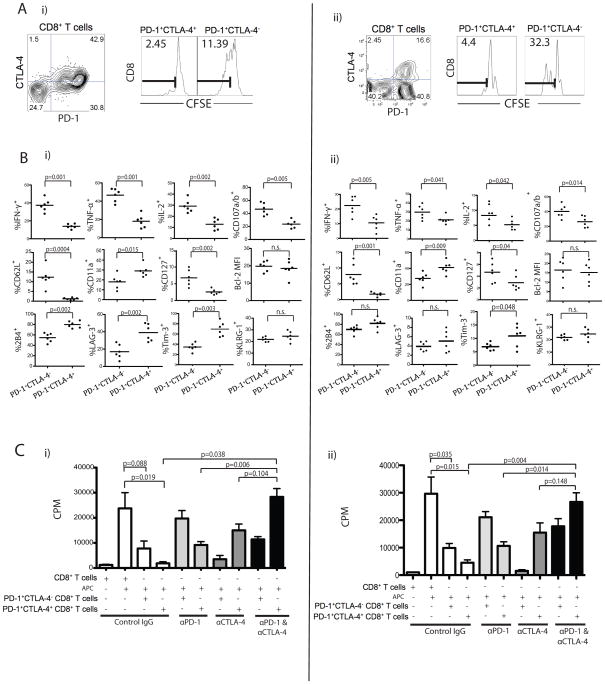
Given the strong association of PD-1 expression and TEM phenotype, we hypothesized that most tumor-reactive TIL are present in the PD-1+ population. To address this, we used AH-1 (gp70423–431) and H2-Kb folate receptor (FR161–169), CD8+ T cell specific epitopes expressed by CT26 and ID8-VEGF tumor cells, respectively (45–46). We hypothesized that the PD-1+CTLA-4+ cells exhibit greater dysfunction in response to cognate antigens than the single-positive (PD-1+) counterparts. To address this, we sorted PD-1+CTLA-4+ and PD-1+CTLA-4− CD8+ TILs, labeled them with CFSE, and co-cultured for 3 days with peptide-pulsed APCs. CFSE dilution was observed only in the PD-1+CTLA-4− population but not in the PD-1+CTLA-4+ population (11.39% vs. 2.45% and 32.3% vs. 4.4% CT26 or ID8-VEGF CFSElo cells, respectively), indicating a profound suppressed state of the double-positive cells (Fig. 2A).
Figure 2. Co-expression of PD-1 and CTLA-4 correlates with more severe dysfunction of CT26 and ID8-VEGF antigen-specific CD8+ T cells.
A) PD-1+CTLA-4+ and PD-1+CTLA-4− CD8+ TIL populations from either CT26 (i) or ID8-VEGF (ii) tumors were sorted, labeled with CFSE, and stimulated with AH-1 or FR peptide, respectively for 3 days in the presence of irradiated splenic CD45.1− APCs. CD8+ T cell proliferation was determined by dilution of CFSE; numbers indicate the percentage of CFSElo (AH-1 or FR reactive cells). B) Frequency of CT26 (i) or ID8-VEGF (ii)-specific PD-1+CTLA-4− and PD1+CTLA-4+ CD8+ T cells showing cytokine and degranulation after peptide stimulation (n=6). PD-1+CTLA-4− and PD1+CTLA-4+ CD8+ TILs cells were further analyzed for differential phenotype and expression of other inhibitory receptors. C) PD-1+CTLA-4+ or PD-1+CTLA-4− CD8+ TILs from CT26 (i) or ID8-VEGF (ii) were stimulated with AH-1 or FR peptide, respectively and cultured with blocking antibodies for 3 days. Proliferation was determined by 3[H]-thymidine incorporation. Statistical significance was determined by Student’s t-test.
Next, we directly compared the functional properties of sorted PD-1+CTLA-4− or PD1+CTLA-4+ CD8 TILs by measuring cytokine secretion in response to peptide stimulation. The frequency of IFN-γ+, TNF-α+, IL-2+ or CD107a/b+ cells was approximately 2-fold higher in the PD-1+CTLA-4− subset compared with the PD1+CTLA-4+ subset (Fig. 2B; p<0.05). The PD1+CTLA-4+ CD8 T cells displayed higher levels of other inhibitory receptors such as 2B4, LAG-3, and TIM-3, along with lower levels of CD62L, and CD127 (Fig. 2B; p<0.01). These results therefore indicate that dysfunction of CD8+ T cells in tumor was associated with coexpression of PD-1 and CTLA-4.
Next, we tested whether checkpoint blockade could rescue the proliferation of double-positive cells and/or enhance the proliferation of single-positive cells. We isolated PD-1+CTLA-4+ and PD-1+CTLA-4− CD8+ CT26 and ID8-VEGF TIL subsets and co-cultured them with AH-1 or FR peptide pulsed splenocytes (APCs) ± αPD-1 and/or αCTLA-4 antibodies. Again, in the absence of any neutralizing antibodies we observed modest proliferation of the PD-1+CTLA-4− CD8+ TIL and no proliferation of the PD-1+CTLA-4+ CD8+ TIL (Fig. 2C; p=0.019 and p=0.015 respectively). PD-1 blockade alone was able to enhance the proliferation of both PD-1+CTLA-4− and PD-1+CTLA-4+ CD8+ TIL, although it was unable to completely restore the proliferation of double-positive cells. CTLA-4 blockade alone was able to enhance the proliferation only of PD-1+CTLA-4+ CD8+ TIL, and had no effect on CTLA-4− cells. However, dual blockade fully restored the proliferation of PD-1+CTLA-4+ CD8+ TIL (Fig. 2C; p=0.038 and p=0.004, respectively). Thus, the double-positive population represents cells with severely dysfunctional status, but their function can be rescued by double blockade.
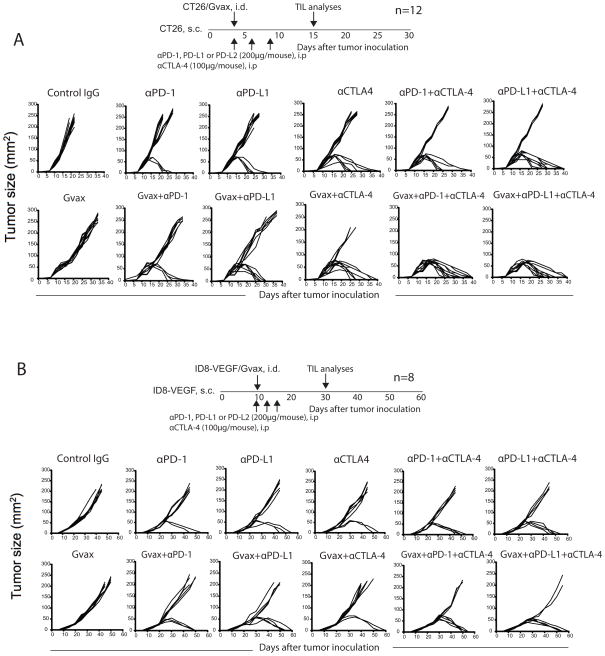
Combination therapy promotes CT26 and ID8-VEGF tumor rejection
Given that a significant proportion of tumor-reactive CD8+ TILs were found to be PD-1+CTLA-4+, and given that their proliferation in response to cognate tumor antigen was restored by double blockade in vitro, we tested whether simultaneous blockade of PD-1 and CTLA-4 could promote tumor rejection in mice. We found that treatment with αPD-1 or αPD-L1 showed CT26 tumor regression in 25% and 33% of the mice, respectively (Fig. 3A; Table 1). CTLA-4 blockade alone led to tumor regression in 50% of the mice. Further, we noticed that combination of either αPD-1 or αPD-L1 with αCTLA-4 induced tumor regression in 75% of mice (p<0.01). Similarly, we found that treatment with αPD-1, αPD-L1 or αCTLA-4 showed ID8-VEGF tumor regression in 25%, 37.5%, and 25% of the mice, respectively (Fig. 3B; Table 1). Combined blockade induced tumor regression in 50% of mice. In contrast, αPD-L2 blockade did not induce tumor regression in both models. Further, in vivo CD4+ and CD8+ depletion completely abolished tumor regression (Supplementary Fig. 3).
Figure 3. In vivo PD-1 and CTLA-4 blockade promotes CT26 and ID8-VEGF tumor rejection in mice.
Regression and survival (<200 mm2) of CT26 (A) and ID8-VEGF (B) inoculated mice following treatment as described in the Methods section. Figures represent one of the 5 (CT26) and 3 (ID8-VEGF) independent experiments.
Table 1.
Cumulative results of all mice inoculated with either CT26 and ID8-VEGF tumors.
| Treatment Groups | CT26 | ID8-VEGF |
|---|---|---|
| Control IgG | 0 | 0 |
| αPD-1 | 25 | 25 |
| αPD-L1 | 33 | 37.5 |
| αCTLA-4 | 50 | 25 |
| αPD-1+αCTLA-4 | 75* | 50 |
| αPD-L1+αCTLA-4 | 75* | 50 |
| GVAX | 0 | 0 |
| GVAX + αPD-1 | 42 | 37.5 |
| GVAX + αPD-L1 | 50 | 50 |
| GVAX + αCTLA-4 | 75* | 37.5 |
| GVAX + αPD-1+ αCTLA-4 | 100* | 75** |
| GVAX + αPD-L1+ αCTLA-4 | 100* | 75** |
p values among different treatments were calculated using the log-rank test
P ≤ 0.05;
P ≤ 0.01.
Hypothesizing that the effects of inhibitory receptor blockade could be enhanced by increasing the frequency of tumor-reactive T cells, we combined our treatment with GM-CSF transduced whole tumor cells (GVAX) (50). Remarkably, 100% of CT26 bearing mice and 75% of ID8-VEGF bearing mice in the triple combination groups (GVAX/αPD-1/αCTLA-4) rejected their tumors (Fig. 3A & B; Table. 1). Thus, double checkpoint blockade has the potential to induce significant tumor rejection, which could be maximized by boosting antitumor immune response with vaccine.
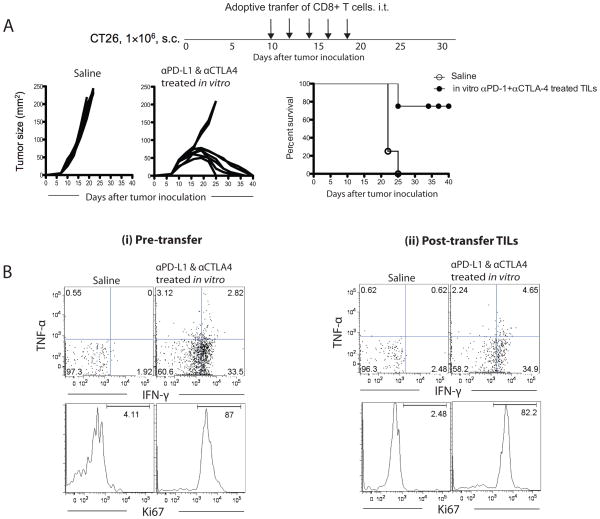
Adoptive transfer of in vitro pre-treated CD8+CTLA-4+PD-1+ TILs with αPD-1 and αCTLA-4 antibodies cause regression of CT26 tumor in mice
Next we tested whether CD8+CTLA-4+PD-1+ TILs treated in vitro with αPD-1 and αCTLA-4 would kill the tumor cells in vivo. We sorted CD8+CTLA-4+PD-1+ TILs from CT26 tumor and cultured in the presence of α-PD-1 and α-CTLA-4 blocking antibodies and AH-1 peptide for 7 days. We found that in vitro treated cells showed profound increase in IFN-γ cytokine production and Ki-67 expression (Fig. 4B; left panel). These in vitro treated cells were adoptively transferred into CT26 tumor bearing mice as described in the Methods. We found that adoptive therapy resulted in tumor regression in 75% of mice (Fig. 4A). In addition, we found that the adoptively transferred TILs were still functional (IFN-γ+Ki67+) in the regressing tumors a week after the treatment (Fig. 4B; right panel).
Figure 4. Therapeutic adoptive transfer of in vitro α-PD-1 and α-CTLA-4 pre-treated TILs cause regression of CT26 tumors in mice.
A) Tumor regression in mice transferred with in vitro expanded CT26 antigen-specific CD8+CTLA-4+PD-1+ CT26 TILs. (B) The percent of IFN-γ+ and Ki67+ of in vitro pre-treated CD8+ T cells just before adoptive transfer (left panel) and the TILs recovered from tumor one week after the final transfer (right panel) are shown.
Combination blockade increases TIL activation and antigen-specific inflammatory cytokine production
Next, we tested whether tumor regression following in vivo blockade was associated with activation of TIL. We resected and analyzed tumors (n=8) that showed evidence of rejection from 13–15 day onwards. As expected, Ki-67 expression on TIL was markedly increased following PD-1, PD-L1, or CTLA-4 monotherapy (p=0.001). Confirming our observation in vitro (Fig. 2) combination of αPD-1 with αCTLA-4 doubled the frequency of Ki-67+CD8+ T cells (Fig. 5A; p=0.002), while αPD-L1 and αCTLA-4 combination increased the frequency of Ki-67+CD8+ T cells by 50% (p=0.012) compared with single antibody blockade (Fig. 5A). Combined blockade also increased granzyme B expression, suggesting an enhancement in cytolytic potential of effector T cells (Fig. 5B; p=0.01).
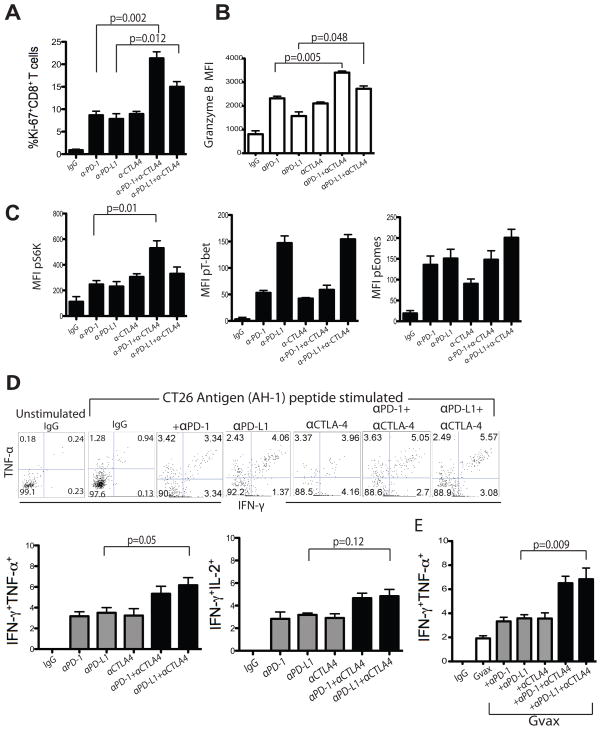
Figure 5. In vivo PD-1 and CTLA-4 blockade increases TIL activation and CT26 antigen-specific inflammatory cytokine production.
Summary data showing percentage of Ki-67+ CD8+ TILs (A) and mean fluorescence intensity (MFI) of granzyme B+ CD8+ TILs (B) from mice treated with various antibodies, as indicated. C) Flow cytometry analysis showing MFI of pT-bet, pEomes and pS6K expression by CD8+ TIL from treated mice. Representative and summary data showing IFN-γ and TNF-α production by CD8+CD45+ TIL from antibody (D) as well as GVAX ± antibody (E) treated mice 13–15 days following tumor inoculation. Statistical significance was determined by Student’s t-test.
We corroborated the above findings by testing the phosphorylation status of key transcription factors by flowcytometry (51–52). The ribosomal S6 kinase (S6K) is implicated in IL-2 induced T cell proliferation (53). Validating the increased expression of Ki-67 by TIL, we observed an increase in the expression of phosphorylated (p)-S6K in CD8+ T cells following single antibody (αPD-1) blockade, and combined PD-1 and CTLA-4 blockade further enhanced pS6K levels (p=0.01; Fig. 5C). T-bet and Eomes are two T-box transcription factors regulating Th1 and cytolytic function of CD8+ cells (54–56). αPD-L1 antibody alone induced high levels of pT-bet, whereas αPD-1 or αCTLA-4 alone or combined showed moderate increases in pT-bet levels (Fig. 5C). Validating the enhanced granzyme B expression, blocking antibodies increased pEomes expression levels (Fig. 5C). Thus, checkpoint blockade induced TIL activation, and combined αPD-1 and αCTLA-4 therapy significantly increased proliferation and cytolytic function of TIL relative to monotherapy.
We next tested whether the functional capacity of tumor-reactive CD8+ TIL increased following single versus double blockade. CT26 tumors from the various treatment groups were dissociated, and mixed cells from these tumors were seeded in primary co-cultures with AH-1 peptide. Cultures derived from single antibody (PD-1, PD-L1, or CTLA-4) treated mice showed modestly increased IFN-γ+TNF-α+ or IFN-γ+IL-2+ CD8+ TILs (Fig. 5D; p=0.05). Cultures from combined αPD-1 and αCTLA-4 treatment, exhibited increased numbers of IFN-γ+TNF-α+ (Fig. 5D; p=0.05), but not IFN-γ+IL-2+ CD8+ TILs (Fig. 5D;p=0.12). These data suggest that combination blockade induces polyfunctional activation of tumor antigen-specific CD8+ T cells. As expected, combining GVAX with αPD-1, αPD-L1, or αCTLA-4 inhibitory blockade further increased the frequency of CT26-specific IFN-γ+TNF-α+ CD8+ TIL (Fig. 5E; p=0.009).
in vivo PD-1 and CTLA-4 blockade enhances infiltration of T cells and reduces Tregs in tumors
As expected based on the above experiments, combined blockade significantly increased total inflammatory infiltration and CD8+ cells in tumors, but also CD4+ cell infiltration (Fig. 6A; p=0.002, p=0.025, and p=0.001 respectively). Then we tested whether the effects of inhibitory receptor blockade could be further enhanced by depleting Tregs. Interestingly, we could not find any additional benefit by combining αCD25 antibody with blocking antibodies (Supplementary Fig. 4). This suggested that blocking antibodies could independently affect Treg accumulation in tumors as well as tumor draining lymph nodes (Supplementary Fig. 5).
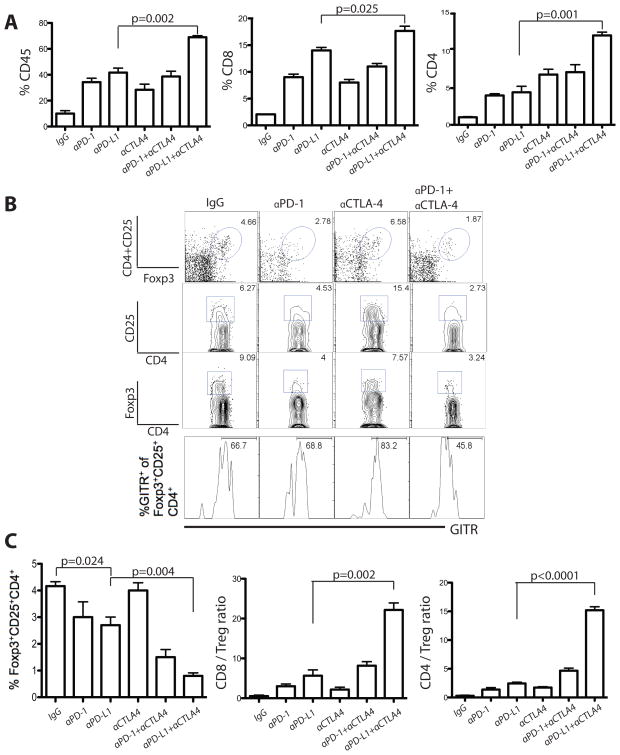
Figure 6. PD-1 and CTLA-4 blockade enhance lymphocyte infiltration and reduce the frequency of Tregs in CT26 tumors.
A) CD45+ leukocyte infiltration, and CD8+ and CD4+ TIL as percent of total CD45+ cells 13–15 days after tumor inoculation. B) Representative data showing the percentage of Tregs and expression of GITR among Tregs. C) Percentage of CD45+ TILs of Tregs, and the ratios of CD8+ or CD4+ effector T cells to Tregs in the tumors of treated mice with indicated blocking antibodies. Values are shown for mice analyzed independently and the sum of 3 independent experiments with 6–12 mice per group. Statistical significance was determined by Student’s t-test.
To test this hypothesis, we analyzed the frequency of Tregs in tumors of mice following single or double blockade. PD-1 or PD-L1 blockade alone produced a moderate reduction in Treg levels in the tumor (Fig. 6B and 6C; p=0.024), while CTLA-4 blockade did not affect Treg numbers. However, combined blockade profoundly reduced the frequency of Tregs (from 9.06% to 3.24% of CD4+; Fig. 6B and 6C; p=0.004). As a result, the CD8/Treg and CD4/Treg ratios increased markedly, especially after combined αPD-L1 and αCTLA-4 treatment.
We asked whether the reduction in Treg frequency was due to apoptosis. None of the blocking antibodies affected the apoptosis rate in Tregs (based on Annexin-V and 7-AAD staining - data not shown). Importantly, combined αCTLA-4 and αPD-1 reduced GITR (a functional marker of activated Tregs) expression from 66.7% to 45.8% of Tregs (Fig. 6B; p<0.001), indicating that double blockade could affect not only the number but also the function of tumor-infiltrating Tregs.
Co-expression of PD-1 and CTLA-4 by Tregs is associated with heightened T cell dysfunction in tumor
Since we found approximately one-fourth of the CD4+ TIL expressing PD-1 and/or CTLA-4 (Fig. 1), we first examined whether these CD4+ TIL are effector cells or Tregs. We found that Tregs were primarily comprised in the CTLA-4+ CD4+ population. Importantly, among the CTLA-4+ cells, most were Foxp3+CD25+ cells, representing activated effector Tregs (30, 35, 37), and were PD-1+CTLA-4+ double-positive cells (Fig. 7A). Notably, splenic Tregs expressed much lower levels of PD-1 and CTLA-4 (Fig. 7A). We also observed not only increased expression of CTLA-4, but also of inducible T cell co-stimulator (ICOS) and folate receptor 4 (FR-4) expression on PD-1hi Treg compared to PD-1lo Treg (Fig. 7B; p=0.001, p=0.001 and p=0.034, respectively), indicating an activated functional status. Thus, tumor Treg were mostly CTLA-4+ and among them, the PD-1+ Tregs appeared to be activated Tregs.
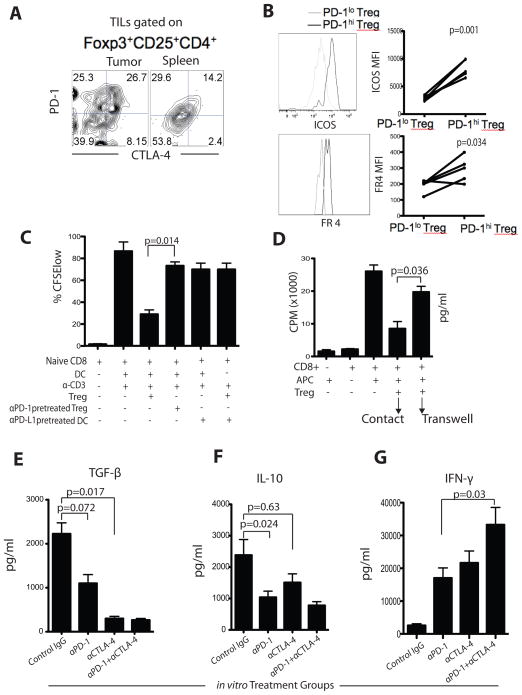
Figure 7. PD-1 and CTLA-4 pathway blockade reduces Treg-mediated suppression of CD8+ T cells in vitro and secretion of regulatory cytokines TGF-β and IL-10.
A) Representative expression of PD-1 and CTLA-4 by intra-tumoral and splenic Tregs in the same mouse. B) ICOS and folate receptor 4 (FR4) expression and MFI in PD-1hi and PD-1lo Tregs. C) TIL Tregs (± αPD-1) were co-cultured with CFSE-labeled CD8+ T cells and DCs (pretreated) in the presence of αCD3. Dividing cells were quantified after 4-days of culture by gating on the CFSElo population. D) [3H]-thymidine incorporation into effector T cells in the presence or absence of TIL Tregs. Tregs were either in direct contact with the effector cells or were separated by a transwell membrane. The whole tumor leucocytes were isolated and cultured ex vivo for 72 hours in the presence of AH-1 peptide and PD-1 and CTLA-4 blocking antibodies as indicated. After culture, supernatants were analyzed for secretion of TGF-β (E), IL-10 (F), and IFN-γ (G). Results are from 3 representative experiments. Bar graphs show mean ± SD.
To understand the specific contribution of PD-1+ Treg to the suppressive program, CD4+CD25+CTLA-4+ Tregs were preincubated with PD-1 blocking antibody or control IgG. Control Tregs or PD-1-neutralized Tregs were then incubated with CD8+ T cells stimulated by immobilized αCD3 antibody (responder cells) in the presence of splenic APCs (CD11c+ cells) or PD-L1 neutralized tumor-derived APCs. Responder T cell proliferation was assessed by CFSE dilution. Whereas control Tregs were able to suppress responder cell proliferation, PD-1 neutralized Tregs as well as PD-L1-neutralized tumor-derived APCs were unable to suppress CD8+ T cell proliferation (Fig. 7C, p=0.0014). Thus, the suppressive function of tumor-derived Tregs is critically dependent on PD-1 signaling, compatible with the notion that the PD-1+CTLA-4+ Tregs are responsible for the suppressor function.
Next, we tested whether PD-1 blockade could attenuate the suppressive phenotype of Tregs and their regulatory cytokine programming (Fig. 7E–G). The whole tumor digests from various in vivo treatment groups were co-cultured with AH-1 peptide for 5 hours, and we measured TGF-β, IL-10 and IFN-γ in the supernatants. We detected significant amounts of TGF-β and IL-10 and minimal IFN-γ in leukocyte co-cultures form untreated mice, indicating ongoing activity of Tregs and no reactivity of antitumor effector cells. TGF-β secretion was suppressed ex vivo following αPD-1 treatment, and more effectively after αCTLA-4 treatment in vivo (Fig. 7E; p=0.017). In contrast, IL-10 secretion was decreased ex vivo following αPD-1 treatment, but not after αCTLA-4 treatment in vivo (Fig. 7F; p=0.024). Furthermore, dual αPD-1 and αCTLA-4 blockade elevated AH-1-specific IFN-γ induction (Fig. 7G; p=0.03). Thus, checkpoint blockade reduces the ability of Tregs to secrete immunoregulatory cytokines.
Discussion
In this study, we demonstrated a non-redundant regulation of CD8+ T cell exhaustion by PD-1 and CTLA-4 in CT26 colon carcinoma and ID8-VEGF ovarian carcinoma models. About one fourth of CD8+ TIL expressed both PD-1 and CTLA-4, and this was associated with more severe CD8+ T cell exhaustion. Blockade of either PD-1 or CTLA-4 pathways enhanced effector T cell infiltration into the tumor. However, simultaneous blockade of both pathways had synergistic effects in activation of tumor antigen specific CD8+ and CD4+ effector T cells as well as affecting Tregs, ultimately improving long-term survival rates. Adoptive transfer of CD8+CTLA-4+PD-1+ TILs that had been pre-treated in vitro with αPD-1 and αCTLA-4 antibodies eliminated CT26 tumors in vivo, further demonstrating the role of the PD-1 and CTLA-4 pathways in the tumor.
These effects were further augmented through combination with a GVAX vaccine. Combining blockade therapy with a single dose of GVAX vaccination resulted in enhanced intratumoral CD45+ and CD8+ T cells and as a result, the CD8/Treg ratios increased markedly. Moreover, mice treated with αPD-1/CTLA-4 combined with GVAX further increased IFN-γ+ TNF-α+ CD8+ TILs (Fig. 5E) resulting in enhanced tumor rejection. Hence our results suggest that the mechanisms underlying the synergistic effects of vaccine could possibly be due to the capacity of vaccine to increase the frequency of tumor-reactive CD8+ TILs, which could be activated by CTLA-4 and PD-1 blockade. The levels of GM-CSF produced by CT26/GVAX and ID8-VEGF/GVAX vaccines are comparable to the previously published preclinical studies using B16/GVAX and Glioma/GVAX (2, 57). The lack of in vivo efficacy of PD-1/PD-L1 blockade with GVAX in B16 melanoma seen by Curran et al. (2) may be due to the fact that the B16 tumor line does not express PD-L1, whereas CT26 and ID8-VEGF cells express high levels. Our results are also in line with previous observations that higher dose of GVAX recruited MDSCs into the tumor (2, 57).
The PD-1/PD-L1 pathway mediates T cell exhaustion by antagonizing activation signaling pathways (28–29, 58–62). We investigated our therapy’s ability to counteract these effects by comparing the levels of key signaling molecules in different treatment groups. First we looked at T-bet, which is expressed preferentially by type 1 effector T cells, and Eomes, which is expressed in memory T cells (61–62). We also monitored the S6K which, when phosphorylated, acts downstream of mTOR to activate cell proliferation, protein translation, and survival (53). In untreated mice, we found that tumor infiltrating T cells showed no or very low expression of pT-bet, pEomes, or pS6K. However, upon single αPD-L1 antibody treatment we observed a marked increase in pT-bet expression. αPD-L1 combined with αCTLA-4 antibody further increased pEomes, and αPD-1 combined with αCTLA-4 blockade further increased pS6K. That our treatments led to increased levels of these phosphorylated signaling molecules supports the contention that we were activating classical anti-tumor effector T cells. However, the differential effects of combining αCTLA-4 with αPD-1 versus αPD-L1 could be because αPD-1 blocks the inhibitory contributions of PD-1 binding to PD-L1 and PD-L2 but leaves the PD-L1/B7-1 inhibitory interaction unaffected (24–25). In contrast, αPD-L1 blocks both the PD-L1/PD-1 and the PD-L1/B7-1 inhibitory pathways but leaves the PD-1/PD-L2 inhibitory interaction unaffected (24–25).
Our experiments with sorted PD-1+CTLA-4− and PD-1+CTLA-4+ cells showed that CT26 or ID8-VEGF antigen-specific CD8+ T cells were exclusively located in the double-positive population and were highly dysfunctional, highlighting the relevance of these receptors in escape from the anti-tumor T cell response. Studies report that PD-1 mediates inhibition by blocking PI3K activation, whereas CTLA-4 functions through binding to the phosphatase PP2A, leading to inhibition of Akt phosphorylation (29, 59, 63–64). Our study proves that double blockade targets both pathways resulting in an additive response by targeting both PD-1 and CTLA-4 signaling molecules allowing more effective T-cell activation, proliferation and function of TILs, and further augmentation of therapeutic effect. Taken together, our results indicate that PD-1 and CTLA-4 pathways act non-redundantly to inhibit TIL function. This is an important lesson to bring to the clinic, highlighting why blockade of both pathways may be essential to completely regain T cell function.
Our therapy’s ability to limit the Treg population in vivo is highly desirable. Here we found simultaneous expression of PD-1 and CTLA-4 on Tregs and distinct effects of blocking each pathway. The importance of the PD-1 pathway for Tregs, however, is not well characterized, although one recent study described PD-L1 expression by Tregs (36). While PD-1 enhanced in vivo Treg function, CTLA-4 blockade did not impact the suppressive function of Tregs in CT26 in vivo (Figs. 6). However, our findings indicate that complete restoration of CD8+ T cell proliferation required combined αPD-1 and αCTLA-4 blockade both in vivo as well as in vitro (Figs. 6 and 7). In our models, PD-L1 on Tregs might bind directly to PD-1 on CD8+ T cells (cell-autonomous), or it may indirectly activate nearby DCs by reversing suppression through PD-1, allowing the induction of a stronger adaptive immune response (non-autonomous). We investigated which of these mechanisms was occurring. We found that blocking either PD-1 or PD-L1 on Tregs led to an increase in CD8+ T cell proliferation (Fig. 7C). Also, in the absence of PD-L1 depleted APCs, the Tregs were not able to suppress CD8+ T cell proliferation (Fig. 7C). This demonstrates that PD-1 expression by effector T cells and Tregs, as well as PD-L1 expression by APCs, are all involved in the suppression of CD8+ TIL responses. We also found that dual blockade reduces production of other immunoinhibitory cytokines including TGF-β and IL-10. This provides further novel insights into the mechanisms of dual blockade.
αCTLA-4 has already been approved by FDA for treatment of melanoma. The antitumor activity of αPD-1 and αPD-L1 are evident in animal models, and αPD-1 and αPD-L1 antibodies are now under intense clinical evaluation (65–67). It will be critical to understand the direct effects of blockade on effector T cells as well as Tregs. Our work suggests that administration of αPD-1 antagonists may harness the therapeutic potential of effector T cells in vivo while concomitantly suppressing the functions of activated Tregs. Our study provides the scientific basis for a clinical trial that would involve combination of tumor-cell vaccination and simultaneous PD-1 and CTLA-4 blockade for sustained tumor control in cancer patients.
Supplementary Material
Acknowledgments
We thank Drs. James Allison and Michael Curran for providing GM-CSF plasmids. This work was supported by NIH grant 2P50-CA083638 (GC), SPORE-Pilot grant (JD), and NIH-P01 AI056299 (GF).
Abbreviations
- PD-1
programmed death-1
- PD-L
PD-1 ligand
- CTLA-4
cytotoxic T-lymphocyte antigen-4
- CT26
colon carcinoma
- ID8
ovarian carcinoma
- Tregs
regulatory CD4+ T cells
- GVAX
Tumor cells transduced to express GM-CSF
Footnotes
Disclosures
G.F. draws royalties from patents regarding PD-1.
Contributions
J.D. and G.C. designed the experiments; G.F. provided the reagents; J.D. performed the experiments, analyzed the data and wrote the manuscript; G.C, G.F, and K.K. edited the manuscript.
References
- 1.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2010;439:682–87. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 2.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: Their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 5.Brown JA, Dorfman DD, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of PD-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 6.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 7.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA-4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709–15. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 9.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–65. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–57. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 11.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of αCTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mumprecht S, Schürch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–36. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107:7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–30. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandalaft LE, Motz GT, Duraiswamy J, Coukos G. Tumor immune surveillance and ovarian cancer: lessons on immune mediated tumor rejection or tolerance. Cancer Metastasis Rev. 2011;30:141–51. doi: 10.1007/s10555-011-9289-9. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Stolina M, Lin Y, Gardner B, Miller PW, Kronenberg M, Dubinett SM. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol. 1999;163:5020–28. [PubMed] [Google Scholar]
- 18.Chen WJ, Jin W, Wahl SM. Engagement of CTLA-4 induces TGF-β production by murine CD4+ T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function . Science. 2008;322:271–75. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 23.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–27. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7–1 and B7–2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;3:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 28.Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, et al. Modulation of TCR induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci USA. 2002;99:11790–95. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curiel TJ. 2007 Treg cells and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–74. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsui J, Nishikawa H, Muraoka D, Wang L, Noguchi T, Sato E, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–91. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 32.Hodi FS, Butler M, Oble DA, Wang L, Noguchi T, Sato E, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karman J, Jiang JL, Gumlaw N, Zhao H, Campos-Rivera J, Sancho J, et al. Ligation of cytotoxic T lymphocyte antigen-4 to the TCR inhibits T cell activation and directs differentiation into FOXP3+ regulatory T cells. J Biol Chem. 2012;287:11098–107. doi: 10.1074/jbc.M111.283705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q, Munger ME, Highfill SE, Tolar J, Weigel BJ, Riddle M, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–93. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–77. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 38.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–71. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: Implications for CD8 T-cell exhaustion. J Virol. 2010;84:2078–89. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–38. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, Restifo NP. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154(9):4685–92. [PMC free article] [PubMed] [Google Scholar]
- 44.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21(4):585–91. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Yang N, Garcia JR, Mohamed A, Benencia F, Rubin SC, Allman D, Coukos G. Generation of a syngeneic mouse model to study the effects of vascular endothelial growth factor in ovarian carcinoma. Am J Pathol. 2002;161(6):2295–309. doi: 10.1016/s0002-9440(10)64505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, Wagner DS, Katsaros D, Caroll R, Coukos G. 2004 Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–8. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 47.Costa GL, Benson JM, Seroogy CM, et al. Targeting rare populations of murine antigen-specific T lymphocytes by retroviral transduction for potential application in gene therapy for autoimmune disease. J Immunol. 2000;164:3581–90. doi: 10.4049/jimmunol.164.7.3581. [DOI] [PubMed] [Google Scholar]
- 48.Huang AY, Gulden PH, Woods AS, Achacoso P, Fathman CG, Nolan GP. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730–35. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, Goode EL, Kalli KR, Knutson KL. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol. 2011 Jun 15;186(12):6905–13. doi: 10.4049/jimmunol.1100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene. 2003;22:3188–92. doi: 10.1038/sj.onc.1206459. [DOI] [PubMed] [Google Scholar]
- 51.Krutzik PO, Clutter MR, Nolan GP. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J Immunol. 2005;175:2357–65. doi: 10.4049/jimmunol.175.4.2357. [DOI] [PubMed] [Google Scholar]
- 52.Irish JM, Kotecha N, Nolan GP. Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nat Rev Cancer. 2006;6:146–55. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- 53.Saitoh M, Pullen M, Brennan P, Cantrell D, Dennis PB, Thomas G. Regulation of an activated S6 kinase 1 variant reveals a novel mammalian target of rapamycin phosphorylation site. J Biol Chem. 2002;277:20104–12. doi: 10.1074/jbc.M201745200. [DOI] [PubMed] [Google Scholar]
- 54.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–71. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 56.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–19. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 57.Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT., Jr Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012;35:385–9. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 59.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–25. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang SF, Fouquet S, Chapon M, Salmon H, Regnier F, Labroquère K, et al. Early T cell signalling is reversibly altered in PD-1+ T lymphocytes infiltrating human tumors. PLoS One. 2011 Mar 7;6(3):e17621. doi: 10.1371/journal.pone.0017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wherry EJ, Ha S-J, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Quigly M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–51. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci USA. 2012;109:6187–92. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37:430–39. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brahmer JR, Tykodu SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.