Abstract
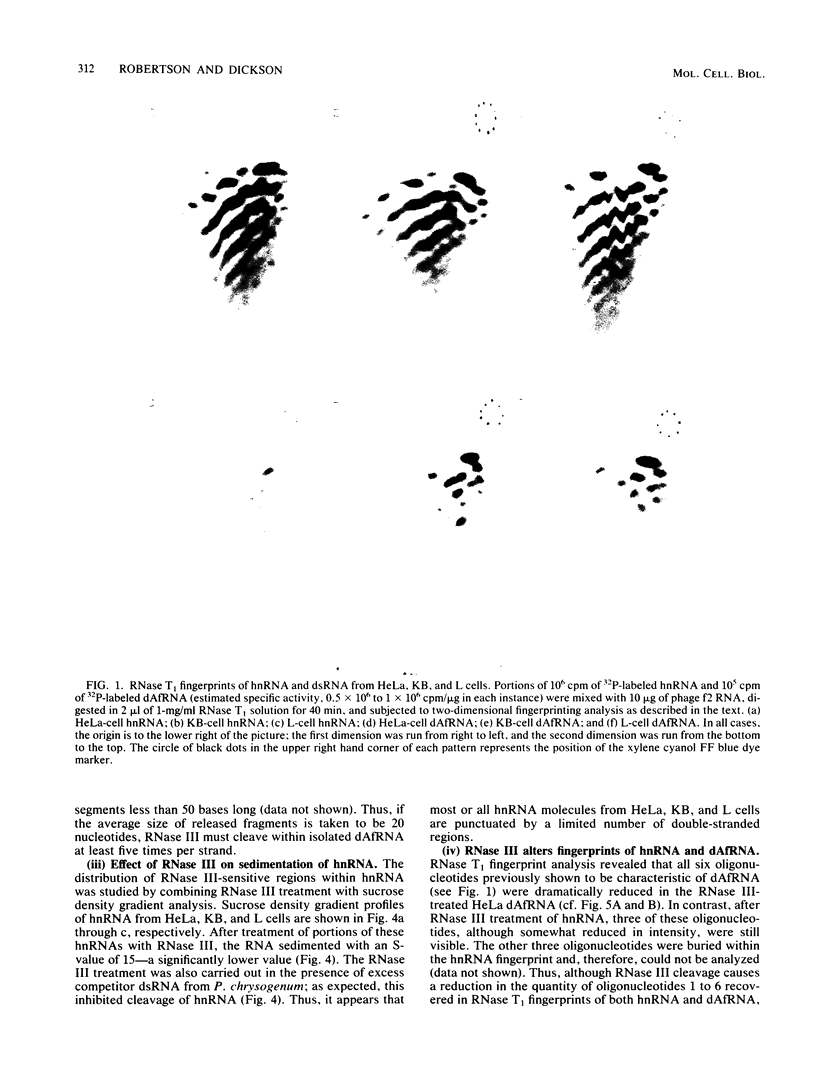
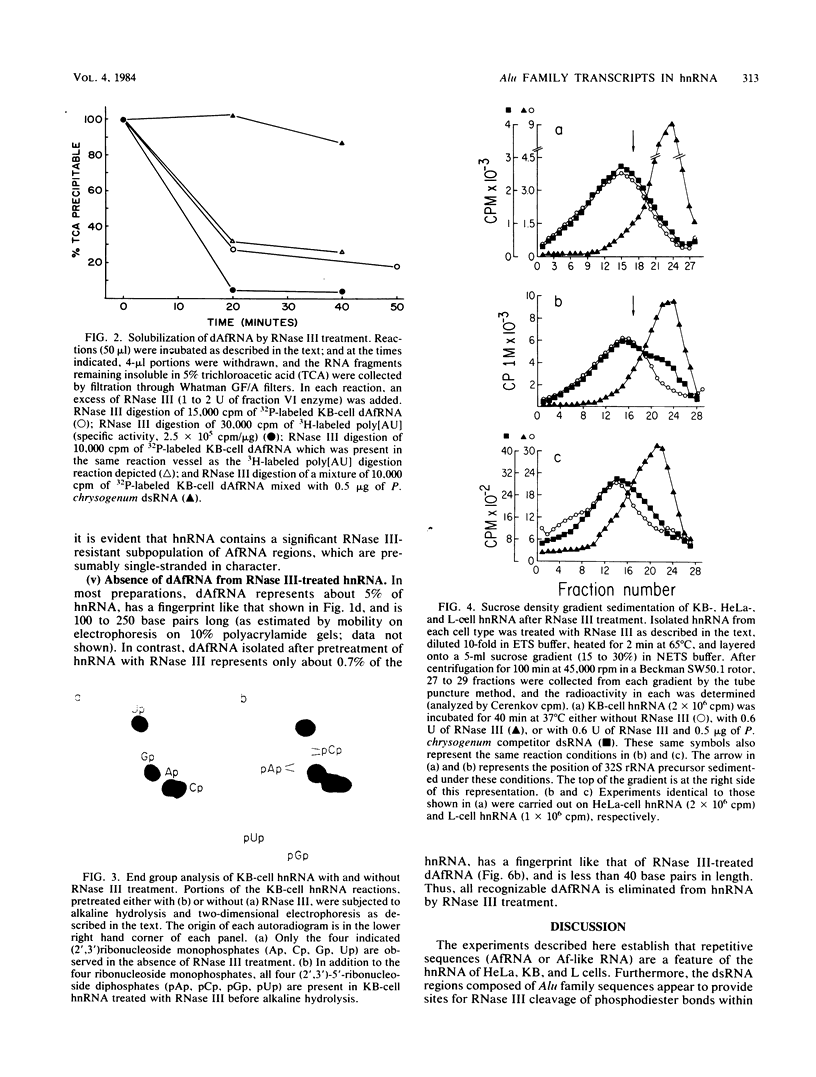
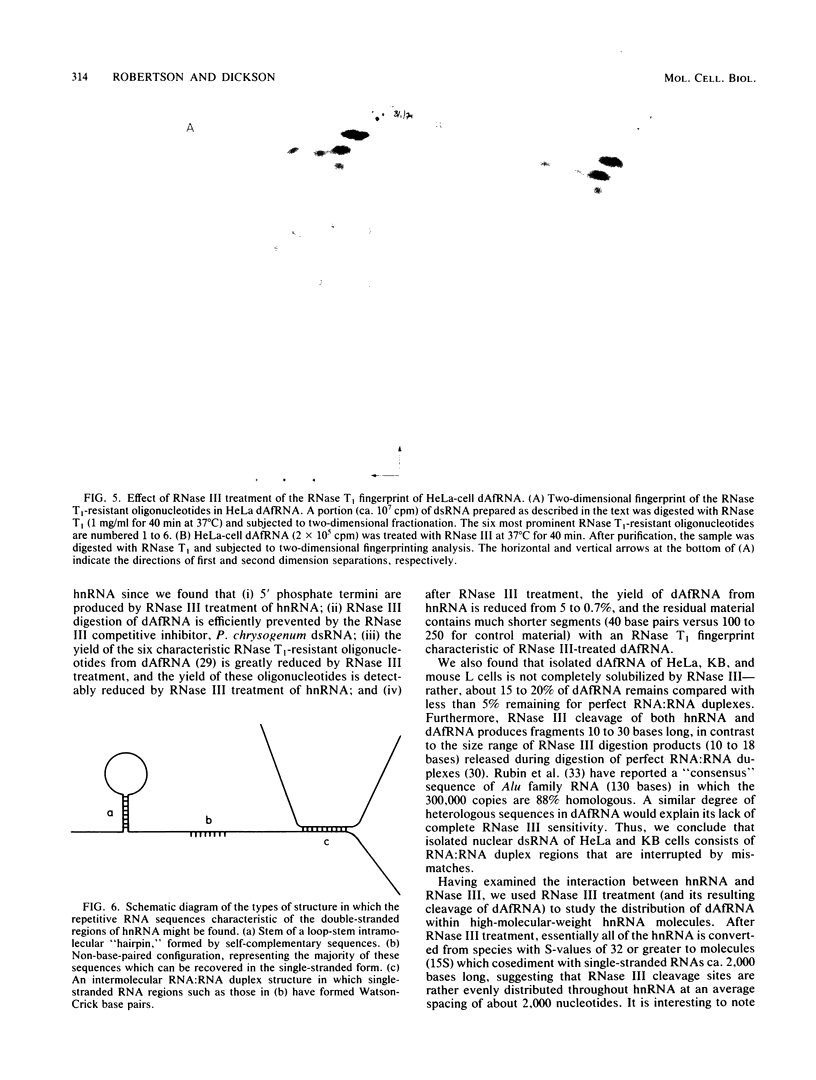
We studied the distribution of repetitive sequence elements capable of forming double-stranded regions in nuclear RNA of HeLa, KB, and L cells. In human RNA populations, we called these regions duplex Alu family RNA (dAfRNA) because they represent transcripts of the highly reiterated family of DNA regions known as "Alu family DNA" (Rubin et al., Nature (London) 284:372-374, 1980). Although the dAfRNA populations of both human cell lines (HeLa and KB) have low sequence complexity, they represent 5% of the total heterogeneous nuclear RNA and have identical fingerprints; mouse L-cell dAf-like RNA (which has a similar complexity) represents only 2% of the total heterogeneous nuclear RNA and has an entirely different fingerprint. We utilized Escherichia coli RNase III as a highly specific reagent for the recognition of RNA:RNA duplex structure. This enzyme cleaves within the six characteristic RNase T1-resistant oligonucleotides of HeLa- and KB-cell dAfRNA (Robertson et al., J. Mol. Biol. 115:571-589, 1977). In addition, the size of heterogeneous nuclear RNA from all three cell types is reduced from greater than 32S to about 15S after RNase III treatment. We conclude that this size shift is a result of cleavage within dAfRNA regions and that such regions are present in most or all of the large RNA transcripts of these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Nucleoprotein organization of inverted repeat DNA transcripts in heterogeneous nuclear RNA-ribonucleoprotein particles from HeLa cells. J Mol Biol. 1978 Jul 5;122(3):361–378. doi: 10.1016/0022-2836(78)90195-x. [DOI] [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Deininger P. L., Schmid C. W. A study of the evolution of repeated DNA sequences in primates and the existence of a new class of repetitive sequences in primates. J Mol Biol. 1979 Feb 5;127(4):437–460. doi: 10.1016/0022-2836(79)90231-6. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N., Wellauer P. K., Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977 Apr;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Yehle C. O., Robertson H. D., Dickson E. Specific RNA-cleaving activities from HeLa cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2395–2399. doi: 10.1073/pnas.77.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel L., Montagnier L. Homology of double stranded RNA from rat liver cells with cellular genome. Nat New Biol. 1971 Jan 27;229(4):106–108. doi: 10.1038/newbio229106a0. [DOI] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R. Inverted repeated DNA from Chinese hamster ovary cells studied with cloned DNA fragments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2679–2683. doi: 10.1073/pnas.75.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Evans R., Wilson M., Salditt-Georgieff M., Darnell J. E. Oligonucleotides in heterogeneous nuclear RNA: similarity of inverted repeats and RNA from repetitious DNA sites. Biochemistry. 1978 Jul 11;17(14):2776–2783. doi: 10.1021/bi00607a012. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Molloy G., Fernandez-Munoz R., Salditt M., Darnell J. E. Secondary structure in heterogeneous nuclear RNA: involvement of regions from repeated DNA sites. J Mol Biol. 1974 Jan 25;82(3):361–370. doi: 10.1016/0022-2836(74)90597-x. [DOI] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Kronenberg L. H., Humphreys T. Double-stranded ribonucleic acid in sea urchin embryos. Biochemistry. 1972 May 23;11(11):2020–2026. doi: 10.1021/bi00761a005. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Robertson H. D. Initiator regions from the small size class of reovirus messenger RNA protected by rabbit reticulocyte ribosomes. J Biol Chem. 1977 Nov 10;252(21):7842–7849. [PubMed] [Google Scholar]
- Montagnier L. Présence d'un acide ribonucléique en double chaîne dans des cellules animales. C R Acad Sci Hebd Seances Acad Sci D. 1968 Oct 21;267(17):1417–1420. [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Robertson H. D., Baglioni C. Heterogeneous nuclear RNA promotes synthesis of (2',5')oligoadenylate and is cleaved by the (2',5')oligoadenylate-activated endoribonuclease. Mol Cell Biol. 1982 Feb;2(2):154–160. doi: 10.1128/mcb.2.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieczenik G., Model P., Robertson H. D. Sequence and symmetry in ribosome binding sites of bacteriophage f1 RNA. J Mol Biol. 1974 Dec 5;90(2):191–124. doi: 10.1016/0022-2836(74)90368-4. [DOI] [PubMed] [Google Scholar]
- Planterose D. N., Birch P. J., Pilch D. J., Sharpe T. J. Antiviral activity of double stranded RNA and virus-like particles from Penicillium stoloniferum. Nature. 1970 Aug 1;227(5257):504–505. doi: 10.1038/227504a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Jelinek W. Determination of nucleotide sequences from double-stranded regions of HeLa cell nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):571–589. doi: 10.1016/0022-2836(77)90103-6. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Robertson H. D., Hunter T. Sensitive methods for the detection and characterization of double helical ribonucleic acid. J Biol Chem. 1975 Jan 25;250(2):418–425. [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]